A Cross-Sectional Exploratory Study of Rat Sarcoid (Ras) Activation in Women with and Without Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dargham, S.R.; Ahmed, L.; Kilpatrick, E.S.; Atkin, S.L. The prevalence and metabolic characteristics of polycystic ovary syndrome in the Qatari population. PLoS ONE 2017, 12, e0181467. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Atkin, S.L. Recent advances in cardiovascular aspects of polycystic ovary syndrome. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2012, 166, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Malakar, P.; Chartarifsky, L.; Hija, A.; Leibowitz, G.; Glaser, B.; Dor, Y.; Karni, R. Insulin receptor alternative splicing is regulated by insulin signaling and modulates beta cell survival. Sci. Rep. 2016, 6, 31222. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert. Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Fan, H.Y.; Liu, Z.; Mullany, L.K.; Richards, J.S. Consequences of RAS and MAPK activation in the ovary: The good, the bad and the ugly. Mol. Cell. Endocrinol. 2012, 356, 74–79. [Google Scholar] [CrossRef]
- Fan, H.Y.; Richards, J.S. Minireview: Physiological and pathological actions of RAS in the ovary. Mol. Endocrinol. 2010, 24, 286–298. [Google Scholar] [CrossRef]
- Heidarzadehpilehrood, R.; Pirhoushiaran, M.; Binti Osman, M.; Ling, K.H.; Abdul Hamid, H. Unveiling Key Biomarkers and Therapeutic Drugs in Polycystic Ovary Syndrome (PCOS) Through Pathway Enrichment Analysis and Hub Gene-miRNA Networks. Iran. J. Pharm. Res. 2023, 22, e139985. [Google Scholar] [CrossRef]
- Till, J.E.; McDaniel, L.; Chang, C.; Long, Q.; Pfeiffer, S.M.; Lyman, J.P.; Padrón, L.J.; Maurer, D.M.; Yu, J.X.; Spencer, C.N.; et al. Circulating KRAS G12D but not G12V is associated with survival in metastatic pancreatic ductal adenocarcinoma. Nat. Commun. 2024, 15, 5763. [Google Scholar] [CrossRef]
