Efficacy of Deferoxamine Mesylate in Serum and Serum-Free Media: Adult Ventral Root Schwann Cell Survival Following Hydrogen Peroxide-Induced Cell Death
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation and Expansion of Schwann Cell Culture
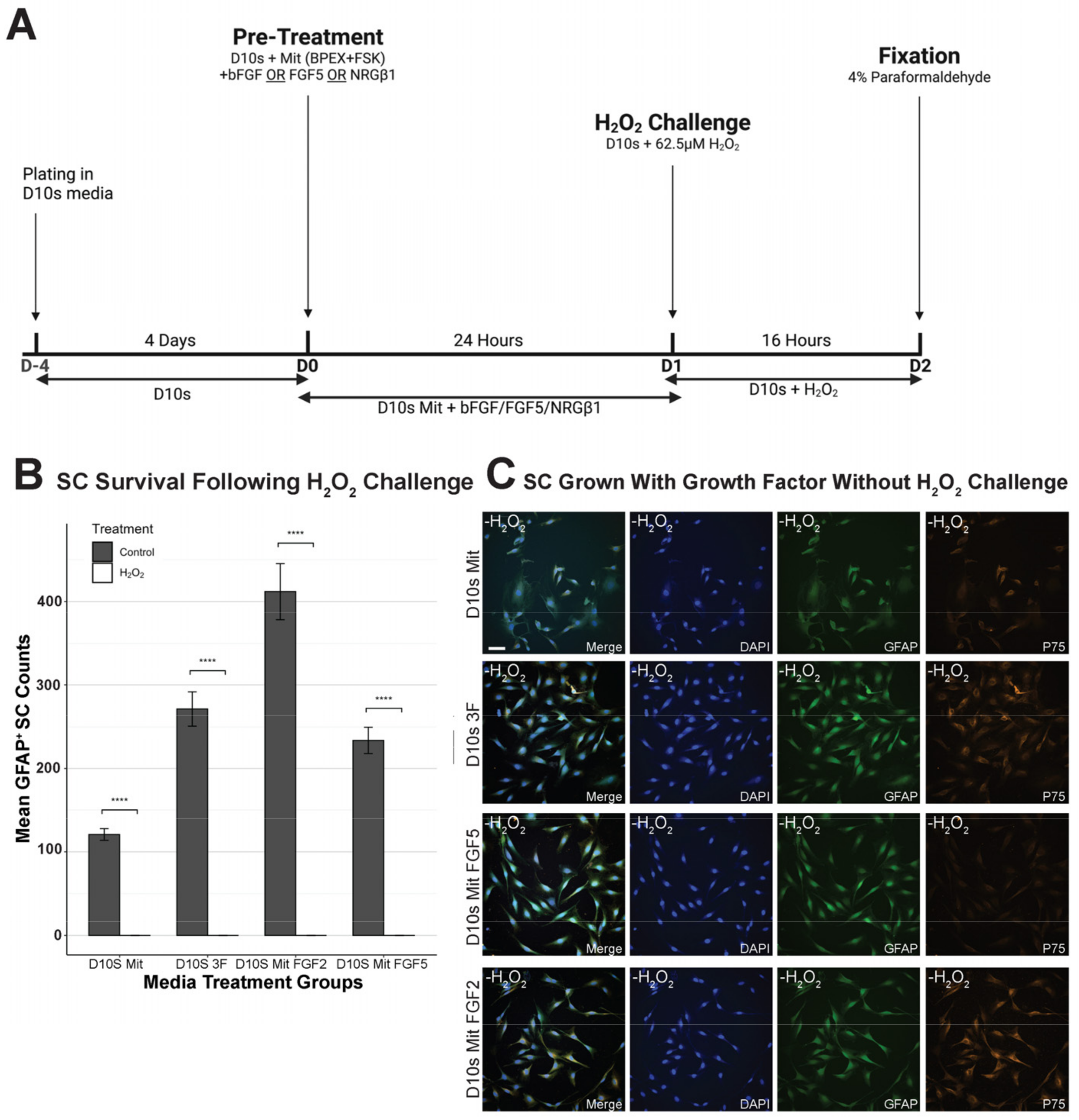
2.2. Experimental Treatments and Timeline
2.3. Immunofluorescence Staining
2.4. Confocal Microscopy
2.5. Survival Quantification
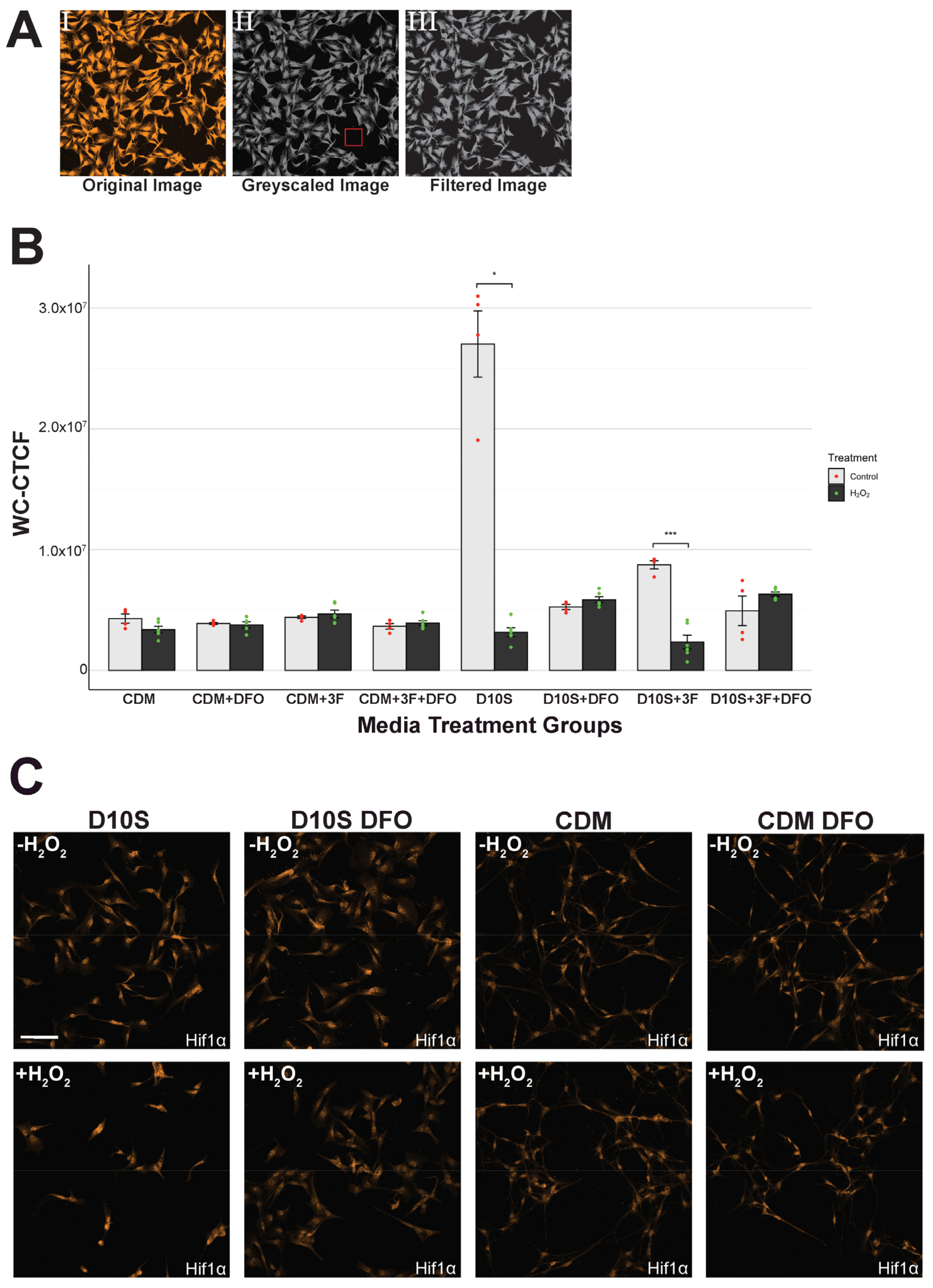
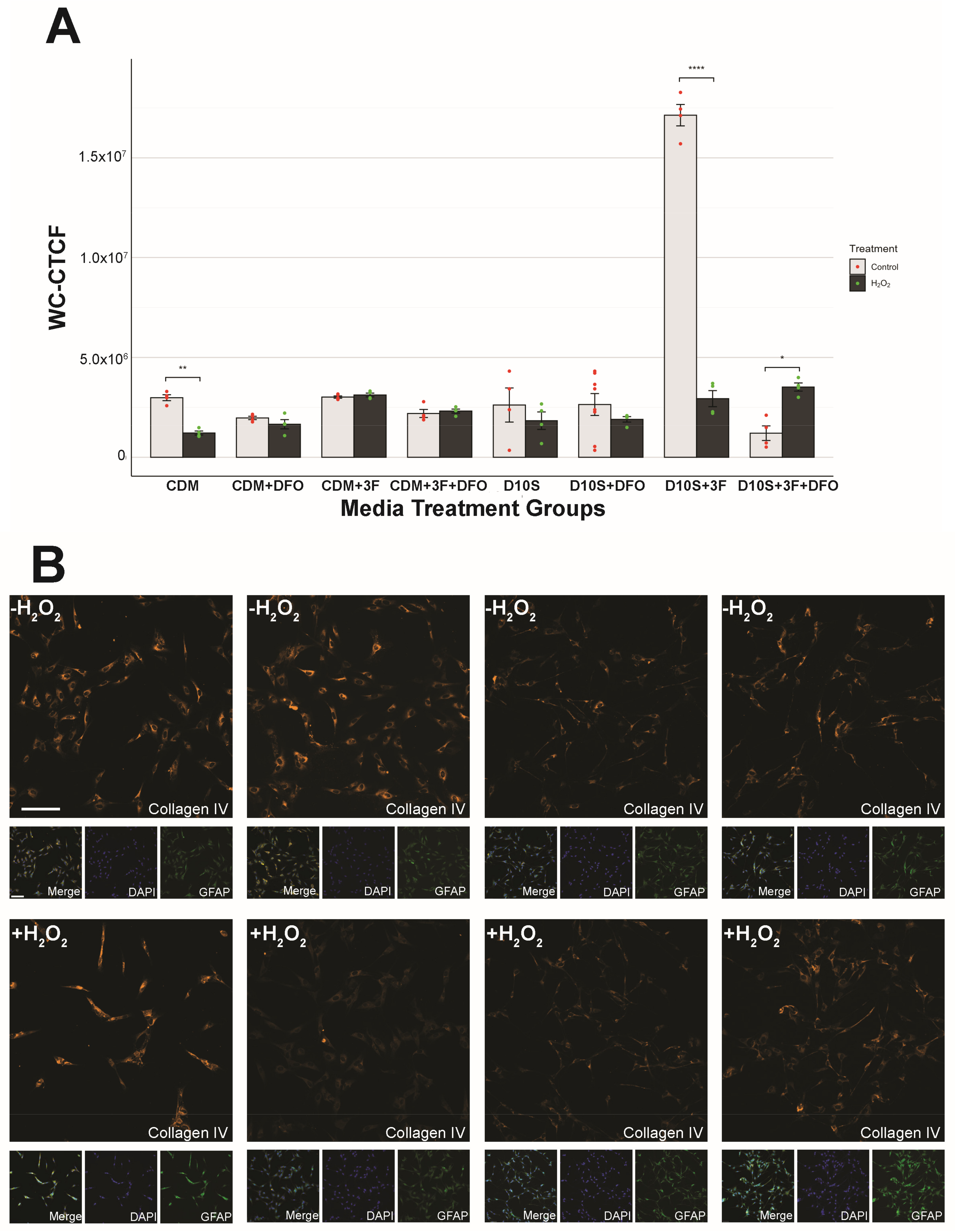
2.6. Hif1α and Collagen IV Quantification
2.7. Statistical Analysis
2.8. Cell Death Transcriptomics
2.8.1. RNA Isolation
2.8.2. cDNA Synthesis
2.8.3. qPCR
2.8.4. Gene Ontology Analysis
3. Results
3.1. Variation in SC Morphology Between Serum-Containing and Serum-Free Conditions
3.2. Growth Factors Failed to Promote SC Survival Following H2O2 Exposure
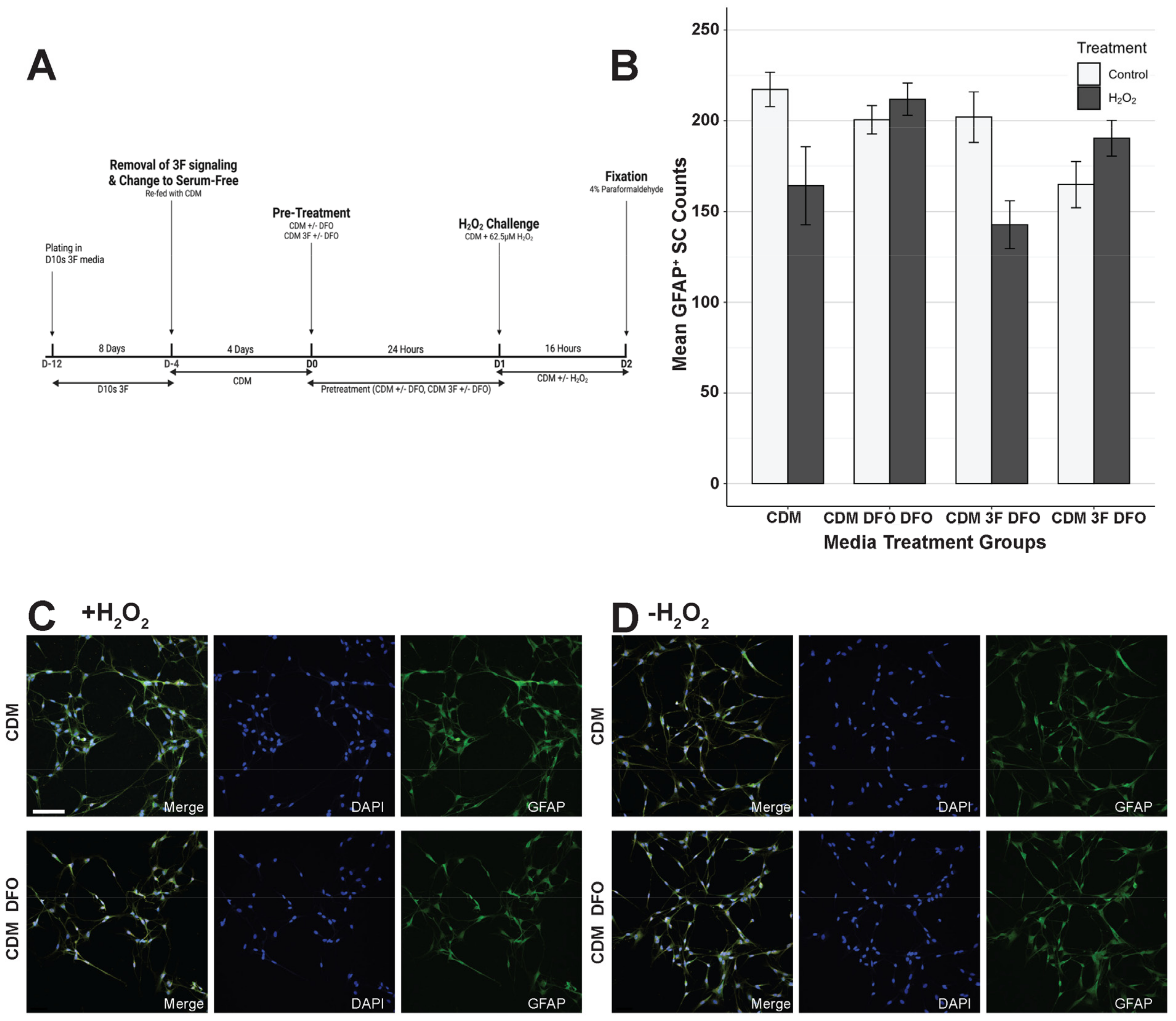
3.3. Deferoxamine Mesylate (DFO) Increased SC Survival Following H2O2 Challenge in Serum-Containing Conditions
3.4. Serum-Free Media Protected SCs Against H2O2-Induced Cell Death Without DFO
3.5. Hif1α Expression Consistent in Serum-Free Conditions but Inconsistent in Serum Conditions
3.6. Serum Drives High Variability in Collagen IV Expression
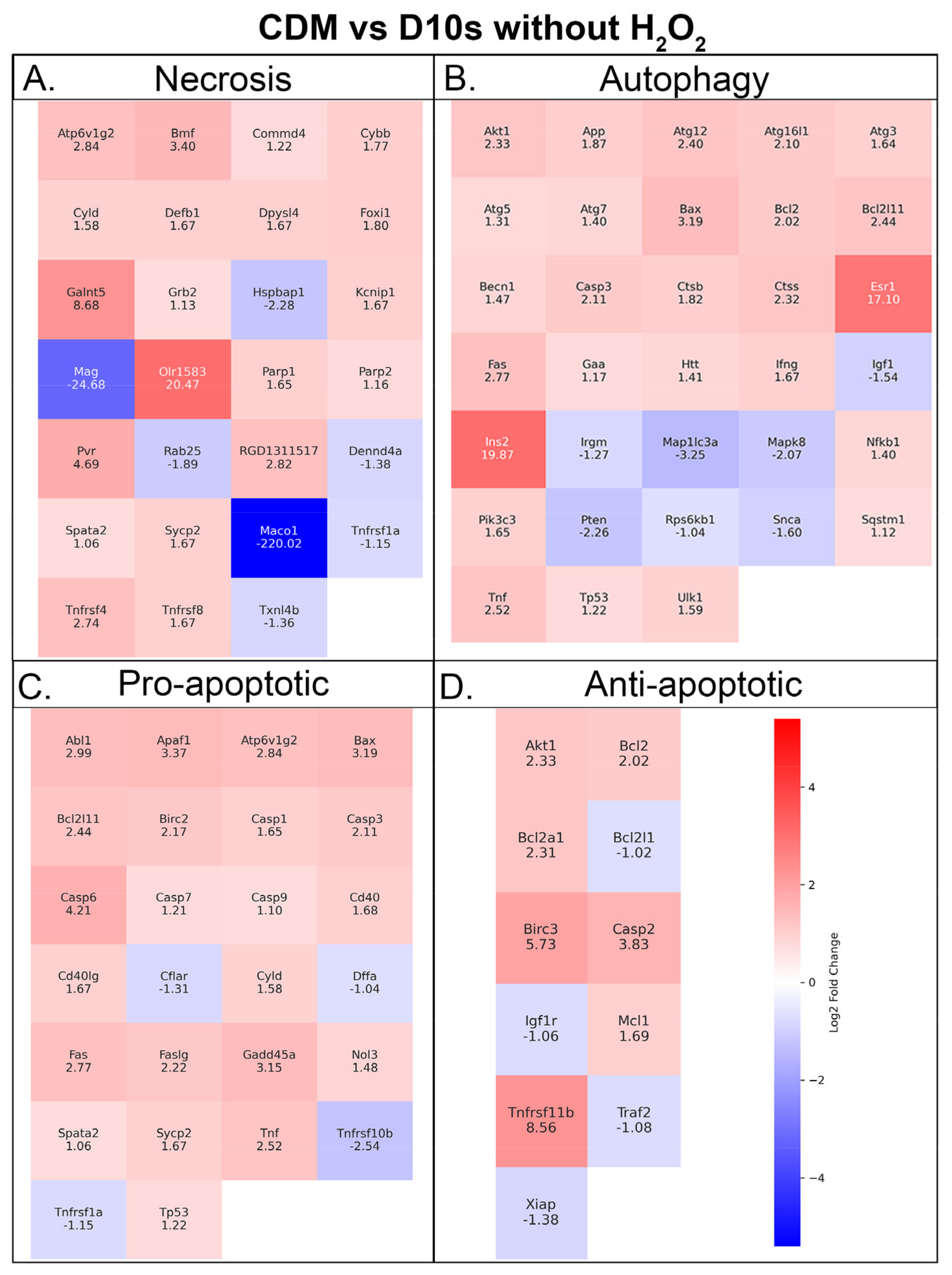
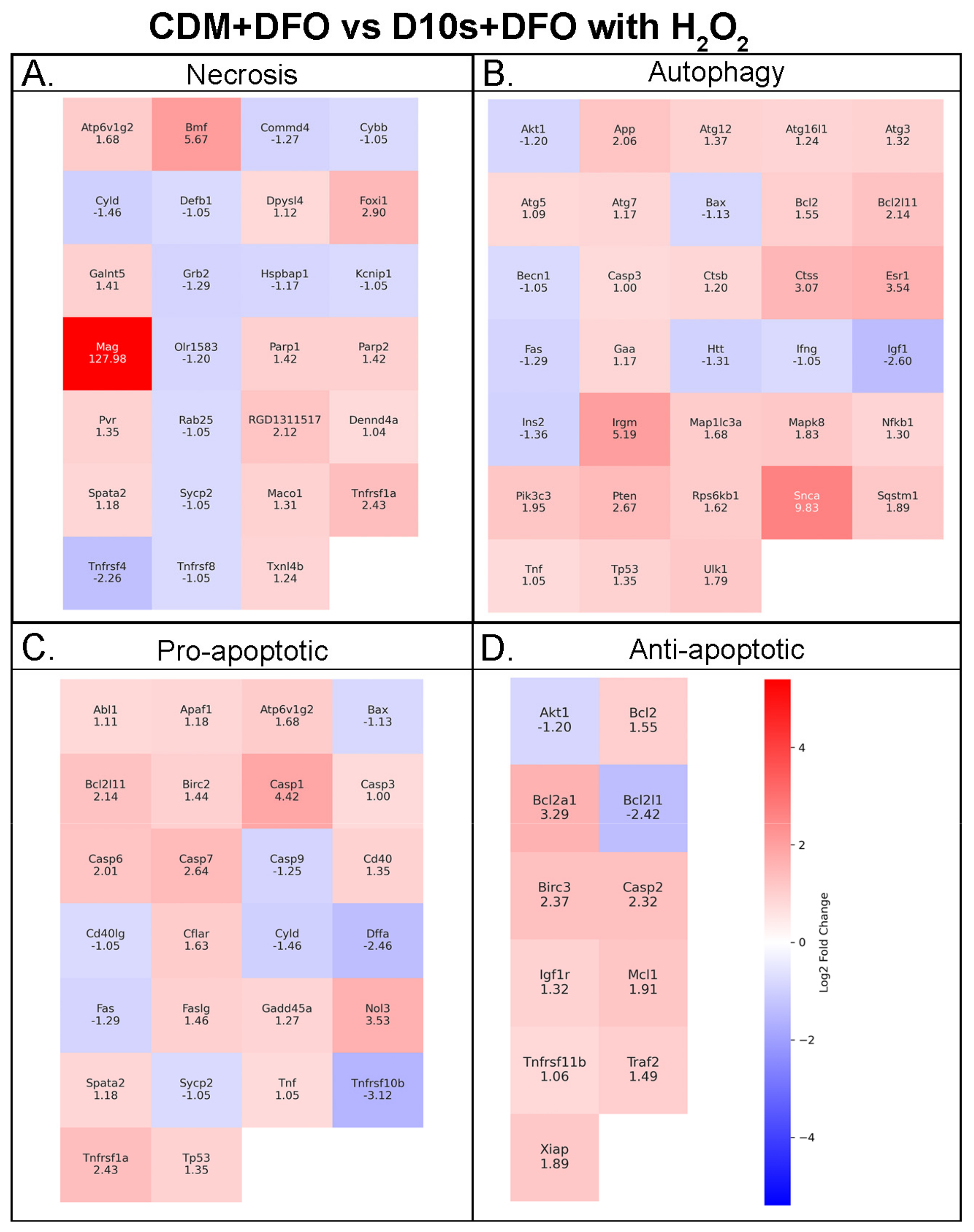
3.7. Serum vs. Serum-Free Conditions Show Differential Transcriptional Responses in Cell Death Pathways
4. Discussion
4.1. Distinctive SC Morphology in Serum and Serum-Free Conditions
4.2. Growth Factors Provide No Protection Against H2O2-Induced Cell Death
4.3. DFO Efficacy in Serum and Serum-Free Conditions
4.4. Transferrin in CDM Medium May Influence HiF-PhD to Override DFO Effects in SCs
4.5. Nuclear Expression of Hif1α Facilitates Protection in Serum-Free Cultured or DFO Pre-Treated SCs
4.6. Collagen IV Expression Reveals Proliferative SCs in Serum Media and Pro-Regenerative SCs in Serum-Free Media
4.7. Serum-Free, Chemically Defined Media Shifts SCs Towards Heightened Metabolism and Cellular Recycling Phenotype Without the Presence of H2O2 and DFO
4.8. Chemically Defined Media Facilitates Pro-Regenerative States and Cell Metabolism in the Presence of Deferoxamine Mesylate and 62.5 mM H2O2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, J.; Das, J.M.; Emmady, P.D. Spinal Cord Injuries. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560721/ (accessed on 23 August 2024).
