Human Pericardial Fluid-Derived Cells Exhibit Mesothelial-like Properties and Exert Proangiogenic Effects on Endothelial Cells
Highlights
- Pericardial fluid-derived mesothelial cells (MCs) can be easily obtained from human pericardial fluid and exhibit a characteristic epithelial-like morphology and phenotype, alongside robust proliferation in vitro.
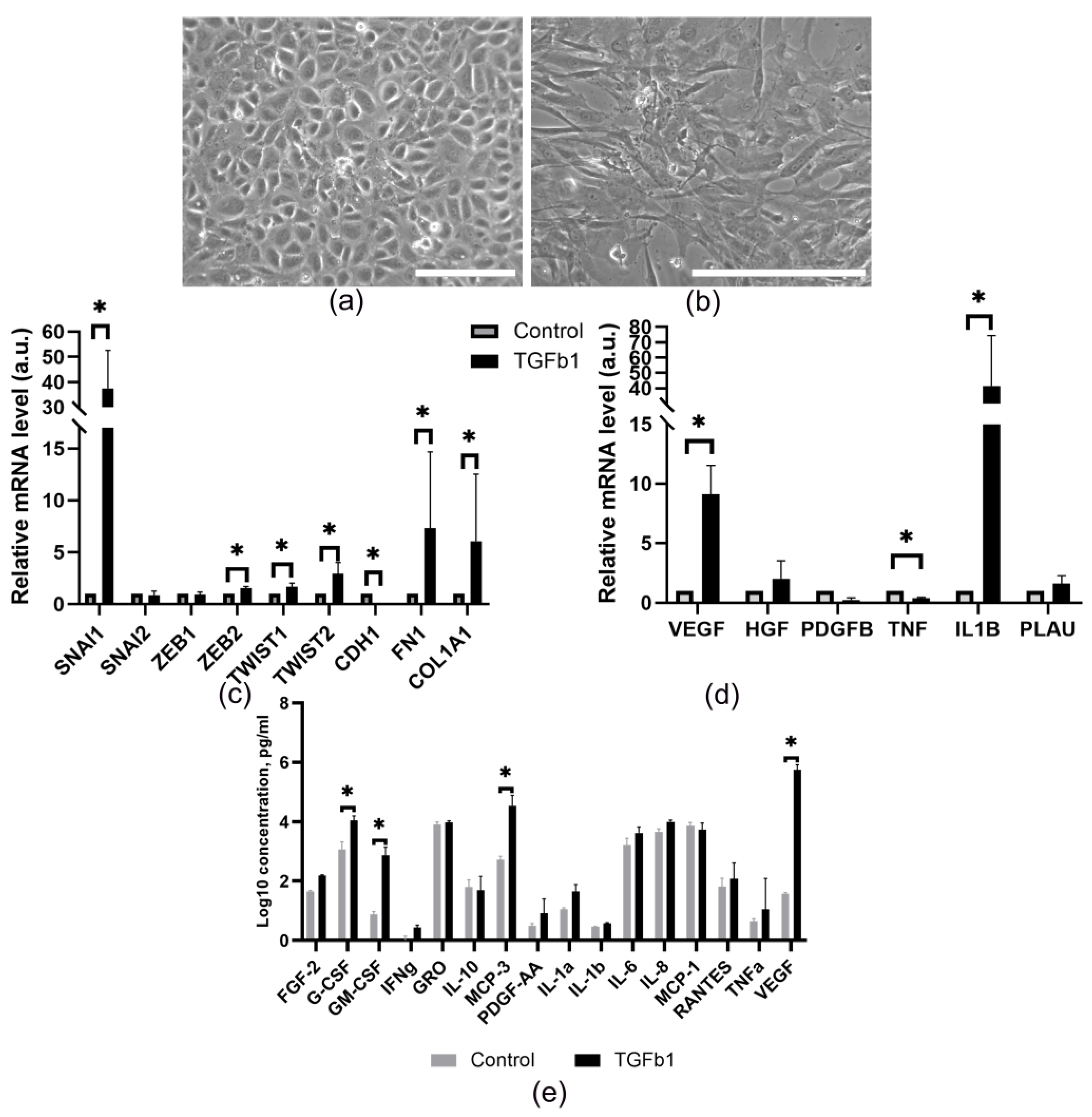
- Pericardial fluid-derived MCs can undergo epithelial-mesenchymal transition (EMT) upon stimulation with TGF-β1, while enhancing the secretion of VEGF, G-CSF, GM-CSF and MCP-3, as well as their proangiogenic properties.
- These findings establish a foundation for further investigation into the role of the mesothelium in regulating angiogenesis and cardiac repair mechanisms.
Abstract
1. Introduction
2. Materials and Methods
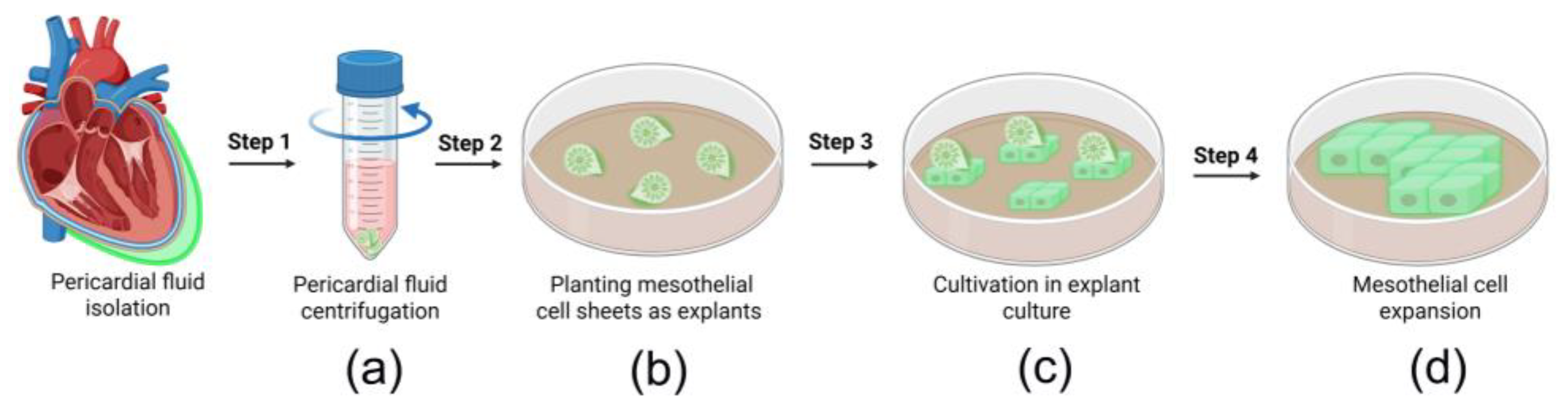
2.1. Acquisition of Pericardial Fluid Samples
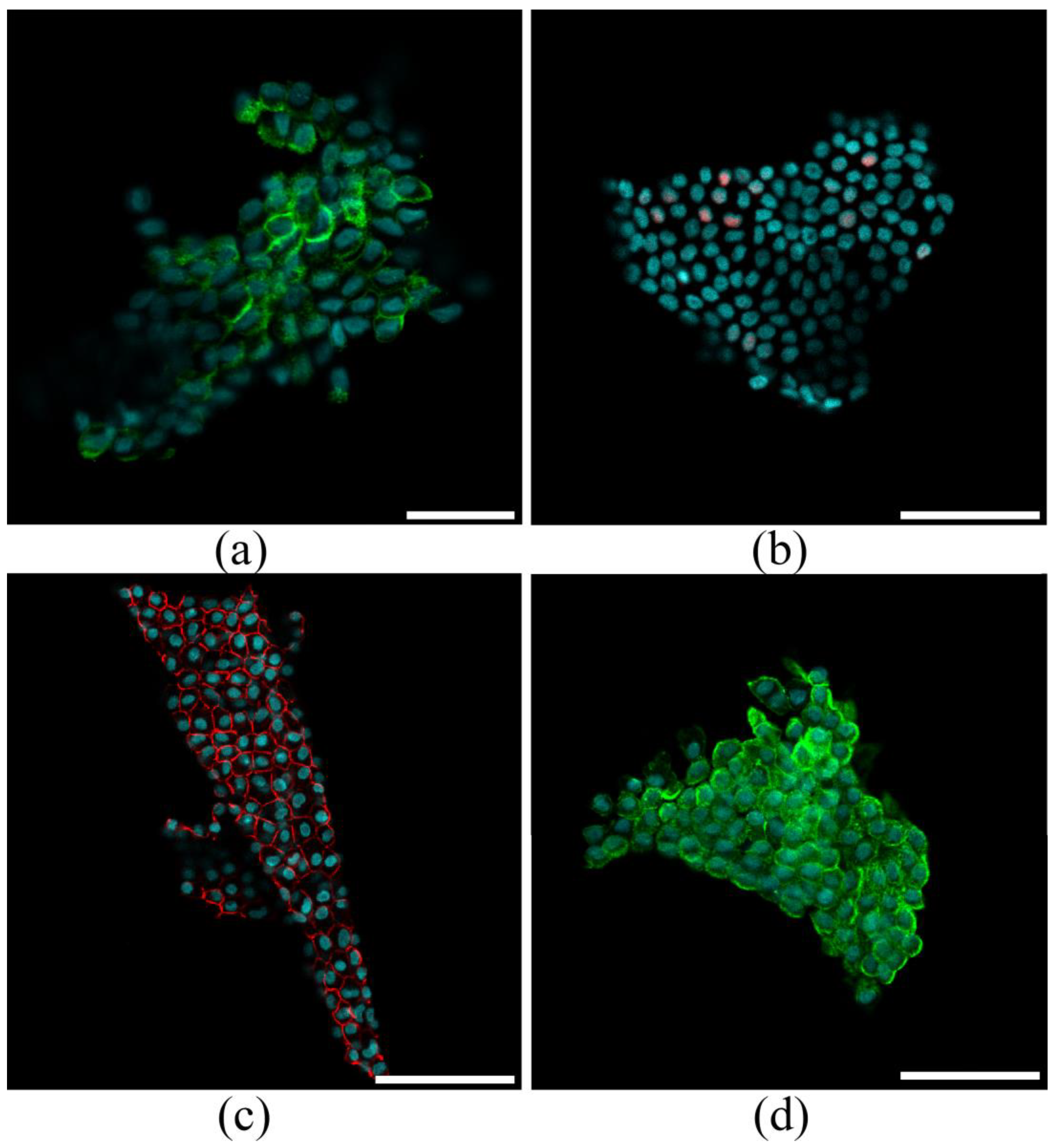
2.2. Characterization of Pericardial Fluid-Derived Cell Sheets
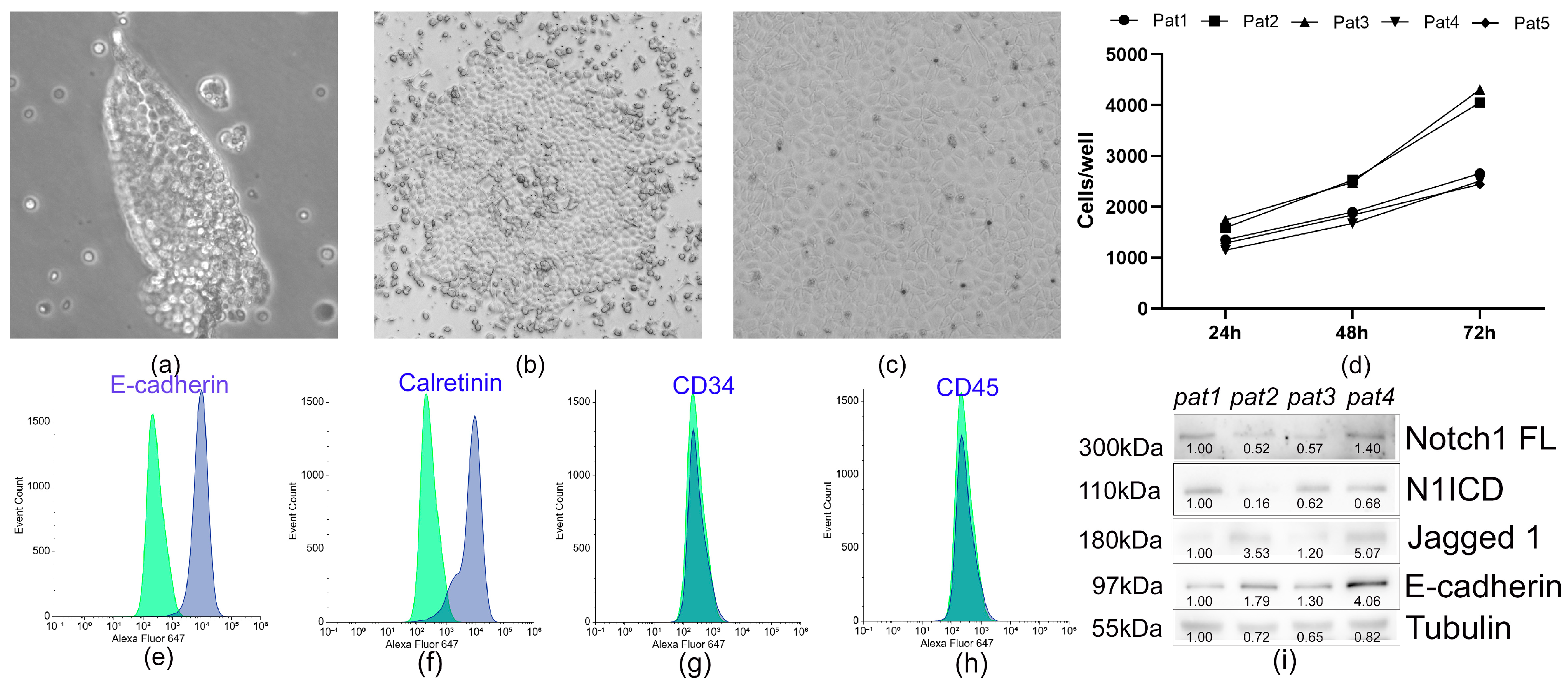
2.3. Flow Cytometry Analysis
2.4. Mesothelial Cell Proliferation Assay
2.5. RNA Isolation, Reverse Transcription and Real-Time Quantitative PCR
2.6. Protein Extraction and Western Blotting
2.7. Condition Medium Harvesting
2.8. Magpix Analysis
2.9. In Vitro Angiogenesis Assay
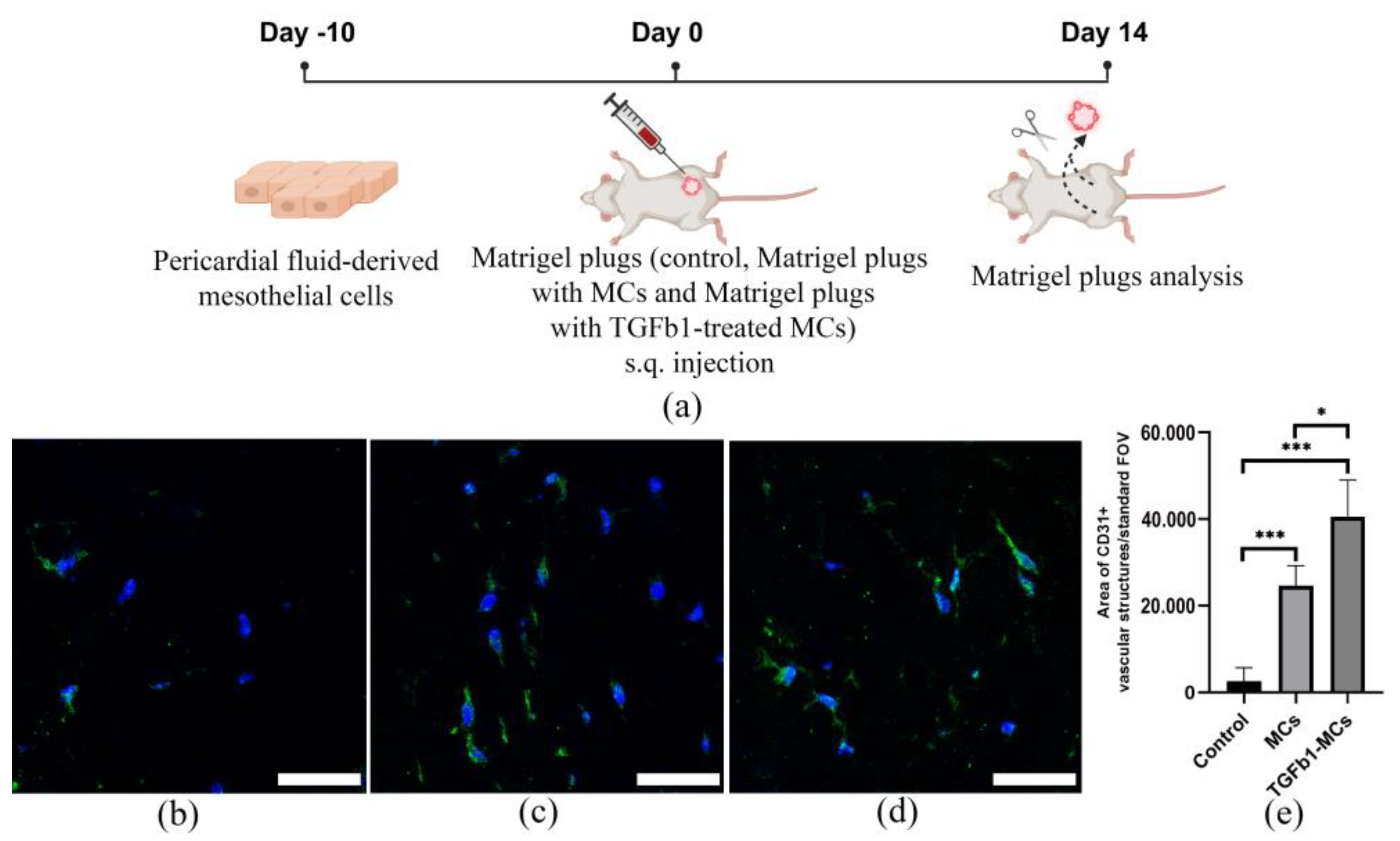
2.10. MatrigelTM Plug Assay
2.11. Statistical Analysis
3. Results
3.1. Isolation of Epicardial MCs Derived from Pericardial Fluid Samples
3.2. Characterization of Pericardial Fluid-Derived MCs
3.3. Pericardial Fluid-Derived MCs Exhibit an Ability to Undergo EMT and Modulate Their Secretion
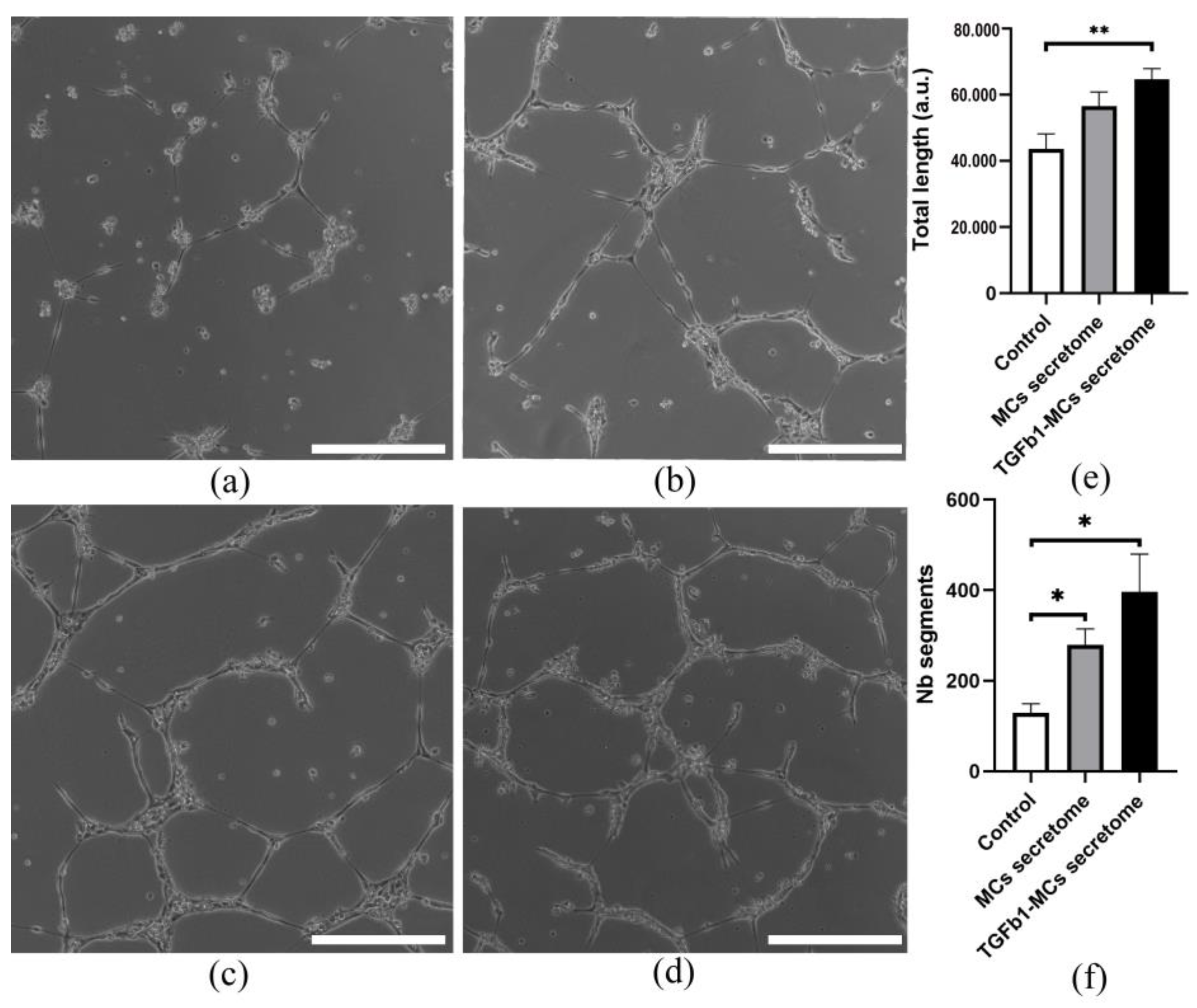
3.4. The Proangiogenic Potential of Pericardial Fluid-Derived MCs in Vascular Network Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BSA | Bovine serum albumin |
| CM | Condition medium |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DM | Diabetes mellitus |
| DPBS | Dulbecco’s Phosphate-Buffered Saline |
| ECM | Extracellular matrix |
| EGF | Epidermal Growth Factor |
| EMT | Epithelial-to-mesenchymal transition |
| EPDCs | Epicardium-derived cells |
| ET-1 | Endothelin-1 |
| FGF | Fibroblast growth factor |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GRO-1 | Growth-Regulated oncogene 1 |
| HC | Hypercholesterolemia |
| HGF | Hepatocyte growth factor |
| HT | Hypertension |
| HUVEC | Human umbilical vein endothelial cells |
| IFN-γ | Interferon gamma |
| IL-1α | Interleukin-1alpha |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-9 | Interleukin-9 |
| IP-10 | Interferon Gamma-induced Protein 10 |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| KGF | Keratinocyte growth factor |
| MC | Mesothelial cell |
| MCAD | Multivessel coronary artery disease |
| MCP-3 | Monocyte chemotactic protein-3 |
| MDC | Macrophage-derived chemokine |
| MIP-1α | Macrophage inflammatory protein 1-alpha |
| mTOR | Mammalian target of rapamycin |
| NICD | Notch Intracellular Domain |
| NF-κB | Nuclear factor kappa-B |
| Pat | Patient |
| PBS | Phosphate-Buffered Saline |
| PDGF | Platelet-derived growth factor |
| PTEN | Phosphatase and Tensin homolog |
| RANTES | Regulated on activation, normal T-cell expressed and secreted |
| TGFb | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
References
