How Do Peripheral Neurons and Glial Cells Participate in Pain Alleviation by Physical Activity?
Abstract
1. Introduction—Peripheral Versus Central Nervous System in Pain Research
Notes on the Terminology
2. Method
3. Physical Exercise and Pain
3.1. Confounding Factors Related to EIA
3.2. Neurochemical Mechanisms of EIA
3.3. Myokines, Exercise, and Pain
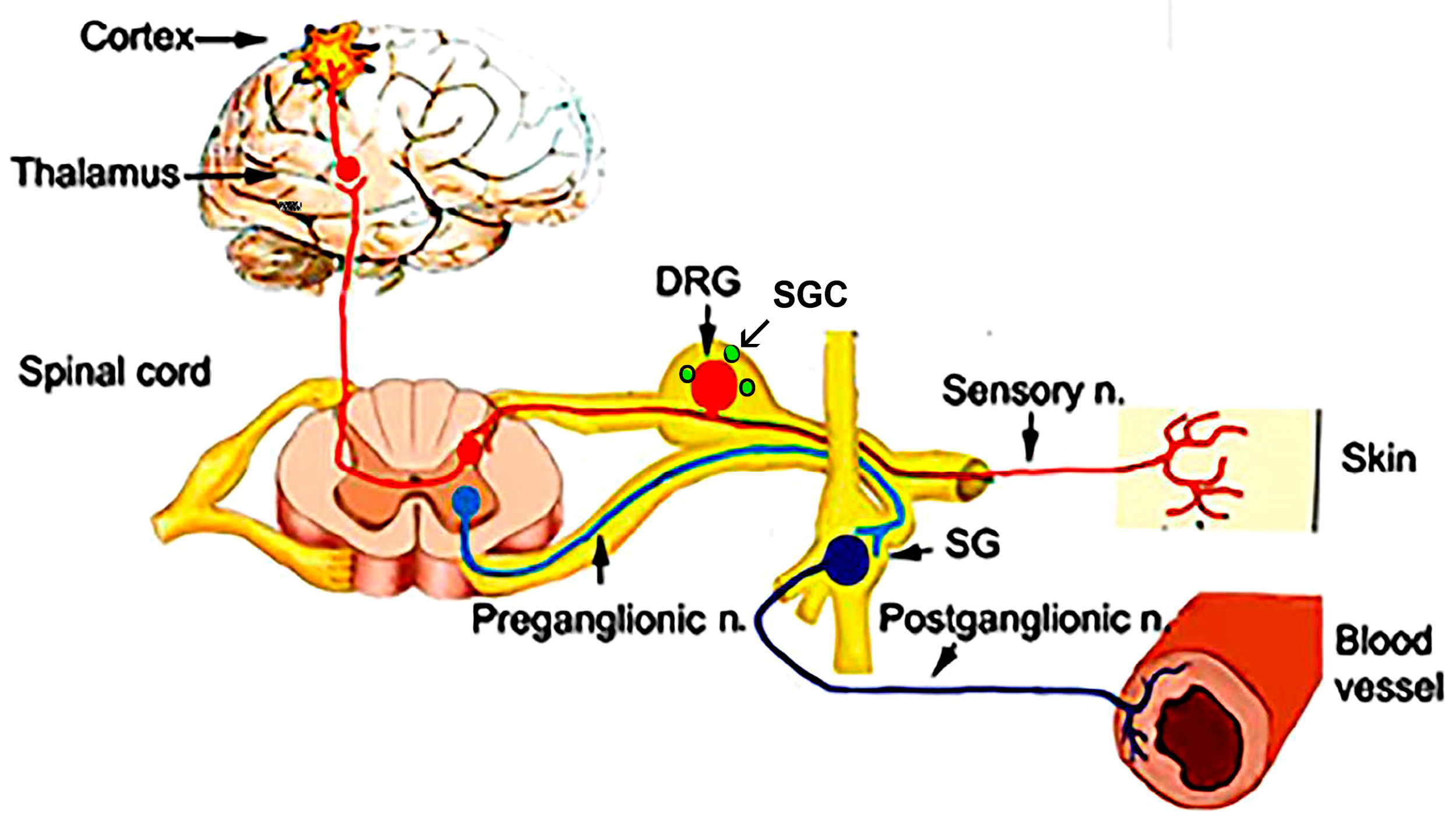
4. Sensory Ganglia and Exercise
4.1. Satellite Glial Cells (SGCs)
SGCs and Immune Disease
5. Axons and Schwann Cells (SCs)
5.1. Charcot–Marie–Tooth Disease
5.2. Guillain–Barré Syndrome
6. The Enteric Nervous System
7. The Peripheral Sympathetic Nervous System
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| CMT | Charcot–Marie–Tooth disease |
| CNS | Central nervous system |
| CRPS | Complex Regional Pain Syndrome |
| Cx43 | Connexin 43 |
| DM | Diabetic mellitus |
| DPN | Diabetic peripheral neuropathy |
| DRG | Dorsal root ganglion |
| ECB | Endocannabinoids |
| EAE | Experimental autoimmune encephalomyelitis |
| EGC | Enteric glial cell |
| EIA | Exercise-induced analgesia |
| ENS | Enteric nervous system |
| GBS | Guillain–Barré syndrome |
| GFAP | Glial fibrillary acidic protein |
| GI | Gastrointestinal |
| IL-6 | Interleukin-6 |
| iNOS | Inducible NO synthase |
| LPS | Lipopolisaccharide |
| NCV | Nerve conduction velocity |
| MS | Multiple sclerosis |
| NO | Nitric oxide |
| PD | Parkinson’s disease |
| PNS | Peripheral nervous system |
| RA | Rheumatoid arthritis |
| SC | Schwann cells |
| SGC | Satellite glial cell |
| SNI | Spared nerve injury |
| SNS | Sympathetic nervous system |
| STZ | Streptozotocin |
| TLR4 | Toll-like-receptor-4 |
| TNF-α | Tumor necrosis factor-α |
| TRPA1 | Transient receptor potential ankyrin1 channel |
| WNT | Wingless and Int-1 |
References
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Smith, P.A. Neuropathic Pain; What We Know and What We Should Do About It. Front. Pain Res. 2023, 4, 1220034. [Google Scholar] [CrossRef] [PubMed]
- Párraga, J.P.; Castellanos, A. A Manifesto in Defense of Pain Complexity: A Critical Review of Essential Insights in Pain Neuroscience. J. Clin. Med. 2023, 12, 7080. [Google Scholar] [CrossRef] [PubMed]
- Abboud, C.; Duveau, A.; Bouali-Benazzouz, R.; Massé, K.; Mattar, J.; Brochoire, L.; Fossat, P.; Boué-Grabot, E.; Hleihel, W.; Landry, M. Animal Models of Pain: Diversity and Benefits. J. Neurosci. Methods 2021, 348, 108997. [Google Scholar] [CrossRef]
- Brum, E.S.; Becker, G.; Fialho, M.F.P.; Oliveira, S.M. Animal Models of Fibromyalgia: What is the Best Choice? Pharmacol. Ther. 2022, 230, 107959. [Google Scholar] [CrossRef]
- Clark, J.D. Preclinical Pain Research: Can We Do Better? Anesthesiology 2016, 125, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Rostock, C.; Schrenk-Siemens, K.; Pohle, J.; Siemens, J. Human vs. Mouse Nociceptors—Similarities and Differences. Neuroscience 2018, 387, 13–27. [Google Scholar] [CrossRef]
- Macionis, V. Nociplastic pain: Controversy of the concept. Korean J. Pain. 2025, 38, 4–13. [Google Scholar] [CrossRef]
- Raja, S.N.; Ringkamp, M.; Guan, Y.; Campbell, J.N.; John, J. Bonica Award Lecture: Peripheral Neuronal Hyperexcitability: The “Low-Hanging” Target for Safe Therapeutic Strategies in Neuropathic Pain. Pain 2020, 161 (Suppl. S1), S14–S26. [Google Scholar] [CrossRef]
- Uniyal, A.; Tiwari, V.; Tsukamoto, T.; Dong, X.; Guan, Y.; Raja, S.N. Targeting Sensory Neuron GPCRs for Peripheral Neuropathic Pain. Trends Pharmacol. Sci. 2023, 44, 1009–1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.N.; Michaelis, M.; Amir, R.; Devor, M. Spinal Nerve Injury Enhances Subthreshold Membrane Potential Oscillations in DRG Neurons: Relation to Neuropathic Pain. J. Neurophysiol. 2000, 84, 205–215. [Google Scholar] [CrossRef]
