Glioblastoma Cell Migration, Invasion and Vasculogenic Mimicry Downmodulated by Novel uPAcyclin Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Peptide Synthesis
2.3. NMR Conformational Analysis
2.4. Binding Assay
2.5. Migration and Invasion Assays
2.6. Analysis of Cytoskeleton
2.7. Vasculogenic Mimicry Assay
2.8. Cell Viability Assay
2.9. Statistical Analyses
3. Results
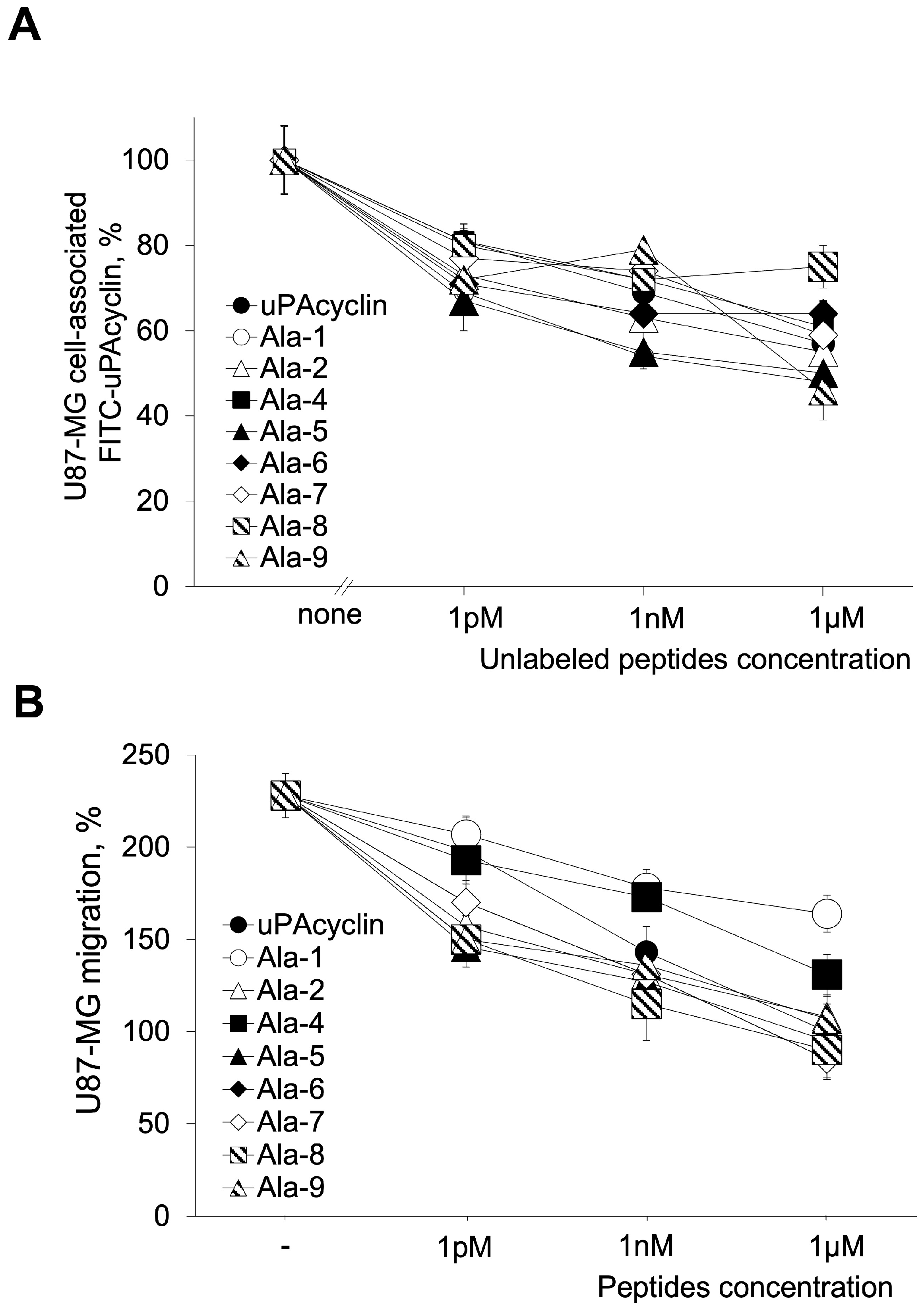
3.1. Specific Binding and Biological Properties of All Ala Peptides
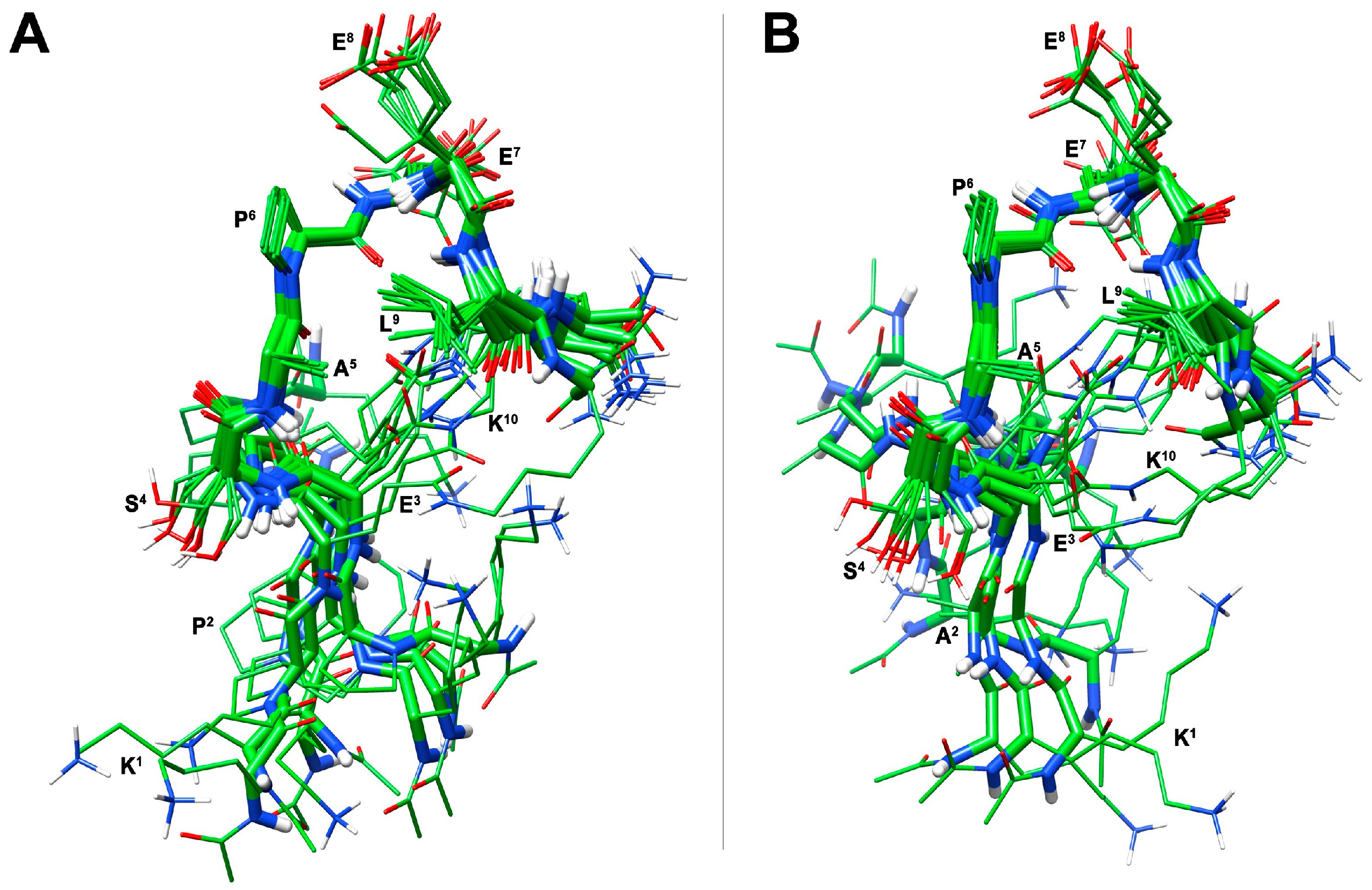
3.2. Conformational Analysis of Selected Peptides
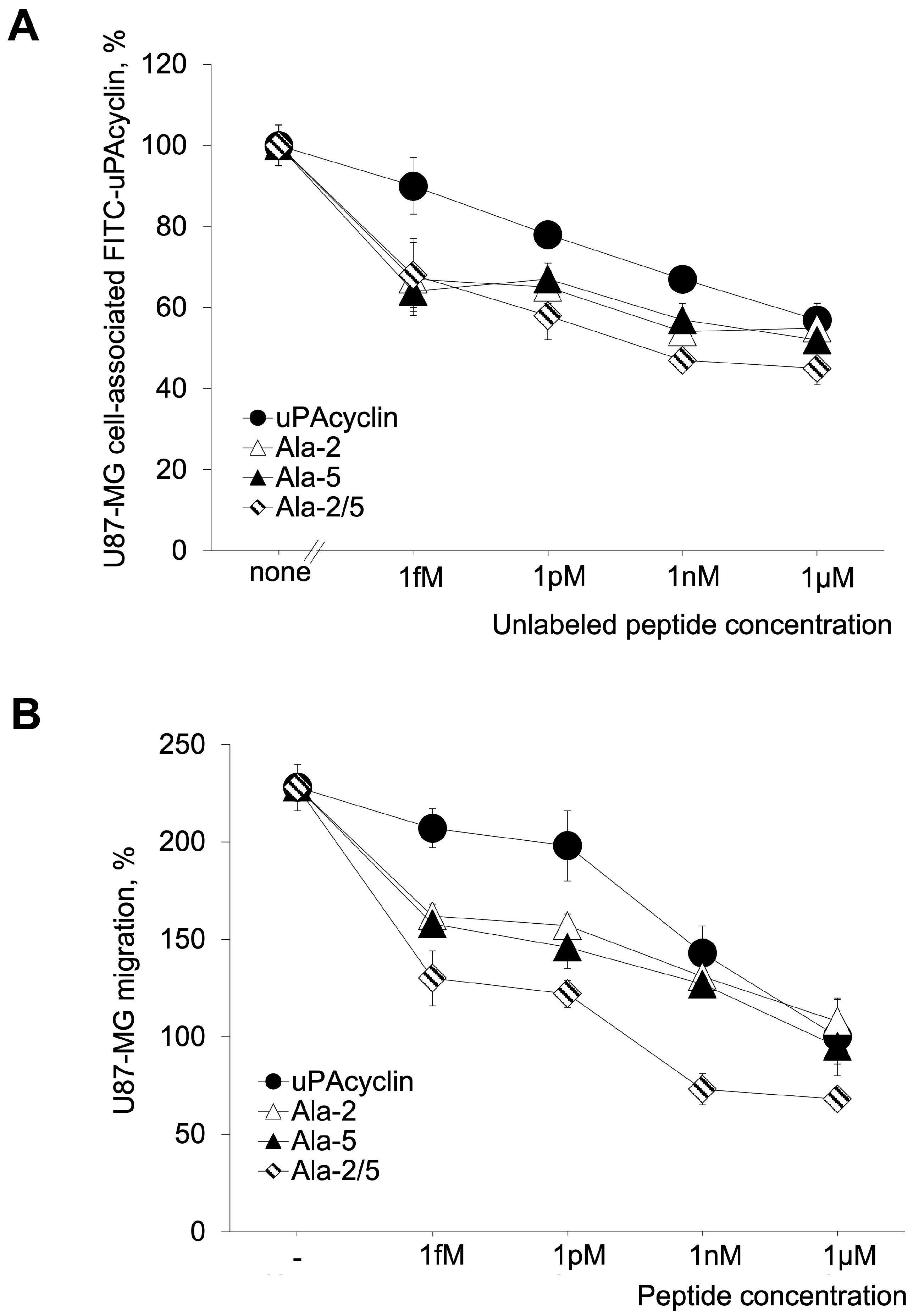
3.3. Development of the Novel Peptide [Ala2, Ala5]uPAcyclin (uPAcyclin-II)
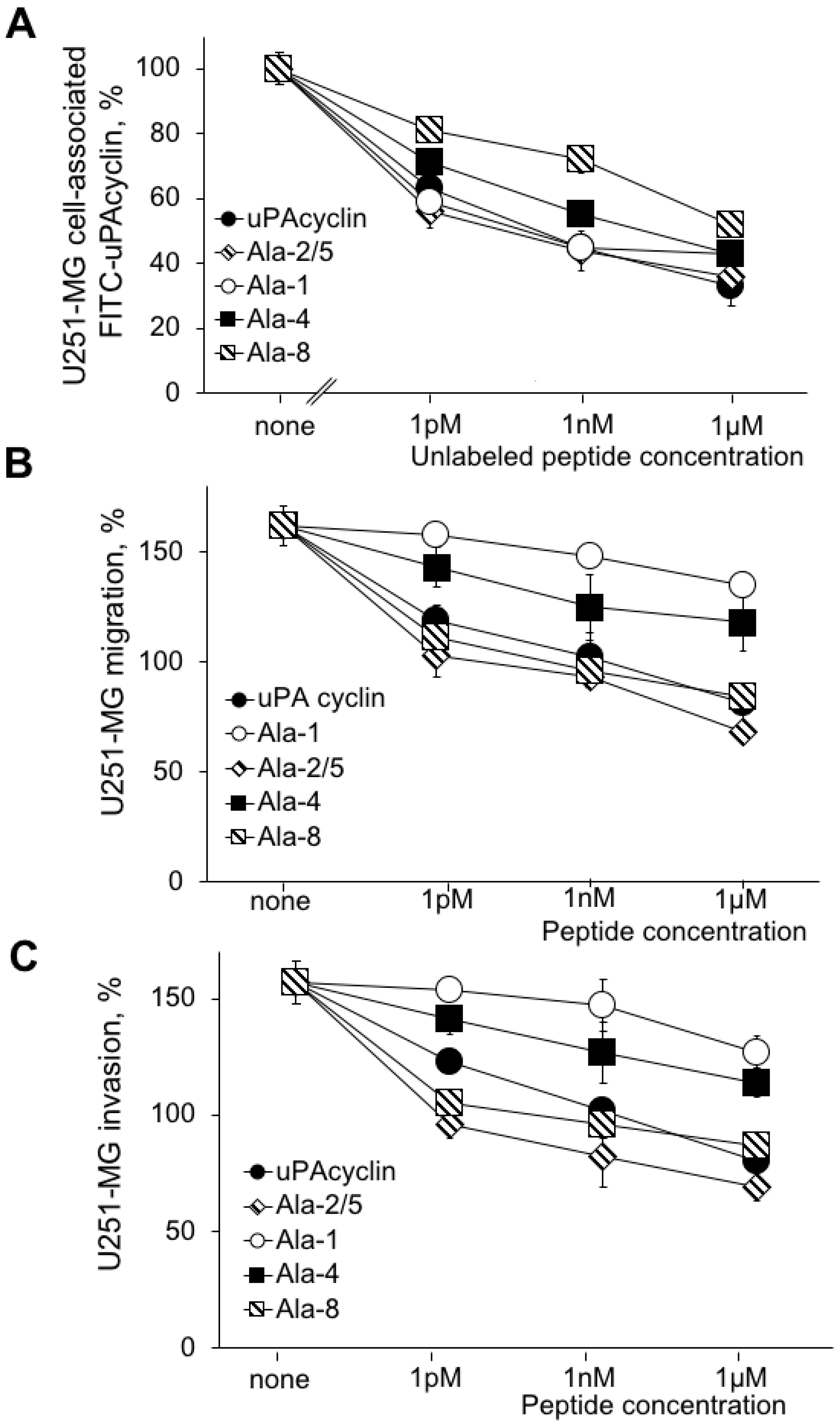
3.4. Effects of the Relevant Peptides on the U251-MG Cell Lines
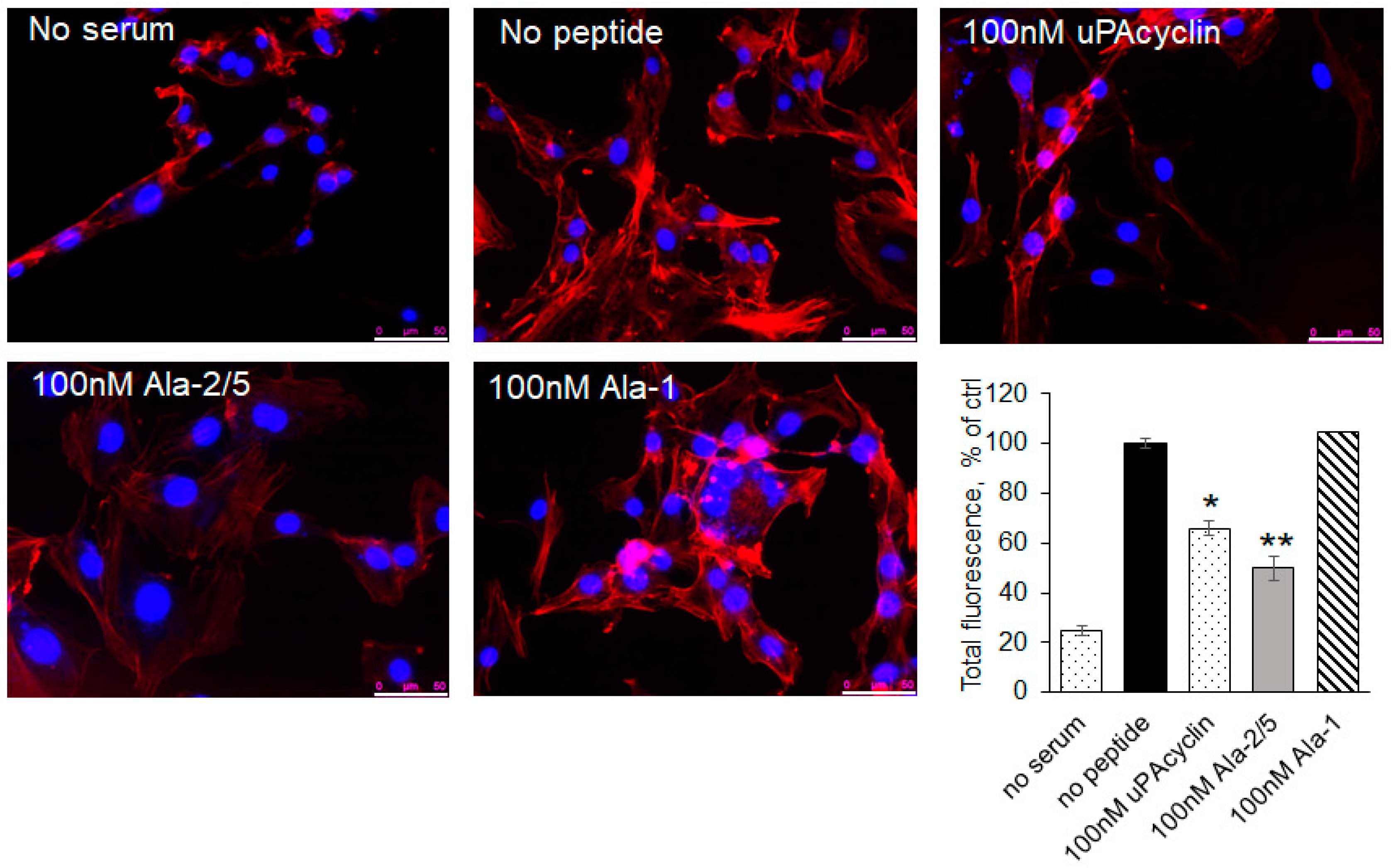
3.5. F-Actin Redistribution in Response to the Novel Peptides
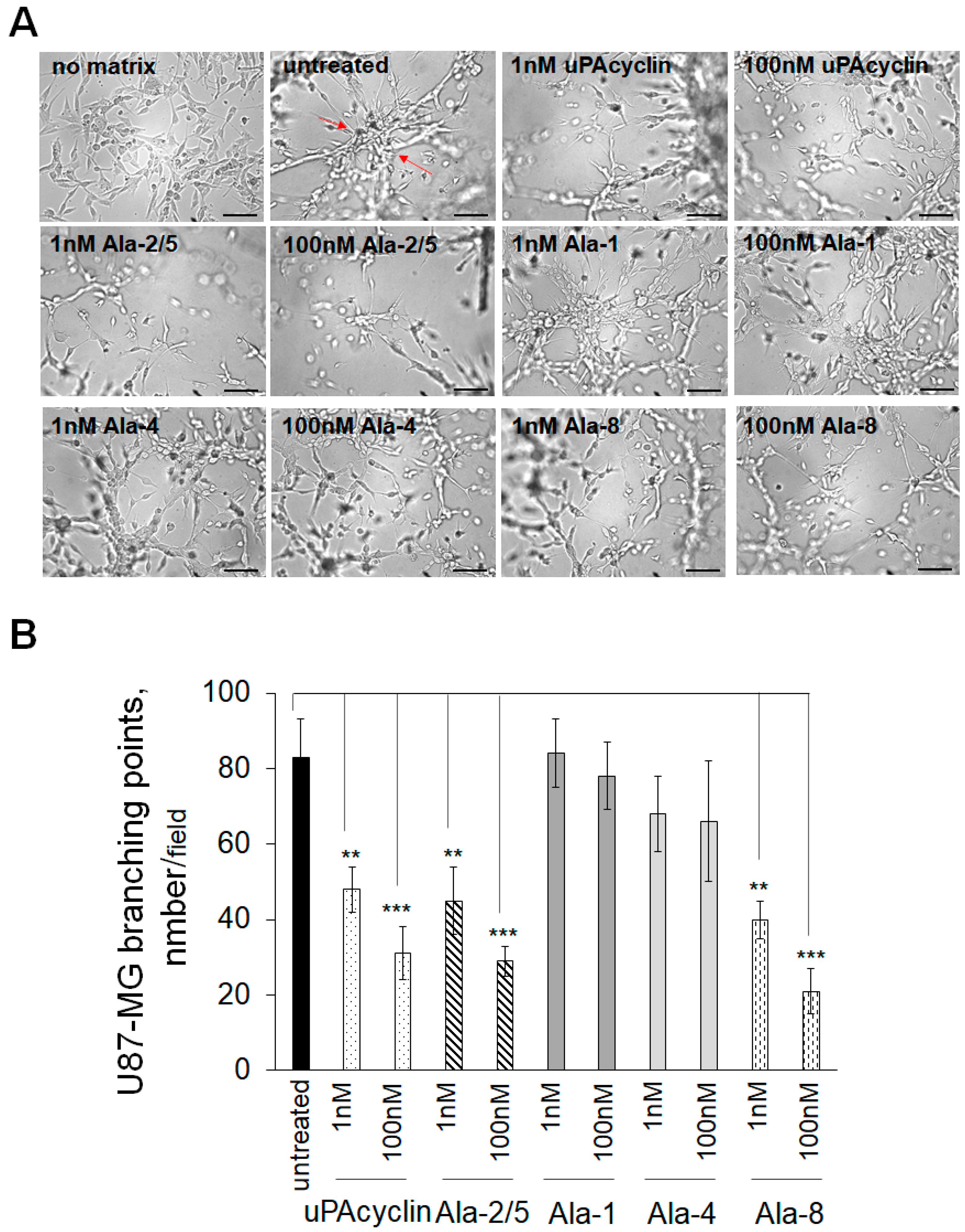
3.6. Inhibition of Vasculogenic Mimicry of U87-MG and U251-MG Cells
3.7. Effects of uPAcyclin and Ala-Peptides on Cell Proliferation
4. Discussion

5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aldape, K.; Zadeh, G.; Mansouri, S.; Reifenberger, G.; von Deimling, A. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Valerius, A.R.; Webb, L.M.; Thomsen, A.; Lehrer, E.J.; Breen, W.G.; Campian, J.L.; Riviere-Cazaux, C.; Burns, T.C.; Sener, U. Review of Novel Surgical, Radiation, and Systemic Therapies and Clinical Trials in Glioblastoma. Int. J. Mol. Sci. 2024, 25, 10570. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Åke, S.; Hartelius, L.; Jakola, A.S.; Antonsson, M. Experiences of language and communication after brain-tumour treatment: A long-term follow-up after glioma surgery. Neuropsychol. Rehabil. 2023, 33, 1225–1261. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Maddison, K.; Bowden, N.A.; Graves, M.C.; Tooney, P.A. Characteristics of vasculogenic mimicry and tumour to endothelial transdifferentiation in human glioblastoma: A systematic review. BMC Cancer 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Echavidre, W.; Picco, V.; Faraggi, M.; Montemagno, C. Integrin-αVβ3 as a Therapeutic Target in Glioblastoma: Back to the Future? Pharmaceutics 2022, 14, 1053. [Google Scholar] [CrossRef]
