Prenatal Exposure to Metals Is Associated with Placental Decelerated Epigenetic Gestational Age in a Sex-Dependent Manner in Infants Born Extremely Preterm
Abstract
1. Introduction
2. Materials and Methods
2.1. The ELGAN Study
2.2. Chronologcial Gestational Age Ascertainment
2.3. Placental Sampling and DNA Methylation Quantification
2.4. Placental Epigenetic Gestational Age Acceleration Estimations
2.5. Quantification of Metals and Metalloids in Umbilical Cord Tissue
2.6. Covariate Selection
2.7. Statistical Analysis
3. Results
3.1. Study Population
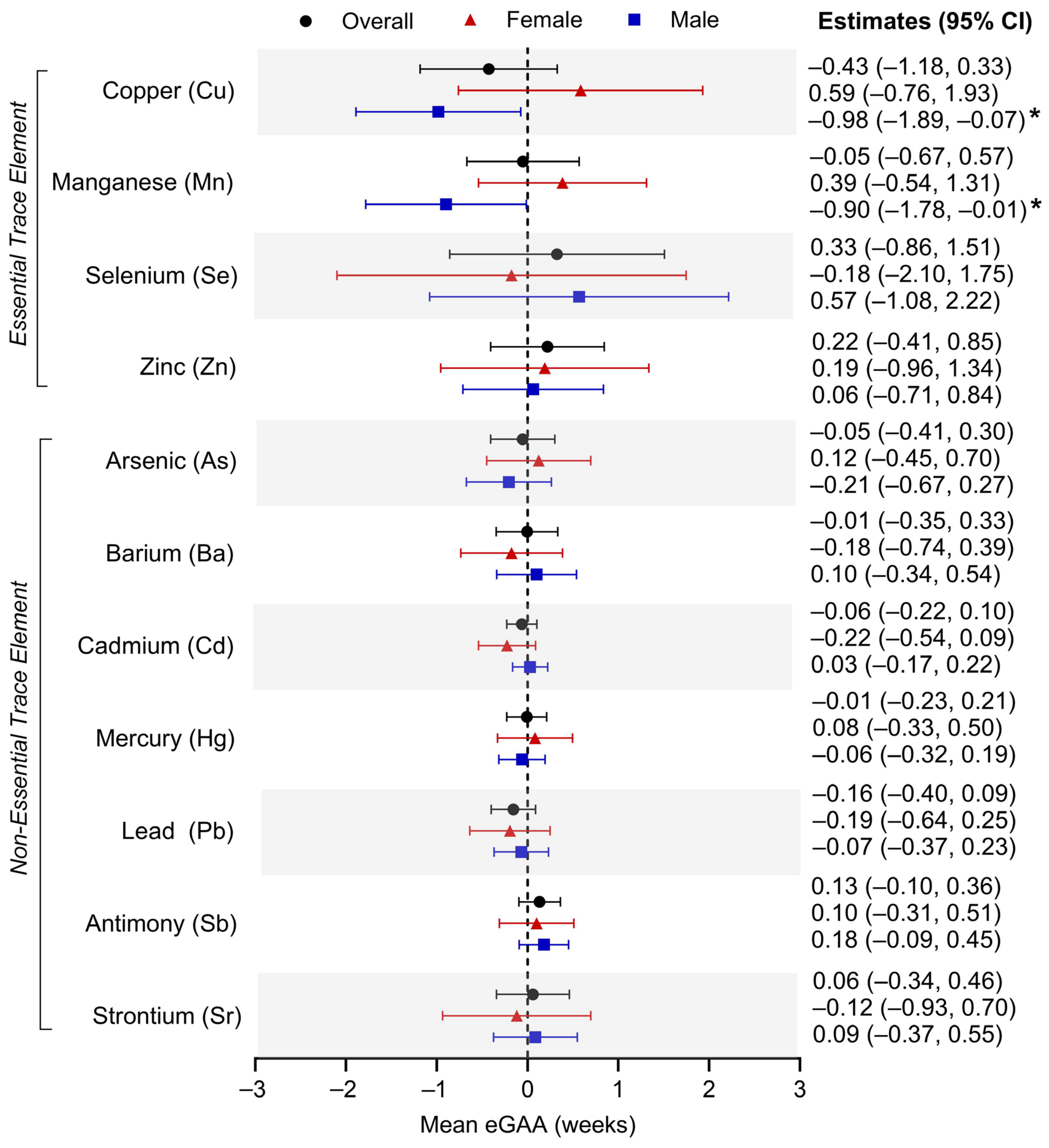
3.2. Individual Umbilical Cord Metal Levels and Placental eGAA
3.3. Individual Umbilical Cord Metal Levels and RPC CpG Methylation
3.4. Umbilical Cord Metal Mixtures and Placental eGAA
3.5. Sensitivity Analyses by Study Site Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic life and Human Health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). ATSDR’s Substance Priority List; ATSDR: Atlanta, GA, USA, 2022. [Google Scholar]
- World Health Organization (WHO). Arsenic; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Eaves, L.A.; Keil, A.P.; Rager, J.E.; George, A.; Fry, R.C. Analysis of the novel NCWELL database highlights two decades of co-occurrence of toxic metals in North Carolina private well water: Public health and environmental justice implications. Sci. Total Environ. 2022, 812, 151479. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Manganese; ATSDR: Atlanta, GA, USA, 2012. [Google Scholar]
- Ma, J.; Zhou, Y.; Wang, D.; Guo, Y.; Wang, B.; Xu, Y.; Chen, W. Associations between essential metals exposure and metabolic syndrome (MetS): Exploring the mediating role of systemic inflammation in a general Chinese population. Environ. Int. 2020, 140, 105802. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Garcia, N.Y.; Cipriano Ramirez, A.I.; Juarez, K.; Brand Galindo, J.; Briceno, G.; Calderon Martinez, E. Maternal Exposure to Arsenic and Its Impact on Maternal and Fetal Health: A Review. Cureus 2023, 15, e49177. [Google Scholar] [CrossRef]
- Borghese, M.M.; Fisher, M.; Ashley-Martin, J.; Fraser, W.D.; Trottier, H.; Lanphear, B.; Johnson, M.; Helewa, M.; Foster, W.; Walker, M.; et al. Individual, Independent, and Joint Associations of Toxic Metals and Manganese on Hypertensive Disorders of Pregnancy: Results from the MIREC Canadian Pregnancy Cohort. Environ. Health Perspect. 2023, 131, 47014. [Google Scholar] [CrossRef]
- Rahman, M.L.; Oken, E.; Hivert, M.F.; Rifas-Shiman, S.; Lin, P.D.; Colicino, E.; Wright, R.O.; Amarasiriwardena, C.; Claus Henn, B.G.; Gold, D.R.; et al. Early pregnancy exposure to metal mixture and birth outcomes—A prospective study in Project Viva. Environ. Int. 2021, 156, 106714. [Google Scholar] [CrossRef]
- Caserta, D.; Graziano, A.; Lo Monte, G.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2198–2206. [Google Scholar] [PubMed]
- Gundacker, C.; Hengstschlager, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Chen, Z.; Myers, R.; Wei, T.; Bind, E.; Kassim, P.; Wang, G.; Ji, Y.; Hong, X.; Caruso, D.; Bartell, T.; et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2020, 14, 47–50. [Google Scholar] [CrossRef]
- Young, J.L.; Cai, L.; States, J.C. Impact of prenatal arsenic exposure on chronic adult diseases. Syst. Biol. Reprod. Med. 2018, 64, 469–483. [Google Scholar] [CrossRef]
- Appleton, A.A.; Jackson, B.P.; Karagas, M.; Marsit, C.J. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics 2017, 12, 607–615. [Google Scholar] [CrossRef]
