Dielectric Responses of Cytosolic Water Change with Aging of Circulating Red Blood Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. RBCs Preparation
2.3. Dielectric Measurements
2.4. RBC Deformability Measurements
2.5. Characterization of Hematological Indices
3. Results
3.1. Spectral Characteristics of the Cells in the Fractions (Figure 1 and Figure 2 Modified with the Respective Controls and Parameters)
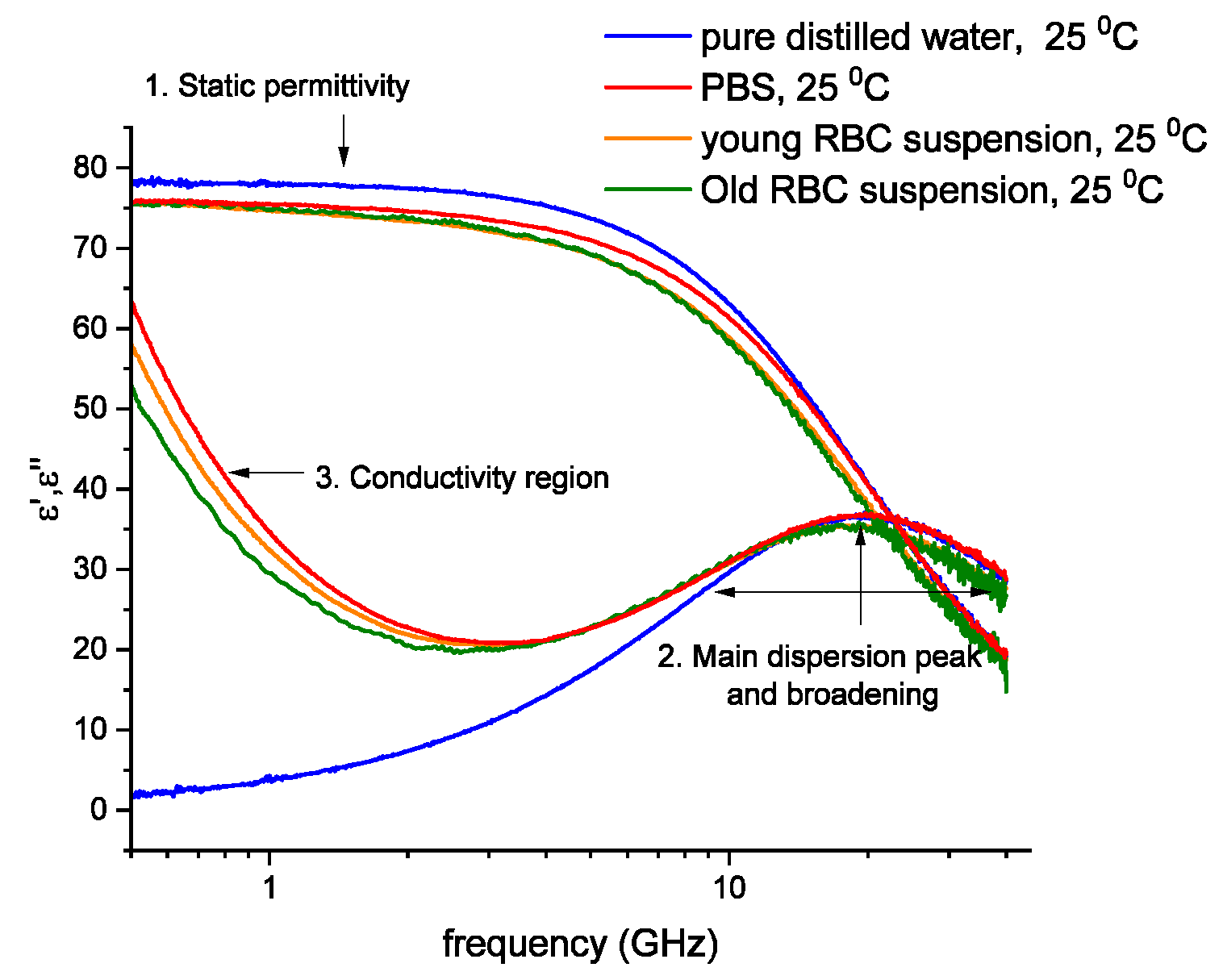
3.2. Does the Separation of RBCs in Percoll