- Kang, K.; Wang, Q.; Li, Y.; Liu, C.; Yu, H.; Li, N. Global and Chinese Perspectives on the Growing Burden of Heart Failure: Trends, Gender, and Age-Related Differences (1990–2021) Based on GBD 2021 Data. BMC Cardiovasc. Disord. 2025, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of Heart Failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Wu, B.; Constanty, F.; Beisaw, A. Cardiac Regeneration: Unraveling the Complex Network of Intercellular Crosstalk. Semin. Cell Dev. Biol. 2025, 171, 103619. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, I.; Frantz, S.; Frangogiannis, N.G. Repair of the Infarcted Heart: Cellular Effectors, Molecular Mechanisms, and Therapeutic Opportunities. Circ. Res. 2024, 134, 1718–1751. [Google Scholar] [CrossRef]
- van der Laan, A.M.; Piek, J.J.; van Royen, N. Targeting Angiogenesis to Restore the Microcirculation after Reperfused MI. Nat. Rev. Cardiol. 2009, 6, 515–523. [Google Scholar] [CrossRef]
- Shah, A.M.; Mann, D.L. In Search of New Therapeutic Targets and Strategies for Heart Failure: Recent Advances in Basic Science. Lancet 2011, 378, 704–712. [Google Scholar] [CrossRef]
- Distefano, G.; Sciacca, P. Molecular Pathogenesis of Myocardial Remodeling and New Potential Therapeutic Targets in Chronic Heart Failure. Ital. J. Pediatr. 2012, 38, 41. [Google Scholar] [CrossRef]
- Smart, N.; Riley, P.R. The Epicardium As a Candidate for Heart Regeneration. Future Cardiol. 2012, 8, 53–69. [Google Scholar] [CrossRef]
- Wong, D.; Martinez, J.; Quijada, P. Exploring the Function of Epicardial Cells Beyond the Surface. Circ. Res. 2024, 135, 353–371. [Google Scholar] [CrossRef]
- Niderla-Bielińska, J.; Jankowska-Steifer, E.; Flaht-Zabost, A.; Gula, G.; Czarnowska, E.; Ratajska, A. Proepicardium: Current Understanding of Its Structure, Induction, and Fate. Anat. Rec. 2019, 302, 893–903. [Google Scholar] [CrossRef]
- Peralta, M.; Steed, E.; Harlepp, S.; González-Rosa, J.M.; Monduc, F.; Ariza-Cosano, A.; Cortés, A.; Rayón, T.; Gómez-Skarmeta, J.-L.; Zapata, A.; et al. Heartbeat-Driven Pericardiac Fluid Forces Contribute to Epicardium Morphogenesis. Curr. Biol. 2013, 23, 1726–1735. [Google Scholar] [CrossRef]
- Plavicki, J.S.; Hofsteen, P.; Yue, M.S.; Lanham, K.A.; Peterson, R.E.; Heideman, W. Multiple Modes of Proepicardial Cell Migration Require Heartbeat. BMC Dev. Biol. 2014, 14, 18. [Google Scholar] [CrossRef]
- Hirose, T.; Karasawa, M.; Sugitani, Y.; Fujisawa, M.; Akimoto, K.; Ohno, S.; Noda, T. PAR3 Is Essential for Cyst-Mediated Epicardial Development by Establishing Apical Cortical Domains. Development 2006, 133, 1389–1398. [Google Scholar] [CrossRef]
- Cai, C.-L.; Martin, J.C.; Sun, Y.; Cui, L.; Wang, L.; Ouyang, K.; Yang, L.; Bu, L.; Liang, X.; Zhang, X.; et al. A Myocardial Lineage Derives from Tbx18 Epicardial Cells. Nature 2008, 454, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Braitsch, C.M.; Combs, M.D.; Quaggin, S.E.; Yutzey, K.E. Pod1/Tcf21 Is Regulated by Retinoic Acid Signaling and Inhibits Differentiation of Epicardium-Derived Cells into Smooth Muscle in the Developing Heart. Dev. Biol. 2012, 368, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Vrancken Peeters, M.-P.F.M.; Mentink, M.M.T.; Gourdie, R.G.; Poelmann, R.E. Epicardium-Derived Cells Contribute a Novel Population to the Myocardial Wall and the Atrioventricular Cushions. Circ. Res. 1998, 82, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, T.; Gourdie, R.G. Pericardial Mesoderm Generates a Population of Coronary Smooth Muscle Cells Migrating into the Heart along with Ingrowth of the Epicardial Organ. Dev. Biol. 1996, 174, 221–232. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C.; Winter, E.M.; Poelmann, R.E. Epicardium Derived Cells (EPDCs) in Development, Cardiac Disease and Repair of Ischemia. J. Cell. Mol. Med. 2010, 14, 1056–1060. [Google Scholar] [CrossRef]
