Beta-3 Adrenoceptor Agonism Protects the Enteric Nervous Tissue Against Hyperoxia-Induced Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model and Drug Administration
2.2. Sample Collection and Process
2.3. Immunofluorescent Analysis
2.4. Statistical Analysis
3. Results
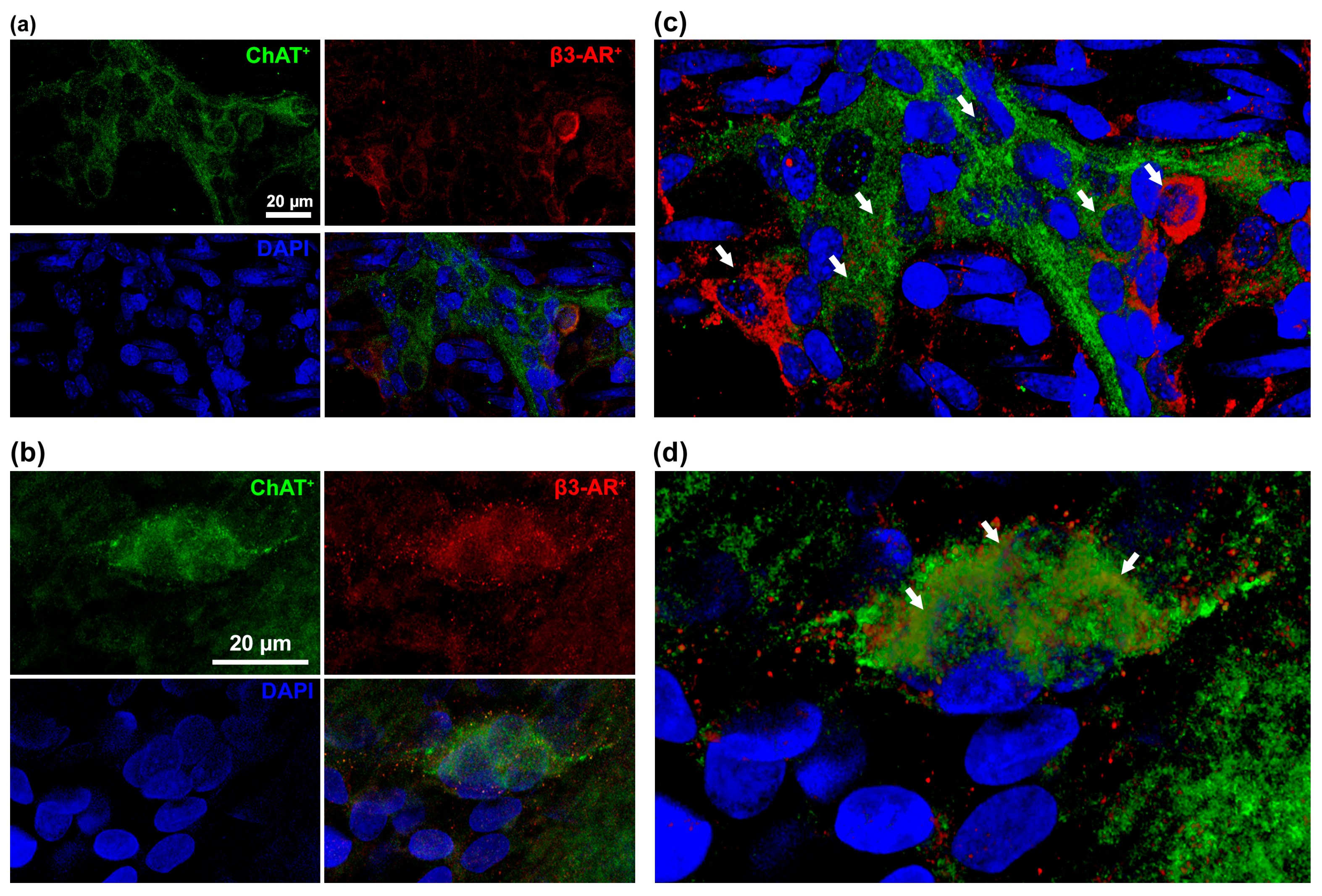
3.1. β3-AR Expression on the ENS
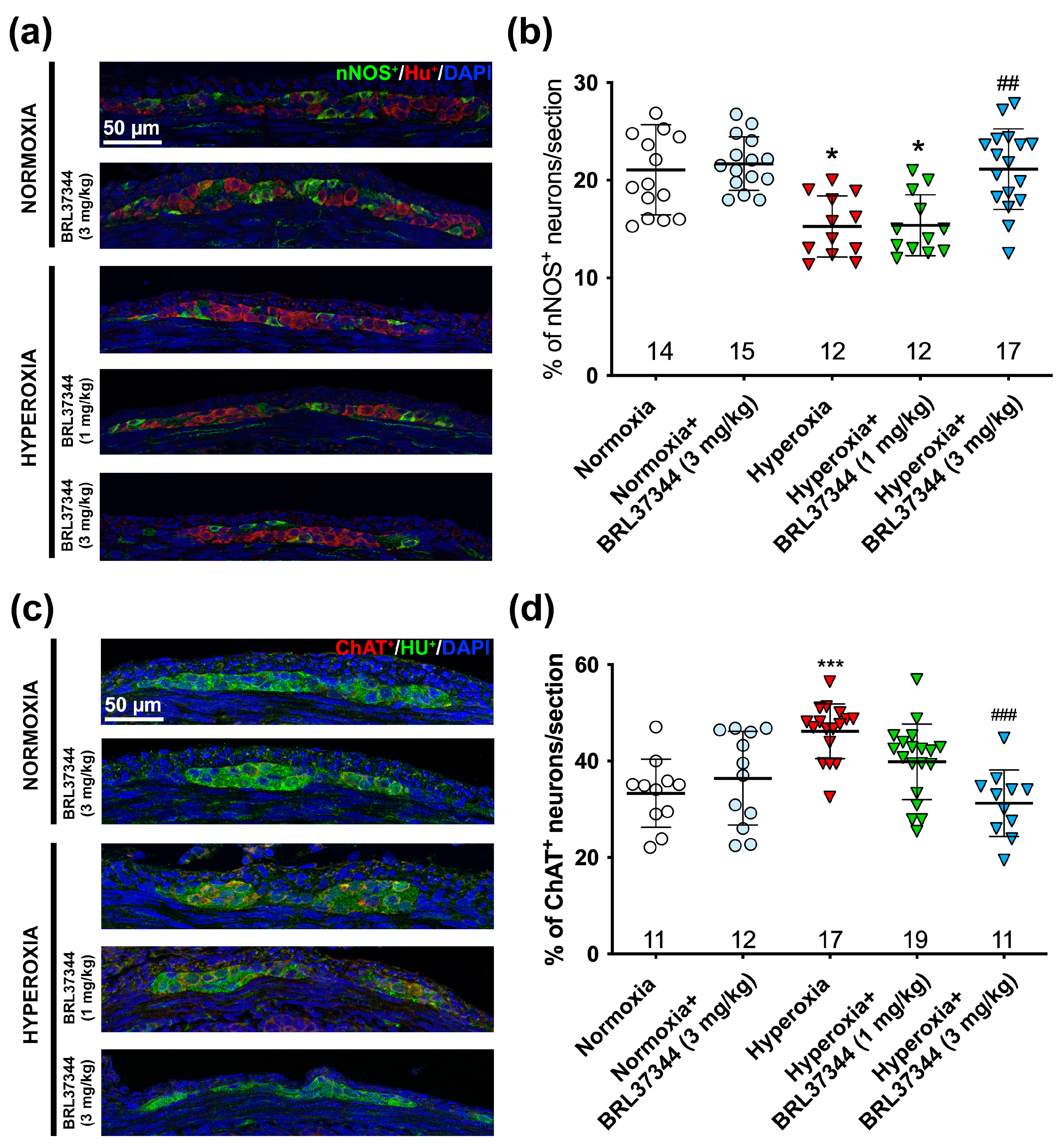
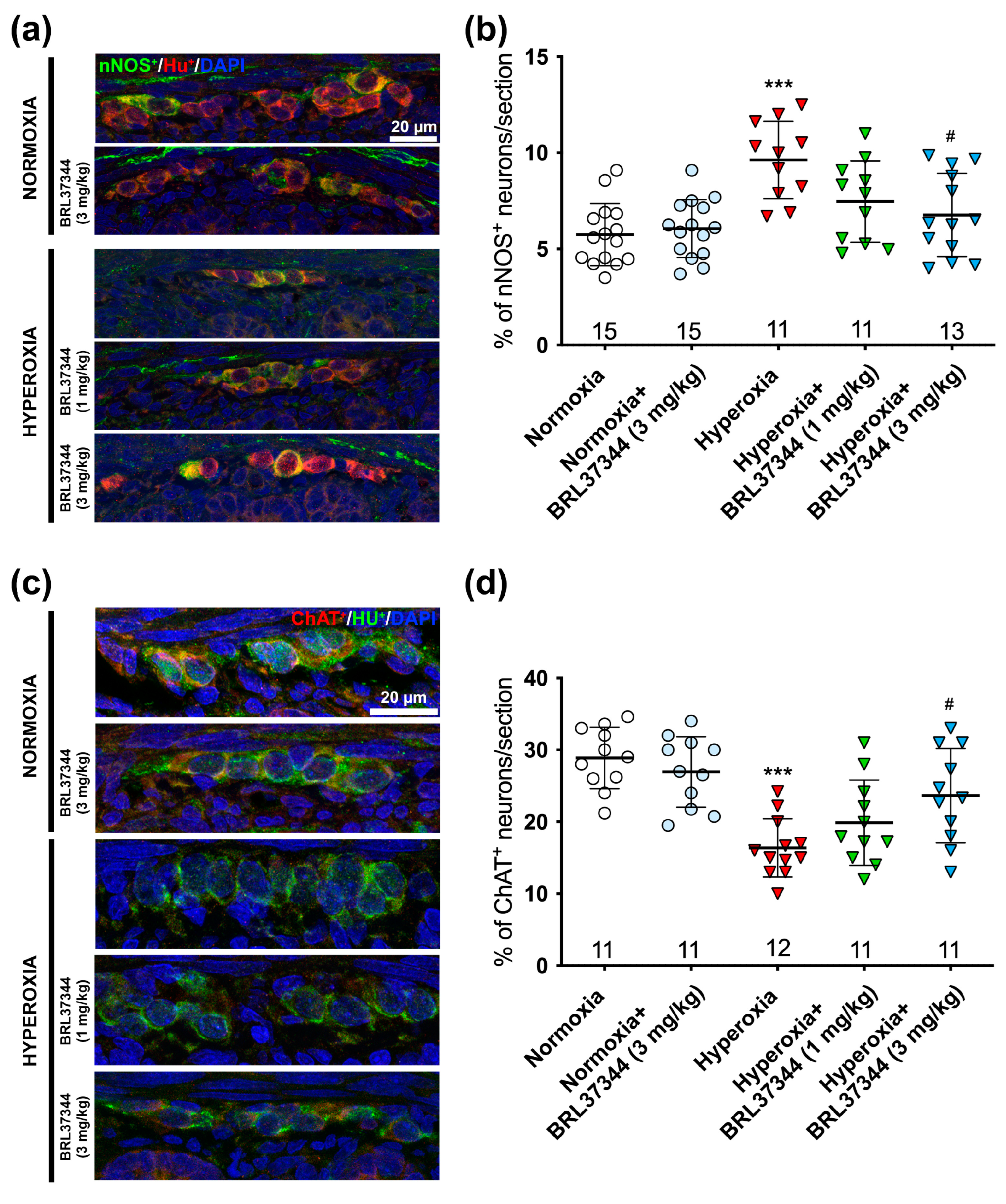
3.2. Protective Effect of BRL37344 on the Hyperoxia-Exposed Myenteric Plexus
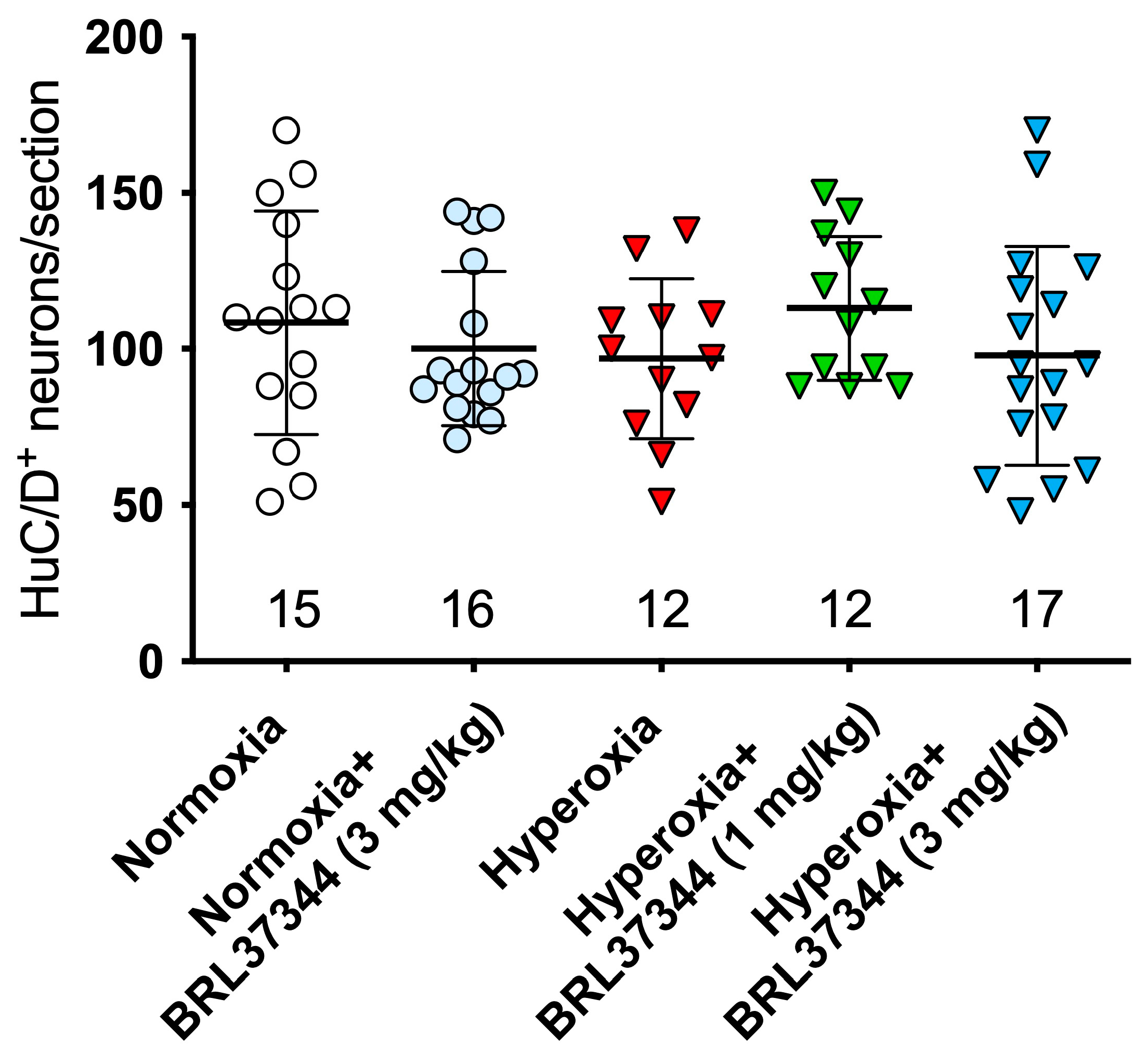
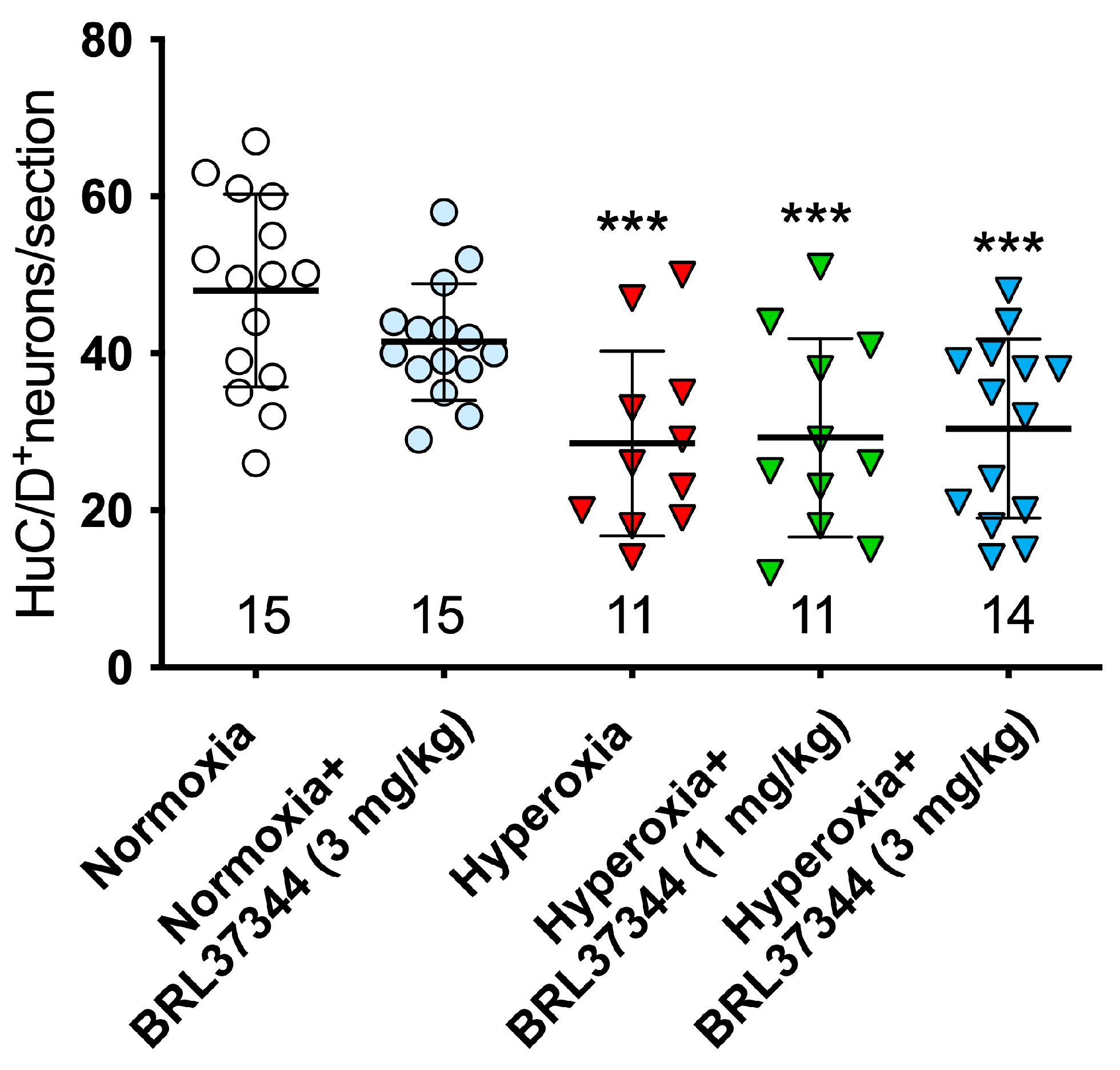
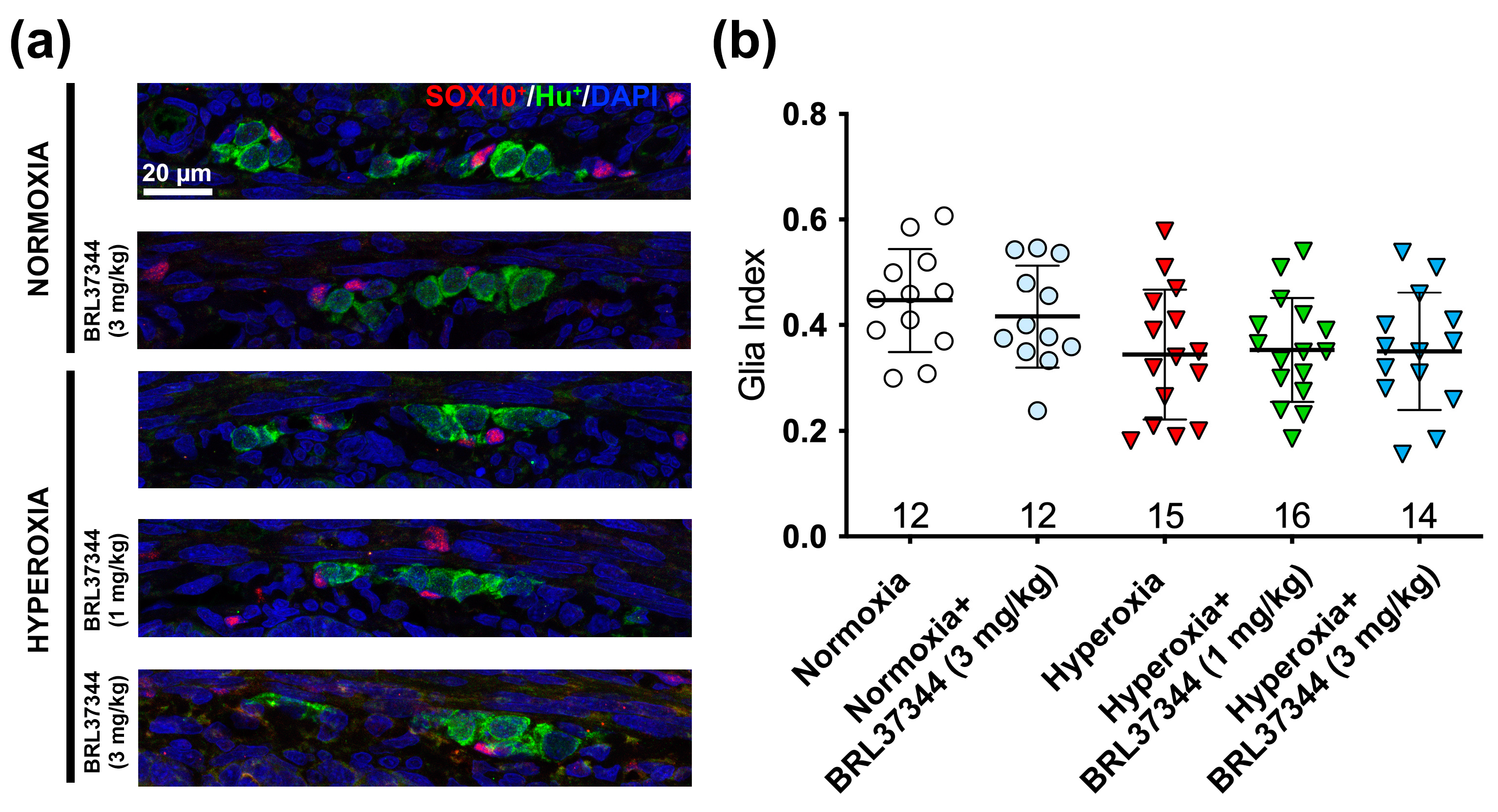
3.3. Protective Effect of BRL37344 on the Submucosal Plexus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular Characterization of the Human Beta 3-Adrenergic Receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Simard, P.M.; Atgié, C.; Mauriège, P.; D’Allaire, F.; Bukowiecki, L.J. Comparison of the Lipolytic Effects of Norepinephrine and BRL 37344 in Rat Brown and White Adipocytes. Obes. Res. 1994, 2, 424–431. [Google Scholar] [CrossRef]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about β(3)-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef]
- Pini, A.; Fazi, C.; Nardini, P.; Calvani, M.; Fabbri, S.; Guerrini, A.; Forni, G.; La Marca, G.; Rosa, A.C.; Filippi, L. Effect of Beta 3 Adrenoreceptor Modulation on Patency of the Ductus Arteriosus. Cells 2020, 9, 2625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, Y.; Chen, J.; Qin, C.; Liu, J.; Guo, D.; Wang, R.; Hu, J.; Zou, Q.; Yang, J.; et al. Beta3-Adrenergic Receptor Activation Alleviates Cardiac Dysfunction in Cardiac Hypertrophy by Regulating Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 3417242. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Cavallini, L.; Tondo, A.; Spinelli, V.; Ricci, L.; Pasha, A.; Bruno, G.; Buonvicino, D.; Bigagli, E.; Vignoli, M.; et al. Β3-Adrenoreceptors Control Mitochondrial Dormancy in Melanoma and Embryonic Stem Cells. Oxid. Med. Cell Longev. 2018, 2018, 6816508. [Google Scholar] [CrossRef]
- de Almeida, V.O.; Pereira, R.A.; Amantéa, S.L.; Rhoden, C.R.; Colvero, M.O. Neonatal Diseases and Oxidative Stress in Premature Infants: An Integrative Review. J. Pediatr. (Rio J.) 2022, 98, 455–462. [Google Scholar] [CrossRef]
- Melecchi, A.; Canovai, A.; Amato, R.; Dal Monte, M.; Filippi, L.; Bagnoli, P.; Cammalleri, M. Agonism of Β3-Adrenoceptors Inhibits Pathological Retinal Angiogenesis in the Model of Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 34. [Google Scholar] [CrossRef]
- Filippi, L.; Nardini, P.; Zizi, V.; Molino, M.; Fazi, C.; Calvani, M.; Carrozzo, F.; Cavallaro, G.; Giuseppetti, G.; Calosi, L.; et al. Β3 Adrenoceptor Agonism Prevents Hyperoxia-Induced Colonic Alterations. Biomolecules 2023, 13, 1755. [Google Scholar] [CrossRef]
- Nardini, P.; Zizi, V.; Molino, M.; Fazi, C.; Calvani, M.; Carrozzo, F.; Giuseppetti, G.; Calosi, L.; Guasti, D.; Biagini, D.; et al. Protective Effects of Beta-3 Adrenoceptor Agonism on Mucosal Integrity in Hyperoxia-Induced Ileal Alterations. Antioxidants 2024, 13, 863. [Google Scholar] [CrossRef]
