MAP Kinase Phosphatase-5 Deficiency Improves Endurance Exercise Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Exercise Training Protocol
2.3. Immunoblotting
2.4. Antibodies and Reagents
2.5. RNA Extraction Followed by Quantitative Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
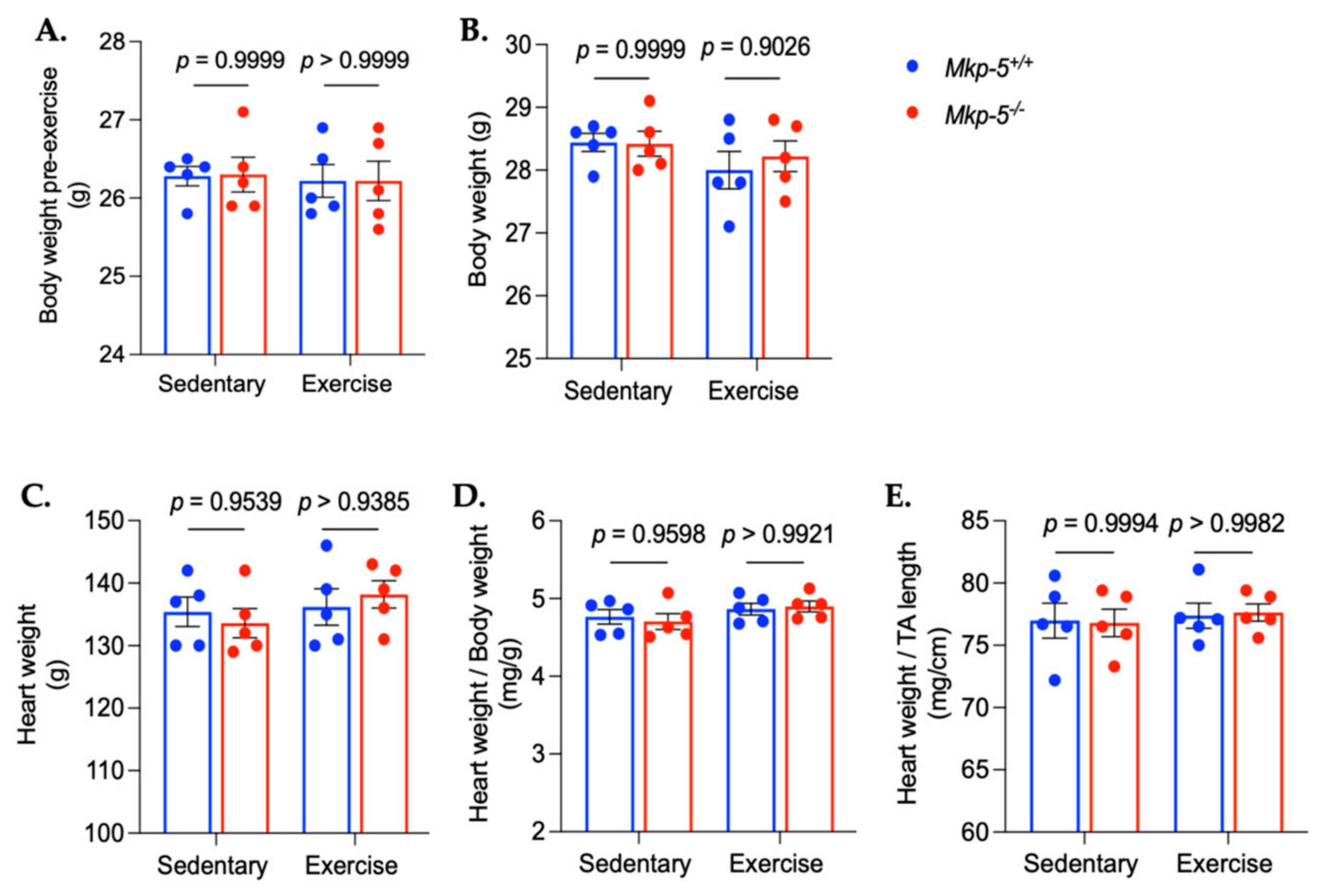
3.1. MKP-5 Deficiency Improves Endurance Exercise Capacity in Response to Aerobic Exercise Training
3.2. MKP-5 Regulates MAPKs in Cardiac Muscle in Response to Aerobic Exercise
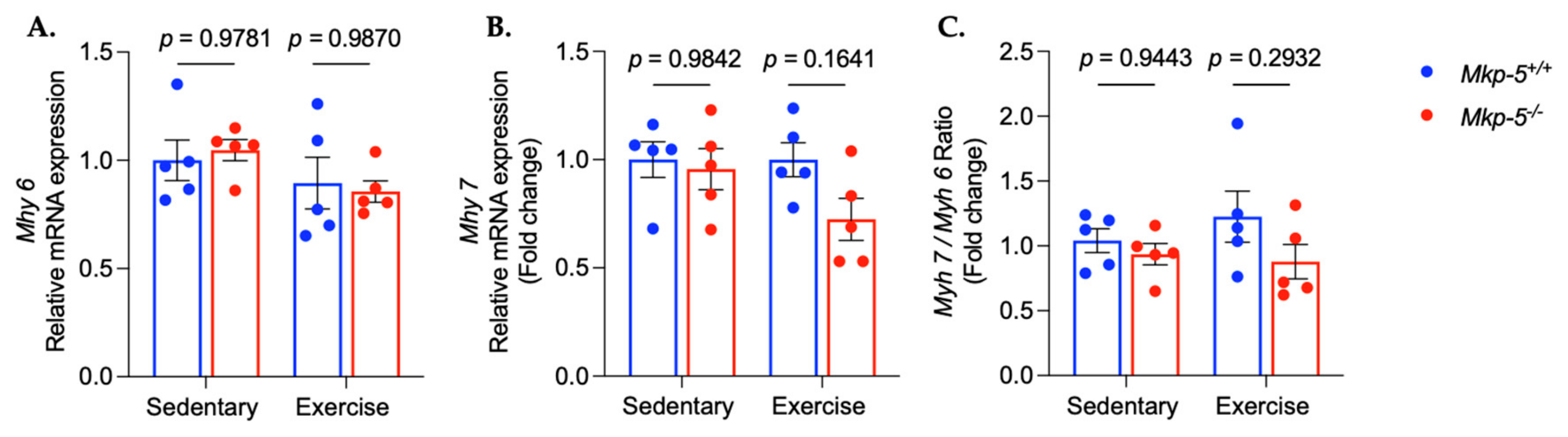
3.3. MKP-5 Deficiency Promotes Protein Synthesis in Cardiac Muscle Following Aerobic Exercise
3.4. MKP-5 Deficiency Promotes Aerobic Exercise-Induced Mitochondrial Biogenesis
3.5. MKP-5 Deficiency Promotes Cardiomyocyte Proliferation in Response to Aerobic Exercise
4. Discussion
5. Limitations and Future Directions of Experimental Approach
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruegsegger, G.N.; Booth, F.W. Health benefits of exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.K.; Heath, G.W.; Schmidt, M.D.; Lee, I.-M. Physical Activity Epidemiology; Human Kinetics: Champaign, IL, USA, 2021. [Google Scholar]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Campbell, W.W.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med. Sci. Sports Exerc. 2019, 51, 1270. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Hu, H.-Y.; Chou, Y.-C.; Huang, N.; Chou, Y.-J.; Li, C.-P. The association of physical activity with all-cause, cardiovascular, and cancer mortalities among older adults. Prev. Med. 2015, 72, 23–29. [Google Scholar] [CrossRef]
- Jeong, S.-W.; Kim, S.-H.; Kang, S.-H.; Kim, H.-J.; Yoon, C.-H.; Youn, T.-J.; Chae, I.-H. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur. Heart J. 2019, 40, 3547–3555. [Google Scholar] [CrossRef]
- Rognmo, Ø.; Moholdt, T.; Bakken, H.; Hole, T.; Mølstad, P.; Myhr, N.E.; Grimsmo, J.; Wisløff, U. Cardiovascular risk of high-versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012, 126, 1436–1440. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, S.; Hsue, C.; Dai, X.; Liu, L.; Miller, J.D.; Fang, Z.; Feng, J.; Huang, Y.; Wang, X. Effects of aerobic training and resistance training in reducing cardiovascular disease risk for patients with prediabetes: A multi-center randomized controlled trial. Prim. Care Diabetes 2021, 15, 1063–1070. [Google Scholar] [CrossRef]
- Fernandes, T.; Baraúna, V.G.; Negrão, C.E.; Phillips, M.I.; Oliveira, E.M. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H543–H552. [Google Scholar] [CrossRef]