- Red-Horse, K.; Ueno, H.; Weissman, I.L.; Krasnow, M.A. Coronary Arteries Form by Developmental Reprogramming of Venous Cells. Nature 2010, 464, 549–553. [Google Scholar] [CrossRef]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct Compartments of the Proepicardial Organ Give Rise to Coronary Vascular Endothelial Cells. Dev. Cell 2012, 22, 639–650. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; Von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial Progenitors Contribute to the Cardiomyocyte Lineage in the Developing Heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.R.M.; Bussmann, J.; Huang, Y.; Zhao, L.; Osorio, A.; Burns, C.G.; Burns, C.E.; Sucov, H.M.; Siekmann, A.F.; Lien, C.-L. Chemokine-Guided Angiogenesis Directs Coronary Vasculature Formation in Zebrafish. Dev. Cell 2015, 33, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, A.; Fujita, M.; Ikemoto, M.; Hasegawa, K.; Nohara, R.; Sasayama, S.; Miyamoto, S.; Yamazato, A.; Tambara, K.; Komeda, M. Myocardial Ischemia Enhances the Expression of Acidic Fibroblast Growth Factor in Human Pericardial Fluid. Heart Vessel. 2000, 15, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ikemoto, M.; Kishishita, M.; Otani, H.; Nohara, R.; Tanaka, T.; Tamaki, S.; Yamazato, A.; Sasayama, S. Elevated Basic Fibroblast Growth Factor in Pericardial Fluid of Patients with Unstable Angina. Circulation 1996, 94, 610–613. [Google Scholar] [CrossRef]
- Sharma, B.; Ho, L.; Ford, G.H.; Chen, H.I.; Goldstone, A.B.; Woo, Y.J.; Quertermous, T.; Reversade, B.; Red-Horse, K. Alternative Progenitor Cells Compensate to Rebuild the Coronary Vasculature in Elabela- and Apj-Deficient Hearts. Dev. Cell 2017, 42, 655–666.e3. [Google Scholar] [CrossRef]
- Schlueter, J.; Brand, T. Epicardial Progenitor Cells in Cardiac Development and Regeneration. J. Cardiovasc. Transl. Res. 2012, 5, 641–653. [Google Scholar] [CrossRef]
- Lien, C.-L.; Schebesta, M.; Makino, S.; Weber, G.J.; Keating, M.T. Gene Expression Analysis of Zebrafish Heart Regeneration. PLoS Biol. 2006, 4, e260. [Google Scholar] [CrossRef]
- Chen, H.I.; Sharma, B.; Akerberg, B.N.; Numi, H.J.; Kivelä, R.; Saharinen, P.; Aghajanian, H.; McKay, A.S.; Bogard, P.E.; Chang, A.H.; et al. The Sinus Venosus Contributes to Coronary Vasculature through VEGFC-Stimulated Angiogenesis. Development 2014, 141, 4500–4512. [Google Scholar] [CrossRef]
- del Monte, G.; Casanova, J.C.; Guadix, J.A.; MacGrogan, D.; Burch, J.B.E.; Pérez-Pomares, J.M.; de la Pompa, J.L. Differential Notch Signaling in the Epicardium Is Required for Cardiac Inflow Development and Coronary Vessel Morphogenesis. Circ. Res. 2011, 108, 824–836. [Google Scholar] [CrossRef]
- Wu, S.-P.; Dong, X.-R.; Regan, J.N.; Su, C.; Majesky, M.W. Tbx18 Regulates Development of the Epicardium and Coronary Vessels. Dev. Biol. 2013, 383, 307–320. [Google Scholar] [CrossRef]