- Zheng, Q.; Dong, X.; Green, D.P.; Dong, X. Peripheral Mechanisms of Chronic Pain. Med. Rev. 2022, 2, 251–270. [Google Scholar] [CrossRef]
- Vaso, A.; Adahan, H.M.; Gjika, A.; Zahaj, S.; Zhurda, T.; Vyshka, G.; Devor, M. Peripheral Nervous System Origin of Phantom Limb Pain. Pain 2014, 155, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Haroutounian, S.; Nikolajsen, L.; Bendtsen, T.F.; Finnerup, N.B.; Kristensen, A.D.; Hasselstrøm, J.B.; Jensen, T.S. Primary Afferent Input Critical for Maintaining Spontaneous Pain in Peripheral Neuropathy. Pain 2014, 155, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Ringkamp, M.; Raja, S.N. A Sore Spot: Central or Peripheral Generation of Chronic Neuropathic Spontaneous Pain? Pain 2014, 155, 1189–1191. [Google Scholar] [CrossRef]
- Voute, M.; Morel, V.; Pickering, G. Topical Lidocaine for Chronic Pain Treatment. Drug Des. Devel. Ther. 2021, 15, 4091–4103. [Google Scholar] [CrossRef]
- Wilson, A.T.; Pinette, J.; Lyons, K.; Hanney, W.J. Exercise-Induced Hypoalgesia during Different Intensities of a Dynamic Resistance Exercise: A Randomized Controlled Trial. PLoS ONE 2024, 19, e0299481. [Google Scholar] [CrossRef]
- Song, J.S.; Seffrin, A.; Yamada, Y.; Kataoka, R.; Hammert, W.B.; Spitz, R.W.; Wong, V.; Kang, A.; Loenneke, J.P. Can We Improve Exercise-Induced Hypoalgesia with Exercise Training? An Overview and Suggestions for Future Studies. Phys. Ther. Sport 2023, 63, 67–72. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of Chronic Pain in Europe: Prevalence, Impact on Daily Life, and Treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Kinnunen, M.; Verbrugghe, J.; Cunningham, C.; Meeus, M.; Armijo-Olivo, S.; Bandholm, T.; Fullen, B.M.; Wittink, H.; Morlion, B.; et al. Physical Activity Should Be the Primary Intervention for Individuals Living with Chronic Pain: A Position Paper from the European Pain Federation (EFIC) ‘On the Move’ Task Force. Eur. J. Pain 2024, 28, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Spray, D.C. Emerging Importance of Satellite Glia in Nervous System Function and Dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef]
- Milligan, E.D.; Watkins, L.R. Pathological and Protective Roles of Glia in Chronic Pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef]
- Zhu, C.C.; Zheng, Y.L.; Gong, C.; Chen, B.L.; Guo, J.B. Role of Exercise on Neuropathic Pain in Preclinical Models: Perspectives for Neuroglia. Mol. Neurobiol. 2024, 62, 3684–3696. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous Systems Involved in Exercise-Induced Analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar] [CrossRef]
- do Espírito-Santo, R.F.; Margerison, S.M.; Zhang, Y.; Pak, J.; Ro, J.Y.; Da Silva, J.T. Age- and Sex-Dependent Effects of Moderate Exercise on Endogenous Pain Inhibition in Rats. Biomedicines 2024, 12, 1122. [Google Scholar] [CrossRef]
- Cooper, M.A.; Kluding, P.M.; Wright, D.E. Emerging Relationships between Exercise, Sensory Nerves, and Neuropathic Pain. Front. Neurosci. 2016, 10, 372. [Google Scholar] [CrossRef]
- Figueiredo, C.; Padilha, C.S.; Dorneles, G.P.; Peres, A.; Krüger, K.; Rosa-Neto, J.C.; Lira, F.S. Type and Intensity as Key Variables of Exercise in Metainflammation Diseases: A Review. Int. J. Sports Med. 2022, 43, 743–767. [Google Scholar] [CrossRef]
- Ninneman, J.V.; Roberge, G.A.; Stegner, A.J.; Cook, D.B. Exercise Training for Chronic Pain: Available Evidence, Current Recommendations, and Potential Mechanisms. Curr. Top. Behav. Neurosci. 2024, 67, 329–366. [Google Scholar] [CrossRef]
