Depletion of MGO or Its Derivatives Ameliorate CUMS-Induced Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Experiment
2.2. Chronic Unpredictable Mild Stress (CUMS)
2.3. Cell Culture and Treatment
2.4. Primary Culture of Microglia
2.5. FPS-ZM1 Treatment
2.6. Morris Water Maze (MWM)
2.7. Novel Object Recognition Test
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Glucose Measurement of Hippocampus
2.10. Immunofluorescence (IF) Staining
2.11. Quantitative Real-Time PCR (qRT-PCR)
2.12. Western Blotting
2.13. RNA Interference
2.14. Virus
2.15. Statistical Analysis
3. Results
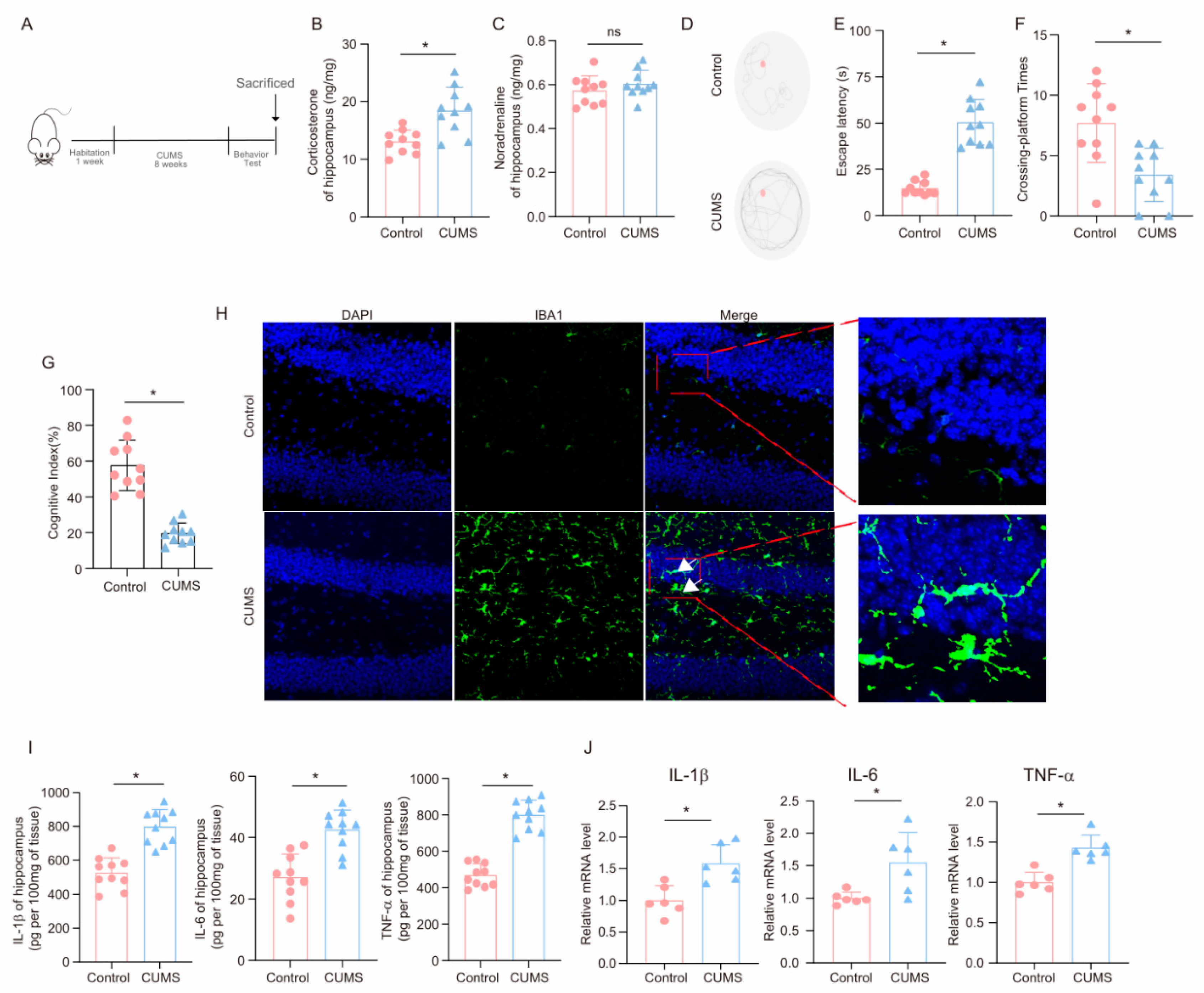
3.1. CUMS Induces Cognitive Decline and Activates Neuroinflammation in Mice
3.2. CUMS Induces the High Level of MGO and Its Derivatives in the Mouse Hippocampus
3.3. Corticosterone Induces Neuroinflammation in Microglia and the High Level of MGO and Its Derivatives
3.4. MGO Depletion Ameliorates Corticosterone-Induced Neuroinflammation in BV2 Cells
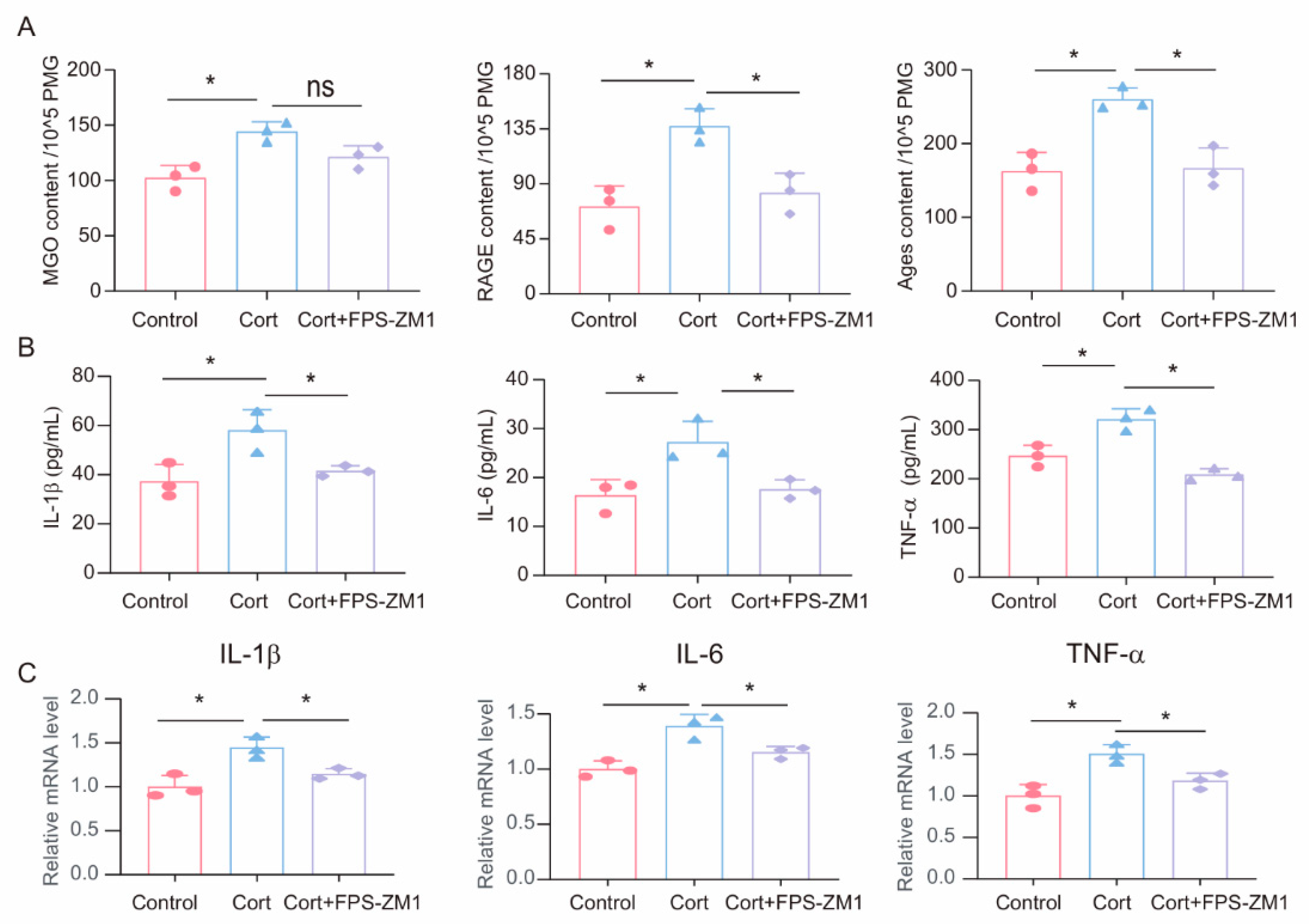
3.5. FPS-ZM1 Ameliorates Corticosterone-Induced Microglia Inflammation and MGO and Its Derivatives
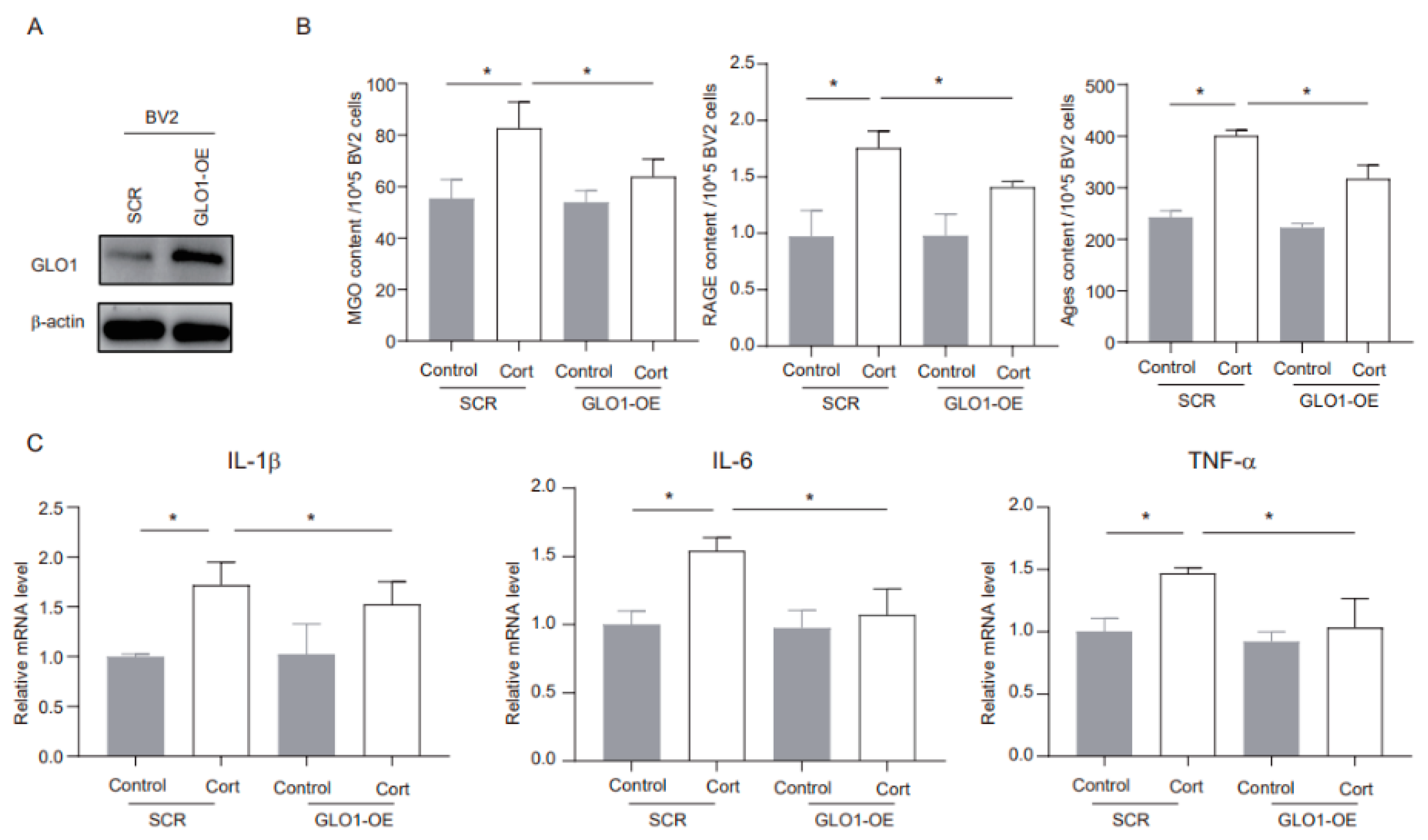
3.6. GLO1-Specific Overexpression of Hippocampal Microglia Ameliorates CUMS-Induced Cognitive Impairment, Inflammatory Response, and Production of MGO and Its Derivatives
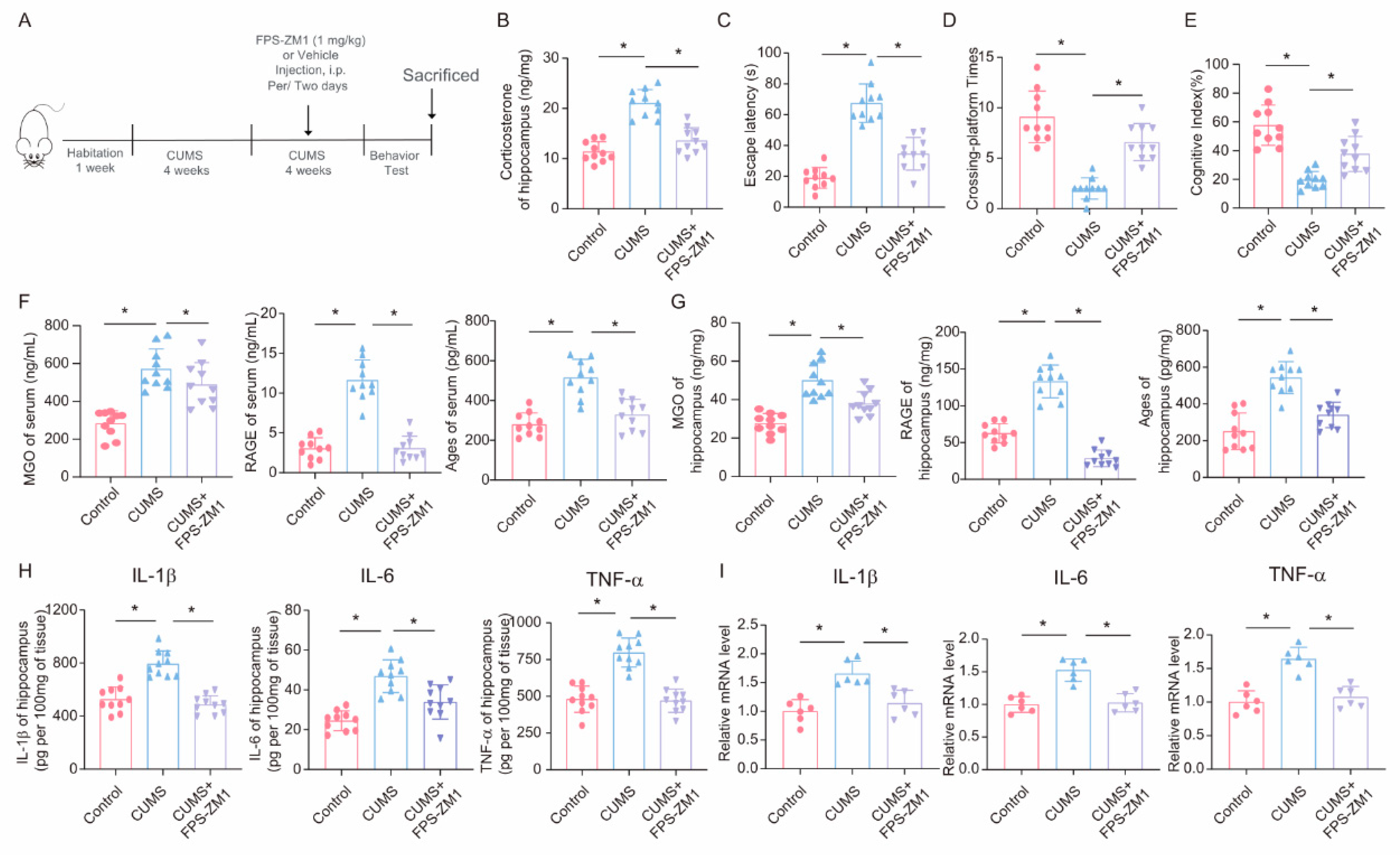
3.7. FPS-ZM1 Ameliorates CUMS-Induced Cognitive Impairment, Neuroinflammation, and MGO and Its Derivatives in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
References
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019, 99, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Gao, X.; Lu, Q.; Zhao, T.; Lu, X.; Zhang, F.; Pei, Y.; Lang, J.; Liu, H.; Song, J.; et al. CUMS induces depressive-like behaviors and cognition impairment by activating the ERS-NLRP3 signaling pathway in mice. J. Affect. Disord. 2025, 369, 547–558. [Google Scholar] [CrossRef]