- Ha, C.P.; Hua, T.N.M.; Vo, V.T.A.; Om, J.; Han, S.; Cha, S.K.; Park, K.S.; Jeong, Y. Humanin activates integrin αV–TGFβ axis and leads to glioblastoma progression. Cell Death Dis. 2024, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.M.; Kelly, M.; Odde, D.J. Glioblastoma Cells Use an Integrin- and CD44-Mediated Motor-Clutch Mode of Migration in Brain Tissue. Cell. Mol. Bioeng. 2024, 17, 121–135. [Google Scholar] [CrossRef]
- Degrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L. Cilengitide in glioblastoma: When did it fail? Lancet Oncol. 2014, 15, 1044–1045. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.J.; Hathley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Belli, S.; Franco, P.; Iommelli, F.; De Vincenzo, A.; Brancaccio, D.; Telesca, M.; Merlino, F.; Novellino, E.; Ranson, M.; Del Vecchio, S.; et al. Breast Tumor Cell Invasion and Pro-Invasive Activity of Cancer-Associated Fibroblasts Co-Targeted by Novel Urokinase-Derived Decapeptides. Cancers 2020, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Alfano, D.; Franco, P.; Stoppelli, M.P. Modulation of Cellular Function by the Urokinase Receptor Signalling: A Mechanistic View. Front. Cell Dev. Biol. 2022, 10, 818616. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Vocca, I.; Carriero, M.V.; Alfano, D.; Cito, L.; Longanesi-Cattani, I.; Grieco, P.; Ossowski, L.; Stoppelli, M.P. Activation of urokinase receptor by a novel interaction between the connecting peptide region of urokinase and alpha v beta 5 integrin. J. Cell Sci. 2006, 119, 3424–3434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franco, P.; Carotenuto, A.; Marcozzi, C.; Votta, G.; Sarno, C.; Iaccarino, I.; Brancaccio, D.; De Vincenzo, A.; Novellino, E.; Grieco, P.; et al. Opposite modulation of cell migration by distinct subregions of urokinase connecting peptide. Chembiochem 2013, 14, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Vocca, I.; Franco, P.; Alfano, D.; Votta, G.; Carriero, M.V.; Estrada, Y.; Caputi, M.; Netti, P.A.; Ossowski, L.; Stoppelli, M.P. Inhibition of migration and invasion of carcinoma cells by urokinase-derived antagonists of alphavbeta5 integrin activation. Int. J. Cancer 2009, 124, 316–325. [Google Scholar] [CrossRef]
