Targeted Inhibition of CHD1L by OTI-611 Reprograms Chemotherapy and Targeted Therapy-Induced Cell Cycle Arrest and Suppresses Proliferation to Produce Synergistic Antitumor Effects in Breast and Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture
2.3. Lentivirus Transduction
2.4. Drug Treatment Concentrations
2.5. Real-Time Cell Cycle Measurements
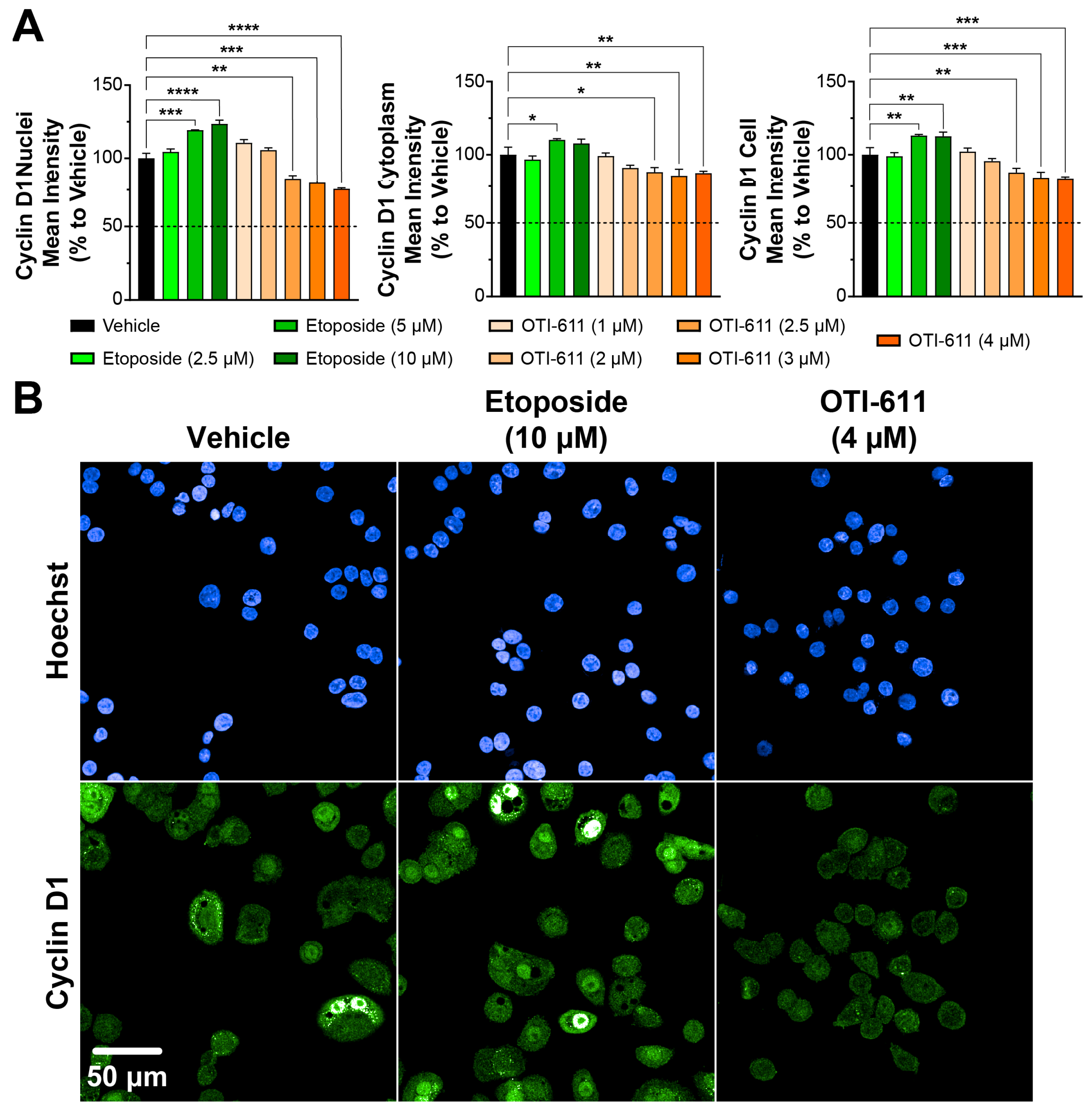
2.6. Cyclin D1, Ki-67, and CDK4 Immunofluorescence
2.7. Statistical Analysis
3. Results
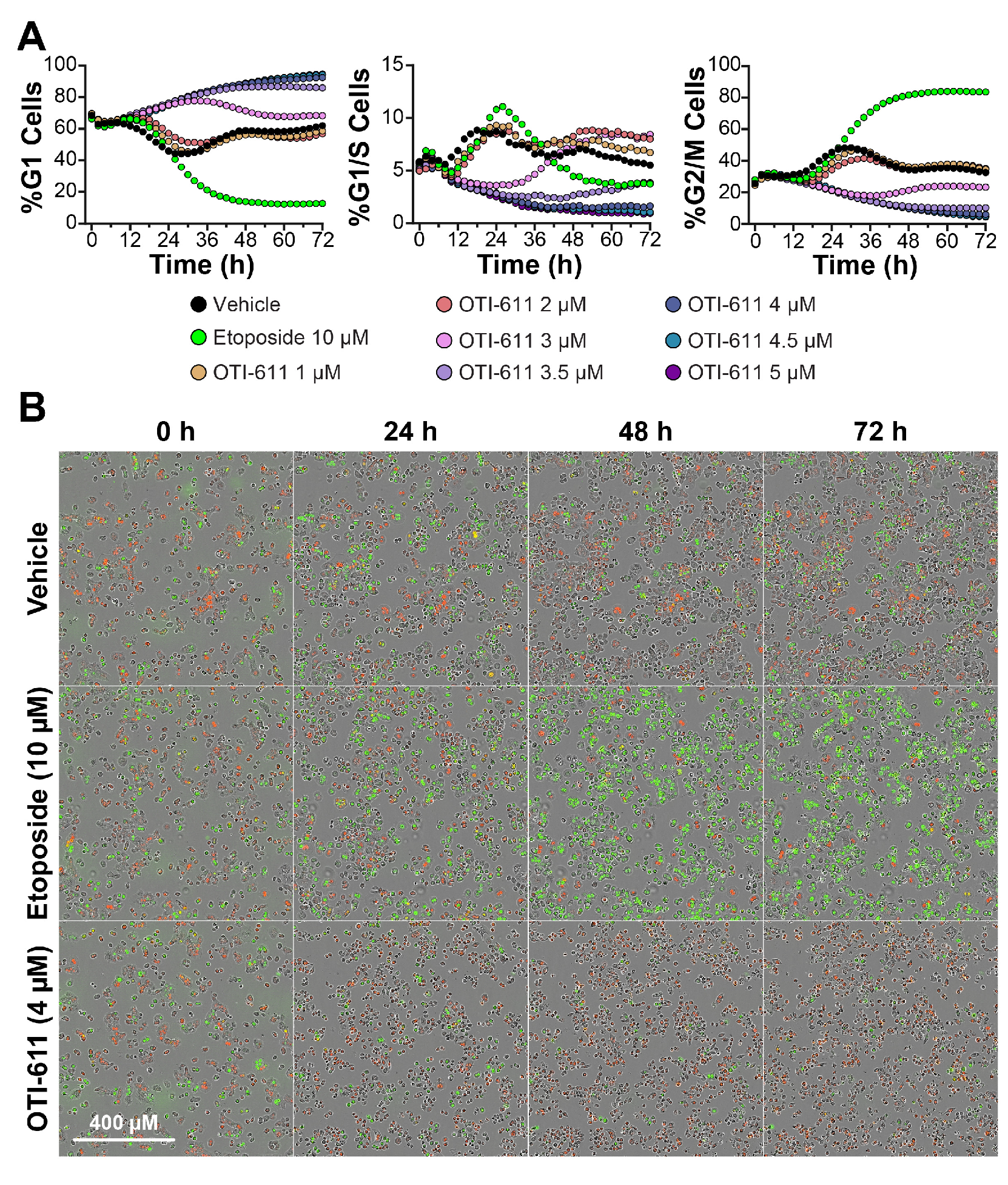
3.1. CHD1L Inhibition with OTI-611 Disrupts Cyclin D1 Dynamics and Induces G1 Cell Cycle Arrest in Colo678 Cells
3.2. OTI-611 Induces G1 Arrest in BC and CRC Cell Lines with Distinct Cell Cycle Dynamics
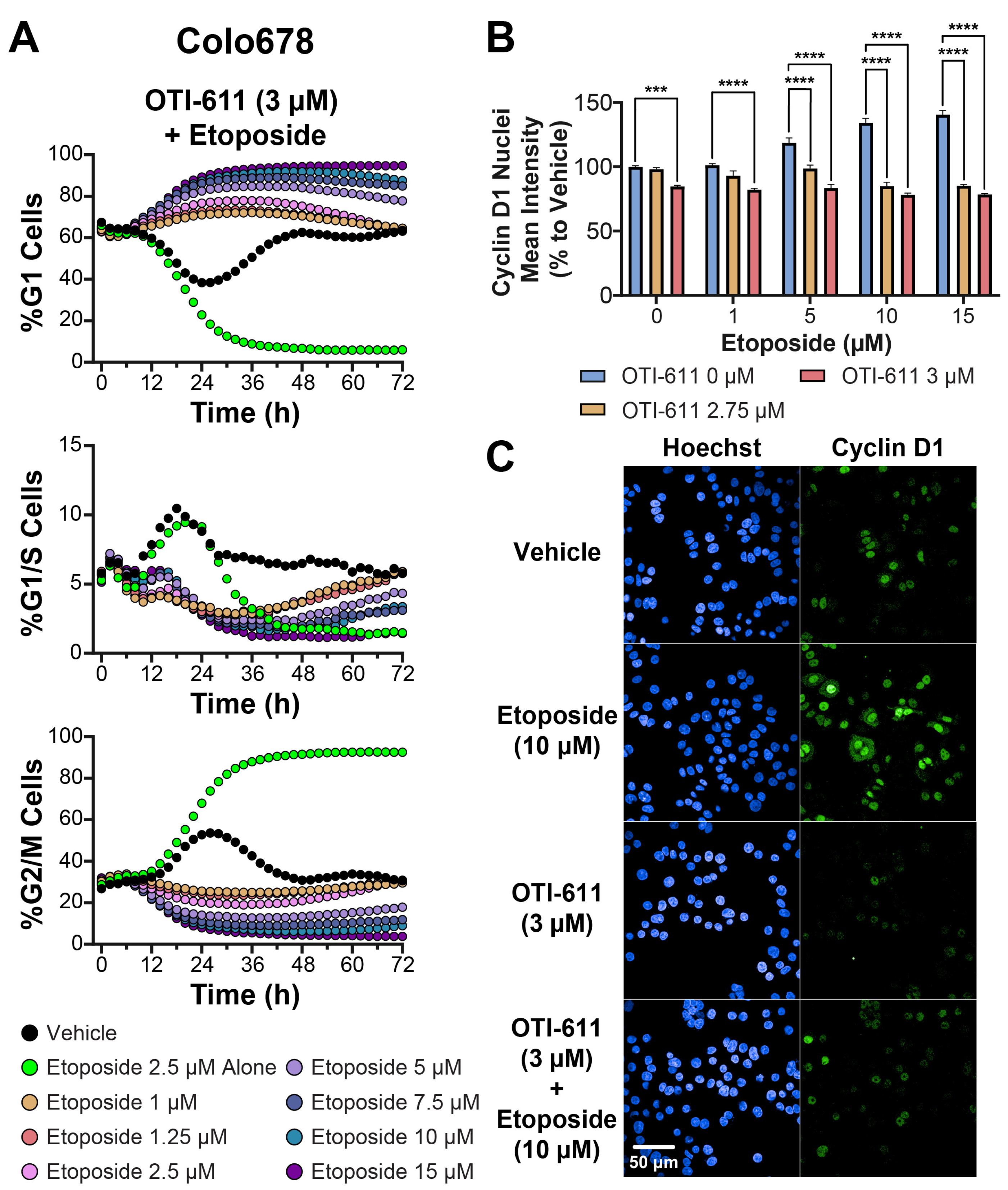
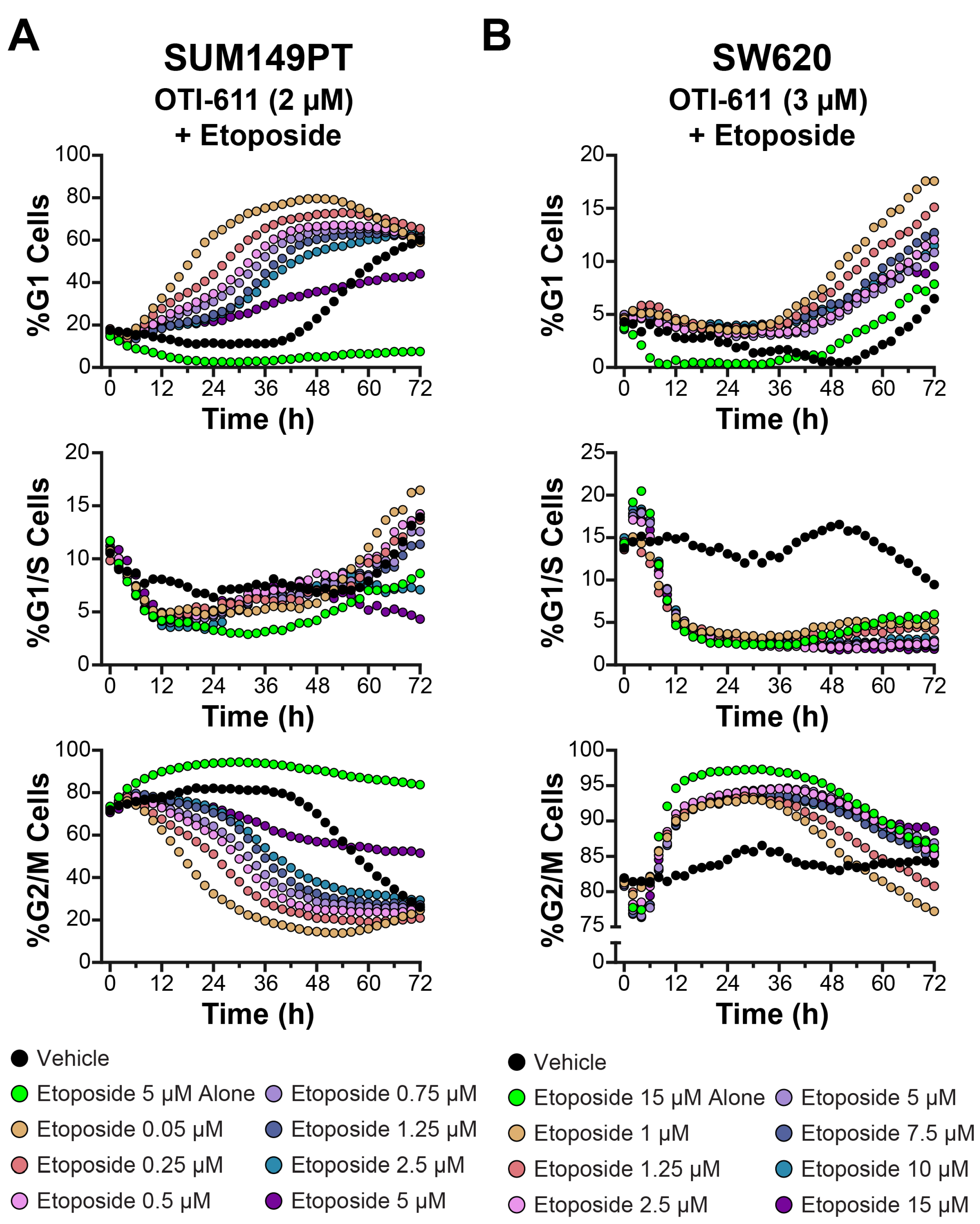
3.3. CHD1Li OTI-611 Reprograms Etoposide’s G2/M Arrest Toward G1 Arrest in Colo678, SUM149PT, and SW620 Cells
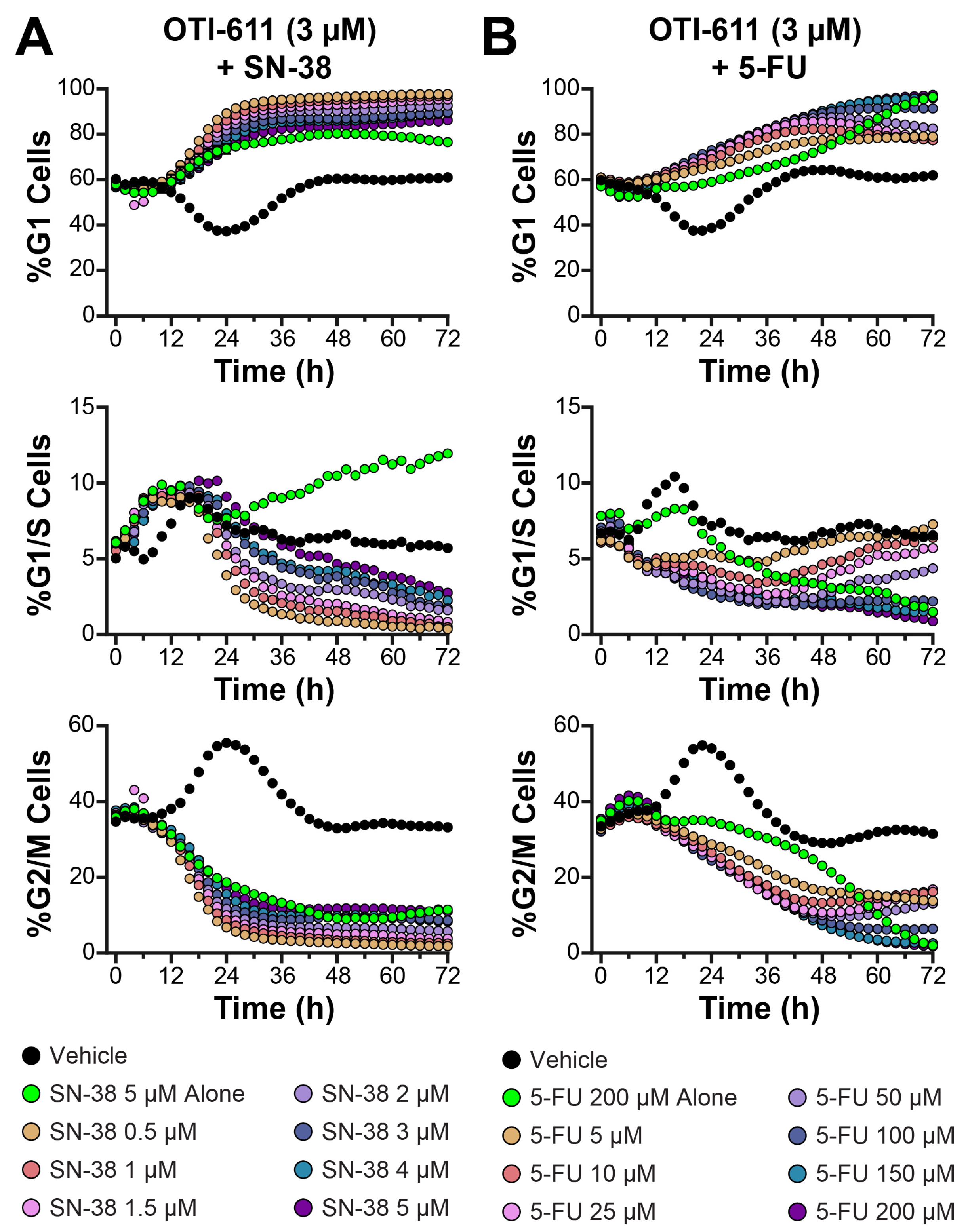
3.4. Colo678 Cells Are Arrested in G1 Phase in Combination with SN-38 and 5-FU
3.5. Olaparib-Induced G2/M Arrest in SUM149PT Cells Is Reprogrammed Toward G1 Arrest When Combined with OTI-611
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Abbott, J.M.; Zhou, Q.; Esquer, H.; Pike, L.; Broneske, T.P.; Rinaldetti, S.; Abraham, A.D.; Ramirez, D.A.; Lunghofer, P.J.; Pitts, T.M.; et al. First-in-Class Inhibitors of Oncogenic CHD1L with Preclinical Activity Against Colorectal Cancer. Mol. Cancer Ther. 2020, 19, 1598–1612. [Google Scholar] [CrossRef]
