Enhanced BDNF and ROS in Mucosa of Lower Motor Neuron Lesioned Dog Bladder Following Somatic Motor Nerve Transfer
Abstract
1. Introduction
2. Materials and Methods
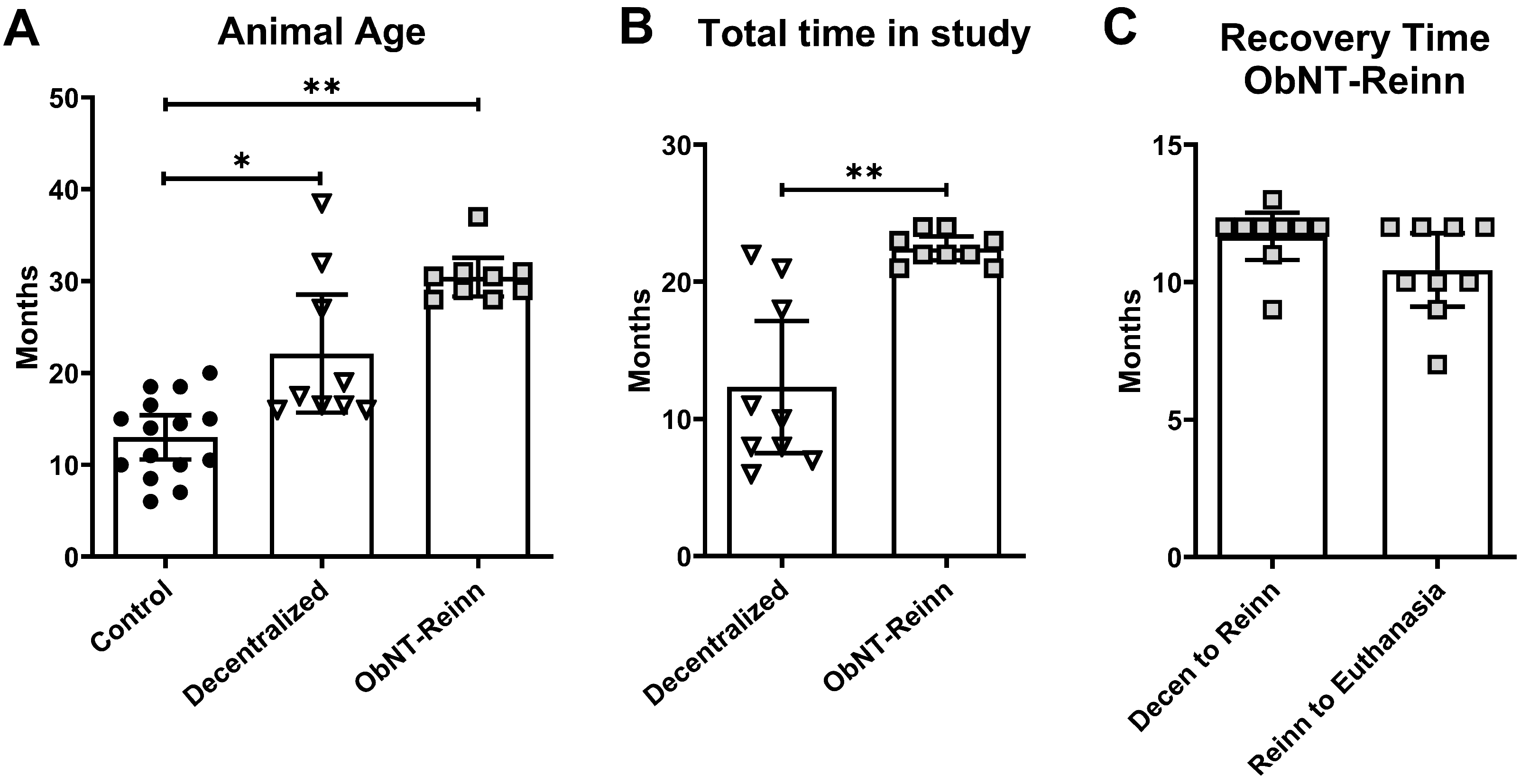
2.1. Animals
2.2. Bladder Decentralization Surgery
2.3. Bladder Reinnervation Surgery Using Nerve Transfer
2.4. Postoperative Care
2.5. Bladder Tissue Collection for Biochemical Assays
2.6. Neurotrophic Factor’ mRNA Gene Expression
2.7. Enzyme-Linked Immunosorbent Assays (ELISA)
2.8. Measurement of ROS Superoxide Production
2.9. Immunohistochemistry on Full Thickness Bladder Specimens
2.10. Dihydroethidium (DHE) Detection of ROS in Tissue Sections
2.11. Statistical Analyses
3. Results
3.1. Neurotrophic Factors Were Differentially Expressed in Dog Bladder Tissues
3.2. Enhanced BDNF Protein in Mucosa of ObNT-Reinn Bladders, and Reduced NT-3 Protein in Mucosa of ObNT-Reinn and Decentralized Bladders
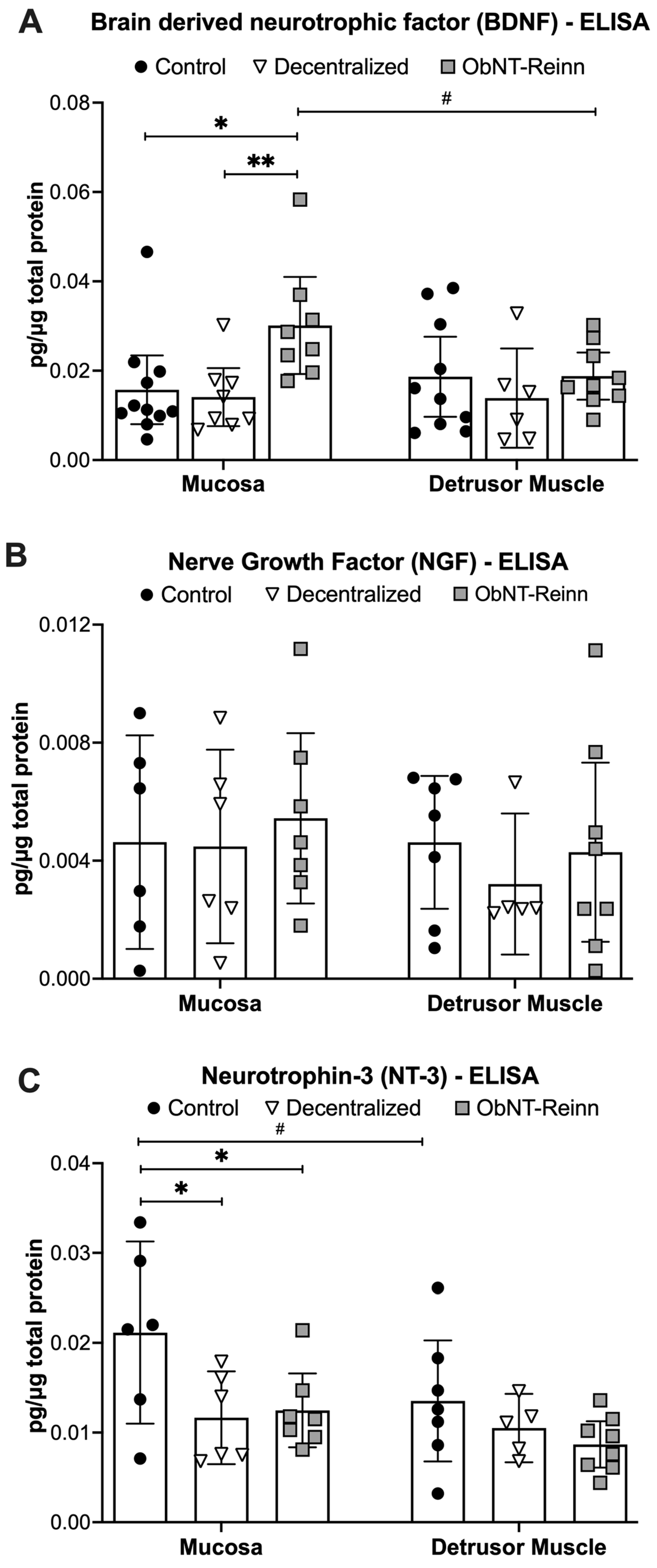
3.3. Reduced Artemin Levels in the Mucosa of Decentralized and ObNT-Reinn Bladders, and Reduced GDNF Protein in Mucosa of Decentralized Bladders

3.4. Similar CNTF Levels in Bladders of the Three Dog Groups
3.5. Reduced TNF-α Levels in the Mucosa of Decentralized Bladders
3.6. Enhanced ROS Production in the Mucosa of ObNT-Reinn Bladders

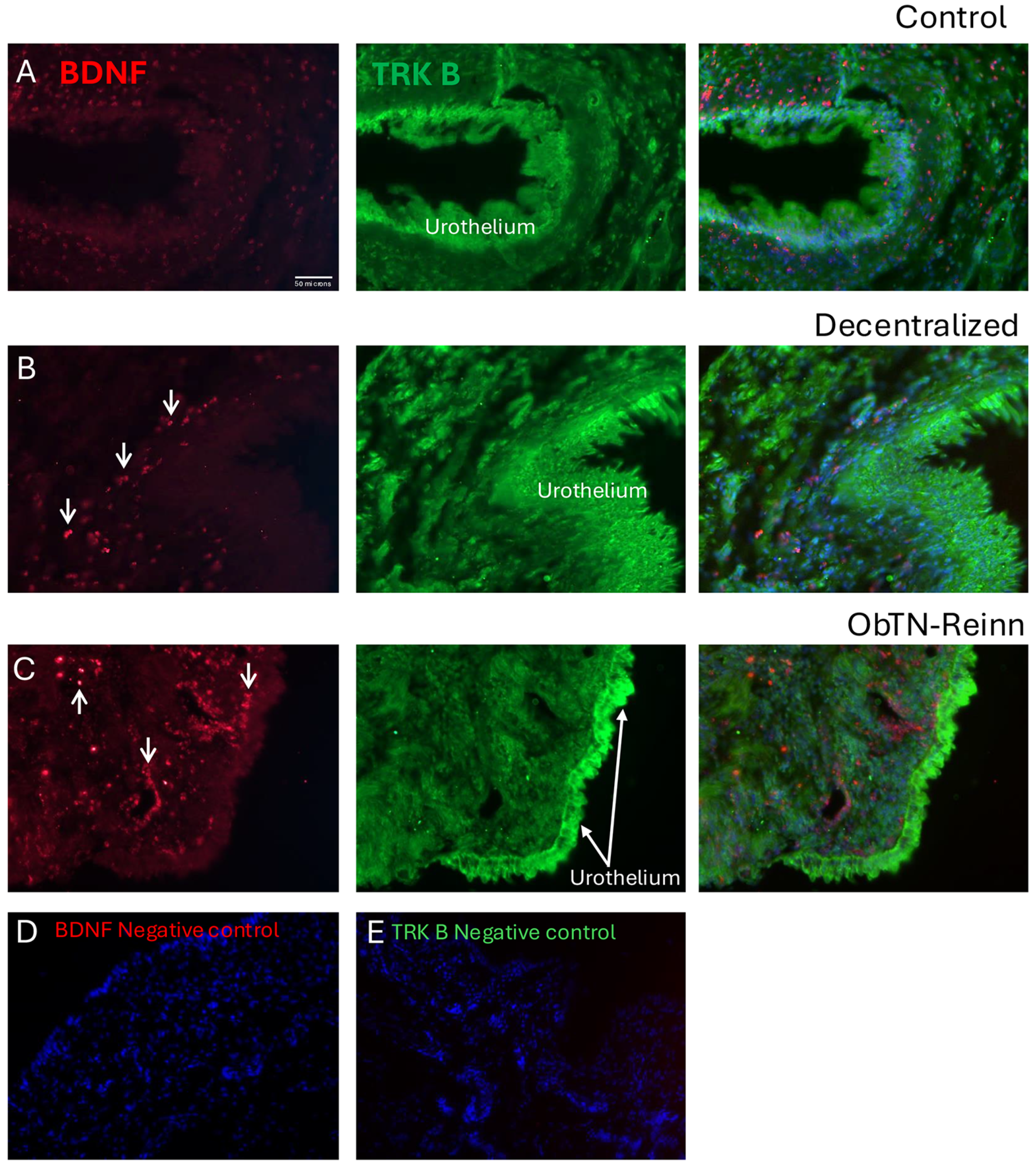
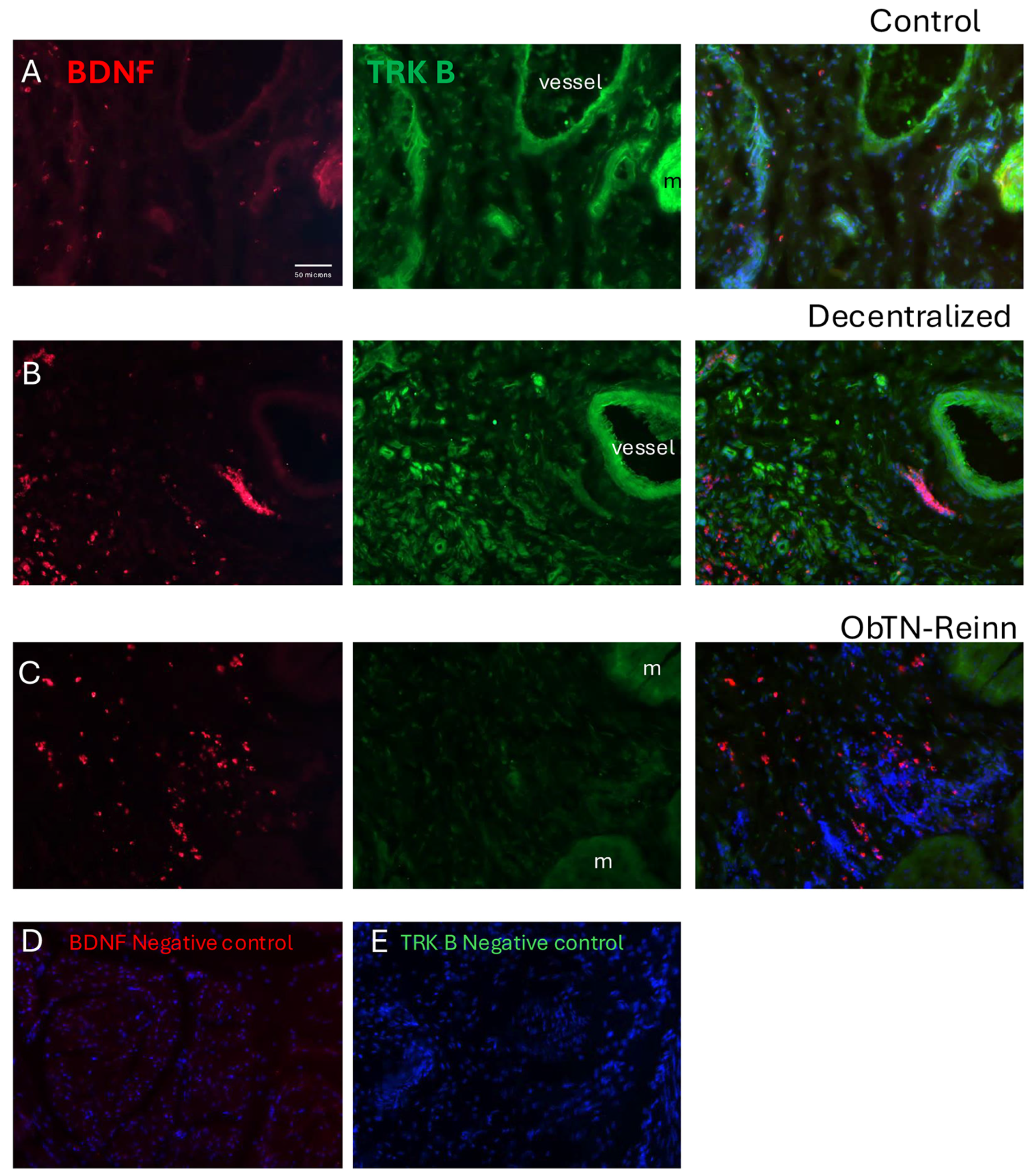
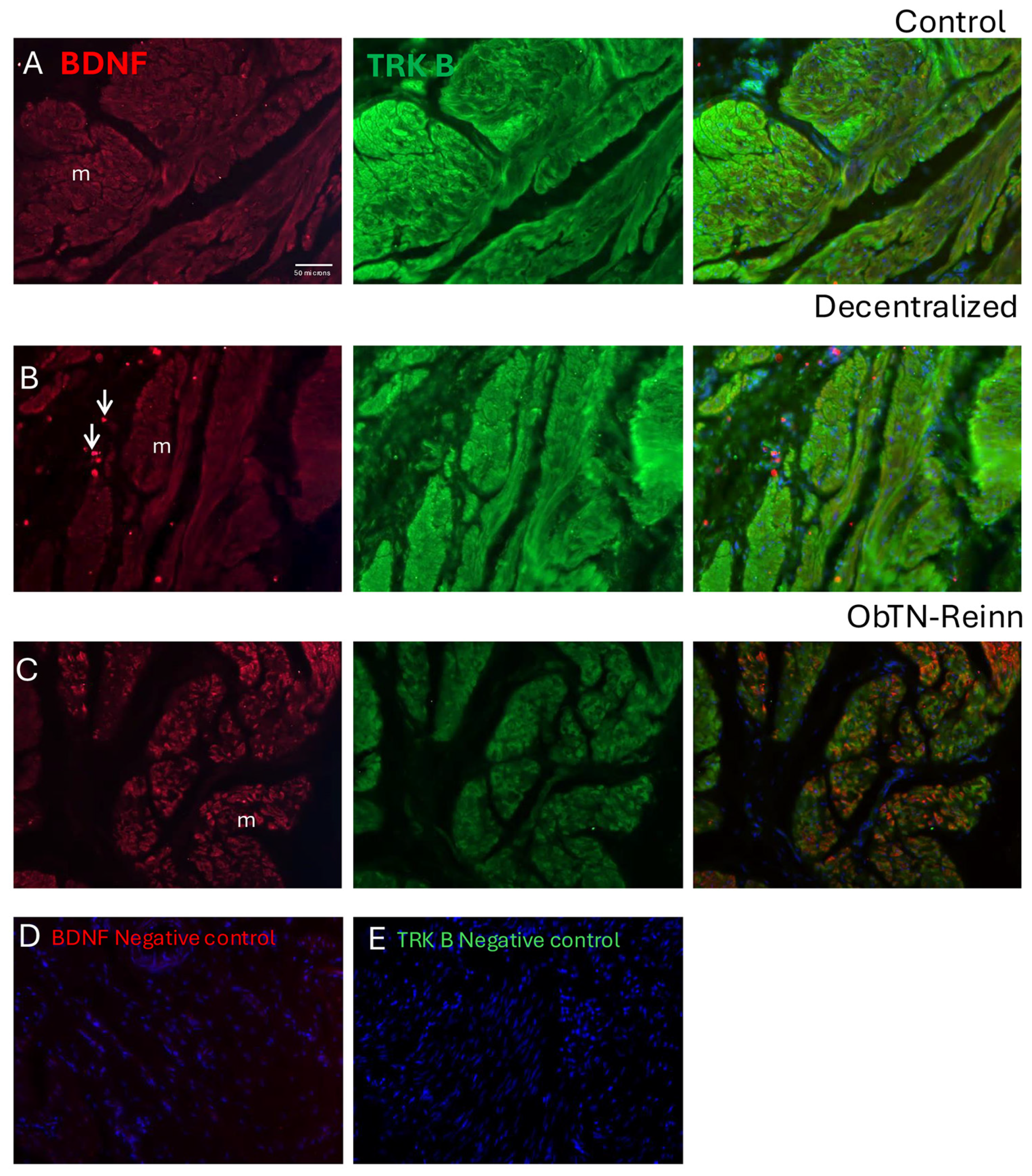
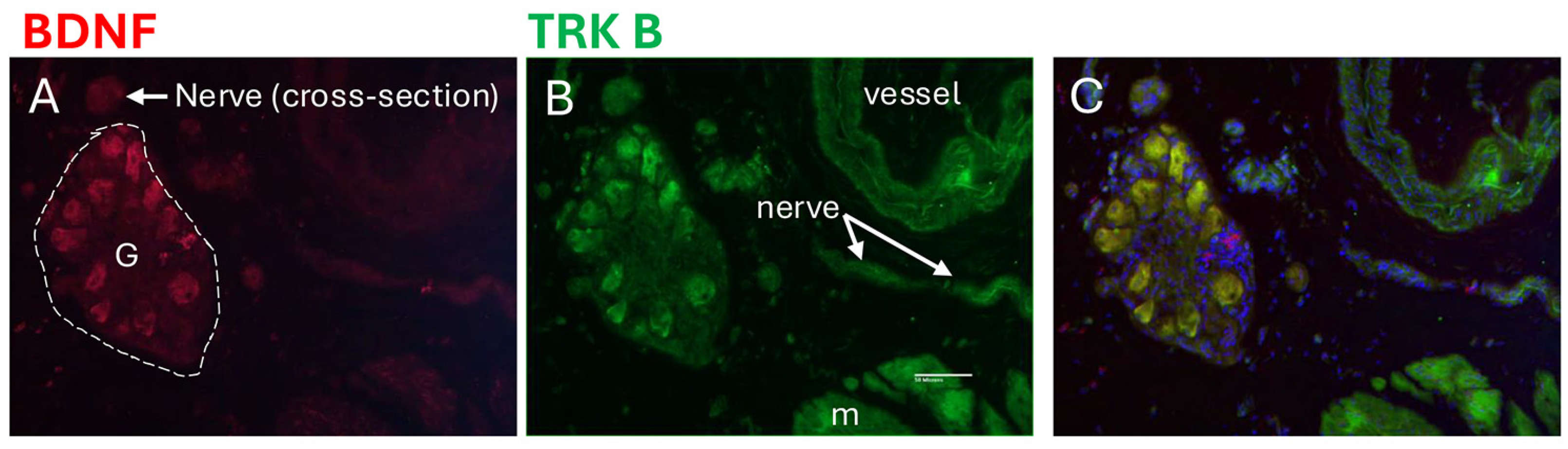
3.7. Localization of BDNF and TRK B in Bladder Wall Using Immunohistochemistry
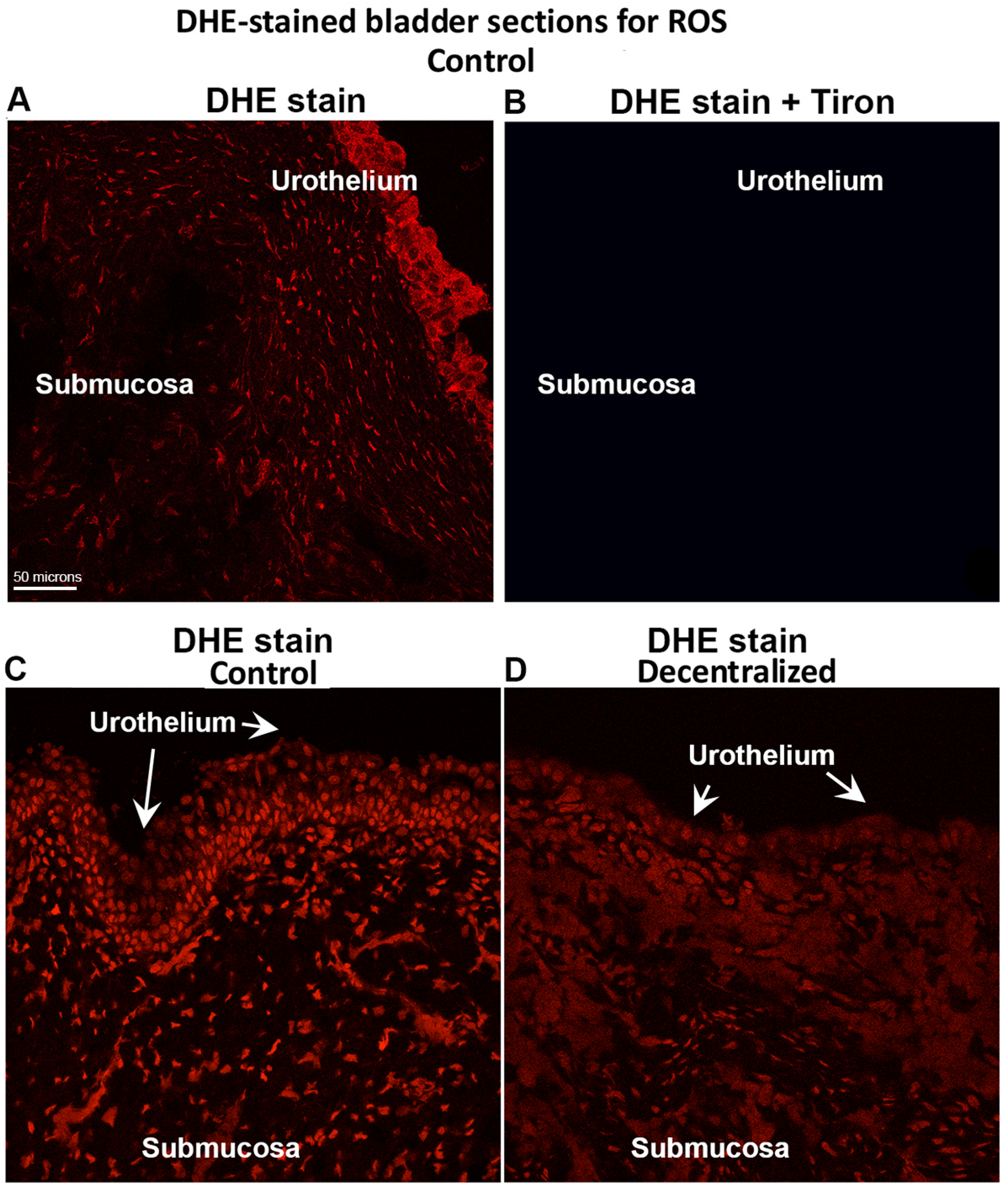
3.8. DHE Detection of ROS
3.9. Correlations Between Biomarker Levels
3.10. Correlations Between Biomarker Levels and Histological Outcomes
3.11. Correlations Between Biomarker Levels and Functional Outcomes
3.12. Correlations Between Biomarker Levels and Age of Animals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apodaca, G.; Kiss, S.; Ruiz, W.; Meyers, S.; Zeidel, M.; Birder, L. Disruption of bladder epithelium barrier function after spinal cord injury. Am. J. Physiol. Ren. Physiol. 2003, 284, F966–F976. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Li, H.; Xie, Q.Q.; Siau, C.S.; Xie, Z.; Zhu, M.T.; Zhou, B.; Li, Z.P.; Wang, S.B. Rehabilitation care of patients with neurogenic bladder after spinal cord injury: A literature review. World J. Clin. Cases 2023, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- DeWitt-Foy, M.E.; Elliott, S.P. Neurogenic bladder: Assessment and operative management. Urol. Clin. N. Am. 2022, 49, 519–532. [Google Scholar] [CrossRef]
- Gomez-Amaya, S.M.; Barbe, M.F.; Brown, J.M.; Lamarre, N.S.; Braverman, A.S.; Massicotte, V.S.; Ruggieri, M.R. Bladder reinnervation using a primarily motor donor nerve (femoral nerve branches) is functionally superior to using a primarily sensory donor nerve (genitofemoral nerve). J. Urol. 2015, 193, 1042–1051. [Google Scholar] [CrossRef]
- Ruggieri, M.R.; Brown, J.M.; Braverman, A.S.; Bernal, R.M.; Pontari, M.A.; Dean, G.E.; Lamarre, N.S.; Barbe, M.F. Transfer of femoral nerve branches to pudendal nerve branches reinnervates the urethral and anal sphincters in a canine model and is feasible in a cadaver study. J. Urol. 2012, 187, e42. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.-Y.; Xu, W.-D.; Hua, X.-Y.; Xu, J.-G.; Gu, Y.-D. Sensory restoration in cortical level after a contralateral C7 nerve transfer to an injured arm in rats. Neurosurgery 2010, 67, 136–143. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.-S.; Zeng, X.-D.; Feng, J.-T.; Xu, W.-D. Contralateral cervical nerve transfer for arm paralysis. N. Engl. J. Med. 2018, 378, 1460–1461. [Google Scholar] [CrossRef]
- Blits, B.; Carlstedt, T.P.; Ruitenberg, M.J.; de Winter, F.; Hermens, W.T.; Dijkhuizen, P.A.; Claasens, J.W.; Eggers, R.; van der Sluis, R.; Tenenbaum, L.; et al. Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor. Exp. Neurol. 2004, 189, 303–316. [Google Scholar] [CrossRef]
- Greensmith, L.; Vrbová, G. Motoneurone survival: A functional approach. Trends Neurosci. (Regul. Ed.) 1996, 19, 450–455. [Google Scholar] [CrossRef]
- Kassar-Duchossoy, L.; Duchossoy, Y.; Rhrich-Haddout, F.; Horvat, J.-C. Reinnervation of a denervated skeletal muscle by spinal axons regenerating through a collagen channel directly implanted into the rat spinal cord. Brain Res. 2001, 908, 25–34. [Google Scholar] [CrossRef]
- Ramer, M.S.; Priestley, J.V.; McMahon, S.B. Functional regeneration of sensory axons into the adult spinal cord. Nature 2000, 403, 312–316. [Google Scholar] [CrossRef]