- Tian, F.Y.; Everson, T.M.; Lester, B.; Punshon, T.; Jackson, B.P.; Hao, K.; Lesseur, C.; Chen, J.; Karagas, M.R.; Marsit, C.J. Selenium-associated DNA methylation modifications in placenta and neurobehavioral development of newborns: An epigenome-wide study of two U.S. birth cohorts. Environ. Int. 2020, 137, 105508. [Google Scholar] [CrossRef]
- Knight, A.K.; Craig, J.M.; Theda, C.; Bækvad-Hansen, M.; Bybjerg-Grauholm, J.; Hansen, C.S.; Hollegaard, M.V.; Hougaard, D.M.; Mortensen, P.B.; Weinsheimer, S.M.; et al. An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biol. 2016, 17, 206. [Google Scholar] [CrossRef]
- Bohlin, J.; Håberg, S.E.; Magnus, P.; Reese, S.E.; Gjessing, H.K.; Magnus, M.C.; Parr, C.L.; Page, C.M.; London, S.J.; Nystad, W. Prediction of gestational age based on genome-wide differentially methylated regions. Genome Biol. 2016, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choufani, S.; Weksberg, R.; Wilson, S.L.; Yuan, V.; Burt, A.; Marsit, C.; Lu, A.T.; Ritz, B.; Bohlin, J.; et al. Placental epigenetic clocks: Estimating gestational age using placental DNA methylation levels. Aging 2019, 11, 4238–4253. [Google Scholar] [CrossRef]
- Mayne, B.T.; Leemaqz, S.Y.; Smith, A.K.; Breen, J.; Roberts, C.T.; Bianco-Miotto, T. Accelerated placental aging in early onset preeclampsia pregnancies identified by DNA methylation. Epigenomics 2017, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Song, A.Y.; Feinberg, J.I.; Bakulski, K.M.; Croen, L.A.; Fallin, M.D.; Newschaffer, C.J.; Hertz-Picciotto, I.; Schmidt, R.J.; Ladd-Acosta, C.; Volk, H.E. Prenatal Exposure to Ambient Air Pollution and Epigenetic Aging at Birth in Newborns. Front. Genet. 2022, 13, 929416. [Google Scholar] [CrossRef]
- Bozack, A.K.; Rifas-Shiman, S.L.; Baccarelli, A.A.; Wright, R.O.; Gold, D.R.; Oken, E.; Hivert, M.F.; Cardenas, A. Associations of prenatal one-carbon metabolism nutrients and metals with epigenetic aging biomarkers at birth and in childhood in a US cohort. Aging 2024, 16, 3107–3136. [Google Scholar] [CrossRef]
- Dieckmann, L.; Lahti-Pulkkinen, M.; Kvist, T.; Lahti, J.; DeWitt, P.E.; Cruceanu, C.; Laivuori, H.; Sammallahti, S.; Villa, P.M.; Suomalainen-König, S.; et al. Characteristics of epigenetic aging across gestational and perinatal tissues. Clin. Epigenet. 2021, 13, 97. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Allred, E.N.; Dammann, O.; Hirtz, D.; Kuban, K.C.; Paneth, N.; Leviton, A.; Investigators, E.s. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum. Dev. 2009, 85, 719–725. [Google Scholar] [CrossRef]
- McElrath, T.F.; Hecht, J.L.; Dammann, O.; Boggess, K.; Onderdonk, A.; Markenson, G.; Harper, M.; Delpapa, E.; Allred, E.N.; Leviton, A.; et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: An epidemiologic approach to classification. Am. J. Epidemiol. 2008, 168, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Smeester, L.; Bommarito, P.A.; Grace, M.R.; Boggess, K.; Kuban, K.; Karagas, M.R.; Marsit, C.J.; O’Shea, T.M.; Fry, R.C. Sexual epigenetic dimorphism in the human placenta: Implications for susceptibility during the prenatal period. Epigenomics 2017, 9, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Bulka, C.M.; Martin, C.L.; Roell, K.; Santos, H.P.; O’Shea, T.M.; Smeester, L.; Fry, R.; Dhingra, R. Placental epigenetic gestational aging in relation to maternal sociodemographic factors and smoking among infants born extremely preterm: A descriptive study. Epigenetics 2022, 17, 2389–2403. [Google Scholar] [CrossRef]