- Lu, Q.; Mouri, A.; Yang, Y.; Kunisawa, K.; Teshigawara, T.; Hirakawa, M.; Mori, Y.; Yamamoto, Y.; Libo, Z.; Nabeshima, T.; et al. Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav. Brain Res. 2019, 372, 112053. [Google Scholar] [CrossRef]
- Teng, T.; Shively, C.A.; Li, X.; Jiang, X.; Neigh, G.N.; Yin, B.; Zhang, Y.; Fan, L.; Xiang, Y.; Wang, M.; et al. Chronic unpredictable mild stress produces depressive-like behavior, hypercortisolemia, and metabolic dysfunction in adolescent cynomolgus monkeys. Transl. Psychiatry 2021, 11, 9. [Google Scholar] [CrossRef]
- Geng, C.; Guo, Y.; Wang, C.; Liao, D.; Han, W.; Zhang, J.; Jiang, P. Systematic impacts of chronic unpredictable mild stress on metabolomics in rats. Sci. Rep. 2020, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2020, 25, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Mbiydzenyuy, N.E.; Qulu, L.A. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab. Brain Dis. 2024, 39, 1613–1636. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.J.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2014, 8, 456. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Sekar, P.; Hsiao, G.; Hsu, S.H.; Huang, D.Y.; Lin, W.W.; Chan, C.M. Metformin inhibits methylglyoxal-induced retinal pigment epithelial cell death and retinopathy via AMPK-dependent mechanisms: Reversing mitochondrial dysfunction and upregulating glyoxalase 1. Redox Biol. 2023, 64, 102786. [Google Scholar] [CrossRef]