- Wang, S.; Moise, A.R. Recent Insights on the Role and Regulation of Retinoic Acid Signaling during Epicardial Development. Genesis 2019, 57, e23303. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.; Männer, J.; Ruiz-Lozano, P. Epicardium-Derived Progenitor Cells Require β-Catenin for Coronary Artery Formation. Proc. Natl. Acad. Sci. USA 2007, 104, 18109–18114. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Baek, S.T.; Sung, C.Y.; Tallquist, M.D. Epicardial-Derived Cell Epithelial-to-Mesenchymal Transition and Fate Specification Require PDGF Receptor Signaling. Circ. Res. 2011, 108, e15–e26. [Google Scholar] [CrossRef] [PubMed]
- Molin, D.G.M.; Bartram, U.; Van der Heiden, K.; Van Iperen, L.; Speer, C.P.; Hierck, B.P.; Poelmann, R.E.; Gittenberger-de-Groot, A.C. Expression Patterns of Tgfbeta1-3 Associate with Myocardialisation of the Outflow Tract and the Development of the Epicardium and the Fibrous Heart Skeleton. Dev. Dyn. 2003, 227, 431–444. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, J. Covering and Re-Covering the Heart: Development and Regeneration of the Epicardium. J. Cardiovasc. Dev. Dis. 2018, 6, 3. [Google Scholar] [CrossRef]
- Vogiatzidis, K.; Zarogiannis, S.G.; Aidonidis, I.; Solenov, E.I.; Molyvdas, P.-A.; Gourgoulianis, K.I.; Hatzoglou, C. Physiology of Pericardial Fluid Production and Drainage. Front. Physiol. 2015, 6, 62. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Shinfeld, A.; Kachel, E.; Chetrit, A.; Livneh, A. The Composition of Normal Pericardial Fluid and Its Implications for Diagnosing Pericardial Effusions. Am. J. Med. 2005, 118, 636–640. [Google Scholar] [CrossRef]
- Buoro, S.; Tombetti, E.; Ceriotti, F.; Simon, C.; Cugola, D.; Seghezzi, M.; Innocente, F.; Maestroni, S.; Del Carmen Baigorria Vaca, M.; Moioli, V.; et al. What Is the Normal Composition of Pericardial Fluid? Heart 2021, 107, 1584–1590. [Google Scholar] [CrossRef]
- Gülaştı, S.; Mutlu, B.; Zencir, C. Prognostic Factors in Moderate-to-Large Pericardial Effusion Requiring Pericardiocentesis. A Single-Center Retrospective Study. Kardiologiia 2025, 65, 42–47. [Google Scholar] [CrossRef]
- Fatehi Hassanabad, A.; Turnbull, J.; Hall, C.; Schoettler, F.I.; Fatehi Hassanabad, M.; Love, E.; De Chantal, E.; Dundas, J.A.; Isidoro, C.A.; Kim, S.; et al. Acute Pericardial Postischemic Inflammatory Responses: Characterization Using a Preclinical Porcine Model. Cardiovasc. Pathol. 2024, 73, 107686. [Google Scholar] [CrossRef]
- Dragoescu, E.A.; Liu, L. Pericardial Fluid Cytology: An Analysis of 128 Specimens over a 6-year Period. Cancer Cytopathol. 2013, 121, 242–251. [Google Scholar] [CrossRef] [PubMed]
- El-Diasty, M.M.; Rodríguez, J.; Pérez, L.; Eiras, S.; Fernández, A.L. Accumulation of Inflammatory Mediators in the Normal Pericardial Fluid. Int. J. Mol. Sci. 2023, 25, 157. [Google Scholar] [CrossRef] [PubMed]
- Kuosmanen, S.M.; Hartikainen, J.; Hippeläinen, M.; Kokki, H.; Levonen, A.-L.; Tavi, P. MicroRNA Profiling of Pericardial Fluid Samples from Patients with Heart Failure. PLoS ONE 2015, 10, e0119646. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kawaguchi, S.; Nakahara, H.; Hachimaru, T. The Roles of Natriuretic Peptides in Pericardial Fluid in Patients with Heart Failure. Clin. Cardiol. 2009, 32, 159–163. [Google Scholar] [CrossRef]
- Fujita, M.; Komeda, M.; Hasegawa, K.; Kihara, Y.; Nohara, R.; Sasayama, S. Pericardial Fluid as a New Material for Clinical Heart Research. Int. J. Cardiol. 2001, 77, 113–118. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Fu, Z.; Dong, Y.; Yu, Y.; Liu, Y.; Liu, Z.; Chen, J.; Yao, Y.; Chen, Y.; et al. Intrapericardial Administration of Human Pericardial Fluid Cells Improves Cardiac Functions in Rats with Heart Failure. Stem Cells Dev. 2024, 33, 616–629. [Google Scholar] [CrossRef]