- Guo, Y.; Mazar, A.P.; Lebrun, J.; Rabbani, S.A. An antiangiogenic urokinase-derived pepide combined with tamoxifen decreases tumor growth and metastasis in a syngeneic model of breast cancer. Cancer Res. 2002, 62, 4678–4684. [Google Scholar] [PubMed]
- Ghamande, S.A.; Silverman, M.H.; Huh, W.; Behbakht, K.; Ball, G.; Cuasay, L.; Würtz, S.O.; Brunner, N.; Gold, M.A. A phase 2, randomized, double-blind, placebo-controlled trial of clinical activity and safety of subcutaneous Å6 in women with asymptomatic CA125 progression after first-line chemotherapy of epithelial ovarian cancer. Gynecol. Oncol. 2008, 111, 89–94. [Google Scholar] [CrossRef]
- Gold, M.A.; Brady, W.E.; Lankes, H.A.; Rose, P.G.; Kelley, J.L.; De Geest, K.; Crispens, M.A.; Resnick, K.E.; Howell, S.B. A phase II study of a urokinase-derived peptide (Å6) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinomas: A Gynecologic Oncology Group study. Gynecol. Oncol. 2012, 125, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Camerino, I.; Merlino, F.; D’Angelo, M.; Cimmino, A.; Carotenuto, A.; Colucci-D’Amato, L.; Stoppelli, M.P. αV-Integrin-Dependent Inhibition of Glioblastoma Cell Migration, Invasion and Vasculogenic Mimicry by the uPAcyclin Decapeptide. Cancers 2023, 15, 4775. [Google Scholar] [CrossRef]
- Merlino, F.; Tomassi, S.; Yousif, A.M.; Messere, A.; Marinelli, L.; Grieco, P.; Novellino, E.; Cosconati, S.; Di Maro, S. Boosting Fmoc solid-phase peptide synthesis by ultrasonication. Org. Lett. 2019, 21, 6378–6382. [Google Scholar] [CrossRef]
- Carotenuto, A.; Merlino, F.; Cai, M.; Brancaccio, D.; Yousif, A.M.; Novellino, E.; Hruby, V.J.; Grieco, P. Discovery of novel potent and selective agonists at the melanocortin-3 receptor. J. Med. Chem. 2015, 58, 9773–9778. [Google Scholar] [CrossRef] [PubMed]
