Development of Novel Peptides That Target the Ninjurin 1 and 2 Pathways to Inhibit Cell Growth and Survival via p53
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Plasmids
2.4. Cell Viability Assay
2.5. LDH Release Assay
2.6. Immunoprecipitation and Western Blot Analysis
2.7. Colony Formation Assay
2.8. Peptide Synthesis
2.9. Statistical Analysis
3. Results
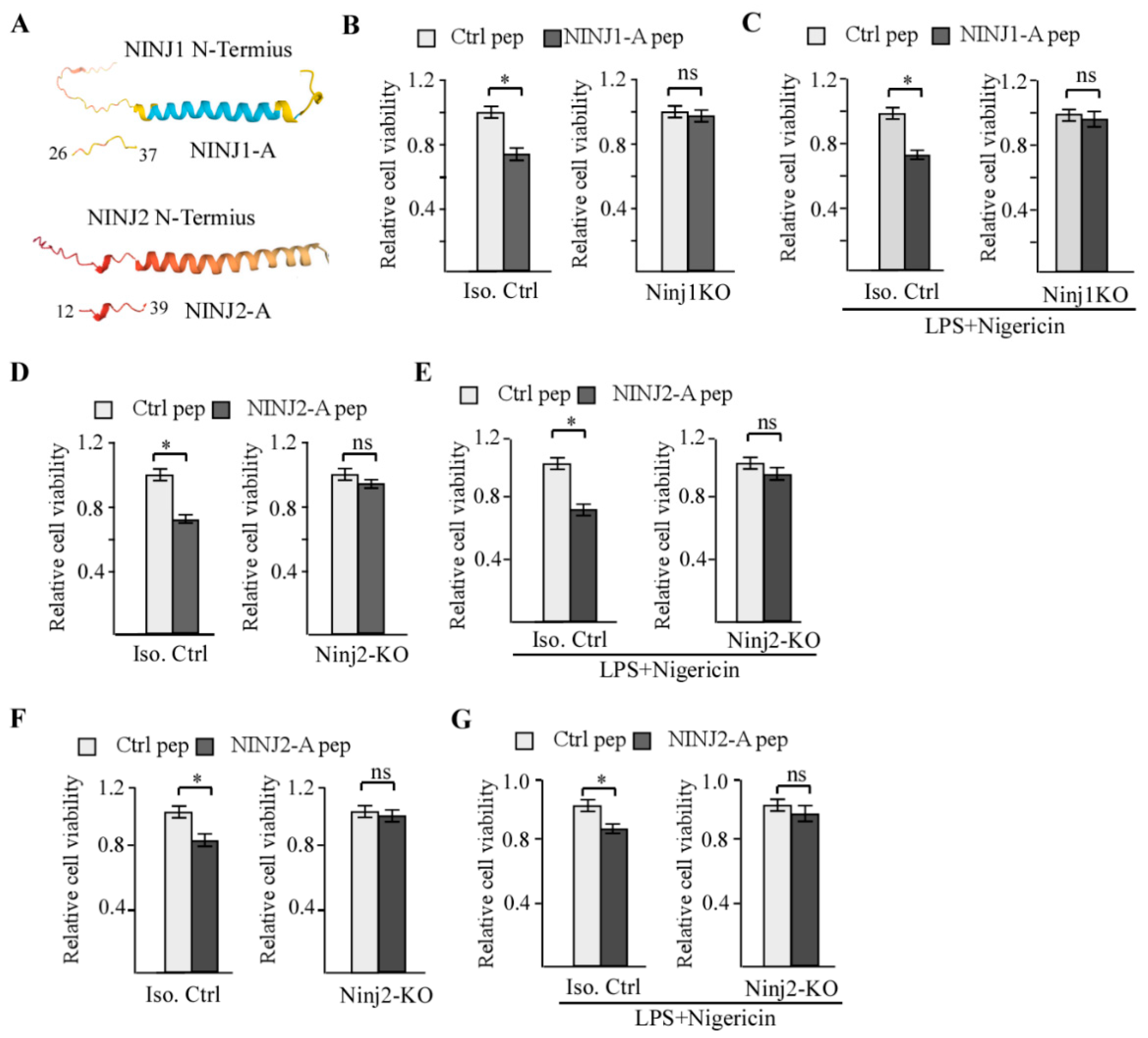
3.1. NINJ2-A Peptide, Which Is Derived from the N-Terminal Adhesion Motif of NINJ2, Inhibits Cell Growth in a NINJ2-Dependent Manner
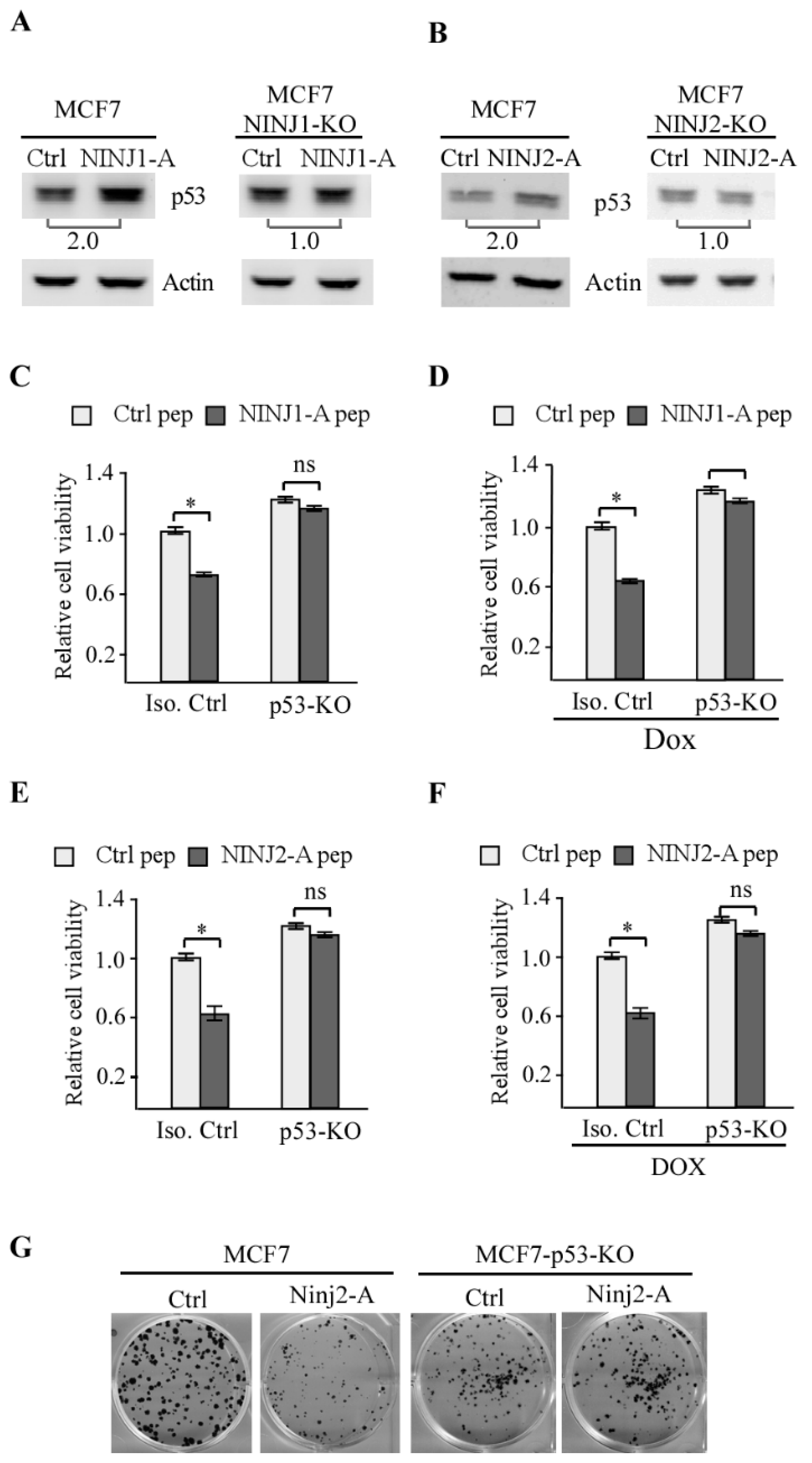
3.2. NINJ2-A Peptide Inhibits Cell Growth in a p53-Dependent Manner
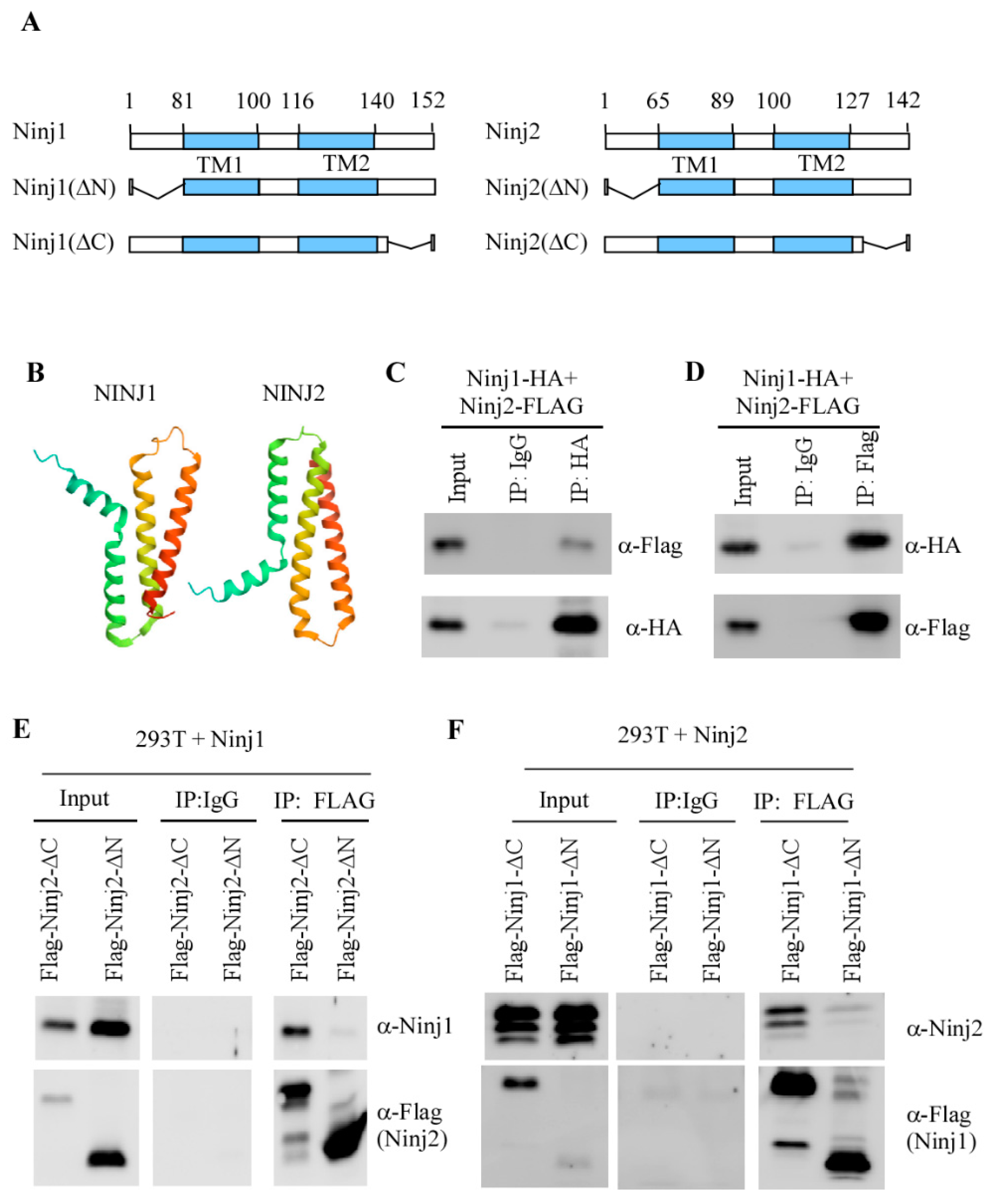
3.3. NINJ1 and NINJ2 Interact via Their N-Termini
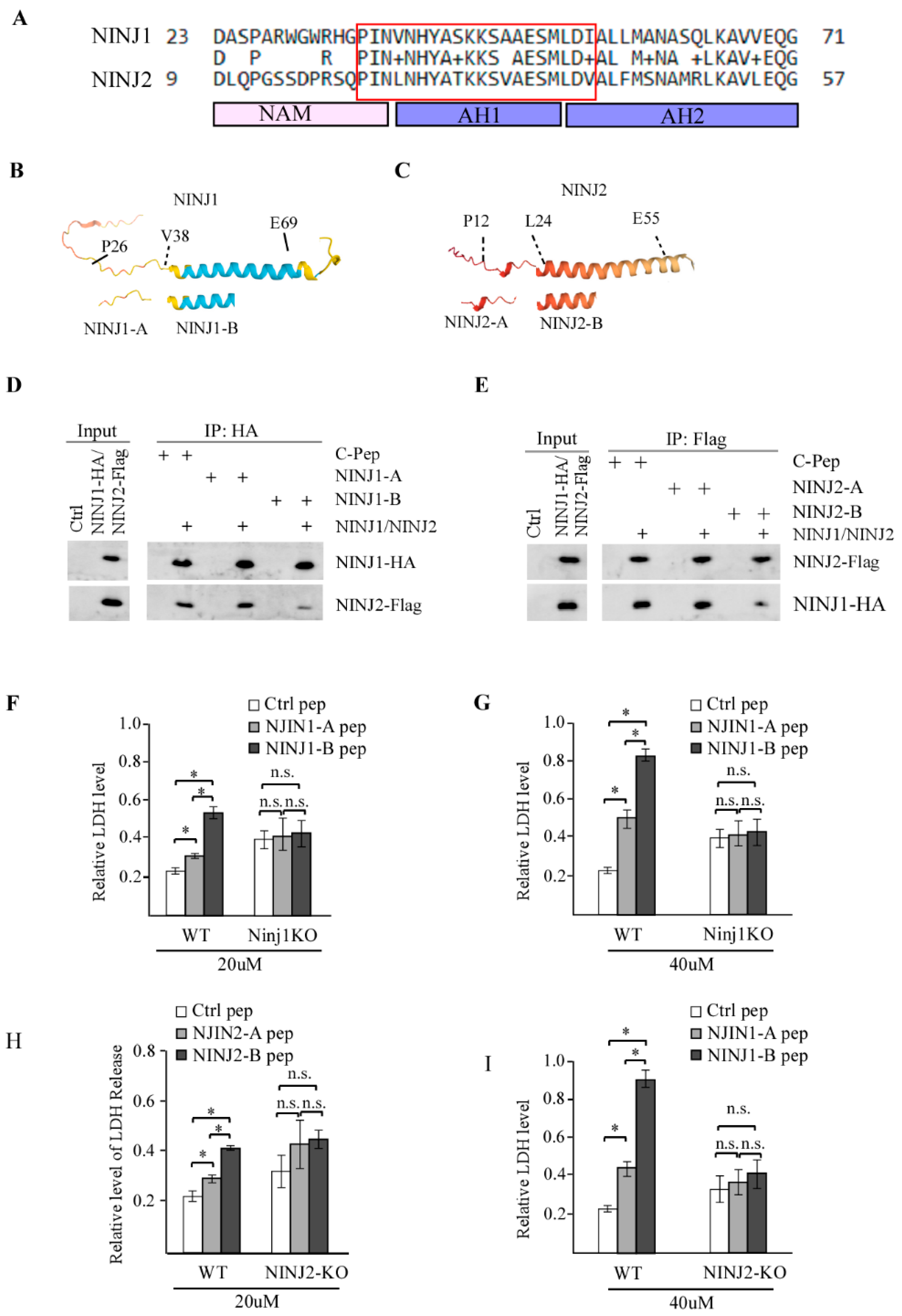
3.4. NINJ1-B and NINJ2-B Peptides, Which Are Derived from the N-Terminal Amphipathic Helices of NINJ1 and NINJ2, Respectively, Are Capable of Disrupting NINJ1-NINJ2 Interaction
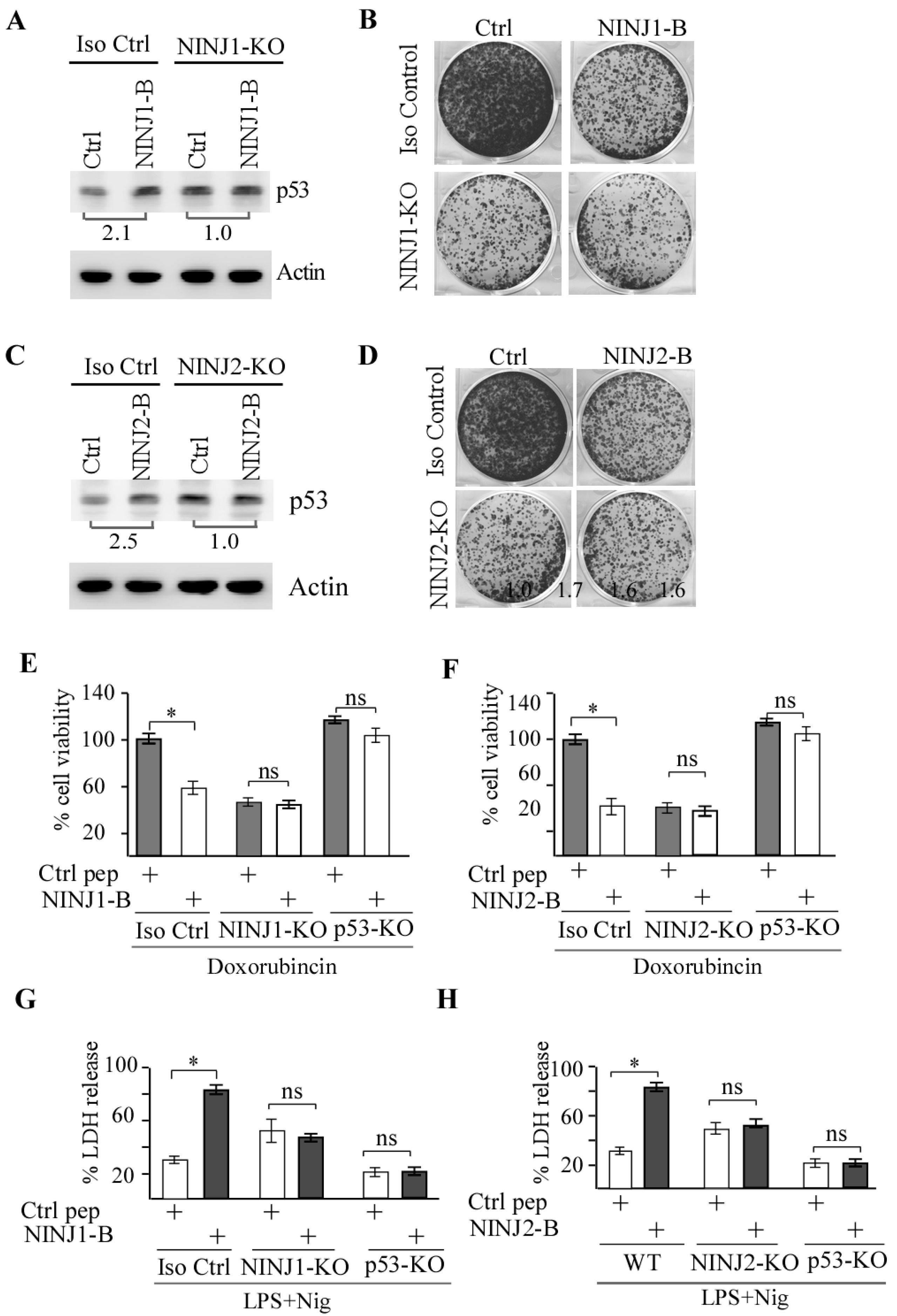
3.5. p53 Is Required for NINJ1-B and NINJ2-B Peptides to Induce Cell Death
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierschbacher, M.D.; Ruoslahti, E. Cell Attachment Activity of Fibronectin Can Be Duplicated by Small Synthetic Fragments of the Molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Hayman, E.G.; Pierschbacher, M.D. Extracellular Matrices and Cell-Adhesion. Arteriosclerosis 1985, 5, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Okegawa, T.; Pong, R.C.; Li, Y.M.; Hsieh, J.T. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim. Pol. 2004, 51, 445–457. [Google Scholar] [CrossRef]
- Okegawa, T.; Li, Y.M.; Pong, R.C.; Hsieh, J.T. Cell adhesion proteins as tumor suppressors. J. Urol. 2002, 167, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Zecchini, S.; Cavallaro, U. Neural Cell Adhesion Molecule in Cancer: Expression and Mechanisms. Struct. Funct. Neural Cell Adhes. Mol. Ncam 2010, 663, 319–333. [Google Scholar] [CrossRef]
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy-Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef]
- Jensen, M.; Berthold, F. Targeting the neural cell adhesion molecule in cancer. Cancer Lett. 2007, 258, 9–21. [Google Scholar] [CrossRef]
- Araki, T.; Milbrandt, J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron 1996, 17, 353–361. [Google Scholar] [CrossRef]
- Araki, T.; Milbrandt, J. Ninjurin2, a novel homophilic adhesion molecule, is expressed in mature sensory and enteric neurons and promotes neurite outgrowth. J. Neurosci. 