c-FOS Confers Stem Cell-like Features to Multiple Myeloma Cells in a Bone Marrow Microenvironment
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Cells and Cell Culture
2.3. In Vitro Co-Culture System with BMSCs to Mimic the BM Microenvironment
2.4. Quantitative Real-Time Reverse Transcription-PCR (qPCR)
2.5. Immunoblotting
2.6. Cell Proliferation Assay
2.7. Chromatin Immunoprecipitation (ChIP) Assay
2.8. Construction and Production of Lentiviral Expression Vector
2.9. Murine Xenograft MM Model
2.10. Statistics
3. Results
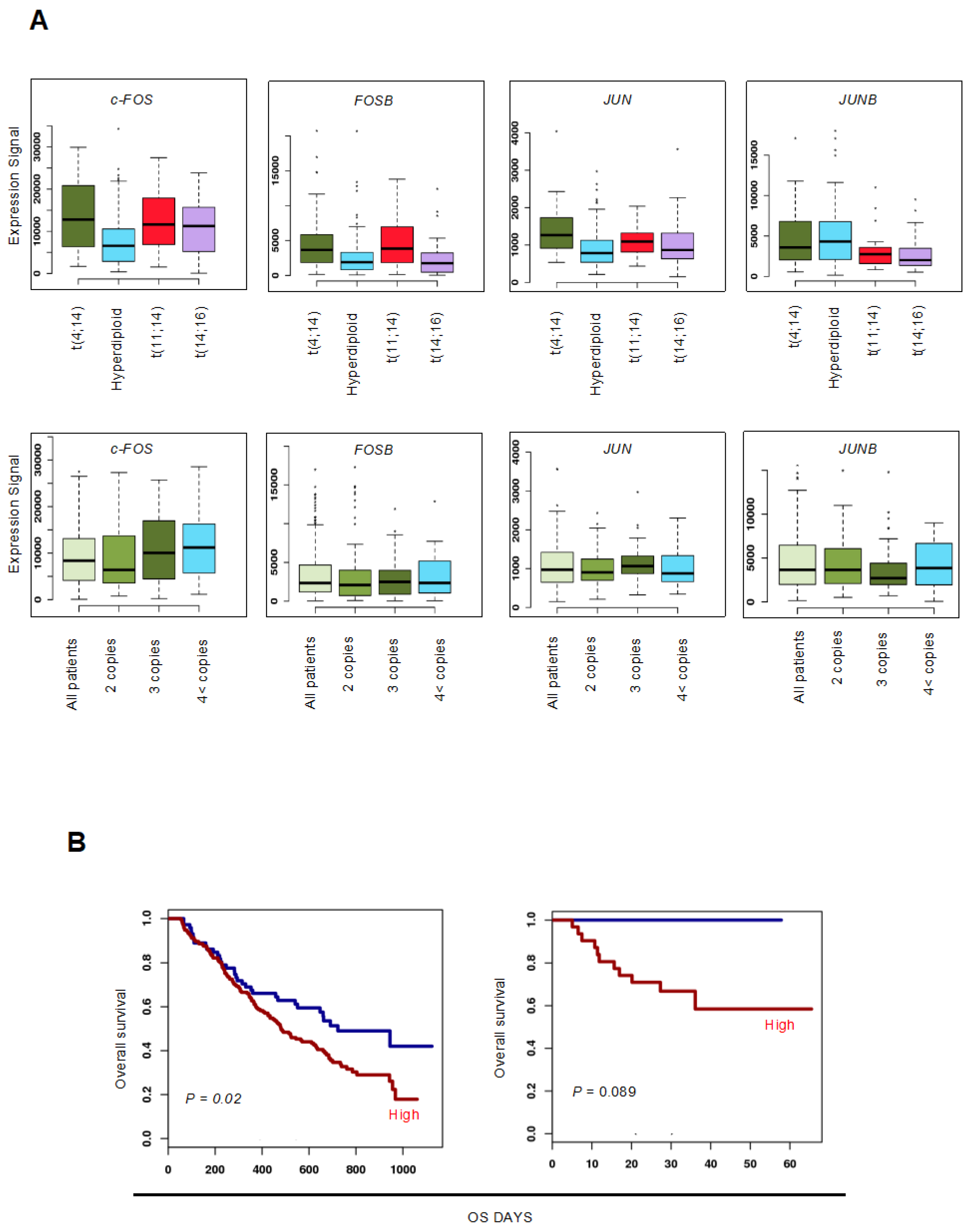
3.1. Upregulation of c-FOS Expression Confers Poor Prognosis in Patients with MM
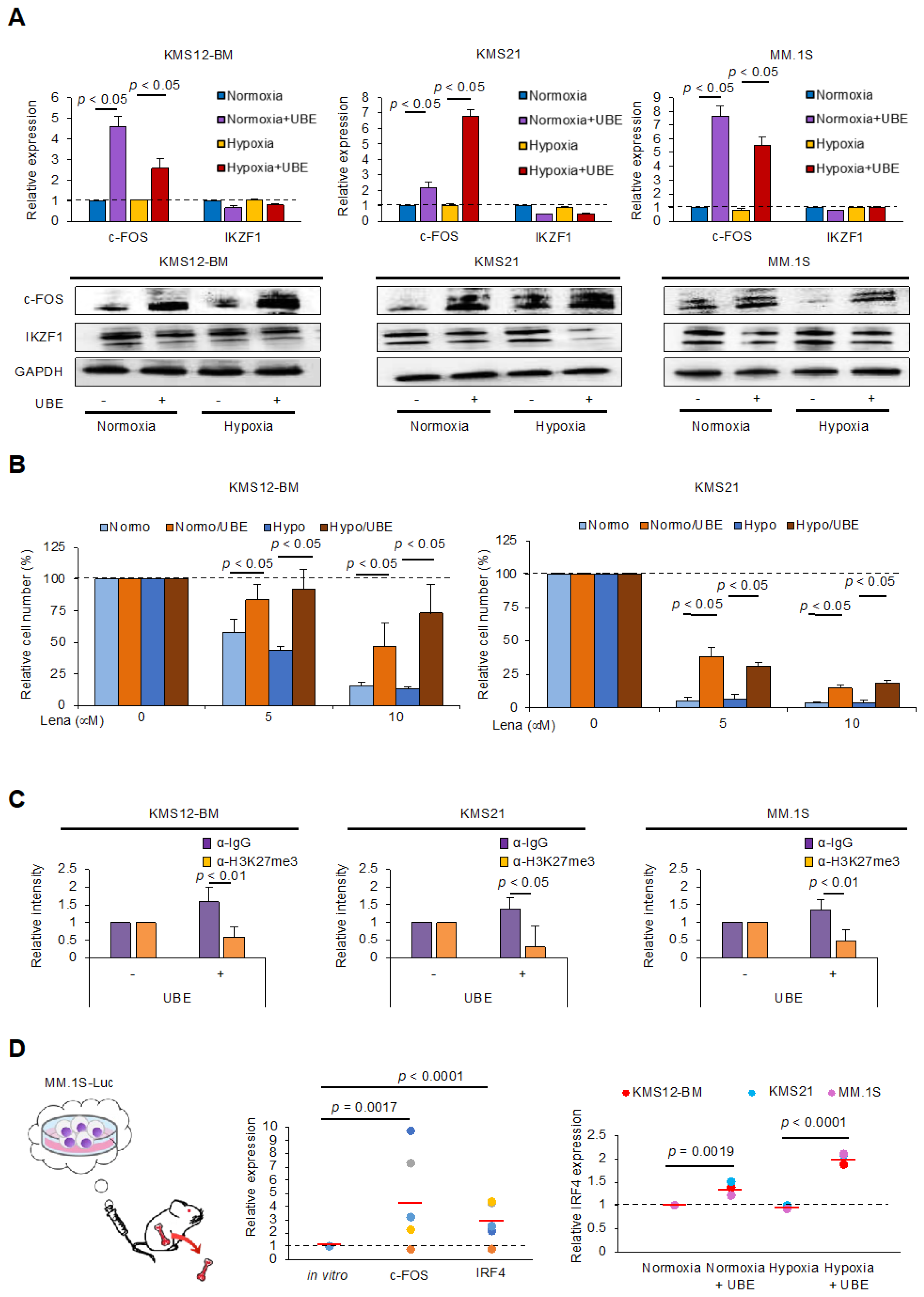
3.2. Upregulation of c-FOS Expression in MM Cells Under Co-Cultured with BMSCs and in the BMME of a Xenograft Murine Model
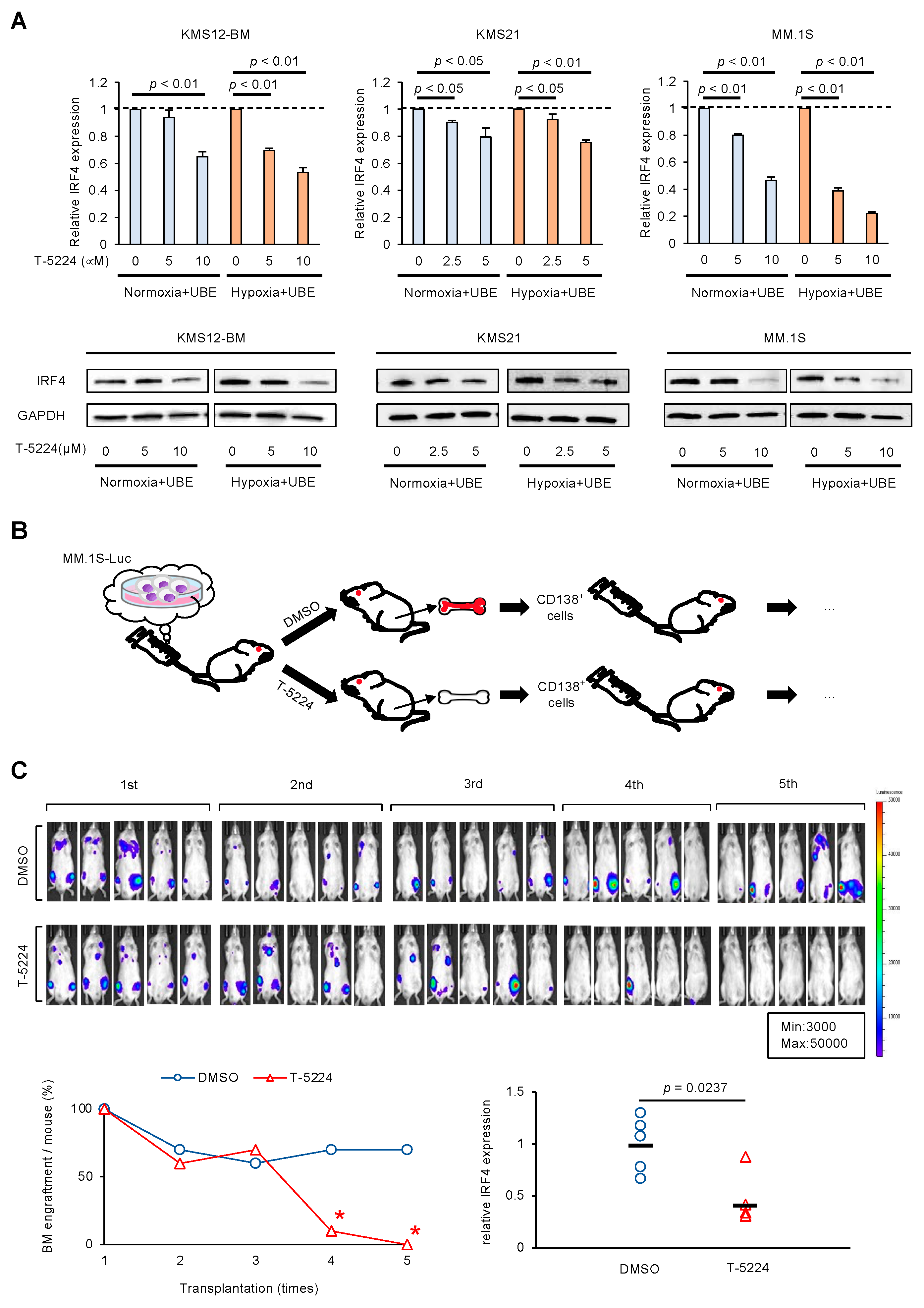
3.3. AP-1 Inhibitor Prevents Regeneration of MM Cells in BMME via IRF4 Downregulation
3.4. AP-1 Inhibitor Reduces the Expression of SLAMF7 in MM Cells Under Adhesion to BMSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MM | multiple myeloma |
| MRD | Minimal residual disease |
| BMME | Bone marrow microenvironment |
| AP-1 | Activator protein-1 |
References
- Minakata, D.; Fujiwara, S.; Yokoyama, D.; Noguchi, A.; Aoe, S.; Oyama, T.; Koyama, S.; Murahashi, R.; Nakashima, H.; Hyodo, K.; et al. Relapsed and refractory multiple myeloma: A systematic review and network meta-analysis of the efficacy of novel therapies. Br. J. Haematol. 2023, 200, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.A.; McDonald, M.M.; Kovacic, N.; Hua Khoo, W.; Terry, R.L.; Down, J. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 2015, 6, 8983. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Prior, T.J.; Masterson, T.; Heuck, C.; Bueno, O.F.; Dash, A.B.; Einsele, H.; Goldschmidt, H.; Knop, S.; Li, C.; et al. EVIDENCE meta-analysis: Evaluating minimal residual disease as an intermediate clinical end point for multiple myeloma. Blood 2024, 144, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, J.; Koyama, D.; Wada, T.; Izumi, T.; Hofgaard, P.O.; Bogen, B.; Furukawa, Y. Phosphorylation-mediated EZH2 inactivation promotes drug resistance in multiple myeloma. J. Clin. Investig. 2015, 125, 4375–4390. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.K.; Hu, J.; Quang, P.; Azab, F.; Pitsillides, C.; Awwad, R.; Thompson, B.; Maiso, P.; Sun, J.D.; Hart, C.P.; et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood 2012, 119, 5782. [Google Scholar] [CrossRef]
- Nefedova, Y.; Landowski, T.H.; Dalton, W.S. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia 2003, 17, 1175–1182. [Google Scholar] [CrossRef]
- Mondala, P.K.; Vora, A.A.; Zhou, T.; Lazzari, E.; Ladel, L.; Luo, X.; Kim, Y.; Costello, C.; MacLeod, A.R.; Jamieson, C.H.; et al. Selective antisense oligonucleotide inhibition of human IRF4 prevents malignant myeloma regeneration via cell cycle disruption. Cell Stem Cell 2021, 28, 623–636. [Google Scholar] [CrossRef]
- Nara, M.; Teshima, K.; Watanabe, A.; Ito, M.; Iwamoto, K.; Kitabayashi, A.; Kume, M.; Hatano, Y.; Takahashi, N.; Iida, S.; et al. Bortezomib reduces the tumorigenicity of multiple myeloma via downregulation of upregulated targets in clonogenic side population cells. PLoS ONE 2013, 8, e56954. [Google Scholar] [CrossRef]