- Merlino, F.; Daniele, S.; La Pietra, V.; Di Maro, S.; Di Leva, F.S.; Brancaccio, D.; Tomassi, S.; Giuntini, S.; Cerofolini, L.; Fragai, M.; et al. Simultaneous targeting of RGD-integrins and dual murine double minute proteins in glioblastoma multiforme. J. Med. Chem. 2018, 61, 4791–4809. [Google Scholar] [CrossRef] [PubMed]
- Piantini, U.; Sorensen, O.W.; Ernst, R.R. Multiple quantum filters for elucidating NMR coupling network. J. Am. Chem. Soc. 1982, 104, 6800–6801. [Google Scholar] [CrossRef]
- Marion, D.; Wüthrich, K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem. Biophys. Res. Commun. 1983, 113, 967–997. [Google Scholar] [CrossRef] [PubMed]
- Braunschweiler, L.; Ernst, R.R. Coherence transfer by isotropic mixing: Application to proton correlation spectroscopy. J. Magn. Reson. 1983, 53, 521–528. [Google Scholar] [CrossRef]
- Jenner, J.; Meyer, B.H.; Bachman, P.; Ernst, R.R. Investigation of exchange processes by two-dimensional NMR spectroscopy. J. Chem. Phys. 1979, 71, 4546–4553. [Google Scholar] [CrossRef]
- States, D.J.; Haberkorn, R.A.; Ruben, D.J. A Two-dimensional nuclear Overhauser experiment with pure absorption phase in four quadrants. J. Magn. Reson. 1982, 48, 286–292. [Google Scholar] [CrossRef]
- Bartels, C.; Xia, T.; Billeter, M.; Guentert, P.; Wüthrich, K. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR 1995, 6, 1–10. [Google Scholar] [CrossRef]
- Wüthrich, K. NMR of Proteins and Nucleic Acids; John Wiley & Sons: New York, NY, USA, 1986; pp. 11–13. [Google Scholar]
- Güntert, P.; Buchner, L. Combined automated NOE assignment and structure calculation with CYANA. J. Biomol. NMR 2015, 62, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Maple, J.R.; Dinur, U.; Hagler, A.T. Derivation of force fields for molecular mechanics and dynamics from ab initio energy surfaces. Proc. Natl. Acad. Sci. USA 1988, 85, 5350–5354. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Stoppelli, M.P.; Tacchetti, C.; Cubellis, M.V.; Corti, A.; Hearing, V.J.; Cassani, G.; Appella, E.; Blasi, F. Autocrin saturation of pro-urokinase receptors on human A431 cells.The receptor for urokinase plasminogen activator. Cell 1986, 45, 675–684. [Google Scholar] [CrossRef]
