The Role of Perineuronal Nets in Physiology and Disease: Insights from Recent Studies
Abstract
1. Introduction
2. Distribution of PNNs in the Mammalian CNS
3. Molecular Structure of PNNs
4. Development and Plasticity
5. Function of PNNs
5.1. Stabilization of Synaptic Sites and Regulation of Synaptic Transmission
5.2. Regulation of Neural Plasticity
5.3. Protection Against Oxidative Stress
5.4. Regulation of Neural Circuity Activity
6. Scientific Methods to Study PNNs in the Mammalian Brain
6.1. Immunohistochemistry and Imaging Techniques
6.2. Knocking out Different PNN Components in Rodents
6.3. Enzymatic Degradation of PNN Components (ChABC, Hyaluronidase)
6.4. Proteomic Studies
7. PNNs and Their Implications for Neurological Disorders
7.1. Schizophrenia and Bipolar Disorder
7.2. Alzheimer’s Disease (AD)
7.3. Epilepsy
8. Therapeutic Potential and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PNN | Perineuronal net |
| CNS | Central nervous system |
| ECM | Extracellular matrix |
| PV+ | Parvalbumin expressing |
| GABA | Gamma-aminobutyric acid |
| HA | Hyaluronic acid |
| CSPG | Chondroitin sulfate proteoglycan |
| CA | Cornu ammonis |
| BA | Brodmann area |
| TNR | Tenascin R |
| CRTL1 | Cartilage link protein 1 |
| HAPLN | Hyaluronan and proteoglycan link protein |
| BRAL2 | Brain link protein 2 |
| CD | Cluster of differentiation |
| HAS | Hyaluronan synthase |
| GAG | Glycosaminoglycan |
| GlcA | Glucoronic acid |
| GalNAc | N-acetlyl-galactosamine |
| RPTP | Receptor protein tyrosine phosphatase |
| SEMA3A | Semaphorin 3A |
| OTX2 | Orthodenticle homeobox 2 |
| MMP | Matrix metalloproteinase |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| TNC | Tenascin C |
| TIMP | Tissue inhibitor of metalloproteinases |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| NMDAR | N-methyl-D-aspartate-receptor |
| LTP | Long-term potentiation |
| ROS | Reactive oxygen species |
| E/I | Excitatory/inhibitory |
| SZ | Schizophrenia |
| ASD | Autism spectrum disorder |
| BPD | Bipolar disorder |
| AD | Alzheimer’s disease |
| ChABC | Chondroitinase ABC |
| WFA | Wisteria floribunda agglutinin |
| TEM | Transmission electron microscopy |
| DAB | 3,3′-diaminobenzidine |
| IHC | Immunohistochemistry |
| IF | Immunofluorescence |
| DAPI | 4′,6-diamidin-2-phenylindol |
| Cmd | Cartilage matrix deficiency |
| ICC | Immunocytochemistry |
| KO | Knock-out |
| MNTB | Medial nucleus of the trapezoid body |
| MTLE | Mesial temporal lobe epilepsy |
| SE | Status epilepticus |
| TLE | Temporal lobe epilepsy |
References
- Nicholson, C.; Hrabětová, S. Brain Extracellular Space: The Final Frontier of Neuroscience. Biophys. J. 2017, 113, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Syková, E.; Nicholson, C. Diffusion in Brain Extracellular Space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef] [PubMed]
- Lehmenkühler, A.; Sykova, E.; Svoboda, Q.J.; Zilles, K.; Nicholson, C. Extracellular space parameters in the rat neocortex and subcortical white matter during postnatal development determined by diffusion analysis. Neuroscience 1993, 55, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Härtig, W.; Brauer, K.; Bigl, V.; Brückner, G. Chondroitin Sulfate Proteoglycan-Immunoreactivity of Lectin-Labeled Perineuronal Nets around Parvalbumin-Containing Neurons. Brain Res. 1994, 635, 307–311. [Google Scholar] [CrossRef]
- Härtig, W.; Brauer, K.; Brückner, G. Wisteria Floribunda Agglutinin-Labelled Nets Surround Parvalbumin-Containing Neurons. Neuroreport 1992, 3, 869–872. [Google Scholar] [CrossRef]
- Dityatev, A.; Schachner, M. Extracellular Matrix Molecules and Synaptic Plasticity. Nat. Rev. Neurosci. 2003, 4, 456–468. [Google Scholar] [CrossRef]
- Frischknecht, R.; Heine, M.; Perrais, D.; Seidenbecher, C.I.; Choquet, D.; Gundelfinger, E.D. Brain Extracellular Matrix Affects AMPA Receptor Lateral Mobility and Short-Term Synaptic Plasticity. Nat. Neurosci. 2009, 12, 897–904. [Google Scholar] [CrossRef]
- Sorg, B.A.; Berretta, S.; Blacktop, J.M.; Fawcett, J.W.; Kitagawa, H.; Kwok, J.C.F.; Miquel, M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef]
- Wingert, J.C.; Sorg, B.A. Impact of Perineuronal Nets on Electrophysiology of Parvalbumin Interneurons, Principal Neurons, and Brain Oscillations: A Review. Front. Synaptic Neurosci. 2021, 13, 673210. [Google Scholar] [CrossRef]
- Balmer, T.S. Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro 2016, 3, 745–751. [Google Scholar] [CrossRef]
- Cabungcal, J.H.; Steullet, P.; Morishita, H.; Kraftsik, R.; Cuenod, M.; Hensch, T.K.; Do, K.Q. Perineuronal Nets Protect Fast-Spiking Interneurons against Oxidative Stress. Proc. Natl. Acad. Sci. USA 2013, 110, 9130–9135. [Google Scholar] [CrossRef] [PubMed]
- Morawski, M.; Brückner, M.K.; Riederer, P.; Brückner, G.; Arendt, T. Perineuronal Nets Potentially Protect against Oxidative Stress. Exp. Neurol. 2004, 188, 309–315. [Google Scholar] [CrossRef]
- Wen, T.H.; Binder, D.K.; Ethell, I.M.; Razak, K.A. The Perineuronal ‘Safety’ Net? Perineuronal Net Abnormalities in Neurological Disorders. Front. Mol. Neurosci. 2018, 11, 270. [Google Scholar] [CrossRef]
- Chaunsali, L.; Tewari, B.P.; Sontheimer, H. Perineuronal Net Dynamics in the Pathophysiology of Epilepsy. Epilepsy Curr. 2021, 21, 273–281. [Google Scholar] [CrossRef]
- Scarlett, J.M.; Hu, S.J.; Alonge, K.M. The “Loss” of Perineuronal Nets in Alzheimer’s Disease: Missing or Hiding in Plain Sight? Front. Integr. Neurosci. 2022, 16, 896400. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Berretta, S. In Sickness and in Health: Perineuronal Nets and Synaptic Plasticity in Psychiatric Disorders. Neural Plast. 2016, 2016, 9847696. [Google Scholar] [CrossRef]