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.E. A view from the ending: Axonal dieback and regeneration following SCI. Neurosci. Lett. 2017, 652, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Bunge, M.B.; Pearse, D.D. Transplantation strategies to promote repair of the injured spinal cord. J. Rehabil. Res. Dev. 2003, 40 (Suppl. 1), 55–62. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Ribeiro, B.F.; da Cruz, B.C.; de Sousa, B.M.; Correia, P.D.; David, N.; Rocha, C.; Almeida, R.D.; Ribeiro da Cunha, M.; Marques Baptista, A.A.; Vieira, S.I. Cell therapies for spinal cord injury: A review of the clinical trials and cell-type therapeutic potential. Brain 2023, 146, 2672–2693, Erratum in Brain 2023, 146, e128. https://doi.org/10.1093/brain/awad204. [Google Scholar] [CrossRef]
- Deng, L.X.; Walker, C.; Xu, X.M. Schwann cell transplantation and descending propriospinal regeneration after spinal cord injury. Brain Res. 2015, 1619, 104–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbour, H.R.; Plant, C.D.; Harvey, A.R.; Plant, G.W. Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model of spinal cord contusion. BMC Neurosci. 2013, 14, 106. [Google Scholar] [CrossRef]
- Marquardt, L.M.; Doulames, V.M.; Wang, A.T.; Dubbin, K.; Suhar, R.A.; Kratochvil, M.J.; Medress, Z.A.; Plant, G.W.; Heilshorn, S.C. Designer, injectable gels to prevent transplanted Schwann cell loss during spinal cord injury therapy. Sci. Adv. 2020, 6, eaaz1039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bartlett Bunge, M.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef]
- Bunge, M.B.; Monje, P.V.; Khan, A.; Wood, P.M. From transplanting Schwann cells in experimental rat spinal cord injury to their transplantation into human injured spinal cord in clinical trials. Prog. Brain Res. 2017, 231, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Santamaria, A.J.; Benavides, F.D. Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr. Opin. Organ. Transplant. 2013, 18, 682–689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- David, S.; Aguayo, A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981, 214, 931–933. [Google Scholar] [CrossRef]