- de Almeida, G.R.L.; Szczepanik, J.C.; Selhorst, I.; Cunha, M.P.; Dafre, A.L. The expanding impact of methylglyoxal on behavior-related disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 120, 110635. [Google Scholar] [CrossRef] [PubMed]
- Berdowska, I.; Matusiewicz, M.; Fecka, I. Methylglyoxal in Cardiometabolic Disorders: Routes Leading to Pathology Counterbalanced by Treatment Strategies. Molecules 2023, 28, 7742. [Google Scholar] [CrossRef]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Teraa, M.; Scheijen, J.; Van de Waarenburg, M.; Gremmels, H.; Stehouwer, C.D.A.; Verhaar, M.C.; Schalkwijk, C.G. Plasma Methylglyoxal Levels Are Associated With Amputations and Mortality in Severe Limb Ischemia Patients With and Without Diabetes. Diabetes Care 2021, 44, 157–163. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and modulation of the AGEs-RAGE axis: Implications for inflammatory pathologies and therapeutic interventions-A review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Heimfarth, L.; Loureiro, S.O.; Pierozan, P.; de Lima, B.O.; Reis, K.P.; Torres, E.B.; Pessoa-Pureur, R. Methylglyoxal-induced cytotoxicity in neonatal rat brain: A role for oxidative stress and MAP kinases. Metab. Brain Dis. 2013, 28, 429–438. [Google Scholar] [CrossRef]
- Md, S.; Hong, S.M.; Lee, J.H.; Park, H.; Chang, K.A.; Kim, H.B.; Park, M.G.; Eo, H.; Oh, M.S.; Kim, S.Y. Depression like-behavior and memory loss induced by methylglyoxal is associated with tryptophan depletion and oxidative stress: A new in vivo model of neurodegeneration. Biol. Res. 2024, 57, 87. [Google Scholar] [CrossRef]
- Vitorakis, N.; Piperi, C. Pivotal role of AGE-RAGE axis in brain aging with current interventions. Ageing Res. Rev. 2024, 100, 102429. [Google Scholar] [CrossRef]