- Luna, A.; Zapata, M.G.; Vicente, V.; Osuna, E. A Cytologic Study of the Sediments in Pericardial Fluid as It Relates to a Diagnosis of the Mechanism of Death. Z. Rechtsmed. 1987, 99, 129–134. [Google Scholar] [CrossRef]
- Zubkova, E.; Dergilev, K.; Beloglazova, I.; Kalinin, A.; Guseva, A.; Andreev, A.; Partigulov, S.; Lepilin, M.; Menshikov, M.; Parfyonova, Y. Paracrine Responses of Cardiosphere-Derived Cells to Cytokines and TLR Ligands: A Comparative Analysis. Int. J. Mol. Sci. 2023, 24, 17278. [Google Scholar] [CrossRef]
- Beloglazova, I.; Zubkova, E.; Dergilev, K.; Goltseva, Y.; Parfyonova, Y. New Insight on 2D In Vitro Angiogenesis Models: All That Stretches Is Not a Tube. Cells 2022, 11, 3278. [Google Scholar] [CrossRef]
- Dergilev, K.V.; Tsokolaeva, Z.I.; Beloglazova, I.B.; Vasilets, Y.D.; Traktuev, D.O.; Kulbitsky, N.B.; Parfenova, E.V. Role of Urokinase-Type Plasminogen Activator Receptor in the Regulation of Angiogenic Properties of Sca1+ Vasculogenic Progenitor Cells. Obs. Reanimatol. 2022, 18, 76–82. [Google Scholar] [CrossRef]
- Gauthier, T.; Chen, W. IFN-γ and TGF-β, Crucial Players in Immune Responses: A Tribute to Howard Young. J. Interferon Cytokine Res. 2022, 42, 643–654. [Google Scholar] [CrossRef]
- Massagué, J.; Sheppard, D. TGF-β Signaling in Health and Disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef] [PubMed]
- Fatehi Hassanabad, A.; Belke, D.D.; Gordon, P.M.K.; Teng, G.; Dundas, J.A.; Zarzycki, A.N.; Turnbull, J.; Deniset, J.F.; Fedak, P.W.M. Pericardial Fluid of Patients With Coronary Artery Disease Can Drive Fibrosis Via TGF-Beta Pathway. JACC Basic Transl. Sci. 2024, 9, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Ristić, A.D.; Pankuweit, S.; Maksimović, R.; Moosdorf, R.; Maisch, B. Pericardial Cytokines in Neoplastic, Autoreactive, and Viral Pericarditis. Heart Fail. Rev. 2013, 18, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Namiguchi, K.; Sakaue, T.; Okazaki, M.; Kanno, K.; Komoda, Y.; Shikata, F.; Kurata, M.; Ota, N.; Kubota, Y.; Kurobe, H.; et al. Unique Angiogenesis From Cardiac Arterioles During Pericardial Adhesion Formation. Front. Cardiovasc. Med. 2021, 8, 761591. [Google Scholar] [CrossRef]
- Janus, P.; Kuś, P.; Jaksik, R.; Vydra, N.; Toma-Jonik, A.; Gramatyka, M.; Kurpas, M.; Kimmel, M.; Widłak, W. Transcriptional Responses to Direct and Indirect TGFB1 Stimulation in Cancerous and Noncancerous Mammary Epithelial Cells. Cell Commun. Signal 2024, 22, 522. [Google Scholar] [CrossRef]
- Taylor, M.A.; Parvani, J.G.; Schiemann, W.P. The Pathophysiology of Epithelial-Mesenchymal Transition Induced by Transforming Growth Factor-β in Normal and Malignant Mammary Epithelial Cells. J. Mammary Gland. Biol. Neoplasia 2010, 15, 169–190. [Google Scholar] [CrossRef]
- Lee, J.; Travis, N.; King, B.; Fernandez, A.L.; Hassanabad, A.F.; Fedak, P.W.M.; Pelletier, M.; El-Diasty, M. Inflammatory Mediators in Pericardial Fluid in Patients Undergoing Cardiac Surgery. CJC Open 2025, 7, 193–202. [Google Scholar] [CrossRef]
- El-Diasty, M.M.; Rodríguez, J.; Pérez, L.; Souaf, S.; Eiras, S.; Fernández, A.L. Compartmentalization of the Inflammatory Response in the Pericardial Cavity in Patients Undergoing Cardiac Surgery. Int. J. Mol. Sci. 2024, 25, 13720. [Google Scholar] [CrossRef]