- Ceto, S.; Sekiguchi, K.J.; Takashima, Y.; Nimmerjahn, A.; Tuszynski, M.H. Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury. Cell Stem Cell 2020, 27, 430–440.e5. [Google Scholar] [CrossRef] [PubMed]
- Dulin, J.N.; Lu, P. Bridging the injured spinal cord with neural stem cells. Neural Regen. Res. 2014, 9, 229–231. [Google Scholar] [CrossRef]
- Swijnenburg, R.J.; Schrepfer, S.; Govaert, J.A.; Cao, F.; Ransohoff, K.; Sheikh, A.Y.; Haddad, M.; Connolly, A.J.; Davis, M.M.; Robbins, R.C.; et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc. Natl. Acad. Sci. USA 2008, 105, 12991–12996. [Google Scholar] [CrossRef]
- Steward, O.; Sharp, K.G.; Yee, K.M.; Hatch, M.N.; Bonner, J.F. Characterization of ectopic colonies that form in widespread areas of the nervous system with neural stem cell transplants into the site of a severe spinal cord injury. J. Neurosci. 2014, 34, 14013–14021. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, B.; Gong, G.; Yin, J. ROS: Executioner of regulating cell death in spinal cord injury. Front. Immunol. 2024, 15, 1330678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aparicio, G.I.; Monje, P.V. Human Schwann Cells in vitro I. Nerve Tissue Processing, Pre-degeneration, Isolation, and Culturing of Primary Cells. Bio-Protocol 2023, 13, e4748. [Google Scholar] [CrossRef]
- Monje, P.V. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells 2020, 9, 1848. [Google Scholar] [CrossRef]
- David, B.T.; Curtin, J.J.; Brown, J.L.; Coutts, D.J.C.; Boles, N.C.; Hill, C.E. Treatment with hypoxia-mimetics protects cultured rat Schwann cells against oxidative stress-induced cell death. Glia 2021, 69, 2215–2234. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.Y.; Lin, J.A.; Ye, J.C.; Hwang, J.M.; Ting, W.J.; Huang, C.Y.; Liu, J.Y. Attenuation of Oxidative Stress-Induced Cell Apoptosis in Schwann RSC96 Cells by Ocimum gratissimum Aqueous Extract. Int. J. Med. Sci. 2017, 14, 764–771. [Google Scholar] [CrossRef]
- Fideles, S.O.M.; de Cássia Ortiz, A.; Buchaim, D.V.; de Souza Bastos Mazuqueli Pereira, E.; Parreira, M.J.B.M.; de Oliveira Rossi, J.; da Cunha, M.R.; de Souza, A.T.; Soares, W.C.; Buchaim, R.L. Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System. Antioxidants 2023, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Siriphorn, A.; Chompoopong, S.; Floyd, C.L. 17β-estradiol protects Schwann cells against H2O2-induced cytotoxicity and increases transplanted Schwann cell survival in a cervical hemicontusion spinal cord injury model. J. Neurochem. 2010, 115, 864–872. [Google Scholar] [CrossRef] [PubMed]