Cause Alteration in Cell Features?
3.3. Hematological Indices of RBC in Fractions of Cells of Different Age
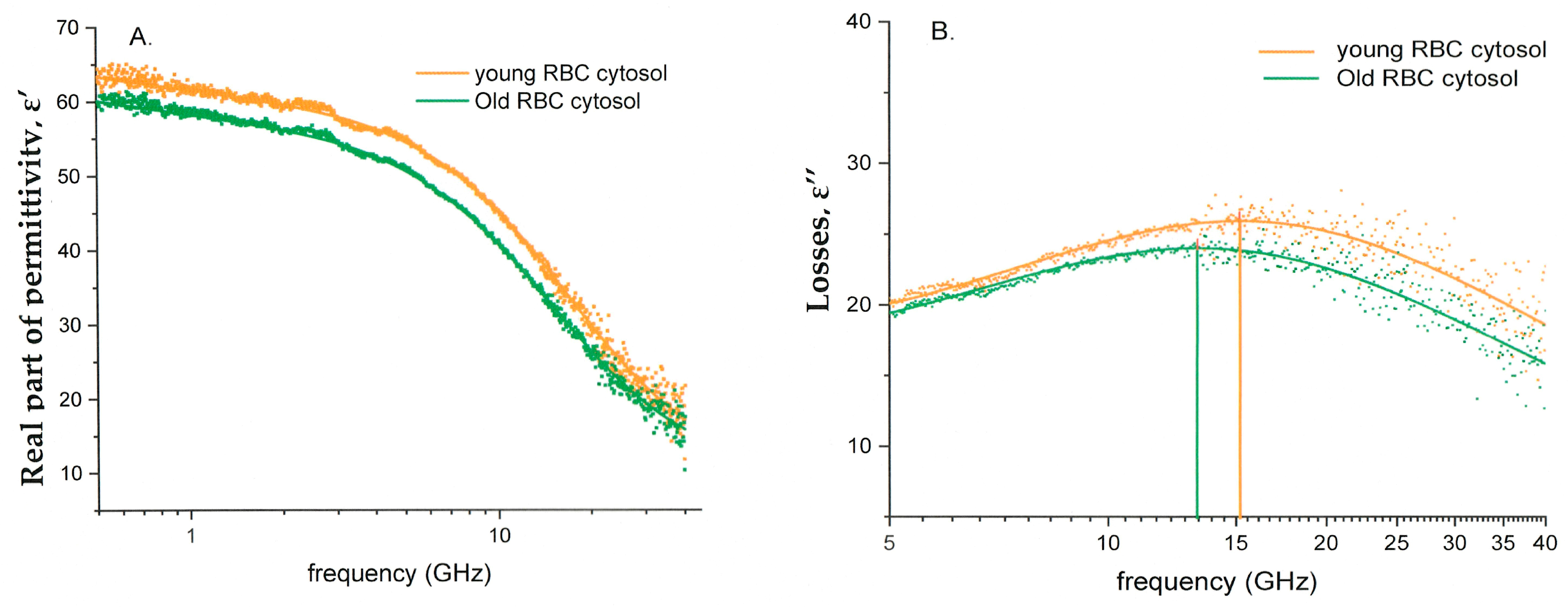
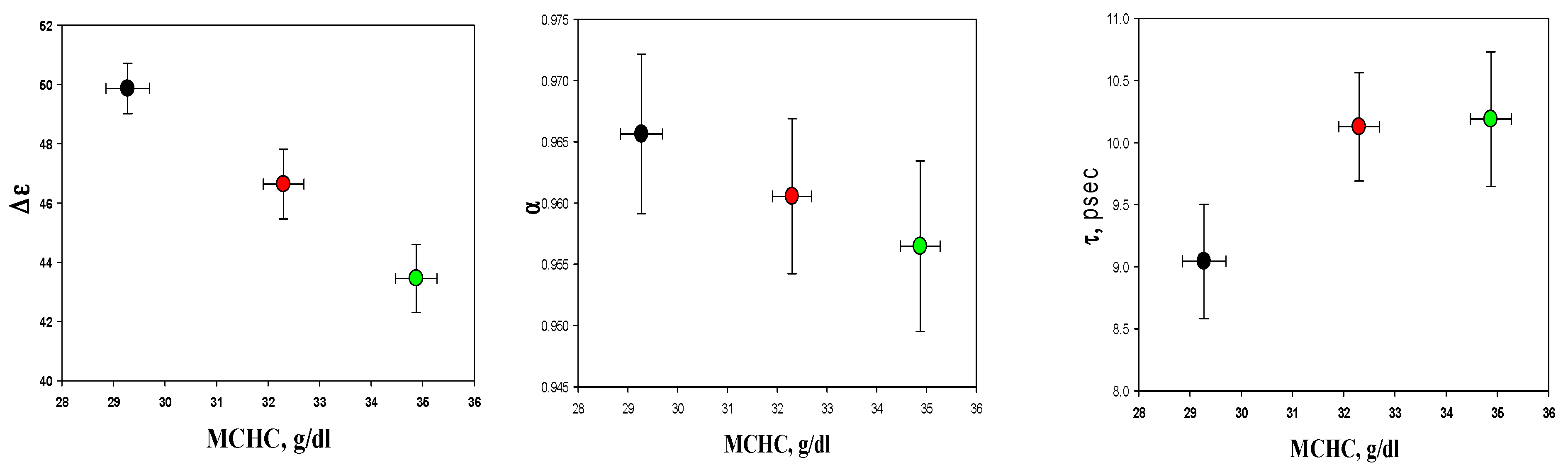
3.4. Dielectric Properties of Cytoplasmic Water
