Diabetic Retinopathy (DR): Mechanisms, Current Therapies, and Emerging Strategies
Abstract
1. Introduction
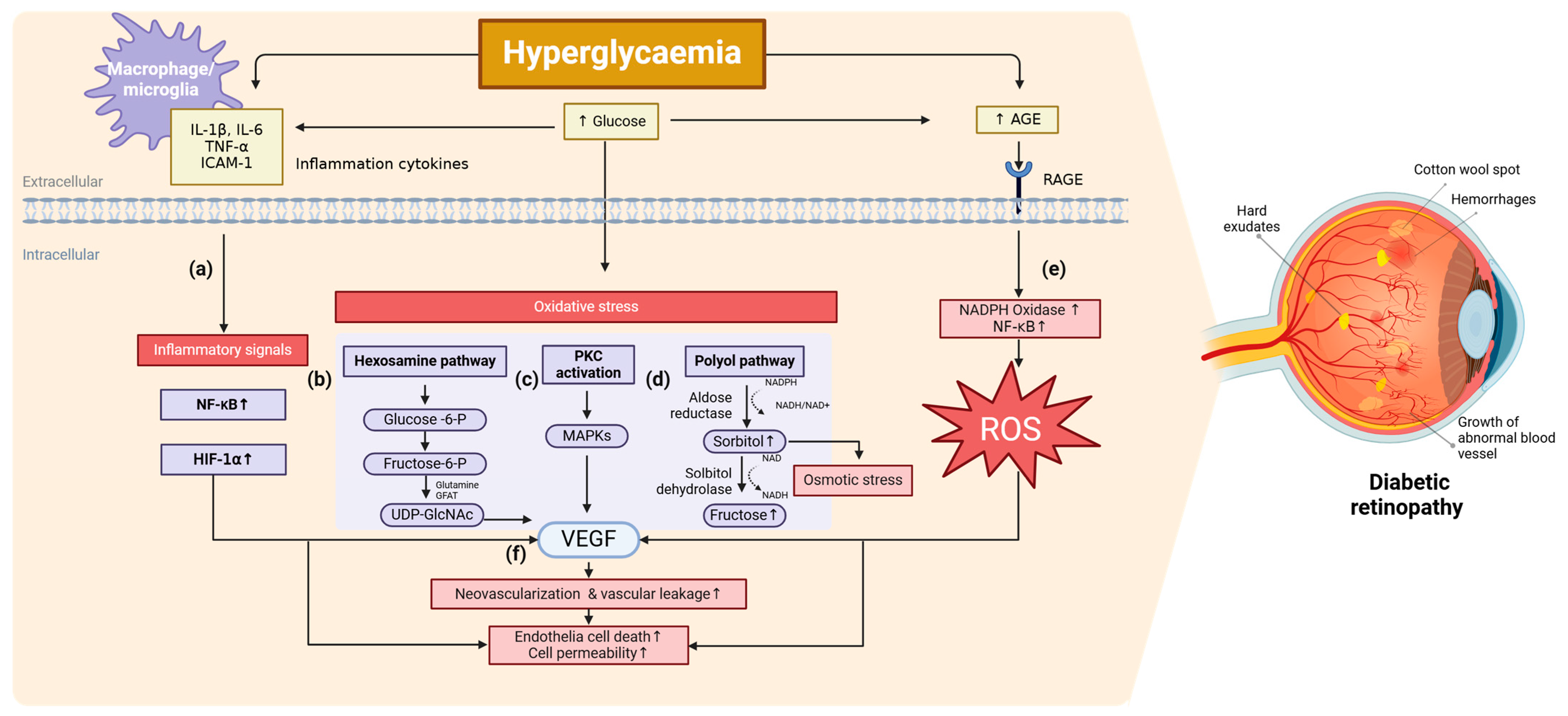
2. Molecular Mechanism of DR
2.1. Inflammation
2.2. Oxidative Stress
2.3. Other Related Pathways
3. Current Clinical Therapies
4. Emerging Therapeutics
4.1. Natural Compounds and Supplements
4.2. Lipid Modulators
4.3. Epigenetic Modulators
4.4. Other Targeted Therapies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korkmaz, H.A.; Dogan, B.; Devebacak, A.; Değirmenci, C.; Afrashi, F. The Relationship of Serum Diabetes Antibodies With the Development of Early Diabetic Retinopathy Findings in Children With Type 1 Diabetes Mellitus. J. Pediatr. Ophthalmol. Strabismus 2024, 0, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rees, G.; Xie, J.; Fenwick, E.K.; Sturrock, B.A.; Finger, R.; Rogers, S.L.; Lim, L.; Lamoureux, E.L. Association Between Diabetes-Related Eye Complications and Symptoms of Anxiety and Depression. JAMA Ophthalmol. 2016, 134, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Al-Namaeh, M. Common causes of visual impairment in the elderly. Med. Hypothesis Discov. Innov. Ophthalmol. 2021, 10, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Cummings, D.M.; Morrissey, S.; Barondes, M.J.; Rogers, L.; Gustke, S. Screening for Diabetic Retinopathy in Rural Areas: The Potential of Telemedicine. J. Rural Health 2001, 17, 25–31. [Google Scholar] [CrossRef]
- Piyasena, M.; Murthy, G.V.S.; Yip, J.L.Y.; Gilbert, C.; Zuurmond, M.; Peto, T.; Gordon, I.; Hewage, S.; Kamalakannan, S. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS ONE 2019, 14, e0198979. [Google Scholar] [CrossRef]
- Patel, H.; Allen, A.; Karl, J.; Weng, P.; Stinnett, S.S.; Rosdahl, J.A.; Schuman, S.G. Diabetic Retinopathy Disease Burden in Patients With Lower Household Incomes vs Higher Household Incomes. J. Vitr. Dis. 2025, 0, 24741264241309683. [Google Scholar] [CrossRef]
- Oncel, D.; Minaker, S.; Shepherd, E.A.; Rezaei, S.; Boucher, N.; Aggarwal, N.; MacCumber, M. Risk Factors for Proliferative Vitreoretinopathy in a Large Clinical Database. Retina 2024, 9900. [Google Scholar] [CrossRef]
- Han, G.; Hu, K.; Luo, T.; Wang, W.; Zhang, D.; Ouyang, L.; Liu, X.; Liu, J.; Wu, Y.; Liang, J.; et al. Research progress of non-coding RNA regulating the role of PANoptosis in diabetes mellitus and its complications. Apoptosis 2025. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, X.; Wang, M.; Li, F.; Sun, T.; Liu, W.; Xu, Z.; Shen, D.; Wang, L.; Li, M.; et al. Risk factors for diabetic retinopathy in young and middle-aged patients: A retrospective study. BMC Ophthalmol. 2024, 24, 544. [Google Scholar] [CrossRef]
- Behnoush, A.H.; Samavarchitehrani, A.; Shirazi Ghaleno, A.M.; Klisic, A. Fetuin-A levels in diabetic retinopathy: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2025, 24, 31. [Google Scholar] [CrossRef] [PubMed]