- Berends, E.; van Oostenbrugge, R.J.; Foulquier, S.; Schalkwijk, C.G. Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity. Fluids Barriers CNS 2023, 20, 75. [Google Scholar] [CrossRef]
- Distler, M.G.; Palmer, A.A. Role of Glyoxalase 1 (Glo1) and methylglyoxal (MG) in behavior: Recent advances and mechanistic insights. Front. Genet. 2012, 3, 250. [Google Scholar] [CrossRef]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase system: A systematic review of its biological activity, related-diseases, screening methods and small molecule regulators. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 131, 110663. [Google Scholar] [CrossRef]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef]
- Hong, Y.; Shen, C.; Yin, Q.; Sun, M.; Ma, Y.; Liu, X. Effects of RAGE-Specific Inhibitor FPS-ZM1 on Amyloid-β Metabolism and AGEs-Induced Inflammation and Oxidative Stress in Rat Hippocampus. Neurochem. Res. 2016, 41, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ma, Y.; Zeng, Z.; Yin, Q.; Hong, Y.; Hou, X.; Liu, X. RAGE-Specific Inhibitor FPS-ZM1 Attenuates AGEs-Induced Neuroinflammation and Oxidative Stress in Rat Primary Microglia. Neurochem. Res. 2017, 42, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lou, S.; Huang, Z.H.; Wang, Z.; Shan, Q.H.; Wang, Y.; Yang, Y.; Li, X.; Gong, H.; Jin, Y.; et al. Prefrontal Cortex Corticotropin-Releasing Factor Neurons Control Behavioral Style Selection under Challenging Situations. Neuron 2020, 106, 301–315.e307. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.W.; Wang, X.; Zhao, Y.; Sun, Z.X.; Wu, Y.H.; Hu, H.; Zhang, L.; Wang, S.D.; Li, F.; Wei, A.J.; et al. Blood-brain barrier dysfunction mediated by the EZH2-Claudin-5 axis drives stress-induced TNF-α infiltration and depression-like behaviors. Brain Behav. Immun. 