- Livshits, A.; Catz, A.; Folman, Y.; Witz, M.; Livshits, V.; Baskov, A.; Gepstein, R. Reinnervation of the neurogenic bladder in the late period of the spinal cord trauma. Spinal Cord 2004, 42, 211–217. [Google Scholar] [CrossRef]
- Conzen, M.A.; Sollmann, H. Reinnervation of the urinary bladder after microsurgical reconstruction of transsected caudal fibres–An experimental study in pigs. Urol. Res. 1982, 10, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.R.; Bruce, A.W.; Lywood, D.W.; Robertson, D.M. Reinnervation of the neurogenic bladder with somatic motor nerves. Investig. Urol. 1971, 9, 59–63. [Google Scholar]
- Vorstman, B.; Schlossberg, S.; Kass, L. Investigations on urinary bladder reinnervation: Historical perspective and review. Urology 1987, 30, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Vorstman, B.; Schlossberg, S.M.; Kass, L.; Devine, C.J. Urinary bladder reinnervation. J. Urol. 1986, 136, 964–969. [Google Scholar] [CrossRef]
- Ruggieri, M.R.; Braverman, A.S.; D’Andrea, L.; Simpkiss, B.; Kozin, S.H.; Pontari, M.A.; Betz, R.; Barbe, M.F. Functional reinnervation of the canine bladder after spinal root transection and immediate end-on-end repair. J. Neurotrauma 2006, 23, 1125–1136. [Google Scholar] [CrossRef]
- Ruggieri, M.R.; Braverman, A.S.; D’Andrea, L.; Betz, R.; Barbe, M.F. Functional reinnervation of the canine bladder after spinal root transection and genitofemoral nerve transfer at one and three months after denervation. J. Neurotrauma 2008, 25, 401–409. [Google Scholar] [CrossRef]
- Tiwari, E.; Porreca, D.S.; Braverman, A.S.; Holt-Bright, L.; Frara, N.A.; Brown, J.M.; Johnston, B.R.; Bazarek, S.F.; Hilliard, B.A.; Mazzei, M.; et al. Nerve transfer for restoration of lower motor neuron-lesioned bladder, urethral and anal sphincter function. Part 4: Effectiveness of the motor reinnervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 326, R528–R551. [Google Scholar] [CrossRef]
- Xiao, C.G.; Godec, C.J. A possible new reflex pathway for micturition after spinal cord injury. Paraplegia 1994, 32, 300–307. [Google Scholar] [CrossRef]
- Chuang, D.C.; Chang, P.L.; Cheng, S.Y. Root reconstruction for bladder reinnervation: An experimental study in rats. Microsurgery 1991, 12, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Testa, C.L.; Cruz, G.E.; Frara, N.A.; Tiwari, E.; Hobson, L.J.; McIntyre, B.S.; Porreca, D.S.; Giaddui, D.; Braverman, A.S.; et al. Nerve transfer for restoration of lower motor neuron-lesioned bladder function. Part 2: Correlation between histological changes and nerve evoked contractions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R897–R915. [Google Scholar] [CrossRef]
- Gomez-Amaya, S.M.; Barbe, M.F.; de Groat, W.C.; Brown, J.M.; Tuite, G.F.; Corcos, J.; Fecho, S.B.; Braverman, A.S.; Ruggieri, M.R., Sr. Neural reconstruction methods of restoring bladder function. Nat. Rev. Urol. 2015, 12, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Vanden Noven, S.; Wallace, N.; Muccio, D.; Turtz, A.; Pinter, M.J. Adult spinal motoneurons remain viable despite prolonged absence of functional synaptic contact with muscle. Exp. Neurol. 1993, 123, 147–156. [Google Scholar] [CrossRef]
- Ma, J.; Novikov, L.N.; Wiberg, M.; Kellerth, J.-O. Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: A quantitative morphological study. Exp. Brain Res. 2001, 139, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Lais, A.C.; Dyck, P.J. Axonal atrophy from permanent peripheral axotomy in adult cat. J. Neuropathol. Exp. Neurol. 1979, 38, 579–585. [Google Scholar] [CrossRef]
- Swett, J.E.; Hong, C.Z.; Miller, P.G. All peroneal motoneurons of the rat survive crush injury but some fail to reinnervate their original targets. J. Comp. Neurol. 1991, 304, 234–252. [Google Scholar] [CrossRef]
- Barbe, M.F.; Ruggieri, M.R., Sr. Innervation of parasympathetic postganglionic neurons and bladder detrusor muscle directly after sacral root transection and repair using nerve transfer. Neurourol. Urodyn. 2011, 30, 599–605. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kihara, K.; Kageyama, Y.; Yamada, T.; Liu, S.; Sato, K. Spontaneous reconstruction of the canine hypogastric nerve over a long period after removing half of its length. Auton. Neurosci. Basic Clin. 2001, 86, 151–162. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kihara, K.; Hyochi, N.; Masuda, H.; Sato, K. Spontaneous regeneration of the seriously injured sympathetic pathway projecting to the prostate over a long period in the dog. BJU Int. 2003, 91, 868–872. [Google Scholar] [CrossRef]