- Almahbobi, G.; Misajon, A.; Hutchinson, P.; Lolatgis, N.; Trounson, A.O. Hyperexpression of epidermal growth factor receptors in granulosa cells from women with polycystic ovary syndrome. Fertil. Steril. 1998, 70, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhan, L.; Zhao, M.Y.; Wang, J.J.; Xie, F.F.; Xu, Z.Y.; Xu, Q.; Cao, Y.X.; Liu, Q.W. Role of EGFR expressed on the granulosa cells in the pathogenesis of polycystic ovarian syndrome. Front. Endocrinol. 2022, 13, 971564. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.-J.; Zhang, H.; Zhou, H.; Liu, L.-H.; Denhard, L.A.; Cooney, A.J. Oocyte-Specific Expression of Fibroblast Growth Factor 8 and Its Functional Role During Ovarian Development. Biol. Reprod. 2008, 78 (Suppl. S1), 223. [Google Scholar] [CrossRef]
- Schmahl, J.; Rizzolo, K.; Soriano, P. The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 2008, 22, 3255–3267. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, L. Association between VEGF gene polymorphisms (11 sites) and polycystic ovary syndrome risk. Biosci. Rep. 2020, 40, BSR20191691. [Google Scholar] [CrossRef]
- Li, X.; Lin, S.; Yang, X.; Chen, C.; Cao, S.; Zhang, Q.; Ma, J.; Zhu, G.; Fang, Q.; Zheng, C.; et al. When IGF-1 Meets Metabolic Inflammation and Polycystic Ovary Syndrome. Int. Immunopharmacol. 2024, 138, 112529. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Al-Qaissi, A.; Kilpatrick, E.S.; Dargham, S.R.; Atkin, S.L. Anti-Mullerian hormone measurement for the diagnosis of polycystic ovary syndrome. Clin. Endocrinol. 2018, 88, 258–262. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Cunningham, T.K.; Allgar, V.; Dargham, S.R.; Kilpatrick, E.; Sathyapalan, T.; Maguiness, S.; Mokhtar Rudin, H.R.; Abdul Ghani, N.M.; Latiff, A.; Atkin, S.L. Association of Vitamin D Metabolites With Embryo Development and Fertilization in Women With and Without PCOS Undergoing Subfertility Treatment. Front. Endocrinol. 2019, 10, 13. [Google Scholar] [CrossRef]
- Kahal, H.; Halama, A.; Aburima, A.; Bhagwat, A.M.; Butler, A.E.; Graumann, J.; Suhre, K.; Sathyapalan, T.; Atkin, S.L. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 2020, 10, 4750. [Google Scholar] [CrossRef]
