Sex Differences in Circulating Inflammatory, Immune, and Tissue Growth Markers Associated with Fabry Disease-Related Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection and Storage
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
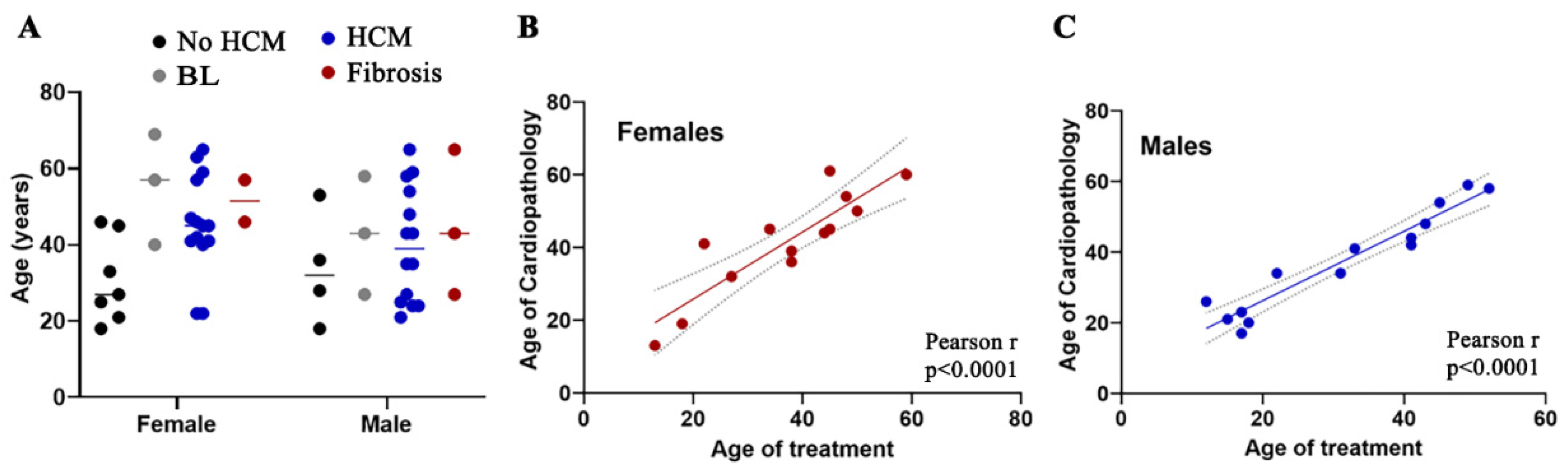
3.1. Early Onset of HCM Is Frequently Encountered in Female Patients with FD
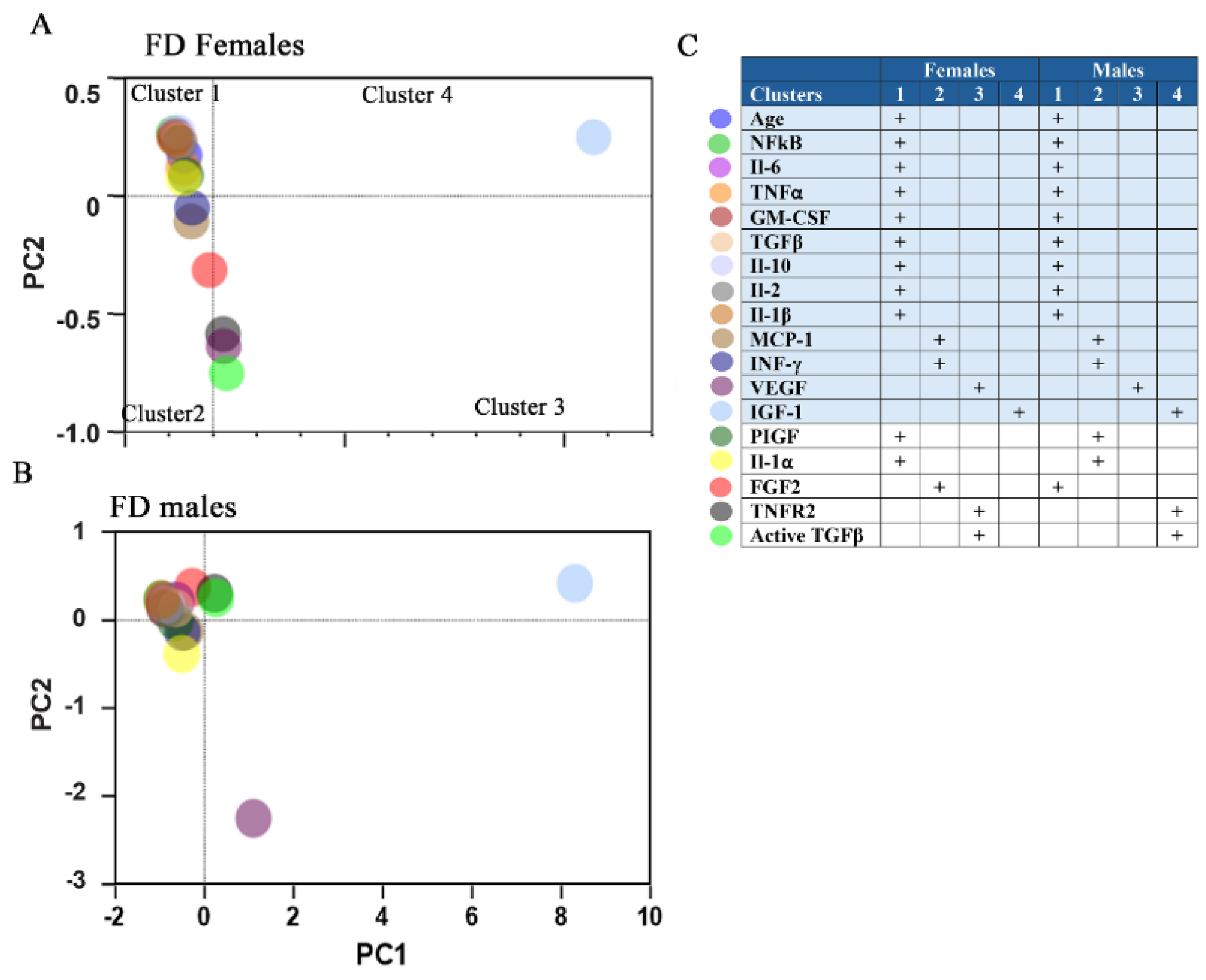
3.2. Principal Component Analysis (PCA) Reveals a Distinct Separation of Inflammatory Biomarkers and Growth Factors Between Male and Female Patients with FD
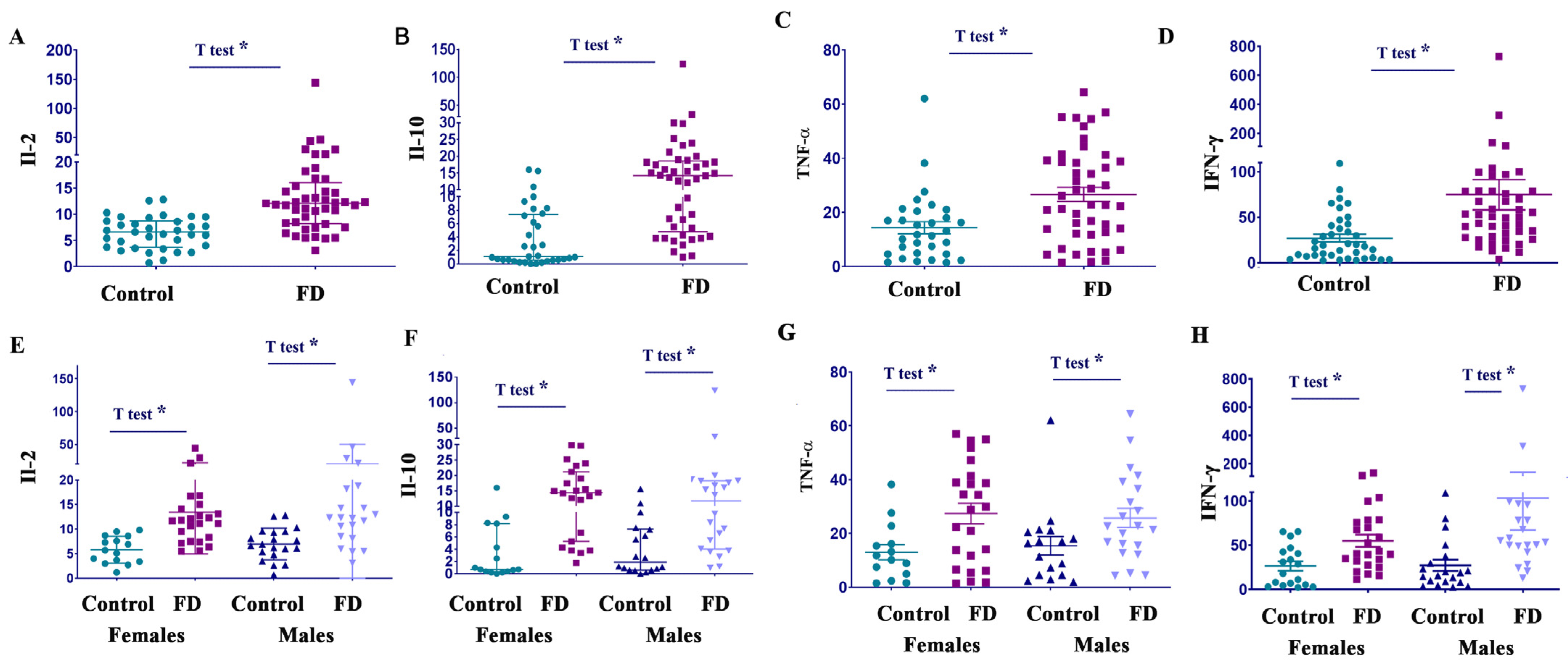
3.3. Il-2, Il-10, TNF-α, and IFN-γ Are Elevated in Both Female and Male Patients with FD
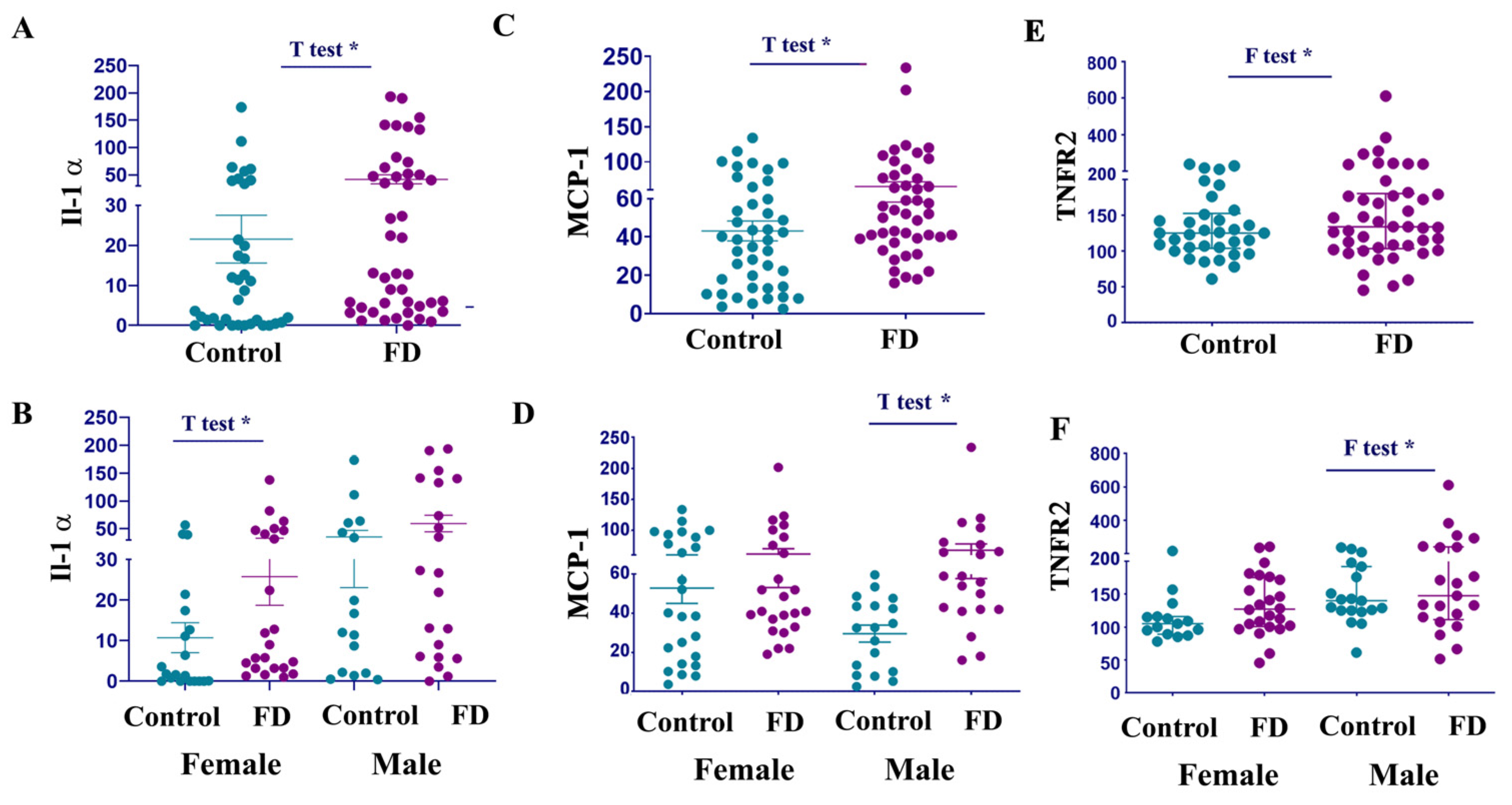
3.4. IL-1α Levels Are Elevated in Female Patients with FD
3.5. MCP-1 and TNFR2 Are Elevated in Males with FD
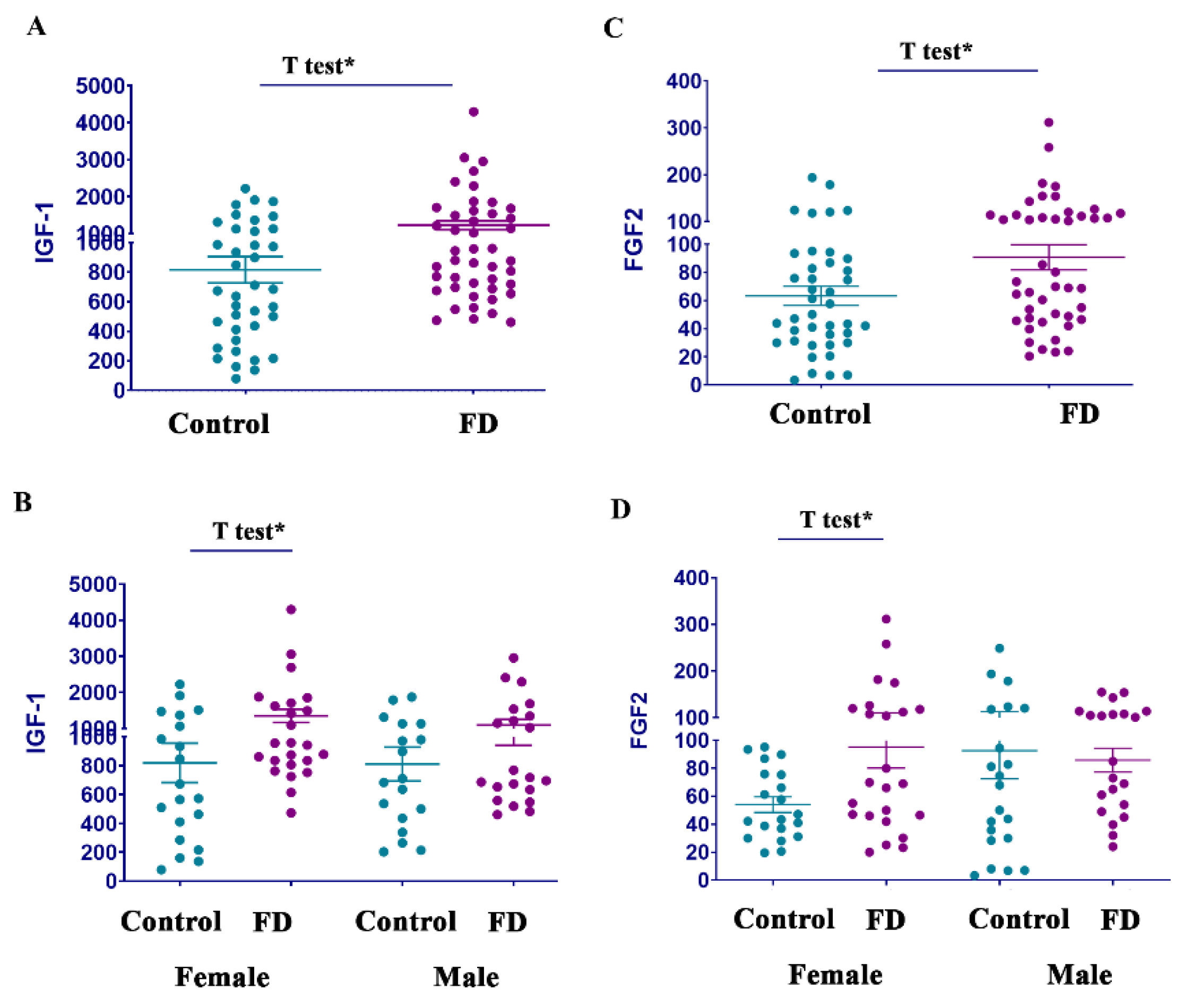
3.6. Growth Factors FGF2 and IGF-1, but Not PIGF, Are Elevated in FD
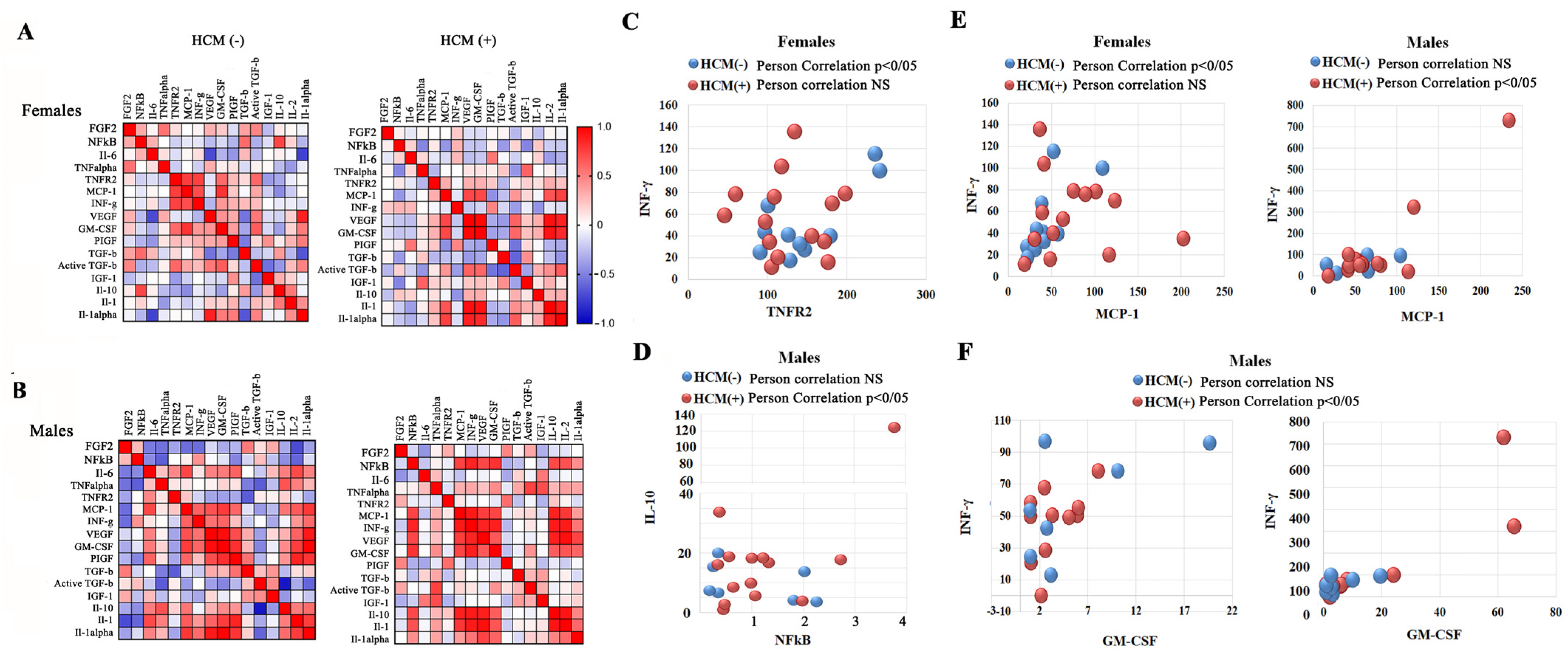
3.7. Associations of Inflammatory Biomarkers and Growth Factors with HCM in FD
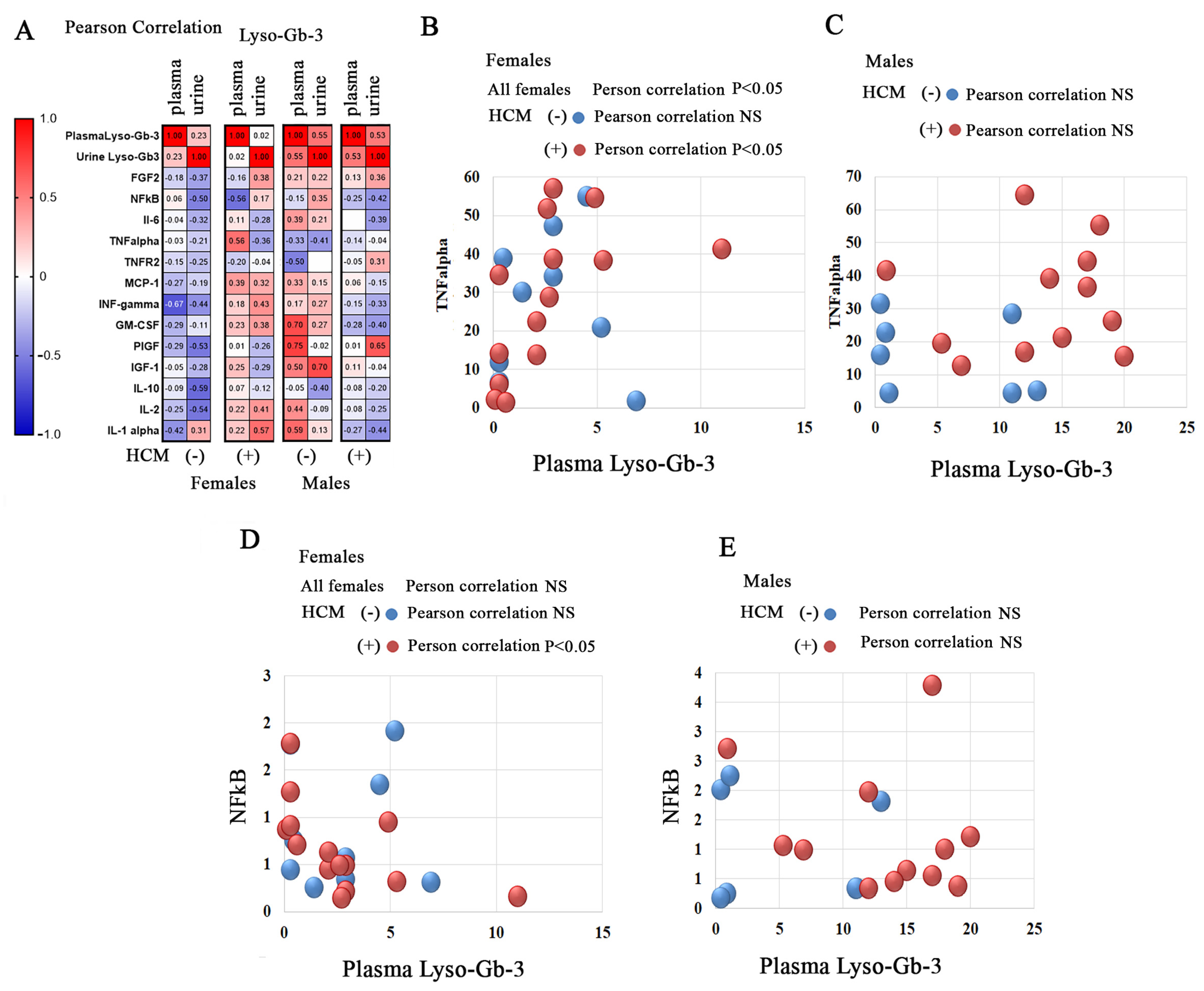
3.8. Correlation of Lyso-Gb-3 and Inflammatory Biomarkers or Growth Factors in FD Patients with Cardiac Involvement
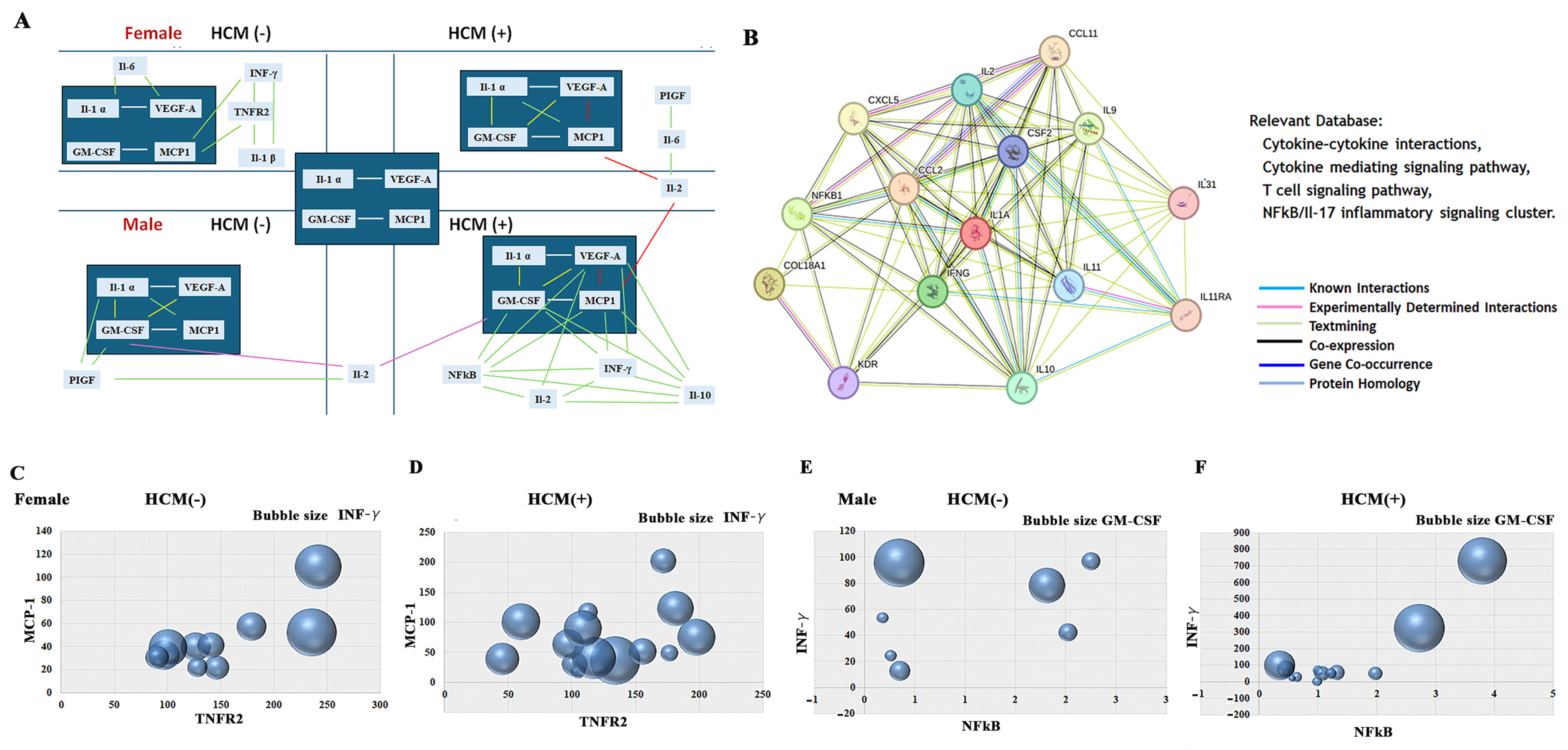
3.9. Sex-Related Association Between HCM and the NF-kB-INFγ Pathways, and TNFα-TNFR2 Signaling Cluster
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IL-2 | Interleukin-2 |
| IL-10 | Interleukin-10 |
| IFN-γ | Interferon gamma |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1α | Interleukin-1 alpha |
| MCP-1 | Monocyte chemoattractant protein-1, chemokine ligand 2 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| TNFR2 | Tumor necrosis factor receptor 2 |
| IL-6 | Interleukin-6 |
| NF-kB | Nuclear factor-kappa B |
| IL-1β | Interleukin-1 betta |
| IGF-1 | Insulin-like growth factor 1 |
| FGF2 | Fibroblast growth factor 2 |
| PIGF | Placental growth factor |
References
- Germain, D.P.; Shabbeer, J.; Cotigny, S.; Desnick, R.J. Fabry disease: Twenty novel alpha-galactosidase A mutations and genotype-phenotype correlations in classical and variant phenotypes. Mol. Med. 2002, 8, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Lerario, S.; Monti, L.; Ambrosetti, I.; Luglio, A.; Pietra, A.; Aiello, V.; Montanari, F.; Bellasi, A.; Zaza, G.; Galante, A.; et al. Fabry disease: A rare disorder calling for personalized medicine. Int. Urol. Nephrol. 2024, 56, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, T.; White, J.A.; Fine, N.M. Anderson-Fabry disease cardiomyopathy: An update on epidemiology, diagnostic approach, management and monitoring strategies. Front. Cardiovasc. Med. 2023, 10, 1152568. [Google Scholar] [CrossRef]
- Echevarria, L.; Benistan, K.; Toussaint, A.; Dubourg, O.; Hagege, A.A.; Eladari, D.; Jabbour, F.; Beldjord, C.; De Mazancourt, P.; Germain, D.P. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016, 89, 44–54. [Google Scholar] [CrossRef]
