Trapped in the NETs: Multiple Roles of Platelets in the Vascular Complications Associated with Neutrophil Extracellular Traps
Abstract
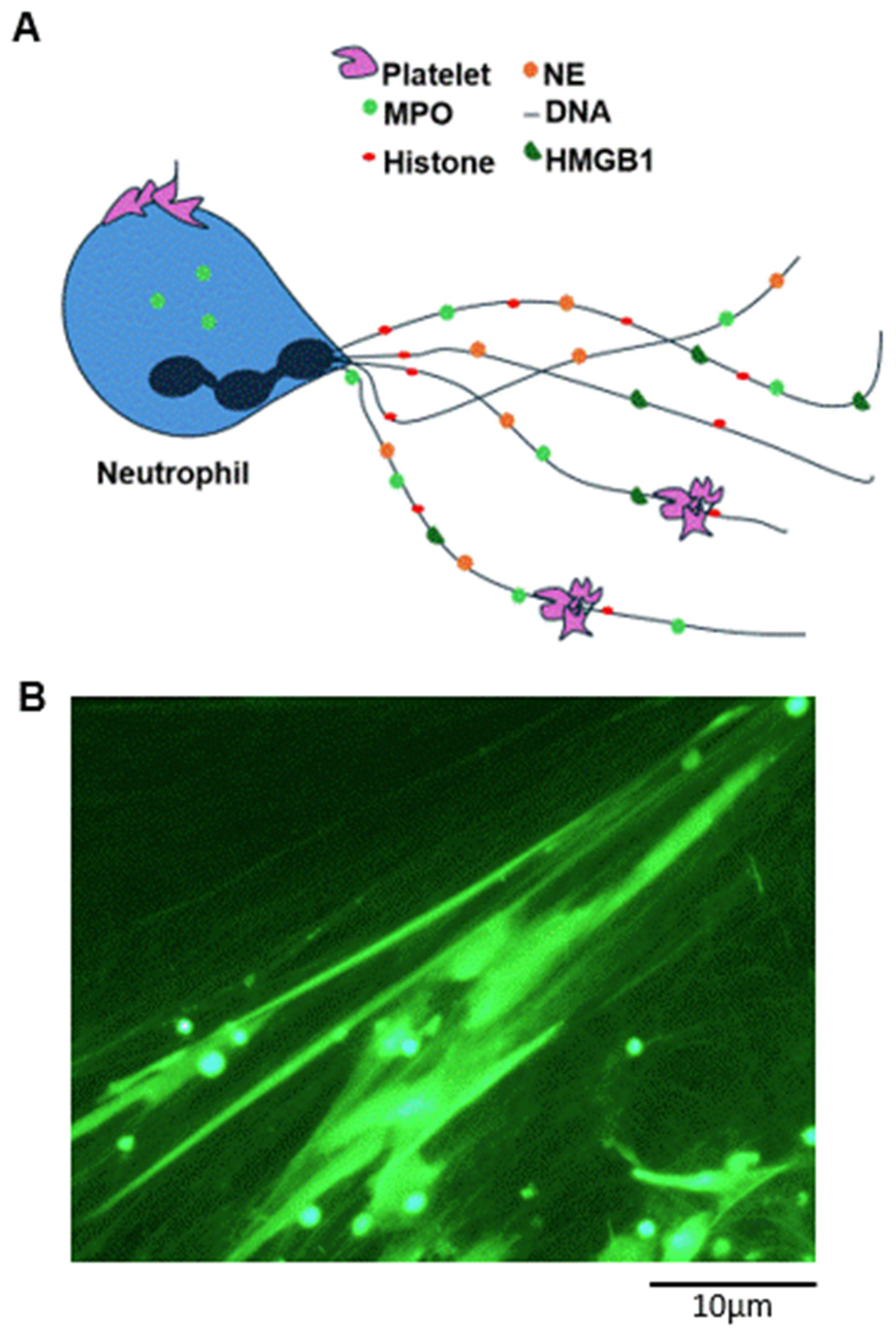
1. Neutrophil Extracellular Traps (NETs): Essential Components of the Innate Immune Response or Pathological Alterations of the Vascular System?
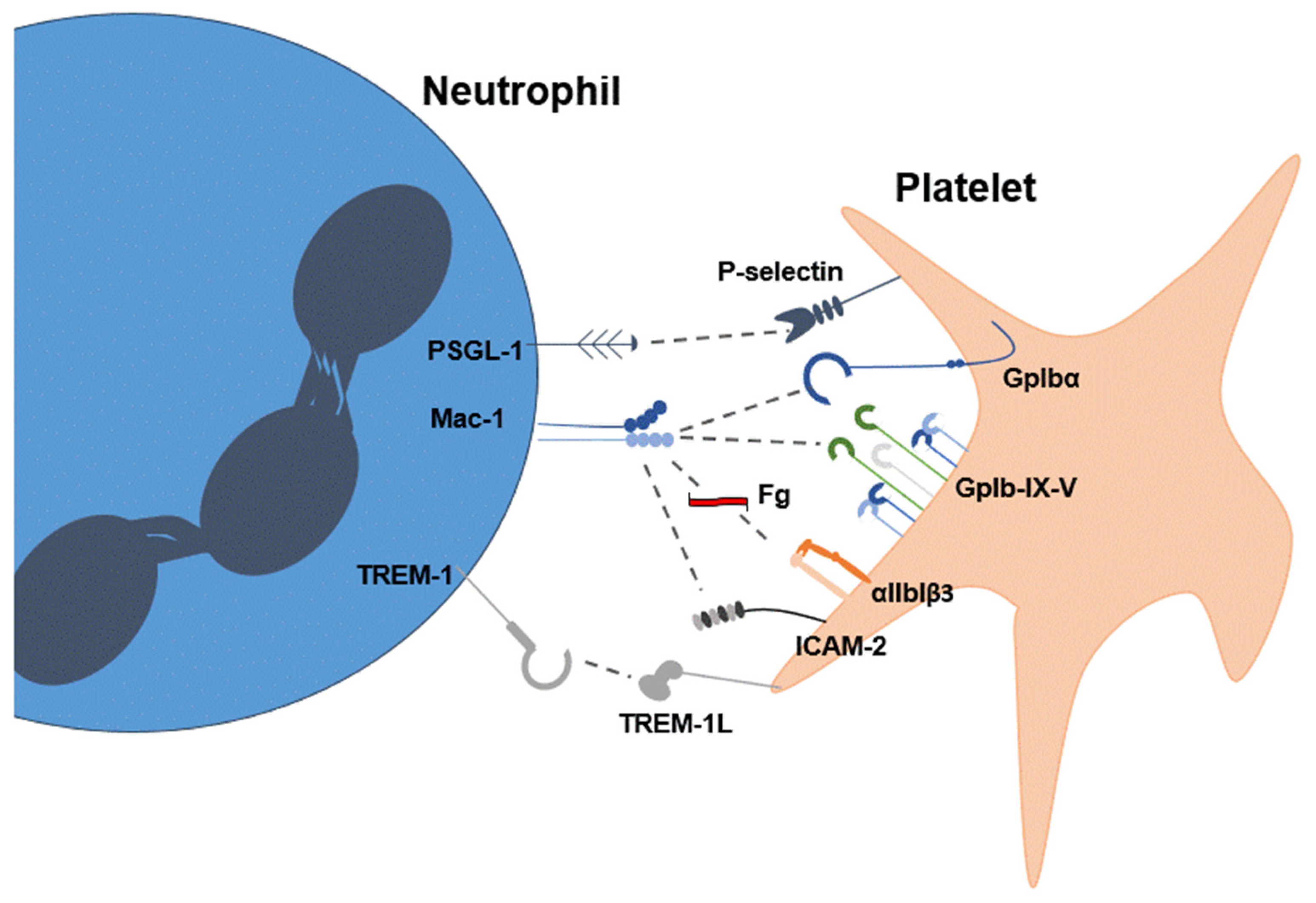
2. Platelets Stimulate the Formation of NETs
3. Platelets Are Activated by NETs
4. The Platelet–NET Involvement in Disease
5. Pharmacological Approaches Targeting NETs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berliner, N.; Coates, T.D. Introduction to a review series on human neutrophils. Blood 2019, 133, 2111–2112. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Tang, H.; Lin, C.; Shen, Y.; Yan, D.; Tang, X.; Guo, D. Extracellular traps and the role in thrombosis. Front. Cardiovasc. Med. 2022, 9, 951670. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Borregaard, N. Neutrophil extracellular traps—The dark side of neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Biermann, M.H.; Brauner, J.M.; Liu, Y.; Zhao, Y.; Herrmann, M. New insights into neutrophil extracellular traps: Mechanisms of formation and role in inflammation. Front. Immunol. 2016, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The role of neutrophils in the immune system: An overview. Methods Mol. Biol. 2014, 1124, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.; Hlushchuk, R.; Simon, D.; Walls, A.F.; Obata-Ninomiya, K.; Karasuyama, H.; Djonov, V.; Eggel, A.; Kaufmann, T.; Simon, H.-U.; et al. NADPH Oxidase–Independent Formation of Extracellular DNA Traps by Basophils. J. Immunol. 2014, 192, 5314–5323. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef]
- Joshi, M.B.; Lad, A.; Prasad, A.S.B.; Balakrishnan, A.; Ramachandra, L.; Satyamoorthy, K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013, 587, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Mitroulis, I.; Kambas, K.; Chrysanthopoulou, A.; Skendros, P.; Apostolidou, E.; Kourtzelis, I.; Drosos, G.I.; Boumpas, D.T.; Ritis, K. Neutrophil Extracellular Trap Formation Is Associated with IL-1β and Autophagy-Related Signaling in Gout. PLoS ONE 2011, 6, e29318. [Google Scholar] [CrossRef]
- Zukas, K.; Cayford, J.; Serneo, F.; Atteberry, B.; Retter, A.; Eccleston, M.; Kelly, T.K. Rapid high-throughput method for investigating physiological regulation of neutrophil extracellular trap formation. J. Thromb. Haemost. 2024, 22, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yasuoka, H.; Yoshimoto, K.; Suzuki, K.; Takeuchi, T. Platelet CXCL4 mediates neutrophil extracellular traps formation in ANCA-associated vasculitis. Sci. Rep. 2021, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Ling, Y.; Deng, Q.; Qiu, Y.; Shen, J.; Lai, H.; Chen, Z.; Huang, C.; Liang, L.; Li, X.; et al. HMGB1-Mediated neutrophil extracellular trap formation exacerbates intestinal ischemia/reperfusion-induced acute lung injury. J. Immunol. 2022, 208, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.E.; Feldman, H.A.; Young, V.; Soule-Albridge, E.L.; Kerper, R.; Kinlay, S.; Nolton, E.; Denorme, F.; Cody, M.; de Menezes, E.M.; et al. Effects of Platelet Transfusion on Plasma Cytokine Levels and Neutrophil Extracellular Traps (NETs) in Neonates. Blood 2024, 144, 20. [Google Scholar] [CrossRef]
- Wienkamp, A.-K.; Erpenbeck, L.; Rossaint, J. Platelets in the NETworks interweaving inflammation and thrombosis. Front. Immunol. 2022, 13, 953129. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, W.; Wang, W.; Tong, X.; Xia, R.; Fan, J.; Du, J.; Zhang, C.; Shi, X. Platelet-derived exosomes promote neutrophil extracellular trap formation during septic shock. Crit. Care 2020, 24, 380. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Mulay, S.R.; Nakazawa, D.; Anders, H.-J. Matters of life and death. How neutrophils die or survive along NET release and is “NETosis”= necroptosis? Cell. Mol. Life Sci. 2016, 73, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood J. Am. Soc. Hematol. 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.; Prikhodko, A.; Galkin, I.; Pletjushkina, O.; Zinovkin, R.; Sud’ina, G.; Chernyak, B.; Pinegin, B. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur. J. Cell Biol. 2017, 96, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Pinegin, B.; Vorobjeva, N.; Pashenkov, M.; Chernyak, B. The role of mitochondrial ROS in antibacterial immunity. J. Cell. Physiol. 2018, 233, 3745–3754. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.; Galkin, I.; Pletjushkina, O.; Golyshev, S.; Zinovkin, R.; Prikhodko, A.; Pinegin, V.; Kondratenko, I.; Pinegin, B.; Chernyak, B. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Basis Dis. 2020, 1866, 165664. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Köckritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFN s induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef]
- Fousert, E.; Toes, R.; Desai, J. Neutrophil Extracellular Traps (NETs) Take the Central Stage in Driving Autoimmune Responses. Cells 2020, 9, 915. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun. Signal. 2019, 17, 147. [Google Scholar] [CrossRef]
- Ganesh, K.; Joshi, M.B. Neutrophil sub-types in maintaining immune homeostasis during steady state, infections and sterile inflammation. Inflamm. Res. 2023, 72, 1175–1192. [Google Scholar] [CrossRef]