- Clune, S.; Awolade, P.; Zhou, Q.; Esquer, H.; Matter, B.; Kearns, J.T.; Kellett, T.; Akintayo, D.C.; Kompella, U.B.; LaBarbera, D.V. The validation of new CHD1L inhibitors as a therapeutic strategy for cancer. Biomed. Pharmacother. 2024, 170, 116037. [Google Scholar] [CrossRef]
- Prigaro, B.J.; Esquer, H.; Zhou, Q.; Pike, L.A.; Awolade, P.; Lai, X.-H.; Abraham, A.D.; Abbott, J.M.; Matter, B.; Kompella, U.B.; et al. Design, Synthesis, and Biological Evaluation of the First Inhibitors of Oncogenic CHD1L. J. Med. Chem. 2022, 65, 3943–3961. [Google Scholar] [CrossRef]
- Sala, R.; Esquer, H.; Kellett, T.; Clune, S.; Awolade, P.; Pike, L.A.; Zhou, Q.; Messersmith, W.A.; LaBarbera, D.V. CHD1L Inhibitor OTI-611 Synergizes with Chemotherapy to Enhance Antitumor Efficacy and Prolong Survival in Colorectal Cancer Mouse Models. Int. J. Mol. Sci. 2024, 25, 13160. [Google Scholar] [CrossRef]
- Sala, R.; Esquer, H.; Kellett, T.; Kearns, J.T.; Awolade, P.; Zhou, Q.; LaBarbera, D.V. CHD1L Regulates Cell Survival in Breast Cancer and Its Inhibition by OTI-611 Impedes the DNA Damage Response and Induces PARthanatos. Int. J. Mol. Sci. 2024, 25, 8590. [Google Scholar] [CrossRef]
- Xiong, X.; Lai, X.; Li, A.; Liu, Z.; Ma, N. Diversity roles of CHD1L in normal cell function and tumorigenesis. Biomark. Res. 2021, 9, 16. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Jiao, P.; Deng, X.; Xie, Y. The high expression of CHD1L and its clinical significance in human solid tumors: A meta-analysis. Medicine 2021, 100, e24851. [Google Scholar] [CrossRef]