- Collins, J.M.; Hyland, N.P.; Clarke, G.; Fitzgerald, P.; Julio-Pieper, M.; Bulmer, D.C.; Dinan, T.G.; Cryan, J.F.; O’Mahony, S.M. Beta 3-Adrenoceptor Agonism Ameliorates Early-Life Stress-Induced Visceral Hypersensitivity in Male Rats. J. Neurochem. 2023, 168, 3813–3826. [Google Scholar] [CrossRef] [PubMed]
- Cellek, S.; Thangiah, R.; Bassil, A.K.; Campbell, C.A.; Gray, K.M.; Stretton, J.L.; Lalude, O.; Vivekanandan, S.; Wheeldon, A.; Winchester, W.J.; et al. Demonstration of Functional Neuronal Beta3-Adrenoceptors within the Enteric Nervous System. Gastroenterology 2007, 133, 175–183. [Google Scholar] [CrossRef]
- Seguella, L.; Gulbransen, B.D. Enteric Glial Biology, Intercellular Signalling and Roles in Gastrointestinal Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Parathan, P.; Wang, Y.; Leembruggen, A.J.; Bornstein, J.C.; Foong, J.P. The Enteric Nervous System Undergoes Significant Chemical and Synaptic Maturation during Adolescence in Mice. Dev. Biol. 2020, 458, 75–87. [Google Scholar] [CrossRef]
- Giannone, P.J.; Bauer, J.A.; Schanbacher, B.L.; Reber, K.M. Effects of Hyperoxia on Postnatal Intestinal Development. Biotech. Histochem. 2007, 82, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Chou, H.-C. Hyperoxia Disrupts the Intestinal Barrier in Newborn Rats. Exp. Mol. Pathol. 2016, 101, 44–49. [Google Scholar] [CrossRef]
- Hoffmann, C.; Leitz, M.R.; Oberdorf-Maass, S.; Lohse, M.J.; Klotz, K.-N. Comparative Pharmacology of Human Beta-Adrenergic Receptor Subtypes--Characterization of Stably Transfected Receptors in CHO Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 151–159. [Google Scholar] [CrossRef]