- Osada, N.; Kikuchi, J.; Iha, H.; Yasui, H.; Ikeda, S.; Takahashi, N.; Furukawa, Y. c-FOS is an integral component of the IKZF1 transactivator complex and mediates lenalidomide resistance in multiple myeloma. Clin. Transl. Med. 2023, 13, e1364. [Google Scholar] [CrossRef]
- Binder, M.; Szalat, R.E.; Talluri, S.; Fulciniti, M.; Avet-Loiseau, H.; Parmigiani, G.; Samur, M.K.; Munshi, N.C. Bone marrow stromal cells induce chromatin remodeling in multiple myeloma cells leading to transcriptional changes. Nat. Commun. 2024, 15, 4139. [Google Scholar] [CrossRef]
- Okada, S.; Fukuda, T.; Inada, K.; Tokuhisa, T. Prolonged expression of c-fos suppresses cell cycle entry of dormant hematopoietic stem cells. Blood 1999, 93, 816. [Google Scholar]
- Ibneeva, L.; Singh, S.P.; Sinha, A.; Eski, S.E.; Wehner, R.; Rupp, L.; Kovtun, I.; Pérez-Valencia, J.A.; Gerbaulet, A.; Reinhardt, S.; et al. CD38 promotes hematopoietic stem cell dormancy. PLoS Biol. 2024, 22, e3002517. [Google Scholar]
- Kikuchi, J.; Kodama, N.; Takeshita, M.; Ikeda, S.; Kobayashi, T.; Kuroda, Y.; Uchiyama, M.; Osada, N.; Bogen, B.; Yasui, H.; et al. Extramedullary multiple myeloma originates from hyaluronan-induced homophilic cell-cell interactions of CD44 variant-expressing myeloma cells under shear stress. Blood Adv. 2023, 7, 508–524. [Google Scholar] [PubMed]