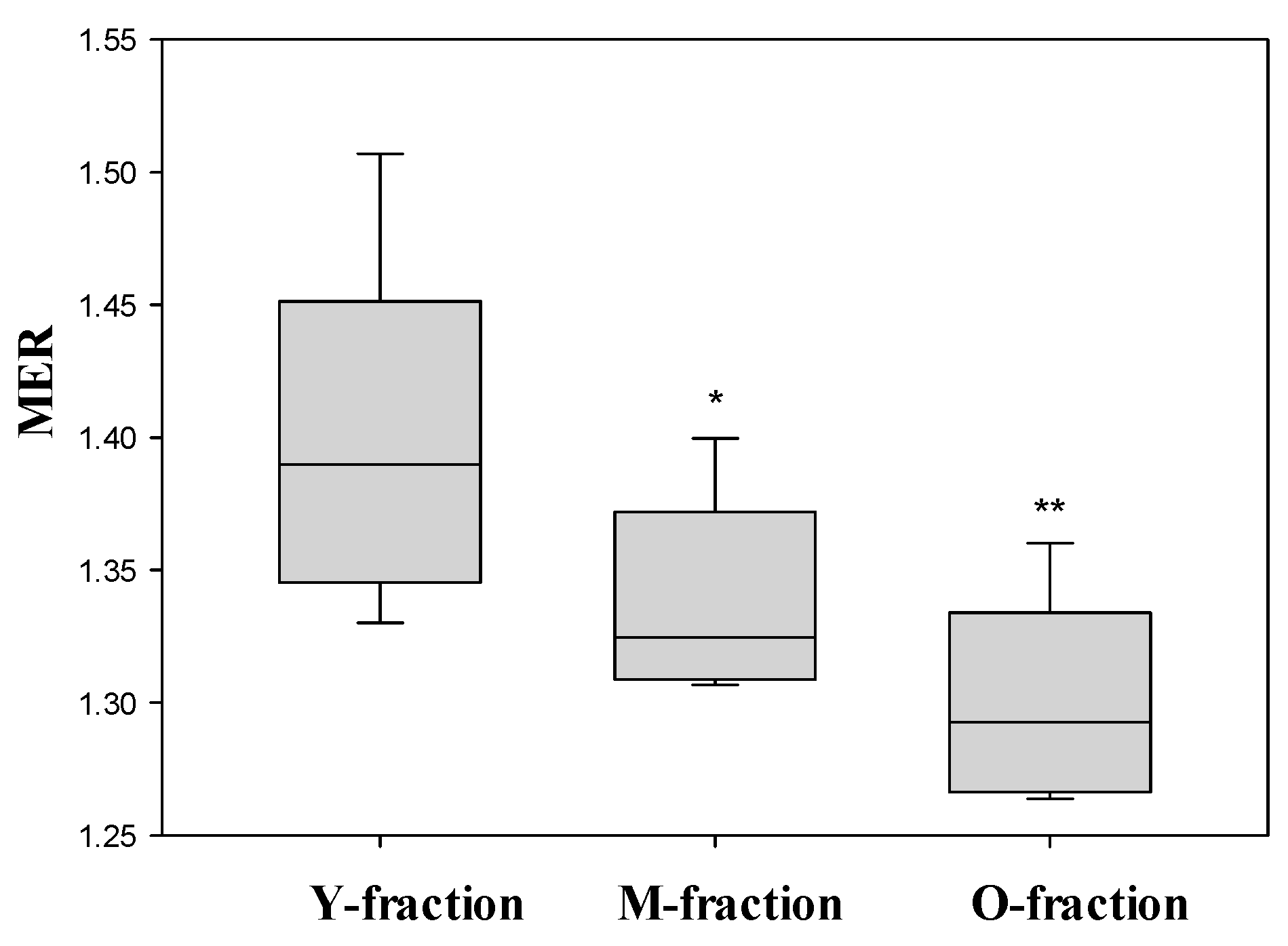
3.5. Deformability of Separated RBCs
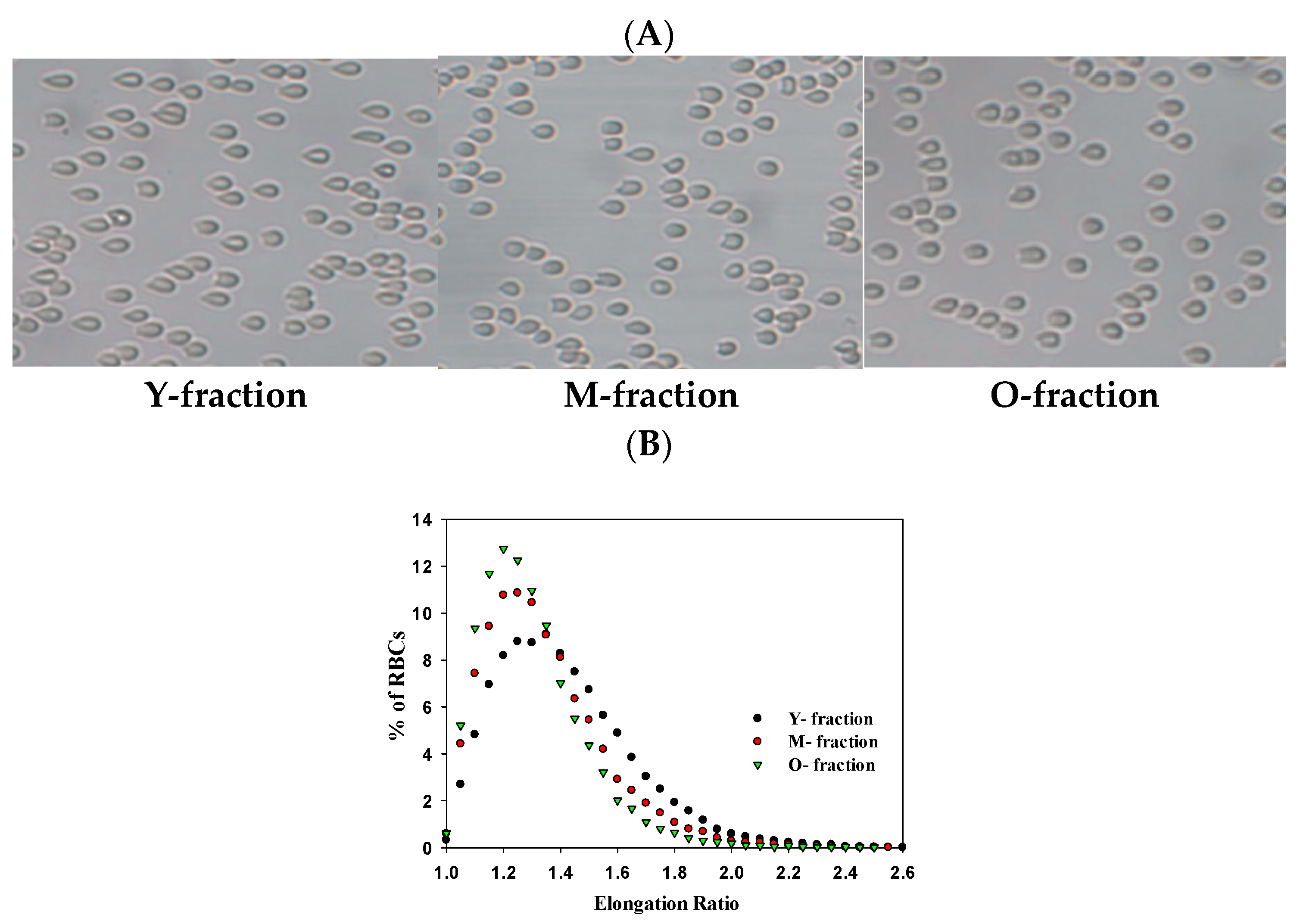
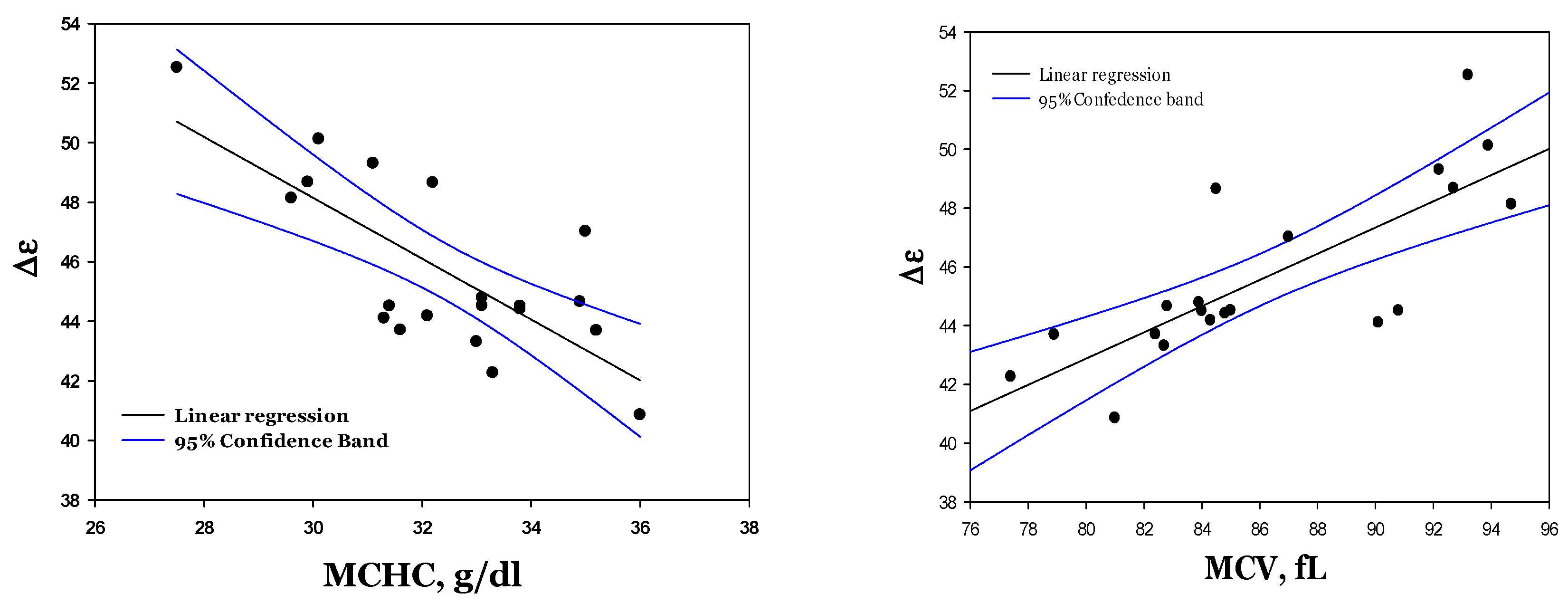
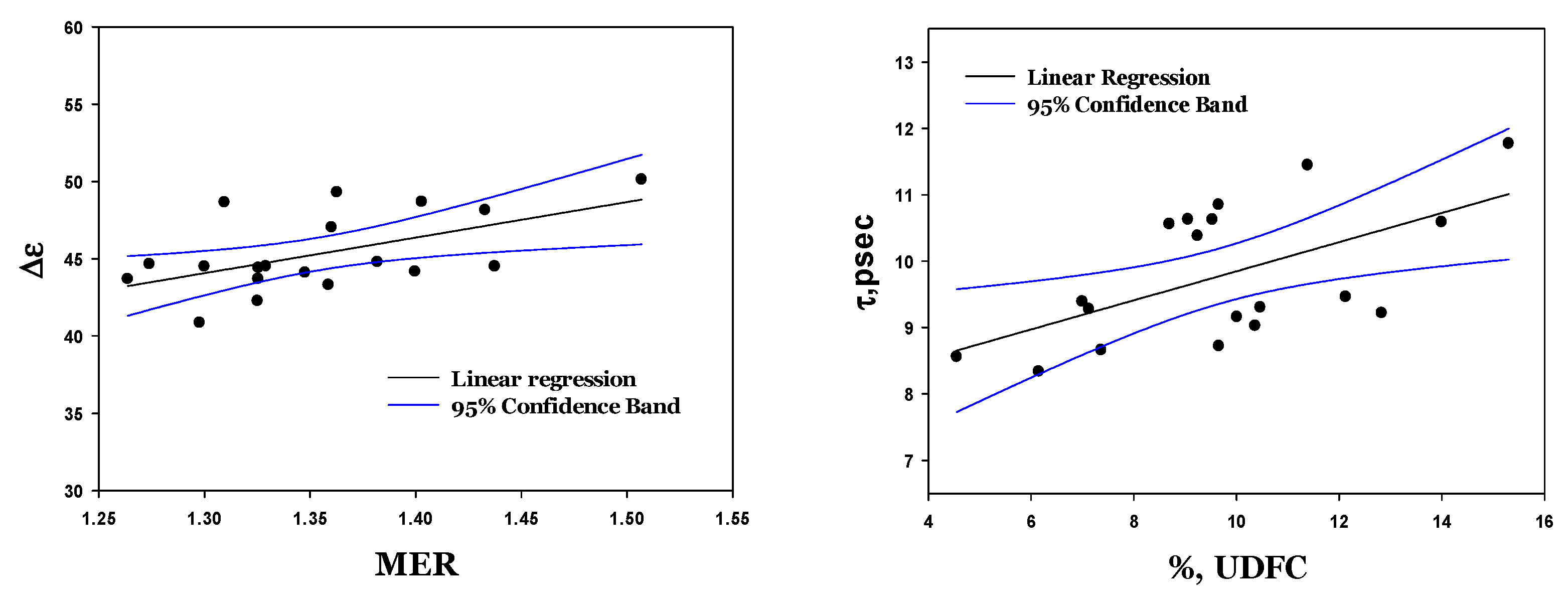
3.6. Dielectric Fitting Parameters of Cytosol Water vs. Hematological Indices
3.7. Dielectric Fitting Parameters vs. RBC Deformability
4. Discussion
5. Conclusions
- Density separation using a standard set-up (based on the Percoll gradient) does not affect the dielectric parameters of cytosolic water.