2024, 115, 143–156. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, K.; Wang, F.; Wu, W.; Mai, W.; Qiu, L.; Luo, Y.; Ge, W.P.; Sun, B.; Shi, L.; et al. Dual roles of hexokinase 2 in shaping microglial function by gating glycolytic flux and mitochondrial activity. Nat. Metab. 2022, 4, 1756–1774. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Landucci, E.; Mango, D.; Carloni, S.; Mazzantini, C.; Pellegrini-Giampietro, D.E.; Saidi, A.; Balduini, W.; Schiavi, E.; Tigli, L.; Pioselli, B.; et al. Beneficial effects of CHF6467, a modified human nerve growth factor, in experimental neonatal hypoxic-ischaemic encephalopathy. Br. J. Pharmacol. 2024, 182, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Tian, Y.; Hu, H.; Zhao, Y.; Xue, B.; Sun, Z.; Wei, A.; Xie, F.; Qian, L.J. GLUT1-mediated microglial proinflammatory activation contributes to the development of stress-induced spatial learning and memory dysfunction in mice. Cell Biosci. 2024, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Xie, J.; Li, X.; Li, Y.; Thirupathi, A.; Zhang, J.; Yu, P.; Gao, G.; Chang, Y.; Shi, Z. Ferritinophagy-Mediated Ferroptosis Involved in Paraquat-Induced Neurotoxicity of Dopaminergic Neurons: Implication for Neurotoxicity in PD. Oxidative Med. Cell. Longev. 2021, 2021, 9961628. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.; Fan, Y.; Liu, M.; Han, B.; Meng, Y.; Xu, X.; Song, Z.; Liu, X.; Hao, Q.; et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br. J. Pharmacol. 2021, 178, 1182–1199. [Google Scholar] [CrossRef]
- Agorastos, A.; Chrousos, G.P. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef]