- Moreira, J.B.; Wohlwend, M.; Wisløff, U. Exercise and cardiac health: Physiological and molecular insights. Nat. Metab. 2020, 2, 829–839. [Google Scholar] [CrossRef]
- Soulsby, M.; Bennett, A.M. Physiological signaling specificity by protein tyrosine phosphatases. Physiology 2009, 24, 281–289. [Google Scholar] [CrossRef]
- Shi, H.; Verma, M.; Zhang, L.; Dong, C.; Flavell, R.A.; Bennett, A.M. Improved regenerative myogenesis and muscular dystrophy in mice lacking Mkp5. J. Clin. Investig. 2013, 123, 2064–2077. [Google Scholar] [CrossRef]
- Lawan, A.; Min, K.; Zhang, L.; Canfran-Duque, A.; Jurczak, M.J.; Camporez, J.P.G.; Nie, Y.; Gavin, T.P.; Shulman, G.I.; Fernandez-Hernando, C. Skeletal muscle–specific deletion of MKP-1 reveals a p38 MAPK/JNK/Akt signaling node that regulates obesity-induced insulin resistance. Diabetes 2018, 67, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Lawan, A.; Bennett, A.M. Loss of MKP-5 promotes myofiber survival by activating STAT3/Bcl-2 signaling during regenerative myogenesis. Skelet. Muscle 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Min, K.; Zhao, Z.; Zhang, C.; Gao, E.; Huang, Y.; Zhang, X.; Baldini, M.; Roy, R.; Yang, X. MAP kinase phosphatase-5 deficiency protects against pressure overload-induced cardiac fibrosis. Front. Immunol. 2021, 12, 790511. [Google Scholar] [CrossRef]
- Xylourgidis, N.; Min, K.; Ahangari, F.; Yu, G.; Herazo-Maya, J.D.; Karampitsakos, T.; Aidinis, V.; Binzenhöfer, L.; Bouros, D.; Bennett, A.M. Role of dual-specificity protein phosphatase DUSP10/MKP-5 in pulmonary fibrosis. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2019, 317, L678–L689. [Google Scholar] [CrossRef]
- Zhang, Y.; Blattman, J.N.; Kennedy, N.J.; Duong, J.; Nguyen, T.; Wang, Y.; Davis, R.J.; Greenberg, P.D.; Flavell, R.A.; Dong, C. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 2004, 430, 793–797. [Google Scholar] [CrossRef]
- Schaefer, V.I.; Talan, M.I.; Shechtman, O. The effect of exercise training on cold tolerance in adult and old C57BL/6J mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1996, 51, B38–B42. [Google Scholar] [CrossRef]
- Dougherty, J.P.; Springer, D.A.; Gershengorn, M.C. The Treadmill Fatigue Test: A Simple, High-throughput Assay of Fatigue-like Behavior for the Mouse. J. Vis. Exp. 2016, 54052. [Google Scholar] [CrossRef]
- Chung, Y.; Hsiao, Y.T.; Huang, W.C. Physiological and Psychological Effects of Treadmill Overtraining Implementation. Biology 2021, 10, 515. [Google Scholar] [CrossRef]
- Ellison, G.M.; Waring, C.D.; Vicinanza, C.; Torella, D. Physiological cardiac remodelling in response to endurance exercise training: Cellular and molecular mechanisms. Heart 2012, 98, 5–10. [Google Scholar] [CrossRef]