2000, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, W.W.; Feng, Z.H. The Unrecognized Role of Ninjurin 2 in Inflammation, Metabolism, and Pyroptosis. Am. J. Pathol. 2024, 194, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ahn, B.J.; Shin, M.W.; Choi, J.H.; Kim, K.W. Ninjurin1: A potential adhesion molecule and its role in inflammation and tissue remodeling. Mol. Cells 2010, 29, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Hartenian, E.; Broz, P. Programmed cell death: NINJ1 and mechanisms of plasma membrane rupture. Trends Biochem. Sci. 2024, 49, 717–728. [Google Scholar] [CrossRef]
- Kayagaki, N.; Kornfeld, O.S.; Lee, B.L.; Stowe, I.B.; O’Rourke, K.; Li, Q.L.; Sandoval, W.; Yan, D.H.; Kang, J.; Xu, M.; et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 2021, 591, 131–136. [Google Scholar] [CrossRef]
- Ramos, S.; Hartenian, E.; Santos, J.C.; Walch, P.; Broz, P. NINJ1 induces plasma membrane rupture and release of damage-associated molecular pattern molecules during ferroptosis. EMBO J. 2024, 43, 1164–1186. [Google Scholar] [CrossRef]
- Chen, S.Y.; Wu, J.L.; Chen, Y.B.; Wang, Y.E.; Setayeshpour, Y.; Federico, C.; Mestre, A.A.; Lin, C.C.; Chi, J.T. NINJ1 regulates ferroptosis via xCT antiporter interaction and CoA modulation. Cell Death Dis. 2024, 15, 755. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.M.D.; Yang, H.J.; Zhang, W.C.; Chen, M.Y.; Chen, X.B. Ninjurin 2 Modulates Tumorigenesis, In fl ammation, and Metabolism via Pyroptosis. Am. J. Pathol. 2024, 194, 849–860. [Google Scholar] [CrossRef]