- Dobrovolny, R.; Dvorakova, L.; Ledvinova, J.; Magage, S.; Bultas, J.; Lubanda, J.C.; Elleder, M.; Karetova, D.; Pavlikova, M.; Hrebicek, M. Relationship between X-inactivation and clinical involvement in Fabry heterozygotes. Eleven novel mutations in the alpha-galactosidase A gene in the Czech and Slovak population. J. Mol. Med. 2005, 83, 647–654. [Google Scholar] [CrossRef]
- Germain, D.P.; Altarescu, G.; Barriales-Villa, R.; Mignani, R.; Pawlaczyk, K.; Pieruzzi, F.; Terryn, W.; Vujkovac, B.; Ortiz, A. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol. Genet. Metab. 2022, 137, 49–61. [Google Scholar] [CrossRef]
- Kolter, T.; Sandhoff, K. Sphingolipid metabolism diseases. Biochim. Biophys. Acta 2006, 1758, 2057–2079. [Google Scholar] [CrossRef]
- van Eijk, M.; Ferraz, M.J.; Boot, R.G.; Aerts, J. Lyso-glycosphingolipids: Presence and consequences. Essays Biochem. 2020, 64, 565–578. [Google Scholar] [CrossRef]
- Machann, W.; Breunig, F.; Weidemann, F.; Sandstede, J.; Hahn, D.; Kostler, H.; Neubauer, S.; Wanner, C.; Beer, M. Cardiac energy metabolism is disturbed in Fabry disease and improves with enzyme replacement therapy using recombinant human galactosidase A. Eur. J. Heart Fail. 2011, 13, 278–283. [Google Scholar] [CrossRef]
- Rozenfeld, P.; Feriozzi, S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol. Genet. Metab. 2017, 122, 19–27. [Google Scholar] [CrossRef]
- Mauhin, W.; Lidove, O.; Masat, E.; Mingozzi, F.; Mariampillai, K.; Ziza, J.M.; Benveniste, O. Innate and Adaptive Immune Response in Fabry Disease. JIMD Rep. 2015, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.M.; Dao, J.; Slayeh, O.A.; Friedman, A.; Goker-Alpan, O. Circulated TGF-beta1 and VEGF-A as Biomarkers for Fabry Disease-Associated Cardiomyopathy. Cells 2023, 12, 2102. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Vernon, J.; Kopach, O.; Minett, M.S.; Mills, K.; Clayton, P.T.; Meert, T.; Wood, J.N. The Fabry disease-associated lipid Lyso-Gb3 enhances voltage-gated calcium currents in sensory neurons and causes pain. Neurosci. Lett. 2015, 594, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, H.; Lavalle, L.; Moon, J.C.C.; Hughes, D. Inflammation in Fabry disease: Stages, molecular pathways, and therapeutic implications. Front. Cardiovasc. Med. 2024, 11, 1420067. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Nishikawa, K.; Yoshida, M.; Kusuhara, M.; Ishigami, N.; Isoda, K.; Miyazaki, K.; Ohsuzu, F. Left ventricular hypertrophy in mice with a cardiac-specific overexpression of interleukin-1. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H176–H183. [Google Scholar] [CrossRef]
- Honsho, S.; Nishikawa, S.; Amano, K.; Zen, K.; Adachi, Y.; Kishita, E.; Matsui, A.; Katsume, A.; Yamaguchi, S.; Nishikawa, K.; et al. Pressure-mediated hypertrophy and mechanical stretch induces IL-1 release and subsequent IGF-1 generation to maintain compensative hypertrophy by affecting Akt and JNK pathways. Circ. Res. 2009, 105, 1149–1158. [Google Scholar] [CrossRef]