- Vega, R.B.; Konhilas, J.P.; Kelly, D.P.; Leinwand, L.A. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 2017, 25, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, H.A. Exercise-induced signaling pathways to counteracting cardiac apoptotic processes. Front. Cell Dev. Biol. 2022, 10, 950927. [Google Scholar] [CrossRef]
- Vujic, A.; Lerchenmüller, C.; Wu, T.-D.; Guillermier, C.; Rabolli, C.P.; Gonzalez, E.; Senyo, S.E.; Liu, X.; Guerquin-Kern, J.-L.; Steinhauser, M.L.; et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat. Commun. 2018, 9, 1659. [Google Scholar] [CrossRef]
- Liao, J.; Li, Y.; Zeng, F.; Wu, Y. Regulation of mTOR Pathway in Exercise-induced Cardiac Hypertrophy. Int. J. Sports Med. 2015, 36, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Al-Horani, R.A.; Janaydeh, S.; Al-Trad, B.; Aljanabi, M.M.; Muhaidat, R. Acute Exercise Promptly Normalizes Myocardial Myosin Heavy-Chain Isoform mRNA Composition in Diabetic Rats: Implications for Diabetic Cardiomyopathy. Medicina 2023, 59, 2193. [Google Scholar] [CrossRef] [PubMed]
- Al-Horani, R.A.; Mohammad, M.A.; Haifawi, S.; Ihsan, M. Changes in myocardial myosin heavy chain isoform composition with exercise and post-exercise cold-water immersion. J. Muscle Res. Cell Motil. 2021, 42, 183–191. [Google Scholar] [CrossRef]
- Wan, W.; Xu, X.; Zhao, W.; Garza, M.A.; Zhang, J.Q. Exercise training induced myosin heavy chain isoform alteration in the infarcted heart. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2014, 39, 226–232. [Google Scholar] [CrossRef]
- Dent, J.R.; Stocks, B.; Campelj, D.G.; Philp, A. Transient changes to metabolic homeostasis initiate mitochondrial adaptation to endurance exercise. Semin. Cell Dev. Biol. 2023, 143, 3–16. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Li, C.; Zhang, X.; Shan, Y.; Zhang, Z.; Bo, H.; Zhang, Y. Endurance exercise-induced histone methylation modification involved in skeletal muscle fiber type transition and mitochondrial biogenesis. Sci. Rep. 2024, 14, 21154. [Google Scholar] [CrossRef]
- Gu, C.; Yan, J.; Zhao, L.; Wu, G.; Wang, Y.L. Regulation of Mitochondrial Dynamics by Aerobic Exercise in Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 788505. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Zhang, J.; Jia, D. Exercise Alleviates Cardiovascular Diseases by Improving Mitochondrial Homeostasis. J. Am. Heart Assoc. 2024, 13, e036555. [Google Scholar] [CrossRef] [PubMed]
- Rowe, G.C.; El-Khoury, R.; Patten, I.S.; Rustin, P.; Arany, Z. PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS ONE 2012, 7, e41817. [Google Scholar] [CrossRef] [PubMed]