- Ohlsson, M.; Nieto, J.H.; Christe, K.L.; Havton, L.A. Long-term effects of a lumbosacral ventral root avulsion injury on axotomized motor neurons and avulsed ventral roots in a non-human primate model of cauda equina injury. Neuroscience 2013, 250, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kepper, M.E.; Keast, J.R. Specific targeting of ganglion cell sprouts provides an additional mechanism for restoring peripheral motor circuits in pelvic ganglia after spinal nerve damage. J. Neurosci. 1998, 18, 7987–7995. [Google Scholar] [CrossRef]
- Sundin, T.; Dahlström, A. The Sympathetic Innervation of the Urinary Bladder and Urethra in the Normal State and After Parasympathetic Denervation at the Spinal Root Level: An Experimental Study in Cats. Scand. J. Urol. Nephrol. 1973, 7, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Caroni, P.; Grandes, P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J. Cell Biol. 1990, 110, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, D.-h.; Zhang, H.-y.; Wang, J.; Li, X.-k.; Xiao, J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300. [Google Scholar] [CrossRef]
- Skundric, D.S.; Lisak, R.P. Role of neuropoietic cytokines in development and progression of diabetic polyneuropathy: From glucose metabolism to neurodegeneration. Exp. Diabesity Res. 2003, 4, 303–312. [Google Scholar] [CrossRef]
- Okan, A.; Ali Ersin, Z.; Cihan, T.; Hülya, A.; Zafer, A. The use of biomarkers in the diagnosis and treatment of overactive bladder: Can we predict the patients who will be resistant to treatment?: Biomarkers As Predictive Tools in Overactive Bladder. Neurourol. Urodyn. 2015, 36, 390–393. [Google Scholar] [CrossRef]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003, 27, 277–323. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Song, L.; Zhu, J.; Zhang, X.; Cui, Z.; Fu, Q.; Huang, J.; Lu, H. BDNF-hypersecreting human umbilical cord blood mesenchymal stem cells promote erectile function in a rat model of cavernous nerve electrocautery injury. Int. Urol. Nephrol. 2016, 48, 37–45. [Google Scholar] [CrossRef]
- Kishino, A.; Ishige, Y.; Tatsuno, T.; Nakayama, C.; Noguchi, H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp. Neurol. 1997, 144, 273–286. [Google Scholar] [CrossRef]
- Li, L.; Wu, W.; Lin, L.-F.H.; Lei, M.; Oppenheim, R.W.; Houenou, L.J. Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 9771–9775. [Google Scholar] [CrossRef] [PubMed]
- Novikov, L.; Novikova, L.; Kellerth, J.O. Brain-derived neurotrophic factor promotes axonal regeneration and long-term survival of adult rat spinal motoneurons in vivo. Neuroscience 1997, 79, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Novikova, L.; Novikov, L.; Kellerth, J.-O. Effects of neurotransplants and BDNF on the survival and regeneration of injured adult spinal motoneurons. Eur. J. Neurosci. 1997, 9, 2774–2777. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. The neurotrophin family of neurotrophic factors: An overview. Methods Mol. Biol. 2012, 846, 1–12. [Google Scholar] [CrossRef]
- More, J.Y.; Bruna, B.A.; Lobos, P.E.; Galaz, J.L.; Figueroa, P.L.; Namias, S.; Sánchez, G.L.; Barrientos, G.C.; Valdés, J.L.; Paula-Lima, A.C.; et al. Calcium release mediated by redox-sensitive RyR2 channels has a central role in hippocampal structural plasticity and spatial memory. Antioxid. Redox Signal. 2018, 29, 1125–1146. [Google Scholar] [CrossRef]
- Bruna, B.; Lobos, P.; Herrera-Molina, R.; Hidalgo, C.; Paula-Lima, A.; Adasme, T. The signaling pathways underlying BDNF-induced Nrf2 hippocampal nuclear translocation involve ROS, RyR-Mediated Ca2+ signals, ERK and PI3K. Biochem. Biophys. Res. Commun. 2018, 505, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Campese, V.M.; Ye, S.; Zhong, H.; Yanamadala, V.; Ye, Z.; Chiu, J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H695–H703. [Google Scholar] [CrossRef] [PubMed]
- Frara, N.; Giaddui, D.; Braverman, A.S.; Porreca, D.S.; Brown, J.M.; Mazzei, M.; Wagner, I.J.; Pontari, M.A.; Tiwari, E.; Testa, C.L.; et al. Nerve transfer for restoration of lower motor neuron-lesioned bladder function. Part 1: Attenuation of purinergic bladder smooth muscle contractions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R885–R896. [Google Scholar] [CrossRef]
- Frara, N.; Barbe, M.F.; Giaddui, D.; Porreca, D.S.; Braverman, A.S.; Tiwari, E.; Ahmad, A.; Brown, J.M.; Johnston, B.R.; Bazarek, S.F.; et al. Nerve transfer for restoration of lower motor neuron-lesioned bladder, urethral, and anal sphincter function in a dog model. Part 3. nicotinic receptor characterization. Am. J. Physiol Regul. Integr. Comp. Physiol. 2023, 325, R344–R358. [Google Scholar] [CrossRef] [PubMed]