- Yang, H.J.; Zhang, J.; Yan, W.; Cho, S.J.; Lucchesi, C.; Chen, M.; Huang, E.C.; Scoumanne, A.; Zhang, W.; Chen, X. Ninjurin 1 has two opposing functions in tumorigenesis in a p53-dependent manner. Proc. Natl. Acad. Sci. USA 2017, 114, 11500–11505. [Google Scholar] [CrossRef]
- Cho, S.J.; Rossi, A.; Jung, Y.S.; Yan, W.; Liu, G.; Zhang, J.; Zhang, M.; Chen, X. Ninjurin1, a target of p53, regulates p53 expression and p53-dependent cell survival, senescence, and radiation-induced mortality. Proc. Natl. Acad. Sci. USA 2013, 110, 9362–9367. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Yang, H.J.; Mohibi, S.; Lucchesi, C.A.; Zhang, W.; Chen, X. Ninjurin 2, a Cell Adhesion Molecule and a Target of p53, Modulates Wild-Type p53 in Growth Suppression and Mutant p53 in Growth Promotion. Cancers 2024, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, C.A.; Zhang, J.; Ma, B.; Chen, M.; Chen, X. Disruption of the Rbm38-eIF4E Complex with a Synthetic Peptide Pep8 Increases p53 Expression. Cancer Res. 2019, 79, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Dohn, M.; Zhang, S.; Chen, X. p63alpha and DeltaNp63alpha can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene 2001, 20, 3193–3205. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Zimonjic, D.B.; Popescu, N.C.; Milbrandt, J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J. Biol. Chem. 1997, 272, 21373–21380. [Google Scholar] [CrossRef]
- Tezcan, G.; Garanina, E.E.; Alsaadi, M.; Gilazieva, Z.E.; Martinova, E.V.; Markelova, M.I.; Arkhipova, S.S.; Hamza, S.; McIntyre, A.; Rizvanov, A.A.; et al. Therapeutic Potential of Pharmacological Targeting NLRP3 Inflammasome Complex in Cancer. Front. Immunol. 2020, 11, 607881. [Google Scholar] [CrossRef]
- Huang, C.C.; Hou, Y.; Woods, L.K.; Moore, G.E.; Minowada, J. Cytogenetic Study of Human Lymphoid T-Cell Lines Derived from Lymphocytic Leukemia. J. Natl. Cancer Inst. 1974, 53, 655–660. [Google Scholar] [CrossRef][Green Version]
- Lin, R.W.; Ho, C.J.; Chen, H.W.; Pao, Y.H.; Chen, L.E.; Yang, M.C.; Huang, S.B.; Wang, S.; Chen, C.H.; Wang, C.H. P53 enhances apoptosis induced by doxorubicin only under conditions of severe DNA damage. Cell Cycle 2018, 17, 2175–2186. [Google Scholar] [CrossRef]
- Lukin, D.J.; Carvajal, L.A.; Liu, W.J.; Resnick-Silverman, L.; Manfredi, J.J. p53 Promotes Cell Survival due to the Reversibility of Its Cell-Cycle Checkpoints. Mol. Cancer Res. 2015, 13, 16–28. [Google Scholar] [CrossRef]