- Fang, L.; Ellims, A.H.; Beale, A.L.; Taylor, A.J.; Murphy, A.; Dart, A.M. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am. J. Transl. Res. 2017, 9, 5063–5073. [Google Scholar]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Musigk, N.; Heidecker, B.; Lyle, M.A.; Cooper, L.T., Jr.; Bruno, K.A. Sex and gender differences in myocarditis and dilated cardiomyopathy: An update. Front. Cardiovasc. Med. 2023, 10, 1129348. [Google Scholar] [CrossRef]
- Olivotto, I.; Maron, M.S.; Adabag, A.S.; Casey, S.A.; Vargiu, D.; Link, M.S.; Udelson, J.E.; Cecchi, F.; Maron, B.J. Gender-Related Differences in the Clinical Presentation and Outcome of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 480–487. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Beale, A.; Martyn, T.; Agarwal, A.; Elkayam, U.; Lam, C.S.P.; Hsich, E. Heart Failure Subtypes and Cardiomyopathies in Women. Circ. Res. 2022, 130, 436–454. [Google Scholar] [CrossRef]
- Niemann, M.; Herrmann, S.; Hu, K.; Breunig, F.; Strotmann, J.; Beer, M.; Machann, W.; Voelker, W.; Ertl, G.; Wanner, C.; et al. Differences in Fabry cardiomyopathy between female and male patients: Consequences for diagnostic assessment. JACC Cardiovasc. Imaging 2011, 4, 592–601. [Google Scholar] [CrossRef]
- Geske, J.B.; Ong, K.C.; Siontis, K.C.; Hebl, V.B.; Ackerman, M.J.; Hodge, D.O.; Miller, V.M.; Nishimura, R.A.; Oh, J.K.; Schaff, H.V.; et al. Women with hypertrophic cardiomyopathy have worse survival. Eur. Heart J. 2017, 38, 3434–3440. [Google Scholar] [CrossRef]
- Butters, A.; Lakdawala, N.K.; Ingles, J. Sex Differences in Hypertrophic Cardiomyopathy: Interaction With Genetics and Environment. Curr. Heart Fail. Rep. 2021, 18, 264–273. [Google Scholar] [CrossRef]
- Eng, C.M.; Fletcher, J.; Wilcox, W.R.; Waldek, S.; Scott, C.R.; Sillence, D.O.; Breunig, F.; Charrow, J.; Germain, D.P.; Nicholls, K.; et al. Fabry disease: Baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J. Inherit. Metab. Dis. 2007, 30, 184–192. [Google Scholar] [CrossRef]
- Azevedo, O.; Gago, M.F.; Miltenberger-Miltenyi, G.; Robles, A.R.; Costa, M.A.; Pereira, O.; Vide, A.T.; Castelo Branco, G.; Simoes, S.; Guimaraes, M.J.; et al. Natural history of the late-onset phenotype of Fabry disease due to the p.F113L mutation. Mol. Genet. Metab. Rep. 2020, 22, 100565. [Google Scholar] [CrossRef]
- Uceyler, N.; Urlaub, D.; Mayer, C.; Uehlein, S.; Held, M.; Sommer, C. Tumor necrosis factor-alpha links heat and inflammation with Fabry pain. Mol. Genet. Metab. 2019, 127, 200–206. [Google Scholar] [CrossRef]
- Rosa Neto, N.S.; Bento, J.C.B.; Caparbo, V.F.; Pereira, R.M.R. Increased Serum Interleukin-6 and Tumor Necrosis Factor Alpha Levels in Fabry Disease: Correlation with Disease Burden. Clinics 2021, 76, e2643. [Google Scholar] [CrossRef]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Broderick, L.; Hoffman, H.M. IL-1 and autoinflammatory disease: Biology, pathogenesis and therapeutic targeting. Nat. Rev. Rheumatol. 2022, 18, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef] [PubMed]