- De Vincenzo, A.; Belli, S.; Franco, P.; Telesca, M.; Iaccarino, I.; Botti, G.; Carriero, M.V.; Ranson, M.; Stoppelli, M.P. Paracrine recruitment and activation of fibroblasts by c-Myc expressing breast epithelial cells through the IGFs/IGF-1R axis. Int. J. Cancer 2019, 145, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, O.; Gentile, M.T.; Mancini, A.; Del Gaudio, N.; Di Costanzo, A.; Bajetto, A.; Franco, P.; Altucci, L.; Florio, T.; Stoppelli, M.P.; et al. Histone Deacetylase Inhibitors Impair Vasculogenic Mimicry from Glioblastoma Cells. Cancers 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Dehghani, F. The Cytoskeleton-A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef]
- Diaz, R.J.; Ali, S.; Qadir, M.G.; De La Fuente, M.I.; Ivan, M.E.; Komotar, R.J. The role of bevacizumab in the treatment of glioblastoma. J. Neuro-oncol. 2017, 133, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Buccarelli, M.; Castellani, G.; Ricci-Vitiani, L. Glioblastoma-Specific Strategies of Vascularization: Implications in Anti-Angiogenic Therapy Resistance. J. Personal. Med. 2022, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.J.; Mas-Moruno, C.; et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [PubMed]
- Folberg, R.; Maniotis, A.J. Vasculogenic mimicry. APMIS 2004, 112, 508–525. [Google Scholar] [CrossRef]
- Angara, K.; Rashid, M.H.; Shankar, A.; Ara, R.; Iskander, A.; Borin, T.F.; Jain, M.; Achyut, B.R.; Arbab, A.S. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol. Histopathol. 2017, 32, 917–928. [Google Scholar] [PubMed]
- Wang, R.; Chadalavada, K.; Wilshire, J.; Kowalik, U.; Hovinga, K.E.; Geber, A.; Fligelman, B.; Leversha, M.; Brennan, C.; Tabar, V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010, 468, 829–833. [Google Scholar] [CrossRef]
- Soda, Y.; Marumoto, T.; Friedmann-Morvinski, D.; Soda, M.; Liu, F.; Michiue, H.; Pastorino, S.; Yang, M.; Hoffman, R.M.; Kesari, S.; et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4274–4280. [Google Scholar] [CrossRef]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef]