- Kraemer, S.; Vaught, J.D.; Bock, C.; Gold, L.; Katilius, E.; Keeney, T.R.; Kim, N.; Saccomano, N.A.; Wilcox, S.K.; Zichi, D.; et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE 2011, 6, e26332. [Google Scholar] [CrossRef]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef]
- Xu, N.; Geller, D.H.; Jones, M.R.; Funari, V.A.; Azziz, R.; Goodarzi, M.O. Comprehensive assessment of expression of insulin signaling pathway components in subcutaneous adipose tissue of women with and without polycystic ovary syndrome. J. Clin. Transl. Endocrinol. 2015, 2, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kyohara, M.; Shirakawa, J.; Okuyama, T.; Togashi, Y.; Inoue, R.; Li, J.; Miyashita, D.; Terauchi, Y. Soluble EGFR, a hepatokine, and adipsin, an adipokine, are biomarkers correlated with distinct aspects of insulin resistance in type 2 diabetes subjects. Diabetol. Metab. Syndr. 2020, 12, 83. [Google Scholar] [CrossRef]
- Rogers, C.; Moukdar, F.; McGee, M.A.; Davis, B.; Buehrer, B.M.; Daniel, K.W.; Collins, S.; Barakat, H.; Robidoux, J. EGF receptor (ERBB1) abundance in adipose tissue is reduced in insulin-resistant and type 2 diabetic women. J. Clin. Endocrinol. Metab. 2012, 97, E329–E340. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.J.; Torres, I.J.; Lam, R.W.; Yatham, L.N. Serum epidermal growth factor, clinical illness course, and limbic brain volumes in early-stage bipolar disorder. J. Affect. Disord. 2020, 270, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, X.; Beeraka, N.M.; Zhou, R.; Zhang, H.; Liu, Y.; Zhang, Y.; Zhang, Y.; Qin, G.; Liu, J. High Body Mass Index Was Associated With Human Epidermal Growth Factor Receptor 2-Positivity, Histological Grade and Disease Progression Differently by Age. World J. Oncol. 2023, 14, 75–83. [Google Scholar] [CrossRef]