- He, L.R.; Ma, N.F.; Chen, J.W.; Li, B.K.; Guan, X.Y.; Liu, M.Z.; Xie, D. Overexpression of CHD1L is positively associated with metastasis of lung adenocarcinoma and predicts patients poor survival. Oncotarget 2015, 6, 31181–31190. [Google Scholar] [CrossRef]
- Wu, J.; Zong, Y.; Fei, X.; Chen, X.; Huang, O.; He, J.; Chen, W.; Li, Y.; Shen, K.; Zhu, L. Presence of CHD1L over-expression is associated with aggressive tumor biology and is a novel prognostic biomarker for patient survival in human breast cancer. PLoS ONE 2014, 9, e98673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Zhang, Q.; Ding, Y.J.; Ren, Y.H.; Yang, H.P.; Xi, Q.; Cheng, Y.N.; Miao, G.L.; Liu, H.K.; Li, C.X.; et al. Overexpression of CHD1L is associated with poor survival and aggressive tumor biology in esophageal carcinoma. Oncotarget 2017, 8, 74178–74187. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, J.; Zhu, L.; Cai, J.; Zhang, J.; Qu, Y.; Zhang, H.; Liu, B.; Zhao, R.; Zhu, Z. CHD1L promotes tumor progression and predicts survival in colorectal carcinoma. J. Surg. Res. 2013, 185, 84–91. [Google Scholar] [CrossRef]
- Hyeon, J.; Ahn, S.; Park, C.K. CHD1L Is a Marker for Poor Prognosis of Hepatocellular Carcinoma after Surgical Resection. Korean J. Pathol. 2013, 47, 9–15. [Google Scholar] [CrossRef]
- Gottschalk, A.J.; Timinszky, G.; Kong, S.E.; Jin, J.; Cai, Y.; Swanson, S.K.; Washburn, M.P.; Florens, L.; Ladurner, A.G.; Conaway, J.W.; et al. Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc. Natl. Acad. Sci. USA 2009, 106, 13770–13774. [Google Scholar] [CrossRef]