- Triche, T.J.; Weisenberger, D.J.; Van Den Berg, D.; Laird, P.W.; Siegmund, K.D. Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res. 2013, 41, e90. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.P.; Triche, T.J.; Hansen, K.D. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 2017, 33, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Bulka, C.M.; Eaves, L.A.; Gardner, A.J.; Parsons, P.J.; Galusha, A.L.; Roell, K.R.; Smeester, L.; O’Shea, T.M.; Fry, R.C. Prenatal exposure to multiple metallic and metalloid trace elements and the risk of bacterial sepsis in extremely low gestational age newborns: A prospective cohort study. Front. Epidemiol. 2022, 2, 8389. [Google Scholar] [CrossRef] [PubMed]
- Eaves, L.A.; Bulka, C.M.; Rager, J.E.; Gardner, A.J.; Galusha, A.L.; Parsons, P.J.; O’Shea, T.M.; Fry, R.C. Metal mixtures modeling identifies birth weight-associated gene networks in the placentas of children born extremely preterm. Chemosphere 2023, 313, 137469. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (EPA). Guidance for Data Quality Assessment: Practical Methods for Data Analysis; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2000. [Google Scholar]
- Bulka, C.M.; Rajkotwala, H.M.; Eaves, L.A.; Gardner, A.J.; Parsons, P.J.; Galusha, A.L.; O’Shea, T.M.; Fry, R.C. Placental cellular composition and umbilical cord tissue metal(loid) concentrations: A descriptive molecular epidemiology study leveraging DNA methylation. Placenta 2024, 147, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Yuan, V.; Hui, D.; Yin, Y.; Peñaherrera, M.S.; Beristain, A.G.; Robinson, W.P. Cell-specific characterization of the placental methylome. BMC Genom. 2021, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Keil, A. The qgcomp Package: G-Computation on Exposure Quantiles. 2023. Available online: https://cran.r-project.org/package=qgcomp (accessed on 9 September 2024).
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Born, G.; Breuer, D.; Wang, S.; Rohlmann, A.; Coulon, P.; Vakili, P.; Reissner, C.; Kiefer, F.; Heine, M.; Pape, H.C.; et al. Modulation of synaptic function through the alpha-neurexin-specific ligand neurexophilin-1. Proc. Natl. Acad. Sci. USA 2014, 111, E1274–E1283. [Google Scholar] [CrossRef]
- Wang, Y.; Du, W.; Sun, Y.; Zhang, J.; Ma, C.; Jin, X. CRTC1 is a potential target to delay aging-induced cognitive deficit by protecting the integrity of the blood-brain barrier via inhibiting inflammation. J. Cereb. Blood Flow Metab. 2023, 43, 1042–1059. [Google Scholar] [CrossRef]
- Finsterwald, C.; Fiumelli, H.; Cardinaux, J.R.; Martin, J.L. Regulation of dendritic development by BDNF requires activation of CRTC1 by glutamate. J. Biol. Chem. 2010, 285, 28587–28595. [Google Scholar] [CrossRef]
- Schaiff, W.T.; Bildirici, I.; Cheong, M.; Chern, P.L.; Nelson, D.M.; Sadovsky, Y. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J. Clin. Endocrinol. Metab. 2005, 90, 4267–4275. [Google Scholar] [CrossRef]
- Tarrade, A.; Schoonjans, K.; Guibourdenche, J.; Bidart, J.M.; Vidaud, M.; Auwerx, J.; Rochette-Egly, C.; Evain-Brion, D. PPAR gamma/RXR alpha heterodimers are involved in human CG beta synthesis and human trophoblast differentiation. Endocrinology 2001, 142, 4504–4514. [Google Scholar] [CrossRef]
- Sakamoto, M.; Yasutake, A.; Domingo, J.L.; Chan, H.M.; Kubota, M.; Murata, K. Relationships between trace element concentrations in chorionic tissue of placenta and umbilical cord tissue: Potential use as indicators for prenatal exposure. Environ. Int. 2013, 60, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jin, L.; Yu, J.; Su, Z.; Sun, Y.; Liu, Y.; Xie, Q.; Li, Z.; Wang, L.; Ren, A. Essential trace elements in umbilical cord tissue and risk for neural tube defects. Reprod. Toxicol. 2020, 98, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Li, Y.; Xu, F.; Wang, M.; Lin, X.; Li, Y.; Yang, C.; Cao, Z.; Xia, W.; et al. Sex-specific associations of early postnatal blood copper levels with neurodevelopment at 2 years of age. J. Trace Elem. Med. Biol. 2022, 74, 127072. [Google Scholar] [CrossRef]