- Cao, J.; Poss, K.D. The Epicardium as a Hub for Heart Regeneration. Nat. Rev. Cardiol. 2018, 15, 631–647. [Google Scholar] [CrossRef]
- Meyers, D.G.; Meyers, R.E.; Prendergast, T.W. The Usefulness of Diagnostic Tests on Pericardial Fluid. Chest 1997, 111, 1213–1221. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Lie-Venema, H.; Gittenberger-de Groot, A.C. The Role of the Epicardium and Neural Crest as Extracardiac Contributors to Coronary Vascular Development. Tex. Heart Inst. J. 2002, 29, 255–261. [Google Scholar]
- Pires-Gomes, A.A.S.; Pérez-Pomares, J.M. The Epicardium and Coronary Artery Formation. J. Dev. Biol. 2013, 1, 186–202. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Z.; Lui, W.; Chen, X.; Wang, Y.; Chamberlain, A.A.; Moreno-Rodriguez, R.A.; Markwald, R.R.; O’Rourke, B.P.; Sharp, D.J.; et al. Endocardial Cells Form the Coronary Arteries by Angiogenesis through Myocardial-Endocardial VEGF Signaling. Cell 2012, 151, 1083–1096. [Google Scholar] [CrossRef]
- Lupu, I.-E.; De Val, S.; Smart, N. Coronary Vessel Formation in Development and Disease: Mechanisms and Insights for Therapy. Nat. Rev. Cardiol. 2020, 17, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Naemura, K.; Arima, Y.; Uchijima, Y.; Nagao, H.; Yoshihara, K.; Singh, M.K.; Uemura, A.; Matsuzaki, F.; Yoshida, Y.; et al. Semaphorin3E-PlexinD1 Signaling in Coronary Artery and Lymphatic Vessel Development with Clinical Implications in Myocardial Recovery. iScience 2021, 24, 102305. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, E.; Cadenas, V.; Temiño, S.; Oliver, G.; Torres, M. Epicardial VEGFC/D Signaling Is Essential for Coronary Lymphangiogenesis. EMBO Rep. 2025, 26, 2803–2818. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villalba, A.; Guadix, J.A.; Pérez-Pomares, J.M. Epicardium and Coronary Vessels. Adv. Exp. Med. Biol. 2024, 1441, 155–166. [Google Scholar] [CrossRef]
- Dergilev, K.V.; Tsokolaeva, Z.I.; Beloglazova, I.B.; Ratner, E.I.; Parfenova, E.V. Transforming Growth Factor Beta (TGF-Β1) Induces Pro-Reparative Phenotypic Changes in Epicardial Cells in Mice. Bull. Exp. Biol. Med. 2021, 170, 565–570. [Google Scholar] [CrossRef]
- Zhou, B.; Pu, W.T. Epicardial Epithelial-to-Mesenchymal Transition in Injured Heart. J. Cell Mol. Med. 2011, 15, 2781–2783. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Álvarez, V.; et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, K.; Nitta, K.; Yamato, M.; Okano, T. Therapeutic Applications of Mesothelial Cell Sheets. Ther. Apher. Dial. 2015, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, X.; Guo, R.; Sun, X.; Zhao, Z.; Guo, H.; Wang, M.; Li, S.; Li, T.; Lv, D.; et al. Notch-1 Regulates Collective Breast Cancer Cell Migration by Controlling Intercellular Junction and Cytoskeletal Organization. Cell Prolif. 2025, 58, e13754. [Google Scholar] [CrossRef] [PubMed]
- Kachanova, O.; Lobov, A.; Malashicheva, A. The Role of the Notch Signaling Pathway in Recovery of Cardiac Function after Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 12509. [Google Scholar] [CrossRef]
- Cai, S.; Dai, Q. Research Advances in Myocardial Infarction Repair and Cardiac Regenerative Medicine via the Notch Signaling Pathway. Rev. Cardiovasc. Med. 2025, 26, 26587. [Google Scholar] [CrossRef]
- van Tuyn, J.; Atsma, D.E.; Winter, E.M.; van der Velde-van Dijke, I.; Pijnappels, D.A.; Bax, N.A.M.; Knaän-Shanzer, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; van der Laarse, A.; et al. Epicardial Cells of Human Adults Can Undergo an Epithelial-to-Mesenchymal Transition and Obtain Characteristics of Smooth Muscle Cells in Vitro. Stem Cells 2007, 25, 271–278. [Google Scholar] [CrossRef]
