Metabolic Astrocytic Support with Decanoic Acid Enhances Energy Metabolism in Alzheimer’s Disease Models
Highlights
- Medium-chain fatty acid decanoic acid (C10) is efficiently metabolized in both wild-type and 5xFAD mouse brain slices, particularly in astrocytes, supporting mitochondrial function.
- Astrocytic acetate metabolism is impaired in 5xFAD mice and familial AD astrocytes exhibit genotype-dependent metabolic responses to C10, with partial alterations in oxidative glucose metabolism in APP and PSEN1 variants.
- Altered astrocytic metabolism might occur before glucose hypometabolism in the 5xFAD mouse model.
- C10 supplementation may provide an auxiliary fuel source capable of supporting brain energy metabolism in Alzheimer’s disease, in part by promoting oxidative metabolism in astrocytes.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Brain Slice Incubations
2.4. Cell Lines
2.5. Neural Progenitor Cells Generation and Cell Differentiation
2.6. Astrocytic Differentiation
2.7. HiPSC Derived Astrocyte Incubations
2.8. Metabolic Mapping Using Gas Chromatography Coupled to Mass Spectrometry (GC–MS)
2.9. Statistical Analysis
3. Results
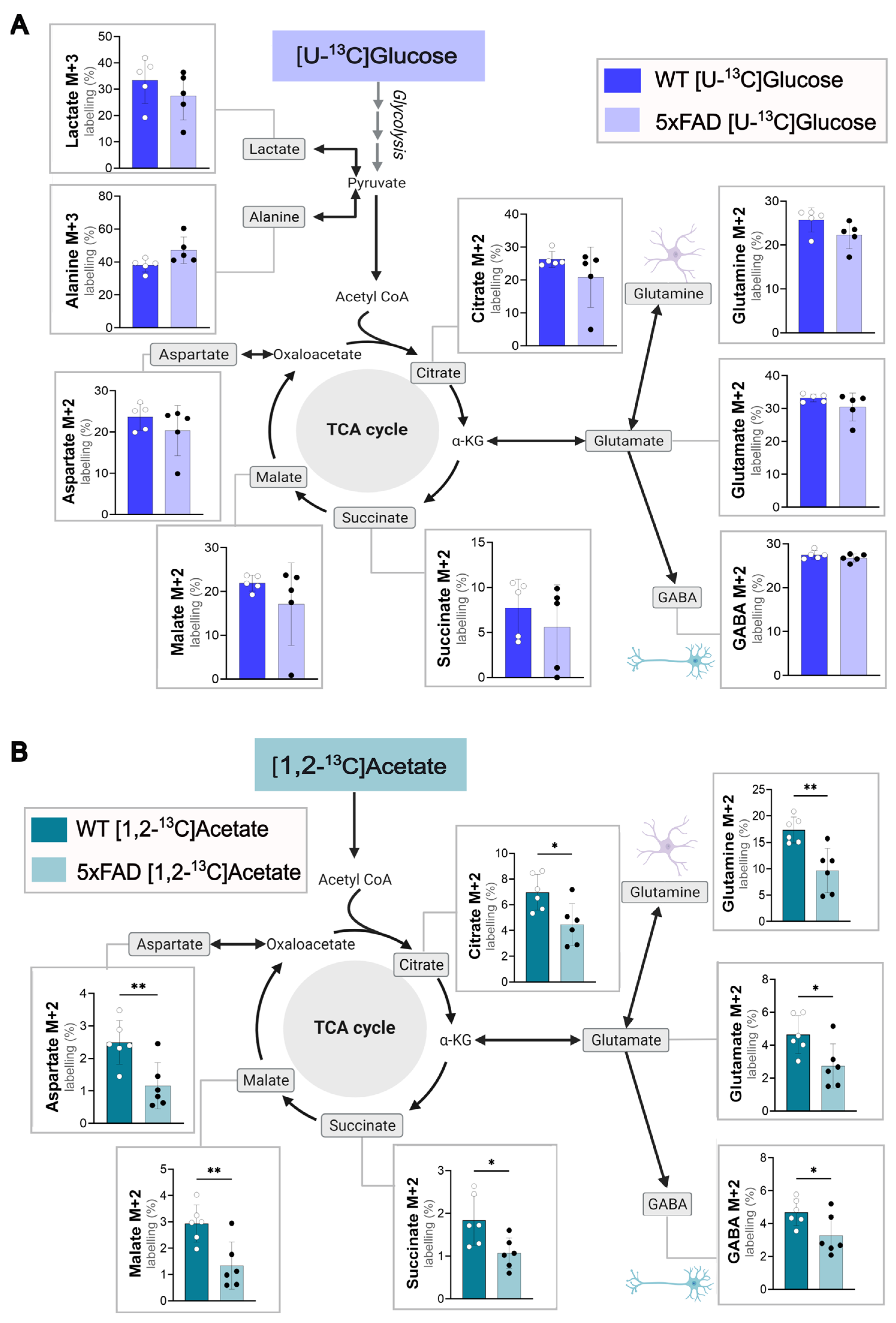
3.1. Acetate Metabolism Is Impaired in Cerebral Cortical Slices of 5xFAD Mice While Glucose Metabolism Is Maintained
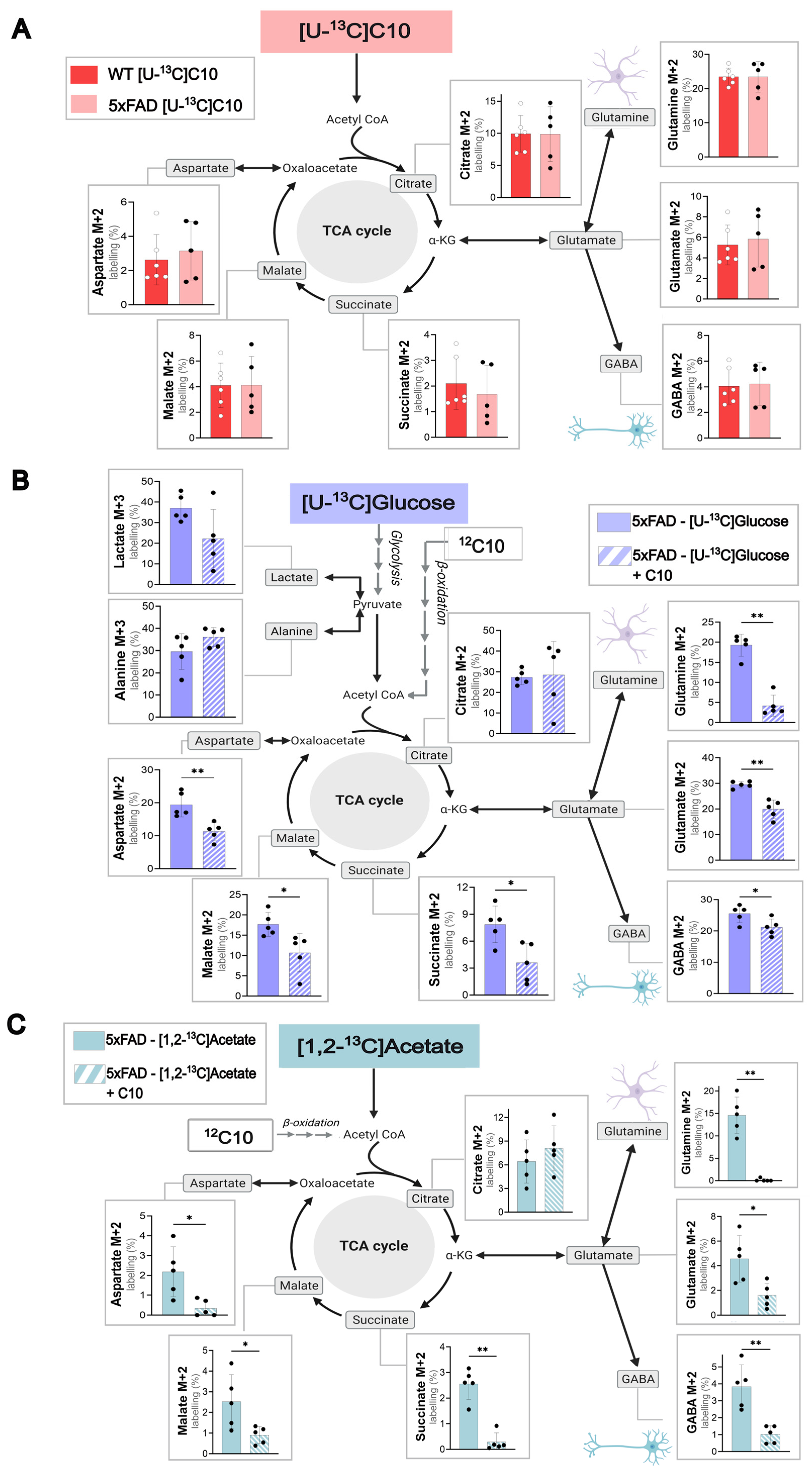
3.2. Decanoic Acid Can Be Used as a Brain Energy Substrate and Can Compete with Glucose and Acetate to Support Brain Energy Metabolism
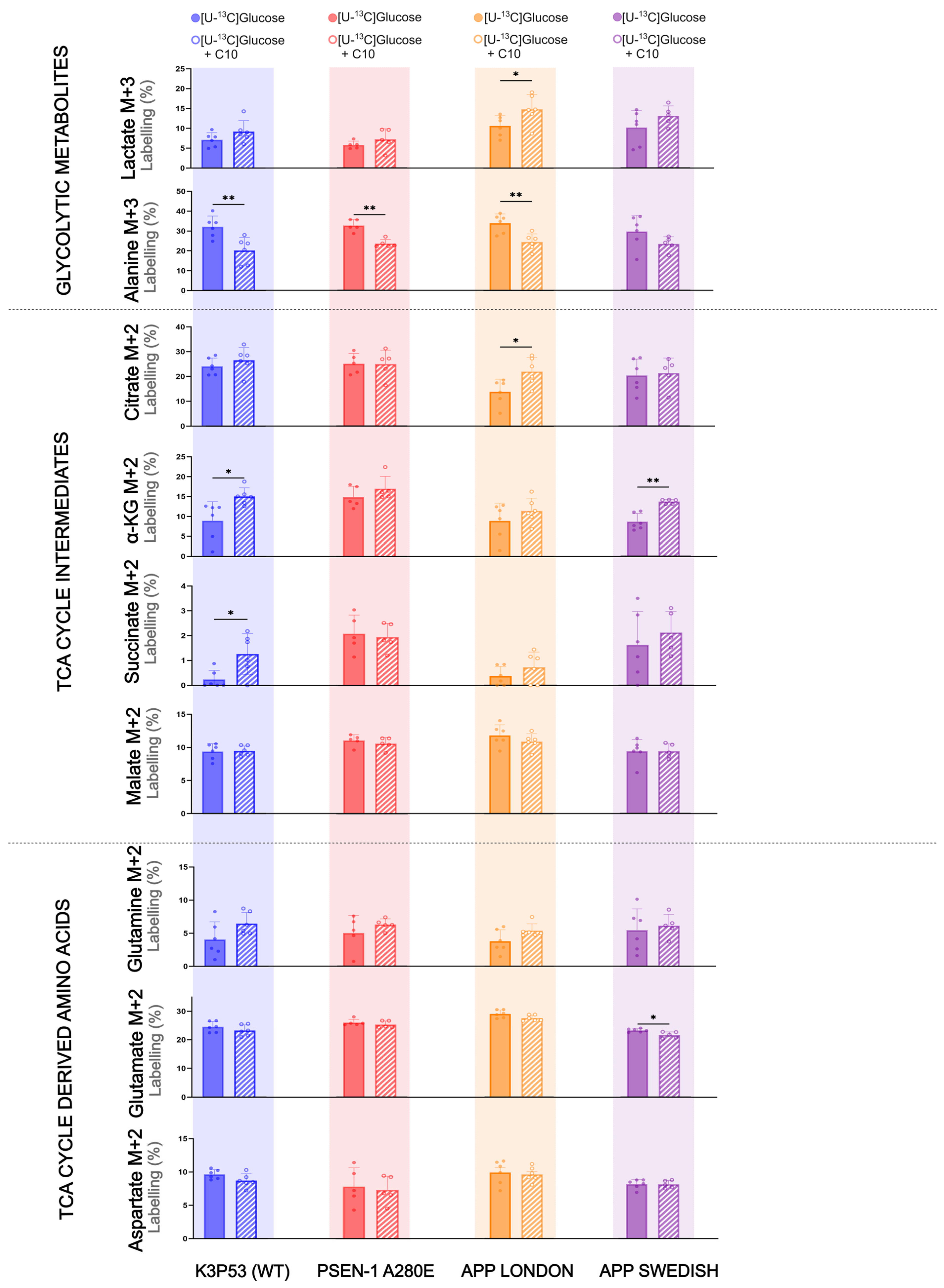
3.3. The Effect of C10 on Glucose Metabolism Is AD Mutation-Dependent in hiPSC-Derived Astrocytes
4. Discussion
4.1. Regional Changes in 5xFAD Brain Energy Metabolism
4.2. C10 as a Brain Substrate in AD and Its Effect on Glucose and Acetate Metabolism
4.3. Effect of C10 on Glucose Metabolism in hiPSC Derived Astrocytes Carrying AD Mutations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 13C | Carbon-13 isotope |
| ACSF | Artificial cerebrospinal fluid |
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| APOE | Apolipoprotein E |
| C10 | Decanoic acid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FAD | Familial Alzheimer’s disease |
| GABA | Gamma-aminobutyric acid |