- Bertolotto, A.; Manzardo, E.; Guglielmone, R. Immunohistochemical Mapping of Perineuronal Nets Containing Chondroitin Unsulfate Proteoglycan in the Rat Central Nervous System. Cell Tissue Res. 1996, 283, 283–295. [Google Scholar] [CrossRef]
- Lensjø, K.K.; Christensen, A.C.; Tennøe, S.; Fyhn, M.; Hafting, T. Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Lupori, L.; Totaro, V.; Cornuti, S.; Ciampi, L.; Carrara, F.; Grilli, E.; Viglione, A.; Tozzi, F.; Putignano, E.; Mazziotti, R.; et al. A Comprehensive Atlas of Perineuronal Net Distribution and Colocalization with Parvalbumin in the Adult Mouse Brain. Cell Rep. 2023, 42, 112788. [Google Scholar] [CrossRef]
- Carstens, K.E.; Phillips, M.L.; Pozzo-Miller, L.; Weinberg, R.J.; Dudek, S.M. Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons. J. Neurosci. 2016, 36, 6312–6320. [Google Scholar] [CrossRef]
- Yamada, J.; Jinno, S. Molecular Heterogeneity of Aggrecan-Based Perineuronal Nets around Five Subclasses of Parvalbumin-Expressing Neurons in the Mouse Hippocampus. J. Comp. Neurol. 2017, 525, 1234–1249. [Google Scholar] [CrossRef] [PubMed]
- Hausen, D.; Brückner, G.; Drlicek, M.; Härtig, W.; Brauer, K.; Bigl, V. Pyramidal Cells Ensheathed by Perineuronal Nets in Human Motor and Somatosensory Cortex. Neurochemistry 1996, 7, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Seeger, G.; Lüth, H.J.; Winkelmann, E.; Brauer, K. Distribution Patterns of Wisteria Floribunda Agglutinin Binding Sites and Parvalbumin-Immunoreactive Neurons in the Human Visual Cortex: A Double-Labelling Study. J. Hirnforsch. 1996, 37, 351–366. [Google Scholar] [PubMed]
- Lehner, A.; Hoffmann, L.; Rampp, S.; Coras, R.; Paulsen, F.; Frischknecht, R.; Hamer, H.; Walther, K.; Brandner, S.; Hofer, W.; et al. Age-Dependent Increase of Perineuronal Nets in the Human Hippocampus and Precocious Aging in Epilepsy. Epilepsia Open 2024, 9, 1372–1381. [Google Scholar] [CrossRef]
- Celio, M.R.; Blümcke, I. Perineuronal Nets-a Specialized Form of Extracellular Matrix in the Adult Nervous System. Brain Res. Rev. 1994, 19, 12–14. [Google Scholar] [CrossRef]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Weigel, P.H.; Hascall, V.C.; Tammi, M. Hyaluronan Synthases. J. Biol. Chem. 1997, 272, 13997–14000. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Lecticans: Organizers of the Brain Extracellular Matrix. CMLS Cell. Mol. Life Sci. 2000, 57, 276–289. [Google Scholar] [CrossRef]
- Rauch, U.; Karthikeyan, L.; Maurel, P.; Margolis, R.U.; Margolis, R.K. Cloning and Primary Structure of Neurocan, a Developmentally Regulated, Aggregating Chondroitin Sulfate Proteoglycan of Brain. J. Biol. Chem. 1992, 267, 19536–19547. [Google Scholar] [CrossRef]
- Doegesq, K.J.; Sasakill, M.; Kimurajj, T.; Yamada, Y. Complete Coding Sequence and Deduced Primary Structure of the Human Cartilage Large Aggregating Proteoglycan, Aggrecan. J. Biol. Chem. 1991, 266, 894–902. [Google Scholar]
- Yamada, H.; Watanabe, K.; Shimonaka, M.; Yamaguchis, Y. Molecular Cloning of Brevican, a Novel Brain Proteoglycan of the Aggrecadersican Family. J. Biol. Chem. 1994, 269, 10119–10126. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, D.R.; Ruoslahti, E. Multiple Domains of the Large Fibroblast Proteoglycan, Versican. EMBO J. 1989, 8, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Testa, D.; Prochiantz, A.; Di Nardo, A.A. Perineuronal Nets in Brain Physiology and Disease. Semin. Cell Dev. Biol. 2019, 89, 125–135. [Google Scholar] [CrossRef]
- Maurel, P.; Rauch, U.; Flad, M.; Margolis, R.K.; Margolis, R.U. Phosphacan, a Chondroitin Sulfate Proteoglycan of Brain That Interacts with Neurons and Neural Cell-Adhesion Molecules, Is an Extracellular Variant of a Receptor-Type Protein Tyrosine Phosphatase. Biochemistry 1994, 91, 2512–2516. [Google Scholar] [CrossRef]
- Eill, G.J.; Sinha, A.; Morawski, M.; Viapiano, M.S.; Matthews, R.T. The Protein Tyrosine Phosphatase RPTPξ/Phosphacan Is Critical for Perineuronal Net Structure. J. Biol. Chem. 2020, 295, 955–968. [Google Scholar] [CrossRef]
- Spicer, A.P.; Joo, A.; Bowling, R.A. A Hyaluronan Binding Link Protein Gene Family Whose Members Are Physically Linked Adjacent to Chrondroitin Sulfate Proteoglycan Core Protein Genes. The Missing Links. J. Biol. Chem. 2003, 278, 21083–21091. [Google Scholar] [CrossRef]
- Oohashi, T.; Edamatsu, M.; Bekku, Y.; Carulli, D. The Hyaluronan and Proteoglycan Link Proteins: Organizers of the Brain Extracellular Matrix and Key Molecules for Neuronal Function and Plasticity. Exp. Neurol. 2015, 274, 134–144. [Google Scholar] [CrossRef]
- Aspberg, A.; Miura, R.; Bourdoulous, S.; Shimonaka, M.; Heinegård, D.; Schachner, M.; Ruoslahti, E.; Yamaguchi, Y.U. The C-Type Lectin Domains of Lecticans, a Family of Aggregating Chondroitin Sulfate Proteoglycans, Bind Tenascin-R by Protein-Protein Interactions Independent of Carbohydrate Moiety. Proc. Natl. Acad. Sci. USA 1997, 94, 10116–10121. [Google Scholar] [CrossRef]
- Jakovljević, A.; Tucić, M.; Blažiková, M.; Korenić, A.; Missirlis, Y.; Stamenković, V.; Andjus, P. Structural and Functional Modulation of Perineuronal Nets: In Search of Important Players with Highlight on Tenascins. Cells 2021, 10, 1345. [Google Scholar] [CrossRef]