- Dessy, C.; Moniotte, S.; Ghisdal, P.; Havaux, X.; Noirhomme, P.; Balligand, J.L. Endothelial Beta3-Adrenoceptors Mediate Vasorelaxation of Human Coronary Microarteries through Nitric Oxide and Endothelium-Dependent Hyperpolarization. Circulation 2004, 110, 948–954. [Google Scholar] [CrossRef]
- Afeli, S.A.Y.; Rovner, E.S.; Petkov, G.V. BRL37344, a Β3-Adrenergic Receptor Agonist, Decreases Nerve-Evoked Contractions in Human Detrusor Smooth Muscle Isolated Strips: Role of BK Channels. Urology 2013, 82, 744.e1-7. [Google Scholar] [CrossRef]
- Mitidieri, E.; Tramontano, T.; Gurgone, D.; Imbimbo, C.; Mirone, V.; Fusco, F.; Cirino, G.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R. β(3) Adrenergic Receptor Activation Relaxes Human Corpus Cavernosum and Penile Artery through a Hydrogen Sulfide/cGMP-Dependent Mechanism. Pharmacol. Res. 2017, 124, 100–104. [Google Scholar] [CrossRef]
- Takeda, M.; Obara, K.; Mizusawa, T.; Tomita, Y.; Arai, K.; Tsutsui, T.; Hatano, A.; Takahashi, K.; Nomura, S. Evidence for Beta3-Adrenoceptor Subtypes in Relaxation of the Human Urinary Bladder Detrusor: Analysis by Molecular Biological and Pharmacological Methods. J. Pharmacol. Exp. Ther. 1999, 288, 1367–1373. [Google Scholar]
- Pott, C.; Brixius, K.; Bundkirchen, A.; Bölck, B.; Bloch, W.; Steinritz, D.; Mehlhorn, U.; Schwinger, R.H.G. The Preferential Beta3-Adrenoceptor Agonist BRL 37344 Increases Force via Beta1-/Beta2-Adrenoceptors and Induces Endothelial Nitric Oxide Synthase via Beta3-Adrenoceptors in Human Atrial Myocardium. Br. J. Pharmacol. 2003, 138, 521–529. [Google Scholar] [CrossRef]