- Degen, M.; Santos, J.C.; Pluhackova, K.; Cebrero, G.; Ramos, S.; Jankevicius, G.; Hartenian, E.; Guillerm, U.; Mari, S.A.; Kohl, B.; et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature 2023, 618, 1065–1071. [Google Scholar] [CrossRef]
- Moore, D.T.; Berger, B.W.; DeGrado, W.F. Protein-protein interactions in the membrane: Sequence, structural, and biological motifs. Structure 2008, 16, 991–1001. [Google Scholar] [CrossRef]
- Segrest, J.P.; Deloof, H.; Dohlman, J.G.; Brouillette, C.G.; Anantharamaiah, G.M. Amphipathic Helix Motif—Classes and Properties. Proteins 1990, 8, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Segrest, J.P.; Jones, M.K.; Deloof, H.; Brouillette, C.G.; Venkatachalapathi, Y.V.; Anantharamaiah, G.M. The Amphipathic Helix in the Exchangeable Apolipoproteins—A Review of Secondary Structure and Function. J. Lipid Res. 1992, 33, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Legrand, C.; Bour, J.M.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D.; et al. Lactate-Dehydrogenase (Ldh) Activity of the Number of Dead Cells in the Medium of Cultured Eukaryotic Cells as Marker. J. Biotechnol. 1993, 31, 234. [Google Scholar] [CrossRef]