- Law, L.F.; Sluka, K.A. How Does Physical Activity Modulate Pain? Pain 2017, 158, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; Arbat-Plana, A.; Lopez-Alvarez, V.M.; Navarro, X. Neuroprotective Effects of Exercise Treatments After Injury: The Dual Role of Neurotrophic Factors. Curr. Neuropharmacol. 2017, 15, 495–518. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Fabisiak, T.J.; Green-Fulgham, S.M.; Anderson, N.D.; Strand, K.A.; Kwilasz, A.J.; Galer, E.L.; Walker, F.R.; Greenwood, B.N.; Maier, S.F. Prior Voluntary Wheel Running Attenuates Neuropathic Pain. Pain 2016, 157, 2012–2023. [Google Scholar] [CrossRef]
- Detloff, M.R.; Smith, E.J.; Quiros Molina, D.; Ganzer, P.D.; Houlé, J.D. Acute Exercise Prevents the Development of Neuropathic Pain and the Sprouting of Non-Peptidergic (GDNF- and Artemin Responsive) C-Fibers after Spinal Cord Injury. Exp. Neurol. 2014, 255, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.V.; Abner, T.S.S.; Sluka, K.A. Does Exercise Increase or Decrease Pain? Central Mechanisms Underlying These Two Phenomena. J. Physiol. 2017, 595, 4141–4150. [Google Scholar] [CrossRef]
- Liao, P.; He, Q.; Zhou, X.; Ma, K.; Wen, J.; Chen, H.; Li, Q.; Qin, D.; Wang, H. Repetitive Bouts of Exhaustive Exercise Induces a Systemic Inflammatory Response and Multi-Organ Damage in Rats. Front. Physiol. 2020, 11, 685. [Google Scholar] [CrossRef]
- Festa, R.R.; Monsalves-Álvarez, M.; Cancino, J.; Jannas-Vela, S. Prescription of High-Intensity Aerobic Interval Training Based on Oxygen Uptake Kinetics. Int. J. Sports Med. 2023, 44, 159–168. [Google Scholar] [CrossRef]
- Gibala, M.J.; MacInnis, M.J. Physiological Basis of Brief, Intense Interval Training to Enhance Maximal Oxygen Uptake: A Mini-Review. Am. J. Physiol. Cell Physiol. 2022, 323, C1410–C1416. [Google Scholar] [CrossRef]
- Botta, R.M.; Palermi, S.; Tarantino, D. High-intensity interval training for chronic pain conditions: A narrative review. J Exerc. Rehabil. 2022, 18, 10–19. [Google Scholar] [CrossRef]
- Cuenca-Martínez, F.; Sempere-Rubio, N.; Varangot-Reille, C.; Fernández-Carnero, J.; Suso-Martí, L.; Al-ba-Quesada, P.; Touche, R. Effects of High-Intensity Interval Training (HIIT) on Patients with Musculo-skeletal Disorders: A Systematic Review and Meta-Analysis with a Meta-Regression and Mapping Report. Diagnostics 2022, 12, 2532. [Google Scholar] [CrossRef]
- De la Corte-Rodriguez, H.; Roman-Belmonte, J.M.; Resino-Luis, C.; Madrid-Gonzalez, J.; Rodriguez-Merchan, E.C. The Role of Physical Exercise in Chronic Musculoskeletal Pain: Best Medicine-A Narrative Review. Healthcare 2024, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Colt, E.W.; Wardlaw, S.L.; Frantz, A.G. The Effect of Running on Plasma Beta-Endorphin. Life Sci. 1981, 28, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Janal, M.N.; Colt, E.W.D.; Clark, W.C.; Glusman, M. Pain Sensitivity, Mood and Plasma Endocrine Levels in Man Following Long-Distance Running: Effects of Naloxone. Pain 1984, 19, 13–25. [Google Scholar] [CrossRef]
- Brito, R.G.; Rasmussen, L.A.; Sluka, K.A. Regular Physical Activity Prevents Development of Chronic Muscle Pain through Modulation of Supraspinal Opioid and Serotonergic Mechanisms. Pain Rep. 2017, 2, e618. [Google Scholar] [CrossRef] [PubMed]
- Crombie, K.M.; Brellenthin, A.G.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and Opioid System Interactions in Exercise-Induced Hypoalgesia. Pain Med. 2018, 19, 118–123. [Google Scholar] [CrossRef]
- Goldfarb, A.H.; Kraemer, R.R.; Baiamonte, B.A. Endogenous Opioids and Exercise-Related Hypoalgesia: Modern Models, Measurement, and Mechanisms of Action. Adv. Neurobiol. 2024, 35, 137–155. [Google Scholar] [CrossRef]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and Analgesia: The Dual Effect of Nitric Oxide in the Nociceptive System. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef]