- Miyoshi, T.; Otsuka, F.; Yamashita, M.; Inagaki, K.; Nakamura, E.; Tsukamoto, N.; Takeda, M.; Suzuki, J.; Makino, H. Functional relationship between fibroblast growth factor-8 and bone morphogenetic proteins in regulating steroidogenesis by rat granulosa cells. Mol. Cell. Endocrinol. 2010, 325, 84–92. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. Inflammatory Markers in Non-Obese Women with Polycystic Ovary Syndrome Are Not Elevated and Show No Correlation with Vitamin D Metabolites. Nutrients 2022, 14, 3540. [Google Scholar] [CrossRef]
- Schreiber, N.B.; Spicer, L.J. Effects of fibroblast growth factor 9 (FGF9) on steroidogenesis and gene expression and control of FGF9 mRNA in bovine granulosa cells. Endocrinology 2012, 153, 4491–4501. [Google Scholar] [CrossRef]
- Drummond, A.E.; Tellbach, M.; Dyson, M.; Findlay, J.K. Fibroblast growth factor-9, a local regulator of ovarian function. Endocrinology 2007, 148, 3711–3721. [Google Scholar] [CrossRef]
- Schütz, L.F.; Batalha, I.M. Granulosa Cells: Central Regulators of Female Fertility. Endocrines 2024, 5, 547–565. [Google Scholar] [CrossRef]
- Patil, K.; Hinduja, I.; Mukherjee, S. Alteration in angiogenic potential of granulosa-lutein cells and follicular fluid contributes to luteal defects in polycystic ovary syndrome. Hum. Reprod. 2021, 36, 1052–1064. [Google Scholar] [CrossRef]
- Sircar, A.; Singh, S.; Xu-Monette, Z.Y.; Coyle, K.M.; Hilton, L.K.; Chavdoula, E.; Ranganathan, P.; Jain, N.; Hanel, W.; Tsichlis, P.; et al. Exploiting the fibroblast growth factor receptor-1 vulnerability to therapeutically restrict the MYC-EZH2-CDKN1C axis-driven proliferation in Mantle cell lymphoma. Leukemia 2023, 37, 2094–2106. [Google Scholar] [CrossRef]
- Stamou, M.I.; Chiu, C.J.; Jadhav, S.V.; Lopes, V.F.; Salnikov, K.B.; Plummer, L.; Lippincott, M.F.; Lee, H.; Seminara, S.B.; Balasubramanian, R. Defective FGFR1 Signaling Disrupts Glucose Regulation: Evidence From Humans With FGFR1 Mutations. J. Endocr. Soc. 2024, 8, bvae118. [Google Scholar] [CrossRef]
- Dambala, K.; Vavilis, D.; Bili, E.; Goulis, D.G.; Tarlatzis, B.C. Serum visfatin, vascular endothelial growth factor and matrix metalloproteinase-9 in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2017, 33, 529–533. [Google Scholar] [CrossRef]
