RHOX Homeobox Transcription Factor Regulation of Ins2 in Rodent Granulosa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Plasmids and siRNA
2.3. Superovulation and Granulosa Cell Cultures
2.4. Real-Time Quantitative RT-PCR (qPCR) Analysis
2.5. Immunohistochemistry
2.6. In Situ Hybridization
2.7. Statistical Analysis
3. Results
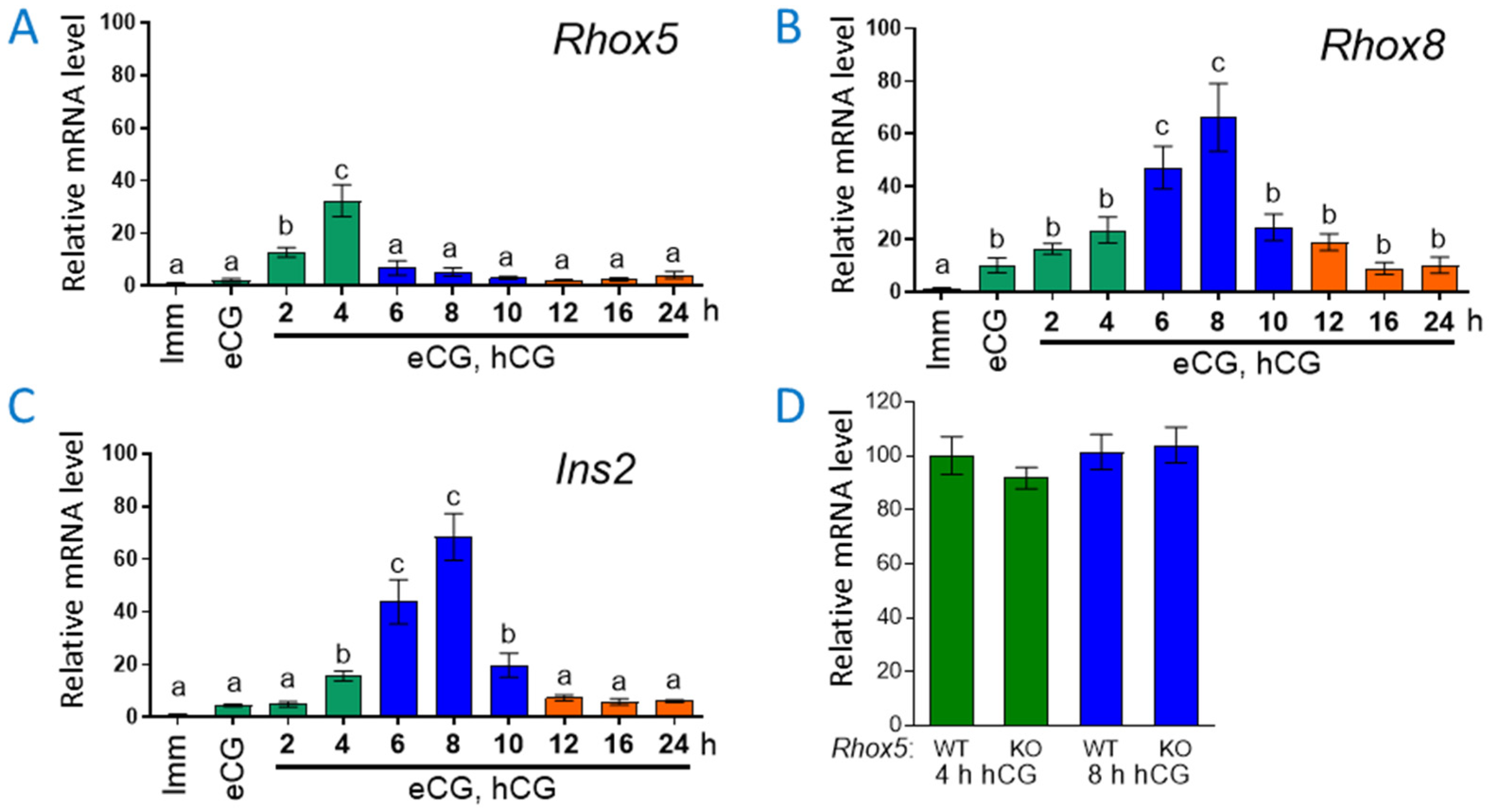
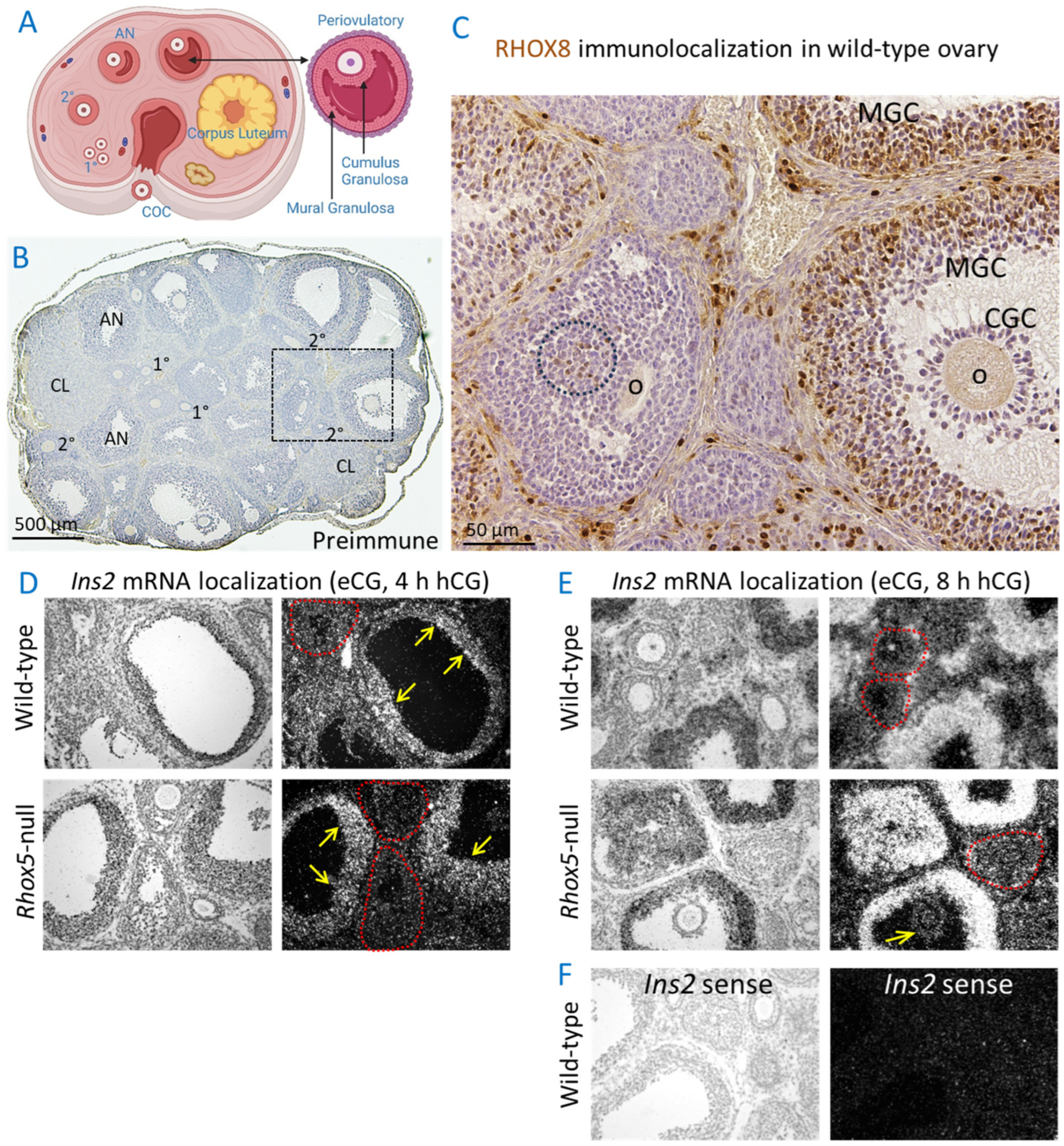
3.1. Ins2 Expression Tracks with Rhox8 Expression in the Ovary
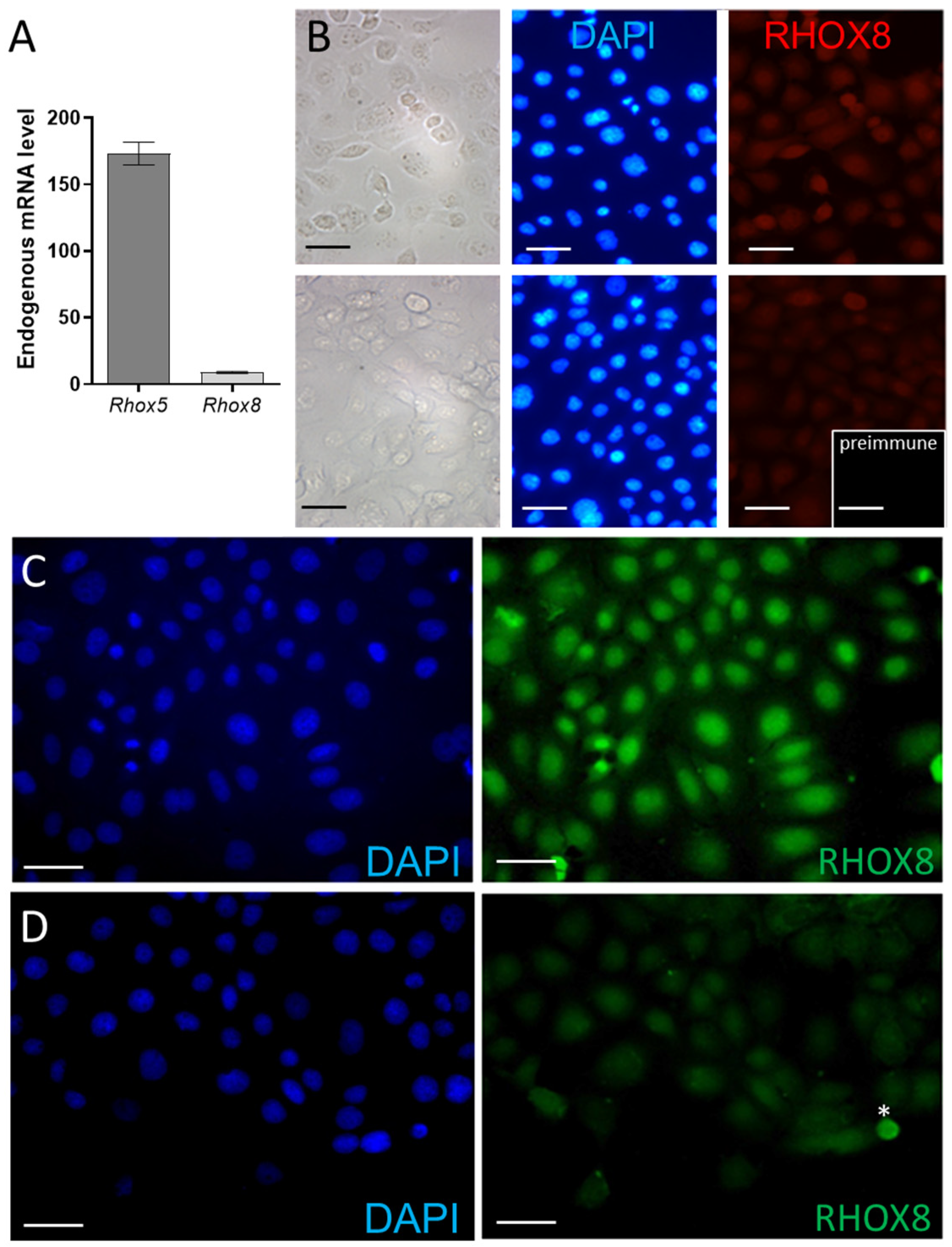
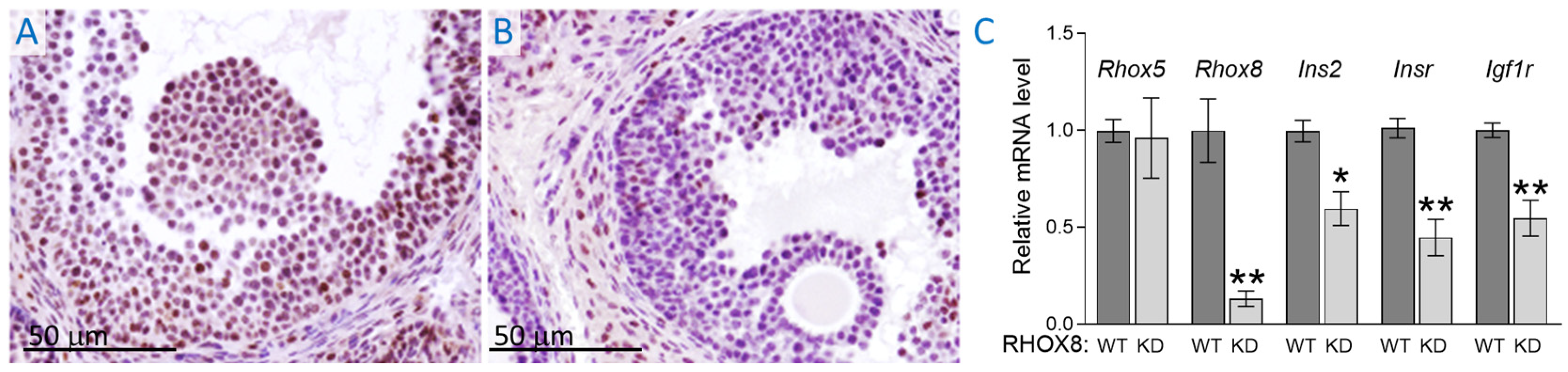
3.2. Regulation of Ins2 in Spontaneously Immortalized Rat Granulosa Cells (SIGC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIGC | Spontaneously immortalized granulosa cells |
| PGR | Progesterone receptor |
| LH | Luteinizing hormone |
| qPCR | Quantitative real-time reverse transcription polymerase chain reaction |
References
- Jo, M.; Brannstrom, M.; Akins, J.W.; Curry, T.E., Jr. New insights into the ovulatory process in the human ovary. Hum. Reprod. Update 2025, 31, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.R. Rerouting of follicle-stimulating hormone secretion and gonadal function. Fertil. Steril. 2023, 119, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Ackert, C.L.; Gittens, J.E.; O’Brien, M.J.; Eppig, J.J.; Kidder, G.M. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 2001, 233, 258–270. [Google Scholar] [CrossRef]
- Chang, R.J.; Cook-Andersen, H. Disordered follicle development. Mol. Cell. Endocrinol. 2013, 373, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Pangas, S.A.; Choi, Y.; Ballow, D.J.; Zhao, Y.; Westphal, H.; Matzuk, M.M.; Rajkovic, A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc. Natl. Acad. Sci. USA 2006, 103, 8090–8095. [Google Scholar] [CrossRef]
- Qin, Y.; Choi, Y.; Zhao, H.; Simpson, J.L.; Chen, Z.J.; Rajkovic, A. NOBOX homeobox mutation causes premature ovarian failure. Am. J. Hum. Genet. 2007, 81, 576–581. [Google Scholar] [CrossRef]
- Rajkovic, A.; Pangas, S.A.; Ballow, D.; Suzumori, N.; Matzuk, M.M. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004, 305, 1157–1159. [Google Scholar] [CrossRef]
- Jorgensen, J.S.; Gao, L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr. Patterns 2005, 5, 756–762. [Google Scholar] [CrossRef]