- Viloria, M.A.D.; Li, Q.; Lu, W.; Nhu, N.T.; Liu, Y.; Cui, Z.Y.; Cheng, Y.J.; Lee, S.D. Effect of exercise training on cardiac mitochondrial respiration, biogenesis, dynamics, and mitophagy in ischemic heart disease. Front. Cardiovasc. Med. 2022, 9, 949744. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Close, G.L.; Drust, B.; Morton, J.P. The emerging role of p53 in exercise metabolism. Sports Med. 2014, 44, 303–309. [Google Scholar] [CrossRef]
- Saleem, A.; Carter, H.N.; Iqbal, S.; Hood, D.A. Role of p53 within the regulatory network controlling muscle mitochondrial biogenesis. Exerc. Sport Sci. Rev. 2011, 39, 199–205. [Google Scholar] [CrossRef]
- Margolis, L.M.; Pasiakos, S.M. Optimizing intramuscular adaptations to aerobic exercise: Effects of carbohydrate restriction and protein supplementation on mitochondrial biogenesis. Adv. Nutr. 2013, 4, 657–664. [Google Scholar] [CrossRef]
- Craig, D.M.; Ashcroft, S.P.; Belew, M.Y.; Stocks, B.; Currell, K.; Baar, K.; Philp, A. Utilizing small nutrient compounds as enhancers of exercise-induced mitochondrial biogenesis. Front. Physiol. 2015, 6, 296. [Google Scholar] [CrossRef]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Mitochondrial Med. 2012, 942, 3–37. [Google Scholar]
- Zhang, G.-l.; Sun, M.-l.; Zhang, X.-a. Exercise-induced adult cardiomyocyte proliferation in mammals. Front. Physiol. 2021, 12, 729364. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Mann, N.; Wu, J.; Quintero, P.A.; Plovie, E.R.; Panáková, D.; Gupta, R.K.; Xiao, C.; MacRae, C.A.; Rosenzweig, A. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 2010, 143, 1072–1083. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Zhu, H.; Wei, X.; Platt, C.; Damilano, F.; Xiao, C.; Bezzerides, V.; Boström, P.; Che, L. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015, 21, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, Y.; Guan, L.; Elkin, K.; Xiao, J. Targets identified from exercised heart: Killing multiple birds with one stone. npj Regen. Med. 2021, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, R.; Feng, Y.; Cheng, L. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct. Target. Ther. 2022, 7, 383. [Google Scholar] [CrossRef]
- Rowe, G.C.; Safdar, A.; Arany, Z. Running forward: New frontiers in endurance exercise biology. Circulation 2014, 129, 798–810. [Google Scholar] [CrossRef]
- Contrepois, K.; Wu, S.; Moneghetti, K.J.; Hornburg, D.; Ahadi, S.; Tsai, M.S.; Metwally, A.A.; Wei, E.; Lee-McMullen, B.; Quijada, J.V.; et al. Molecular Choreography of Acute Exercise. Cell 2020, 181, 1112–1130.e1116. [Google Scholar] [CrossRef]