- Chiu, Y.M.; Claus Henn, B.; Hsu, H.L.; Pendo, M.P.; Coull, B.A.; Austin, C.; Cagna, G.; Fedrighi, C.; Placidi, D.; Smith, D.R.; et al. Sex differences in sensitivity to prenatal and early childhood manganese exposure on neuromotor function in adolescents. Environ. Res. 2017, 159, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Inkster, A.M.; Yuan, V.; Konwar, C.; Matthews, A.M.; Brown, C.J.; Robinson, W.P. A cross-cohort analysis of autosomal DNA methylation sex differences in the term placenta. Biol. Sex Differ. 2021, 12, 38. [Google Scholar] [CrossRef]
- Bulka, C.M.; Everson, T.M.; Burt, A.A.; Marsit, C.J.; Karagas, M.R.; Boyle, K.E.; Niemiec, S.; Kechris, K.; Davidson, E.J.; Yang, I.V.; et al. Sex-based differences in placental DNA methylation profiles related to gestational age: An NIH ECHO meta-analysis. Epigenetics 2023, 18, 2179726. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.Y.; Kim, Y.M.; Kim, G.; Kim, E.H.; Lee, B.K.; So, H.; Kwon, Y.; Shin, J.; Kim, M. Prevalence of asthma in preterm and associated risk factors based on prescription data from the Korean National Health Insurance database. Sci. Rep. 2023, 13, 4484. [Google Scholar] [CrossRef]
- Christensen, R.; Chau, V.; Synnes, A.; Guo, T.; Ufkes, S.; Grunau, R.E.; Miller, S.P. Preterm Sex Differences in Neurodevelopment and Brain Development from Early Life to 8 Years of Age. J. Pediatr. 2025, 276, 114271. [Google Scholar] [CrossRef]
- Nwanaji-Enwerem, J.C.; Colicino, E.; Specht, A.J.; Gao, X.; Wang, C.; Vokonas, P.; Weisskopf, M.G.; Boyer, E.W.; Baccarelli, A.A.; Schwartz, J. Individual species and cumulative mixture relationships of 24-hour urine metal concentrations with DNA methylation age variables in older men. Environ. Res. 2020, 186, 109573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zan, G.; Feng, X.; Bao, Y.; Huang, S.; Luo, X.; Xu, X.; Zhang, Z.; Yang, X. The associations of multiple metals mixture with accelerated DNA methylation aging. Environ. Pollut. 2021, 269, 116230. [Google Scholar] [CrossRef]
- Boyer, K.; Domingo-Relloso, A.; Jiang, E.; Haack, K.; Goessler, W.; Zhang, Y.; Umans, J.G.; Belsky, D.W.; Cole, S.A.; Navas-Acien, A.; et al. Metal mixtures and DNA methylation measures of biological aging in American Indian populations. Environ. Int. 2023, 178, 108064. [Google Scholar] [CrossRef]
- McArdle, H.J.; Andersen, H.S.; Jones, H.; Gambling, L. Copper and iron transport across the placenta: Regulation and interactions. J. Neuroendocrinol. 2008, 20, 427–431. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef]
- Lien, Y.C.; Zhang, Z.; Cheng, Y.; Polyak, E.; Sillers, L.; Falk, M.J.; Ischiropoulos, H.; Parry, S.; Simmons, R.A. Human Placental Transcriptome Reveals Critical Alterations in Inflammation and Energy Metabolism with Fetal Sex Differences in Spontaneous Preterm Birth. Int. J. Mol. Sci. 2021, 22, 7899. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.; Everson, T.M.; Punshon, T.; Jackson, B.P.; Hao, K.; Lambertini, L.; Chen, J.; Karagas, M.R.; Marsit, C.J. Copper associates with differential methylation in placentae from two US birth cohorts. Epigenetics 2020, 15, 215–230. [Google Scholar] [CrossRef]
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. Met. Ions Life Sci. 2013, 13, 199–227. [Google Scholar] [CrossRef]
- Evans, G.R.; Masullo, L.N. Manganese Toxicity. 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560903/ (accessed on 29 August 2024).
- Arbuckle, T.E.; Liang, C.L.; Morisset, A.S.; Fisher, M.; Weiler, H.; Cirtiu, C.M.; Legrand, M.; Davis, K.; Ettinger, A.S.; Fraser, W.D.; et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 2016, 163, 270–282. [Google Scholar] [CrossRef]