| GC–MS | Gas chromatography–mass spectrometry |
| hiPSC | Human induced pluripotent stem cell |
| MCI | Mild cognitive impairment |
| MCFA | Medium-chain fatty acid |
| MCT | Medium-chain triglyceride |
| PSEN1 | Presenilin 1 |
| SMAD | Small mothers against decapentaplegic |
| TCA | Tricarboxylic acid cycle |
References
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 486–510. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Frontzkowski, L.; Ewers, M.; Brendel, M.; Biel, D.; Ossenkoppele, R.; Hager, P.; Steward, A.; Dewenter, A.; Römer, S.; Rubinski, A.; et al. Earlier Alzheimer’s disease onset is associated with tau pathology in brain hub regions and facilitated tau spreading. Nat. Commun. 2022, 13, 4899. [Google Scholar] [CrossRef]
- Patel, V.; Mill, J.; Okonkwo, O.C.; Salamat, S.; Li, L.; Raife, T. Global Energy Metabolism Deficit in Alzheimer Disease Brain. J. Prev. Alzheimer’s Dis. 2024, 11, 171–178. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Ameen, A.O.; Freude, K.; Aldana, B.I. Fats, Friends or Foes: Investigating the Role of Short- and Medium-Chain Fatty Acids in Alzheimer’s Disease. Biomedicines 2022, 10, 2778. [Google Scholar] [CrossRef]
- Sun, L.; Ye, K.X.; Wong, H.L.K.; Wang, L.; Lim, S.L.; Chao, Y.X.; Zhang, C.; Yap, K.Z.; Feng, L. The Effects of Medium Chain Triglyceride for Alzheimer’s Disease Related Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2023, 94, 441–456. [Google Scholar] [CrossRef]
- Giannos, P.; Prokopidis, K.; Lidoriki, I.; Triantafyllidis, K.K.; Kechagias, K.S.; Celoch, K.; Candow, D.G.; Ostojic, S.M.; Forbes, S.C. Medium-chain triglycerides may improve memory in non-demented older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2022, 22, 817. [Google Scholar] [CrossRef]
- Croteau, E.; Castellano, C.A.; Richard, M.A.; Fortier, M.; Nugent, S.; Lepage, M.; Duchesne, S.; Whittingstall, K.; E Turcotte, É.; Bocti, C.; et al. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 551–561. [Google Scholar] [CrossRef]