- Di Pilato, M.; Palomino-Segura, M.; Mejías-Pérez, E.; Gómez, C.E.; Rubio-Ponce, A.; D’antuono, R.; Pizzagalli, D.U.; Pérez, P.; Kfuri-Rubens, R.; Benguría, A.; et al. Neutrophil subtypes shape HIV-specific CD8 T-cell responses after vaccinia virus infection. npj Vaccines 2021, 6, 52. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Domerecka, W.; Homa-Mlak, I.; Mlak, R.; Michalak, A.; Wilińska, A.; Kowalska-Kępczyńska, A.; Dreher, P.; Cichoż-Lach, H.; Małecka-Massalska, T. Indicator of Inflammation and NETosis—Low-Density Granulocytes as a Biomarker of Autoimmune Hepatitis. J. Clin. Med. 2022, 11, 2174. [Google Scholar] [CrossRef]
- Sharma, S.; Hofbauer, T.M.; Ondracek, A.S.; Chausheva, S.; Alimohammadi, A.; Artner, T.; Panzenboeck, A.; Rinderer, J.; Shafran, I.H.; Mangold, A.; et al. Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood 2021, 137, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Yaykasli, K.O.; Schauer, C.; Muñoz, L.E.; Mahajan, A.; Knopf, J.; Schett, G.; Herrmann, M. Neutrophil Extracellular Trap-Driven Occlusive Diseases. Cells 2021, 10, 2208. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rahmy, S.; Liu, Z.; Zhang, C.; Lu, X. Rational targeting of immunosuppressive neutrophils in cancer. Pharmacol. Ther. 2020, 212, 107556. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Demers, M.; Fuchs, T.A.; Wong, S.L.; Brill, A.; Gallant, M.; Hu, J.; Wang, Y.; Wagner, D.D. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 8674–8679. [Google Scholar] [CrossRef] [PubMed]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; von Bernuth, H.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, Y.; Sun, R.; Hu, H.; Liu, Y.; Herrmann, M.; Zhao, Y.; Muñoz, L.E. Receptor-Mediated NETosis on Neutrophils. Front. Immunol. 2021, 12, 775267. [Google Scholar] [CrossRef] [PubMed]
- Wallis, S.; Wolska, N.; Englert, H.; Posner, M.; Upadhyay, A.; Renné, T.; Eggleston, I.; Bagby, S.; Pula, G. A peptide from the staphylococcal protein Efb binds P-selectin and inhibits the interaction of platelets with leukocytes. J. Thromb. Haemost. 2022, 20, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef] [PubMed]
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights Into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85. [Google Scholar] [CrossRef]
- Jin, R.; Yu, S.; Song, Z.; Zhu, X.; Wang, C.; Yan, J.; Wu, F.; Nanda, A.; Granger, D.N.; Li, G. Soluble CD40 ligand stimulates CD40-Dependent activation of the β2 Integrin Mac-1 and protein kinase C Zeda (PKCζ) in neutrophils: Implications for neutrophil-platelet interactions and neutrophil oxidative burst. PLoS ONE 2013, 8, e64631. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Shi, C.; Erhardt, P.W.; Pavlovsky, A.; Soloviev, D.A.; Bledzka, K.; Ustinov, V.; Zhu, L.; Qin, J. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nat. Commun. 2017, 8, 15559. [Google Scholar] [CrossRef]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nácher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef]
- Diacovo, T.G.; Defougerolles, A.R.; Bainton, D.F.; Springer, T.A. A functional integrin ligand on the surface of platelets: Intercellular adhesion molecule-2. J. Clin. Investig. 1994, 94, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Spertini, C.; Baïsse, B.; Spertini, O. Ezrin-Radixin-Moesin-binding Sequence of PSGL-1 glycoprotein regulates leukocyte rolling on selectins and activation of extracellular signal-regulated kinases. J. Biol. Chem. 2012, 287, 10693–10702. [Google Scholar] [CrossRef]
- Stadtmann, A.; Germena, G.; Block, H.; Boras, M.; Rossaint, J.; Sundd, P.; Lefort, C.; Fisher, C.I.; Buscher, K.; Gelschefarth, B.; et al. The PSGL-1–L-selectin signaling complex regulates neutrophil adhesion under flow. J. Exp. Med. 2013, 210, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Abadier, M.; Ley, K. P-selectin glycoprotein ligand-1 in T cells. Curr. Opin. Hematol. 2017, 24, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, Z.; Díaz-Godínez, C.; Mora, N.; Alemán, O.R.; Uribe-Querol, E.; Carrero, J.C.; Rosales, C. Entamoeba histolytica Induce Signaling via Raf/MEK/ERK for Neutrophil Extracellular Trap (NET) Formation. Front. Cell. Infect. Microbiol. 2018, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; De Luca, M.; McNally, T.; Michelson, A.D.; Andrews, R.K.; Berndt, M.C. Regulation of P-selectin binding to the neutrophil P-selectin counter-receptor P-selectin glycoprotein ligand-1 by neutrophil elastase and cathepsin G. Blood 2001, 98, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Boulaftali, Y.; Hess, P.R.; Getz, T.M.; Cholka, A.; Stolla, M.; Mackman, N.; Owens, A.P.; Ware, J.; Kahn, M.L.; Bergmeier, W. Platelet ITAM signaling is critical for vascular integrity in inflammation. J. Clin. Investig. 2013, 123, 908–916. [Google Scholar] [CrossRef]
- Claushuis, T.A.M.; de Vos, A.F.; Nieswandt, B.; Boon, L.; Roelofs, J.J.T.H.; de Boer, O.J.; Veer, C.J.v.; van der Poll, T. Platelet glycoprotein VI aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood 2018, 131, 864–876. [Google Scholar] [CrossRef]
- Stark, K.; Philippi, V.; Stockhausen, S.; Busse, J.; Antonelli, A.; Miller, M.; Schubert, I.; Hoseinpour, P.; Chandraratne, S.; von Brühl, M.-L.; et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 2016, 128, 2435–2449. [Google Scholar] [CrossRef] [PubMed]