- Lai, S.W.T.; Lopez Gonzalez, E.J.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and Its Adducts: Induction, Repair, and Association with Disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Lu, H.; Cai, S. Methylglyoxal in the Brain: From Glycolytic Metabolite to Signalling Molecule. Molecules 2022, 27, 7905. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef]
- Sasaki, N.; Fukatsu, R.; Tsuzuki, K.; Hayashi, Y.; Yoshida, T.; Fujii, N.; Koike, T.; Wakayama, I.; Yanagihara, R.; Garruto, R.; et al. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am. J. Pathol. 1998, 153, 1149–1155. [Google Scholar] [CrossRef]
- Wetzels, S.; Vanmierlo, T.; Scheijen, J.; van Horssen, J.; Amor, S.; Somers, V.; Schalkwijk, C.G.; Hendriks, J.J.A.; Wouters, K. Methylglyoxal-Derived Advanced Glycation Endproducts Accumulate in Multiple Sclerosis Lesions. Front. Immunol. 2019, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Agur, T.; Steinmetz, T.; Goldman, S.; Zingerman, B.; Bielopolski, D.; Nesher, E.; Fattal, I.; Meisel, E.; Rozen-Zvi, B. The impact of metformin on kidney disease progression and mortality in diabetic patients using SGLT2 inhibitors: A real-world cohort study. Cardiovasc. Diabetol. 2025, 24, 97. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Yang, Q.; Xu, C.; Zheng, Y.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis. 2022, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Burstein, A.H.; Sabbagh, M.; Andrews, R.; Valcarce, C.; Dunn, I.; Altstiel, L. Development of Azeliragon, an Oral Small Molecule Antagonist of the Receptor for Advanced Glycation Endproducts, for the Potential Slowing of Loss of Cognition in Mild Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2018, 5, 149–154. [Google Scholar] [CrossRef]
