The Beneficial Role of the Thyroid Hormone Receptor Beta 2 (thrb2) in Facilitating the First Feeding and Subsequent Growth in Medaka as Fish Larval Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mutants
2.3. First-Feeding Analysis of Larvae
2.4. Growth and Survival Analyses
2.5. RNA Isolation and Quantitative RT-PCR
2.6. Hematoxylin–Eosin (HE) Staining
2.7. Movement Behaviour Analysis
2.8. Statistical Analysis
3. Results
3.1. Construction of thrb2 Mutant in Medaka
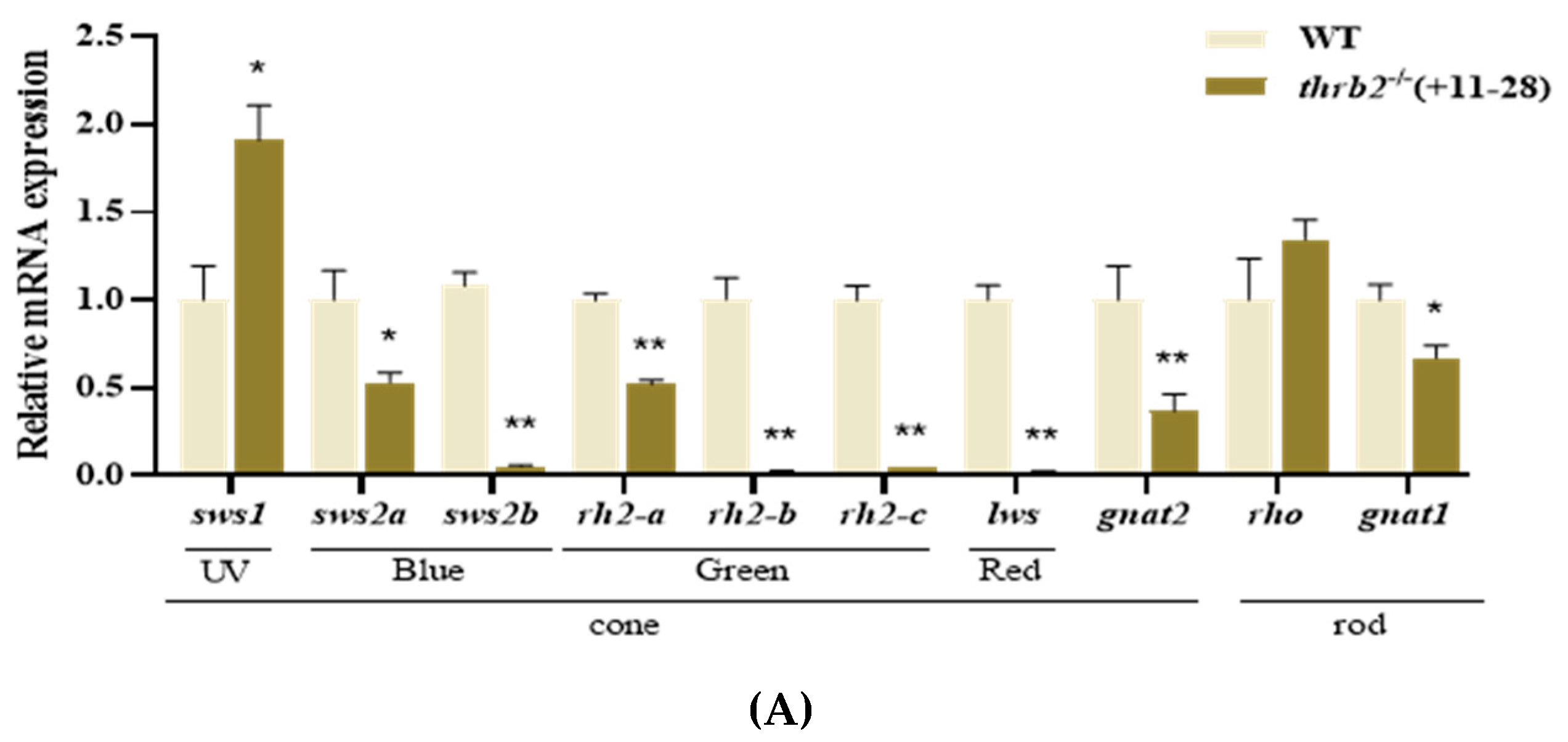
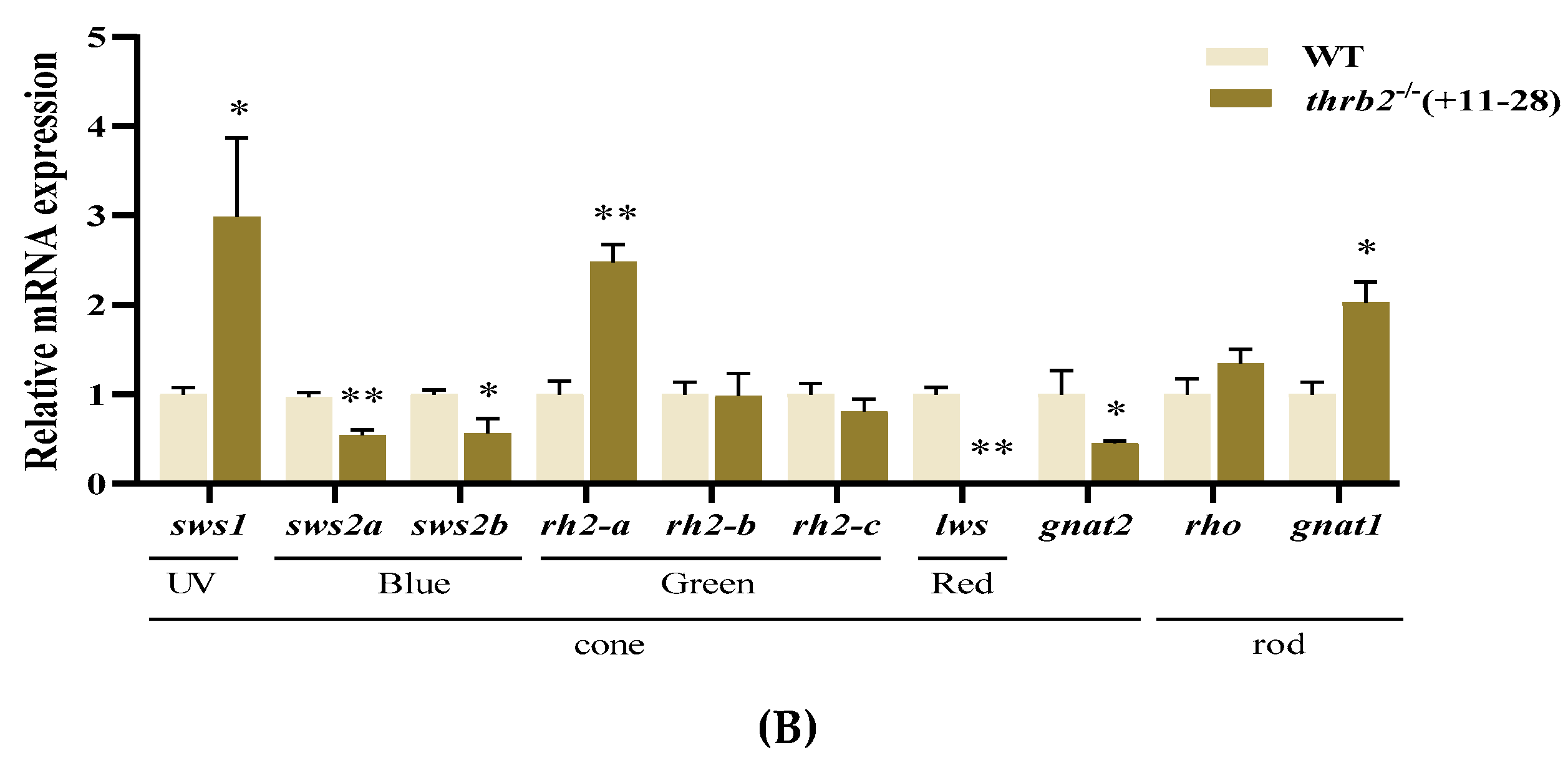
3.2. Analysis of Opsin Expression After thrb2 Knockout
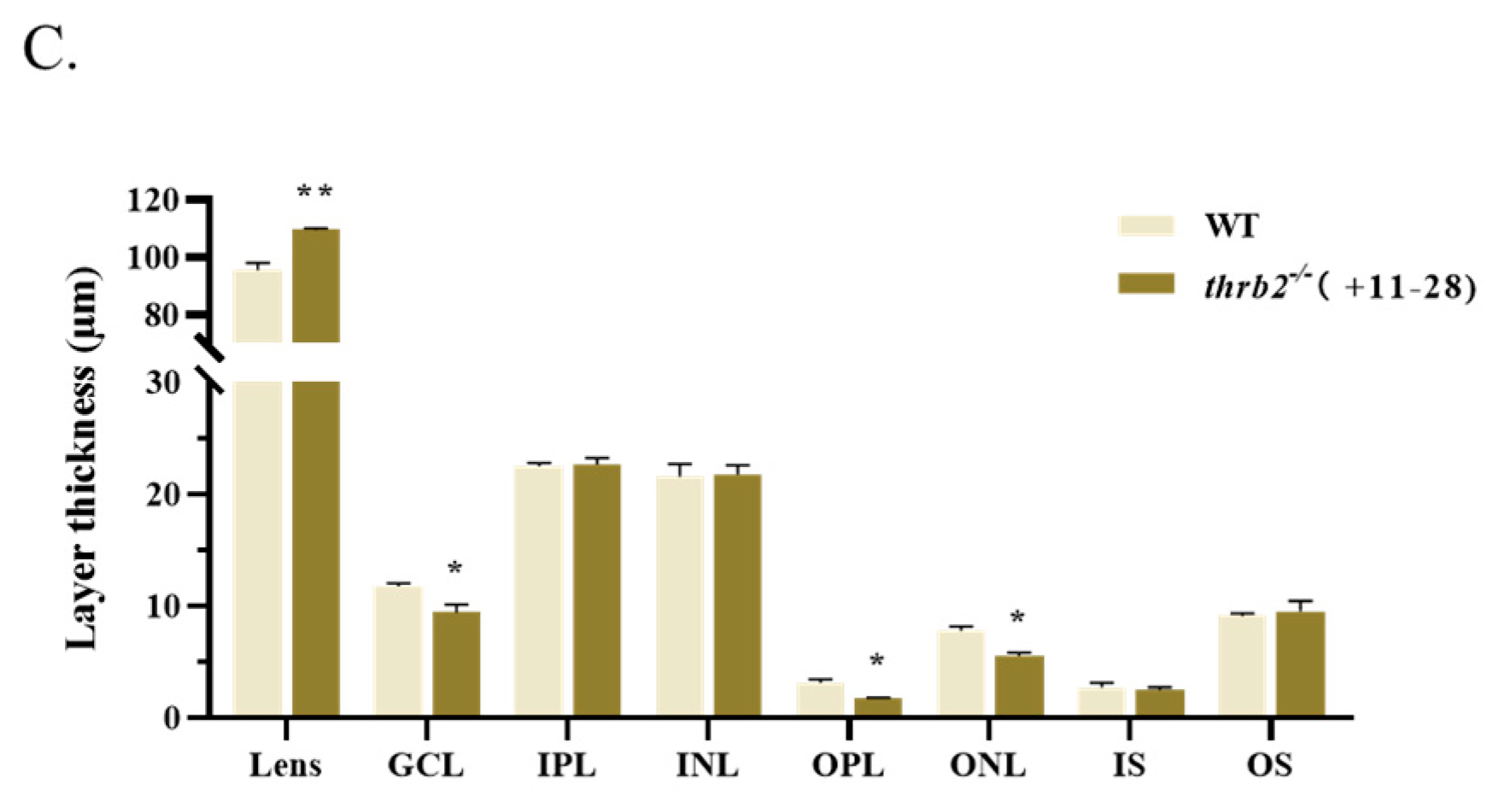
3.3. Analysis of the Effects of Retinal Development
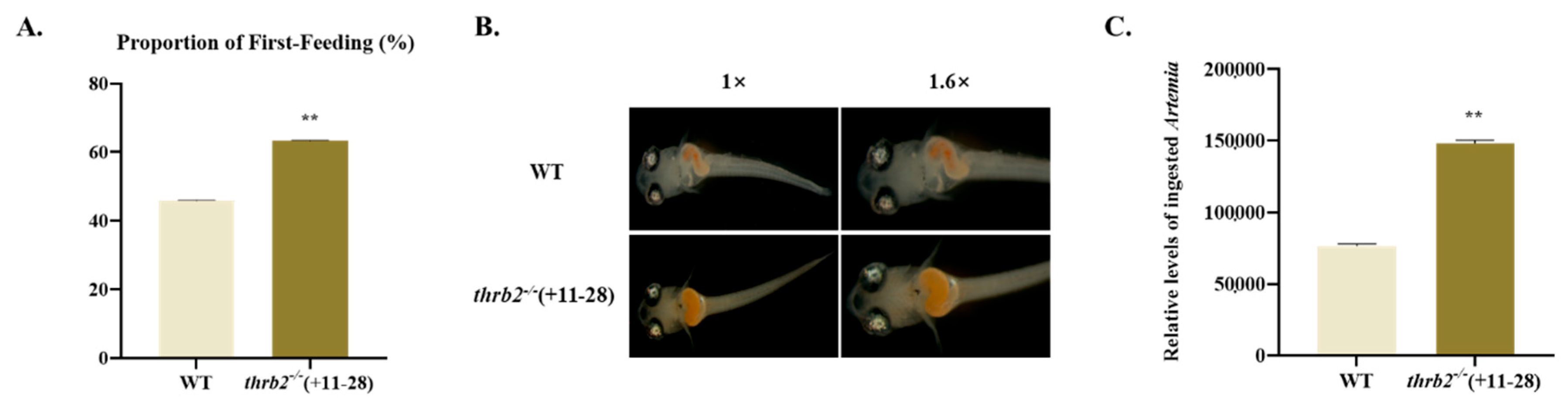
3.4. First-Feeding Test
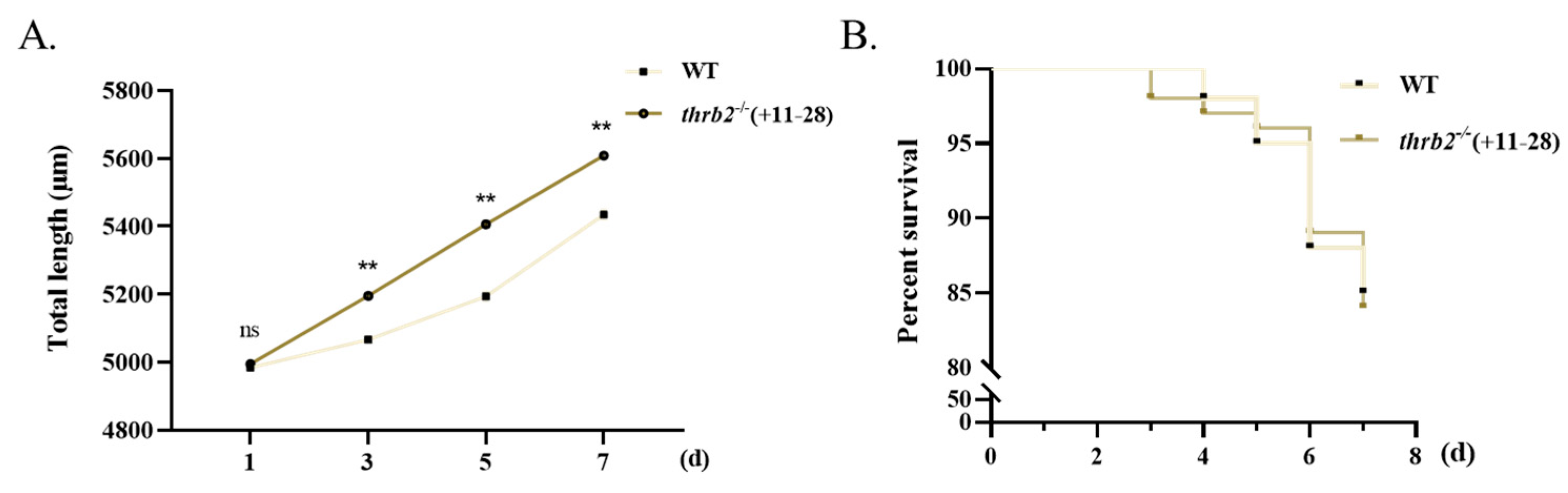
3.5. Growth and Survival Analysis
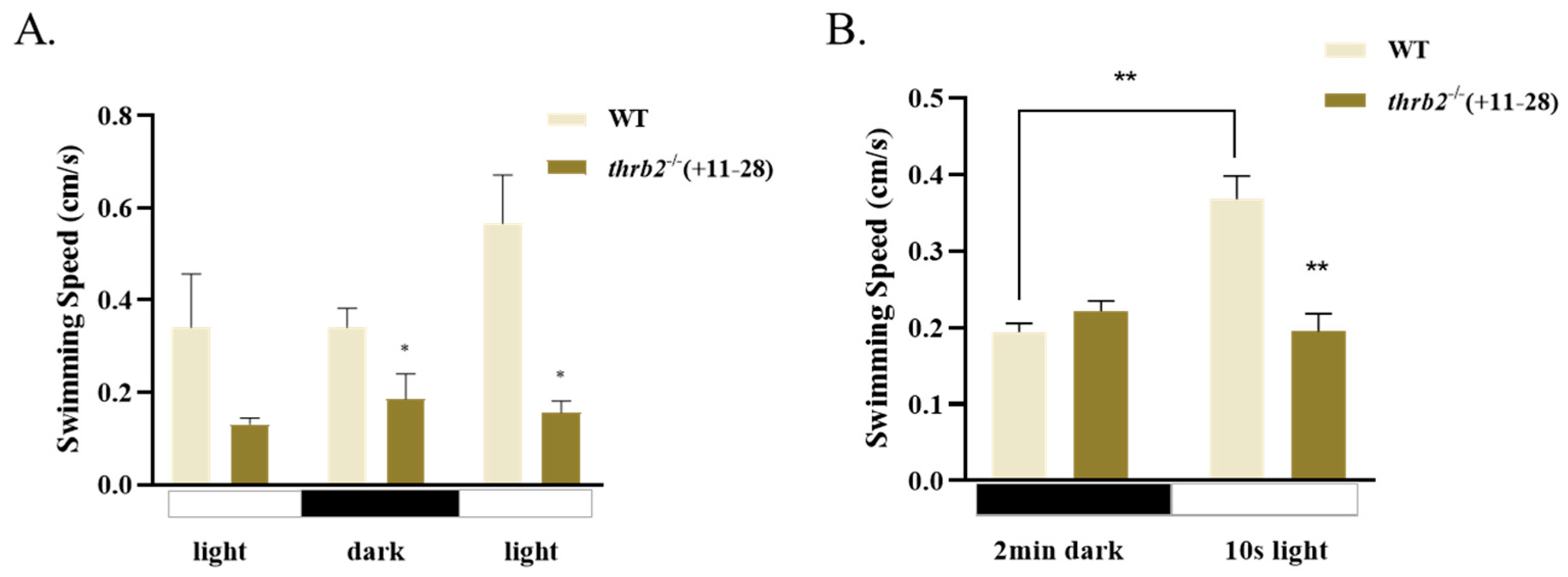
3.6. Locomotor Behaviour of Larvae
3.7. Expression Analysis of Genes Related to Phototransduction Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofmann, C.M.; Carleton, K.L. Gene duplication and differential gene expression play an important role in the diversification of visual pigments in fish. Integr. Comp. Biol. 2009, 49, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Musilova, Z.; Cortesi, F.; Matschiner, M.; Davies, W.I.L.; Patel, J.S.; Stieb, S.M.; de Busserolles, F.; Malmstrøm, M.; Tørresen, O.K.; Brown, C.J.; et al. Vision using multiple distinct rod opsins in deep-sea fishes. Science 2019, 364, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-S.; Mukai, Y. Morphogenesis of sense organs and behavioural changes in larvae of the brown-marbled grouper Epinephelus fuscoguttatus (Forsskål). Mar. Freshw. Behav. Physiol. 2014, 47, 313–327. [Google Scholar] [CrossRef]