- Microwave dielectric spectroscopy (MDS) can distinguish between subpopulations enriched with old, mature, and young RBCs (obtained by a Percoll gradient) due to the redistribution of bulk and bound water in the cytoplasm and the changes in its mobility.
- Dielectric parameters are associated with physiological properties of RBCs, such as deformability in age-separated fractions of cells.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, M. The state of water in living systems: From the liquid to the jellyfish. Cell Mol. Biol. 2005, 51, 677–702. [Google Scholar] [PubMed]
- Levy, E.; Puzenko, A.; Kaatze, U.; Ben Ishai, P.; Feldman, Y. Dielectric spectra broadening as the signature of dipole-matrix interaction. II. Water in ionic solutions. J. Chem. Phys. 2012, 136, 114503. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Puzenko, A.; Kaatze, U.; Ishai, P.B.; Feldman, Y. Dielectric spectra broadening as the signature of dipole-matrix interaction. I. Water in nonionic solutions. J. Chem. Phys. 2012, 136, 114502–114505. [Google Scholar] [CrossRef]
- Puzenko, A.; Levy, E.; Shendrik, A.; Talary, M.S.; Caduff, A.; Feldman, Y. Dielectric spectra broadening as a signature for dipole-matrix interaction. III. Water in adenosine monophosphate/adenosine-5′-triphosphate solutions. J. Chem. Phys. 2012, 137, 194502–194508. (In English) [Google Scholar] [CrossRef] [PubMed]
- Chaplin, M. A proposal for the structuring of water. Biophys. Chem. 2000, 83, 211–221. [Google Scholar]
- Ruffle, S.V.; Michalarias, I.; Li, J.C.; Ford, R.C. Inelastic incoherent neutron scattering studies of water interacting with biological macromolecules. J. Am. Chem. Soc. 2002, 124, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Stadler, A.M.; van Eijck, L.; Demmel, F.; Artmann, G. Macromolecular dynamics in red blood cells investigated using neutron spectroscopy. J. R. Soc. Interface 2011, 8, 590–600. [Google Scholar] [CrossRef]
- Clark, M.R.; Mohandas, N.; Shohet, S.B. Osmotic gradient ektacytometry: Comprehensive characterization of red cell volume and surface maintenance. Blood 1983, 61, 899–910. [Google Scholar] [CrossRef]
- Clark, M.R. Computation of the average shear-induced deformation of red blood cells as a function of osmolality. Blood Cells 1989, 15, 427–439; discussion 440–442. [Google Scholar]
- Dey, K.; van Cromvoirt, A.M.; Hegemann, I.; Goede, J.S.; Bogdanova, A. Role of Piezo1 in Terminal Density Reversal of Red Blood Cells. Cells 2024, 13, 1363. [Google Scholar] [CrossRef]
- Lutz, H.U.; Stammler, P.; Fasler, S.; Ingold, M.; Fehr, J. Density separation of human red blood cells on self forming Percoll gradients: Correlation with cell age. Biochim. Biophys. Acta 1992, 1116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.H.; Werre, J.M.; Roerdinkholder-Stoelwinder, B.; Huls, T.H.; Willekens, F.L.; Halie, M.R. Characteristics of red blood cell populations fractionated with a combination of counterflow centrifugation and Percoll separation. Blood 1992, 79, 254–260. [Google Scholar] [PubMed]
- Peretz, S.; Livshits, L.; Pretorius, E.; Makhro, A.; Bogdanova, A.; Gassmann, M.; Koren, A.; Levin, C. The protective effect of the spleen in sickle cell patients. A comparative study between patients with asplenia/hyposplenism and hypersplenism. Front. Physiol. 2022, 13, 796837. [Google Scholar] [CrossRef]
- Barshtein, G.; Arbell, D.; Gural, A.; Livshits, L. Distribution of Red Blood Cells Deformability: Study on Density-Separated Cell Subpopulations. Colloid Interface 2024, 8, 47. (In English) [Google Scholar] [CrossRef]