- Kummerle, D.; Beals, D.; Simon, L.; Rogers, F.; Pogroszewski, S. Revolutionizing Diabetic Retinopathy Screening: Integrating AI-Based Retinal Imaging in Primary Care. J. CME 2025, 14, 2437294. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.Y.; Lo, M.T.; Chen, T.C.; Huang, C.H.; Huang, A.; Wang, P.C. A deep learning-based ADRPPA algorithm for the prediction of diabetic retinopathy progression. Sci. Rep. 2024, 14, 31772. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.H.; Campbell, J.; Holekamp, N.M.; Kiss, S.; Loewenstein, A.; Augustin, A.J.; Ma, J.; Ho, A.C.; Patel, V.; Whitcup, S.M.; et al. Early and Long-Term Responses to Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema: Analysis of Protocol I Data. Am. J. Ophthalmol. 2016, 172, 72–79. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Kishikawa, H.; Araki, E.; Miyata, T.; Isami, S.; Motoyoshi, S.; Kojima, Y.; Furuyoshi, N.; Shichiri, M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Res. Clin. Pract. 1995, 28, 103–117. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- Kumar, J.; Malaviya, P.; Kowluru, R.A. Long noncoding RNAs and metabolic memory associated with continued progression of diabetic retinopathy. J. Diabetes 2024, 16, e70009. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.B.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Yun, J.H. Interleukin-1β induces pericyte apoptosis via the NF-κB pathway in diabetic retinopathy. Biochem. Biophys. Res. Commun. 2021, 546, 46–53. [Google Scholar] [CrossRef]
- Qiu, A.-W.; Wang, N.-Y.; Yin, W.-J.; Zhu, Z.-Q.; Liu, Q.-H.; Zhang, W.-W. Retinal Müller Cell-Released Exosomal MiR-92a-3p Delivers Interleukin-17A Signal by Targeting Notch-1 to Promote Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2025, 66, 1. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, J.; Zhou, Y.; Wang, S.; Zhang, Z.; Jiang, Q.; Li, K. Macrophage/microglia polarization for the treatment of diabetic retinopathy. Front. Endocrinol. 2023, 14, 1276225. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- De, I.; Nikodemova, M.; Steffen, M.D.; Sokn, E.; Maklakova, V.I.; Watters, J.J.; Collier, L.S. CSF1 overexpression has pleiotropic effects on microglia In Vivo. Glia 2014, 62, 1955–1967. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, W.; Wang, C.; Dong, X.; Yu, F.; Su, Y.; Huang, J.; Huo, L.; Wan, P. ALKBH5-Mediated m(6)A Modification of A20 Regulates Microglia Polarization in Diabetic Retinopathy. Front. Immunol. 2022, 13, 813979. [Google Scholar] [CrossRef]