- Flamarique, I.N.; Fujihara, R.; Yazawa, R.; Bolstad, K.; Gowen, B.; Yoshizaki, G. Disrupted eye and head development in rainbow trout with reduced ultraviolet (sws1) opsin expression. J. Comp. Neurol. 2021, 529, 3013–3031. [Google Scholar] [CrossRef]
- Aavani, T.; Tachibana, N.; Wallace, V.; Biernaskie, J.; Schuurmans, C. Temporal profiling of photoreceptor lineage gene expression during murine retinal development. Gene Expr. Patterns 2017, 23–24, 32–44. [Google Scholar] [CrossRef]
- Bonvini, E.; Parma, L.; Gatta, P.P.; Mandrioli, L.; Sirri, R.; Martelli, G.; Nannoni, E.; Mordenti, A.; Bonaldo, A. Effects of light intensity on growth, feeding activity and development in common sole (Solea solea L.) larvae in relation to sensory organ ontogeny. Aquac. Res. 2014, 47, 1809–1819. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zeng, C.; Jerry, D.R.; Cobcroft, J.M. Recent advances of marine ornamental fish larviculture: Broodstock reproduction, live prey and feeding regimes, and comparison between demersal and pelagic spawners. Rev. Aquac. 2019, 12, 1518–1541. [Google Scholar] [CrossRef]
- Bowmaker, J.K. Evolution of vertebrate visual pigments. Vis. Res. 2008, 48, 2022–2041. [Google Scholar] [CrossRef]
- Marshall, J.; Carleton, K.L.; Cronin, T. Colour vision in marine organisms. Curr. Opin. Neurobiol. 2015, 34, 86–94. [Google Scholar] [CrossRef]
- Ogawa, Y.; Shiraki, T.; Fukada, Y.; Kojima, D. Foxq2 determines blue cone identity in zebrafish. Sci. Adv. 2021, 7, eabi9784. [Google Scholar] [CrossRef]