- Gottschalk, A.J.; Trivedi, R.D.; Conaway, J.W.; Conaway, R.C. Activation of the SNF2 family ATPase ALC1 by poly(ADP-ribose) in a stable ALC1.PARP1.nucleosome intermediate. J. Biol. Chem. 2012, 287, 43527–43532. [Google Scholar] [CrossRef]
- Lehmann, L.C.; Hewitt, G.; Aibara, S.; Leitner, A.; Marklund, E.; Maslen, S.L.; Maturi, V.; Chen, Y.; van der Spoel, D.; Skehel, J.M.; et al. Mechanistic Insights into Autoinhibition of the Oncogenic Chromatin Remodeler ALC1. Mol. Cell 2017, 68, 847–859.e7. [Google Scholar] [CrossRef]
- Lehmann, L.C.; Bacic, L.; Hewitt, G.; Brackmann, K.; Sabantsev, A.; Gaullier, G.; Pytharopoulou, S.; Degliesposti, G.; Okkenhaug, H.; Tan, S.; et al. Mechanistic Insights into Regulation of the ALC1 Remodeler by the Nucleosome Acidic Patch. Cell Rep. 2020, 33, 108529. [Google Scholar] [CrossRef]
- Juhasz, S.; Smith, R.; Schauer, T.; Spekhardt, D.; Mamar, H.; Zentout, S.; Chapuis, C.; Huet, S.; Timinszky, G. The chromatin remodeler ALC1 underlies resistance to PARP inhibitor treatment. Sci. Adv. 2020, 6, eabb8626. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Chan, T.H.; Liu, M.; Kong, K.L.; Qiu, J.L.; Li, Y.; Yuan, Y.F.; Guan, X.Y. SPOCK1 is regulated by CHD1L and blocks apoptosis and promotes HCC cell invasiveness and metastasis in mice. Gastroenterology 2013, 144, 179–191.e4. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Zhao, H.; Qiu, X.; Chen, W.; Wang, D.; Ban, N.; Fan, S.; Shen, C.; Xia, X.; et al. CHD1L regulates cell cycle, apoptosis, and migration in glioma. Cell. Mol. Neurobiol. 2016, 36, 565–576. [Google Scholar] [CrossRef]