- Semino, C.; Carta, S.; Gattorno, M.; Sitia, R.; Rubartelli, A. Progressive waves of IL-1β release by primary human monocytes via sequential activation of vesicular and gasdermin D-mediated secretory pathways. Cell Death Dis. 2018, 9, 1088. [Google Scholar] [CrossRef]
- van Deuren, M.; van der Ven-Jongekrijg, J.; Vannier, E.; van Dalen, R.; Pesman, G.; Bartelink, A.K.M.; Dinarello, C.A.; van der Meer, J.W.M. The Pattern of Interleukin-1β (IL-1β) and Its Modulating Agents IL-1 Receptor Antagonist and IL-1 Soluble Receptor Type II in Acute Meningococcal Infections. Blood 1997, 90, 1101–1108. [Google Scholar] [CrossRef]
- Georgakis, M.K.; de Lemos, J.A.; Ayers, C.; Wang, B.; Bjorkbacka, H.; Pana, T.A.; Thorand, B.; Sun, C.; Fani, L.; Malik, R.; et al. Association of Circulating Monocyte Chemoattractant Protein-1 Levels With Cardiovascular Mortality: A Meta-analysis of Population-Based Studies. JAMA Cardiol. 2021, 6, 587–592. [Google Scholar] [CrossRef]
- Chen, K.H.; Chien, Y.; Wang, K.L.; Leu, H.B.; Hsiao, C.Y.; Lai, Y.H.; Wang, C.Y.; Chang, Y.L.; Lin, S.J.; Niu, D.M.; et al. Evaluation of Proinflammatory Prognostic Biomarkers for Fabry Cardiomyopathy With Enzyme Replacement Therapy. Can. J. Cardiol. 2016, 32, 1221.e1–1221.e9. [Google Scholar] [CrossRef]
- Yogasundaram, H.; Nikhanj, A.; Putko, B.N.; Boutin, M.; Jain-Ghai, S.; Khan, A.; Auray-Blais, C.; West, M.L.; Oudit, G.Y. Elevated Inflammatory Plasma Biomarkers in Patients With Fabry Disease: A Critical Link to Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e009098. [Google Scholar] [CrossRef]
- Khosravi, F.; Ahmadvand, N.; Bellusci, S.; Sauer, H. The Multifunctional Contribution of FGF Signaling to Cardiac Development, Homeostasis, Disease and Repair. Front. Cell Dev. Biol. 2021, 9, 672935. [Google Scholar] [CrossRef]
- Levstek, T.; Vujkovac, B.; Trebusak Podkrajsek, K. Biomarkers of Fabry Nephropathy: Review and Future Perspective. Genes 2020, 11, 1091. [Google Scholar] [CrossRef]
- Nowak, A.; Mechtler, T.P.; Desnick, R.J.; Kasper, D.C. Plasma LysoGb3: A useful biomarker for the diagnosis and treatment of Fabry disease heterozygotes. Mol. Genet. Metab. 2017, 120, 57–61. [Google Scholar] [CrossRef]
- Piccolo, S.; Casal, M.; Rossi, V.; Ferrigni, F.; Piccoli, A.; Bolzan, B.; Setti, M.; Butturini, C.; Benfari, G.; Ferrero, V.; et al. Ventricular arrhythmias and primary prevention of sudden cardiac death in Anderson-Fabry disease. Int. J. Cardiol. 2024, 415, 132444. [Google Scholar] [CrossRef] [PubMed]
- Paelinck, B.P.; Bondue, A.; Robyns, T.; Eyskens, F. Left ventricular hypertrophy: Do not forget Fabry disease. Diagnostic work-up and differential diagnosis. Acta Cardiol. 2024, 79, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Del Franco, A.; Iannaccone, G.; Meucci, M.C.; Lillo, R.; Cappelli, F.; Zocchi, C.; Pieroni, M.; Graziani, F.; Olivotto, I. Clinical staging of Anderson-Fabry cardiomyopathy: An operative proposal. Heart Fail. Rev. 2024, 29, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Heidary, S.; Patel, H.; Chung, J.; Yokota, H.; Gupta, S.N.; Bennett, M.V.; Katikireddy, C.; Nguyen, P.; Pauly, J.M.; Terashima, M.; et al. Quantitative tissue characterization of infarct core and border zone in patients with ischemic cardiomyopathy by magnetic resonance is associated with future cardiovascular events. J. Am. Coll. Cardiol. 2010, 55, 2762–2768. [Google Scholar] [CrossRef]