- Morote, E.; Olivar, M.P.; Bozzano, A.; Villate, F.; Uriarte, I. Feeding selectivity in larvae of the European hake (Merluccius merluccius) in relation to ontogeny and visual capabilities. Mar. Biol. 2011, 158, 1349–1361. [Google Scholar] [CrossRef]
- Yahaya, S.; Lim, L.-S.; Shaleh, S.R.M.; Mukai, Y.; Anraku, K.; Kawamura, G. Ontogenetic eye development and related behavioural changes in larvae and juveniles of barramundi Lates calcarifer (Bloch). Mar. Freshw. Behav. Physiol. 2011, 44, 339–348. [Google Scholar] [CrossRef]
- Sampson, J.A.; Duston, J.; Croll, R.P. Superficial neuromasts facilitate non-visual feeding by larval striped bass (Morone saxatilis). J. Exp. Biol. 2013, 216, 3522–3530. [Google Scholar] [CrossRef] [PubMed]
- Cronin, T.W.; Bok, M.J. Photoreception and vision in the ultraviolet. J. Exp. Biol. 2016, 219, 2790–2801. [Google Scholar] [CrossRef]
- Fuller, R.C.; Fleishman, L.J.; Leal, M.; Travis, J.; Loew, E. Intraspecific variation in retinal cone distribution in the bluefin killifish, Lucania goodei. J. Comp. Physiol. 2003, 189, 609–616. [Google Scholar] [CrossRef]
- Flamarique, I.N. Opsin switch reveals function of the ultraviolet cone in fish foraging. Proc. R. Soc. B: Biol. Sci. 2013, 280, 20122490. [Google Scholar] [CrossRef]
- Isayama, T.; Makino, C.L. Pigment mixtures and other determinants of spectral sensitivity of vertebrate retinal photoreceptors. In Photoreceptors: Physiology, Types and Abnormalities, 1st ed.; Nova Science Publishers: New York, NY, USA, 2012; Volume 1, pp. 1–31. [Google Scholar]