- Rajkovic, A.; Yan, C.; Yan, W.; Klysik, M.; Matzuk, M.M. Obox, a family of homeobox genes preferentially expressed in germ cells. Genomics 2002, 79, 711–717. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, E.Y.; Kim, K.H.; Moon, J.; Park, K.S.; Kim, K.S.; Lee, K.A. Obox4 critically regulates cAMP-dependent meiotic arrest and MI-MII transition in oocytes. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2314–2324. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, K.H.; Kim, E.Y.; Lee, S.Y.; Ko, J.J.; Lee, K.A. Obox4-silencing-activated STAT3 and MPF/MAPK signaling accelerate nuclear membrane breakdown in mouse oocytes. Reproduction 2016, 151, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Neirijnck, Y.; Papaioannou, M.D.; Nef, S. The Insulin/IGF System in Mammalian Sexual Development and Reproduction. Int. J. Mol. Sci. 2019, 20, 4440. [Google Scholar] [CrossRef]
- Bengtsson, M.; Stahlberg, A.; Rorsman, P.; Kubista, M. Gene expression profiling in single cells from the pancreatic islets of Langerhans reveals lognormal distribution of mRNA levels. Genome Res. 2005, 15, 1388–1392. [Google Scholar]
- Deltour, L.; Leduque, P.; Blume, N.; Madsen, O.; Dubois, P.; Jami, J.; Bucchini, D. Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc. Natl. Acad. Sci. USA 1993, 90, 527–531. [Google Scholar]
- Heath, V.L.; Moore, N.C.; Parnell, S.M.; Mason, D.W. Intrathymic expression of genes involved in organ specific autoimmune disease. J. Autoimmun. 1998, 11, 309–318. [Google Scholar] [CrossRef]
- MacLean, J.A., 2nd; Hu, Z.; Welborn, J.P.; Song, H.W.; Rao, M.K.; Wayne, C.M.; Wilkinson, M.F. The RHOX homeodomain proteins regulate the expression of insulin and other metabolic regulators in the testis. J. Biol. Chem. 2013, 288, 34809–34825. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, E.L.; Albanna, G.; Frolova, A.I.; Moley, K.H. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes 2012, 61, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, B.M.; Schaefer, I.M.; Villa-Komaroff, L.; Chirgwin, J.M. Characterization of the two nonallelic genes encoding mouse preproinsulin. J. Mol. Evol. 1986, 23, 305–312. [Google Scholar]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar]
- Izumi, T.; Yokota-Hashimoto, H.; Zhao, S.; Wang, J.; Halban, P.A.; Takeuchi, T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes 2003, 52, 409–416. [Google Scholar]