- Yoon, M.; Nong, A.; Clewell, H.J., 3rd; Taylor, M.D.; Dorman, D.C.; Andersen, M.E. Evaluating placental transfer and tissue concentrations of manganese in the pregnant rat and fetuses after inhalation exposures with a PBPK model. Toxicol. Sci. 2009, 112, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Schroeter, J.D.; Nong, A.; Taylor, M.D.; Dorman, D.C.; Andersen, M.E.; Clewell, H.J., 3rd. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: Describing manganese homeostasis during development. Toxicol. Sci. 2011, 122, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hivert, M.F.; Rifas-Shiman, S.L.; Rahman, M.L.; Oken, E.; Cardenas, A.; Mueller, N.T. Prospective Association Between Manganese in Early Pregnancy and the Risk of Preeclampsia. Epidemiology 2020, 31, 677–680. [Google Scholar] [CrossRef]
- Wu, A.; Li, J.; Yuan, J.; Zhang, N.; Zhang, Y.; Li, M.; Zhu, T. Association of Blood Manganese and Preeclampsia: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2024, 202, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Martin, J.; Dodds, L.; Arbuckle, T.E.; Ettinger, A.S.; Shapiro, G.D.; Fisher, M.; Monnier, P.; Morisset, A.S.; Fraser, W.D.; Bouchard, M.F. Maternal and cord blood manganese (Mn) levels and birth weight: The MIREC birth cohort study. Int. J. Hyg. Environ. Health 2018, 221, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, G.; Gao, Y.; Wang, P.; Shi, R.; Huang, H.; Tian, Y. Manganese concentrations in maternal-infant blood and birth weight. Environ. Sci. Pollut. Res. Int. 2014, 21, 6170–6175. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tang, W.Y.; Wills-Karp, M.; Ji, H.; Bartell, T.R.; Ji, Y.; Hong, X.; Pearson, C.; Cheng, T.L.; Wang, X. A Nonlinear Relation Between Maternal Red Blood Cell Manganese Concentrations and Child Blood Pressure at Age 6–12 y: A Prospective Birth Cohort Study. J. Nutr. 2021, 151, 570–578. [Google Scholar] [CrossRef]
- Rhee, C.; Lee, B.K.; Beck, S.; Anjum, A.; Cook, K.R.; Popowski, M.; Tucker, H.O.; Kim, J. Arid3a is essential to execution of the first cell fate decision via direct embryonic and extraembryonic transcriptional regulation. Genes. Dev. 2014, 28, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Edwards, M.; Dang, C.; Harris, J.; Brown, M.; Kim, J.; Tucker, H.O. ARID3A is required for mammalian placenta development. Dev. Biol. 2017, 422, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Grundeken, M.; Gustin, K.; Vahter, M.; Delaval, M.; Barman, M.; Sandin, A.; Sandberg, A.S.; Wold, A.E.; Broberg, K.; Kippler, M. Toxic metals and essential trace elements in placenta and their relation to placental function. Environ. Res. 2024, 248, 118355. [Google Scholar] [CrossRef]
- Diez-Ahijado, L.; Cilleros-Portet, A.; Fernandez-Jimenez, N.; Fernandez, M.F.; Guxens, M.; Julvez, J.; Llop, S.; Lopez-Espinosa, M.J.; Subiza-Perez, M.; Lozano, M.; et al. Evaluating the association between placenta DNA methylation and cognitive functions in the offspring. Transl. Psychiatry 2024, 14, 383. [Google Scholar] [CrossRef]
- Parra-Damas, A.; Valero, J.; Chen, M.; Espana, J.; Martin, E.; Ferrer, I.; Rodriguez-Alvarez, J.; Saura, C.A. Crtc1 activates a transcriptional program deregulated at early Alzheimer’s disease-related stages. J. Neurosci. 2014, 34, 5776–5787. [Google Scholar] [CrossRef]
- Uchida, S.; Shumyatsky, G.P. Epigenetic regulation of Fgf1 transcription by CRTC1 and memory enhancement. Brain Res. Bull. 2018, 141, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C., Jr.; Morcillo, P.; Ijomone, O.M.; Venkataramani, V.; Harrison, F.E.; Lee, E.; Bowman, A.B.; Aschner, M. New Insights on the Role of Manganese in Alzheimer’s Disease and Parkinson’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 3546. [Google Scholar] [CrossRef]