- Suliman, T.; Flamarique, I.N. Visual pigments and opsin expression in the juveniles of three species of fish (rainbow trout, zebrafish, and killifish) following prolonged exposure to thyroid hormone or retinoic acid. J. Comp. Neurol. 2013, 522, 98–117. [Google Scholar] [CrossRef]
- Leech, D.M.; Johnsen, S. Ultraviolet vision and foraging in juvenile bluegill (Lepomis macrochirus). Can. J. Fish. Aquat. Sci. 2006, 63, 2183–2190. [Google Scholar] [CrossRef]
- Flamarique, I.N.; Wachowiak, M. Functional segregation of retinal ganglion cell projections to the optic tectum of rainbow trout. J. Neurophysiol. 2015, 114, 2703–2717. [Google Scholar] [CrossRef][Green Version]
- Rick, I.P.; Bloemker, D.; Bakker, T.C.M. Spectral composition and visual foraging in the three-spined stickleback (Gasterosteidae: Gasterosteus aculeatus L.): Elucidating the role of ultraviolet wavelengths. Biol. J. Linn. Soc. 2011, 105, 359–368. [Google Scholar] [CrossRef]
- Flamarique, I.N. Diminished foraging performance of a mutant zebrafish with reduced population of ultraviolet cones. Proc. R. Soc. B: Biol. Sci. 2016, 283, 20160058. [Google Scholar] [CrossRef]
- Chan, S.; Kilby, M.D. Thyroid hormone and central nervous system development. J. Endocrinol. 2000, 165, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Simonides, W.S.; Dentice, M.; Zavacki, A.M.; Larsen, P.R. Thyroid hormones and skeletal muscle—new insights and potential implications. Nat. Rev. Endocrinol. 2013, 10, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Al-Mendalawi, M.D. Pulmonary consequences of hypothyroidism. Ann. Thorac. Med. 2018, 13, 62–63. [Google Scholar] [CrossRef]