- Chang, A.S.; Dale, A.N.; Moley, K.H. Maternal diabetes adversely affects preovulatory oocyte maturation, development, and granulosa cell apoptosis. Endocrinology 2005, 146, 2445–2453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.; Moley, K.H. Maternal diabetes and oocyte quality. Mitochondrion 2010, 10, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Le Beulze, M.; Daubech, C.; Balde-Camara, A.; Ghieh, F.; Vialard, F. Mammal Reproductive Homeobox (Rhox) Genes: An Update of Their Involvement in Reproduction and Development. Genes 2023, 14, 1685. [Google Scholar] [CrossRef]
- Borgmann, J.; Tuttelmann, F.; Dworniczak, B.; Ropke, A.; Song, H.W.; Kliesch, S.; Wilkinson, M.F.; Laurentino, S.; Gromoll, J. The human RHOX gene cluster: Target genes and functional analysis of gene variants in infertile men. Hum. Mol. Genet. 2016, 25, 4898–4910. [Google Scholar] [CrossRef] [PubMed]
- Wilming, L.G.; Boychenko, V.; Harrow, J.L. Comprehensive comparative homeobox gene annotation in human and mouse. Database 2015, 2015, bav091. [Google Scholar] [CrossRef]
- Kazemi-Oula, G.; Ghafouri-Fard, S.; Mobasheri, M.B.; Geranpayeh, L.; Modarressi, M.H. Upregulation of RHOXF2 and ODF4 Expression in Breast Cancer Tissues. Cell J. 2015, 17, 471–477. [Google Scholar] [CrossRef]
- Shibata-Minoshima, F.; Oki, T.; Doki, N.; Nakahara, F.; Kageyama, S.; Kitaura, J.; Fukuoka, J.; Kitamura, T. Identification of RHOXF2 (PEPP2) as a cancer-promoting gene by expression cloning. Int. J. Oncol. 2012, 40, 93–98. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Guo, H.; Liu, M.; Wang, L. LOXL1-AS1 Contributes to Non-Small Cell Lung Cancer Progression by Regulating miR-3128/RHOXF2 Axis. Onco Targets Ther. 2020, 13, 6063–6071. [Google Scholar] [CrossRef] [PubMed]
- Song, H.W.; Anderson, R.A.; Bayne, R.A.; Gromoll, J.; Shimasaki, S.; Chang, R.J.; Parast, M.M.; Laurent, L.C.; de Rooij, D.G.; Hsieh, T.C.; et al. The RHOX homeobox gene cluster is selectively expressed in human oocytes and male germ cells. Hum. Reprod. 2013, 28, 1635–1646. [Google Scholar] [CrossRef]
- Niu, A.L.; Wang, Y.Q.; Zhang, H.; Liao, C.H.; Wang, J.K.; Zhang, R.; Che, J.; Su, B. Rapid evolution and copy number variation of primate RHOXF2, an X-linked homeobox gene involved in male reproduction and possibly brain function. BMC Evol. Biol. 2011, 11, 298. [Google Scholar] [CrossRef]