- Quijada, P.; Trembley, M.A.; Misra, A.; Myers, J.A.; Baker, C.D.; Pérez-Hernández, M.; Myers, J.R.; Dirkx, R.A.; Cohen, E.D.; Delmar, M.; et al. Coordination of Endothelial Cell Positioning and Fate Specification by the Epicardium. Nat. Commun. 2021, 12, 4155. [Google Scholar] [CrossRef]
- Boezio, G.L.M.; Zhao, S.; Gollin, J.; Priya, R.; Mansingh, S.; Guenther, S.; Fukuda, N.; Gunawan, F.; Stainier, D.Y.R. The Developing Epicardium Regulates Cardiac Chamber Morphogenesis by Promoting Cardiomyocyte Growth. Dis. Model. Mech. 2023, 16, dmm049571. [Google Scholar] [CrossRef]
- Bax, N.A.M.; Duim, S.N.; Kruithof, B.P.T.; Smits, A.M.; Bouten, C.V.C.; Goumans, M.J. In Vivo and in Vitro Approaches Reveal Novel Insight Into the Ability of Epicardium-Derived Cells to Create Their Own Extracellular Environment. Front. Cardiovasc. Med. 2019, 6, 81. [Google Scholar] [CrossRef]
- Bouchey, D.; Drake, C.J.; Wunsch, A.M.; Little, C.D. Distribution of Connective Tissue Proteins during Development and Neovascularization of the Epicardium. Cardiovasc. Res. 1996, 31, E104–E115. [Google Scholar] [CrossRef]
- Lavine, K.J.; Yu, K.; White, A.C.; Zhang, X.; Smith, C.; Partanen, J.; Ornitz, D.M. Endocardial and Epicardial Derived FGF Signals Regulate Myocardial Proliferation and Differentiation in Vivo. Dev. Cell 2005, 8, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, D.J.; Mikawa, T. Normal Patterning of the Coronary Capillary Plexus Is Dependent on the Correct Transmural Gradient of FGF Expression in the Myocardium. Dev. Biol. 2005, 279, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Masters, M.; Riley, P.R. The Epicardium Signals the Way towards Heart Regeneration. Stem Cell Res. 2014, 13 Pt B, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Page, E.; Upshaw-Earley, J.; Goings, G. Permeability of Rat Atrial Endocardium, Epicardium, and Myocardium to Large Molecules. Stretch-Dependent Effects. Circ. Res. 1992, 71, 159–173. [Google Scholar] [CrossRef]
- Michailova, K.N.; Usunoff, K.G. Serosal Membranes (Pleura, Pericardium, Peritoneum). Normal Structure, Development and Experimental Pathology. In Advances in Anatomy Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2006; Volume 183, pp. 1–144. [Google Scholar]
- Neufeld, G.; Tessler, S.; Gitay-Goren, H.; Cohen, T.; Levi, B.Z. Vascular Endothelial Growth Factor and Its Receptors. Prog. Growth Factor Res. 1994, 5, 89–97. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R. Hematopoietic Growth Factors and Tumor Angiogenesis. Cancer Lett. 2019, 440–441, 47–53. [Google Scholar] [CrossRef]
- Bousquenaud, M.; Schwartz, C.; Léonard, F.; Rolland-Turner, M.; Wagner, D.; Devaux, Y. Monocyte Chemotactic Protein 3 Is a Homing Factor for Circulating Angiogenic Cells. Cardiovasc. Res. 2012, 94, 519–525. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Anwar, M.; Vilahur, G.; Martino, F.; Kyriazis, P.G.; de Winter, N.; Punjabi, P.P.; Angelini, G.D.; Sattler, S.; Emanueli, C. Small Extracellular Vesicles in the Pericardium Modulate Macrophage Immunophenotype in Coronary Artery Disease. JACC Basic Transl. Sci. 2024, 9, 1057–1072. [Google Scholar] [CrossRef]