- Sharma, S.; Chawla, S.; Kumar, P.; Ahmad, R.; Kumar Verma, P. The chronic unpredictable mild stress (CUMS) Paradigm: Bridging the gap in depression research from bench to bedside. Brain Res. 2024, 1843, 149123. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Jiang, M.; Wang, M.; Ji, M.; Xie, X.; Sheng, H. Chronic stress induces depression-like behavior in rats through affecting brain mitochondrial function and inflammation. Psychoneuroendocrinology 2025, 172, 107261. [Google Scholar] [CrossRef]
- Charles-Messance, H.; Sheedy, F.J. Train to Lose: Innate Immune Memory in Metaflammation. Mol. Nutr. Food Res. 2021, 65, e1900480. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P., Jr.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, M.; Li, X.; Shao, Y. The Metaflammatory and Immunometabolic Role of Macrophages and Microglia in Diabetic Retinopathy. Hum. Cell 2021, 34, 1617–1628. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Dong, K.; Zhao, Y.; Wang, X.; Sun, Z.; Xie, F.; Qian, L. Depletion of MGO or Its Derivatives Ameliorate CUMS-Induced Neuroinflammation. Cells 2025, 14, 397. https://doi.org/10.3390/cells14060397
Liu B, Dong K, Zhao Y, Wang X, Sun Z, Xie F, Qian L. Depletion of MGO or Its Derivatives Ameliorate CUMS-Induced Neuroinflammation. Cells. 2025; 14(6):397. https://doi.org/10.3390/cells14060397
Chicago/Turabian StyleLiu, Bing, Ke Dong, Yun Zhao, Xue Wang, Zhaowei Sun, Fang Xie, and Lingjia Qian. 2025. "Depletion of MGO or Its Derivatives Ameliorate CUMS-Induced Neuroinflammation" Cells 14, no. 6: 397. https://doi.org/10.3390/cells14060397
APA StyleLiu, B., Dong, K., Zhao, Y., Wang, X., Sun, Z., Xie, F., & Qian, L. (2025). Depletion of MGO or Its Derivatives Ameliorate CUMS-Induced Neuroinflammation. Cells, 14(6), 397. https://doi.org/10.3390/cells14060397