- David, B.T.; Curtin, J.J.; Goldberg, D.C.; Scorpio, K.; Kandaswamy, V.; Hill, C.E. Hypoxia-Inducible Factor 1α (HIF-1α) Counteracts the Acute Death of Cells Transplanted into the Injured Spinal Cord. eNeuro 2020, 7, ENEURO.0092-19.2019. [Google Scholar] [CrossRef]
- Singhal, R.; Mitta, S.R.; Das, N.K.; Kerk, S.A.; Sajjakulnukit, P.; Solanki, S.; Andren, A.; Kumar, R.; Olive, K.P.; Banerjee, R.; et al. HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J. Clin. Investig. 2021, 131, e143691. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, G.; Hou, B.; Song, E.; Wen, J.; Ba, Y.; Zhu, D.; Wang, G.; Qin, F. Effects of ECM proteins (laminin, fibronectin, and type IV collagen) on the biological behavior of Schwann cells and their roles in the process of remyelination after peripheral nerve injury. Front. Bioeng. Biotechnol. 2023, 11, 1133718. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Hao, J.; Duan, H.Q.; Zhao, C.X.; Sun, C.; Li, B.O.; Fan, B.Y.; Wang, X.U.; Li, W.X.; et al. Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regen. Res. 2019, 14, 532–541, Erratum in Neural Regen. Res. 2019, 14, 1068. https://doi.org/10.4103/1673-5374.250633. [Google Scholar] [CrossRef]
- De Mello, T.; Dunlop, S.; Plant, G.W. Myelination of axons by olfactory ensheathing glia. In Proceedings of the Fourteenth Annual Combined Biological Sciences Meeting, Copenhagen, The Netherlands, 15–18 April 2023; Newberry, J.A., Quail, E.A., Watson, M., Eds.; BioMed Chem: London, UK, 2003; Volume 1, p. 15. [Google Scholar]
- Wang, X.; Wei, L.; Li, Q.; Lai, Y. HIF-1α protects osteoblasts from ROS-induced apoptosis. Free Radic. Res. 2022, 56, 143–153. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Zheng, X.; Baker, H.; Hancock, W.S.; Fawaz, F.; McCaman, M.; Pungor, E., Jr. Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol. Prog. 2006, 22, 1294–1300. [Google Scholar] [CrossRef]
- Nakamura, M.; Okano, H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013, 23, 70–80. [Google Scholar] [CrossRef]