- Hu, Y.; Mauri, A.; Donahue, J.; Singh, R.; Acosta, B.; McMenamin, S. Thyroid hormone coordinates developmental trajectories but does not underlie developmental truncation in danionins. Dev. Dyn. 2019, 248, 1144–1154. [Google Scholar] [CrossRef]
- Volkov, L.I.; Kim-Han, J.S.; Saunders, L.M.; Poria, D.; Hughes, A.E.O.; Kefalov, V.J.; Parichy, D.M.; Corbo, J.C. Thyroid hormone receptors mediate two distinct mechanisms of long-wavelength vision. Proc. Natl. Acad. Sci. USA 2020, 117, 15262–15269. [Google Scholar] [CrossRef]
- Yavuz, S.; del Prado, S.S.N.; Celi, F.S. Thyroid Hormone Action and Energy Expenditure. J. Endocr. Soc. 2019, 3, 1345–1356. [Google Scholar] [CrossRef]
- Szél, Á.; Lukáts, Á.; Fekete, T.; Szepessy, Z.; Röhlich, P. Photoreceptor distribution in the retinas of subprimate mammals. J. Opt. Soc. Am. 2000, 17, 568–579. [Google Scholar] [CrossRef]
- Bassett, E.A.; Wallace, V.A. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012, 35, 565–573. [Google Scholar] [CrossRef]
- Kelley, M.W.; Turner, J.K.; Reh, T.A. Regulation of proliferation and photoreceptor differentiation in fetal human retinal cell cultures. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1280–1289. [Google Scholar]
- Kelley, M.W.; Turner, J.K.; Reh, T.A. Ligands of steroid/thyroid receptors induce cone photoreceptors in vertebrate retina. Development 1995, 121, 3777–3785. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Sun, Y.; Li, S.; Chen, Y.; Li, L.; Fang, M.; Shi, R.; Tong, D.; Chen, J.; Ma, Y.; et al. Single-cell profiling reveals Müller glia coordinate retinal intercellular communication during light/dark adaptation via thyroid hormone signaling. Protein Cell 2023, 14, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Hodin, R.A.; Lazar, M.A.; Wintman, B.I.; Darling, D.S.; Koenig, R.J.; Larsen, P.R.; Moore, D.D.; Chin, W.W. Identification of a Thyroid Hormone Receptor That Is Pituitary-Specific. Science 1989, 244, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, M.; Vennström, B.; Forrest, D. Thyroid hormone receptors in chick retinal development: Differential expression of mRNAs for α and N-terminal variant β receptors. Development 1992, 114, 39–47. [Google Scholar] [CrossRef]
- Ng, L.; Liu, H.; Liu, Y.; Forrest, D. Biphasic expression of thyroid hormone receptor TRβ1 in mammalian retina and anterior ocular tissues. Front. Endocrinol. 2023, 14, 1174600. [Google Scholar] [CrossRef]
- Porazinski, S.R.; Wang, H.; Furutani-Seiki, M. Microinjection of Medaka Embryos for use as a Model Genetic Organism. J. Vis. Exp. 2010, 46, e1937. [Google Scholar] [CrossRef]
- Shimada, Y.; Hirano, M.; Nishimura, Y.; Tanaka, T. A High-Throughput Fluorescence-Based Assay System for Appetite-Regulating Gene and Drug Screening. PLoS ONE 2012, 7, 52549. [Google Scholar] [CrossRef]
- Huang, H.; Huang, C.; Wang, L.; Ye, X.; Bai, C.; Simonich, M.T.; Tanguay, R.L.; Dong, Q. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat. Toxicol. 2010, 98, 139–147. [Google Scholar] [CrossRef]
- Cai, S.; Chen, Y.; Shang, Y.; Cui, J.; Li, Z.; Li, Y. Knockout of zebrafish interleukin 7 receptor (IL7R) by the CRISPR/Cas9 system delays retinal neurodevelopment. Cell Death Dis. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Yang, F.; Ma, H.; Ding, X.-Q. Thyroid Hormone Signaling in Retinal Development, Survival, and Disease. Vitam. Horm. 2018, 106, 333–349. [Google Scholar] [CrossRef]