- Frainais, C.; Kannengiesser, C.; Albert, M.; Molina-Gomes, D.; Boitrelle, F.; Bailly, M.; Grandchamp, B.; Selva, J.; Vialard, F. RHOXF2 gene, a new candidate gene for spermatogenesis failure. Basic Clin. Androl. 2014, 24, 3. [Google Scholar] [CrossRef][Green Version]
- Tan, K.; Kim, M.E.; Song, H.W.; Skarbrevik, D.; Babajanian, E.; Bedrosian, T.A.; Gage, F.H.; Wilkinson, M.F. The Rhox gene cluster suppresses germline LINE1 transposition. Proc. Natl. Acad. Sci. USA 2021, 118, e2024785118. [Google Scholar] [CrossRef]
- Brown, R.M.; Davis, M.G.; Hayashi, K.; MacLean, J.A. Regulated expression of Rhox8 in the mouse ovary: Evidence for the role of progesterone and RHOX5 in granulosa cells. Biol. Reprod. 2013, 88, 126. [Google Scholar] [CrossRef] [PubMed]
- Daggag, H.; Svingen, T.; Western, P.S.; van den Bergen, J.A.; McClive, P.J.; Harley, V.R.; Koopman, P.; Sinclair, A.H. The rhox homeobox gene family shows sexually dimorphic and dynamic expression during mouse embryonic gonad development. Biol. Reprod. 2008, 79, 468–474. [Google Scholar] [CrossRef]
- MacLean, J.A., 2nd; Rao, M.K.; Doyle, K.M.; Richards, J.S.; Wilkinson, M.F. Regulation of the Rhox5 homeobox gene in primary granulosa cells: Preovulatory expression and dependence on SP1/SP3 and GABP. Biol. Reprod. 2005, 73, 1126–1134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Welborn, J.P.; Davis, M.G.; Ebers, S.D.; Stodden, G.R.; Hayashi, K.; Cheatwood, J.L.; Rao, M.K.; MacLean, J.A., 2nd. Rhox8 Ablation in the Sertoli Cells Using a Tissue-Specific RNAi Approach Results in Impaired Male Fertility in Mice. Biol. Reprod. 2015, 93, 8. [Google Scholar] [CrossRef]
- Maclean, J.A., 2nd; Chen, M.A.; Wayne, C.M.; Bruce, S.R.; Rao, M.; Meistrich, M.L.; Macleod, C.; Wilkinson, M.F. Rhox: A new homeobox gene cluster. Cell 2005, 120, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Kasu, M.; Bottoms, C.J.; Douglas, J.C.; Sekulovski, N.; Hayashi, K.; MacLean, J.A., II. Rhox8 homeobox gene ablation leads to rete testis abnormality and male subfertility in mice. Biol. Reprod. 2023, 109, 520–532. [Google Scholar] [CrossRef]
- Pitman, J.L.; Lin, T.P.; Kleeman, J.E.; Erickson, G.F.; MacLeod, C.L. Normal reproductive and macrophage function in Pem homeobox gene-deficient mice. Dev. Biol. 1998, 202, 196–214. [Google Scholar] [CrossRef][Green Version]