- Andersen, J.V.; Westi, E.W.; Neal, E.S.; Aldana, B.I.; Borges, K. β-Hydroxybutyrate and Medium-Chain Fatty Acids are Metabolized by Different Cell Types in Mouse Cerebral Cortex Slices. Neurochem. Res. 2023, 48, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Westi, E.W.; Jakobsen, E.; Urruticoechea, N.; Borges, K.; Aldana, B.I. Astrocyte metabolism of the medium-chain fatty acids octanoic acid and decanoic acid promotes GABA synthesis in neurons via elevated glutamine supply. Mol. Brain 2021, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Christensen, S.K.; Westi, E.W.; Diaz-delCastillo, M.; Tanila, H.; Schousboe, A.; Aldana, B.I.; Waagepetersen, H.S. Deficient astrocyte metabolism impairs glutamine synthesis and neurotransmitter homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 148, 105198. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Adamson, D.C. Neuronal-Astrocyte Metabolic Interactions: Understanding the Transition Into Abnormal Astrocytoma Metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Mett, J.; Müller, U. The medium-chain fatty acid decanoic acid reduces oxidative stress levels in neuroblastoma cells. Sci. Rep. 2021, 11, 6135. [Google Scholar] [CrossRef]
- Tan, K.N.; Carrasco-Pozo, C.; McDonald, T.S.; Puchowicz, M.; Borges, K. Tridecanoin is anticonvulsant, antioxidant, and improves mitochondrial function. J. Cereb. Blood Flow Metab. 2017, 37, 2035–2048. [Google Scholar] [CrossRef]
- Warren, E.C.; Dooves, S.; Lugarà, E.; Damstra-Oddy, J.; Schaf, J.; Heine, V.M.; Walker, M.C.; Williams, R.S.B. Decanoic acid inhibits mTORC1 activity independent of glucose and insulin signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 23617–23625. [Google Scholar] [CrossRef]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef]
- Abghari, M.; Vu, J.T.C.M.; Eckberg, N.; Aldana, B.I.; Kohlmeier, K.A. Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules 2023, 13, 1461. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129. [Google Scholar] [CrossRef]
- Westi, E.W.; Molhemi, S.; Hansen, C.T.; Skoven, C.S.; Knopper, R.W.; Ahmad, D.A.; Rindshøj, M.B.; Ameen, A.O.; Hansen, B.; Kohlmeier, K.A.; et al. Comprehensive Analysis of the 5xFAD Mouse Model of Alzheimer’s Disease Using dMRI, Immunohistochemistry, and Neuronal and Glial Functional Metabolic Mapping. Biomolecules 2024, 14, 1294. [Google Scholar] [CrossRef] [PubMed]