- Nhan, N.T.T.; Yamada, T.; Yamada, K.H. Peptide-Based Agents for Cancer Treatment: Current Applications and Future Directions. Int. J. Mol. Sci. 2023, 24, 12931. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef]
- David, L.; Borges, J.P.; Hollingsworth, L.R.; Volchuk, A.; Jansen, I.; Garlick, E.; Steinberg, B.E.; Wu, H. NINJ1 mediates plasma membrane rupture by cutting and releasing membrane disks. Cell 2024, 187, 2224–2235. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Kong, X.; Chen, X. Development of Novel Peptides That Target the Ninjurin 1 and 2 Pathways to Inhibit Cell Growth and Survival via p53. Cells 2025, 14, 401. https://doi.org/10.3390/cells14060401
Zhang J, Kong X, Chen X. Development of Novel Peptides That Target the Ninjurin 1 and 2 Pathways to Inhibit Cell Growth and Survival via p53. Cells. 2025; 14(6):401. https://doi.org/10.3390/cells14060401
Chicago/Turabian StyleZhang, Jin, Xiangmudong Kong, and Xinbin Chen. 2025. "Development of Novel Peptides That Target the Ninjurin 1 and 2 Pathways to Inhibit Cell Growth and Survival via p53" Cells 14, no. 6: 401. https://doi.org/10.3390/cells14060401
APA StyleZhang, J., Kong, X., & Chen, X. (2025). Development of Novel Peptides That Target the Ninjurin 1 and 2 Pathways to Inhibit Cell Growth and Survival via p53. Cells, 14(6), 401. https://doi.org/10.3390/cells14060401