- Fleenor, D.E.; Freemark, M. Prolactin induction of insulin gene transcription: Roles of glucose and signal transducer and activator of transcription 5. Endocrinology 2001, 142, 2805–2810. [Google Scholar]
- Sekulovski, N.; Whorton, A.E.; Shi, M.; Hayashi, K.; MacLean, J.A., 2nd. Periovulatory insulin signaling is essential for ovulation, granulosa cell differentiation, and female fertility. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.S.; Stoica, G.; Tilley, R.; Burghardt, R.C. Rat ovarian granulosa cell culture: A model system for the study of cell-cell communication during multistep transformation. Cancer Res. 1991, 51, 696–706. [Google Scholar]
- Chen, X.; Fang, F.; Liou, Y.C.; Ng, H.H. Zfp143 regulates Nanog through modulation of Oct4 binding. Stem Cells 2008, 26, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck-Guillem, V.; Vanacker, J.M.; Verger, A.; Tomavo, N.; Stehelin, D.; Laudet, V.; Duterque-Coquillaud, M. Mutual repression of transcriptional activation between the ETS-related factor ERG and estrogen receptor. Oncogene 2003, 22, 8072–8084. [Google Scholar] [CrossRef]
- Hippenmeyer, S.; Youn, Y.H.; Moon, H.M.; Miyamichi, K.; Zong, H.; Wynshaw-Boris, A.; Luo, L. Genetic mosaic dissection of Lis1 and Ndel1 in neuronal migration. Neuron 2010, 68, 695–709. [Google Scholar] [CrossRef]
- Tasic, B.; Hippenmeyer, S.; Wang, C.; Gamboa, M.; Zong, H.; Chen-Tsai, Y.; Luo, L. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc. Natl. Acad. Sci. USA 2011, 108, 7902–7907. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Coumoul, X.; Deng, C.X. RNAi-based conditional gene knockdown in mice using a U6 promoter driven vector. Int. J. Biol. Sci. 2007, 3, 91–99. [Google Scholar]
- Shukla, V.; Coumoul, X.; Wang, R.H.; Kim, H.S.; Deng, C.X. RNA interference and inhibition of MEK-ERK signaling prevent abnormal skeletal phenotypes in a mouse model of craniosynostosis. Nat. Genet. 2007, 39, 1145–1150. [Google Scholar] [CrossRef]
- Jamin, S.P.; Arango, N.A.; Mishina, Y.; Hanks, M.C.; Behringer, R.R. Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol. Cell Endocrinol. 2003, 211, 15–19. [Google Scholar]