- Cladis, D.P.; Kleiner, A.C.; Santerre, C.R. Mercury content in commercially available finfish in the United States. J. Food Prot. 2014, 77, 1361–1366. [Google Scholar] [CrossRef]
- Real, M.I.H.; Azam, H.M.; Majed, N. Consumption of heavy metal contaminated foods and associated risks in Bangladesh. Environ. Monit. Assess. 2017, 189, 651. [Google Scholar] [CrossRef] [PubMed]
- Kozikowska, I.; Binkowski, L.J.; Szczepanska, K.; Slawska, H.; Miszczuk, K.; Sliwinska, M.; Laciak, T.; Stawarz, R. Mercury concentrations in human placenta, umbilical cord, cord blood and amniotic fluid and their relations with body parameters of newborns. Environ. Pollut. 2013, 182, 256–262. [Google Scholar] [CrossRef]
- Kuivenhoven, M.; Mason, K. Arsenic Toxicity; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Eaves, L.A.; Fry, R.C. Invited Perspective: Toxic Metals and Hypertensive Disorders of Pregnancy. Environ. Health Perspect. 2023, 131, 41303. [Google Scholar] [CrossRef]
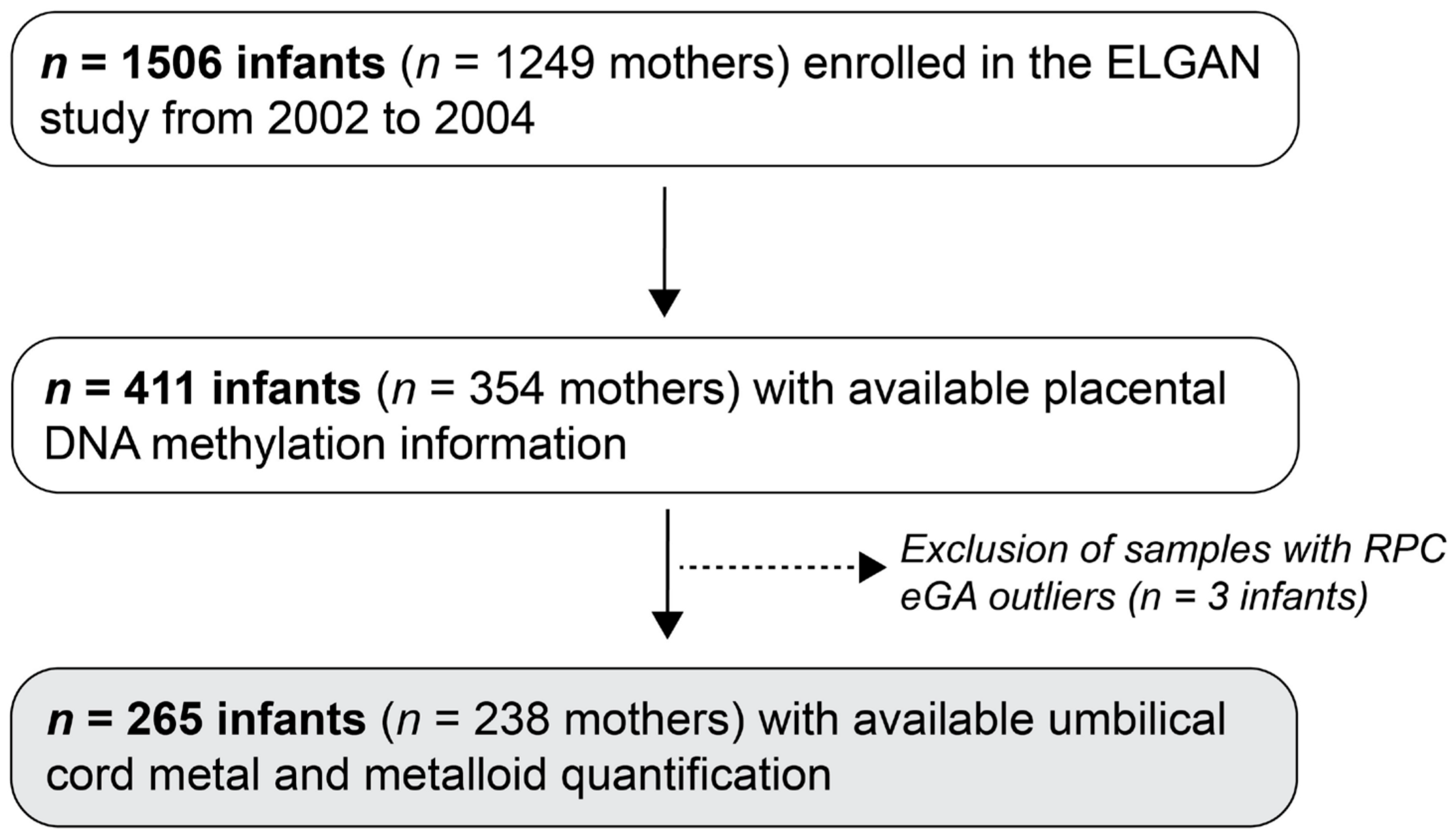
| Maternal Characteristics | Median (IQR) or n (%) |
|---|---|
| Age, years | 29.40 (24.70, 34.00) |
| Pre-pregnancy body mass index (BMI) | 23.19 (20.49, 29.27) |
| Self-reported race | |
| White | 165 (62.98%) |
| Black | 74 (28.24%) |
| Other | 23 (8.78%) |
| Missing | 3 |
| Self-reported ethnicity | |
| Non-Hispanic | 243 (91.70%) |
| Hispanic | 22 (8.30%) |
| Educational attainment | |
| High school diploma or less | 101 (38.85%) |
| At least some college | 55 (21.15%) |
| College degree or greater | 104 (40.00%) |
| Missing | 5 |
| Parity | |
| 0 | 147 (55.47%) |
| 1 | 74 (27.92%) |
| 2+ | 44 (16.60%) |
| Smoking during pregnancy | |
| No | 239 (91.57%) |
| Yes | 22 (8.43%) |
| Missing | 4 |
| Public insurance | |
| No | 176 (67.18%) |
| Yes | 86 (32.82%) |
| Missing | 3 |
| Study site region | |
| New England | 120 (45.28%) |
| North Carolina | 81 (30.57%) |
| Midwest | 64 (24.15%) |
| Infant Characteristics | Median (IQR) or n (%) |
| Gestational age, weeks | 26.29 (25.14, 27.29) |
| Epigenetic gestational age (eGA), weeks | 28.17 (27.05, 29.32) |
| eGA acceleration (eGAA) residual, weeks | −0.03 (−0.52, 0.48) |
| Sex | |
| Female | 120 (45.28%) |
| Male | 145 (54.82%) |
| Birthweight, grams | |
| ≤750 | 101 (38.11%) |
| 751–1000 | 116 (43.77%) |
| 1001–1250 | 40 (15.09%) |
| >1250 | 8 (3.02%) |
| Metal | Unit | Sample | Mean (SD) | Range | Median (IQR) | p 1 |
|---|---|---|---|---|---|---|
| Copper (Cu) | μg/g | Full | 3.83 (1.37) | 1.62–14.31 | 3.48 (2.97, 4.32) | 0.08 |
| Female | 3.67 (1.15) | 1.96–7.93 | 3.42 (2.88, 4.09) | |||
| Male | 3.96 (1.52) | 1.62–14.31 | 3.59 (3.02, 4.53) | |||
| Manganese (Mn) | μg/g | Full | 0.40 (0.37) | 0.10–5.58 | 0.34 (0.30, 0.42) | >0.90 |
| Female | 0.43 (0.51) | 0.10–5.58 | 0.34 (0.30, 0.42) | |||
| Male | 0.38 (0.18) | 0.19–1.70 | 0.34 (0.30, 0.42) | |||
| Selenium (Se) | μg/g | Full | 0.88 (0.18) | 0.44–1.98 | 0.86 (0.78, 0.97) | 0.80 |
| Female | 0.87 (0.18) | 0.44–1.61 | 0.87 (0.77, 0.97) | |||
| Male | 0.89 (0.17) | 0.44–1.98 | 0.85 (0.79, 0.96) | |||
| Zinc (Zn) | μg/g | Full | 69.22 (44.40) | 29.87–487.50 | 59.90 (52.20, 71.50) | 0.13 |
| Female | 66.70 (41.72) | 29.87–445.50 | 57.40 (51.00, 69.90) | |||
| Male | 71.31 (46.54) | 33.40–487.50 | 61.50 (53.80, 71.70) | |||
| Antimony (Sb) | ng/g | Full | 8.30 (27.53) | 0.62–360.93 | 3.09 (1.80, 5.71) | >0.90 |
| Female | 8.47 (34.05) | 0.62–360.93 | 3.13 (1.76, 5.60) | |||
| Male | 8.17 (20.77) | 0.62–149.21 | 3.08 (1.85, 5.93) | |||
| Arsenic (As) | ng/g | Full | 7.20 (9.44) | 1.26–77.70 | 4.70 (3.31, 7.48) | 0.30 |