- Frara, N.; Giaddui, D.; Braverman, A.S.; Jawawdeh, K.; Wu, C.; Ruggieri, M.R., Sr.; Barbe, M.F. Mechanisms involved in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox)-derived reactive oxygen species (ROS) modulation of muscle function in human and dog bladders. PLoS ONE 2023, 18, e0287212. [Google Scholar] [CrossRef]
- Vollert, J.; Macleod, M.; Dirnagl, U.; Kas, M.J.; Michel, M.C.; Potschka, H.; Riedel, G.; Wever, K.E.; Würbel, H.; Steckler, T.; et al. The EQIPD framework for rigor in the design, conduct, analysis and documentation of animal experiments. Nat. Methods 2022, 19, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Jiang, Y.-H.; Kuo, H.-C. Urinary biomarkers in patients with detrusor underactivity with and without bladder function recovery. Int. Urol. Nephrol. 2017, 49, 1763–1770. [Google Scholar] [CrossRef]
- Koven, N.S.; Collins, L.R. Urinary brain-derived neurotrophic factor as a biomarker of executive functioning. Neuropsychobiology 2014, 69, 227–234. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Braun, A.; Mannsfeldt, A.; Botchkarev, V.A.; Botchkareva, N.V.; Paus, R.; Fischer, A.; Lewin, G.R.; Renz, H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia: Implications for paracrine and target-derived neurotrophic functions. Am. J. Pathol. 1999, 155, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Oddiah, D.; Anand, P.; McMahon, S.B.; Rattray, M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 1998, 9, 1455–1458. [Google Scholar] [CrossRef]
- Murray, E.; Malley, S.E.; Qiao, L.Y.; Hu, V.Y.; Vizzard, M.A. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J. Urol. 2004, 172, 2434–2439. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Tomoyoshi, T.; Tooyama, I.; Kitahama, K.; Kim, S.U.; Maeda, T. Low-affinity nerve growth factor receptor immunoreactivity in the human urinary bladder. Neurosci. Lett. 1995, 186, 9–12. [Google Scholar] [CrossRef]
- Keast, J.R.; Kepper, M.E. Differential regulation of trkA and p75 in noradrenergic pelvic autonomic ganglion cells after deafferentation of their cholinergic neighbours. Eur. J. Neurosci. 2001, 13, 211–220. [Google Scholar] [CrossRef]
- Kashyap, M.P.; Pore, S.K.; de Groat, W.C.; Chermansky, C.J.; Yoshimura, N.; Tyagi, P. BDNF overexpression in the bladder induces neuronal changes to mediate bladder overactivity. Am. J. Physiol. Ren. Physiol. 2018, 315, F45–F56. [Google Scholar] [CrossRef] [PubMed]
- Grosheva, M.; Nohroudi, K.; Schwarz, A.; Rink, S.; Bendella, H.; Sarikcioglu, L.; Klimaschewski, L.; Gordon, T.; Angelov, D.N. Comparison of trophic factors’ expression between paralyzed and recovering muscles after facial nerve injury. A quantitative analysis in time course. Exp. Neurol. 2016, 279, 137–148. [Google Scholar] [CrossRef]
- Srinivasan, P.; Zervantonakis, I.K.; Kothapalli, C.R. Synergistic effects of 3D ECM and chemogradients on neurite outgrowth and guidance: A simple modeling and microfluidic framework. PLoS ONE 2014, 9, e99640. [Google Scholar] [CrossRef]
- Gonzalez, M.; Collins, W.F., III. Modulation of motoneuron excitability by brain-derived neurotrophic factor. J. Neurophysiol. 1997, 77, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Mendell, L.M. Neurotrophins and sensory neurons: Role in development, maintenance and injury. A thematic summary. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1996, 351, 463–467. [Google Scholar] [CrossRef]
- Braun, A.; Lommatzsch, M.; Mannsfeldt, A.; Neuhaus-Steinmetz, U.; Fischer, A.; Schnoy, N.; Lewin, G.R.; Renz, H. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am. J. Respir. Cell Mol. Biol. 1999, 21, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Frias, B.; Allen, S.; Dawbarn, D.; McMahon, S.B.; Cruz, F.; Cruz, C.D. Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience 2010, 166, 907–916. [Google Scholar] [CrossRef]
- Kobayashi, N.R.; Bedard, A.M.; Hincke, M.T.; Tetzlaff, W. Increased expression of BDNF and trkB mRNA in rat facial motoneurons after axotomy. Eur. J. Neurosci. 1996, 8, 1018–1029. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Krishnan, K.R.; Mansour, P.; Soni, B.M.; McDicken, I. p75 nerve growth factor receptor in the vesical urothelium of patients with neuropathic bladder: An immunohistochemical study. Spinal Cord 1998, 36, 541–547. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.Y.; Luo, X.G.; Xian, C.J.; Liu, Z.H.; Zhou, X.F. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur. J. Neurosci. 2000, 12, 4171–4180. [Google Scholar] [CrossRef]