- Douglas, J.C.; Sekulovski, N.; Arreola, M.R.; Oh, Y.; Hayashi, K.; MacLean, J.A., 2nd. Normal Ovarian Function in Subfertile Mouse with Amhr2-Cre-Driven Ablation of Insr and Igf1r. Genes 2024, 15, 616. [Google Scholar] [CrossRef]
- Kim, S.T.; Moley, K.H. Paternal effect on embryo quality in diabetic mice is related to poor sperm quality and associated with decreased glucose transporter expression. Reproduction 2008, 136, 313–322. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.C.; Kim, S.Y.; Cho, G.J.; Woodruff, T.K. Poorly-Controlled Type 1 Diabetes Mellitus Impairs LH-LHCGR Signaling in the Ovaries and Decreases Female Fertility in Mice. Yonsei Med. J. 2019, 60, 667–678. [Google Scholar] [CrossRef]
- Yu, J.; Yaba, A.; Kasiman, C.; Thomson, T.; Johnson, J. mTOR controls ovarian follicle growth by regulating granulosa cell proliferation. Plos One 2011, 6, e21415. [Google Scholar] [CrossRef]
- Li, Q.; O’Malley, M.E.; Bartlett, D.L.; Guo, Z.S. Homeobox gene Rhox5 is regulated by epigenetic mechanisms in cancer and stem cells and promotes cancer growth. Mol. Cancer 2011, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bartlett, D.L.; Gorry, M.C.; O’Malley, M.E.; Guo, Z.S. Three epigenetic drugs up-regulate homeobox gene Rhox5 in cancer cells through overlapping and distinct molecular mechanisms. Mol. Pharmacol. 2009, 76, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.S.; Stein, D.W.; Echols, J.; Burghardt, R.C. Concomitant alterations of desmosomes, adhesiveness, and diffusion through gap junction channels in a rat ovarian transformation model system. Exp. Cell Res. 1993, 207, 19–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Willis, D.; Franks, S. Insulin action in human granulosa cells from normal and polycystic ovaries is mediated by the insulin receptor and not the type-I insulin-like growth factor receptor. J. Clin. Endocrinol. Metab. 1995, 80, 3788–3790. [Google Scholar] [CrossRef]
- Willis, D.; Mason, H.; Gilling-Smith, C.; Franks, S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J. Clin. Endocrinol. Metab. 1996, 81, 302–309. [Google Scholar] [CrossRef][Green Version]
- Ipsa, E.; Cruzat, V.F.; Kagize, J.N.; Yovich, J.L.; Keane, K.N. Growth Hormone and Insulin-Like Growth Factor Action in Reproductive Tissues. Front. Endocrinol. 2019, 10, 777. [Google Scholar] [CrossRef]