- Dambala, K.; Paschou, S.A.; Michopoulos, A.; Siasos, G.; Goulis, D.G.; Vavilis, D.; Tarlatzis, B.C. Biomarkers of Endothelial Dysfunction in Women With Polycystic Ovary Syndrome. Angiology 2019, 70, 797–801. [Google Scholar] [CrossRef]
- Agrawal, R.; Sladkevicius, P.; Engmann, L.; Conway, G.S.; Payne, N.N.; Bekis, J.; Tan, S.L.; Campbell, S.; Jacobs, H.S. Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum. Reprod. 1998, 13, 651–655. [Google Scholar] [CrossRef]
- Agrawal, R.; Jacobs, H.; Payne, N.; Conway, G. Concentration of vascular endothelial growth factor released by cultured human luteinized granulosa cells is higher in women with polycystic ovaries than in women with normal ovaries. Fertil. Steril. 2002, 78, 1164–1169. [Google Scholar] [CrossRef]
- Ferrara, N.; Frantz, G.; LeCouter, J.; Dillard-Telm, L.; Pham, T.; Draksharapu, A.; Giordano, T.; Peale, F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am. J. Pathol. 2003, 162, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Skrzynska, K.; Zachurzok, A.; Pietrusik, A.; Jakubowska-Kowal, K.; Gawlik-Starzyk, A. Visfatin and VEGF levels are not increased in adolescent girls with polycystic ovary syndrome. Front. Endocrinol. 2024, 15, 1488249. [Google Scholar] [CrossRef]
- Iwashita, M.; Mimuro, T.; Watanabe, M.; Setoyama, T.; Matsuo, A.; Adachi, T.; Takeda, Y.; Sakamoto, S. Plasma levels of insulin-like growth factor-I and its binding protein in polycystic ovary syndrome. Horm. Res. 1990, 33 (Suppl. S2), 21–26. [Google Scholar] [CrossRef] [PubMed]
- Thierry van Dessel, H.J.; Lee, P.D.; Faessen, G.; Fauser, B.C.; Giudice, L.C. Elevated serum levels of free insulin-like growth factor I in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1999, 84, 3030–3035. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Liu, C.; Zhang, Y.; Yang, H.; Fu, S.; Lv, H. Association of Insulin-Like Growth Factor-1 With Polycystic Ovarian Syndrome: A Systematic Review and Meta-analysis. Endocr. Pract. 2023, 29, 388–397. [Google Scholar] [CrossRef]