- Eldred, K.C.; Hadyniak, S.E.; Hussey, K.A.; Brenerman, B.; Zhang, P.-W.; Chamling, X.; Sluch, V.M.; Welsbie, D.S.; Hattar, S.; Taylor, J.; et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science 2018, 362, eaau6348. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Hurley, J.B.; Dierks, B.; Srinivas, M.; Saltó, C.; Vennström, B.; Reh, T.A.; Forrest, D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 2001, 27, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Y.; Huang, C.; Hu, B.; Hu, C.; Zhou, B. Acute exposure to DE-71 causes alterations in visual behavior in zebrafish larvae. Environ. Toxicol. Chem. 2013, 32, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Gregory-Evans, K.; Bhattacharya, S.S. Genetic blindness: Current concepts in the pathogenesis of human outer retinal dystrophies. Trends Genet. 1998, 14, 103–108. [Google Scholar] [CrossRef]
- Lu, K.; Wu, J.; Tang, S.; Wang, Y.; Zhang, L.; Chai, F.; Liang, X.-F. Altered Visual Function in Short-Wave-Sensitive 1 (sws1) Gene Knockout Japanese Medaka (Oryzias latipes) Larvae. Cells 2023, 12, 2157. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Z.; Chen, L.; Fu, J.; Han, J.; Hu, B.; Zhou, B. Optical toxicity of triphenyl phosphate in zebrafish larvae. Aquat. Toxicol. 2019, 210, 139–147. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Wang, Z.; Magnuson, J.T.; Volz, D.C.; Schlenk, D.; Jiang, J.; Wang, C. Environmentally relevant concentrations of boscalid exposure affects the neurobehavioral response of zebrafish by disrupting visual and nervous systems. J. Hazard. Mater. 2021, 404, 124083. [Google Scholar] [CrossRef]