- Barbieri, A.; Bursi, F.; Mantovani, F.; Valenti, C.; Quaglia, M.; Berti, E.; Marino, M.; Modena, M.G. Left ventricular hypertrophy reclassification and death: Application of the Recommendation of the American Society of Echocardiography/European Association of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 109–117. [Google Scholar] [CrossRef]
- Militaru, S.; Ginghina, C.; Popescu, B.A.; Saftoiu, A.; Linhart, A.; Jurcut, R. Multimodality imaging in Fabry cardiomyopathy: From early diagnosis to therapeutic targets. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1313–1322. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Poggesi, C.; Yacoub, M.H. Patterns of disease progression in hypertrophic cardiomyopathy: An individualized approach to clinical staging. Circ. Heart Fail. 2012, 5, 535–546. [Google Scholar] [CrossRef]
- Deegan, P.B.; Bahner, F.; Barba, M.; Hughes, D.A.; Beck, M. Fabry disease in females: Clinical characteristics and effects of enzyme replacement therapy. In Fabry Disease: Perspectives from 5 Years of FOS; Mehta, A., Beck, M., Sunder-Plassmann, G., Eds.; Oxford PharmaGenesis Ltd.: Oxford, UK, 2006. [Google Scholar]
- Michaud, M.; Mauhin, W.; Belmatoug, N.; Garnotel, R.; Bedreddine, N.; Catros, F.; Ancellin, S.; Lidove, O.; Gaches, F. When and How to Diagnose Fabry Disease in Clinical Pratice. Am. J. Med. Sci. 2020, 360, 641–649. [Google Scholar] [CrossRef]
- Faro, D.C.; Losi, V.; Rodolico, M.S.; Torrisi, E.M.; Colomba, P.; Duro, G.; Monte, I.P. Sex Differences in Anderson-Fabry Cardiomyopathy: Clinical, Genetic, and Imaging Analysis in Women. Genes 2023, 14, 1804. [Google Scholar] [CrossRef]
- Izhar, R.; Borriello, M.; La Russa, A.; Di Paola, R.; De, A.; Capasso, G.; Ingrosso, D.; Perna, A.F.; Simeoni, M. Fabry Disease in Women: Genetic Basis, Available Biomarkers, and Clinical Manifestations. Genes 2024, 15, 37. [Google Scholar] [CrossRef]
- Beirao, I.; Cabrita, A.; Torres, M.; Silva, F.; Aguiar, P.; Laranjeira, F.; Gomes, A.M. Biomarkers and Imaging Findings of Anderson-Fabry Disease-What We Know Now. Diseases 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Núñez, A.; Pérez-Márquez, T.; Alves-Villar, M.; Fernández-Pereira, C.; Fernández-Martín, J.; Rivera-Gallego, A.; Melcón-Crespo, C.; San Millán-Tejado, B.; Ruz-Zafra, A.; Garofano-López, R.; et al. Inflammatory and Cardiovascular Biomarkers to Monitor Fabry Disease Progression. Int. J. Mol. Sci. 2024, 25, 6024. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; De Gori, C.; Faggioni, L.; Parisella, M.L.; Cioni, D.; Lencioni, R.; Neri, E. Diagnostic and prognostic role of late gadolinium enhancement in cardiomyopathies. Eur. Heart J. Suppl. 2023, 25, C130–C136. [Google Scholar] [CrossRef]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-α Signaling in Heart Disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef]