- Premoli, A.C.; Santana, L.F.; Ferriani, R.A.; Moura, M.D.; De Sá, M.F.; Reis, R.M. Growth hormone secretion and insulin-like growth factor-1 are related to hyperandrogenism in nonobese patients with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1852–1855. [Google Scholar] [CrossRef]
- Xu, H.; Han, Y.; Lou, J.; Zhang, H.; Zhao, Y.; Győrffy, B.; Li, R. PDGFRA, HSD17B4 and HMGB2 are potential therapeutic targets in polycystic ovarian syndrome and breast cancer. Oncotarget 2017, 8, 69520–69526. [Google Scholar] [CrossRef]


| Baseline Demographics | PCOS (n = 147) | Controls (n = 97) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years) | 29.1 ± 6.1 | 29.6 ± 6.5 |
| BMI (kg/m2) | 34.1 ± 7.5 | 26.7 ± 6.6 *** |
| Insulin (IU/mL) | 10.2 ± 6.1 | 6.2 ± 3.2 *** |
| HOMA-IR | 3.8 ± 0.6 | 1.6 ± 0.2 *** |
| Testosterone (nmol/L) | 1.6 ± 1.0 | 1.05 ± 0.48 *** |
| SHBG (nmol/L) | 42.5 ± 39.6 | 77.5 ± 78.4 *** |
| Free androgen index (FAI) | 4.5 ± 3.9 | 2.1 ± 1.4 *** |
| CRP (mg/L) | 4.4 ± 4.2 | 2.4 ± 3.9 *** |
| Systolic blood pressure (mmHg) | 121 ± 14 | 114 ± 11 *** |
| Diastolic blood pressure (mmHg) | 77 ± 10 | 73 ± 11 *** |
| Fasting blood glucose (mmol/L) | 4.9 ± 1.1 | 4.6 ± 0.6 ** |
| Cholesterol (mmol/L) | 4.8 ± 1.0 | 4.6 ± 0.7 |
| High density lipoprotein (mmol/L) | 1.22 ± 0.30 | 1.43 ± 0.31 *** |
| Low density lipoprotein 9 mmol/L) | 2.90 ± 0.86 | 2.72 ± 0.6 |
| AMH (ng/mL) | 40 ± 31 | 18 ± 18 *** |
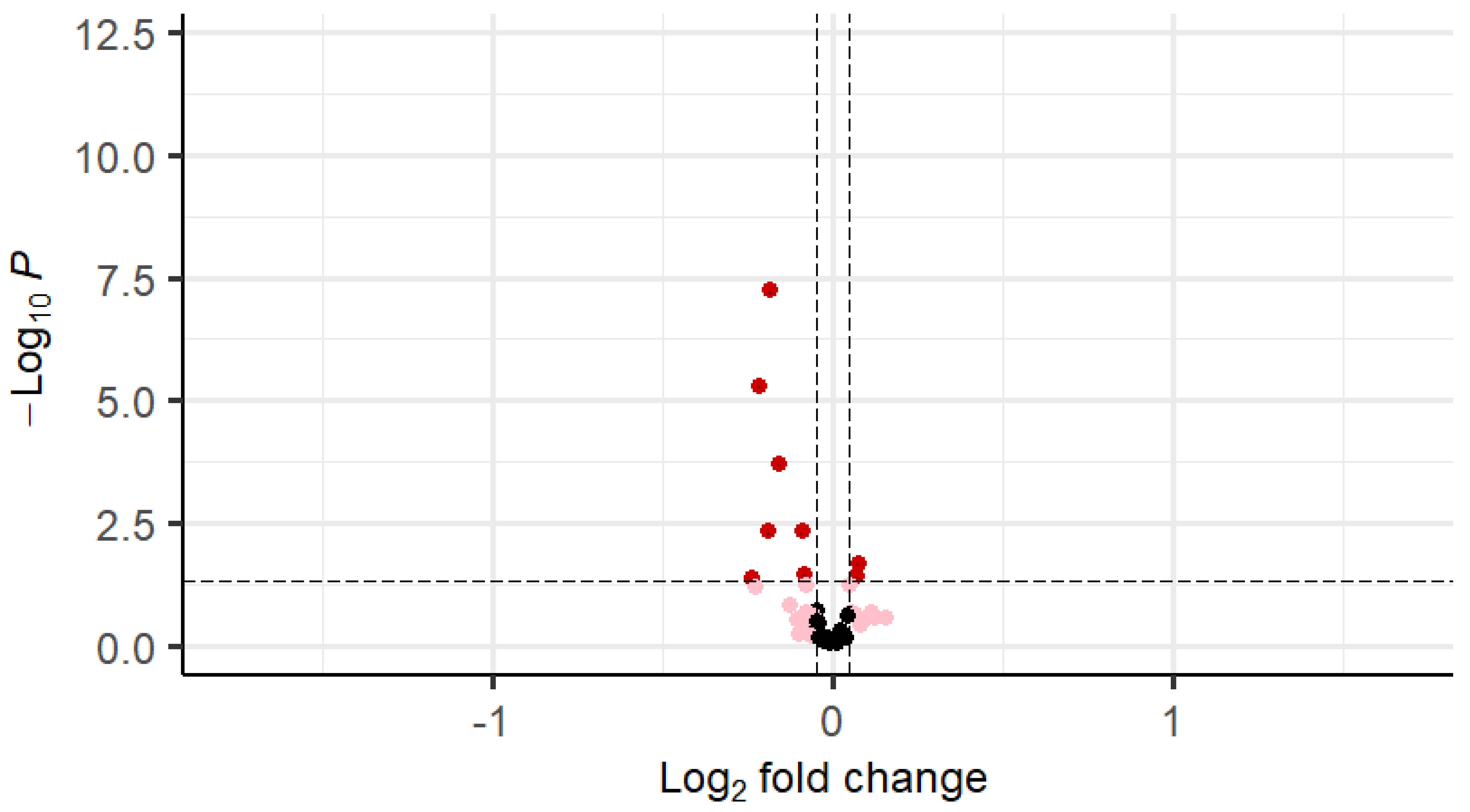
| Gene | logFC | Average Expression | t | p Value |
|---|---|---|---|---|
| FGFR1 (bFGF-R) | −0.18 | 9.36 | −5.60 | <0.001 |
| IGF-1 | −0.22 | 9.58 | −4.68 | <0.001 |
| VEGF-D | −0.16 | 8.85 | −3.79 | <0.001 |
| PDGFRA | −0.19 | 9.72 | −2.87 | <0.001 |
| EGFRvIII | −0.09 | 14.72 | −2.86 | <0.001 |
| FGF-8 | 0.07 | 9.53 | 2.33 | 0.02 |
| IGF-I sR | −0.09 | 12.49 | −2.14 | 0.02 |
| FGF9 | 0.07 | 8.93 | 2.10 | 0.03 |