- Schepici, G.; Gugliandolo, A.; Mazzon, E. Serum-Free Cultures: Could They Be a Future Direction to Improve Neuronal Differentiation of Mesenchymal Stromal Cells? Int. J. Mol. Sci. 2022, 23, 6391. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.D.; Srinivas, S.; Piñero, G.; Monje, P.V. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci. Rep. 2016, 6, 31781. [Google Scholar] [CrossRef] [PubMed]
- Ravelo, K.M.; Andersen, N.D.; Monje, P.V. Magnetic-Activated Cell Sorting for the Fast and Efficient Separation of Human and Rodent Schwann Cells from Mixed Cell Populations. Methods Mol. Biol. 2018, 1739, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Manthorpe, M.; Adler, R.; Varon, S. Survival, proliferation and morphological specialization of mouse Schwann cells in a serum-free, fully defined medium. J. Neurocytol. 1980, 9, 683–697. [Google Scholar] [CrossRef]
- Yan, H.; Bunge, M.B.; Wood, P.M.; Plant, G.W. Mitogenic response of adult rat olfactory ensheathing glia to four growth factors. Glia 2001, 33, 334–342. [Google Scholar] [CrossRef]
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol.-Ren. Physiol. 2010, 299, F1–F13. [Google Scholar] [CrossRef]
- Kang, M.; Yang, Y.; Zhang, H.; Zhang, Y.; Wu, Y.; Denslin, V.; Othman, R.B.; Yang, Z.; Han, J. Comparative Analysis of Serum and Serum-Free Medium Cultured Mesenchymal Stromal Cells for Cartilage Repair. Int. J. Mol. Sci. 2024, 25, 10627. [Google Scholar] [CrossRef]
- Chase, L.G.; Lakshmipathy, U.; Solchaga, L.A.; Rao, M.S.; Vemuri, M.C. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res. Ther. 2010, 1, 8. [Google Scholar] [CrossRef]
- Ito, D.; Fujita, N.; Ibanez, C.; Sasaki, N.; Franklin, R.J.; Jeffery, N.D. Serum-free medium provides a clinically relevant method to increase olfactory ensheathing cell numbers in olfactory mucosa cell culture. Cell Transplant. 2008, 16, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hu, G.; Fan, G.; Mei, Z.; Deng, L.; Du, J. Protective Effect of bFGF on Bone Marrow Mesenchymal Stem Cell against Hydrogen Peroxide-Induced Apoptosis. Open Access Libr. J. 2016, 3, 68251. [Google Scholar] [CrossRef]
- David, B.T.; Curtin, J.J.; Brown, J.L.; Scorpio, K.; Kandaswamy, V.; Coutts, D.J.; Vivinetto, A.; Bianchimano, P.; Karuppagounder, S.S.; Metcalfe, M.; et al. Temporary induction of hypoxic adaptations by preconditioning fails to enhance Schwann cell transplant survival after spinal cord injury. Glia 2023, 71, 648–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, W.; Li, Y.; Sun, C. Fetal bovine serum, an important factor affecting the reproducibility of cell experiments. Sci. Rep. 2023, 13, 1942. [Google Scholar] [CrossRef]