- Frias, B.; Santos, J.; Morgado, M.; Sousa, M.M.; Gray, S.M.Y.; McCloskey, K.D.; Allen, S.; Cruz, F.; Cruz, C.D. The Role of Brain-Derived Neurotrophic Factor (BDNF) in the Development of Neurogenic Detrusor Overactivity (NDO). J. Neurosci. 2015, 35, 2146–2160. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.L.; Payne, S.C.; Keast, J.R.; Osborne, P.B. Peripheral injury of pelvic visceral sensory nerves alters GFRα (GDNF family receptor alpha) localization in sensory and autonomic pathways of the sacral spinal cord. Front. Neuroanat. 2015, 9, 43. [Google Scholar] [CrossRef]
- Höke, A.; Gordon, T.; Zochodne, D.W.; Sulaiman, O.A. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp. Neurol. 2002, 173, 77–85. [Google Scholar] [CrossRef]
- Naveilhan, P.; ElShamy, W.M.; Ernfors, P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFRα after sciatic nerve lesion in the mouse. Eur. J. Neurosci. 1997, 9, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Trupp, M.; Belluardo, N.; Funakoshi, H.; Ibanez, C.F. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J. Neurosci. 1997, 17, 3554–3567. [Google Scholar] [CrossRef] [PubMed]
- Buj-Bello, A.; Buchman, V.L.; Horton, A.; Rosenthal, A.; Davies, A.M. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron 1995, 15, 821–828. [Google Scholar] [CrossRef]
- Henderson, C.E.; Phillips, H.S.; Pollock, R.A.; Davies, A.M.; Lemeulle, C.; Armanini, M.; Simmons, L.; Moffet, B.; Vandlen, R.A.; Koliatsos, V.E.; et al. GDNF: A potent survival factor for motoneurons present in peripheral nerve and muscle. Science 1994, 266, 1062–1064. [Google Scholar] [CrossRef]
- Heuckeroth, R.O.; Lampe, P.A.; Johnson, E.M.; Milbrandt, J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev. Biol. 1998, 200, 116–129. [Google Scholar] [CrossRef]
- Trupp, M.; Rydén, M.; Jörnvall, H.; Funakoshi, H.; Timmusk, T.; Arenas, E.; Ibáñez, C.F. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J. Cell Biol. 1995, 130, 137–148. [Google Scholar] [CrossRef]
- Okragly, A.J.; Niles, A.L.; Saban, R.; Schmidt, D.; Hoffman, R.L.; Warner, T.F.; Moon, T.D.; Uehling, D.T.; Haak-Frendscho, M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J. Urol. 1999, 161, 438–441, discussion 441–432. [Google Scholar] [CrossRef]
- Ekman, M.; Zhu, B.; Swärd, K.; Uvelius, B. Neurite outgrowth in cultured mouse pelvic ganglia–Effects of neurotrophins and bladder tissue. Auton. Neurosci. 2017, 205, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Ryals, J.M.; Wright, D.E. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J. Neurosci. 2004, 24, 9405–9413. [Google Scholar] [CrossRef] [PubMed]
- Tonyan, S.; Pospelova, M.; Krasnikova, V.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Vavilova, T.; Vasilieva, E.; Makhanova, A.; et al. Neurotrophin-3 (NT-3) as a potential biomarker of the peripheral nervous system damage following breast cancer treatment. Pathophysiology 2023, 30, 110–122. [Google Scholar] [CrossRef]
- Funakoshi, H.; Frisén, J.; Barbany, G.; Timmusk, T.; Zachrisson, O.; Valerie, M.K.V.; Persson, H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993, 123, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, O.; Parsadanian, A.S.; Sendtner, M.; Thoenen, H. Expression of neurotrophins in skeletal muscle: Quantitative comparison and significance for motoneuron survival and maintenance of function. J. Neurosci. Res. 1995, 42, 21–33. [Google Scholar] [CrossRef]
- Schaller, S.; Buttigieg, D.; Alory, A.; Jacquier, A.; Barad, M.; Merchant, M.; Gentien, D.; de la Grange, P.; Haase, G. Novel combinatorial screening identifies neurotrophic factors for selective classes of motor neurons. Proc. Natl. Acad. Sci. USA 2017, 114, E2486–E2493. [Google Scholar] [CrossRef]
- Baloh, R.H.; Tansey, M.G.; Lampe, P.A.; Fahrner, T.J.; Enomoto, H.; Simburger, K.S.; Leitner, M.L.; Araki, T.; Johnson, E.M., Jr.; Milbrandt, J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron 1998, 21, 1291–1302. [Google Scholar] [CrossRef]
- Abrams, M.; Widenfalk, J. Emerging strategies to promote improved functional outcome after peripheral nerve injury. Restor. Neurol. Neurosci. 2005, 23, 367–382. [Google Scholar] [CrossRef]
- Bennett, D.L.; Boucher, T.J.; Armanini, M.P.; Poulsen, K.T.; Michael, G.J.; Priestley, J.V.; Phillips, H.S.; McMahon, S.B.; Shelton, D.L. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J. Neurosci. 2000, 20, 427–437. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J. Neurosci. 1995, 15, 3876–3885. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J. Neurosci. 1995, 15, 3886–3895. [Google Scholar] [CrossRef]