- McNair, L.F.; Kornfelt, R.; Walls, A.B.; Andersen, J.V.; Aldana, B.I.; Nissen, J.D.; Schousboe, A.; Waagepetersen, H.S. Metabolic Characterization of Acutely Isolated Hippocampal and Cerebral Cortical Slices Using [U-(13)C]Glucose and [1,2-(13)C]Acetate as Substrates. Neurochem. Res. 2017, 42, 810–826. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.R.; Holst, B.; Ramakrishna, S.; Muddashetty, R.; Schmid, B.; Freude, K. Generation of two iPSC lines with either a heterozygous V717I or a heterozygous KM670/671NL mutation in the APP gene. Stem Cell Res. 2019, 34, 101368. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.R.; Holst, B.; Mau-Holzmann, U.A.; Freude, K.; Schmid, B. Generation of two isogenic iPSC lines with either a heterozygous or a homozygous E280A mutation in the PSEN1 gene. Stem Cell Res. 2019, 35, 101403. [Google Scholar] [CrossRef]
- Dittlau, K.S.; Chandrasekaran, A.; Freude, K.; Van Den Bosch, L. Generation of Human Induced Pluripotent Stem Cell (hiPSC)-Derived Astrocytes for Amyotrophic Lateral Sclerosis and Other Neurodegenerative Disease Studies. Bio-Protocol 2024, 14, e4936. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Avci, H.X.; Ochalek, A.; Rösingh, L.N.; Molnár, K.; László, L.; Bellák, T.; Téglási, A.; Pesti, K.; Mike, A.; et al. Comparison of 2D and 3D neural induction methods for the generation of neural progenitor cells from human induced pluripotent stem cells. Stem Cell Res. 2017, 25, 139–151. [Google Scholar] [CrossRef]
- Shaltouki, A.; Peng, J.; Liu, Q.; Rao, M.S.; Zeng, X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells 2013, 31, 941–952. [Google Scholar] [CrossRef]
- Salcedo, C.; Pozo Garcia, V.; García-Adán, B.; Ameen, A.O.; Gegelashvili, G.; Waagepetersen, H.S.; Freude, K.K.; Aldana, B.I. Increased glucose metabolism and impaired glutamate transport in human astrocytes are potential early triggers of abnormal extracellular glutamate accumulation in hiPSC-derived models of Alzheimer’s disease. J. Neurochem. 2023, 168, 822–840. [Google Scholar] [CrossRef]
- Wlaź, P.; Socała, K.; Nieoczym, D.; Żarnowski, T.; Żarnowska, I.; Czuczwar, S.J.; Gasior, M. Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 57, 110–116. [Google Scholar] [CrossRef]