- Miclescu, A.; Gordh, T. Nitric Oxide and Pain: ’Something Old, Something New’. Acta Anaesthesiol. Scand. 2009, 53, 1107–1120. [Google Scholar] [CrossRef]
- Belzer, V.; Hanani, M. Nitric Oxide as a Messenger between Neurons and Satellite Glial Cells in Dorsal Root Ganglia. Glia 2019, 67, 1296–1307. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, J.E.; Seo, T.B. Effect of Treadmill Exercise on Pain-Related Wnt/Beta-Catenin Signaling Pathway in Dorsal Root Ganglion Neurons at the Early Phase Regeneration of the Injured Sciatic Nerve. J. Exerc. Rehabil. 2021, 17, 96–102. [Google Scholar] [CrossRef]
- Secondulfo, C.; Mazzeo, F.; Pastorino, G.M.G.; Vicidomini, A.; Meccariello, R.; Operto, F.F. Opioid and Cannabinoid Systems in Pain: Emerging Molecular Mechanisms and Use in Clinical Practice, Health, and Fitness. Int. J. Mol. Sci. 2024, 25, 9407. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, S.G.; Chapman, V.; Finn, D.P.; Hohmann, A.G.; Neugebauer, V. The Cannabinoid System and Pain. Neuropharmacology 2017, 124, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise Activates the Endocannabinoid System. Neuroreport 2003, 14, 2209–2211. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Amital, H.; Shoenfeld, Y. Physical Activity and Autoimmune Diseases: Get Moving and Manage the Disease. Autoimmun. Rev. 2018, 17, 53–72. [Google Scholar] [CrossRef]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef]
- Zheng, Y.N.; Zheng, Y.L.; Wang, X.Q.; Chen, P.J. Role of Exercise on Inflammation Cytokines of Neuropathic Pain in Animal Models. Mol. Neurobiol. 2024, 61, 10288–10301. [Google Scholar] [CrossRef]
- Balchin, C.; Tan, A.L.; Golding, J.; Bissell, L.A.; Wilson, O.J.; McKenna, J.; Stavropoulos-Kalinoglou, A. Acute effects of exercise on pain symptoms, clinical inflammatory markers and inflammatory cytokines in people with rheumatoid arthritis: A systematic literature review. Ther. Adv. Musculoskelet Dis. 2022, 14, 1759720X221114104. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Z.; Zhang, X.A.; Ning, K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise-A Narrative Review. Biomolecules 2024, 14, 1205. [Google Scholar] [CrossRef]
- Slate-Romano, J.J.; Yano, N.; Zhao, T.C. Irisin Reduces Inflammatory Signaling Pathways in Inflammation-Mediated Metabolic Syndrome. Mol. Cell. Endocrinol. 2022, 552, 111676. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hwang, S.M.; Go, E.J.; Kim, Y.H.; Park, C.K. Irisin Alleviates CFA-Induced Inflammatory Pain by Modulating Macrophage Polarization and Spinal Glial Cell Activation. Biomed. Pharmacother. 2024, 178, 117157. [Google Scholar] [CrossRef]
- Takeda, M.; Takahashi, M.; Matsumoto, S. Contribution of the Activation of Satellite Glia in Sensory Ganglia to Pathological Pain. Neurosci. Biobehav. Rev. 2009, 33, 784–792. [Google Scholar] [CrossRef]
- Sankaranarayanan, I.; Tavares-Ferreira, D.; He, L.; Kume, M.; Mwirigi, J.M.; Madsen, T.M.; Petersen, K.A.; Munro, G.; Price, T.J. Meteorin Alleviates Paclitaxel-Induced Peripheral Neuropathic Pain in Mice. J. Pain 2023, 24, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Warwick, R.A.; Hanani, M. The Contribution of Satellite Glial Cells to Chemotherapy-Induced Neuropathic Pain. Eur. J. Pain 2013, 17, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Medrado, L.N.; Oliveira-Junior, S.A.; Martinez, P.F. The Mediating Role of Kinesiophobia in Pain Intensity, Physical Function, and Physical Activity Level in Inflammatory Arthritis. Int. J. Rehabil. Res. 2024, 47, 252–259. [Google Scholar] [CrossRef]
- Hanani, M. Satellite Glial Cells in Sensory Ganglia: From Form to Function. Brain Res. Brain Res. Rev. 2005, 48, 457–476. [Google Scholar] [CrossRef]