| Female | 7.44 (11.67) | 1.26–77.70 | 4.58 (3.32, 6.76) | |||
| Male | 7.00 (7.13) | 1.28–66.84 | 4.73 (3.30, 8.11) | |||
| Barium (Ba) | ng/g | Full | 117.97 (207.75) | 17.50–3131.90 | 81.90 (56.40, 128.40) | >0.90 |
| Female | 127.77 (288.90) | 19.80–3131.90 | 87.05 (58.58, 124.45) | |||
| Male | 109.85 (100.05) | 17.50–789.30 | 81.00 (56.40, 131.00) | |||
| Cadmium (Cd) | ng/g | Full | 24.05 (253.35) | 0.23–4057.21 | 1.28 (0.75, 2.86) | 0.20 |
| Female | 5.01 (12.06) | 0.23–74.77 | 1.20 (0.71, 2.29) | |||
| Male | 39.80 (342.06) | 0.23–4057.21 | 1.33 (0.80, 3.24) | |||
| Lead (Pb) | ng/g | Full | 32.95 (67.79) | 2.60–708.70 | 16.10 (9.30, 29.90) | 0.06 |
| Female | 26.73 (47.11) | 2.60–350.20 | 14.45 (8.83, 25.83) | |||
| Male | 38.10 (80.82) | 2.90–708.70 | 17.80 (10.60, 33.60) | |||
| Mercury (Hg) | ng/g | Full | 14.67 (19.09) | 0.56–174.11 | 8.13 (3.63, 17.53) | 0.20 |
| Female | 12.07 (13.97) | 0.56–93.36 | 7.46 (3.35, 15.07) | |||
| Male | 16.82 (22.29) | 0.56–174.11 | 8.51 (4.02, 20.20) | |||
| Strontium (Sr) | μg/g | Full | 0.70 (0.48) | 0.17–3.33 | 0.55 (0.39, 0.86) | 0.60 |
| Female | 0.65 (0.43) | 0.19–3.33 | 0.55 (0.41, 0.79) | |||
| Male | 0.74 (0.53) | 0.17–3.00 | 0.56 (0.38, 0.90) |
| Prenatal Copper (Cu): 21 RPC CpGs | ||||||
|---|---|---|---|---|---|---|
| CpG Probe | Chromosome | Genomic Region a | Gene | β (95% CI) b | p | q c |
| cg14654324 | 9 | Body | RXRA | 1.56 (0.58, 2.53) | 0.00 | 0.03 * |
| cg15909725 | 11 | −1.35 (−2.4, −0.3) | 0.01 | 0.12 | ||
| cg01958723 | 20 | −0.64 (−1.16, −0.13) | 0.02 | 0.14 | ||
| cg08493294 | 2 | Body | DNMT3A | 0.82 (0.15, 1.5) | 0.02 | 0.15 |
| cg15488978 | 10 | Body | C10orf41 | −1.36 (−2.48, −0.24) | 0.02 | 0.15 |
| cg22947322 | 17 | Body | IGF2BP1 | 0.54 (0.09, 0.99) | 0.02 | 0.16 |
| cg00035630 | 11 | TSS1500 | DEPDC7 | −0.95 (−1.75, −0.16) | 0.02 | 0.17 |
| cg20327163 | 15 | Body;5′UTR | NR2F2 | −0.6 (−1.1, −0.09) | 0.02 | 0.17 |
| cg10482057 | 6 | Body | KIAA0319 | −0.93 (−1.72, −0.15) | 0.02 | 0.17 |
| cg15862128 | 14 | TSS200 | MIR494 | −0.91 (−1.75, −0.07) | 0.03 | 0.25 |
| cg18752527 | 2 | Body | HECW2 | 0.85 (0.07, 1.64) | 0.03 | 0.25 |
| cg12551957 | 7 | Body | FBXL18 | 0.78 (0.06, 1.51) | 0.03 | 0.26 |
| cg14415214 | 11 | 1stExon | PHLDA2 | −0.97 (−1.88, −0.06) | 0.04 | 0.26 |
| cg21467614 | 6 | 1stExon | TNF | −0.62 (−1.2, −0.04) | 0.04 | 0.27 |
| cg24244478 | 11 | Body | MAP6 | −0.59 (−1.14, −0.03) | 0.04 | 0.27 |
| cg07634706 | 16 | TSS200 | CCL17 | −0.68 (−1.34, −0.03) | 0.04 | 0.28 |
| cg22755932 | 15 | Body | ARRDC4 | −0.94 (−1.84, −0.04) | 0.04 | 0.29 |
| cg02388882 | 10 | 5′UTR | AGAP11 | −1.64 (−3.25, −0.03) | 0.05 | 0.31 |
| cg02603128 | 9 | Body | FRMD3 | 0.79 (0.01, 1.57) | 0.05 | 0.32 |
| cg12077698 | 1 | Body | TRIM62 | −0.56 (−1.11, 0) | 0.05 | 0.32 |
| cg03543893 | 20 | −1.52 (−3.02, −0.01) | 0.05 | 0.32 | ||
| Prenatal Manganese (Mn): 25 RPC CpGs | ||||||
| CpG Probe | Chromosome | Genomic Region | Gene | β (95% CI) | p | q |
| cg25298189 | 19 | Body | ARID3A | 1.22 (0.41, 2.04) | 0.00 | 0.04 * |
| cg16673477 | 19 | Body | CRTC1 | −0.95 (−1.59, −0.32) | 0.00 | 0.04 * |
| cg14620572 | 9 | Body | GPR144 | −1.98 (−3.32, −0.65) | 0.00 | 0.04 * |
| cg03836586 | 7 | Body | NXPH1 | −1.62 (−2.71, −0.52) | 0.00 | 0.05 * |
| cg26502666 | 2 | 5′UTR | SPDYA | −2.08 (−3.65, −0.5) | 0.01 | 0.10 |
| cg17452301 | 10 | Body | PWWP2B | 0.95 (0.21, 1.7) | 0.01 | 0.12 |
| cg08493294 | 2 | Body | DNMT3A | 0.8 (0.16, 1.43) | 0.02 | 0.14 |
| cg02012338 | 4 | Body | CYP4V2 | 0.55 (0.1, 0.99) | 0.02 | 0.14 |
| cg21523908 | 1 | TSS200 | FAM5B | −1.45 (−2.63, −0.27) | 0.02 | 0.14 |
| cg22193385 | 12 | Body | KRT7 | 1.47 (0.27, 2.66) | 0.02 | 0.15 |