- França, G.O.; Frantz, E.D.C.; Magliano, D.C.; Bargut, T.C.L.; Sepúlveda-Fragoso, V.; Silvares, R.R.; Daliry, A.; Nascimento, A.R.D.; Borges, J.P. Effects of short-term high-intensity interval and continuous exercise training on body composition and cardiac function in obese sarcopenic rats. Life Sci. 2020, 256, 117920. [Google Scholar] [CrossRef]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, J.H.; Seo, D.Y.; Han, J.; Jung, S.J.; Kwak, H.B. Effects of Acute Exercise on Mitochondrial Function, Dynamics, and Mitophagy in Rat Cardiac and Skeletal Muscles. Int. Neurourol. J. 2019, 23, S22-31. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Chen, Z.; Gibson, T.B.; Robinson, F.; Silvestro, L.; Pearson, G.; Xu, B.-e.; Wright, A.; Vanderbilt, C.; Cobb, M.H. MAP kinases. Chem. Rev. 2001, 101, 2449–2476. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, A.T.; Gratidao, L.; Ludlow, L.W.; Spangenburg, E.E.; Roth, S.M. Acute exercise activates p38 MAPK and increases the expression of telomere-protective genes in cardiac muscle. Exp. Physiol. 2017, 102, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.D.; Muller, B.N.; Krenz, M.; Hanft, L.M.; McDonald, K.S.; Dellsperger, K.C.; Emter, C.A. Heart failure with preserved ejection fraction: Chronic low-intensity interval exercise training preserves myocardial O2 balance and diastolic function. J. Appl. Physiol. 2013, 114, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Chengji, W.; Shoujun, H. Aerobic exercise can ameliorate heart function in patients with myocardial infarction through up-regulating M3 receptor. IJC Metab. Endocr. 2016, 13, 1–5. [Google Scholar] [CrossRef][Green Version]
- Kondoh, K.; Nishida, E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1227–1237. [Google Scholar] [CrossRef]
- Maillet, M.; Van Berlo, J.H.; Molkentin, J.D. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 2013, 14, 38–48. [Google Scholar] [CrossRef]
- Fulghum, K.; Hill, B.G. Metabolic mechanisms of exercise-induced cardiac remodeling. Front. Cardiovasc. Med. 2018, 5, 127. [Google Scholar] [CrossRef]
- Duncker, D.J.; Bache, R.J. Regulation of coronary blood flow during exercise. Physiol. Rev. 2008, 88, 1009–1086. [Google Scholar] [CrossRef]
- Roach, R.C.; Koskolou, M.D.; Calbet, J.A.; Saltin, B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H438–H445. [Google Scholar] [CrossRef]
- DeBosch, B.; Treskov, I.; Lupu, T.S.; Weinheimer, C.; Kovacs, A.; Courtois, M.; Muslin, A.J. Akt1 is required for physiological cardiac growth. Circulation 2006, 113, 2097–2104. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Lamming, D.W. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016, 23, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Baar, K. mTOR and the health benefits of exercise. Semin. Cell Dev. Biol. 2014, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, B.; Zhang, Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle 2016, 15, 781–786. [Google Scholar] [CrossRef]