- Matsumori, A. Nuclear Factor-κB is a Prime Candidate for the Diagnosis and Control of Inflammatory Cardiovascular Disease. Eur. Cardiol. 2023, 18, e40. [Google Scholar] [CrossRef]
- De Francesco, P.N.; Mucci, J.M.; Ceci, R.; Fossati, C.A.; Rozenfeld, P.A. Fabry disease peripheral blood immune cells release inflammatory cytokines: Role of globotriaosylceramide. Mol. Genet. Metab. 2013, 109, 93–99. [Google Scholar] [CrossRef]
- Kuusisto, J.; Kärjä, V.; Sipola, P.; Kholová, I.; Peuhkurinen, K.; Jääskeläinen, P.; Naukkarinen, A.; Ylä-Herttuala, S.; Punnonen, K.; Laakso, M. Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart 2012, 98, 1007–1013. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Coelho-Ribeiro, B.; Silva, H.G.; Sampaio-Marques, B.; Fraga, A.G.; Azevedo, O.; Pedrosa, J.; Ludovico, P. Inflammation and Exosomes in Fabry Disease Pathogenesis. Cells 2024, 13, 654. [Google Scholar] [CrossRef]
- López-Valverde, L.; Vázquez-Mosquera, M.E.; Colón-Mejeras, C.; Bravo, S.B.; Barbosa-Gouveia, S.; Álvarez, J.V.; Sánchez-Martínez, R.; López-Mendoza, M.; López-Rodríguez, M.; Villacorta-Argüelles, E.; et al. Characterization of the plasma proteomic profile of Fabry disease: Potential sex- and clinical phenotype-specific biomarkers. Transl. Res. 2024, 269, 47–63. [Google Scholar] [CrossRef]
- Fang, J.Y.; Ayyadurai, S.; Pybus, A.F.; Sugimoto, H.; Qian, M.G. Exploring the diagnostic potential of miRNA signatures in the Fabry disease serum: A comparative study of automated and manual sample isolations. PLoS ONE 2024, 19, e0301733. [Google Scholar] [CrossRef] [PubMed]
- Maier, N.; Gatterer, C.; Haider, P.; Salzmann, M.; Kaun, C.; Speidl, W.S.; Sunder-Plassmann, G.; Podesser, B.K.; Wojta, J.; Graf, S.; et al. MiRNA Let-7a and Let-7d Are Induced by Globotriaosylceramide via NF-kB Activation in Fabry Disease. Genes 2021, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Salamon, I.; Biagini, E.; Kunderfranco, P.; Roncarati, R.; Ferracin, M.; Taglieri, N.; Nardi, E.; Laprovitera, N.; Tomasi, L.; Santostefano, M.; et al. Circulating miR-184 is a potential predictive biomarker of cardiac damage in Anderson-Fabry disease. Cell Death Dis. 2021, 12, 1150. [Google Scholar] [CrossRef]
- Han, M.; Zhang, D.; Ji, J.; Zhang, J.; Qin, M. Downregulating miR-184 relieves calcium oxalate crystal-mediated renal cell damage via activating the Rap1 signaling pathway. Aging 2023, 15, 14749–14763. [Google Scholar] [CrossRef]
- Chen, B. The miRNA-184 drives renal fibrosis by targeting HIF1AN in vitro and in vivo. Int. Urol. Nephrol. 2019, 51, 543–550. [Google Scholar] [CrossRef]
- Ivanova, M.M.; Changsila, E.; Iaonou, C.; Goker-Alpan, O. Impaired autophagic and mitochondrial functions are partially restored by ERT in Gaucher and Fabry diseases. PLoS ONE 2019, 14, e0210617. [Google Scholar] [CrossRef]