- Wlaź, P.; Socała, K.; Nieoczym, D.; Łuszczki, J.J.; Żarnowska, I.; Żarnowski, T.; Czuczwar, S.J.; Gasior, M. Anticonvulsant profile of caprylic acid, a main constituent of the medium-chain triglyceride (MCT) ketogenic diet, in mice. Neuropharmacology 2012, 62, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.B.; Bak, L.K.; Sonnewald, U.; Schousboe, A.; Waagepetersen, H.S. Metabolic Mapping of Astrocytes and Neurons in Culture Using Stable Isotopes and Gas Chromatography-Mass Spectrometry (GC-MS). In Brain Energy Metabolism; Hirrlinger, J., Waagepetersen, H.S., Eds.; Springer: New York, NY, USA, 2014; pp. 73–105. [Google Scholar]
- Sonnewald, U.; Westergaard, N.; Schousboe, A.; Svendsen, J.S.; Unsgård, G.; Petersen, S.B. Direct demonstration by [13C]NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem. Int. 1993, 22, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Schousboe, A. Glial Glutamine Homeostasis in Health and Disease. Neurochem. Res. 2023, 48, 1100–1128. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, N.; Waagepetersen, H.S.; Belhage, B.; Schousboe, A. Citrate, a Ubiquitous Key Metabolite with Regulatory Function in the CNS. Neurochem. Res. 2017, 42, 1583–1588. [Google Scholar] [CrossRef]
- Macdonald, I.R.; DeBay, D.R.; Reid, G.A.; O’Leary, T.P.; Jollymore, C.T.; Mawko, G.; Burrell, S.; Martin, E.; Bowen, C.V.; Brown, R.E.; et al. Early detection of cerebral glucose uptake changes in the 5XFAD mouse. Curr. Alzheimer Res. 2014, 11, 450–460. [Google Scholar] [CrossRef]
- Choi, H.; Choi, Y.; Lee, E.J.; Kim, H.; Lee, Y.; Kwon, S.; Hwang, D.W.; Lee, D.S. Hippocampal glucose uptake as a surrogate of metabolic change of microglia in Alzheimer’s disease. J. Neuroinflamm. 2021, 18, 190. [Google Scholar] [CrossRef]
- Gordon, B.A.; Blazey, T.M.; Su, Y.; Hari-Raj, A.; Dincer, A.; Flores, S.; Christensen, J.; McDade, E.; Wang, G.; Xiong, C.; et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol. 2018, 17, 241–250. [Google Scholar] [CrossRef]
- Ashraf, A.; Fan, Z.; Brooks, D.J.; Edison, P. Cortical hypermetabolism in MCI subjects: A compensatory mechanism? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 447–458. [Google Scholar] [CrossRef]
- Xiang, X.; Wind, K.; Wiedemann, T.; Blume, T.; Shi, Y.; Briel, N.; Beyer, L.; Biechele, G.; Eckenweber, F.; Zatcepin, A.; et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci. Transl. Med. 2021, 13, eabe5640. [Google Scholar] [CrossRef]