- Dick, G.; Liktan, C.; Alves, J.N.; Ehlert, E.M.E.; Miller, G.M.; Hsieh-Wilson, L.C.; Sugahara, K.; Oosterhof, A.; Van Kuppevelt, T.H.; Verhaagen, J.; et al. Semaphorin 3A Binds to the Perineuronal Nets via Chondroitin Sulfate Type E Motifs in Rodent Brains. J. Biol. Chem. 2013, 288, 27384–27395. [Google Scholar] [CrossRef]
- Beurdeley, M.; Spatazza, J.; Lee, H.H.C.; Sugiyama, S.; Bernard, C.; Di Nardo, A.A.; Hensch, T.K.; Prochiantz, A. Otx2 Binding to Perineuronal Nets Persistently Regulates Plasticity in the Mature Visual Cortex. J. Neurosci. 2012, 32, 9429–9437. [Google Scholar] [CrossRef] [PubMed]
- Lander, C.; Zhang, H.; Hockfield, S. Neurons Produce a Neuronal Cell Surface-Associated Chondroitin Sulfate Proteoglycan. J. Neurosci. 1998, 18, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Nishimura, Y.; Hayashi, N.; Oohira, A. Construction of Perineuronal Net-like Structure by Cortical Neurons in Culture. Neuroscience 2005, 136, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Giamanco, K.A.; Matthews, R.T. Deconstructing the Perineuronal Net: Cellular Contributions and Molecular Composition of the Neuronal Extracellular Matrix. Neuroscience 2012, 218, 367–384. [Google Scholar] [CrossRef]
- Carulli, D.; Rhodes, K.E.; Brown, D.J.; Bonnert, T.P.; Pollack, S.J.; Oliver, K.; Strata, P.; Fawcett, J.W. Composition of Perineuronal Nets in the Adult Rat Cerebellum and the Cellular Origin of Their Components. J. Comp. Neurol. 2006, 494, 559–577. [Google Scholar] [CrossRef]
- Bartsch, U. The extracellular matrix molecule tenascin-C: Expression in vivo and functional characterization in vitro. Pergamon Prog. Neurobiol. 1996, 49, 145–168. [Google Scholar] [CrossRef]
- Wiese, S.; Karus, M.; Faissner, A. Astrocytes as a Source for Extracellular Matrix Molecules and Cytokines. Front. Pharmacol. 2012, 3, 120. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) Family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Larsen, P.H.; Yong, V.W. The Expression of Matrix Metalloproteinase-12 by Oligodendrocytes Regulates Their Maturation and Morphological Differentiation. J. Neurosci. 2004, 24, 7597–7603. [Google Scholar] [CrossRef]
- Gorter, R.P.; Baron, W. Matrix Metalloproteinases Shape the Oligodendrocyte (Niche) during Development and upon Demyelination. Neurosci. Lett. 2020, 729, 134980. [Google Scholar] [CrossRef]
- Hamel, M.G.; Mayer, J.; Gottschall, P.E. Altered Production and Proteolytic Processing of Brevican by Transforming Growth Factor β in Cultured Astrocytes. J. Neurochem. 2005, 93, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Dityatev, A. Crosstalk between Glia, Extracellular Matrix and Neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bosiacki, M.; Gąssowska-Dobrowolska, M.; Kojder, K.; Fabiańska, M.; Jeżewski, D.; Gutowska, I.; Lubkowska, A. Perineuronal Nets and Their Role in Synaptic Homeostasis. Int. J. Mol. Sci. 2019, 20, 4108. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Takao, K.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Okamoto, M.; Aoki, S.; Ishihara, T. Age-Dependent and Region-Specific Alteration of Parvalbumin Neurons and Perineuronal Nets in the Mouse Cerebral Cortex. Neurochem. Int. 2018, 112, 59–70. [Google Scholar] [CrossRef]
- Mafi, A.M.; Hofer, L.N.; Russ, M.G.; Young, J.W.; Mellott, J.G. The Density of Perineuronal Nets Increases With Age in the Inferior Colliculus in the Fischer Brown Norway Rat. Front. Aging Neurosci. 2020, 12, 27. [Google Scholar] [CrossRef]
- Gogolla, N.; Caroni, P.; Lüthi, A.; Herry, C. Perineuronal Nets Protect Fear Memories from Erasure. Science 2009, 325, 1258–1261. [Google Scholar] [CrossRef]
- Rogers, S.L.; Rankin-Gee, E.; Risbud, R.M.; Porter, B.E.; Marsh, E.D. Normal Development of the Perineuronal Net in Humans; In Patients with and without Epilepsy. Neuroscience 2018, 384, 350–360. [Google Scholar] [CrossRef]
- Gao, R.; Wang, M.; Lin, J.; Hu, L.; Li, Z.; Chen, C.; Yuan, L. Spatiotemporal Expression Patterns of Chondroitin Sulfate Proteoglycan MRNAs in the Developing Rat Brain. Neuroreport 2018, 29, 517–523. [Google Scholar] [CrossRef]
- Fung, S.J.; Webster, M.J.; Sivagnanasundaram, S.; Duncan, C.; Elashoff, M.; Weickert, C.S. Interneuron Markers in the Developing Human and in Schizophrenia. Am. J. Psychiatry 2010, 167, 1479–1488. [Google Scholar] [CrossRef]
- Pizzorusso, T.; Medini, P.; Berardi, N.; Chierzi, S.; Fawcett, J.W.; Maffei, L. Reactivation of Ocular Dominance Plasticity in the Adult Visual Cortex. Science 2002, 298, 1248–1251. [Google Scholar] [CrossRef]
- Carulli, D.; Pizzorusso, T.; Kwok, J.C.F.; Putignano, E.; Poli, A.; Forostyak, S.; Andrews, M.R.; Deepa, S.S.; Glant, T.T.; Fawcett, J.W. Animals Lacking Link Protein Have Attenuated Perineuronal Nets and Persistent Plasticity. Brain 2010, 133, 2331–2347. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Di Nardo, A.A.; Aizawa, S.; Matsuo, I.; Volovitch, M.; Prochiantz, A.; Hensch, T.K. Experience-Dependent Transfer of Otx2 Homeoprotein into the Visual Cortex Activates Postnatal Plasticity. Cell 2008, 134, 508–520. [Google Scholar] [CrossRef] [PubMed]
- De Winter, F.; Kwok, J.C.F.; Fawcett, J.W.; Vo, T.T.; Carulli, D.; Verhaagen, J. The Chemorepulsive Protein Semaphorin 3A and Perineuronal Net-Mediated Plasticity. Neural Plast. 2016, 2016, 3679545. [Google Scholar] [CrossRef]
- Boggio, E.M.; Ehlert, E.M.; Lupori, L.; Moloney, E.B.; De Winter, F.; Vander Kooi, C.W.; Baroncelli, L.; Mecollari, V.; Blits, B.; Fawcett, J.W.; et al. Inhibition of Semaphorin3A Promotes Ocular Dominance Plasticity in the Adult Rat Visual Cortex. Mol. Neurobiol. 2019, 56, 5987–5997. [Google Scholar] [CrossRef]