- Hu, X. Microglia/macrophage polarization: Fantasy or evidence of functional diversity? J. Cereb. Blood Flow. Metab. 2020, 40, S134–S136. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, C.; Lu, L.; Tian, H.; Liu, K.; Luo, D.; Qiu, Q.; Xu, G.T.; Zhang, J. Melatonin Maintains Inner Blood-Retinal Barrier by Regulating Microglia via Inhibition of PI3K/Akt/Stat3/NF-κB Signaling Pathways in Experimental Diabetic Retinopathy. Front. Immunol. 2022, 13, 831660. [Google Scholar] [CrossRef]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Nerlich, A.G.; Sauer, U.; Kolm-Litty, V.; Wagner, E.; Koch, M.; Schleicher, E.D. Expression of Glutamine: Fructose-6-Phosphate Amidotransferase in Human Tissues: Evidence for High Variability and Distinct Regulation in Diabetes. Diabetes 1998, 47, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Dierschke, S.K.; Miller, W.P.; Favate, J.S.; Shah, P.; Imamura Kawasawa, Y.; Salzberg, A.C.; Kimball, S.R.; Jefferson, L.S.; Dennis, M.D. O-GlcNAcylation alters the selection of mRNAs for translation and promotes 4E-BP1–dependent mitochondrial dysfunction in the retina. J. Biol. Chem. 2019, 294, 5508–5520. [Google Scholar] [CrossRef] [PubMed]
- Donovan, K.; Alekseev, O.; Qi, X.; Cho, W.; Azizkhan-Clifford, J. O-GlcNAc modification of transcription factor Sp1 mediates hyperglycemia-induced VEGF-A upregulation in retinal cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7862–7873. [Google Scholar] [CrossRef]
- Gurel, Z.; Sieg, K.M.; Shallow, K.D.; Sorenson, C.M.; Sheibani, N. Retinal O-linked N-acetylglucosamine protein modifications: Implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Mol. Vis. 2013, 19, 1047–1059. [Google Scholar] [PubMed]
- Gurel, Z.; Sheibani, N. O-Linked β-N-acetylglucosamine (O-GlcNAc) modification: A new pathway to decode pathogenesis of diabetic retinopathy. Clin. Sci. 2018, 132, 185–198. [Google Scholar] [CrossRef]
- Donnelly, R.; Idris, I.; Forrester, J.V. Protein kinase C inhibition and diabetic retinopathy: A shot in the dark at translational research. Br. J. Ophthalmol. 2004, 88, 145–151. [Google Scholar] [CrossRef]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed. Res. Int. 2014, 2014, 801269. [Google Scholar] [CrossRef]
- Thorne, C.A.; Grey, A.C.; Lim, J.C.; Donaldson, P.J. The Synergistic Effects of Polyol Pathway-Induced Oxidative and Osmotic Stress in the Aetiology of Diabetic Cataracts. Int. J. Mol. Sci. 2024, 25, 9042. [Google Scholar] [CrossRef]
- A randomized trial of sorbinil, an aldose reductase inhibitor, in diabetic retinopathy. Sorbinil Retinopathy Trial Research Group. Arch. Ophthalmol. 1990, 108, 1234–1244. [CrossRef]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diab. Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef]
- Jeon, H.-Y.; Lee, A.-J.; Moon, C.-H.; Ha, K.-S. Regulation of AMPK and GAPDH by Transglutaminase 2 Plays a Pivotal Role in Microvascular Leakage in Diabetic Retinas. Diabetes 2024, 73, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, Z.; Li, Z.; Xu, M.; Tian, A.; An, Z.; Guo, W.; He, C.; Dong, Y.; Wen, J.; et al. MicroRNA-mediated Ets1 repression in retinal endothelial cells: A novel anti-angiogenic mechanism in nonproliferative diabetic retinopathy. Diabetes Obes. Metab. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.H.; Zhang, S.S.; Kong, Y.; Bi, Y.F.; Wang, L.; Zhang, Q. Effects of intensive control of blood glucose and blood pressure on microvascular complications in patients with type II diabetes mellitus. Int. J. Ophthalmol. 2013, 6, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Q.; Gillies, M.C.; Wong, T.Y. Management of diabetic retinopathy: A systematic review. JAMA 2007, 298, 902–916. [Google Scholar] [CrossRef]
- Reddy, S.V.; Husain, D. Panretinal Photocoagulation: A Review of Complications. Semin. Ophthalmol. 2018, 33, 83–88. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Wang, P.-w.; Ruiz, C.Q. Panretinal Photocoagulation for Diabetic Retinopathy in the RIDE and RISE Trials: Not “1 and Done”. Ophthalmology 2021, 128, 1448–1457. [Google Scholar] [CrossRef]
- Striph, G.G.; Hart, W.M.; Olk, R.J. Modified Grid Laser Photocoagulation for Diabetic Macular Edema: The Effect on the Central Visual Field. Ophthalmology 1988, 95, 1673–1679. [Google Scholar] [CrossRef]
- Sims, L.M.; Stoessel, K.; Thompson, J.T.; Hirsch, J. Assessment of Visual-Field Changes before and after Focal Photocoagulation for Clinically Significant Diabetic Macular Edema. Ophthalmologica 2010, 200, 133–141. [Google Scholar] [CrossRef]
- Golden, M.P.; Russell, B.P.; Ingersoll, G.M.; Gray, D.L.; Hummer, K.M. Management of Diabetes Mellitus in Children Younger Than 5 Years of Age. Am. J. Dis. Child. 1985, 139, 448–452. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA 2015, 314, 2137–2146. [Google Scholar] [CrossRef]
- Nishikawa, K.; Murakami, T.; Yoshida, M.; Terada, N.; Ishihara, K.; Mori, Y.; Ito, S.; Tsujikawa, A. Extracellular Mitochondria Exacerbate Retinal Pigment Epithelium Degeneration in Diabetic Retinopathy. Diabetes 2025, 74, 409–415. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Musa, M.; Gagliano, C.; Zeppieri, M. The Treatment of Diabetic Retinal Edema with Intravitreal Steroids: How and When. J. Clin. Med. 2024, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, Q.; Zhang, Y.; Gao, X.; Li, H.; Yuan, Z. Ripasudil alleviated the inflammation of RPE cells by targeting the miR-136-5p/ROCK/NLRP3 pathway. BMC Ophthalmol. 2020, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.N.; Vasiliev, A.S.; Kalinicheva, Y.A.; Maltsev, D.S. Topical bromfenac in VEGF-driven maculopathies: Topical review and meta-analysis. BMC Ophthalmol. 2024, 24, 369. [Google Scholar] [CrossRef] [PubMed]
- Amrite, A.C.; Ayalasomayajula, S.P.; Cheruvu, N.P.; Kompella, U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1149–1160. [Google Scholar] [CrossRef]
- Friedman, S.M.; Almukhtar, T.H.; Baker, C.W.; Glassman, A.R.; Elman, M.J.; Bressler, N.M.; Maker, M.P.; Jampol, L.M.; Melia, M.; Network, D.R.C.R. Topical nepafenec in eyes with noncentral diabetic macular edema. Retina 2015, 35, 944–956. [Google Scholar] [CrossRef]