- Tadie, J.-M.; Bae, H.-B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.-F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Sauter, R.J.; Sauter, M.; Reis, E.S.; Emschermann, F.N.; Nording, H.; Ebenhöch, S.; Kraft, P.; Münzer, P.; Mauler, M.; Rheinlaender, J.; et al. Functional relevance of the anaphylatoxin receptor C3aR for platelet function and arterial thrombus formation marks an Intersection point between innate immunity and thrombosis. Circulation 2018, 138, 1720–1735. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, P.E.; Heemskerk, J.W. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, E.; Amadio, P.; Squellerio, I.; Porro, B.; Sandrini, L.; Turnu, L.; Cavalca, V.; Tremoli, E.; Barbieri, S.S. Role of thromboxane-dependent platelet activation in venous thrombosis: Aspirin effects in mouse model. Pharmacol. Res. 2016, 107, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chi, D.; Ju, S.; Zhao, X.; Li, X.; Zhao, J.; Xie, H.; Li, Y.; Jin, J.; Mang, G.; et al. Platelet factor 4 promotes deep venous thrombosis by regulating the formation of neutrophil extracellular traps. Thromb. Res. 2024, 237, 52–63. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Baetta, R.; Mallia, A.; Banfi, C.; Tremoli, E. Platelets in Healthy and Disease States: From Biomarkers Discovery to Drug Targets Identification by Proteomics. Int. J. Mol. Sci. 2020, 21, 4541. [Google Scholar] [CrossRef] [PubMed]
- Schenk, B.I.; Petersen, F.; Flad, H.-D.; Brandt, E. Platelet-derived chemokines CXC chemokine ligand (CXCL)7, connective tissue-activating peptide III, and CXCL4 differentially affect and cross-regulate neutrophil adhesion and transendothelial migration. J. Immunol. 2002, 169, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, X.-L.; Wei, Y.-Q.; Wei, X.-W. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1871, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Rajarathnam, K.; Desai, U.R. Structural insights into how proteoglycans determine chemokine-CXCR1/CXCR2 interactions: Progress and challenges. Front. Immunol. 2020, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Moussouras, N.A.; Getschman, A.E.; Lackner, E.R.; Veldkamp, C.T.; Dwinell, M.B.; Volkman, B.F. Differences in Sulfotyrosine binding amongst CXCR1 and CXCR2 chemokine ligands. Int. J. Mol. Sci. 2017, 18, 1894. [Google Scholar] [CrossRef] [PubMed]
- Hook, J.S.; Cao, M.; Potera, R.M.; Alsmadi, N.Z.; Schmidtke, D.W.; Moreland, J.G. Nox2 regulates platelet activation and NET formation in the lung. Front. Immunol. 2019, 10, 1472. [Google Scholar] [CrossRef]
- Tsourouktsoglou, T.-D.; Warnatsch, A.; Ioannou, M.; Hoving, D.; Wang, Q.; Papayannopoulos, V. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep. 2020, 31, 107602. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; He, J.; Zhang, H.; Xia, Y.; Hu, Z.; Loughran, P.; Billiar, T.; Huang, H.; Tsung, A. Platelet TLR4-ERK5 Axis Facilitates NET-Mediated Capturing of Circulating Tumor Cells and Distant Metastasis after Surgical Stress. Cancer Res. 2021, 81, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef]
- Campos, J.; Ponomaryov, T.; De Prendergast, A.; Whitworth, K.; Smith, C.W.; Khan, A.O.; Kavanagh, D.; Brill, A. Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv. 2021, 5, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Aubé, F.-A.; Bidias, A.; Pépin, G. Who and how, DNA sensors in NETs-driven inflammation. Front. Immunol. 2023, 14, 1190177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Han, L.; Bo, T.; Qi, Z.; Zhong, H.; Xu, H.; Hu, L.; Chen, S.; Zhang, S. Double-stranded DNA enhances platelet activation, thrombosis, and myocardial injury via cyclic GMP-AMP synthase. Cardiovasc. Res. 2024, cvae218. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Bhandari, A.A.; Wagner, D.D. Histones induce rapid and profound thrombocytopenia in mice. Blood 2011, 118, 3708–3714. [Google Scholar] [CrossRef]
- Colicchia, M.; Perrella, G.; Gant, P.; Rayes, J. Novel mechanisms of thrombo-inflammation during infection: Spotlight on neutrophil extracellular trap-mediated platelet activation. Res. Pr. Thromb. Haemost. 2023, 7, 100116. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.J.; Vu, T.T.; Swystun, L.L.; Dwivedi, D.J.; Mai, S.H.; Weitz, J.I.; Liaw, P.C. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arter. Thromb. Vasc. Biol. 2014, 34, 1977–1984. [Google Scholar] [CrossRef]
- Saravanan, R.; Choong, Y.K.; Lim, C.H.; Lim, L.M.; Petrlova, J.; Schmidtchen, A. Cell-Free DNA Promotes Thrombin Autolysis and Generation of Thrombin-Derived C-Terminal Fragments. Front. Immunol. 2021, 12, 593020. [Google Scholar] [CrossRef]
- Beckmann, L.; Voigtlaender, M.; Rolling, C.C.; Schulenkorf, A.; Bokemeyer, C.; Langer, F. Myeloperoxidase has no effect on the low procoagulant activity of silica-free DNA. Thromb. Res. 2021, 203, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Behzadifard, M.; Soleimani, M. NETosis and SARS-CoV-2 infection related thrombosis: A narrative review. Thromb. J. 2022, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, M.C.L.; Bouwens, B.R.; Wichapong, K.; Suylen, D.P.; Bouwman, F.G.; Hackeng, T.M.; Koenen, R.R. Protein arginine deiminase 4 inactivates tissue factor pathway inhibitor-alpha by enzymatic modification of functional arginine residues. J. Thromb. Haemost. 2023, 21, 1214–1226. [Google Scholar] [CrossRef]