- Wang, T.; Molliver, D.C.; Jing, X.; Schwartz, E.S.; Yang, F.C.; Samad, O.A.; Ma, Q.; Davis, B.M. Phenotypic switching of nonpeptidergic cutaneous sensory neurons following peripheral nerve injury. PLoS ONE 2011, 6, e28908. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Matsuoka, I.; Wetmore, C.; Olson, L.; Thoenen, H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: Different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992, 119, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Sano, M.; Omura, K.; Hasegawa, T.; Doi, M.; Sawada, T.; Nagano, A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J. Peripher. Nerv. Syst. 2005, 10, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hammarberg, H.; Piehl, F.; Risling, M.; Cullheim, S. Differential regulation of trophic factor receptor mRNAs in spinal motoneurons after sciatic nerve transection and ventral root avulsion in the rat. J. Comp. Neurol. 2000, 426, 587–601. [Google Scholar] [CrossRef]
- Fariñas, I.; Jones, K.R.; Backus, C.; Wang, X.-Y.; Reichardt, L.F. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature 1994, 369, 658–661. [Google Scholar] [CrossRef]
- Ruit, K.G.; Elliott, J.L.; Osborne, P.A.; Yan, Q.; Snider, W.D. Selective dependence of mammalian dorsal root ganglion neurons on nerve growth factor during embryonic development. Neuron 1992, 8, 573–587. [Google Scholar] [CrossRef]
- Hoke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef]
- Vizzard, M.A. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp. Neurol. 2000, 161, 273–284. [Google Scholar] [CrossRef]
- Hilliard, B.A.; Amin, M.; Popoff, S.N.; Barbe, M.F. Force dependent effects of chronic overuse on fibrosis-related genes and proteins in skeletal muscles. Connect. Tissue Res. 2021, 62, 133–149. [Google Scholar] [CrossRef]
- Ohtori, S.; Takahashi, K.; Moriya, H.; Myers, R.R. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: Studies in murine DRG and spinal cord. Spine 2004, 29, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Gomez-Amaya, S.; Braverman, A.S.; Brown, J.M.; Lamarre, N.S.; Massicotte, V.S.; Lewis, J.K.S.; Dachert, S.R.; Ruggieri, M.R. Evidence of vagus nerve sprouting to innervate the urinary bladder and clitoris in a canine model of lower motoneuron lesioned bladder. Neurourol. Urodyn. 2017, 36, 91–97. [Google Scholar] [CrossRef]
- Wagner, R.; Myers, R.R. Schwann cells produce tumor necrosis factor alpha: Expression in injured and non-injured nerves. Neuroscience 1996, 73, 625–629. [Google Scholar] [CrossRef]
- Sakuma, Y.; Ohtori, S.; Miyagi, M.; Ishikawa, T.; Inoue, G.; Doya, H.; Koshi, T.; Ito, T.; Yamashita, M.; Yamauchi, K.; et al. Up-regulation of p55 TNF alpha-receptor in dorsal root ganglia neurons following lumbar facet joint injury in rats. Eur. Spine J. 2007, 16, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Buehl, A.; Sommer, C. Tumor necrosis factor receptor 1 and 2 proteins are differentially regulated during Wallerian degeneration of mouse sciatic nerve. Exp. Neurol. 2005, 192, 163–166. [Google Scholar] [CrossRef]
- Shamash, S.; Reichert, F.; Rotshenker, S. The cytokine network of Wallerian degeneration: Tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J. Neurosci. 2002, 22, 3052–3060. [Google Scholar] [CrossRef]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133, 433–447. [Google Scholar] [CrossRef]
- Yu, L.; O’Brien, V.P.; Livny, J.; Dorsey, D.; Bandyopadhyay, N.; Colonna, M.; Caparon, M.G.; Roberson, E.D.; Hultgren, S.J.; Hannan, T.J. Mucosal infection rewires TNFɑ signaling dynamics to skew susceptibility to recurrence. eLife 2019, 8, e46677. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Chang, C.H.; Kuo, H.C. Clinical efficacy and changes of urothelial dysfunction after repeated detrusor botulinum toxin A injections in chronic spinal cord-injured bladder. Toxins 2016, 8, 164. [Google Scholar] [CrossRef]
- de Groat, W.C.; Yoshimura, N. Changes in afferent activity after spinal cord injury. Neurourol. Urodyn. 2010, 29, 63–76. [Google Scholar] [CrossRef]
- Lankelma, J.M.; Belzer, C.; Hoogendijk, A.J.; de Vos, A.F.; de Vos, W.M.; van der Poll, T.; Wiersinga, W.J. Antibiotic-induced gut microbiota disruption decreases TNF-α release by mononuclear cells in healthy adults. Clin. Transl. Gastroenterol. 2016, 7, e186. [Google Scholar] [CrossRef]
- Li, J.K.M.; Chiu, P.K.F.; Ng, C.F. The impact of microbiome in urological diseases: A systematic review. Int. Urol. Nephrol. 2019, 51, 1677–1697. [Google Scholar] [CrossRef] [PubMed]