- Ethell, I.M.; Ethell, D.W. Matrix Metalloproteinases in Brain Development and Remodeling: Synaptic Functions and Targets. J. Neurosci. Res. 2007, 85, 2813–2823. [Google Scholar] [CrossRef]
- Bozdagi, O.; Nagy, V.; Kwei, K.T.; Huntley, G.W. In Vivo Roles for Matrix Metalloproteinase-9 in Mature Hippocampal Synaptic Physiology and Plasticity. J. Neurophysiol. 2007, 98, 334–344. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagi, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, J.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix Metalloproteinase-9 Is Required for Hippocampal Late-Phase Long-Term Potentiation and Memory. J. Neurosci. 2006, 26, 1923–1934. [Google Scholar] [CrossRef]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in Translation: From Molecule to Brain Physiology, Pathology, and Therapy. J. Neurochem. 2016, 139, 91–114. [Google Scholar] [CrossRef]
- Wilczynski, G.M.; Konopacki, F.A.; Wilczek, E.; Lasiecka, Z.; Gorlewicz, A.; Michaluk, P.; Wawrzyniak, M.; Malinowska, M.; Okulski, P.; Kolodziej, L.R.; et al. Important Role of Matrix Metalloproteinase 9 in Epileptogenesis. J. Cell Biol. 2008, 180, 1021–1035. [Google Scholar] [CrossRef]
- Rankin-Gee, E.K.; McRae, P.A.; Baranov, E.; Rogers, S.; Wandrey, L.; Porter, B.E. Perineuronal Net Degradation in Epilepsy. Epilepsia 2015, 56, 1124–1133. [Google Scholar] [CrossRef]
- Hobohm, C.; Günther, A.; Grosche, J.; Roßner, S.; Schneider, D.; Brückner, G. Decomposition and Long-Lasting Downregulation of Extracellular Matrix in Perineuronal Nets Induced by Focal Cerebral Ischemia in Rats. J. Neurosci. Res. 2005, 80, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Cheong Lee, H.H.; Hameed, M.Q.; Pascual-Leone, A.; Hensch, T.K.; Rotenberg, A. Trajectory of Parvalbumin Cell Impairment and Loss of Cortical Inhibition in Traumatic Brain Injury. Cereb. Cortex 2017, 27, 5509–5524. [Google Scholar] [CrossRef] [PubMed]
- Kuno, K.; Kanada, N.; Nakashima, E.; Fujiki, F.; Ichimura, F.; Matsushima, K. Molecular Cloning of a Gene Encoding a New Type of Metalloproteinase-Disintegrin Family Protein with Thrombospondin Motifs as an Inflammation Associated Gene. J. Biol. Chem. 1997, 272, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan Degradation by the ADAMTS Family of Proteinases. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1616–1629. [Google Scholar] [CrossRef]
- Tang, B.L. ADAMTS: A Novel Family of Extracellular Matrix Proteases. Int. J. Biochem. Cell Biol. 2001, 33, 33–44. [Google Scholar] [CrossRef]
- Apte, S.S. A Disintegrin-like and Metalloprotease (Reprolysin Type) with Thrombospondin Type 1 Motifs: The ADAMTS Family. Int. J. Biochem. Cell Biol. 2004, 36, 981–985. [Google Scholar] [CrossRef]
- Lemarchant, S.; Pruvost, M.; Montaner, J.; Emery, E.; Vivien, D.; Kanninen, K.; Koistinaho, J. ADAMTS Proteoglycanases in the Physiological and Pathological Central Nervous System. J. Neuroinflammation 2013, 10, 133. [Google Scholar] [CrossRef]
- Tauchi, R.; Imagama, S.; Natori, T.; Ohgomori, T.; Muramoto, A.; Shinjo, R.; Matsuyama, Y.; Ishiguro, N.; Kadomatsu, K. The Endogenous Proteoglycan-Degrading Enzyme ADAMTS-4 Promotes Functional Recovery after Spinal Cord Injury. J. Neuroinflammation 2012, 9, 53. [Google Scholar] [CrossRef]
- Rolls, A.; Shechter, R.; Schwartz, M. The Bright Side of the Glial Scar in CNS Repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef]
- Arpino, V.; Brock, M.; Gill, S.E. The Role of TIMPs in Regulation of Extracellular Matrix Proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Dankovich, T.M.; Rizzoli, S.O. The Synaptic Extracellular Matrix: Long-Lived, Stable, and Still Remarkably Dynamic. Front. Synaptic Neurosci. 2022, 14, 854956. [Google Scholar] [CrossRef] [PubMed]
- Lev-Ram, V.; Lemieux, S.P.; Deerinck, T.J.; Bushong, E.A.; Perez, A.J.; Pritchard, D.R.; Toyama, B.H.; Park, S.K.R.; McClatchy, D.B.; Savas, J.N.; et al. Do Perineuronal Nets Stabilize the Engram of a Synaptic Circuit? Cells 2024, 13, 1627. [Google Scholar] [CrossRef] [PubMed]
- Dityatev, A.; Schachner, M. The Extracellular Matrix and Synapses. Cell Tissue Res. 2006, 326, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Eggli, P.; Celio, M.R. Relationship between Astrocytic Processes and “Perineuronal Nets” in Rat Neocortex. Glia 1995, 15, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tewari, B.P.; Woo, A.L.M.; Prim, C.E.; Chaunsali, L.; Patel, D.C.; Kimbrough, I.F.; Engel, K.; Browning, J.L.; Campbell, S.L.; Sontheimer, H. Astrocytes Require Perineuronal Nets to Maintain Synaptic Homeostasis in Mice. Nat. Neurosci. 2024, 27, 1475–1488. [Google Scholar] [CrossRef]
- Thomas, P.; Mortensen, M.; Hosie, A.M.; Smart, T.G. Dynamic Mobility of Functional GABAA Receptors at Inhibitory Synapses. Nat. Neurosci. 2005, 8, 889–897. [Google Scholar] [CrossRef]
- Triller, A.; Choquet, D. Synaptic Structure and Diffusion Dynamics of Synaptic Receptors. Biol. Cell 2003, 95, 465–476. [Google Scholar] [CrossRef]
- Lin, B.; Arai, A.C.; Lynch, G.; Gall, C.M. Integrins Regulate NMDA Receptor-Mediated Synaptic Currents. J. Neurophysiol. 2003, 89, 2874–2878. [Google Scholar] [CrossRef]
- Sloan Warren, M.; Bradley, W.D.; Gourley, S.L.; Lin, Y.C.; Simpson, M.A.; Reichardt, L.F.; Greer, C.A.; Taylor, J.R.; Koleske, A.J. Integrin Β1 Signals through Arg to Regulate Postnatal Dendritic Arborization, Synapse Density, and Behavior. J. Neurosci. 2012, 32, 2824–2834. [Google Scholar] [CrossRef]