- Tsuda, M.; Cho, K.; Ooka, M.; Shimizu, N.; Watanabe, R.; Yasui, A.; Nakazawa, Y.; Ogi, T.; Harada, H.; Agama, K.; et al. ALC1/CHD1L, a chromatin-remodeling enzyme, is required for efficient base excision repair. PLoS ONE 2017, 12, e0188320. [Google Scholar] [CrossRef]
- Verma, P.; Zhou, Y.; Cao, Z.; Deraska, P.V.; Deb, M.; Arai, E.; Li, W.; Shao, Y.; Puentes, L.; Li, Y.; et al. ALC1 links chromatin accessibility to PARP inhibitor response in homologous recombination-deficient cells. Nat. Cell Biol. 2021, 23, 160–171. [Google Scholar] [CrossRef]
- Chen, L.; Hu, L.; Chan, T.H.; Tsao, G.S.; Xie, D.; Huo, K.K.; Fu, L.; Ma, S.; Zheng, B.J.; Guan, X.Y. Chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like (CHD1l) gene suppresses the nucleus-to-mitochondria translocation of nur77 to sustain hepatocellular carcinoma cell survival. Hepatology 2009, 50, 122–129. [Google Scholar] [CrossRef]
- Soltan, M.A.; Eldeen, M.A.; Eid, R.A.; Alyamani, N.M.; Alqahtani, L.S.; Albogami, S.; Jafri, I.; Park, M.N.; Alsharif, G.; Fayad, E.; et al. A pan-cancer analysis reveals CHD1L as a prognostic and immunological biomarker in several human cancers. Front. Mol. Biosci. 2023, 10, 1017148. [Google Scholar] [CrossRef]
- Chen, L.; Chan, T.H.; Yuan, Y.F.; Hu, L.; Huang, J.; Ma, S.; Wang, J.; Dong, S.S.; Tang, K.H.; Xie, D.; et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J. Clin. Investig. 2010, 120, 1178–1191. [Google Scholar] [CrossRef]