- Sakumoto, T.; Tokunaga, Y.; Tanaka, H.; Nohara, M.; Motegi, E.; Shinkawa, T.; Nakaza, A.; Higashi, M. Insulin resistance/hyperinsulinemia and reproductive disorders in infertile women. Reprod. Med. Biol. 2010, 9, 185–190. [Google Scholar] [CrossRef]
- Kwon, H.; Choi, D.H.; Bae, J.H.; Kim, J.H.; Kim, Y.S. mRNA expression pattern of insulin-like growth factor components of granulosa cells and cumulus cells in women with and without polycystic ovary syndrome according to oocyte maturity. Fertil. Steril. 2010, 94, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Kayampilly, P.P.; Wanamaker, B.L.; Stewart, J.A.; Wagner, C.L.; Menon, K.M. Stimulatory effect of insulin on 5α-reductase type 1 (SRD5A1) expression through an Akt-dependent pathway in ovarian granulosa cells. Endocrinology 2010, 151, 5030–5037. [Google Scholar] [CrossRef]
- Rice, S.; Pellatt, L.J.; Bryan, S.J.; Whitehead, S.A.; Mason, H.D. Action of metformin on the insulin-signaling pathway and on glucose transport in human granulosa cells. J. Clin. Endocrinol. Metab. 2011, 96, E427–E435. [Google Scholar] [CrossRef]
- Tan, M.; Cheng, Y.; Zhong, X.; Yang, D.; Jiang, S.; Ye, Y.; Ding, M.; Guan, G.; Yang, D.; Zhao, X. LNK promotes granulosa cell apoptosis in PCOS via negatively regulating insulin-stimulated AKT-FOXO3 pathway. Aging 2021, 13, 4617–4633. [Google Scholar] [CrossRef]
- Xu, P.; Huang, B.Y.; Zhan, J.H.; Liu, M.T.; Fu, Y.; Su, Y.Q.; Sun, Q.Y.; Wang, W.H.; Chen, D.J.; Liu, J.Q. Insulin Reduces Reaction of Follicular Granulosa Cells to FSH Stimulation in Women With Obesity-Related Infertility During IVF. J. Clin. Endocrinol. Metab. 2019, 104, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
- Spicer, L.J.; Maylem, E.R.S.; Schutz, L.F. Granulosa cell function in domestic animals: A review on the in vitro effects of FSH, insulin and insulin-like growth factor 1. Domest. Anim. Endocrinol. 2025, 91, 106919. [Google Scholar] [CrossRef]
- Hein, G.J.; Panzani, C.G.; Rodriguez, F.M.; Salvetti, N.R.; Diaz, P.U.; Gareis, N.C.; Benitez, G.A.; Ortega, H.H.; Rey, F. Impaired insulin signaling pathway in ovarian follicles of cows with cystic ovarian disease. Anim. Reprod. Sci. 2015, 156, 64–74. [Google Scholar] [CrossRef]