- Liao, H.; Huang, W.; Schachner, M.; Guan, Y.; Guo, J.; Yan, J.; Qin, J.; Bai, X.; Zhang, L. β 1 Integrin-Mediated Effects of Tenascin-R Domains EGFL and FN6-8 on Neural Stem/Progenitor Cell Proliferation and Differentiation in Vitro. J. Biol. Chem. 2008, 283, 27927–27936. [Google Scholar] [CrossRef]
- Bernard-Trifilo, J.A.; Kramár, E.A.; Torp, R.; Lin, C.Y.; Pineda, E.A.; Lynch, G.; Gall, C.M. Integrin Signaling Cascades Are Operational in Adult Hippocampal Synapses and Modulate NMDA Receptor Physiology. J. Neurochem. 2005, 93, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Kwok, J.C.F.; Patani, R.; Ffrench-Constant, C.; Chandran, S.; Fawcett, J.W. Integrin Activation Promotes Axon Growth on Inhibitory Chondroitin Sulfate Proteoglycans by Enhancing Integrin Signaling. J. Neurosci. 2011, 31, 6289–6295. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, M.; Skupien, A.; Wójtowicz, T.; Konopka, A.; Gorlewicz, A.; Kisiel, M.; Bekisz, M.; Ruszczycki, B.; Dolezyczek, H.; Rejmak, E.; et al. CD44: A Novel Synaptic Cell Adhesion Molecule Regulating Structural and Functional Plasticity of Dendritic Spines. Mol. Biol. Cell 2016, 27, 4055–4066. [Google Scholar] [CrossRef]
- Rogers, J.T.; Rusiana, I.; Trotter, J.; Zhao, L.; Donaldson, E.; Pak, D.T.S.; Babus, L.W.; Peters, M.; Banko, J.L.; Chavis, P.; et al. Reelin Supplementation Enhances Cognitive Ability, Synaptic Plasticity, and Dendritic Spine Density. Learn. Mem. 2011, 18, 558–564. [Google Scholar] [CrossRef]
- De Vivo, L.; Landi, S.; Panniello, M.; Baroncelli, L.; Chierzi, S.; Mariotti, L.; Spolidoro, M.; Pizzorusso, T.; Maffei, L.; Ratto, G.M. Extracellular Matrix Inhibits Structural and Functional Plasticity of Dendritic Spines in the Adult Visual Cortex. Nat. Commun. 2013, 4, 1484. [Google Scholar] [CrossRef]
- Happel, M.F.K.; Niekisch, H.; Castiblanco Rivera, L.L.; Ohl, F.W.; Deliano, M.; Frischknecht, R. Enhanced Cognitive Flexibility in Reversal Learning Induced by Removal of the Extracellular Matrix in Auditory Cortex. Proc. Natl. Acad. Sci. USA 2014, 111, 2800–2805. [Google Scholar] [CrossRef]
- Suttkus, A.; Rohn, S.; Weigel, S.; Glöckner, P.; Arendt, T.; Morawski, M. Aggrecan, Link Protein and Tenascin-R Are Essential Components of the Perineuronal Net to Protect Neurons against Iron-Induced Oxidative Stress. Cell Death Dis. 2014, 5, e1119. [Google Scholar] [CrossRef]
- Treiman, D.M. GABAergic Mechanisms in Epilepsy. Epilepsia 2001, 42, 8–12. [Google Scholar] [CrossRef]
- Ruden, J.B.; Dugan, L.L.; Konradi, C. Parvalbumin Interneuron Vulnerability and Brain Disorders. Neuropsychopharmacology 2021, 46, 279–287. [Google Scholar] [CrossRef]
- Bartos, M.; Vida, I.; Jonas, P. Synaptic Mechanisms of Synchronized Gamma Oscillations in Inhibitory Interneuron Networks. Nat. Rev. Neurosci. 2007, 8, 45–56. [Google Scholar] [CrossRef]
- Guan, A.; Wang, S.; Huang, A.; Qiu, C.; Li, Y.; Li, X.; Wang, J.; Wang, Q.; Deng, B. The Role of Gamma Oscillations in Central Nervous System Diseases: Mechanism and Treatment. Front. Cell Neurosci. 2022, 16, 962957. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Wong, A.H.C. GABAergic Inhibitory Neurons as Therapeutic Targets for Cognitive Impairment in Schizophrenia. Acta Pharmacol. Sin. 2018, 39, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Brückner, G.; Bringmann, A.; Köppe, G.; Härtig, W.; Brauer, K. In vivo and in vitro Labelling of Perineuronal Nets in Rat Brain. Brain Res. 1996, 720, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, C.; Théberge, S.; Netto, S.; Rahimian, R.; Fakhfouri, G.; Hosdey, C.; Davoli, M.A.; Hendrickson, A.; Hao, K.; Giros, B.; et al. Chondroitin Sulfate Glycan Sulfation Patterns Influence Histochemical Labeling of Perineuronal Nets: A Comparative Study of Interregional Distribution in Human and Mouse Brain. Glycobiology 2024, 34, cwae049. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Markota, M.; Jaquet, F.; Ghosh, D.; Wallin, A.; Santos, A.; Caterson, B.; Berretta, S. Aggrecan and Chondroitin-6-Sulfate Abnormalities in Schizophrenia and Bipolar Disorder: A Postmortem Study on the Amygdala. Transl. Psychiatry 2015, 5, e496. [Google Scholar] [CrossRef]
- Köppe, G.; Brückner, G.; Härtig, W.; Delpech, B.; Bigl, V. Characterization of Proteoglycan-Containing Perineuronal Nets by Enzymatic Treatments of Rat Brain Sections. Histochem. J. 1996, 29, 11–20. [Google Scholar] [CrossRef]
- Virgintino, D.; Perissinotto, D.; Girolamo, F.; Mucignat, M.T.; Montanini, L.; Errede, M.; Kaneiwa, T.; Yamada, S.; Sugahara, K.; Roncali, L.; et al. Differential Distribution of Aggrecan Isoforms in Perineuronal Nets of the Human Cerebral Cortex. J. Cell Mol. Med. 2009, 13, 3151–3173. [Google Scholar] [CrossRef]
- Banovac, I.; Prkačin, M.V.; Kirchbaum, I.; Trnski-Levak, S.; Bobić-Rasonja, M.; Sedmak, G.; Petanjek, Z.; Jovanov-Milosevic, N. Morphological and Molecular Characteristics of Perineuronal Nets in the Human Prefrontal Cortex—A Possible Link to Microcircuitry Specialization. Mol. Neurobiol. 2024, 62, 1094–1111. [Google Scholar] [CrossRef]
- Lendvai, D.; Morawski, M.; Négyessy, L.; Gáti, G.; Jäger, C.; Baksa, G.; Glasz, T.; Attems, J.; Tanila, H.; Arendt, T.; et al. Neurochemical Mapping of the Human Hippocampus Reveals Perisynaptic Matrix around Functional Synapses in Alzheimer’s Disease. Acta Neuropathol. 2013, 125, 215–229. [Google Scholar] [CrossRef]
- Härtig, W.; Meinicke, A.; Michalski, D.; Schob, S.; Jäger, C. Update on Perineuronal Net Staining With Wisteria Floribunda Agglutinin (WFA). Front. Integr. Neurosci. 2022, 16, 851988. [Google Scholar] [CrossRef]