- Benzinger, T.L.S.; Blazey, T.; Jack, C.R.; Koeppe, R.A.; Su, Y.; Xiong, C.; Raichle, M.E.; Snyder, A.Z.; Ances, B.M.; Bateman, R.J.; et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2013, 110, E4502–E4509. [Google Scholar] [CrossRef]
- Wyss, M.T.; Magistretti, P.J.; Buck, A.; Weber, B. Labeled acetate as a marker of astrocytic metabolism. J. Cereb. Blood Flow Metab. 2011, 31, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Skotte, N.H.; Christensen, S.K.; Polli, F.S.; Shabani, M.; Markussen, K.H.; Haukedal, H.; Westi, E.W.; Diaz-Delcastillo, M.; Sun, R.C.; et al. Hippocampal disruptions of synaptic and astrocyte metabolism are primary events of early amyloid pathology in the 5xFAD mouse model of Alzheimer’s disease. Cell Death Dis. 2021, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.; Anderson, H.D.; Anderson, C.M. Astrocyte dysfunction in Alzheimer disease. J. Neurosci. Res. 2017, 95, 2430–2447. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Carter, S.F.; Herholz, K.; Rosa-Neto, P.; Pellerin, L.; Nordberg, A.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol. Med. 2019, 25, 77–95. [Google Scholar] [CrossRef]
- Oksanen, M.; Lehtonen, S.; Jaronen, M.; Goldsteins, G.; Hämäläinen, R.H.; Koistinaho, J. Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell Mol. Life Sci. 2019, 76, 2739–2760. [Google Scholar] [CrossRef]
- Steele, M.L.; Robinson, S.R. Reactive astrocytes give neurons less support: Implications for Alzheimer’s disease. Neurobiol. Aging 2012, 33, 423.e1–423.e13. [Google Scholar] [CrossRef]
- Romano, A.; Koczwara, J.B.; Gallelli, C.A.; Vergara, D.; Micioni Di Bonaventura, M.V.; Gaetani, S.; Giudetti, A.M. Fats for thoughts: An update on brain fatty acid metabolism. Int. J. Biochem. Cell Biol. 2017, 84, 40–45. [Google Scholar] [CrossRef]
- Khabbush, A.; Orford, M.; Tsai, Y.C.; Rutherford, T.; O’Donnell, M.; Eaton, S.; Heales, S.J.R. Neuronal decanoic acid oxidation is markedly lower than that of octanoic acid: A mechanistic insight into the medium-chain triglyceride ketogenic diet. Epilepsia 2017, 58, 1423–1429. [Google Scholar] [CrossRef]