- Li, J.; Tong, D.; Song, B.; Xie, F.; Zhang, G.; Hao, X.; Li, W.; Chi, H.; Wang, W.; Shao, Y. Inflammatory cytokines induce neutrophil extracellular traps interaction with activated platelets and endothelial cells exacerbate coagulation in moderate and severe essential hypertension. J. Hypertens. 2022, 40, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Sprenkeler, E.G.G.; Zandstra, J.; van Kleef, N.D.; Goetschalckx, I.; Verstegen, B.; Aarts, C.E.M.; Janssen, H.; Tool, A.T.J.; van Mierlo, G.; van Bruggen, R.; et al. S100A8/A9 Is a Marker for the Release of Neutrophil Extracellular Traps and Induces Neutrophil Activation. Cells 2022, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Gao, Y.; Wang, Q.; Chi, J.; Zhu, Z.; Diao, Q.; Li, X.; Wang, Z.; Qu, M.; Shi, Y. Preliminary clinical analysis and pathway study of S100A8 as a biomarker for the diagnosis of acute deep vein thrombosis. Sci. Rep. 2024, 14, 13298. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Lu, R.; Halkidis, K.; Kocher, N.K.; Cao, W.; Marques, M.B.; Zheng, X.L. Plasma levels of S100A8/A9, histone/DNA complexes, and cell-free DNA predict adverse outcomes of immune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2021, 19, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Colicchia, M.; Schrottmaier, W.C.; Perrella, G.; Reyat, J.S.; Begum, J.; Slater, A.; Price, J.; Clark, J.C.; Zhi, Z.; Simpson, M.J.; et al. S100A8/A9 drives the formation of procoagulant platelets through GPIbα. Blood 2022, 140, 2626–2643. [Google Scholar] [CrossRef]
- Kolarova, H.; Klinke, A.; Kremserova, S.; Adam, M.; Pekarova, M.; Baldus, S.; Eiserich, J.; Kubala, L. Myeloperoxidase induces the priming of platelets. Free. Radic. Biol. Med. 2013, 61, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Selak, M.A. Neutrophil elastase potentiates cathepsin G-induced platelet activation. Thromb. Haemost. 1992, 68, 570–576. [Google Scholar]
- Feige, T.; Bosbach, A.; Krott, K.J.; Mulorz, J.; Chatterjee, M.; Ortscheid, J.; Krüger, E.; Krüger, I.; Salehzadeh, N.; Goebel, S.; et al. GP VI–Mediated Platelet Activation and Procoagulant Activity Aggravate Inflammation and Aortic Wall Remodeling in Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2294–2317. [Google Scholar] [CrossRef] [PubMed]
- Pircher, J.; Czermak, T.; Ehrlich, A.; Eberle, C.; Gaitzsch, E.; Margraf, A.; Grommes, J.; Saha, P.; Titova, A.; Ishikawa-Ankerhold, H. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat. Commun. 2018, 9, 1523. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Efenberger, M.; Brzezińska-Błaszczyk, E. Cathelicidin impact on inflammatory cells. Cent. Eur. J. Immunol. 2015, 40, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Salamah, M.F.; Ravishankar, D.; Kodji, X.; Moraes, L.A.; Williams, H.F.; Vallance, T.M.; Albadawi, D.A.; Vaiyapuri, R.; Watson, K.; Gibbins, J.M.; et al. The endogenous antimicrobial cathelicidin LL37 induces platelet activation and augments thrombus formation. Blood Adv. 2018, 2, 2973–2985. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Eleftheriou, D.; Hussain, A.A.; Price-Kuehne, F.E.; Savage, C.O.; Jayne, D.; Little, M.A.; Salama, A.D.; Klein, N.J.; Brogan, P.A. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J. Am. Soc. Nephrol. 2012, 23, 49–62. [Google Scholar] [CrossRef]
- Wang, Y.; Du, F.; Hawez, A.; Mörgelin, M.; Thorlacius, H. Neutrophil extracellular trap-microparticle complexes trigger neutrophil recruitment via high-mobility group protein 1 (HMGB1)-toll-like receptors(TLR2)/TLR4 signalling. Br. J. Pharmacol. 2019, 176, 3350–3363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, L.; Braun, O.Ö.; Westman, J.; Madhi, R.; Herwald, H.; Mörgelin, M.; Thorlacius, H. Neutrophil extracellular trap-microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci. Rep. 2018, 8, 4020. [Google Scholar] [CrossRef] [PubMed]
- Marki, A.; Buscher, K.; Lorenzini, C.; Meyer, M.; Saigusa, R.; Fan, Z.; Yeh, Y.T.; Hartmann, N.; Dan, J.M.; Kiosses, W.B.; et al. Elongated neutrophil-derived structures are blood-borne microparticles formed by rolling neutrophils during sepsis. J. Exp. Med. 2021, 218, e20200551. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Hally, K.E.; Parker, O.M.; Brunton-O’Sullivan, M.M.; Harding, S.A.; Larsen, P.D. Linking Neutrophil Extracellular Traps and Platelet Activation: A Composite Biomarker Score for Predicting Outcomes after Acute Myocardial Infarction. Thromb. Haemost. 2021, 121, 1637–1649. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, S.; Wu, X.; Li, Y.; Han, X. Neutrophil extracellular traps in acute coronary syndrome. J. Inflamm. 2023, 20, 17. [Google Scholar] [CrossRef]
- Teh, B.W.; Reynolds, G.K.; Mikulska, M.; Mueller, N.J.; Slavin, M.A. Improving infection reporting in hematology treatment trials. Blood Adv. 2024, 8, 5925–5926. [Google Scholar] [CrossRef] [PubMed]
- Mourikis, P.; Polzin, A. Dual-Antiplatelet Therapy After Percutaneous Coronary Intervention: How Short Is Too Short? J. Am. Heart Assoc. 2023, 12, e028775. [Google Scholar] [CrossRef]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef]
- Antoniak, S.; Cardenas, J.C.; Buczek, L.J.; Church, F.C.; Mackman, N.; Pawlinski, R. Protease-Activated Receptor 1 Contributes to Angiotensin II-Induced Cardiovascular Remodeling and Inflammation. Cardiology 2016, 136, 258–268. [Google Scholar] [CrossRef]
- Sia, C.-H.; Tan, S.-H.; Chan, S.-P.; Marchesseau, S.; Sim, H.-W.; Carvalho, L.; Chen, R.; Amin, N.H.M.; Fong, A.Y.-Y.; Richards, A.M.; et al. Enhanced Thrombin Generation Is Associated with Worse Left Ventricular Scarring after ST-Segment Elevation Myocardial Infarction: A Cohort Study. Pharmaceuticals 2022, 15, 718. [Google Scholar] [CrossRef]