- Hiran, H.M.; Kamath, A.; Mendonca, T.M.; Rodrigues, G.R.; Nayak, R.R.; Kamath, G.; Kamath, S.J. Association of serum lipid profile and other systemic risk factors with retinal hard exudates in diabetic retinopathy. Int. Ophthalmol. 2024, 44, 338. [Google Scholar] [CrossRef]
- Chew, E.Y.; Klein, M.L.; Ferris, F.L., III; Remaley, N.A.; Murphy, R.P.; Chantry, K.; Hoogwerf, B.J.; Miller, D. Association of Elevated Serum Lipid Levels With Retinal Hard Exudate in Diabetic Retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch. Ophthalmol. 1996, 114, 1079–1084. [Google Scholar] [CrossRef]
- Davis, M.D.; Fisher, M.R.; Gangnon, R.E.; Barton, F.; Aiello, L.M.; Chew, E.Y.; Ferris, F.; Knatterud, G.L. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report# 18. Investig. Ophthalmol. Vis. Sci. 1998, 39, 233–252. [Google Scholar]
- Ferris, F.L., 3rd; Chew, E.Y.; Hoogwerf, B.J. Serum lipids and diabetic retinopathy. Early Treatment Diabetic Retinopathy Study Research Group. Diabetes Care 1996, 19, 1291–1293. [Google Scholar] [CrossRef]
- Zhang, J.; McGwin, G. Association of statin use with the risk of developing diabetic retinopathy. Arch. Ophthalmol. 2007, 125, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, J.E.; Abbott, A.B.; Chew, E.Y. Fenofibrate and Diabetic Retinopathy. Curr. Diabetes Rep. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.-C.; Liu, L.; Rim, T.H.; Zhang, L.; Majithia, S.; Chee, M.L.; Tan, N.Y.Q.; Wong, K.-H.; Ting, D.S.W.; Sabanayagam, C.; et al. Association of Cataract Surgery With Risk of Diabetic Retinopathy Among Asian Participants in the Singapore Epidemiology of Eye Diseases Study. JAMA Netw. Open 2020, 3, e208035. [Google Scholar] [CrossRef]
- Schachat, A.P.; Oyakawa, R.T.; Michels, R.G.; Rice, T.A. Complications of Vitreous Surgery for Diabetic Retinopathy: II. Postoperative Complications. Ophthalmology 1983, 90, 522–530. [Google Scholar] [CrossRef]
- Ostri, C.; Lux, A.; Lund-Andersen, H.; la Cour, M. Long-term results, prognostic factors and cataract surgery after diabetic vitrectomy: A 10-year follow-up study. Acta Ophthalmol. 2014, 92, 571–576. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, L.; Xu, H.; Fang, J.; Zhong, H. Resveratrol alleviates reactive oxygen species and inflammation in diabetic retinopathy via SIRT1/HMGB1 pathway-mediated ferroptosis. Toxicol. Appl. Pharmacol. 2024, 495, 117214. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, T.; Yang, Y.; Xu, X.; Liu, Y. Oridonin attenuates diabetic retinopathy progression by suppressing NLRP3 inflammasome pathway. Mol. Cell. Endocrinol. 2025, 596, 112419. [Google Scholar] [CrossRef]