- Tebani, A.; Mauhin, W.; Abily-Donval, L.; Lesueur, C.; Berger, M.G.; Nadjar, Y.; Berger, J.; Benveniste, O.; Lamari, F.; Laforêt, P.; et al. A Proteomics-Based Analysis Reveals Predictive Biological Patterns in Fabry Disease. J. Clin. Med. 2020, 9, 1325. [Google Scholar] [CrossRef]
- Zampetti, A.; Gnarra, M.; Borsini, W.; Giurdanella, F.; Antuzzi, D.; Piras, A.; Smaldone, C.; Pieroni, M.; Cadeddu, C.; de Waure, C.; et al. Vascular endothelial growth factor (VEGF-a) in Fabry disease: Association with cutaneous and systemic manifestations with vascular involvement. Cytokine 2013, 61, 933–939. [Google Scholar] [CrossRef]
- Ivanova, M. Altered Sphingolipids Metabolism Damaged Mitochondrial Functions: Lessons Learned From Gaucher and Fabry Diseases. J. Clin. Med. 2020, 9, 1116. [Google Scholar] [CrossRef]
- Amodio, F.; Caiazza, M.; Monda, E.; Rubino, M.; Capodicasa, L.; Chiosi, F.; Simonelli, V.; Dongiglio, F.; Fimiani, F.; Pepe, N.; et al. An Overview of Molecular Mechanisms in Fabry Disease. Biomolecules 2022, 12, 1460. [Google Scholar] [CrossRef]
- Martin, T.G.; Leinwand, L.A. Hearts apart: Sex differences in cardiac remodeling in health and disease. J. Clin. Investig. 2024, 134, jci180074. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Barcena, M.L.; Jeuthe, S.; Niehues, M.H.; Pozdniakova, S.; Haritonow, N.; Kühl, A.A.; Messroghli, D.R.; Regitz-Zagrosek, V. Sex-Specific Differences of the Inflammatory State in Experimental Autoimmune Myocarditis. Front. Immunol. 2021, 12, 686384. [Google Scholar] [CrossRef]
- Di Risi, T.; Vinciguerra, R.; Cuomo, M.; Della Monica, R.; Riccio, E.; Cocozza, S.; Imbriaco, M.; Duro, G.; Pisani, A.; Chiariotti, L. DNA methylation impact on Fabry disease. Clin. Epigenetics 2021, 13, 24. [Google Scholar] [CrossRef]
- Hossain, M.A.; Yanagisawa, H.; Miyajima, T.; Wu, C.; Takamura, A.; Akiyama, K.; Itagaki, R.; Eto, K.; Iwamoto, T.; Yokoi, T.; et al. The severe clinical phenotype for a heterozygous Fabry female patient correlates to the methylation of non-mutated allele associated with chromosome 10q26 deletion syndrome. Mol. Genet. Metab. 2017, 120, 173–179. [Google Scholar] [CrossRef]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; van Roomen, C.; Mirzaian, M.; Wijburg, F.A.; Linthorst, G.E.; et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. USA 2008, 105, 2812–2817. [Google Scholar] [CrossRef]
- Frustaci, A.; Verardo, R.; Grande, C.; Galea, N.; Piselli, P.; Carbone, I.; Alfarano, M.; Russo, M.A.; Chimenti, C. Immune-Mediated Myocarditis in Fabry Disease Cardiomyopathy. J. Am. Heart Assoc. 2018, 7, e009052. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
| CNT vs. FD Females | CNT vs. FD Males | |||||
|---|---|---|---|---|---|---|
| CNT | FD | Statistic * | CNT | FD | Statistic * | |
| Il-2 | 5.8 ± 0.7 | 13.41 ± 1.1 | * p < 0.0005 | 6.9 ± 0.7 | 20.24 ± 6.2 | * p < 0.05 |
| Il-10 | 3.5 ± 1.2 | 14.3 ± 1.7 | * p < 0.0001 | 4.1 ± 1.1 | 16.1 ± 5.3 | * p < 0.05 |
| IFN-γ | 26.4 ± 5.4 | 55.0 ± 6.91 | * p < 0.001 | 27.3 ± 6.5 | 103 ± 36 | * p < 0.05 |
| TNF-α | 13.0 ± 2.8 | 27.3 ± 3.8 | * p < 0.01 | 15.0 ± 3.4 | 25.9 ± 3.5 | * p < 0.05 |