- Enwleft, J.F.; Sanapala, S.; Foglio, A.; Berry, R.; Fish, K.N.; Lewis, D.A. Reduced Labeling of Parvalbumin Neurons and Perineuronal Nets in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia. Neuropsychopharmacology 2016, 41, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Brückner, G.; Grosche, J.; Schmidt, S.; Härtig, W.; Margolis, R.U.; Delpech, B.; Seidenbecher, C.I.; Czaniera, R.; Schachner, M. Postnatal Development of Perineuronal Nets in Wild-Type Mice and in a Mutant Deficient in Tenascin-R. J. Comp. Neurol. 2000, 428, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Brückner, G.; Härtig, W.; Kacza, J.; Seeger, J.; Welt, K.; Brauer, K. Extracellular Matrix Organization in Various Regions of Rat Brain Grey Matter. J. Neurocytol. 1996, 25, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Giamanco, K.A.; Morawski, M.; Matthews, R.T. Perineuronal Net Formation and Structure in Aggrecan Knockout Mice. Neuroscience 2010, 170, 1314–1327. [Google Scholar] [CrossRef]
- Rowlands, D.; Lensjø, K.K.; Dinh, T.; Yang, S.; Andrews, M.R.; Hafting, T.; Fyhn, M.; Fawcett, J.W.; Dick, G. Aggrecan Directs Extracellular Matrix-Mediated Neuronal Plasticity. J. Neurosci. 2018, 38, 10102–10113. [Google Scholar] [CrossRef]
- Brakebusch, C.; Seidenbecher, C.I.; Asztely, F.; Rauch, U.; Matthies, H.; Meyer, H.; Krug, M.; Böckers, T.M.; Zhou, X.; Kreutz, M.R.; et al. Brevican-Deficient Mice Display Impaired Hippocampal CA1 Long-Term Potentiation but Show No Obvious Deficits in Learning and Memory. Mol. Cell Biol. 2002, 22, 7417–7427. [Google Scholar] [CrossRef]
- Favuzzi, E.; Marques-Smith, A.; Deogracias, R.; Winterflood, C.M.; Sánchez-Aguilera, A.; Mantoan, L.; Maeso, P.; Fernandes, C.; Ewers, H.; Rico, B. Activity-Dependent Gating of Parvalbumin Interneuron Function by the Perineuronal Net Protein Brevican. Neuron 2017, 95, 639–655.e10. [Google Scholar] [CrossRef]
- Schmidt, S.; Arendt, T.; Morawski, M.; Sonntag, M. Neurocan Contributes to Perineuronal Net Development. Neuroscience 2020, 442, 69–86. [Google Scholar] [CrossRef]
- Morawski, M.; Dityatev, A.; Hartlage-Rübsamen, M.; Blosa, M.; Holzer, M.; Flach, K.; Pavlica, S.; Dityateva, G.; Dityateva, G.; Brückner, G.; et al. Tenascin-R Promotes Assembly of the Extracellular Matrix of Perineuronal Nets via Clustering of Aggrecan. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20140046. [Google Scholar] [CrossRef]
- Bekku, Y.; Saito, M.; Moser, M.; Fuchigami, M.; Maehara, A.; Nakayama, M.; Kusachi, S.; Ninomiya, Y.; Oohashi, T. Bral2 Is Indispensable for the Proper Localization of Brevican and the Structural Integrity of the Perineuronal Net in the Brainstem and Cerebellum. J. Comp. Neurol. 2012, 520, 1721–1736. [Google Scholar] [CrossRef]
- Sinha, A.; Kawakami, J.; Cole, K.S.; Ladutska, A.; Nguyen, M.Y.; Zalmai, M.S.; Holder, B.L.; Broerman, V.M.; Matthews, R.T.; Bouyain, S. Protein–Protein Interactions between Tenascin-R and RPTPζ/Phosphacan Are Critical to Maintain the Architecture of Perineuronal Nets. J. Biol. Chem. 2023, 299, 104952. [Google Scholar] [CrossRef] [PubMed]
- Gottschling, C.; Wegrzyn, D.; Denecke, B.; Faissner, A. Elimination of the Four Extracellular Matrix Molecules Tenascin-C, Tenascin-R, Brevican and Neurocan Alters the Ratio of Excitatory and Inhibitory Synapses. Sci. Rep. 2019, 9, 13939. [Google Scholar] [CrossRef]
- Mueller-Buehl, C.; Reinhard, J.; Roll, L.; Bader, V.; Winklhofer, K.F.; Faissner, A. Brevican, Neurocan, Tenascin-C, and Tenascin-R Act as Important Regulators of the Interplay Between Perineuronal Nets, Synaptic Integrity, Inhibitory Interneurons, and Otx2. Front. Cell Dev. Biol. 2022, 10, 886527. [Google Scholar] [CrossRef]
- Brückner, G.; Bringmann, A.; Härtig, W.; Köppe, G.; Delpech, B.; Brauer, K. Acute and Long-Lasting Changes in Extracellular-Matrix Chondroitin-Sulphate Proteoglycans Induced by Injection of Chondroitinase ABC in the Adult Rat Brain. Exp. Brain Res. 1998, 121, 300–310. [Google Scholar] [CrossRef]
- Fawcett, J.W. The Extracellular Matrix in Plasticity and Regeneration after CNS Injury and Neurodegenerative Disease. Prog. Brain Res. 2015, 218, 213–226. [Google Scholar] [CrossRef]
- Shi, W.; Wei, X.; Wang, X.; Du, S.; Liu, W.; Song, J.; Wang, Y. Perineuronal Nets Protect Long-Term Memory by Limiting Activity-Dependent Inhibition from Parvalbumin Interneurons. Proc. Natl. Acad. Sci. USA 2019, 116, 27063–27073. [Google Scholar] [CrossRef]
- Willis, A.; Pratt, J.A.; Morris, B.J. Enzymatic Degradation of Cortical Perineuronal Nets Reverses GABAergic Interneuron Maturation. Mol. Neurobiol. 2022, 59, 2874–2893. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Ju, J. Removal of Perineuronal Nets Leads to Altered Neuronal Excitability and Synaptic Transmission in the Visual Cortex with Distinct Time Courses. Neurosci. Lett. 2022, 785, 136763. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Men, S.; Li, X.; Hou, S.T.; Ju, J. Elimination of Perineuronal Nets in CA1 Disrupts GABA Release and Long-Term Contextual Fear Memory Retention. Hippocampus 2023, 33, 862–871. [Google Scholar] [CrossRef]