| cg09163779 | 10 | 1stExon;5′UTR | RPP30 | −0.57 (−1.05, −0.1) | 0.02 | 0.16 |
| cg01152073 | 5 | 5′UTR;TSS1500 | SQSTM1 | 0.71 (0.12, 1.3) | 0.02 | 0.16 |
| cg12816936 | 3 | 0.46 (0.07, 0.85) | 0.02 | 0.17 | ||
| cg04474049 | 7 | TSS1500 | CAV1 | 0.64 (0.1, 1.17) | 0.02 | 0.17 |
| cg23044186 | 5 | 0.64 (0.1, 1.19) | 0.02 | 0.18 | ||
| cg16970628 | 12 | Body | TMEM132C | −0.69 (−1.3, −0.08) | 0.03 | 0.21 |
| cg26140475 | 8 | 0.51 (0.05, 0.97) | 0.03 | 0.23 | ||
| cg04110105 | 3 | 5′UTR;1stExon | CCR2 | 1.07 (0.08, 2.05) | 0.03 | 0.25 |
| cg14789214 | 10 | Body | KCNIP2 | 0.5 (0.03, 0.96) | 0.04 | 0.27 |
| cg15405432 | 5 | TSS200 | FLJ44606 | −1.7 (−3.3, −0.09) | 0.04 | 0.27 |
| cg24998879 | 6 | 0.49 (0.02, 0.96) | 0.04 | 0.28 | ||
| cg11841722 | 5 | TSS1500;5′UTR | LIFR | 0.93 (0.04, 1.83) | 0.04 | 0.28 |
| cg04435320 | 11 | Body | TCP11L1 | 0.58 (0.02, 1.13) | 0.04 | 0.28 |
| cg17160984 | 10 | −1.67 (−3.28, −0.06) | 0.04 | 0.28 | ||
| cg11798873 | 6 | Body | PTPRK | 0.58 (0.01, 1.15) | 0.05 | 0.32 |
| Metal Group | Population | Crude β (95% CI) | Adjusted β (95% CI) | Adjusted Positive Weights | Adjusted Negative Weights |
|---|---|---|---|---|---|
| All Metals | Full | −0.09 (−0.29, 0.11) | −0.02 (−0.23, 0.19) | Se: 0.27 Sr: 0.27 Sb: 0.20 Cd: 0.17 Ba: 0.06 Zn: 0.02 Hg: 0.01 | Cu: −0.47 Mn: −0.30 Pb: −0.13 As: −0.10 |
| All Metals | Female | −0.07 (−0.43, 0.29) | −0.07 (−0.45, 0.31) | Sr: 0.25 Cu: 0.20 As: 0.17 Hg: 0.17 Mn: 0.14 Cd: 0.05 Zn: 0.02 | Se: −0.46 Ba: −0.26 Pb: −0.20 Sb: −0.08 |
| All Metals | Male | −0.10 (−0.33, 0.13) | −0.03 (−0.28, 0.22) | Se: 0.33 Ba: 0.25 Pb: 0.21 Sb: 0.13 Zn: 0.08 | Mn: −0.33 Cu: −0.32 Hg: −0.16 As: −0.13 Cd: −0.04 Sr: −0.02 |
| Essential Metals | Full | −0.11 (−0.27, 0.05) | −0.09 (−0.26, 0.08) | Se: 0.73 Zn: 0.27 | Cu: −0.58 Mn: −0.42 |
| Essential Metals | Female | −0.05 (−0.30, 0.20) | −0.05 (−0.33, 0.23) | Cu: 0.67 Zn: 0.29 Mn: 0.04 | Se: −1.00 |
| Essential Metals | Male | −0.13 (−0.32, 0.06) | −0.11 (−0.34, 0.12) | Se: 0.92 Zn: 0.08 | Cu: −0.50 Mn: −0.50 |
| Non-Essential Metals | Full | −0.04 (−0.20, 0.13) | 0.05 (−0.14, 0.24) | Sr: 0.54 Cd: 0.28 Sb: 0.18 | Pb: −0.49 As: −0.33 Hg: −0.14 Ba: −0.04 |
| Non-Essential Metals | Female | 0 (−0.26, 0.26) | −0.04 (−0.34, 0.26) | Sr: 0.61 Hg: 0.20 As: 0.19 | Ba: −0.56 Pb: −0.33 Sb: −0.09 Cd: −0.02 |
| Non-Essential Metals | Male | −0.04 (−0.24, 0.16) | 0.12 (−0.13, 0.37) | Ba: 0.46 Pb: 0.36 Sb: 0.16 Sr: 0.02 | Hg: −0.69 As: −0.16 Cd: −0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huff, K.K.; Roell, K.R.; Eaves, L.A.; O’Shea, T.M.; Fry, R.C. Prenatal Exposure to Metals Is Associated with Placental Decelerated Epigenetic Gestational Age in a Sex-Dependent Manner in Infants Born Extremely Preterm. Cells 2025, 14, 306. https://doi.org/10.3390/cells14040306
Huff KK, Roell KR, Eaves LA, O’Shea TM, Fry RC. Prenatal Exposure to Metals Is Associated with Placental Decelerated Epigenetic Gestational Age in a Sex-Dependent Manner in Infants Born Extremely Preterm. Cells. 2025; 14(4):306. https://doi.org/10.3390/cells14040306
Chicago/Turabian StyleHuff, Katelyn K., Kyle R. Roell, Lauren A. Eaves, Thomas Michael O’Shea, and Rebecca C. Fry. 2025. "Prenatal Exposure to Metals Is Associated with Placental Decelerated Epigenetic Gestational Age in a Sex-Dependent Manner in Infants Born Extremely Preterm" Cells 14, no. 4: 306. https://doi.org/10.3390/cells14040306
APA StyleHuff, K. K., Roell, K. R., Eaves, L. A., O’Shea, T. M., & Fry, R. C. (2025). Prenatal Exposure to Metals Is Associated with Placental Decelerated Epigenetic Gestational Age in a Sex-Dependent Manner in Infants Born Extremely Preterm. Cells, 14(4), 306. https://doi.org/10.3390/cells14040306