- Doheny, H.C.; Lynch, C.M.; Smith, T.J.; Morrison, J.J. Functional Coupling of Beta3-Adrenoceptors and Large Conductance Calcium-Activated Potassium Channels in Human Uterine Myocytes. J. Clin. Endocrinol. Metab. 2005, 90, 5786–5796. [Google Scholar] [CrossRef] [PubMed]
- Anthony, A.; Schepelmann, S.; Guillaume, J.L.; Strosberg, A.D.; Dhillon, A.P.; Pounder, R.E.; Wakefield, A.J. Localization of the Beta(Beta)3-Adrenoceptor in the Human Gastrointestinal Tract: An Immunohistochemical Study. Aliment. Pharmacol. Ther. 1998, 12, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-N.; Fung, C.; Vanden Berghe, P. Gut Innervation and Enteric Nervous System Development: A Spatial, Temporal and Molecular Tour de Force. Development 2021, 148, dev182543. [Google Scholar] [CrossRef]
- Cossais, F.; Durand, T.; Chevalier, J.; Boudaud, M.; Kermarrec, L.; Aubert, P.; Neveu, I.; Naveilhan, P.; Neunlist, M. Postnatal Development of the Myenteric Glial Network and Its Modulation by Butyrate. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G941–G951. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, R.; Giancola, F.; Boschetti, E.; Abdo, H.; Lardeux, B.; Neunlist, M. Enteric Glia and Neuroprotection: Basic and Clinical Aspects. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G887–G893. [Google Scholar] [CrossRef]
- McClain, J.; Grubišić, V.; Fried, D.; Gomez-Suarez, R.A.; Leinninger, G.M.; Sévigny, J.; Parpura, V.; Gulbransen, B.D. Ca2+ Responses in Enteric Glia Are Mediated by Connexin-43 Hemichannels and Modulate Colonic Transit in Mice. Gastroenterology 2014, 146, 497–507.e1. [Google Scholar] [CrossRef]