- Latypova, L.; Barshtein, G.; Puzenko, A.; Poluektov, Y.; Anashkina, A.; Petrushanko, I.; Fenk, S.; Bogdanova, A.; Feldman, Y. Oxygenation state of hemoglobin defines dynamics of water molecules in its vicinity. J. Chem. Phys. 2020, 153, 135101. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; David, M.; Barshtein, G.; Yedgar, S.; Livshits, L.; Ben Ishai, P.; Feldman, Y. Dielectric Response of Cytoplasmic Water and Its Connection to the Vitality of Human Red Blood Cells. II. The Influence of Storage. J. Phys. Chem. B 2017, 121, 5273–5278. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics. I. Alternating current characteristics. J. Chem. Phys. Eng. Data 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Fuchs, K.; Kaatze, U. Molecular Dynamics of Carbohydrate Aqueous Solutions. Dielectric Relaxation as a Function of Glucose and Fructose Concentration. J. Phys. Chem. B 2001, 105, 2036–2042. [Google Scholar] [CrossRef]
- Noertemann, K.; Hilland, J.; Kaatze, U. Dielectric Properties of Aqueous NaCl Solutions at Microwave Frequencies. J. Phys. Chem. A 1997, 101, 6864–6869. [Google Scholar] [CrossRef]
- Rodríguez-Arteche, I.; Cerveny, S.; Alegría, Á.; Colmenero, J. Dielectric spectroscopy in the GHz region on fully hydrated zwitterionic amino acids. Phys. Chem. Chem. Phys. PCCP 2012, 14, 11352–11362. [Google Scholar] [CrossRef]
- Raicu, V.; Feldman, Y. (Eds.) Dielectric Relaxation in Biological Systems: Physical Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 2015; p. 464. (In English) [Google Scholar]
- Asami, K. Radiofrequency dielectric properties of cell suspensions. In Dielectric Relaxation in Biological Systems: Physical Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 2015; pp. 340–362. [Google Scholar]
- Kraszewski, A.; Kulinski, S.; Matuszewski, M. Dielectric Properties and a Model of Biphase Water Suspension at 9.4 Ghz. J. Appl. Phys. 1976, 47, 1275–1277. (In English) [Google Scholar] [CrossRef]
- Levy, E.; Barshtein, G.; Livshits, L.; Ben Ishai, P.; Feldman, Y. Dielectric Response of Cytoplasmic Water and Its Connection to the Vitality of Human Red Blood Cells: I. Glucose Concentration Influence. J. Phys. Chem. B 2016, 120, 10214–10220. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Goldschmidt, N.; Pries, A.R.; Zelig, O.; Arbell, D.; Yedgar, S. Deformability of transfused red blood cells is a potent effector of transfusion-induced hemoglobin increment: A study with beta-thalassemia major patients. Am. J. Hematol. 2017, 92, E559–E560. [Google Scholar] [CrossRef] [PubMed]
- Feldman, Y.; Puzenko, A.; Ben Ishai, P.; Gutina Greenbaum, A. The dielectric response of interfacial water—From the ordered structures to the single hydrated shell. Colloid Polym. Sci. 2014, 292, 1923–1932. [Google Scholar] [CrossRef]
- Barshtein, G.; Bergelson, L.; Dagan, A.; Gratton, E.; Yedgar, S. Membrane lipid order of human red blood cells is altered by physiological levels of hydrostatic pressure. Am. J. Physiol. 1997, 272, H538–H543. [Google Scholar] [CrossRef]
- Barshtein, G.; Bergelson, L.; Gratton, E.; Yedgar, S. Human red blood cell shape and volume are changed by physiological levels of hydrostatic pressure. J. Basic Clin. Physiol. Pharmacol. 1996, 7, 321–329. [Google Scholar] [CrossRef]
- Orbach, A.; Zelig, O.; Yedgar, S.; Barshtein, G. Biophysical and Biochemical Markers of Red Blood Cell Fragility. Transfus. Med. Hemother. 2017, 44, 183–187. [Google Scholar] [CrossRef]
- Clark, M.R.; Mohandas, N.; Shohet, S.B. Hydration of sickle cells using the sodium ionophore Monensin. A model for therapy. J. Clin. Investig. 1982, 70, 1074–1080. [Google Scholar] [CrossRef]
- Clark, M.R. Calcium extrusion by high-density human red blood cells. Blood Cells 1988, 14, 119–137. [Google Scholar]
- Vettore, L.; De Matteis, M.C.; Zampini, P. A new density gradient system for the separation of human red blood cells. Am. J. Hematol. 1980, 8, 291–297. [Google Scholar] [CrossRef]
- Latypova, L.; Puzenko, A.; Poluektov, Y.; Anashkina, A.; Petrushanko, I.; Bogdanova, A.; Feldman, Y. Hydration of methemoglobin studied by in silico modeling and dielectric spectroscopy. J. Chem. Phys. 2021, 155, 015101. [Google Scholar] [CrossRef] [PubMed]