- Ayton, S.; Lei, P.; Mclean, C.; Bush, A.I.; Finkelstein, D.I. Transferrin protects against Parkinsonian neurotoxicity and is deficient in Parkinson’s substantia nigra. Signal Transduct. Target. Ther. 2016, 1, 16015. [Google Scholar] [CrossRef]
- Tacchini, L.; Bianchi, L.; Bernelli-Zazzera, A.; Cairo, G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J. Biol. Chem. 1999, 274, 24142–24146. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, J.Y.; Kim, H.B.; Yoo, H.I.; Song, D.Y.; Min, S.S.; Baik, T.K.; Woo, R.S. Neuregulin1 Attenuates H2O2-Induced Reductions in EAAC1 Protein Levels and Reduces H2O2-Induced Oxidative Stress. Neurotox. Res. 2019, 35, 401–409. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Tarnawski, A.S. Critical role of hypoxia sensor–HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 2012, 19, 90–97. [Google Scholar] [CrossRef]
- Sondell, M.; Lundborg, G.; Kanje, M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neurosci. 1999, 19, 5731–5740. [Google Scholar] [CrossRef]
- Quarles, R.H. Myelin-associated glycoprotein (MAG): Past, present and beyond. J. Neurochem. 2007, 100, 1431–1448. [Google Scholar] [CrossRef]
- Arellano-Carbajal, F.; Briseño-Roa, L.; Couto, A.; Cheung, B.H.; Labouesse, M.; de Bono, M. Macoilin, a conserved nervous system-specific ER membrane protein that regulates neuronal excitability. PLoS Genet. 2011, 7, e1001341. [Google Scholar] [CrossRef] [PubMed]
- Agthong, S.; Kaewsema, A.; Tanomsridejchai, N.; Chentanez, V. Activation of MAPK ERK in peripheral nerve after injury. BMC Neurosci. 2006, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Koo, J. Olfactory receptors in non-chemosensory tissues. BMB Rep. 2012, 45, 612–622. [Google Scholar] [CrossRef]
- Flegel, C.; Manteniotis, S.; Osthold, S.; Hatt, H.; Gisselmann, G. Expression profile of ectopic olfactory receptors determined by deep sequencing. PLoS ONE 2013, 8, e55368. [Google Scholar] [CrossRef]
- Korolchuk, V.I.; Menzies, F.M.; Rubinsztein, D.C. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010, 584, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Glenn, T.D.; Talbot, W.S. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr. Opin. Neurobiol. 2013, 23, 1041–1048. [Google Scholar] [CrossRef]