- Panov, A.; Orynbayeva, Z.; Vavilin, V.; Lyakhovich, V. Fatty Acids in Energy Metabolism of the Central Nervous System. BioMed Res. Int. 2014, 2014, 472459. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Egan, J.M.; Mattson, M.P.; Kapogiannis, D. Medium Chain Triglycerides induce mild ketosis and may improve cognition in Alzheimer’s disease. A systematic review and meta-analysis of human studies. Ageing Res. Rev. 2020, 58, 101001. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D.; Martinez-Hernandez, A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979, 161, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; McNair, L.F.; Schousboe, A.; Waagepetersen, H.S. Specificity of exogenous acetate and glutamate as astrocyte substrates examined in acute brain slices from female mice using methionine sulfoximine (MSO) to inhibit glutamine synthesis. J. Neurosci. Res. 2017, 95, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Leke, R.; Schousboe, A. The Glutamine Transporters and Their Role in the Glutamate/GABA-Glutamine Cycle. Adv. Neurobiol. 2016, 13, 223–257. [Google Scholar] [CrossRef]
- Hertz, L. The Glutamate-Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front. Endocrinol. 2013, 4, 59. [Google Scholar] [CrossRef]
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.R.; Sun, W.; Goldman, S.; Blekot, S.; Nielsen, M.; et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 2015, 6, 6807. [Google Scholar] [CrossRef]
- Haynes, V.R.; Michael, N.J.; van den Top, M.; Zhao, F.Y.; Brown, R.D.; De Souza, D.; Dodd, G.T.; Spanswick, D.; Watt, M.J. A Neural basis for Octanoic acid regulation of energy balance. Mol. Metab. 2020, 34, 54–71. [Google Scholar] [CrossRef]
- Oksanen, M.; Petersen, A.J.; Naumenko, N.; Puttonen, K.; Lehtonen, Š.; Gubert Olivé, M.; Shakirzyanova, A.; Leskelä, S.; Sarajärvi, T.; Viitanen, M.; et al. PSEN1 Mutant iPSC-Derived Model Reveals Severe Astrocyte Pathology in Alzheimer’s Disease. Stem Cell Rep. 2017, 9, 1885–1897. [Google Scholar] [CrossRef]
- Ryu, W.-I.; Bormann, M.K.; Shen, M.; Kim, D.; Forester, B.; Park, Y.; So, J.; Seo, H.; Sonntag, K.-C.; Cohen, B.M. Brain cells derived from Alzheimer’s disease patients have multiple specific innate abnormalities in energy metabolism. Mol. Psychiatry 2021, 26, 5702–5714. [Google Scholar] [CrossRef]