- Mirzaei, B.; Ghavami Amin, H.; Fadaei Chafy, M.R. Investigating the effect of exercise training in different periods of growth on protein synthesis (4E-BP1) and proliferation of cardiac cells (S6K1) in male rats. J. Exerc. Organ Cross Talk 2023, 3, 116–123. [Google Scholar]
- Hernández, G.; Lal, H.; Fidalgo, M.; Guerrero, A.; Zalvide, J.; Force, T.; Pombo, C.M. A novel cardioprotective p38-MAPK/mTOR pathway. Exp. Cell Res. 2011, 317, 2938–2949. [Google Scholar] [CrossRef]
- Yue, H.-W.; Liu, J.; Liu, P.-P.; Li, W.-J.; Chang, F.; Miao, J.-Y.; Zhao, J. Sphingosylphosphorylcholine protects cardiomyocytes against ischemic apoptosis via lipid raft/PTEN/Akt1/mTOR mediated autophagy. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 1186–1193. [Google Scholar] [CrossRef]
- Rodríguez Huertas, J.F.; Casuso, R.A.; Hernansanz Agustín, P.; Cogliati, S. Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm. Oxidative Med. Cell. Longev. 2021, 2021, 9274841. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Andersen, S.P.; Qadri, K.A.N.; Thøgersen, F.D.; Krag, T.; Ørngreen, M.C.; Vissing, J.; Jeppesen, T.D. Effect of Aerobic Exercise Training and Deconditioning on Oxidative Capacity and Muscle Mitochondrial Enzyme Machinery in Young and Elderly Individuals. J. Clin. Med. 2020, 9, 3113. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, D.M.; Yu, R.R.; Zhang, L.L.; Liu, Y.Z.; Chen, J.X.; Chen, H.C.; Liu, Y.P. The Effect of Aerobic Exercise on the Oxidative Capacity of Skeletal Muscle Mitochondria in Mice with Impaired Glucose Tolerance. J. Diabetes Res. 2022, 2022, 3780156. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1α. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Pohnert, S.C.; Li, P.; Zhang, M.; Gumbs, C.; Rosenberg, P.B.; Williams, R.S.; Yan, Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. 2005, 280, 19587–19593. [Google Scholar] [CrossRef]
- Wright, D.C.; Han, D.-H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Lezza, A.M.S. Regulation of mitochondrial biogenesis through TFAM–mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion 2015, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Beyfuss, K.; Erlich, A.T.; Triolo, M.; Hood, D.A. The role of p53 in determining mitochondrial adaptations to endurance training in skeletal muscle. Sci. Rep. 2018, 8, 14710. [Google Scholar] [CrossRef]