- Neunlist, M.; Van Landeghem, L.; Mahé, M.M.; Derkinderen, P.; des Varannes, S.B.; Rolli-Derkinderen, M. The Digestive Neuronal-Glial-Epithelial Unit: A New Actor in Gut Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 90–100. [Google Scholar] [CrossRef]
- Xue-Jiao, H.; Jian-Hua, F. A Review of the Effects of Early Postnatal Hyperoxia Exposure on the Immature Brain. Exp. Neurol. 2023, 370, 114550. [Google Scholar] [CrossRef]
- Gonzales, J.; Gulbransen, B.D. The Physiology of Enteric Glia. Annu. Rev. Physiol. 2025, 87, 353–380. [Google Scholar] [CrossRef] [PubMed]
- von Boyen, G.B.T.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.-H.; Adler, G.; Kirsch, J. Proinflammatory Cytokines Increase Glial Fibrillary Acidic Protein Expression in Enteric Glia. Gut 2004, 53, 222–228. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K.W. Glial Fibrillary Acidic Protein: From Intermediate Filament Assembly and Gliosis to Neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Lithopoulos, M.A.; Toussay, X.; Zhong, S.; Xu, L.; Mustafa, S.B.; Ouellette, J.; Freitas-Andrade, M.; Comin, C.H.; Bassam, H.A.; Baker, A.N.; et al. Neonatal Hyperoxia in Mice Triggers Long-Term Cognitive Deficits via Impairments in Cerebrovascular Function and Neurogenesis. J. Clin. Investig. 2022, 132, 22. [Google Scholar] [CrossRef]
- Stavely, R.; Ott, L.C.; Sahakian, L.; Rashidi, N.; Sakkal, S.; Nurgali, K. Oxidative Stress and Neural Dysfunction in Gastrointestinal Diseases: Can Stem Cells Offer a Solution? Stem Cells Transl. Med. 2023, 12, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ngala, R.A.; O’Dowd, J.; Wang, S.J.; Stocker, C.; Cawthorne, M.A.; Arch, J.R.S. Beta2-Adrenoceptors and Non-Beta-Adrenoceptors Mediate Effects of BRL37344 and Clenbuterol on Glucose Uptake in Soleus Muscle: Studies Using Knockout Mice. Br. J. Pharmacol. 