- Birder, L.A.; Ruggieri, M.; Takeda, M.; van Koeveringe, G.; Veltkamp, S.; Korstanje, C.; Parsons, B.; Fry, C.H. How does the urothelium affect bladder function in health and disease? ICI-RS 2011. Neurourol. Urodyn. 2012, 31, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Valerie, F.; Saddek, M.-S.; Noelle, H.; Céline, F.; Klaus, P.; Ulrich, E. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: Opposite roles of TNF receptor 1 and TNF receptor 2. J. Neurosci. 2002, 22, RC216. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Hervera, A.; De Virgiliis, F.; Palmisano, I.; Zhou, L.; Tantardini, E.; Kong, G.; Hutson, T.; Danzi, M.C.; Perry, R.B.; Santos, C.X.C.; et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 2018, 20, 307–319. [Google Scholar] [CrossRef]
- Kamsler, A.; Segal, M. Hydrogen peroxide modulation of synaptic plasticity. J. Neurosci. 2003, 23, 269–276. [Google Scholar] [CrossRef]
- Kamsler, A.; Segal, M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol. Neurobiol. 2004, 29, 167–178. [Google Scholar] [CrossRef]
- Min, J.Y.; Park, M.H.; Park, M.K.; Park, K.W.; Lee, N.W.; Kim, T.; Kim, H.J.; Lee, D.H. Staurosporin induces neurite outgrowth through ROS generation in HN33 hippocampal cell lines. J. Neural Transm. 2006, 113, 1821–1826. [Google Scholar] [CrossRef]
- Oswald, M.C.; Brooks, P.S.; Zwart, M.F.; Mukherjee, A.; West, R.J.; Giachello, C.N.; Morarach, K.; Baines, R.A.; Sweeney, S.T.; Landgraf, M. Reactive oxygen species regulate activity-dependent neuronal plasticity in Drosophila. eLife 2018, 7, e39393. [Google Scholar] [CrossRef]
- Rieger, S.; Sagasti, A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol. 2011, 9, e1000621. [Google Scholar] [CrossRef] [PubMed]
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Wang, Y.; Li, R.; Huang, S.; Li, X.; Liu, X. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct. 2017, 8, 4421–4432. [Google Scholar] [CrossRef] [PubMed]
- Rink-Notzon, S.; Reuscher, J.; Wollny, L.; Sarikcioglu, L.; Bilmen, S.; Manthou, M.; Gordon, T.; Angelov, D.N. Appropriate dosage, timing, and site of intramuscular injections of brain-derived neurotrophic factor (BDNF) promote motor recovery after facial nerve injury in rats. Muscle Nerve 2024, 69, 490–497. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, F.; Tao, J.; Tan, S.; Zeng, L.; Peng, X.; Wu, B. Insulin-Like Growth Factor-1 Contributes to Mucosal Repair by beta-Arrestin2-Mediated Extracellular Signal-Related Kinase Signaling in Experimental Colitis. Am. J. Pathol. 2015, 185, 2441–2453. [Google Scholar] [CrossRef]

| Gene | Assay | Reporter Dye |
|---|---|---|
| ACTB (b-Actin) | Cf04931159 | FAM |
| BDNF | Cf02718934 | FAM |
| NTF3 | Cf02700489 | FAM |
| GDNF | Cf02691052 | FAM |
| CNTF | Cf03460095 | FAM |
| HGF (hepatocyte growth factor) | Cf02692661 | FAM |
| NGF | Cf02697134 | FAM |
| Neurotrophin | BDNF | NGF | NT-3 | ARTN | GDNF | CNTF | TNF-α |
|---|---|---|---|---|---|---|---|
| Mucosa vs. muscle | r = 0.4, p = 0.04 * | r = −0.25, p = 0.36 | r = 0.2, p = 0.5 | r = 0.13, p = 0.62 | r = −0.5, p = 0.046 * | r = −0.04, p = 0.7 | r = 0.56, p = 0.005 ** |
| Histological Parameters | Urothelial Integrity Score (0 = Normal, 3 = Detached) | Submucosa, Inflammation Score (0 = Normal, 3 = Abnormal) | Detrusor Layer Thickness (mm) | Detrusor Layer, Inflammation Score (0 = Normal, 3 = Abnormal) | #Neuronal Cell Bodies/ Ganglion (#/mm2) | Axon Density (#/mm2) |
|---|---|---|---|---|---|---|
| Mucosa | ||||||
| BDNF | r = −0.31, p = 0.13 | r = 0.46, p = 0.03 * | r = 0.56, p = 0.03 * | r = 0.46, p = 0.03 * | r = 0.43, p = 0.04 * | r = 0.76, p < 0.0001 ** |
| NGF | r = 0.03, p = 0.46 | r = 0.30, p = 0.17 | r = 0.0002, p = 0.5 | r = 0.3, p = 0.2 | r = −0.08, p = 0.41 | r = 0.31, p = 0.2 |
| NT-3 | r = −0.17, p = 0.3 | r = 0.30, p = 0.17 | r = 0.11, p = 0.4 | r = −0.3, p = 0.2 | r = −0.17, p = 0.33 | r = 0.3, p = 0.2 |
| ARTN | r = −0.24, p = 0.28 | r = −0.43, p = 0.08 | r = 0.01, p = 0.5 | r = −0.5, p = 0.09 | r = −0.23, p = 0.27 | r = 0.46, p = 0.11 |
| GDNF | r = 0.23, p = 0.27 | r = −0.37, p = 0.15 | r = 0.15, p = 0.35 | r = −0.3, p = 0.15 | r = −0.3, p = 0.2 | r = 0.04, p = 0.45 |
| CNTF | r = −0.71, p = 0.02 * | r = 0.23, p = 0.26 | r = 0.59, p = 0.06 | r = 0.23, p = 0.3 | r = 0.6, p = 0.04 * | r = 0.4, p = 0.14 |
| TNF-α | r = −0.12, p = 0.33 | r = −0.04, p = 0.43 | r = −0.04, p = 0.45 | r = − 0.04, p = 0.4 | r = −0.07, p = 0.4 | r = 0.02, p = 0.5 |
| Muscle | ||||||