- Weckbach, L.T.; Grabmaier, U.; Uhl, A.; Gess, S.; Boehm, F.; Zehrer, A.; Pick, R.; Salvermoser, M.; Czermak, T.; Pircher, J.; et al. Midkine Drives Cardiac Inflammation by Promoting Neutrophil Trafficking and NETosis in Myocarditis. J. Exp. Med. 2019, 216, 350–368. [Google Scholar] [CrossRef]
- Silva, E.D.; Pereira-Sousa, D.; Ribeiro-Costa, F.; Cerqueira, R.; Enguita, F.J.; Gomes, R.N.; Dias-Ferreira, J.; Pereira, C.; Castanheira, A.; Pinto-do-Ó, P.; et al. Pericardial Fluid Accumulates microRNAs That Regulate Heart Fibrosis after Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 8329. [Google Scholar] [CrossRef]
- Nafissi, N.A.; DeBenedittis, P.; Thomas, M.C.; Karra, R. Differentiation of Human Induced Pluripotent Stem Cells into Epicardial-Like Cells. Methods Mol. Biol. 2021, 2158, 141–153. [Google Scholar] [CrossRef]
- Ge, Y.; Smits, A.M.; Liu, J.; Zhang, J.; van Brakel, T.J.; Goumans, M.J.T.H.; Jongbloed, M.R.M.; de Vries, A.A.F. Generation, Characterization, and Application of Inducible Proliferative Adult Human Epicardium-Derived Cells. Cells 2021, 10, 2064. [Google Scholar] [CrossRef]
| Gene Name | Forward | Reversed |
|---|---|---|
| SNAI1 | TCGGAAGCCTAACTACAGCGA | AGATGAGCATTGGCAGCGAG |
| SNAI2 | CGAACTGGACACACATACAGTG | CTGAGGATCTCTGGTTGTGGT |
| ZEB1 | TTACACCTTTGCATACAGAACCC | TTTACGATTACACCCAGACTGC |
| ZEB2 | GCGATGGTCATGCAGTCAG | CAGGTGGCAGGTCATTTTCTT |
| TWIST1 | GTCCGCAGTCTTACGAGGAG | GCTTGAGGGTCTGAATCTTGCT |
| TWIST2 | TCTGAAACCTGAACAACCTCAG | CTGCTGTCCCTTCTCTCGAC |
| CDH1 | ATTTTTCCCTCGACACCCGAT | TCCCAGGCGTAGACCAAGA |
| FN1 | GACGCATCACTTGCACTTCT | GCAGGTTTCCTCGATTATCCT |
| COL1A1 | CCAAATCTGTCTCCCCAGAA | TCAAAAACGAAGGGGAGATG |
| VEGFA | CAACATCACCATGCAGATTATGC | GCTTTCGTTTTTGCCCCTTTC |
| HGF | AGGGGCACTGTCAATACCATT | CGTGAGGATACTGAGAATCCCAA |
| PDGFB | TCCCGAGGAGCTTTATGAGA | GGGTCATGTTCAGGTCCAAC |
| TNFA | ATGAGCACTGAAAGCATGATCC | GAGGGCTGATTAGAGAGAGGTC |
| IL1B | ACAGATGAAGTGCTCCTTCCA | GTCGGAGATTCGTAGCTGGAT |
| PLAU | CTCCTGTGCATGGGTGAA | AACCATGGGCCTCACAAAT |
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dergilev, K.; Zubko, A.; Beloglazova, I.; Tsokolaeva, Z.; Azimova, E.; Dolgodvorova, A.; Iarushkina, I.; Andreev, A.; Shiryaev, A.; Docshin, P.; et al. Human Pericardial Fluid-Derived Cells Exhibit Mesothelial-like Properties and Exert Proangiogenic Effects on Endothelial Cells. Cells 2025, 14, 1855. https://doi.org/10.3390/cells14231855
Dergilev K, Zubko A, Beloglazova I, Tsokolaeva Z, Azimova E, Dolgodvorova A, Iarushkina I, Andreev A, Shiryaev A, Docshin P, et al. Human Pericardial Fluid-Derived Cells Exhibit Mesothelial-like Properties and Exert Proangiogenic Effects on Endothelial Cells. Cells. 2025; 14(23):1855. https://doi.org/10.3390/cells14231855
Chicago/Turabian StyleDergilev, Konstantin, Alexander Zubko, Irina Beloglazova, Zoya Tsokolaeva, Ekaterina Azimova, Aleria Dolgodvorova, Irina Iarushkina, Alexander Andreev, Andrey Shiryaev, Pavel Docshin, and et al. 2025. "Human Pericardial Fluid-Derived Cells Exhibit Mesothelial-like Properties and Exert Proangiogenic Effects on Endothelial Cells" Cells 14, no. 23: 1855. https://doi.org/10.3390/cells14231855
APA StyleDergilev, K., Zubko, A., Beloglazova, I., Tsokolaeva, Z., Azimova, E., Dolgodvorova, A., Iarushkina, I., Andreev, A., Shiryaev, A., Docshin, P., Malashicheva, A., & Parfyonova, Y. (2025). Human Pericardial Fluid-Derived Cells Exhibit Mesothelial-like Properties and Exert Proangiogenic Effects on Endothelial Cells. Cells, 14(23), 1855. https://doi.org/10.3390/cells14231855