- Molteni, R.; Zheng, J.Q.; Ying, Z.; Gómez-Pinilla, F.; Twiss, J.L. Voluntary Exercise Increases Axonal Regeneration from Sensory Neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 8473–8478. [Google Scholar] [CrossRef]
- Cho, Y.H.; Seo, T.B. The Role of Walking Exercise on Axonal Regrowth and Neuropathic Pain Markers in Dorsal Root Ganglion after Sciatic Nerve Injury. J. Exerc. Rehabil. 2023, 19, 320–326. [Google Scholar] [CrossRef]
- Almeida, C.; DeMaman, A.; Kusuda, R.; Cadetti, F.; Ravanelli, M.I.; Queiroz, A.L.; Sousa, T.A.; Zanon, S.; Silveira, L.R.; Lucas, G. Exercise Therapy Normalizes BDNF Upregulation and Glial Hyperactivity in a Mouse Model of Neuropathic Pain. Pain 2015, 156, 504–513. [Google Scholar] [CrossRef]
- Hanani, M. Satellite Glial Cells in Human Disease. Cells 2024, 13, 566. [Google Scholar] [CrossRef]
- Huang, L.Y.; Gu, Y.; Chen, Y. Communication between Neuronal Somata and Satellite Glial Cells in Sensory Ganglia. Glia 2013, 61, 1571–1581. [Google Scholar] [CrossRef]
- Lu, J.; Wang, D.; Xu, J.; Zhang, H.; Yu, W. New Insights on the Role of Satellite Glial Cells. Stem Cell Rev. Rep. 2023, 19, 358–367. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, A.; Ji, R.R. The Similar and Distinct Roles of Satellite Glial Cells and Spinal Astrocytes in Neuropathic Pain. Cells 2023, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Pannese, E. The Structure of the Perineuronal Sheath of Satellite Glial Cells (SGCs) in Sensory Ganglia. Neuron Glia Biol. 2010, 6, 3–10. [Google Scholar] [CrossRef]
- Vit, J.P.; Ohara, P.T.; Bhargava, A.; Kelley, K.; Jasmin, L. Silencing the Kir4.1 Potassium Channel Subunit in Satellite Glial Cells of the Rat Trigeminal Ganglion Results in Pain-like Behavior in the Absence of Nerve Injury. J. Neurosci. 2008, 28, 4161–4171. [Google Scholar] [CrossRef]
- Kushnir, R.; Cherkas, P.S.; Hanani, M. Peripheral Inflammation Upregulates P2X Receptor Expression in Satellite Glial Cells of Mouse Trigeminal Ganglia: A Calcium Imaging Study. Neuropharmacology 2011, 61, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Blum, E.; Procacci, P.; Conte, V.; Hanani, M. Systemic Inflammation Alters Satellite Glial Cell Function and Structure: A Possible Contribution to Pain. Neuroscience 2014, 274, 209–217. [Google Scholar] [CrossRef]
- Spray, D.C.; Hanani, M. Gap Junctions, Pannexins and Pain. Neurosci. Lett. 2019, 695, 46–52. [Google Scholar] [CrossRef]
- Dublin, P.; Hanani, M. Satellite Glial Cells in Sensory Ganglia: Their Possible Contribution to Inflammatory Pain. Brain Behav. Immun. 2007, 21, 1033–1037. [Google Scholar] [CrossRef]
- Huang, T.Y.; Belzer, V.; Hanani, M. Gap Junctions in Dorsal Root Ganglia: Possible Contribution to Visceral Pain. Eur. J. Pain 2010, 14, 49.e1–49.e11. [Google Scholar] [CrossRef]
- Ohara, P.T.; Vit, J.P.; Bhargava, A.; Jasmin, L. Evidence for a Role of Connexin 43 in Trigeminal Pain Using RNA Interference In Vivo. J. Neurophysiol. 2008, 100, 3064–3073. [Google Scholar] [CrossRef]
- Goebel, A.; Krock, E.; Gentry, C.; Israel, M.R.; Jurczak, A.; Urbina, C.M.; Sandor, K.; Vastani, N.; Maurer, M.; Cuhadar, U.; et al. Passive Transfer of Fibromyalgia Symptoms from Patients to Mice. J. Clin. Investig. 2021, 131, e144201. [Google Scholar] [CrossRef] [PubMed]
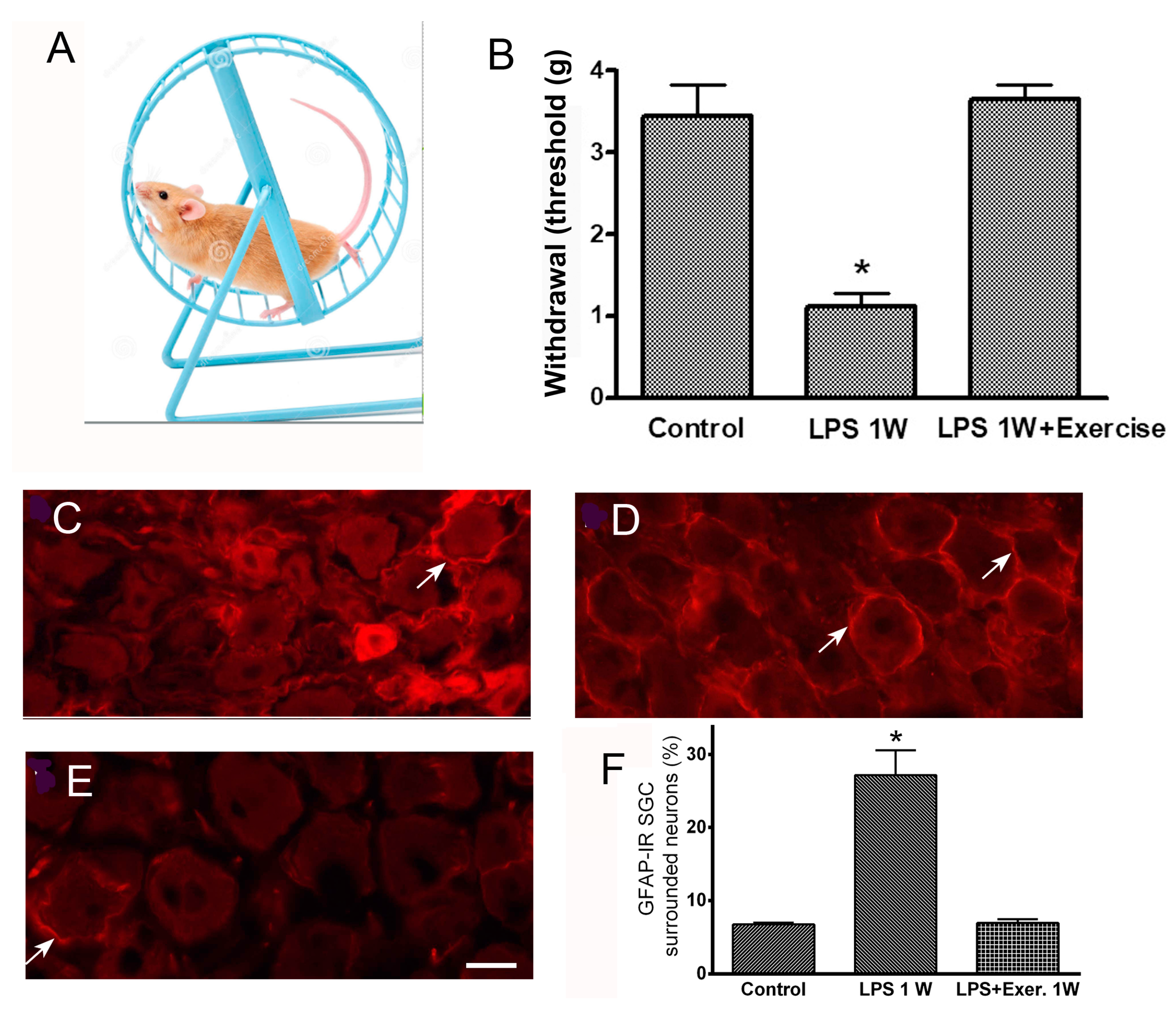
- Feldman-Goriachnik, R.; Blum, E.; Hanani, M. Exercise Reduces Pain Behavior and Pathological Changes in Dorsal Root Ganglia Induced by Systemic Inflammation in Mice. Neurosci. Lett. 2022, 778, 136616. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic Neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Craner, M.J.; Klein, J.P.; Renganathan, M.; Black, J.A.; Waxman, S.G. Changes of Sodium Channel Expression in Experimental Painful Diabetic Neuropathy. Ann. Neurol. 2002, 52, 786–792. [Google Scholar] [CrossRef]