| BDNF | r = −0.004, p = 0.5 | r = 0.12, p = 0.32 | r = −0.14, p = 0.3 | r = 0.12, p = 0.32 | r = 0.08, p = 0.4 | r = 0.3, p = 0.13 |
| NGF | r = 0.2, p = 0.3 | r = −0.32, p = 0.10 | r = −0.4, p = 0.1 | r = −0.32, p = 0.12 | r = −0.26, p = 0.2 | r = −0.2, p = 0.3 |
| NT-3 | r = 0.12, p = 0.4 | r = −0.32, p = 0.12 | r = 0.12, p = 0.4 | r = −0.53, p = 0.04 * | r = −0.3, p = 0.2 | r = −0.15, p = 0.3 |
| ARTN | r = 0.14, p = 0.3 | r = −0.37, p = 0.12 | r = −0.006, p = 0.5 | r = −0.4, p = 0.12 | r = −0.4, p = 0.14 | r = −0.53, p = 0.055 |
| GDNF | r = −0.15, p = 0.36 | r = 0.37, p = 0.13 | r = 0.05, p = 0.45 | r = 0.4, p = 0.13 | r = 0.35, p = 0.2 | r = −0.1, p = 0.4 |
| CNTF | r = 0.3, p = 0.3 | r = 0.13, p = 0.36 | r = 0.1, p = 0.41 | r = 0.13, p = 0.4 | r = −0.2, p = 0.33 | r = 0.16, p = 0.3 |
| TNF-α | r = −0.01, p = 0.5 | r = −0.18, p = 0.25 | r = −0.5, p = 0.046 * | r = −0.2, p = 0.25 | r = −0.11, p = 0.3 | r = −0.2, p = 0.3 |
| Histological Parameters | Urothelial Integrity Score (0 = Normal, 3 = Detached) | Submucosa, Inflammation Score (0 = Normal, 3 = Abnormal) | Detrusor Layer Thickness (mm) | Detrusor Layer, Inflammation Score (0 = Normal, 3 = Abnormal) | # Neuronal Cell Bodies/ Ganglion (#/mm2) | Axon Density (#/mm2) |
|---|---|---|---|---|---|---|
| Mucosa | r = 0.26, p = 0.22 | r = 0.07, p = 0.40 | r = −0.67, p = 0.02 * | r = −0.44, p = 0.04 * | r = 0.11, p = 0.4 | r = 0.3, p = 0.2 |
| Muscle | r = 0.001, p = 0.5 | r = −0.02, p = 0.46 | r = −0.26, p = 0.25 | r = −0.4, p = 0.08 | r = 0.03, p = 0.5 | r = 0.1, p = 0.4 |
| Functional Outcome | Bladder Contraction After Electrical Stimulation of Vesical or Transferred Obturator Nerve | Bladder Contraction After Electrical Stimulation of Spinal Root of Origin (Vesical (L7-S3) or Transferred Obturator (L2-L6)) |
|---|---|---|
| Mucosa | ||
| BDNF | r = 0.31, p = 0.45 | r = 0.09, p = 0.35 |
| NGF | r = −0.03, p = 0.46 | r = 0.07, p = 0.42 |
| NT-3 | r = 0.07, p = 0.37 | r = 0.26, p = 0.24 |
| ARTN | r = 0.44, p = 0.09 | r = −0.15, p = 0.34 |
| GDNF | r = 0.45, p = 0.04 * | r = 0.43, p = 0.08 |
| CNTF | r = −0.31, p = 0.13 | r = 0.13, p = 0.35 |
| TNF-α | r = 0.76, p = 0.003 * | r = 0.34, p = 0.08 |
| ROS | r = 0.56, p = 0.04 * | r = 0.74, p = 0.0005 * |
| Muscle | ||
| BDNF | r = 0.40, p = 0.04 * | r = −0.03, p = 0.45 |
| NGF | r = 0.36, p = 0.06 | r = −0.22, p = 0.22 |
| NT-3 | r = 0.44, p = 0.09 | r = −0.15, p = 0.32 |
| ARTN | r = 0.46, p = 0.67 | r = −0.37, p = 0.12 |
| GDNF | r = −0.75, p = 0.04 * | r = −0.41, p = 0.11 |
| CNTF | r = 0.28, p = 0.15 | r = −0.02, p = 0.47 |
| TNF-α | r = 0.73, p = 0.006 * | r = 0.16, p = 0.26 |
| ROS | r = −0.29, p = 0.18 | r = 0.10, p = 0.35 |
| Neurotrophin | BDNF | NGF | NT-3 | ARTN | GDNF | CNTF | TNF-α | ROS |
|---|---|---|---|---|---|---|---|---|
| Mucosa | r = −0.04, p = 0.4 | r = −0.52, p = 0.01 * | r = 0.1, p = 0.3 | r = 0.1, p = 0.3 | r = 0.2, p = 0.2 | r = −0.1, p = 0.4 | r = −0.3, p = 0.06 | r = 0.003, p = 0.5 |
| Muscle | r = 0.03, p = 0.47 | r = 0.2, p = 0.2 | r = 0.46, p = 0.02 * | r = 0.1, p = 0.3 | r = 0.1, p = 0.3 | r = −0.26, p = 0.16 | r = 0.04, p = 0.4 | r = −0.4, p = 0.04 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frara, N.; Jawawdeh, K.; Giaddui, D.; Tamas, I.P.; Gares, R.P.; McGonagle, E.R.; Hilliard, B.A.; Kolpakov, M.A.; Bright-Rowe, L.; Braverman, A.S.; et al. Enhanced BDNF and ROS in Mucosa of Lower Motor Neuron Lesioned Dog Bladder Following Somatic Motor Nerve Transfer. Cells 2025, 14, 406. https://doi.org/10.3390/cells14060406
Frara N, Jawawdeh K, Giaddui D, Tamas IP, Gares RP, McGonagle ER, Hilliard BA, Kolpakov MA, Bright-Rowe L, Braverman AS, et al. Enhanced BDNF and ROS in Mucosa of Lower Motor Neuron Lesioned Dog Bladder Following Somatic Motor Nerve Transfer. Cells. 2025; 14(6):406. https://doi.org/10.3390/cells14060406
Chicago/Turabian StyleFrara, Nagat, Kais Jawawdeh, Dania Giaddui, Istvan P. Tamas, Ryan P. Gares, Elizabeth R. McGonagle, Brendan A. Hilliard, Mikhail A. Kolpakov, Lewis Bright-Rowe, Alan S. Braverman, and et al. 2025. "Enhanced BDNF and ROS in Mucosa of Lower Motor Neuron Lesioned Dog Bladder Following Somatic Motor Nerve Transfer" Cells 14, no. 6: 406. https://doi.org/10.3390/cells14060406
APA StyleFrara, N., Jawawdeh, K., Giaddui, D., Tamas, I. P., Gares, R. P., McGonagle, E. R., Hilliard, B. A., Kolpakov, M. A., Bright-Rowe, L., Braverman, A. S., Brown, J. M., Ruggieri, M. R., Sr., & Barbe, M. F. (2025). Enhanced BDNF and ROS in Mucosa of Lower Motor Neuron Lesioned Dog Bladder Following Somatic Motor Nerve Transfer. Cells, 14(6), 406. https://doi.org/10.3390/cells14060406