- Barlogie, B.; Anaissie, E.; van Rhee, F.; Haessler, J.; Hollmig, K.; Pineda-Roman, M.; Cottler-Fox, M.; Mohiuddin, A.; Alsayed, Y.; Tricot, G.; et al. Incorporating bortezomib into upfront treatment for multiple myeloma: Early results of total therapy 3. Br. J. Haematol. 2007, 138, 176. [Google Scholar]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269. [Google Scholar]
- Ikeda, S.; Tagawa, H. Impact of hypoxia on the pathogenesis and therapy resistance in multiple myeloma. Cancer Sci. 2021, 112, 3995–4004. [Google Scholar]
- Kawano, Y.; Kikukawa, Y.; Fujiwara, S.; Wada, N.; Okuno, Y.; Mitsuya, H.; Hata, H. Hypoxia reduces CD138 expression and induces an immature and stem cell-like transcriptional program in myeloma cells. Int. J. Oncol. 2013, 43, 1809–1816. [Google Scholar]
- Ishibashi, M.; Soeda, S.; Sasaki, M.; Handa, H.; Imai, Y.; Tanaka, N.; Tanosaki, S.; Ito, S.; Odajima, T.; Sugimori, H.; et al. Clinical impact of serum soluble SLAMF7 in multiple myeloma. Oncotarget 2018, 9, 34784–34793. [Google Scholar]
- Kikuchi, J.; Hori, M.; Osada, N.; Matsuoka, S.; Suzuki, A.; Kakugawa, S.; Yasui, H.; Harada, T.; Tenshin, H.; Abe, M.; et al. Soluble SLAMF7 is generated by alternative splicing in multiple myeloma cells. Haematologica 2024, 109, 3414–3418. [Google Scholar]
- O’Connell, P.; Hyslop, S.; Blake, M.K.; Godbehere, S.; Amalfitano, A.; Aldhamen, Y.A. SLAMF7 signaling reprograms T cells toward exhaustion in the tumor microenvironment. J. Immunol. 2020, 206, 193–205. [Google Scholar]
- Awwad, M.H.S.; Mahmoud, A.; Bruns, H.; Echchannaoui, H.; Kriegsmann, K.; Lutz, R. Selective elimination of immunosuppressive T cells in patients with multiple myeloma. Leukemia 2021, 35, 2602–2615. [Google Scholar] [PubMed]