- Poli, A.; Viglione, A.; Mazziotti, R.; Totaro, V.; Morea, S.; Melani, R.; Silingardi, D.; Putignano, E.; Berardi, N.; Pizzorusso, T. Selective Disruption of Perineuronal Nets in Mice Lacking Crtl1 Is Sufficient to Make Fear Memories Susceptible to Erasure. Mol. Neurobiol. 2023, 60, 4105–4119. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Bozzelli, P.L.; Caccavano, A.; Allen, M.; Balmuth, J.; Vicini, S.; Wu, J.Y.; Conant, K. Disruption of Perineuronal Nets Increases the Frequency of Sharp Wave Ripple Events. Hippocampus 2018, 28, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Carstens, K.E.; Gloss, B.R.; Alexander, G.M.; Dudek, S.M. Modified Adeno-Associated Virus Targets the Bacterial Enzyme Chondroitinase ABC to Select Mouse Neuronal Populations in Vivo Using the Cre-LoxP System. Eur. J. Neurosci. 2021, 53, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Pires, G.; Leitner, D.; Drummond, E.; Kanshin, E.; Nayak, S.; Askenazi, M.; Faustin, A.; Friedman, D.; Debure, L.; Ueberheide, B.; et al. Proteomic Differences in the Hippocampus and Cortex of Epilepsy Brain Tissue. Brain Commun. 2021, 3, fcab021. [Google Scholar] [CrossRef]
- Pokhilko, A.; Brezzo, G.; Handunnetthi, L.; Heilig, R.; Lennon, R.; Smith, C.; Allan, S.M.; Granata, A.; Sinha, S.; Wang, T.; et al. Global Proteomic Analysis of Extracellular Matrix in Mouse and Human Brain Highlights Relevance to Cerebrovascular Disease. J. Cereb. Blood Flow. Metab. 2021, 41, 2423–2438. [Google Scholar] [CrossRef]
- Chmelova, M.; Androvic, P.; Kirdajova, D.; Tureckova, J.; Kriska, J.; Valihrach, L.; Anderova, M.; Vargova, L. A View of the Genetic and Proteomic Profile of Extracellular Matrix Molecules in Aging and Stroke. Front. Cell Neurosci. 2023, 17, 1296455. [Google Scholar] [CrossRef]
- Leitner, D.; Pires, G.; Kavanagh, T.; Kanshin, E.; Askenazi, M.; Ueberheide, B.; Devinsky, O.; Wisniewski, T.; Drummond, E. Similar Brain Proteomic Signatures in Alzheimer’s Disease and Epilepsy. Acta Neuropathol. 2024, 147, 27. [Google Scholar] [CrossRef]
- do Canto, A.M.; Donatti, A.; Geraldis, J.C.; Godoi, A.B.; da Rosa, D.C.; Lopes-Cendes, I. Neuroproteomics Epilepsy: What Do We Know So Far? Front. Mol. Neurosci. 2021, 13, 604158. [Google Scholar] [CrossRef]
- Downs, M.; Zaia, J.; Sethi, M.K. Mass Spectrometry Methods for Analysis of Extracellular Matrix Components in Neurological Diseases. Mass. Spectrom. Rev. 2023, 42, 1848–1875. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Woo, T.U.W.; Lim, M.P.; Lange, N.; Berretta, S. Extracellular Matrix-Glial Abnormalities in the Amygdala and Entorhinal Cortex of Subjects Diagnosed with Schizophrenia. Arch. Gen. Psychiatry 2010, 67, 155–166. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Boyer-Boiteau, A.; Holbrook, E.H.; Jang, W.; Hahn, C.G.; Arnold, S.E.; Berretta, S. Proteoglycan Abnormalities in Olfactory Epithelium Tissue from Subjects Diagnosed with Schizophrenia. Schizophr. Res. 2013, 150, 366–372. [Google Scholar] [CrossRef]
- Mauney, S.A.; Athanas, K.M.; Pantazopoulos, H.; Shaskan, N.; Passeri, E.; Berretta, S.; Woo, T.U.W. Developmental Pattern of Perineuronal Nets in the Human Prefrontal Cortex and Their Deficit in Schizophrenia. Biol. Psychiatry 2013, 74, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Matuszko, G.; Curreli, S.; Kaushik, R.; Becker, A.; Dityatev, A. Extracellular Matrix Alterations in the Ketamine Model of Schizophrenia. Neuroscience 2017, 350, 13–22. [Google Scholar] [CrossRef]
- Steullet, P.; Cabungcal, J.H.; Bukhari, S.A.; Ardelt, M.I.; Pantazopoulos, H.; Hamati, F.; Salt, T.E.; Cuenod, M.; Do, K.Q.; Berretta, S. The Thalamic Reticular Nucleus in Schizophrenia and Bipolar Disorder: Role of Parvalbumin-Expressing Neuron Networks and Oxidative Stress. Mol. Psychiatry 2018, 23, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Cabungcal, J.H.; Steullet, P.; Kraftsik, R.; Cuenod, M.; Do, K.Q. A Developmental Redox Dysregulation Leads to Spatio-Temporal Deficit of Parvalbumin Neuron Circuitry in a Schizophrenia Mouse Model. Schizophr. Res. 2019, 213, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Zhang, P.; Hadjimichael, E.; Walker, R.L.; Chen, C.; Liu, S.; Won, H.; Van Bakel, H.; Varghese, M.; Wang, Y.; et al. Transcriptome-Wide Isoform-Level Dysregulation in ASD, Schizophrenia, and Bipolar Disorder. Science 2018, 362, eaat8127. [Google Scholar] [CrossRef]
- Alcaide, J.; Guirado, R.; Crespo, C.; Blasco-Ibáñez, J.M.; Varea, E.; Sanjuan, J.; Nacher, J. Alterations of Perineuronal Nets in the Dorsolateral Prefrontal Cortex of Neuropsychiatric Patients. Int. J. Bipolar Disord. 2019, 7, 24. [Google Scholar] [CrossRef]
- Bitanihirwe, B.K.Y.; Woo, T.U.W. A Conceptualized Model Linking Matrix Metalloproteinase-9 to Schizophrenia Pathogenesis. Schizophr. Res. 2020, 218, 28–35. [Google Scholar] [CrossRef]
- Kaushik, R.; Lipachev, N.; Matuszko, G.; Kochneva, A.; Dvoeglazova, A.; Becker, A.; Paveliev, M.; Dityatev, A. Fine Structure Analysis of Perineuronal Nets in the Ketamine Model of Schizophrenia. Eur. J. Neurosci. 2021, 53, 3988–4004. [Google Scholar] [CrossRef]
- Fujikawa, R.; Yamada, J.; Jinno, S. Subclass Imbalance of Parvalbumin-Expressing GABAergic Neurons in the Hippocampus of a Mouse Ketamine Model for Schizophrenia, with Reference to Perineuronal Nets. Schizophr. Res. 2021, 229, 80–93. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Katsel, P.; Haroutunian, V.; Chelini, G.; Klengel, T.; Berretta, S. Molecular Signature of Extracellular Matrix Pathology in Schizophrenia. Eur. J. Neurosci. 2021, 53, 3960–3987. [Google Scholar] [CrossRef]
- Sultana, R.; Brooks, C.B.; Shrestha, A.; Ogundele, O.M.; Lee, C.C. Perineuronal Nets in the Prefrontal Cortex of a Schizophrenia Mouse Model: Assessment of Neuroanatomical, Electrophysiological and Behavioral Contributions. Int. J. Mol. Sci. 2021, 22, 11140. [Google Scholar] [CrossRef]
- Liang, Y.R.; Zhang, X.H. Inhibition of GluN2B-Containing NMDA Receptors in Early Life Combined with Social Stress in Adulthood Leads to Alterations in Prefrontal PNNs in Mice. Acta Physiol. Sin. 2024, 76, 1–11. [Google Scholar] [CrossRef]