- Luo, S.; Rubinsztein, D.C. BCL2L11/BIM: A novel molecular link between autophagy and apoptosis. Autophagy 2013, 9, 104–105. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, X.; Ren, J.; Chen, K.; Wang, H.L.; Miao, Y.Y.; Qi, M.M. How does estrogen work on autophagy? Autophagy 2019, 15, 197–211. [Google Scholar] [CrossRef]
- Zhao, Y.; Klionsky, D.J.; Wang, X.; Huang, Q.; Deng, Z.; Xiang, J. The Estrogen-Autophagy Axis: Insights into Cytoprotection and Therapeutic Potential in Cancer and Infection. Int. J. Mol. Sci. 2024, 25, 12576. [Google Scholar] [CrossRef]
- Monje, P.V.; Rendon, S.; Athauda, G.; Bates, M.; Wood, P.M.; Bunge, M.B. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia 2009, 57, 947–961. [Google Scholar] [CrossRef]
- Raschig, J.; Mailänder-Sánchez, D.; Berscheid, A.; Berger, J.; Strömstedt, A.A.; Courth, L.F.; Malek, N.P.; Brötz-Oesterhelt, H.; Wehkamp, J. Ubiquitously expressed Human Beta Defensin 1 (hBD1) forms bacteria-entrapping nets in a redox dependent mode of action. PLoS Pathog. 2017, 13, e1006261. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Alves-Lopes, R.; Neves, K.B.; Strembitska, A.; Harvey, A.P.; Harvey, K.Y.; Yusuf, H.; Haniford, S.; Hepburn, R.T.; Dyet, J.; Beattie, W.; et al. Osteoprotegerin regulates vascular function through syndecan-1 and NADPH oxidase-derived reactive oxygen species. Clin. Sci. 2021, 135, 2429–2444. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The Role of Osteoprotegerin and Its Ligands in Vascular Function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Grant, S. BCL2L11/Bim as a dual-agent regulating autophagy and apoptosis in drug resistance. Autophagy 2015, 11, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.T.; Xu, L. Bcl-2:Beclin 1 complex: Multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012, 2, 214–221. [Google Scholar]
- Gelman, A.; Rawet-Slobodkin, M.; Elazar, Z. Huntingtin facilitates selective autophagy. Nat. Cell Biol. 2015, 17, 214–215. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Jensen, C.C.; Beckerle, M.C. Phosphorylation of the small heat shock protein HspB1 regulates cytoskeletal recruitment and cell motility. Mol. Biol. Cell 2022, 33, ar100. [Google Scholar] [CrossRef]