- Luo, C.; Chen, G.; Li, R.; Peng, S.; Zhang, P.; Wang, F.; Yu, S.; Zhu, Y.; Zhang, J. Juglone suppresses vasculogenic mimicry in glioma through inhibition of HuR-mediated VEGF-A expression. Biochem. Pharmacol. 2024, 227, 116458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Zhao, P.; Zhao, H.; Gao, W.; Wang, L. Celastrol Suppresses Glioma Vasculogenic Mimicry Formation and An-giogenesis by Blocking the PI3K/Akt/mTOR Signaling Pathway. Front. Pharmacol. 2020, 11, 25. [Google Scholar]
- Camerino, I.; Franco, P.; Bajetto, A.; Thellung, S.; Florio, T.; Stoppelli, M.P.; Colucci-D’Amato, L. Ruta graveolens, but Not Rutin, Inhibits Survival, Migration, Invasion, and Vasculogenic Mimicry of Glioblastoma Cells. Int. J. Mol. Sci. 2024, 25, 11789. [Google Scholar] [CrossRef] [PubMed]
- Sada, N.; Fujita, Y.; Mizuta, N.; Ueno, M.; Furukawa, T.; Yamashita, T. Inhibition of HDAC increases BDNF expression and promotes neuronal rewiring and functional recovery after brain injury. Cell Death Dis. 2020, 11, 655. [Google Scholar] [CrossRef]
- Shukla, S.; Shariat-Madar, Z.; Walker, L.A.; Tekwani, B.L. Mechanism for neurotropic action of vorinostat, a pan histone deacetylase inhibitor. Mol. Cell. Neurosci. 2016, 77, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.T.; Ciniglia, C.; Reccia, M.G.; Volpicelli, F.; Gatti, M.; Thellung, S.; Florio, T.; Melone, M.A.; Colucci-D’Amato, L. Ruta graveolens induces death of glioblastoma cells and neural progenitors, but not of neurons, via ERK 1/2 and AKT activation. PLoS ONE 2015, 10, e0118864. [Google Scholar] [CrossRef] [PubMed]
- Campanile, M.; Cuomo, O.; Brancaccio, P.; Vinciguerra, A.; Casamassa, A.; Pastorino, O.; Volpicelli, F.; Gentile, M.T.; Amoroso, S.; Annunziato, L.; et al. Ruta graveolens water extract (RGWE) ameliorates ischemic damage and improves neurological deficits in a rat model of transient focal brain ischemia. Biomed. Pharmacother. 2022, 154, 113587. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, F.; Graziani, G.; Levati, L.; Tentori, L.; D’Atri, S.; Lacal, P.M. Cilengitide downmodulates invasiveness and vasculogenic mimicry of neuropilin 1 expressing melanoma cells through the inhibition of αvβ5 integrin. Inter. J. Cancer 2015, 136, E545–E558. [Google Scholar] [CrossRef]