- Berretta, S.; Pantazopoulos, H.; Markota, M.; Brown, C.; Batzianouli, E.T. Losing the Sugar Coating: Potential Impact of Perineuronal Net Abnormalities on Interneurons in Schizophrenia. Schizophr. Res. 2015, 167, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bitanihirwe, B.K.Y.; Woo, T.U.W. Perineuronal Nets and Schizophrenia: The Importance of Neuronal Coatings. Neurosci. Biobehav. Rev. 2014, 45, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Bitanihirwe, B.K.Y.; Mauney, S.A.; Woo, T.U.W. Weaving a Net of Neurobiological Mechanisms in Schizophrenia and Unraveling the Underlying Pathophysiology. Biol. Psychiatry 2016, 80, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, J.R.F.; Costa, O.; Pakes, G.H.; Colodete, D.A.E.; Gomes, F.V. Perineuronal Net Density in Schizophrenia: A Systematic Review of Postmortem Brain Studies. Schizophr. Res. 2024, 271, 100–109. [Google Scholar] [CrossRef]
- Sethi, M.K.; Zaia, J. Extracellular Matrix Proteomics in Schizophrenia and Alzheimer’s Disease. Anal. Bioanal. Chem. 2017, 409, 379–394. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, G.; Jäger, C.; Seeger, G.; Arendt, T. Neurons Associated with Aggrecan-Based Perineuronal Nets Are Protected against Tau Pathology in Subcortical Regions in Alzheimer’s Disease. Neuroscience 2010, 169, 1347–1363. [Google Scholar] [CrossRef]
- Morawski, M.; Pavlica, S.; Seeger, G.; Grosche, J.; Kouznetsova, E.; Schliebs, R.; Brückner, G.; Arendt, T. Perineuronal Nets Are Largely Unaffected in Alzheimer Model Tg2576 Mice. Neurobiol. Aging 2010, 31, 1254–1256. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, G.; Jäger, C.; Seeger, G.; Matthews, R.T.; Arendt, T. Involvement of Perineuronal and Perisynaptic Extracellular Matrix in Alzheimer’s Disease Neuropathology. Brain Pathol. 2012, 22, 547–561. [Google Scholar] [CrossRef]
- Schmidt, S.; Stapf, C.; Schmutzler, S.; Lachmann, I.; Arendt, T.; Holzer, M.; Sonntag, M.; Morawski, M. Aggrecan Modulates the Expression and Phosphorylation of Tau in a Novel Bigenic TauP301L–Acan Mouse Model. Eur. J. Neurosci. 2021, 53, 3889–3904. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, A.F.; Francis, K.L.; Richardson, N.E.; Hu, S.J.; Faber, C.L.; Phan, B.A.; Nguyen, V.; Setthavongsack, N.; Banks, W.A.; Woltjer, R.L.; et al. Decoding Perineuronal Net Glycan Sulfation Patterns in the Alzheimer’s Disease Brain. Alzheimer’s Dement. 2021, 18, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Holzer, M.; Arendt, T.; Sonntag, M.; Morawski, M. Tau Protein Modulates Perineuronal Extracellular Matrix Expression in the TauP301L-Acan Mouse Model. Biomolecules 2022, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Epilepsy A Public Health Imperative International League Against Epilepsy. Available online: https://www.who.int/publications/i/item/epilepsy-a-public-health-imperative (accessed on 20 January 2025).
- Mcrae, P.A.; Baranov, E.; Rogers, S.L.; Porter, B.E. Persistent Decrease in Multiple Components of the Perineuronal Net Following Status Epilepticus. Eur. J. Neurosci. 2012, 36, 3471–3482. [Google Scholar] [CrossRef]
- Dubey, D.; McRae, P.A.; Rankin-Gee, E.K.; Baranov, E.; Wandrey, L.; Rogers, S.; Porter, B.E. Increased Metalloproteinase Activity in the Hippocampus Following Status Epilepticus. Epilepsy Res. 2017, 132, 50–58. [Google Scholar] [CrossRef]
- Tewari, B.P.; Chaunsali, L.; Campbell, S.L.; Patel, D.C.; Goode, A.E.; Sontheimer, H. Perineuronal Nets Decrease Membrane Capacitance of Peritumoral Fast Spiking Interneurons in a Model of Epilepsy. Nat. Commun. 2018, 9, 4724. [Google Scholar] [CrossRef]
- Pollock, E.; Everest, M.; Brown, A.; Poulter, M.O. Metalloproteinase Inhibition Prevents Inhibitory Synapse Reorganization and Seizure Genesis. Neurobiol. Dis. 2014, 70, 21–31. [Google Scholar] [CrossRef]
- Broekaart, D.W.M.; Bertran, A.; Jia, S.; Korotkov, A.; Senkov, O.; Bongaarts, A.; Mills, J.D.; Anink, J.J.; Seco, J.; Baayen, J.C.; et al. The Matrix Metalloproteinase Inhibitor IPR-179 Has Antiseizure and Antiepileptogenic Effects. J. Clin. Investig. 2021, 131, e138332. [Google Scholar] [CrossRef]
- Duncan, J.A.; Foster, R.; Kwok, J.C.F. The Potential of Memory Enhancement through Modulation of Perineuronal Nets. Br. J. Pharmacol. 2019, 176, 3611–3621. [Google Scholar] [CrossRef]

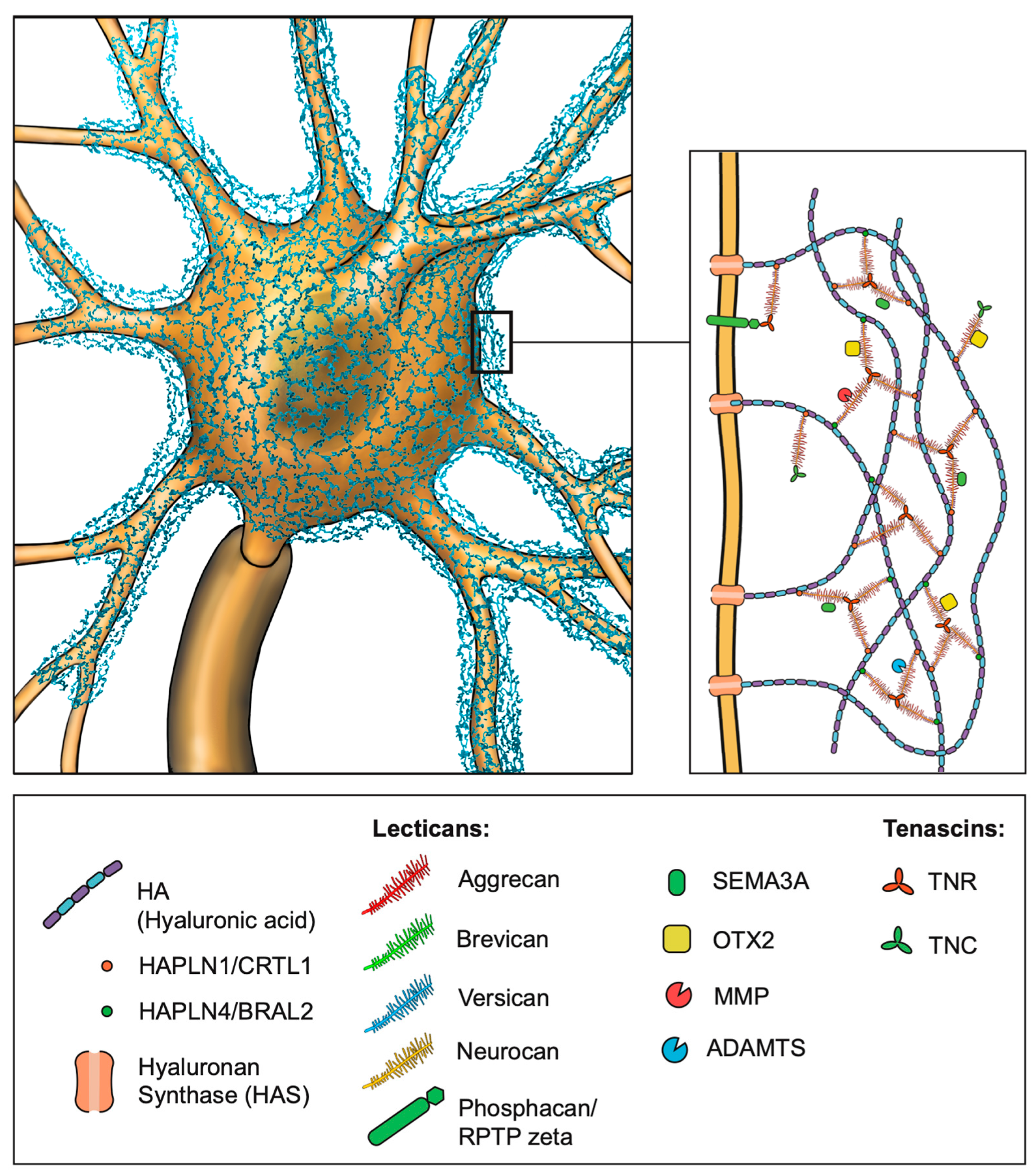

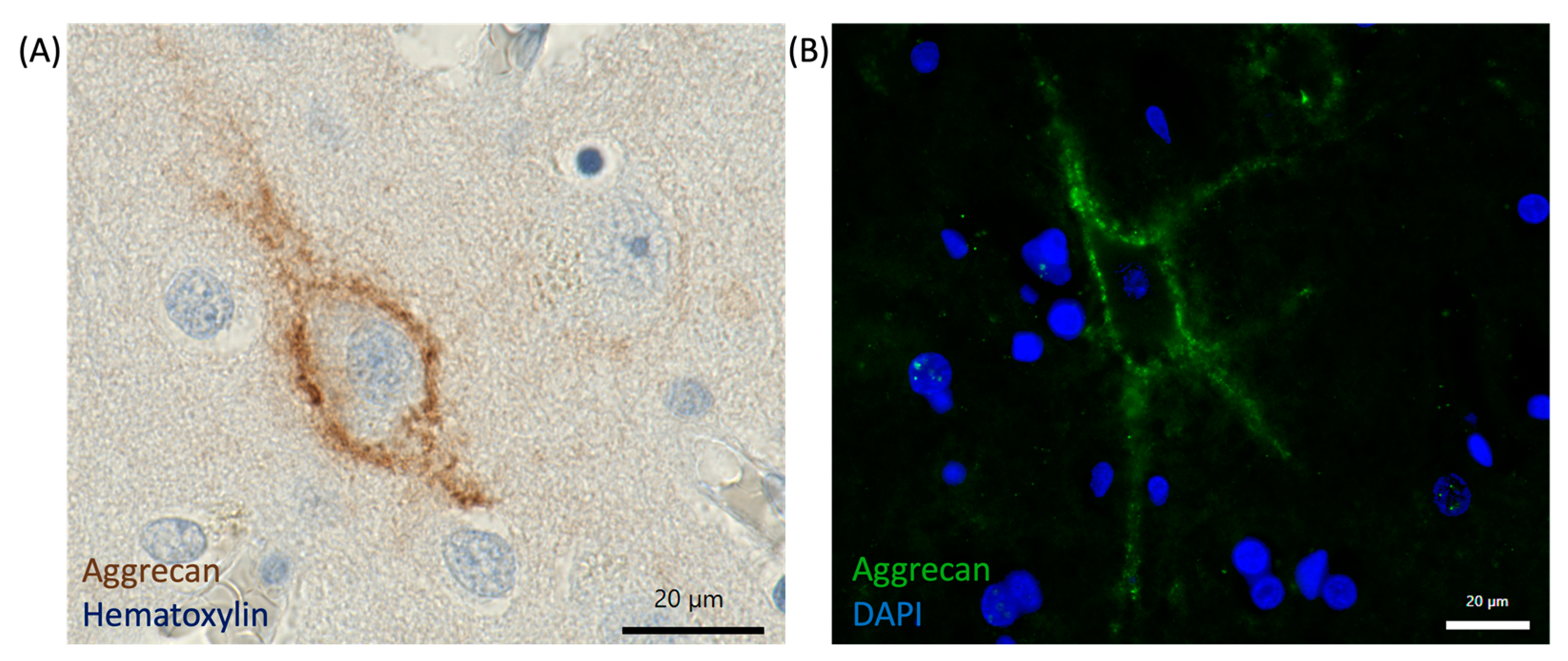
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auer, S.; Schicht, M.; Hoffmann, L.; Budday, S.; Frischknecht, R.; Blümcke, I.; Paulsen, F. The Role of Perineuronal Nets in Physiology and Disease: Insights from Recent Studies. Cells 2025, 14, 321. https://doi.org/10.3390/cells14050321
Auer S, Schicht M, Hoffmann L, Budday S, Frischknecht R, Blümcke I, Paulsen F. The Role of Perineuronal Nets in Physiology and Disease: Insights from Recent Studies. Cells. 2025; 14(5):321. https://doi.org/10.3390/cells14050321
Chicago/Turabian StyleAuer, Sophia, Martin Schicht, Lucas Hoffmann, Silvia Budday, Renato Frischknecht, Ingmar Blümcke, and Friedrich Paulsen. 2025. "The Role of Perineuronal Nets in Physiology and Disease: Insights from Recent Studies" Cells 14, no. 5: 321. https://doi.org/10.3390/cells14050321
APA StyleAuer, S., Schicht, M., Hoffmann, L., Budday, S., Frischknecht, R., Blümcke, I., & Paulsen, F. (2025). The Role of Perineuronal Nets in Physiology and Disease: Insights from Recent Studies. Cells, 14(5), 321. https://doi.org/10.3390/cells14050321