- Miyashita, A.; Kobayashi, M.; Yokota, T.; Zochodne, D.W. Diabetic Polyneuropathy: New Strategies to Target Sensory Neurons in Dorsal Root Ganglia. Int. J. Mol. Sci. 2023, 24, 5977. [Google Scholar] [CrossRef]
- Gonçalves, N.P.; Vægter, C.B.; Pallesen, L.T. Peripheral Glial Cells in the Development of Diabetic Neuropathy. Front. Neurol. 2018, 9, 268. [Google Scholar] [CrossRef]
- Singleton, J.R.; Foster-Palmer, S.; Marcus, R.L. Exercise as Treatment for Neuropathy in the Setting of Diabetes and Prediabetic Metabolic Syndrome: A Review of Animal Models and Human Trials. Curr. Diabetes Rev. 2022, 18, e230921196752. [Google Scholar] [CrossRef]
- Hanani, M.; Blum, E.; Liu, S.; Peng, L.; Liang, S. Satellite Glial Cells in Dorsal Root Ganglia Are Activated in Streptozotocin-Treated Rodents. J. Cell. Mol. Med. 2014, 18, 2367–2371. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Li, L.; Zou, L.; Gong, Y.; Jia, T.; Zhao, S.; Yuan, H.; Shi, L.; Liu, S.; et al. P2Y12 shRNA Treatment Decreases SGC Activation to Relieve Diabetic Neuropathic Pain in Type 2 Diabetes Mellitus Rats. J. Cell Physiol. 2018, 233, 9620–9628. [Google Scholar] [CrossRef]
- Katz, P.; Andonian, B.J.; Huffman, K.M. Benefits and Promotion of Physical Activity in Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2020, 32, 307–314. [Google Scholar] [CrossRef]
- Jurczak, A.; Sandor, K.; Bersellini Farinotti, A.; Krock, E.; Hunt, M.A.; Agalave, N.M.; Barbier, J.; Simon, N.; Wang, Z.; Rudjito, R.; et al. Insights into FcγR Involvement in Pain-like Behavior In-duced by an RA-Derived Anti-Modified Protein Autoantibody. Brain Behav. Immun. 2023, 113, 212–227. [Google Scholar] [CrossRef]
- Bernardes, D.; Brambilla, R.; Bracchi-Ricard, V.; Karmally, S.; Dellarole, A.; Carvalho-Tavares, J.; Bethea, J.R. Prior Regular Exercise Improves Clinical Outcome and Reduces Demyelination and Axonal Injury in Experimental Autoimmune Encephalomyelitis. J. Neurochem. 2016, 136 (Suppl. S1), 63–73. [Google Scholar] [CrossRef]
- Warwick, R.A.; Ledgerwood, C.J.; Brenner, T.; Hanani, M. Satellite Glial Cells in Dorsal Root Ganglia Are Activated in Experimental Autoimmune Encephalomyelitis. Neurosci. Lett. 2014, 569, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, C.; Saveri, P.; Pareyson, D. Challenges in Treating Charcot-Marie-Tooth Disease and Related Neuropathies: Current Management and Future Perspectives. Brain Sci. 2021, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Berciano, J. Axonal Degeneration in Guillain-Barré Syndrome: A Reappraisal. J. Neurol. 2021, 268, 3728–3743. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.; Klein, C.J. Guillain-Barré Syndrome. Mayo Clin. Proc. 2017, 92, 467–479. [Google Scholar] [CrossRef]
- Linan-Rico, A.; Ochoa-Cortes, F.; Schneider, R.; Christofi, F.L. Mini-Review: Enteric Glial Cell Reactions to Inflammation and Potential Therapeutic Implications for GI Diseases, Motility Disorders, and Abdominal Pain. Neurosci. Lett. 2023, 812, 137395. [Google Scholar] [CrossRef]
- Morales-Soto, W.; Gonzales, J.; Jackson, W.F.; Gulbransen, B.D. Enteric Glia Promote Visceral Hypersensitivity during Inflammation through Intercellular Signaling with Gut Nociceptors. Sci. Signal. 2023, 16, eadg1668. [Google Scholar] [CrossRef]
- Montalbán-Rodríguez, A.; Abalo, R.; López-Gómez, L. From the Gut to the Brain: The Role of Enteric Glial Cells and Their Involvement in the Pathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 1294. [Google Scholar] [CrossRef]
- Thomasi, B.; Valdetaro, L.; Ricciardi, M.C.; Gonçalves de Carvalho, M.; Fialho Tavares, I.; Tavares-Gomes, A.L. Enteric Glia as a Player of Gut-Brain Interactions during Parkinson’s Disease. Front. Neurosci. 2023, 17, 1281710. [Google Scholar] [CrossRef]