- Su, F.R.; Ding, J.H.; Bo, L.; Liu, X.G. Chromodomain helicase/ATPase DNA binding protein 1-like protein expression predicts poor prognosis in nasopharyngeal carcinoma. Exp. Ther. Med. 2014, 8, 1745–1750. [Google Scholar] [CrossRef]
- He, W.P.; Zhou, J.; Cai, M.Y.; Xiao, X.S.; Liao, Y.J.; Kung, H.F.; Guan, X.Y.; Xie, D.; Yang, G.F. CHD1L protein is overexpressed in human ovarian carcinomas and is a novel predictive biomarker for patients survival. BMC Cancer 2012, 12, 437. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; De Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Mills, C.C.; Kolb, E.; Sampson, V.B. Development of Chemotherapy with Cell-Cycle Inhibitors for Adult and Pediatric Cancer Therapy. Cancer Res. 2018, 78, 320–325. [Google Scholar] [CrossRef]
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting cell-cycle machinery in cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Fei, X.; Chen, W.; Li, Y.; Shen, K.; Zhu, L. CHD1L promotes cell cycle progression and cell motility by up-regulating MDM2 in breast cancer. Am. J. Transl. Res. 2019, 11, 1581–1592. [Google Scholar]
- Ma, N.-F.; Hu, L.; Fung, J.M.; Xie, D.; Zheng, B.-J.; Chen, L.; Tang, D.-J.; Fu, L.; Wu, Z.; Chen, M.; et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology 2008, 47, 503–510. [Google Scholar] [CrossRef]
- Zielke, N.; Edgar, B.A. FUCCI sensors: Powerful new tools for analysis of cell proliferation. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 469–487. [Google Scholar] [CrossRef]