- Rohlfing, A.-K.; Kolb, K.; Sigle, M.; Ziegler, M.; Bild, A.; Münzer, P.; Sudmann, J.; Dicenta, V.; Harm, T.; Manke, M.-C.; et al. ACKR3 regulates platelet activation and ischemia-reperfusion tissue injury. Nat. Commun. 2022, 13, 1823. [Google Scholar] [CrossRef]
- Massalha, E.; Oren, D.; Goitein, O.; Brodov, Y.; Fardman, A.; Younis, A.; Berkovitch, A.; Raibman-Spector, S.; Konen, E.; Maor, E.; et al. Post–ST-Segment–Elevation Myocardial Infarction Platelet Reactivity Is Associated With the Extent of Microvascular Obstruction and Infarct Size as Determined by Cardiac Magnetic Resonance Imaging. J. Am. Heart Assoc. 2022, 11, e020973. [Google Scholar] [CrossRef]
- Denorme, F.; Portier, I.; Rustad, J.L.; Cody, M.J.; de Araujo, C.V.; Hoki, C.; Alexander, M.D.; Grandhi, R.; Dyer, M.R.; Neal, M.D. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, L.M.; Posch, F.; Martinod, K.; Grilz, E.; Däullary, T.; Hell, L.; Brostjan, C.; Zielinski, C.; Ay, C.; Wagner, D. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Ansari, J.; Senchenkova, E.Y.; Vital, S.A.; Al-Yafeai, Z.; Kaur, G.; Sparkenbaugh, E.M.; Orr, A.W.; Pawlinski, R.; Hebbel, R.P.; Granger, D.N. Targeting the AnxA1/Fpr2/ALX pathway regulates neutrophil function, promoting thromboinflammation resolution in sickle cell disease. Blood J. Am. Soc. Hematol. 2021, 137, 1538–1549. [Google Scholar] [CrossRef]
- Ansari, J.; Gavins, F.N.E. Neutrophils and Platelets: Immune Soldiers Fighting Together in Stroke Pathophysiology. Biomedicines 2021, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Sorvillo, N.; Mizurini, D.M.; Coxon, C.; Martinod, K.; Tilvawala, R.; Cherpokova, D.; Salinger, A.J.; Seward, R.J.; Staudinger, C.; Weerapana, E. Plasma peptidylarginine deiminase IV promotes VWF-platelet string formation and accelerates thrombosis after vessel injury. Circ. Res. 2019, 125, 507–519. [Google Scholar] [CrossRef]
- Claessens, L.A.; Wesselius, J.; van Lummel, M.; Laban, S.; Mulder, F.; Mul, D.; Nikolic, T.; Aanstoot, H.-J.; Koeleman, B.P.; Roep, B.O. Clinical and genetic correlates of islet-autoimmune signatures in juvenile-onset type 1 diabetes. Diabetologia 2020, 63, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, S.R.; Zhou, Z.; Lam, K.S.; Xu, A. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, H.; Mogi, K.; Yasuda, T.; Nakajima, T.; Nakashima, Y.; Mori, S.; Hoshino, T.; Kishi, K. Mammalian deoxyribonucleases I are classified into three types: Pancreas, parotid, and pancreas–parotid (mixed), based on differences in their tissue concentrations. Biochem. Biophys. Res. Commun. 2000, 269, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Popp, S.K.; Fukuda, R.; Parish, C.R.; Bosi, E.; Simeonovic, C.J. The Contribution of Neutrophils and NETs to the Development of Type 1 Diabetes. Front. Immunol. 2022, 13, 930553. [Google Scholar] [CrossRef] [PubMed]
- Popp, S.K.; Vecchio, F.; Brown, D.J.; Fukuda, R.; Suzuki, Y.; Takeda, Y.; Wakamatsu, R.; Sarma, M.A.; Garrett, J.; Giovenzana, A. Circulating platelet-neutrophil aggregates characterize the development of type 1 diabetes in humans and NOD mice. JCI Insight 2022, 7, e153993. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Buono, N.L.; Stabilini, A.; Nigi, L.; Dufort, M.J.; Geyer, S.; Rancoita, P.M.; Cugnata, F.; Mandelli, A.; Valle, A. Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Carestia, A.; Frechtel, G.; Cerrone, G.; Linari, M.A.; Gonzalez, C.D.; Casais, P.; Schattner, M. NETosis before and after Hyperglycemic Control in Type 2 Diabetes Mellitus Patients. PLoS ONE 2016, 11, e0168647. [Google Scholar] [CrossRef]
- Ibrahim, I.; Nuermaimaiti, Y.; Maimaituxun, G.; Luo, X.; Maimaituxun, M.; Akbar, A.; Tuerxun, K.; Wu, Y. Neutrophil Extracellular Traps (NETs) Are Associated with Type 2 Diabetes and Diabetic Foot Ulcer Related Amputation: A Prospective Cohort Study. Diabetes Ther. 2024, 15, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Bryk, A.H.; Prior, S.M.; Plens, K.; Konieczynska, M.; Hohendorff, J.; Malecki, M.T.; Butenas, S.; Undas, A. Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: Associations with a prothrombotic state and hypofibrinolysis. Cardiovasc. Diabetol. 2019, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Mulet, M.; Osuna-Gomez, R.; Zamora, C.; Artesero, I.; Arus, M.; Vera-Artazcoz, P.; Cordon, A.; Vilalta, N.; San-Jose, P.; Abril, A.; et al. Dysregulated neutrophil extracellular traps formation in sepsis. Immunology 2023, 170, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qi, H.; Kan, K.; Chen, J.; Xie, H.; Guo, X.; Zhang, L. Neutrophil Extracellular Traps Promote Hypercoagulability in Patients with Sepsis. Shock 2017, 47, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Folco, E.J.; Mawson, T.L.; Vromman, A.; Bernardes-Souza, B.; Franck, G.; Persson, O.; Nakamura, M.; Newton, G.; Luscinskas, F.W.; Libby, P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1alpha and Cathepsin G. Arter. Thromb. Vasc. Biol. 2018, 38, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Zhao, M.H.; Chen, M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 2015, 181, 518–527. [Google Scholar] [CrossRef]