| IL-1α | 10.7 ± 3.6 | 25.8 ± 7.1 | * p < 0.05 | 35.1 ± 12 | 59.4 ± 14.9 | No |
| MCP-1 | 52.8 ± 7.7 | 61.9 ± 8.7 | p = 0.22 | 29.5 ± 4.3 | 68.05 ± 10.2 | * p = 0.001 |
| GM-CSF | 1.9 ± 0.2 | 2.5 ± 0.8 | # p < 0.0001 | 5.3 ± 1.6 | 11.0 ± 4.1 | # p < 0.0001 |
| TNFR2 | 113 ± 9 | 135.4 ± 10 | * p = 0.06 | 149.8 ± 10 | 191.8 ± 28 | # p < 0.0001 |
| Il-6 | 10.4 ± 1.5 | 7.8 ± 0.9 | p = 0.07 | 9.4 ± 1.3 | 11.4 ± 1.3 | p = 0.17 |
| NF-kB | 0.74 ± 0.1 | 0.76 ± 0.1 | p = 0.49 | 1.04 ± 0.2 | 1.14 ± 0.2 | p = 0.18 |
| IL-1β | 4.3 ± 1.2 | 4.2 ± 0.9 | p = 0.46 | 3.6 ± 0.9 | 3.06 ± 1.2 | p = 0.45 |
| IGF-1 | 819 ± 136 | 1350 ± 183 | * p < 0.05 | 811 ± 117 | 1096 ± 155 | p = 0.08 |
| FGF2 | 54.1 ± 5.5 | 95.2 ± 15.1 | * p < 0.05 | 78.9 ± 14.5 | 85.8 ± 8.5 | p = 0.32 |
| PIGF | 35.0 ± 4.6 | 30.7 ± 3.5 | p = 0.20 | 32.7 ± 6.1 | 38.3 ± 4.1 | p = 0.22 |
| Females | Males | |||||
|---|---|---|---|---|---|---|
| HCM(−) | HCM (+) | Statistic * | HCM(−) | HCM (+) | Statistic * | |
| Il-2 | 14.93+/−2.02 | 12.32+/−1.02 | p = 0.23 | 13.5+/−2.92 | 23.6+/−9.7 | # p = 0.001 |
| Il-10 | 13.7+/−2.9 | 13.9+/−2.3 | p = 0.48 | 10.1+/−2.4 | 21.1+/−8.3 | # p < 0.001 |
| IFN-γ | 50.9+/−10.4 | 57.9+/−9.4 | p = 0.31 | 57.8+/−12.7 | 128+/−54.5 | # p < 0.001 |
| TNF-α | 26.4+/−5.8 | 29.0+/−5.7 | p = 0.32 | 16.4+/−6.7 | 30.1+/−5.2 | p < 0.05 |
| IL-1α | 23.0+/−8.7 | 27.9+/−10.8 | p = 0.36 | 36.7+/−18.4 | 70.7+/−20 | p = 0.14 |
| MCP-1 | 44.7+/−8.06 | 74.2+/−13.2 | * p < 0.05 | 54.4+/−11.1 | 74.8+/−14.3 | p = 0.18 |
| GM-CSF | 1.5+/−0.3 | 3.3+/−1.4 | # p < 0.001 | 5.75+/−2.6 | 13.6+/−5.8 | # p < 0.001 |
| TNFR2 | 149 +/−17.2 | 126+/−12.2 | p = 0.14 | 139+/−31 | 218+/−37.8 | p = 0.09 |
| Il-6 | 6.8+/−1.1 | 8.5+/−1.1 | p = 0.2 | 7.2+/−0.6 | 13.6+/−1.7 | *p < 0.05 |
| NF-kB | 0.8+/−0.2 | 0.67+/−0.1 | p = 0.24 | 1.04+/−0.3 | 1.2+/−0.3 | p = 0.36 |
| IL-1β | 2.9+/−1.0 | 5.4+/−1.94 | # p < 0.05 | 3.9+/−3.1 | 2.6+/−0.9 | # p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, M.M.; Dao, J.; Friedman, A.; Kasaci, N.; Goker-Alpan, O. Sex Differences in Circulating Inflammatory, Immune, and Tissue Growth Markers Associated with Fabry Disease-Related Cardiomyopathy. Cells 2025, 14, 322. https://doi.org/10.3390/cells14050322
Ivanova MM, Dao J, Friedman A, Kasaci N, Goker-Alpan O. Sex Differences in Circulating Inflammatory, Immune, and Tissue Growth Markers Associated with Fabry Disease-Related Cardiomyopathy. Cells. 2025; 14(5):322. https://doi.org/10.3390/cells14050322
Chicago/Turabian StyleIvanova, Margarita M., Julia Dao, Andrew Friedman, Neil Kasaci, and Ozlem Goker-Alpan. 2025. "Sex Differences in Circulating Inflammatory, Immune, and Tissue Growth Markers Associated with Fabry Disease-Related Cardiomyopathy" Cells 14, no. 5: 322. https://doi.org/10.3390/cells14050322
APA StyleIvanova, M. M., Dao, J., Friedman, A., Kasaci, N., & Goker-Alpan, O. (2025). Sex Differences in Circulating Inflammatory, Immune, and Tissue Growth Markers Associated with Fabry Disease-Related Cardiomyopathy. Cells, 14(5), 322. https://doi.org/10.3390/cells14050322