- Zhang, W.; Gou, P.; Dupret, J.M.; Chomienne, C.; Rodrigues-Lima, F. Etoposide, an anticancer drug involved in therapy-related secondary leukemia: Enzymes at play. Transl. Oncol. 2021, 14, 101169. [Google Scholar] [CrossRef]
- Tchakarska, G.; Sola, B. The double dealing of cyclin D1. Cell Cycle 2020, 19, 163–178. [Google Scholar] [CrossRef]
- Dai, M.; Boudreault, J.; Wang, N.; Poulet, S.; Daliah, G.; Yan, G.; Moamer, A.; Burgos, S.A.; Sabri, S.; Ali, S.; et al. Differential Regulation of Cancer Progression by CDK4/6 Plays a Central Role in DNA Replication and Repair Pathways. Cancer Res. 2021, 81, 1332–1346. [Google Scholar] [CrossRef]
- Sobecki, M.; Mrouj, K.; Camasses, A.; Parisis, N.; Nicolas, E.; Llères, D.; Gerbe, F.; Prieto, S.; Krasinska, L.; David, A.; et al. The cell proliferation antigen Ki-67 organises heterochromatin. eLife 2016, 5, e13722. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Bhonde, M.R.; Hanski, M.L.; Stehr, J.; Jebautzke, B.; Peiro-Jordan, R.; Fechner, H.; Yokoyama, K.K.; Lin, W.C.; Zeitz, M.; Hanski, C. Mismatch repair system decreases cell survival by stabilizing the tetraploid G1 arrest in response to SN-38. Int. J. Cancer 2010, 126, 2813–2825. [Google Scholar] [CrossRef]
- te Poele, R.H.; Joel, S.P. Schedule-dependent cytotoxicity of SN-38 in p53 wild-type and mutant colon adenocarcinoma cell lines. Br. J. Cancer 1999, 81, 1285–1293. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Yaffee, P.; Osipov, A.; Tan, C.; Tuli, R.; Hendifar, A. Review of systemic therapies for locally advanced and metastatic rectal cancer. J. Gastrointest. Oncol. 2015, 6, 185–200. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esquer, H.; Zhou, Q.; LaBarbera, D.V. Targeted Inhibition of CHD1L by OTI-611 Reprograms Chemotherapy and Targeted Therapy-Induced Cell Cycle Arrest and Suppresses Proliferation to Produce Synergistic Antitumor Effects in Breast and Colorectal Cancer. Cells 2025, 14, 318. https://doi.org/10.3390/cells14050318
Esquer H, Zhou Q, LaBarbera DV. Targeted Inhibition of CHD1L by OTI-611 Reprograms Chemotherapy and Targeted Therapy-Induced Cell Cycle Arrest and Suppresses Proliferation to Produce Synergistic Antitumor Effects in Breast and Colorectal Cancer. Cells. 2025; 14(5):318. https://doi.org/10.3390/cells14050318
Chicago/Turabian StyleEsquer, Hector, Qiong Zhou, and Daniel V. LaBarbera. 2025. "Targeted Inhibition of CHD1L by OTI-611 Reprograms Chemotherapy and Targeted Therapy-Induced Cell Cycle Arrest and Suppresses Proliferation to Produce Synergistic Antitumor Effects in Breast and Colorectal Cancer" Cells 14, no. 5: 318. https://doi.org/10.3390/cells14050318
APA StyleEsquer, H., Zhou, Q., & LaBarbera, D. V. (2025). Targeted Inhibition of CHD1L by OTI-611 Reprograms Chemotherapy and Targeted Therapy-Induced Cell Cycle Arrest and Suppresses Proliferation to Produce Synergistic Antitumor Effects in Breast and Colorectal Cancer. Cells, 14(5), 318. https://doi.org/10.3390/cells14050318