- Yuen, J.; Pluthero, F.G.; Douda, D.N.; Riedl, M.; Cherry, A.; Ulanova, M.; Kahr, W.H.; Palaniyar, N.; Licht, C. NETosing Neutrophils Activate Complement Both on Their Own NETs and Bacteria via Alternative and Non-alternative Pathways. Front. Immunol. 2016, 7, 137. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Qu, M.; Yu, Y.; Chen, Z.; Zhu, S.; Guo, K.; Chen, W.; Miao, C. Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury. Front. Cell. Infect. Microbiol. 2021, 11, 677902. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, X.; Chatterjee, V.; Meegan, J.E.; Beard, R.S., Jr.; Yuan, S.Y. Role of Neutrophil Extracellular Traps and Vesicles in Regulating Vascular Endothelial Permeability. Front. Immunol. 2019, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Ye, X.; Xu, H.; Liu, S.F. Activation of endothelial intrinsic NF-kappaB pathway impairs protein C anticoagulation mechanism and promotes coagulation in endotoxemic mice. Blood 2009, 114, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Paunel-Gorgulu, A.; Flohe, S.; Hoffmann, A.; Witte, I.; MacKenzie, C.; Baldus, S.E.; Windolf, J.; Logters, T.T. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit Care 2012, 16, R137. [Google Scholar] [CrossRef]
- McDonald, B.; Davis, R.P.; Kim, S.J.; Tse, M.; Esmon, C.T.; Kolaczkowska, E.; Jenne, C.N. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 2017, 129, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Narasaraju, T.; Tang, B.M.; Herrmann, M.; Muller, S.; Chow, V.T.K.; Radic, M. Neutrophilia and NETopathy as Key Pathologic Drivers of Progressive Lung Impairment in Patients With COVID-19. Front. Pharmacol. 2020, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetite, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129. [Google Scholar] [CrossRef] [PubMed]
- Kvietys, P.R.; Fakhoury, H.M.A.; Kadan, S.; Yaqinuddin, A.; Al-Mutairy, E.; Al-Kattan, K. COVID-19: Lung-Centric Immunothrombosis. Front. Cell. Infect. Microbiol. 2021, 11, 679878. [Google Scholar] [CrossRef]
- Strich, J.R.; Ramos-Benitez, M.J.; Randazzo, D.; Stein, S.R.; Babyak, A.; Davey, R.T.; Suffredini, A.F.; Childs, R.W.; Chertow, D.S. Fostamatinib Inhibits Neutrophils Extracellular Traps Induced by COVID-19 Patient Plasma: A Potential Therapeutic. J. Infect Dis. 2021, 223, 981–984. [Google Scholar] [CrossRef]
- Delaveris, C.S.; Wilk, A.J.; Riley, N.M.; Stark, J.C.; Yang, S.S.; Rogers, A.J.; Ranganath, T.; Nadeau, K.C.; Stanford, C.-B.; Blish, C.A.; et al. Synthetic Siglec-9 Agonists Inhibit Neutrophil Activation Associated with COVID-19. ACS Cent Sci. 2021, 7, 650–657. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, H.H.; Park, W.; Kim, H.; Jang, J.G.; Hong, K.S.; Lee, J.Y.; Seo, H.S.; Na, D.H.; Kim, T.H.; et al. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials 2021, 267, 120389. [Google Scholar] [CrossRef]
- Sutanto, H.; Soegiarto, G. Risk of Thrombosis during and after a SARS-CoV-2 Infection: Pathogenesis, Diagnostic Approach, and Management. Hematol. Rep. 2023, 15, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, H.; Zhang, C.; Chen, Z.; Liu, H.; Lei, F.; Qin, J.-J.; Liu, Y.-M.; Zhou, F.; Song, X. The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metab. 2021, 33, 258–269.e3. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Hellebrekers, P.; Chen, N.; van Aalst, C.; Bongers, S.; Hietbrink, F.; Koenderman, L.; Vrisekoop, N. On the origin of low-density neutrophils. J. Leukoc. Biol. 2020, 107, 809–818. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Liu, X. NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond. Front. Immunol. 2022, 13, 838011. [Google Scholar] [CrossRef]
- Cabrera, L.E.; Pekkarinen, P.T.; Alander, M.; Nowlan, K.H.; Nguyen, N.A.; Jokiranta, S.; Kuivanen, S.; Patjas, A.; Mero, S.; Pakkanen, S.H. Characterization of low-density granulocytes in COVID-19. PLoS Pathog. 2021, 17, e1009721. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, J.; Absalón-Aguilar, A.; Nuñez-Aguirre, M.; Pérez-Fragoso, A.; Carrillo-Vázquez, D.A.; Maravillas-Montero, J.L.; Mejía-Domínguez, N.R.; Llorente, L.; Alcalá-Carmona, B.; Lira-Luna, J. Neutrophil extracellular traps contribute to COVID-19 hyperinflammation and humoral autoimmunity. Cells 2021, 10, 2545. [Google Scholar] [CrossRef] [PubMed]
- Staats, L.A.; Pfeiffer, H.; Knopf, J.; Lindemann, A.; Fürst, J.; Kremer, A.E.; Hackstein, H.; Neurath, M.F.; Muñoz, L.E.; Achenbach, S. IgA2 antibodies against SARS-CoV-2 correlate with NET formation and fatal outcome in severely diseased COVID-19 patients. Cells 2020, 9, 2676. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef]
- Hammond, M.E.; Christensen, E.D.; Belenky, M.; Snow, G.L.; Shah, K.; Hammond, M.E.H. Evidence of autoinflammation as a principal mechanism of myocardial injury in SARS-CoV-2 PCR-positive medical examiner cases. Diagn. Pathol. 2023, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.; Uzun, G.; Zlamal, J.; Singh, A.; Bakchoul, T. Platelet-neutrophil interaction in COVID-19 and vaccine-induced thrombotic thrombocytopenia. Front. Immunol. 2023, 14, 1186000. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, S.; Filipczak, N.; Yalamarty, S.S.K.; Shang, H.; Zhang, J.; Zheng, Q. Role and Therapeutic Targeting Strategies of Neutrophil Extracellular Traps in Inflammation. Int. J. Nanomed. 2023, 18, 5265–5287. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm (2020) 2022, 3, e162. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.; Muller, H.S.; Martins, V.P. Unweaving the NET: Microbial strategies for neutrophil extracellular trap evasion. Microb. Pathog. 2022, 171, 105728. [Google Scholar] [CrossRef] [PubMed]