2009, 158, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Nasser, Y.; Ho, W.; Sharkey, K.A. Distribution of Adrenergic Receptors in the Enteric Nervous System of the Guinea Pig, Mouse, and Rat. J. Comp. Neurol. 2006, 495, 529–553. [Google Scholar] [CrossRef]
- Foong, J.P.P. Postnatal Development of the Mouse Enteric Nervous System. Adv. Exp. Med. Biol. 2016, 891, 135–143. [Google Scholar] [CrossRef]
- Sasidharan, A.; Peethambar, B.A.; Kumar, K.S.; Kumar, A.V.; Hiregange, A.; Fawkes, N.; Collins, J.F.; Grosche, A.; Vidyasagar, S. Advancing Peristalsis Deciphering in Mouse Small Intestine by Multi-Parameter Tracking. Commun. Biol. 2023, 6, 1237. [Google Scholar] [CrossRef]
- Grubišić, V.; Gulbransen, B.D. Enteric Glia: The Most Alimentary of All Glia. J. Physiol. 2017, 595, 557–570. [Google Scholar] [CrossRef]
- Keam, S.J. Mirabegron: Pediatric First Approval. Paediatr. Drugs 2021, 23, 411–415. [Google Scholar] [CrossRef] [PubMed]



| Antigen | Species | Source | Catalog Number | Concentration |
|---|---|---|---|---|
| B3-AR | Rabbit | GeneTex (Irvine, CA, USA) | GTX70685 | 1:300 |
| ChAT | Chicken | GeneTex | GTX85450 | 1:200 |
| GFAP | Chicken | Abcam (Cambrige, UK) | Ab4674 | 1:900 |
| nNOS | Rabbit | Genetex | GTX133403 | 1:500 |
| S100β | Rabbit | Genetex | GTX129573 | 1:500 |
| Sox10 | Rabbit | Abcam | Ab227680 | 1:50 |