- De Logu, F.; De Prá, S.D.; de David Antoniazzi, C.T.; Kudsi, S.Q.; Ferro, P.R.; Landini, L.; Rigo, F.K.; de Bem Silveira, G.; Silveira, P.C.L.; Oliveira, S.M.; et al. Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain Behav. Immun. 2020, 88, 535–546. [Google Scholar] [CrossRef]
- Albrecht, P.J.; Hines, S.; Eisenberg, E.; Pud, D.; Finlay, D.R.; Connolly, K.M.; Paré, M.; Davar, G.; Rice, F.L. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain 2006, 120, 244–266. [Google Scholar] [CrossRef]
- Li, J.; Guan, R.; Pan, L. Mechanism of Schwann Cells in Diabetic Peripheral Neuropathy: A Review. Medicine 2023, 102, e32653. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Alonso, J.; Calvo-Enrique, L.; Paricio-Montesinos, R.; Kumar, R.; Zhang, M.D.; Poulet, J.F.A.; Ernfors, P.; Lewin, G.R. Sensory Schwann Cells Set Perceptual Thresholds for Touch and Selectively Regulate Mechanical Nociception. Nat. Commun. 2024, 15, 898. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.L.; Xie, Z.L.; Wu, Y.W.; Duan, W.R.; Xie, Y.K. Early Demyelination of Primary A-Fibers Induces a Rapid-Onset of Neuropathic Pain in Rat. Neuroscience 2012, 200, 186–198. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- Zhou, Y.; Notterpek, L. Promoting Peripheral Myelin Repair. Exp. Neurol. 2016, 283, 573–580. [Google Scholar] [CrossRef]
- Goulart, C.O.; Jürgensen, S.; Souto, A.; Oliveira, J.T.; de Lima, S.; Tonda-Turo, C.; Marques, S.A.; de Almeida, F.M.; Martinez, A.M. A Combination of Schwann-Cell Grafts and Aerobic Exercise Enhances Sciatic Nerve Regeneration. PLoS ONE 2014, 9, e110090. [Google Scholar] [CrossRef]
- Klein, D.; Yuan, X.; Weiß, E.M.; Martini, R. Physical Exercise Mitigates Neuropathic Changes in an Animal Model for Charcot-Marie-Tooth Disease 1X. Exp. Neurol. 2021, 343, 113786. [Google Scholar] [CrossRef]
- Shah, N.; Shrivastava, M.; Kumar, S.; Nagi, R.S. Supervised, Individualised Exercise Reduces Fatigue and Improves Strength and Quality of Life More than Unsupervised Home Exercise in People with Chronic Guillain-Barré Syndrome: A Randomised Trial. J. Physiother. 2022, 68, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Mawe, G.M. The Enteric Nervous System. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef]
- Seguella, L.; Gulbransen, B.D. Enteric Glial Biology, Intercellular Signalling and Roles in Gastrointestinal Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Hanani, M.; Reichenbach, A. Morphology of Horseradish Peroxidase (HRP)-Injected Glial Cells in the Myenteric Plexus of the Guinea-Pig. Cell Tissue Res. 1994, 278, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gabella, G. Enteric Glia: Extent, Cohesion, Axonal Contacts, Membrane Separations, and Mitochondria in Auerbach’s Ganglia of Guinea Pigs. Cell Tissue Res. 2022, 389, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, V.; McClain, J.L.; Fried, D.E.; Grants, I.; Rajasekhar, P.; Csizmadia, E.; Ajijola, O.A.; Watson, R.E.; Poole, D.P.; Robson, S.C.; et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020, 32, 108100. [Google Scholar] [CrossRef]
- Shannon, K.; Vanden Berghe, P. The Enteric Nervous System in PD: Gateway, Bystander Victim, or Source of Solutions. Cell Tissue Res. 2018, 373, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Magierowski, M.; Kwiecien, S.; Wojcik, D.; Ptak-Belowska, A.; Surmiak, M.; Targosz, A.; Magierowska, K.; Brzozowski, T. Exploiting Significance of Physical Exercise in Prevention of Gastrointestinal Disorders. Curr. Pharm. Des. 2018, 24, 1916–1925. [Google Scholar] [CrossRef]