- Oswal, M.; Varghese, R.; Zagade, T.; Dhatrak, C.; Sharma, R.; Kumar, D. Dietary supplements and medicinal plants in urolithiasis: Diet, prevention, and cure. J. Pharm. Pharmacol. 2023, 75, 719–745. [Google Scholar] [CrossRef]
- Karati, D.; Varghese, R.; Mahadik, K.R.; Sharma, R.; Kumar, D. Plant Bioactives in the Treatment of Inflammation of Skeletal Muscles: A Molecular Perspective. Evid. Based Complement. Altern. Med. 2022, 2022, 4295802. [Google Scholar] [CrossRef]
- Sharma, R.; Jadhav, M.; Choudhary, N.; Kumar, A.; Rauf, A.; Gundamaraju, R.; AlAsmari, A.F.; Ali, N.; Singla, R.K.; Sharma, R.; et al. Deciphering the impact and mechanism of Trikatu, a spices-based formulation on alcoholic liver disease employing network pharmacology analysis and in vivo validation. Front. Nutr. 2022, 9, 1063118. [Google Scholar] [CrossRef]
- Hodson, M.E. Aerosolized Dornase Alfa (rhDNase) for Therapy of Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 1995, 151, S70–S74. [Google Scholar] [CrossRef]
- Davis, J.C., Jr.; Manzi, S.; Yarboro, C.; Rairie, J.; McInnes, I.; Averthelyi, D.; Sinicropi, D.; Hale, V.G.; Balow, J.; Austin, H.; et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 1999, 8, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Englert, H.; Göbel, J.; Khong, D.; Omidi, M.; Wolska, N.; Konrath, S.; Frye, M.; Mailer, R.K.; Beerens, M.; Gerwers, J.C.; et al. Targeting NETs using dual-active DNase1 variants. Front. Immunol. 2023, 14, 1181761. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Mohanty, T.; Karlsson, C.A.Q.; Khademi, S.M.H.; Malmström, E.; Frigyesi, A.; Nordenfelt, P.; Malmstrom, J.; Linder, A. Proteome Profiling of Recombinant DNase Therapy in Reducing NETs and Aiding Recovery in COVID-19 Patients. Mol. Cell Proteom. 2021, 20, 100113. [Google Scholar] [CrossRef] [PubMed]
- Veras, F.P.; Gomes, G.F.; Silva, B.M.S.; Caetité, D.B.; Almeida, C.J.L.R.; Silva, C.M.S.; Schneider, A.H.; Corneo, E.S.; Bonilha, C.S.; Batah, S.S.; et al. Targeting neutrophils extracellular traps (NETs) reduces multiple organ injury in a COVID-19 mouse model. Respir. Res. 2023, 24, 66. [Google Scholar] [CrossRef]
- Yadav, R.; Momin, A.; Godugu, C. DNase based therapeutic approaches for the treatment of NETosis related inflammatory diseases. Int. Immunopharmacol. 2023, 124, 110846. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef]
- Adrover, J.M.; Carrau, L.; Daßler-Plenker, J.; Bram, Y.; Chandar, V.; Houghton, S.; Redmond, D.; Merrill, J.R.; Shevik, M.; tenOever, B.R.; et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. JCI Insight 2022, 7, e157342. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, Y.; Chen, L.; Wang, Z.; Chen, J.; Ni, Q.; Guo, X.; Zhang, L.; Xue, G. Disulfiram accelerates diabetic foot ulcer healing by blocking NET formation via suppressing the NLRP3/Caspase-1/GSDMD pathway. Transl. Res. 2023, 254, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Choidas, A.; Burn, G.L.; Habenberger, P.; Di Lucrezia, R.; Kordes, S.; Menninger, S.; Eickhoff, J.; Nussbaumer, P.; Klebl, B. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6689. [Google Scholar] [CrossRef] [PubMed]
- Antonelou, M.; Michaëlsson, E.; Evans, R.D.R.; Wang, C.J.; Henderson, S.R.; Walker, L.S.K.; Unwin, R.J.; Salama, A.D. Therapeutic Myeloperoxidase Inhibition Attenuates Neutrophil Activation, ANCA-Mediated Endothelial Damage, and Crescentic GN. J. Am. Soc. Nephrol. 2020, 31, 350–364. [Google Scholar] [CrossRef]
- Hair, P.S.; Enos, A.I.; Krishna, N.K.; Cunnion, K.M. Inhibition of Immune Complex Complement Activation and Neutrophil Extracellular Trap Formation by Peptide Inhibitor of Complement C1. Front. Immunol. 2018, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Krishna, N.K.; Cunnion, K.M.; Parker, G.A. The EPICC Family of Anti-Inflammatory Peptides: Next Generation Peptides, Additional Mechanisms of Action, and In Vivo and Ex Vivo Efficacy. Front. Immunol. 2022, 13, 752315. [Google Scholar] [CrossRef] [PubMed]
- Cunnion, K.; Goss, J.; Hair, P.; Dell, L.; Roberson, D.; Thienel, U.; Müller, M.; Carstensen-Aurèche, S.; Badorrek, P.; Holz, O.; et al. RLS-0071, a novel anti-inflammatory agent, significantly reduced inflammatory biomarkers in a randomised human evaluation of mechanisms and safety study. ERJ Open Res. 2024, 10, 01006. [Google Scholar] [CrossRef] [PubMed]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Chen, P.-J.; Chang, S.-H.; Weng, Y.-T.; Chang, F.-R.; Chang, K.-Y.; Chen, C.-Y.; Kao, T.-I.; Hwang, T.-L. Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biochem. Pharmacol. 2018, 154, 384–396. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; Vigili de Kreutzeberg, S.; Avogaro, A.; et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018, 55, 593–601. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, M.; Pu, J.; Wang, T. Stachydrine exhibits a novel antiplatelet property and ameliorates platelet-mediated thrombo-inflammation. Biomed. Pharmacother. 2022, 152, 113184. [Google Scholar] [CrossRef]
- Sexton, T.R.; Zhang, G.; Macaulay, T.E.; Callahan, L.A.; Charnigo, R.; Vsevolozhskaya, O.A.; Li, Z.; Smyth, S. Ticagrelor Reduces Thromboinflammatory Markers in Patients With Pneumonia. JACC Basic Transl. Sci. 2018, 3, 435–449. [Google Scholar] [CrossRef]