| HuC/D | Mouse | Invitrogen (Waltham, MA, USA) | A21271 | 1:200 |
| Secondary Antisera | ||||
| Alexa Fluor 488 | Donkey | Jackson ImmunoResearch (Ely, UK) | 711-545-152 | 1:175 |
| Alexa Fluor 488 | Donkey | Jackson ImmunoResearch | 703-545-155 | 1:175 |
| Alexa Fluor 594 | Donkey | Jackson ImmunoResearch | 711-585-152 | 1:175 |
| Alexa Fluor 594 | Sheep | Jackson ImmunoResearch | 515-585-062 | 1:175 |
| Alexa Fluor 488 | Sheep | Jackson ImmunoResearch | 515-545-062 | 1:175 |
| Alexa Fluor 594 | Bovine | Jackson ImmunoResearch | 805-585-180 | 1:175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardini, P.; Filippi, L.; Zizi, V.; Molino, M.; Fazi, C.; Chivetti, M.; Pini, A. Beta-3 Adrenoceptor Agonism Protects the Enteric Nervous Tissue Against Hyperoxia-Induced Damage. Cells 2025, 14, 475. https://doi.org/10.3390/cells14070475
Nardini P, Filippi L, Zizi V, Molino M, Fazi C, Chivetti M, Pini A. Beta-3 Adrenoceptor Agonism Protects the Enteric Nervous Tissue Against Hyperoxia-Induced Damage. Cells. 2025; 14(7):475. https://doi.org/10.3390/cells14070475
Chicago/Turabian StyleNardini, Patrizia, Luca Filippi, Virginia Zizi, Marta Molino, Camilla Fazi, Matteo Chivetti, and Alessandro Pini. 2025. "Beta-3 Adrenoceptor Agonism Protects the Enteric Nervous Tissue Against Hyperoxia-Induced Damage" Cells 14, no. 7: 475. https://doi.org/10.3390/cells14070475
APA StyleNardini, P., Filippi, L., Zizi, V., Molino, M., Fazi, C., Chivetti, M., & Pini, A. (2025). Beta-3 Adrenoceptor Agonism Protects the Enteric Nervous Tissue Against Hyperoxia-Induced Damage. Cells, 14(7), 475. https://doi.org/10.3390/cells14070475