| EGFR | −0.24 | 8.25 | −2.07 | 0.04 |
| EGF | −0.08 | 9.34 | −1.93 | 0.04 |
| FGF-17 | 0.05 | 7.62 | 1.92 | 0.04 |
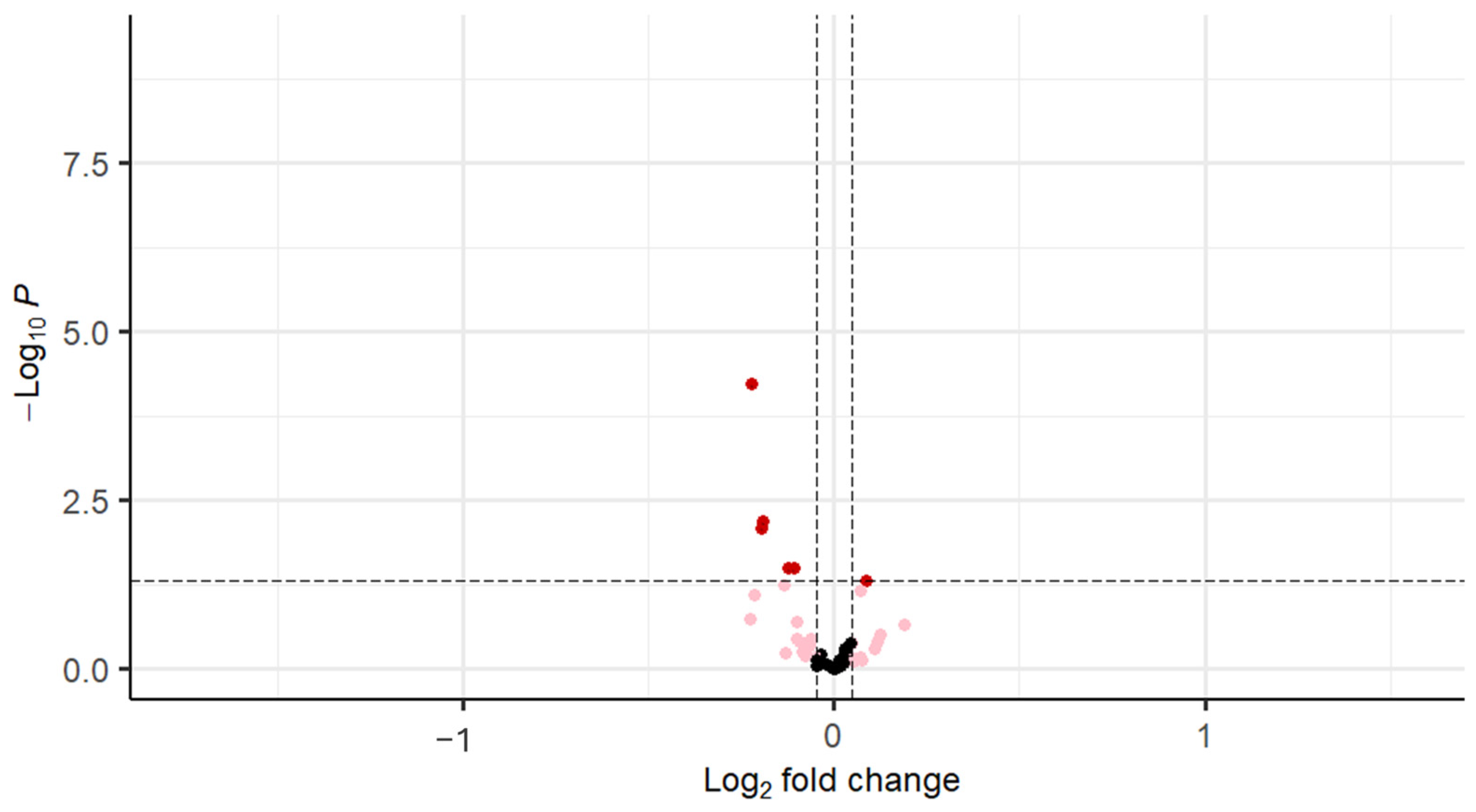
| Gene | logFC | Average Expression | t | p Value |
|---|---|---|---|---|
| FGFR1 (bFGF-R) | −0.22 | 9.39 | −4.17 | <0.001 |
| IGF-1 | −0.19 | 9.62 | −2.77 | 0.01 |
| VEGF-D | −0.20 | 8.90 | −2.70 | 0.01 |
| IGF-I sR | −0.12 | 12.50 | −2.17 | 0.03 |
| EGFR | −0.11 | 14.74 | −2.17 | 0.03 |
| FGF-8 | 0.08 | 9.51 | 1.99 | 0.04 |
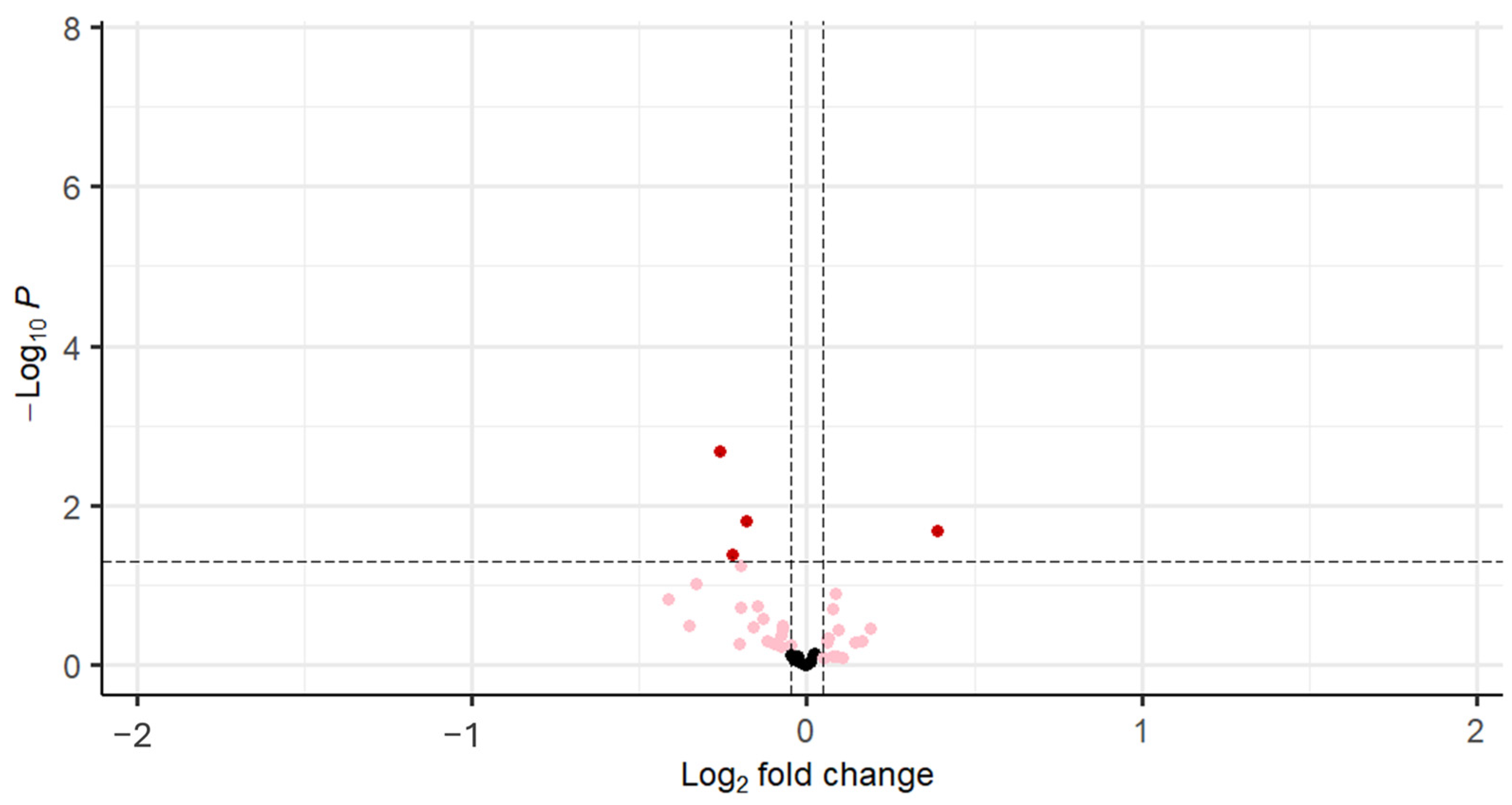
| Gene | logFC | Average Expression | t | p Value |
|---|---|---|---|---|
| FGFR1 (bFGF-R) | −0.26 | 9.42 | −3.20 | <0.001 |
| IGF-I sR | −0.18 | 12.49 | −2.48 | 0.02 |
| IGF-1 | −0.22 | 9.65 | −2.08 | 0.04 |
| VEGF-D | −0.20 | 8.94 | −1.95 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niinuma, S.A.; Habib, H.; Takemoto, A.S.-N.; Das, P.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. A Cross-Sectional Exploratory Study of Rat Sarcoid (Ras) Activation in Women with and Without Polycystic Ovary Syndrome. Cells 2025, 14, 377. https://doi.org/10.3390/cells14050377
Niinuma SA, Habib H, Takemoto AS-N, Das P, Sathyapalan T, Atkin SL, Butler AE. A Cross-Sectional Exploratory Study of Rat Sarcoid (Ras) Activation in Women with and Without Polycystic Ovary Syndrome. Cells. 2025; 14(5):377. https://doi.org/10.3390/cells14050377
Chicago/Turabian StyleNiinuma, Sara Anjum, Haniya Habib, Ashleigh Suzu-Nishio Takemoto, Priya Das, Thozhukat Sathyapalan, Stephen L. Atkin, and Alexandra E. Butler. 2025. "A Cross-Sectional Exploratory Study of Rat Sarcoid (Ras) Activation in Women with and Without Polycystic Ovary Syndrome" Cells 14, no. 5: 377. https://doi.org/10.3390/cells14050377
APA StyleNiinuma, S. A., Habib, H., Takemoto, A. S.-N., Das, P., Sathyapalan, T., Atkin, S. L., & Butler, A. E. (2025). A Cross-Sectional Exploratory Study of Rat Sarcoid (Ras) Activation in Women with and Without Polycystic Ovary Syndrome. Cells, 14(5), 377. https://doi.org/10.3390/cells14050377