- Sapieha, P.; Chen, J.; Stahl, A.; Seaward, M.R.; Favazza, T.L.; Juan, A.M.; Hatton, C.J.; Joyal, J.S.; Krah, N.M.; Dennison, R.J.; et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr. Diabetes 2012, 2, e36. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Park, J.C.; Ma, G.; Farooq, Z.; Baccouche, B.; Sawant, O.B.; McAnany, J.J.; Yao, X.; Kazlauskas, A.; et al. Retinal docosahexaenoic acid is significantly reduced in diabetic humans and mice: Possible relationship to diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 39. [Google Scholar] [CrossRef]
- Rodríguez González-Herrero, M.E.; Ruiz, M.; López Román, F.J.; Marín Sánchez, J.M.; Domingo, J.C. Supplementation with a highly concentrated docosahexaenoic acid plus xanthophyll carotenoid multivitamin in nonproliferative diabetic retinopathy: Prospective controlled study of macular function by fundus microperimetry. Clin. Ophthalmol. 2018, 12, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Yalagala, P.C.R.; Subbaiah, P.V. Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients 2020, 12, 3114. [Google Scholar] [CrossRef] [PubMed]
- Varughese, M.S.; Nayak, A.U.; Jacob, S. PCSK9 levels and diabetic retinopathy: Opportunities for a potential target and novel therapeutic approach in conjunction with treating dyslipidaemia. Eye 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tang, H.; Li, S. Protective effect of evolocumab on Müller cells in the rat retina under hyperglycaemic and hypoxic conditions. J. Diabetes Complicat. 2023, 37, 108593. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Yang, P.; Guo, J.; Liu, L.; Yang, Z.; Nan, K. Genetic Association between Lipid-Regulating Drug Targets and Diabetic Retinopathy: A Drug Target Mendelian Randomization Study. J. Lipids 2024, 2024, 5324127. [Google Scholar] [CrossRef]
- Varughese, M.S.; Nayak, A.U.; Jacob, S. Fenofibrate therapy in reducing the progression of diabetic retinopathy: Revisiting the FIELD and ACCORD-EYE studies through the LENS trial. Eye 2025, 39, 15–17. [Google Scholar] [CrossRef]
- Wong, T.Y.; Simó, R.; Mitchell, P. Fenofibrate—A potential systemic treatment for diabetic retinopathy? Am. J. Ophthalmol. 2012, 154, 6–12. [Google Scholar] [CrossRef]
- Magupalli, V.G.; Negro, R.; Tian, Y.; Hauenstein, A.V.; Di Caprio, G.; Skillern, W.; Deng, Q.; Orning, P.; Alam, H.B.; Maliga, Z.; et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science 2020, 369, eaas8995. [Google Scholar] [CrossRef]
- Kim, J.S.; Jun, J.H.; Lee, J.; Park, S.; Kim, E.; Hwang, S.J.; Moon, H.; Baek, S.H.; Kim, H.K.; Park, J.; et al. HDAC6 mediates NLRP3 inflammasome activation in the pathogenesis of diabetic retinopathy. Metabolism 2024, 164, 156108. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Liu, Y.; Jin, Y.; Yang, H. PRDX4 mitigates diabetic retinopathy by inhibiting reactive gliosis, apoptosis, ER stress, oxidative stress, and mitochondrial dysfunction in Müller cells. J. Biol. Chem. 2024, 301, 108111. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, C.; Zhang, J.; Zhong, X.; Wang, R.; Zeng, X.; Ba, X. The Role of PARPs in Inflammation-and Metabolic-Related Diseases: Molecular Mechanisms and Beyond. Cells 2019, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Auer, B.; Stingl, L.; Berghammer, H.; Haidacher, D.; Schweiger, M.; Wagner, E.F. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes. Dev. 1995, 9, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, G.; Chen, R.; Zhou, J.; Chen, T.; Cheng, Y.; Lou, Q.; Wang, H. PARP1 Is Upregulated by Hyperglycemia Via N6-methyladenosine Modification and Promotes Diabetic Retinopathy. Discov. Med. 2022, 34, 115–129. [Google Scholar] [PubMed]
- Guzyk, M.M.; Tykhomyrov, A.A.; Nedzvetsky, V.S.; Prischepa, I.V.; Grinenko, T.V.; Yanitska, L.V.; Kuchmerovska, T.M. Poly(ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors Reduce Reactive Gliosis and Improve Angiostatin Levels in Retina of Diabetic Rats. Neurochem. Res. 2016, 41, 2526–2537. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Wu, Q.; Jia, L.; Du, X. Minocycline inhibits PARP-1 expression and decreases apoptosis in diabetic retinopathy. Mol. Med. Rep. 2015, 12, 4887–4894. [Google Scholar] [CrossRef]
- Greenwald, S.H.; Pierce, E.A. Parthanatos as a Cell Death Pathway Underlying Retinal Disease. Adv. Exp. Med. Biol. 2019, 1185, 323–327. [Google Scholar] [CrossRef]
| Type | Drug Name | Target of Action | Administration Route |
|---|---|---|---|
| Biologics | Bevacizumab | Anti-VEGF | Intravitreal injection |
| Ranibizumab | |||
| Alfibercept | |||
| Faricimab | |||
| Brolucizumab | |||
| Small molecules | Conbercept | Glucocorticoid receptor agonist | Intravitreal implant |
| Difluprednate | Ophthalmic | ||
| Dexamethason | Intravitreal implant | ||
| Triamcinolone acetonide | Intravitreal injection | ||
| Finerenone | Mineralocorticoid receptor antagonist | Oral | |
| Fenofibrate | Lipoprotein lipase stimulator (PPAR alpha agonist) | Oral | |
| Ocriplasmin | Alpha-2 antiplasmin inhibitor, collagen antagonist, fibronectin inhibitor, laminin antagonist plasmin stimulator | Intravitreal injection | |
| Ripasudil hydrochloride | Rho-associated protein kinase inhibitor | Ophthalmic | |
| Bromfenac sodium | Cyclooxygenase inhibitor | Ophthalmic | |
| Nepafenac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Park, S.-J.; Song, M. Diabetic Retinopathy (DR): Mechanisms, Current Therapies, and Emerging Strategies. Cells 2025, 14, 376. https://doi.org/10.3390/cells14050376
Seo H, Park S-J, Song M. Diabetic Retinopathy (DR): Mechanisms, Current Therapies, and Emerging Strategies. Cells. 2025; 14(5):376. https://doi.org/10.3390/cells14050376
Chicago/Turabian StyleSeo, Hyewon, Sun-Ji Park, and Minsoo Song. 2025. "Diabetic Retinopathy (DR): Mechanisms, Current Therapies, and Emerging Strategies" Cells 14, no. 5: 376. https://doi.org/10.3390/cells14050376
APA StyleSeo, H., Park, S.-J., & Song, M. (2025). Diabetic Retinopathy (DR): Mechanisms, Current Therapies, and Emerging Strategies. Cells, 14(5), 376. https://doi.org/10.3390/cells14050376