- Yan, L.; Li, Z.; Li, C.; Chen, J.; Zhou, X.; Cui, J.; Liu, P.; Shen, C.; Chen, C.; Hong, H.; et al. Hspb1 and Lgals3 in spinal neurons are closely associated with autophagy following excitotoxicity based on machine learning algorithms. PLoS ONE 2024, 19, e0303235. [Google Scholar] [CrossRef]
- Feng, J.; Fu, S.; Cao, X.; Wu, H.; Lu, J.; Zeng, M.; Liu, L.; Yang, X.; Shen, Y. Synaptonemal complex protein 2 (SYCP2) mediates the association of the centromere with the synaptonemal complex. Protein Cell 2017, 8, 538–543. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kuramoto, K.; Wang, N.; Situ, X.; Priyadarshini, M.; Zhang, W.; Cordoba-Chacon, J.; Layden, B.T.; He, C. Autophagy Differentially Regulates Insulin Production and Insulin Sensitivity. Cell Rep. 2018, 23, 3286–3299. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wei, W.; Wang, G.; Gaertig, M.A.; Feng, Y.; Wang, W.; Li, X.J.; Li, S. Mutant huntingtin downregulates myelin regulatory factor-mediated myelin gene expression and affects mature oligodendrocytes. Neuron 2015, 85, 1212–1226. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.H.E.; Putta, A.R.; Chan, C.H.H.; Vidman, S.R.; Monje, P.; Plant, G.W. Efficacy of Deferoxamine Mesylate in Serum and Serum-Free Media: Adult Ventral Root Schwann Cell Survival Following Hydrogen Peroxide-Induced Cell Death. Cells 2025, 14, 461. https://doi.org/10.3390/cells14060461
Ma YHE, Putta AR, Chan CHH, Vidman SR, Monje P, Plant GW. Efficacy of Deferoxamine Mesylate in Serum and Serum-Free Media: Adult Ventral Root Schwann Cell Survival Following Hydrogen Peroxide-Induced Cell Death. Cells. 2025; 14(6):461. https://doi.org/10.3390/cells14060461
Chicago/Turabian StyleMa, Yee Hang Ethan, Abhinay R. Putta, Cyrus H. H. Chan, Stephen R. Vidman, Paula Monje, and Giles W. Plant. 2025. "Efficacy of Deferoxamine Mesylate in Serum and Serum-Free Media: Adult Ventral Root Schwann Cell Survival Following Hydrogen Peroxide-Induced Cell Death" Cells 14, no. 6: 461. https://doi.org/10.3390/cells14060461
APA StyleMa, Y. H. E., Putta, A. R., Chan, C. H. H., Vidman, S. R., Monje, P., & Plant, G. W. (2025). Efficacy of Deferoxamine Mesylate in Serum and Serum-Free Media: Adult Ventral Root Schwann Cell Survival Following Hydrogen Peroxide-Induced Cell Death. Cells, 14(6), 461. https://doi.org/10.3390/cells14060461