- Kulig, P.; Łuczkowska, K.; Machaliński, B.; Baumert, B. Deep hematologic response to RD treatment in patients with multiple myeloma is associated with overexpression of IL-17R in CD138+ plasma cells. Sci. Rep. 2024, 14, 23559. [Google Scholar]
- Chery, J.; Çakan, E.; Lara, O.D.; Szymanowska, A.; Bayraktar, E.; Chavez-Reyes, A.; Lopez-Berestein, G.; Amero, P.; Rodriguez-Aguayo, C. Therapeutic antisense oligonucleotides in oncology: From bench to bedside. Cancers 2024, 16, 2940–2945. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osada, N.; Kikuchi, J.; Matsuoka, S.; Yasui, H.; Ikeda, S.; Takahashi, N.; Furukawa, Y.; Nakasone, H. c-FOS Confers Stem Cell-like Features to Multiple Myeloma Cells in a Bone Marrow Microenvironment. Cells 2025, 14, 474. https://doi.org/10.3390/cells14070474
Osada N, Kikuchi J, Matsuoka S, Yasui H, Ikeda S, Takahashi N, Furukawa Y, Nakasone H. c-FOS Confers Stem Cell-like Features to Multiple Myeloma Cells in a Bone Marrow Microenvironment. Cells. 2025; 14(7):474. https://doi.org/10.3390/cells14070474
Chicago/Turabian StyleOsada, Naoki, Jiro Kikuchi, Sae Matsuoka, Hiroshi Yasui, Sho Ikeda, Naoto Takahashi, Yusuke Furukawa, and Hideki Nakasone. 2025. "c-FOS Confers Stem Cell-like Features to Multiple Myeloma Cells in a Bone Marrow Microenvironment" Cells 14, no. 7: 474. https://doi.org/10.3390/cells14070474
APA StyleOsada, N., Kikuchi, J., Matsuoka, S., Yasui, H., Ikeda, S., Takahashi, N., Furukawa, Y., & Nakasone, H. (2025). c-FOS Confers Stem Cell-like Features to Multiple Myeloma Cells in a Bone Marrow Microenvironment. Cells, 14(7), 474. https://doi.org/10.3390/cells14070474