- Cronin, O.; Molloy, M.G.; Shanahan, F. Exercise, Fitness, and the Gut. Curr. Opin. Gastroenterol. 2016, 32, 67–73. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Crowley, E.K.; Brown, J.R.; O’Sullivan, O.; O’Leary, O.F.; Timmons, S.; Nolan, Y.M.; Clarke, D.J.; Hyland, N.P.; Joyce, S.A.; et al. Nigral Overexpression of Alpha-Synuclein in a Rat Parkinson’s Disease Model Indicates Alterations in the Enteric Nervous System and the Gut Microbiome. Neurogastroenterol. Motil. 2020, 32, e13726. [Google Scholar] [CrossRef]
- Day, M. Sympathetic Blocks: The Evidence. Pain Pract. 2008, 8, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, O.H.; Kenis-Coskun, O. Ganglion Blocks as a Treatment of Pain: Current Perspectives. J. Pain Res. 2017, 10, 2815–2826. [Google Scholar] [CrossRef]
- Duong, S.; Bravo, D.; Todd, K.J.; Finlayson, R.J.; Tran, Q. Treatment of Complex Regional Pain Syndrome: An Updated Systematic Review and Narrative Synthesis. Can. J. Anaesth. 2018, 65, 658–684. [Google Scholar] [CrossRef]
- Shi, X.; Guo, T.; Li, W.; Sahbaie, P.; Rice, K.C.; Sulima, A.; Clark, J.D.; Kingery, W.S. Exercise Reverses Nociceptive Sensitization, Upregulated Neuropeptide Signaling, Inflammatory Changes, Anxiety, and Memory Impairment in a Mouse Tibia Fracture Model. Anesthesiology 2018, 129, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Kiyomoto, K.; Emori, M.; Hanaka, M.; Teramoto, A.; Hayakawa, H.; Takashima, K.; Yamashita, T.; Iba, K. Remission of Hypersensitivity by Simple Weight Load Stimuli in a Complex Regional Pain Syndrome Mouse Model. J. Orthop. Res. 2024, 42, 1020–1032. [Google Scholar] [CrossRef]
- McLachlan, E.M.; Jänig, W.; Devor, M.; Michaelis, M. Peripheral Nerve Injury Triggers Noradrenergic Sprouting within Dorsal Root Ganglia. Nature 1993, 363, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xie, W.; Lückemeyer, D.D.; Lay, M.; Wang, X.W.; Dong, X.; Limjunyawong, N.; Ye, Y.; Zhou, F.Q.; Strong, J.A.; et al. Synchronized Cluster Firing: A Distinct Form of Sensory Neuron Activation Drives Spontaneous Pain. Neuron 2022, 110, 209–220.e6. [Google Scholar] [CrossRef]
- Tank, A.W.; Lee Wong, D. Peripheral and Central Effects of Circulating Catecholamines. Compr. Physiol. 2015, 5, 1–15. [Google Scholar] [CrossRef]
| Name of Disorder | Suggested Peripheral Cells Involved | Comment | References |
|---|---|---|---|
| Fibromyalgia | Satellite glia, nerve fibers | Human immunoglobulins tested in mice | [82] |
| Multiple sclerosis | Satellite glia | Mouse model | [94] |
| Diabetes | Satellite glia | Rat model | [90] |
| Rheumatoid arthritis | Satellite glia | Mouse model | [92] |
| Charcot–Marie–Tooth | Schwann cells | Humans | [95] |
| Guillain–Barre | Axons | Humans | [96,97] |
| Intestinal inflammation | Enteric glia | Mouse models | [98,99] |
| Parkinson’s disease | Enteric neurons and glia | Humans, rodent models | [100,101] |
| Complex Regional Pain | * Schwann cells, * macrophages # Sympathetic nerves | * Mouse model, # humans | [102] *, [103] # |
| Systemic inflammation | Satellite glia | Mouse model | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanani, M. How Do Peripheral Neurons and Glial Cells Participate in Pain Alleviation by Physical Activity? Cells 2025, 14, 462. https://doi.org/10.3390/cells14060462
Hanani M. How Do Peripheral Neurons and Glial Cells Participate in Pain Alleviation by Physical Activity? Cells. 2025; 14(6):462. https://doi.org/10.3390/cells14060462
Chicago/Turabian StyleHanani, Menachem. 2025. "How Do Peripheral Neurons and Glial Cells Participate in Pain Alleviation by Physical Activity?" Cells 14, no. 6: 462. https://doi.org/10.3390/cells14060462
APA StyleHanani, M. (2025). How Do Peripheral Neurons and Glial Cells Participate in Pain Alleviation by Physical Activity? Cells, 14(6), 462. https://doi.org/10.3390/cells14060462