- Nicolini, G.; Casini, G.; Posarelli, C.; Amato, R.; Lulli, M.; Balzan, S.; Forini, F. Thyroid Hormone Signaling in Retinal Development and Function: Implications for Diabetic Retinopathy and Age-Related Macular Degeneration. Int. J. Mol. Sci. 2024, 25, 7364. [Google Scholar] [CrossRef]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Roaf, H.E. The Vertebrate Eye and its Adaptive Radiation. Nature 1943, 151, 236. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Lu, K.; Xie, R.; Zhu, C.; Luo, Q.; Liang, X.-F. The Beneficial Role of the Thyroid Hormone Receptor Beta 2 (thrb2) in Facilitating the First Feeding and Subsequent Growth in Medaka as Fish Larval Model. Cells 2025, 14, 386. https://doi.org/10.3390/cells14050386
Wu J, Lu K, Xie R, Zhu C, Luo Q, Liang X-F. The Beneficial Role of the Thyroid Hormone Receptor Beta 2 (thrb2) in Facilitating the First Feeding and Subsequent Growth in Medaka as Fish Larval Model. Cells. 2025; 14(5):386. https://doi.org/10.3390/cells14050386
Chicago/Turabian StyleWu, Jiaqi, Ke Lu, Ruipeng Xie, Chenyuan Zhu, Qiyao Luo, and Xu-Fang Liang. 2025. "The Beneficial Role of the Thyroid Hormone Receptor Beta 2 (thrb2) in Facilitating the First Feeding and Subsequent Growth in Medaka as Fish Larval Model" Cells 14, no. 5: 386. https://doi.org/10.3390/cells14050386
APA StyleWu, J., Lu, K., Xie, R., Zhu, C., Luo, Q., & Liang, X.-F. (2025). The Beneficial Role of the Thyroid Hormone Receptor Beta 2 (thrb2) in Facilitating the First Feeding and Subsequent Growth in Medaka as Fish Larval Model. Cells, 14(5), 386. https://doi.org/10.3390/cells14050386