- Gong, R.; Jiang, Z.; Zagidullin, N.; Liu, T.; Cai, B. Regulation of cardiomyocyte fate plasticity: A key strategy for cardiac regeneration. Signal Transduct. Target. Ther. 2021, 6, 31. [Google Scholar] [CrossRef]
- Kikuchi, K.; Holdway, J.E.; Werdich, A.A.; Anderson, R.M.; Fang, Y.; Egnaczyk, G.F.; Evans, T.; MacRae, C.A.; Stainier, D.Y.; Poss, K.D. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 2010, 464, 601–605. [Google Scholar] [CrossRef]
- Broderick, T.L.; Parrott, C.R.; Wang, D.; Jankowski, M.; Gutkowska, J. Expression of cardiac GATA4 and downstream genes after exercise training in the db/db mouse. Pathophysiology 2012, 19, 193–203. [Google Scholar] [CrossRef]
- Naderi, N.; Hemmatinafar, M.; Gaeini, A.A.; Bahramian, A.; Ghardashi-Afousi, A.; Kordi, M.R.; Darbandi-Azar, A.; Karimzade, F.; Mohebbi, H.; Barati, M. High-intensity interval training increase GATA4, CITED4 and c-Kit and decreases C/EBPβ in rats after myocardial infarction. Life Sci. 2019, 221, 319–326. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Spanos, M.; Li, G.; Lu, R.; Bei, Y.; Xiao, J. Exercise training maintains cardiovascular health: Signaling pathways involved and potential therapeutics. Signal Transduct. Target. Ther. 2022, 7, 306. [Google Scholar] [CrossRef]





| Primer Name | Sequences |
|---|---|
| Mkp-5 | 5′-ACCGCAGCTAGGAATAATGGA-3′ 5′-ACCAAAAGCCTTGACTCCG-3′ |
| Pgc-1α | 5′-CCCTGCCATTGTTAAGACC-3′ |
| 5′-CTTTTGTCCTTGTCGTCGTC-3′ | |
| Tfam | 5′-ATTCCGAAGTGTTTTTCCAGCA-3′ 5′-TCTGAAAGTTTTGCATCTGGGT-3′ |
| Myh6 | 5′-GTCCCGGACACTGGACCAGGCC-3′ 5′-CTCCTTTTCTTCCAGTTGCCTAGCCAA-3′ |
| Myh7 | 5′-GAGCAAGGCCGAGGAGACGCAGCGT-3′ 5′-GAGCCTCCTTCTCGTCCAGCTGCCGG-3′ |
| 18S | 5′-ACCGCAGCTAGGAATAATGGA-3′ 5′-ACCAAAAGCCTTGACTCCG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perales, J.A.; Lawan, A.; Bajpeyi, S.; Han, S.M.; Bennett, A.M.; Min, K. MAP Kinase Phosphatase-5 Deficiency Improves Endurance Exercise Capacity. Cells 2025, 14, 410. https://doi.org/10.3390/cells14060410
Perales JA, Lawan A, Bajpeyi S, Han SM, Bennett AM, Min K. MAP Kinase Phosphatase-5 Deficiency Improves Endurance Exercise Capacity. Cells. 2025; 14(6):410. https://doi.org/10.3390/cells14060410
Chicago/Turabian StylePerales, Jaime A., Ahmed Lawan, Sudip Bajpeyi, Sung Min Han, Anton M. Bennett, and Kisuk Min. 2025. "MAP Kinase Phosphatase-5 Deficiency Improves Endurance Exercise Capacity" Cells 14, no. 6: 410. https://doi.org/10.3390/cells14060410
APA StylePerales, J. A., Lawan, A., Bajpeyi, S., Han, S. M., Bennett, A. M., & Min, K. (2025). MAP Kinase Phosphatase-5 Deficiency Improves Endurance Exercise Capacity. Cells, 14(6), 410. https://doi.org/10.3390/cells14060410