- Brnjas-Kraljevic, J.; Maricic, S.; Bracika, V. Self-association of oxyhaemoglobin. A nuclear magnetic relaxation study in H2O/D2O solutions. Biophys. Chem. 1977, 6, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Brnjas-Kraljevic, J.; Pifat, G.; Maricic, S. Quaternary structure, hydration, and self-association of hemoglobin. A proton magnetic relaxation study. Physiol. Chem. Phys. 1979, 11, 371–376. [Google Scholar] [PubMed]
- Zhbanov, A.; Lee, Y.S.; Son, M.; Jung, M.H.; Eom, K.; Yang, S. Effect of hemoglobin hydration on the physical properties of erythrocyte cytoplasm and whole blood. Electrochim. Acta 2023, 438, 141560. (In English) [Google Scholar] [CrossRef]
- Basu, A.; Chakrabarti, A. Hemoglobin interacting proteins and implications of spectrin hemoglobin interaction. J. Proteom. 2015, 128, 469–475. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Blasi, B.; D’Amici, G.M.; Marrocco, C.; Zolla, L. Red blood cell subpopulations in freshly drawn blood: Application of proteomics and metabolomics to a decades-long biological issue. Blood Transfus. 2013, 11, 75–87. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, Z.J.; Mehrishi, J.; Huang, B.T.; Chen, X.Y.; Zheng, X.J.; Liu, W.J.; Luo, M. Human red blood cell aging: Correlative changes in surface charge and cell properties. J. Cell Mol. Med. 2011, 15, 2634–2642. [Google Scholar] [CrossRef]
- Kang, L.L.; Huang, Y.X.; Liu, W.J.; Zheng, X.J.; Wu, Z.J.; Luo, M. Confocal Raman microscopy on single living young and old erythrocytes. Biopolymers 2008, 89, 951–959. [Google Scholar] [CrossRef]
- Mohandas, N.; Groner, W. Cell membrane and volume changes during red cell development and aging. Ann. N. Y. Acad. Sci. 1989, 554, 217–224. [Google Scholar] [CrossRef]
- Minetti, G.; Achilli, C.; Perotti, C.; Ciana, A. Continuous Change in Membrane and Membrane-Skeleton Organization During Development From Proerythroblast to Senescent Red Blood Cell. Front. Physiol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Werre, J.M.; Willekens, F.L.; Bosch, F.H.; de Haans, L.D.; van der Vegt, S.G.; van den Bos, A.G.; Bosman, G.J. The red cell revisited—Matters of life and death. Cell Mol. Biol. 2004, 50, 139–145. [Google Scholar] [PubMed]
- McVey, M.J.; Kuebler, W.M.; Orbach, A.; Arbell, D.; Zelig, O.; Barshtein, G.; Yedgar, S. Reduced deformability of stored red blood cells is associated with generation of extracellular vesicles. Transfus. Apher. Sci. 2020, 59, 102851. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Native | O-Fraction | M-Fraction | Y-Fraction | |||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Vs Y, p Value | Mean ± SE | Vs Y, p Value | Mean ± SE | Vs Y, p-Value | Mean ± SE | |
| MCHC, g/dL | 32.8 ± 0.4 | 0.002 | 35.0 ± 0.4 | 0.0001 | 32.5 ± 0.4 | 0.0012 | 28.9 ± 0.5 |
| MCV, fL | 86.3 ± 1.5 | 0.0025 | 81.4 ± 1.6 | 0.0002 | 86.6 ± 1.4 | 0.0015 | 94.7 ± 1.5 |
| MCH, pg | 28.0 ± 0.57 | NS | 28.5 ± 0.74 | NS | 28.1 ± 0.41 | NS | 27.4 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latypova, L.; Galindo, C.; Livshits, L.; Teope, R.V.; Arbell, D.; Barshtein, G.; Bogdanova, A.; Feldman, Y. Dielectric Responses of Cytosolic Water Change with Aging of Circulating Red Blood Cells. Cells 2025, 14, 486. https://doi.org/10.3390/cells14070486
Latypova L, Galindo C, Livshits L, Teope RV, Arbell D, Barshtein G, Bogdanova A, Feldman Y. Dielectric Responses of Cytosolic Water Change with Aging of Circulating Red Blood Cells. Cells. 2025; 14(7):486. https://doi.org/10.3390/cells14070486
Chicago/Turabian StyleLatypova, Larisa, Cindy Galindo, Leonid Livshits, Rodolfo Victor Teope, Dan Arbell, Gregory Barshtein, Anna Bogdanova, and Yuri Feldman. 2025. "Dielectric Responses of Cytosolic Water Change with Aging of Circulating Red Blood Cells" Cells 14, no. 7: 486. https://doi.org/10.3390/cells14070486
APA StyleLatypova, L., Galindo, C., Livshits, L., Teope, R. V., Arbell, D., Barshtein, G., Bogdanova, A., & Feldman, Y. (2025). Dielectric Responses of Cytosolic Water Change with Aging of Circulating Red Blood Cells. Cells, 14(7), 486. https://doi.org/10.3390/cells14070486