- Ziff, O.J.; Parfitt, G.M.; Jolly, S.; Casey, J.M.; Granat, L.; Samra, S.; Setó-Salvia, N.; Alatza, A.; Phadke, L.; Galet, B.; et al. Mutations in PSEN1 predispose inflammation in an astrocyte model of familial Alzheimer’s disease through disrupted regulated intramembrane proteolysis. Mol. Neurodegener. 2025, 20, 73. [Google Scholar] [CrossRef]
- Konttinen, H.; Gureviciene, I.; Oksanen, M.; Grubman, A.; Loppi, S.; Huuskonen, M.T.; Korhonen, P.; Lampinen, R.; Keuters, M.; Belaya, I.; et al. PPARβ/δ-agonist GW0742 ameliorates dysfunction in fatty acid oxidation in PSEN1ΔE9 astrocytes. Glia 2019, 67, 146–159. [Google Scholar] [CrossRef]
- McComish, S.F.; O’Sullivan, J.; Copas, A.M.M.; Imiolek, M.; Boyle, N.T.; Crompton, L.A.; Lane, J.D.; Caldwell, M.A. Reactive astrocytes generated from human iPSC are pro-inflammatory and display altered metabolism. Exp. Neurol. 2024, 382, 114979. [Google Scholar] [CrossRef]
- Afridi, R.; Kim, J.-H.; Rahman, M.H.; Suk, K. Metabolic Regulation of Glial Phenotypes: Implications in Neuron–Glia Interactions and Neurological Disorders. Front. Cell. Neurosci. 2020, 14, 20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, A.O.; Rindshøj, M.B.; Dittlau, K.S.; Borges, K.; Freude, K.K.; Aldana, B.I. Metabolic Astrocytic Support with Decanoic Acid Enhances Energy Metabolism in Alzheimer’s Disease Models. Cells 2025, 14, 2007. https://doi.org/10.3390/cells14242007
Ameen AO, Rindshøj MB, Dittlau KS, Borges K, Freude KK, Aldana BI. Metabolic Astrocytic Support with Decanoic Acid Enhances Energy Metabolism in Alzheimer’s Disease Models. Cells. 2025; 14(24):2007. https://doi.org/10.3390/cells14242007
Chicago/Turabian StyleAmeen, Aishat O., Maja B. Rindshøj, Katarina Stoklund Dittlau, Karin Borges, Kristine K. Freude, and Blanca I. Aldana. 2025. "Metabolic Astrocytic Support with Decanoic Acid Enhances Energy Metabolism in Alzheimer’s Disease Models" Cells 14, no. 24: 2007. https://doi.org/10.3390/cells14242007
APA StyleAmeen, A. O., Rindshøj, M. B., Dittlau, K. S., Borges, K., Freude, K. K., & Aldana, B. I. (2025). Metabolic Astrocytic Support with Decanoic Acid Enhances Energy Metabolism in Alzheimer’s Disease Models. Cells, 14(24), 2007. https://doi.org/10.3390/cells14242007