- Mavria, G.; Vercoulen, Y.; Yeo, M.; Paterson, H.; Karasarides, M.; Marais, R.; Bird, D.; Marshall, C.J. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 2006, 9, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Vestweber, D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog. Mol. Biol. Trans. Sci. 2013, 116, 119–144. [Google Scholar]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- She, Q.; Hu, S.; Pu, X.; Guo, Q.; Mou, C.; Yang, C. The effect of hepatocellular carcinoma-associated fibroblasts on hepatoma vasculogenic mimicry. Am. J. Cancer Res. 2020, 10, 4198–4210. [Google Scholar] [PubMed]
- Yang, D.; Liu, J.; Qian, H.; Zhuang, Q. Cancer-associated fibroblasts: From basic science to anticancer therapy. Exp. Mol. Med. 2023, 55, 1322–1332. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Cicatiello, A.E.; Reccia, M.G.; Volpicelli, F.; Severino, V.; Russo, R.; Sandomenico, A.; Doti, N.; D’Esposito, V.; Formisano, P.; et al. A targeted secretome profiling by multiplexed immunoassay revealed that secreted chemokine ligand 2 (MCP-1/CCL2) affects neural differentiation in mesencephalic neural progenitor cells. Proteomics 2015, 15, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Farina, A.; Vissers, J.P.; Chambery, A. Quantitative neuroproteomics: Classical and novel tools for studying neural differentiation and function. Stem Cell Rev. Rep. 2011, 7, 77–93. [Google Scholar] [CrossRef]
- Van Agthoven, J.F.; Xiong, J.P.; Alonso, J.L.; Rui, X.; Adair, B.D.; Goodman, S.L.; Arnaout, M.A. Structural basis for pure antagonism of integrin alpha V beta 3 by a high-affinity form of fibronectin. Nat. Struct. Mol. Biol. 2014, 21, 383–388. [Google Scholar] [CrossRef]

| Cmpd | Sequence a |
|---|---|
| linear-uPAcyclin b | Ac-Lys1-Pro2-Glu3-Ser4-Pro5-Pro6-Glu7-Glu8-Leu9-Lys10-NH2 |