- Anazawa, M.; Ashibe, S.; Nagao, Y. Gene expression levels in cumulus cells are correlated with developmental competence of bovine oocytes. Theriogenology 2025, 231, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mota, G.B.; e Silva, I.O.; de Souza, D.K.; Tuany, F.; Pereira, M.M.; Camargo, L.S.; e Silva, A.A.M.R. Insulin influences developmental competence of bovine oocytes cultured in α-MEM plus follicle-simulating hormone. Zygote 2015, 23, 563–572. [Google Scholar] [CrossRef]
- Dupont, J.; Scaramuzzi, R.J. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem. J. 2016, 473, 1483–1501. [Google Scholar] [CrossRef]
- Nandi, A.; Wang, X.; Accili, D.; Wolgemuth, D.J. The effect of insulin signaling on female reproductive function independent of adiposity and hyperglycemia. Endocrinology 2010, 151, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Sekulovski, N.; Whorton, A.E.; Shi, M.; Hayashi, K.; MacLean, J.A., 2nd. Insulin signaling is an essential regulator of endometrial proliferation and implantation in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21440. [Google Scholar] [CrossRef]

| Gene | Sequence of Forward and Reverse Primers 5′-3′ | Amplicon Length (bp) | TM | |
|---|---|---|---|---|
| Rhox5 | For | GCCTGGGAGTCAAGGAA | 187 | 60° |
| Rev | AGGACCAGGAGCACCAGGA | |||
| Rhox8 | For | CCTCAAGAAGTCACCCAGTCG | 191 | 60° |
| Rev | ACCTGCGTTCTCCTCTCTCT | |||
| Ins2 | For | CCTGCTGGCCCTGCTCTT | 214 | 60° |
| Rev | CAAGGTCTGAAGGTCACCTG | |||
| Insr | For | GCCCTAAGGTCTGCCAAATC | 114 | 60° |
| Rev | CTCGGATGTTGATGATCAGGCT | |||
| Igf1r | For | GGAGTGTCCCTCAGGCTTCA | 217 | 60° |
| Rev | GTTCTCCAACTCCGAGGCAA | |||
| Rpl19 | For | TGCCTCTAGTGTCCTCCGC | 237 | 60° |
| Rev | ATCCGAGCATTGGCAGTACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; MacLean, J.A., II. RHOX Homeobox Transcription Factor Regulation of Ins2 in Rodent Granulosa Cells. Cells 2025, 14, 478. https://doi.org/10.3390/cells14070478
Hayashi K, MacLean JA II. RHOX Homeobox Transcription Factor Regulation of Ins2 in Rodent Granulosa Cells. Cells. 2025; 14(7):478. https://doi.org/10.3390/cells14070478
Chicago/Turabian StyleHayashi, Kanako, and James A. MacLean, II. 2025. "RHOX Homeobox Transcription Factor Regulation of Ins2 in Rodent Granulosa Cells" Cells 14, no. 7: 478. https://doi.org/10.3390/cells14070478
APA StyleHayashi, K., & MacLean, J. A., II. (2025). RHOX Homeobox Transcription Factor Regulation of Ins2 in Rodent Granulosa Cells. Cells, 14(7), 478. https://doi.org/10.3390/cells14070478