- Mitsios, A.; Chrysanthopoulou, A.; Arampatzioglou, A.; Angelidou, I.; Vidali, V.; Ritis, K.; Skendros, P.; Stakos, D. Ticagrelor Exerts Immune-Modulatory Effect by Attenuating Neutrophil Extracellular Traps. Int. J. Mol. Sci. 2020, 21, 3625. [Google Scholar] [CrossRef]
- Keuters, M.H.; Keksa-Goldsteine, V.; Rõlova, T.; Jaronen, M.; Kettunen, P.; Halkoluoto, A.; Goldsteins, G.; Koistinaho, J.; Dhungana, H. Benserazide is neuroprotective and improves functional recovery after experimental ischemic stroke by altering the immune response. Sci. Rep. 2024, 14, 17949. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Ding, Z.; Rönnow, C.F.; Rahman, M.; Schiopu, A.; Thorlacius, H. S100A9 induces reactive oxygen species-dependent formation of neutrophil extracellular traps in abdominal sepsis. Exp. Cell Res. 2022, 421, 113405. [Google Scholar] [CrossRef]
- Keir, H.R.; Shoemark, A.; Dicker, A.J.; Perea, L.; Pollock, J.; Giam, Y.H.; Suarez-Cuartin, G.; Crichton, M.L.; Lonergan, M.; Oriano, M.; et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: An international, observational, multicohort study. Lancet Respir. Med. 2021, 9, 873–884. [Google Scholar] [CrossRef]
- Garcia Vazquez, E.; Mensa, J.; Martinez, J.A.; Marcos, M.A.; Puig, J.; Ortega, M.; Torres, A. Lower mortality among patients with community-acquired pneumonia treated with a macrolide plus a beta-lactam agent versus a beta-lactam agent alone. Eur. J. Clin. Microbiol Infect Dis. 2005, 24, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Boone, B.A.; Murthy, P.; Miller-Ocuin, J.; Doerfler, W.R.; Ellis, J.T.; Liang, X.; Ross, M.A.; Wallace, C.T.; Sperry, J.L.; Lotze, M.T.; et al. Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer 2018, 18, 678. [Google Scholar] [CrossRef]
- Eisen, D.P.; Leder, K.; Woods, R.L.; Lockery, J.E.; McGuinness, S.L.; Wolfe, R.; Pilcher, D.; Moore, E.M.; Shastry, A.; Nelson, M.R.; et al. Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): A randomised, double-blind, placebo-controlled primary prevention trial. Lancet Respir. Med. 2021, 9, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.A.; Gandhi, A.A.; Meng, H.; Yalavarthi, S.; Vreede, A.P.; Estes, S.K.; Palmer, O.R.; Bockenstedt, P.L.; Pinsky, D.J.; Greve, J.M.; et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019, 10, 1916. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Liu, S.; Sun, J.; Chen, Z.; Jiang, M.; Zhang, Q.; Wei, Y.; Wang, X.; Huang, Y.Y.; et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B 2020, 10, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Holy, E.W.; Akhmedov, A.; Bonetti, N.R.; Nietlispach, F.; Matter, C.M.; Mach, F.; Montecucco, F.; Beer, J.H.; Paneni, F.; et al. Interleukin-1beta Mediates Arterial Thrombus Formation via NET-Associated Tissue Factor. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef]
- Ruiz-Limon, P.; Ladehesa-Pineda, M.L.; Castro-Villegas, M.D.C.; Abalos-Aguilera, M.D.C.; Lopez-Medina, C.; Lopez-Pedrera, C.; Barbarroja, N.; Espejo-Peralbo, D.; Gonzalez-Reyes, J.A.; Villalba, J.M.; et al. Enhanced NETosis generation in radiographic axial spondyloarthritis: Utility as biomarker for disease activity and anti-TNF-alpha therapy effectiveness. J. Biomed. Sci. 2020, 27, 54. [Google Scholar] [CrossRef]
- Perez-Sanchez, C.; Ruiz-Limon, P.; Aguirre, M.A.; Jimenez-Gomez, Y.; Arias-de la Rosa, I.; Abalos-Aguilera, M.C.; Rodriguez-Ariza, A.; Castro-Villegas, M.C.; Ortega-Castro, R.; Segui, P.; et al. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J. Autoimmun. 2017, 82, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Francois, B.; Fiancette, M.; Helms, J.; Mercier, E.; Lascarrou, J.B.; Kayanoki, T.; Tanaka, K.; Fineberg, D.; Vincent, J.L.; Wittebole, X. Efficacy and safety of human soluble thrombomodulin (ART-123) for treatment of patients in France with sepsis-associated coagulopathy: Post hoc analysis of SCARLET. Ann. Intensive Care 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Rietschel, E.; Ballmann, M.; Griese, M.; Worlitzsch, D.; Shute, J.; Chen, C.; Schink, T.; Doring, G.; van Koningsbruggen, S.; et al. Effect of treatment with dornase alpha on airway inflammation in patients with cystic fibrosis. Am. J. Respir. Crit Care Med. 2004, 169, 719–725. [Google Scholar] [CrossRef]
- Maki, C.; Inoue, Y.; Ishihara, T.; Hirano, Y.; Kondo, Y.; Sueyoshi, K.; Okamoto, K.; Tanaka, H. Evaluation of appropriate indications for the use of sivelestat sodium in acute respiratory distress syndrome: A retrospective cohort study. Acute Med. Surg. 2020, 7, e471. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sennett, C.; Pula, G. Trapped in the NETs: Multiple Roles of Platelets in the Vascular Complications Associated with Neutrophil Extracellular Traps. Cells 2025, 14, 335. https://doi.org/10.3390/cells14050335
Sennett C, Pula G. Trapped in the NETs: Multiple Roles of Platelets in the Vascular Complications Associated with Neutrophil Extracellular Traps. Cells. 2025; 14(5):335. https://doi.org/10.3390/cells14050335
Chicago/Turabian StyleSennett, Christopher, and Giordano Pula. 2025. "Trapped in the NETs: Multiple Roles of Platelets in the Vascular Complications Associated with Neutrophil Extracellular Traps" Cells 14, no. 5: 335. https://doi.org/10.3390/cells14050335
APA StyleSennett, C., & Pula, G. (2025). Trapped in the NETs: Multiple Roles of Platelets in the Vascular Complications Associated with Neutrophil Extracellular Traps. Cells, 14(5), 335. https://doi.org/10.3390/cells14050335