| uPAcyclin b,c | Ac-Lys1-Pro2-[Glu3-Ser4-Pro5-Pro6-Glu7-Glu8-Leu9-Lys10]-NH2 |
| [Ala1]uPAcyclin | Ac-Ala1-Pro2-[Glu3-Ser4-Pro5-Pro6-Glu7-Glu8-Leu9-Lys10]-NH2 |
| [Ala2]uPAcyclin | Ac-Lys1-Ala2-[Glu3-Ser4-Pro5-Pro6-Glu7-Glu8-Leu9-Lys10]-NH2 |
| [Ala4]uPAcyclin | Ac-Lys1-Pro2-[Glu3-Ala4-Pro5-Pro6-Glu7-Glu8-Leu9-Lys10]-NH2 |
| [Ala5]uPAcyclin | Ac-Lys1-Pro2-[Glu3-Ser4-Ala5-Pro6-Glu7-Glu8-Leu9-Lys10]-NH2 |
| [Ala6]uPAcyclin | Ac-Lys1-Pro2-[Glu3-Ser4-Pro5-Ala6-Glu7-Glu8-Leu9-Lys10]-NH2 |
| [Ala7]uPAcyclin | Ac-Lys1-Pro2-[Glu3-Ser4-Pro5-Pro6-Ala7-Glu8-Leu9-Lys10]-NH2 |
| [Ala8]uPAcyclin | Ac-Lys1-Pro2-[Glu3-Ser4-Pro5-Pro6-Glu7-Ala8-Leu9-Lys10]-NH2 |
| [Ala9]uPAcyclin | Ac-Lys1-Pro2-[Glu3-Ser4-Pro5-Pro6-Glu7-Glu8-Ala9-Lys10]-NH2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, F.; Merlino, F.; Brancaccio, D.; Camerino, I.; Belli, S.; Cimmino, A.; Grieco, P.; Colucci-D’Amato, L.; Stoppelli, M.P.; Franco, P.; et al. Glioblastoma Cell Migration, Invasion and Vasculogenic Mimicry Downmodulated by Novel uPAcyclin Derivatives. Cells 2025, 14, 259. https://doi.org/10.3390/cells14040259
Santoro F, Merlino F, Brancaccio D, Camerino I, Belli S, Cimmino A, Grieco P, Colucci-D’Amato L, Stoppelli MP, Franco P, et al. Glioblastoma Cell Migration, Invasion and Vasculogenic Mimicry Downmodulated by Novel uPAcyclin Derivatives. Cells. 2025; 14(4):259. https://doi.org/10.3390/cells14040259
Chicago/Turabian StyleSantoro, Federica, Francesco Merlino, Diego Brancaccio, Iolanda Camerino, Stefania Belli, Amelia Cimmino, Paolo Grieco, Luca Colucci-D’Amato, Maria Patrizia Stoppelli, Paola Franco, and et al. 2025. "Glioblastoma Cell Migration, Invasion and Vasculogenic Mimicry Downmodulated by Novel uPAcyclin Derivatives" Cells 14, no. 4: 259. https://doi.org/10.3390/cells14040259
APA StyleSantoro, F., Merlino, F., Brancaccio, D., Camerino, I., Belli, S., Cimmino, A., Grieco, P., Colucci-D’Amato, L., Stoppelli, M. P., Franco, P., & Carotenuto, A. (2025). Glioblastoma Cell Migration, Invasion and Vasculogenic Mimicry Downmodulated by Novel uPAcyclin Derivatives. Cells, 14(4), 259. https://doi.org/10.3390/cells14040259









