Sustained EGFR Signaling Expands Otx2+ and Chx10+ Retinal Progenitors in the Postnatal Mouse Retina
Highlights
- Sustained EGF signaling during postnatal retinal development enhances progenitor proliferation, increases EGFR expression, and expands Otx2+ and Chx10+ progenitor populations.
- The postnatal retinal explant system provides a physiologically relevant model that retains the retinal architecture while enabling controlled manipulation of growth factor signaling.
- These findings indicate that prolonged EGFR activation stabilizes specific progenitor identities, supporting future studies aimed at defining their molecular trajectories and differentiation potential.
- The explant platform offers a practical tool for preclinical assessment of signaling modulators and regenerative strategies, bridging stem cell culture systems and in vivo models.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Retinal Explant Culture
2.3. BrdU Labeling and Immunostaining
2.4. Immunofluorescence
2.5. Imaging and Quantification
2.6. Statistical Analysis
3. Results
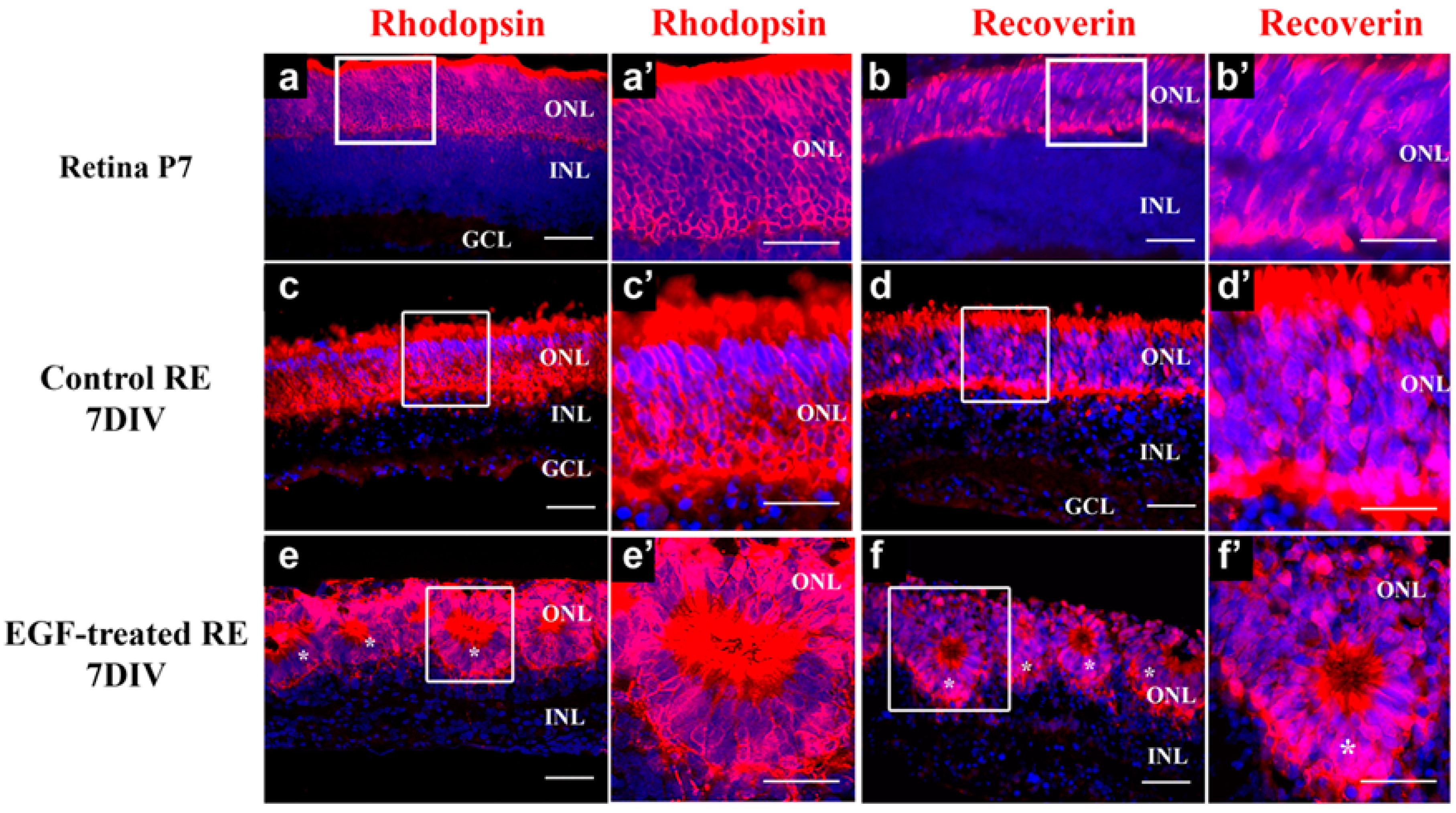
3.1. Characterization of Control and EGF-Treated Retinal Explants
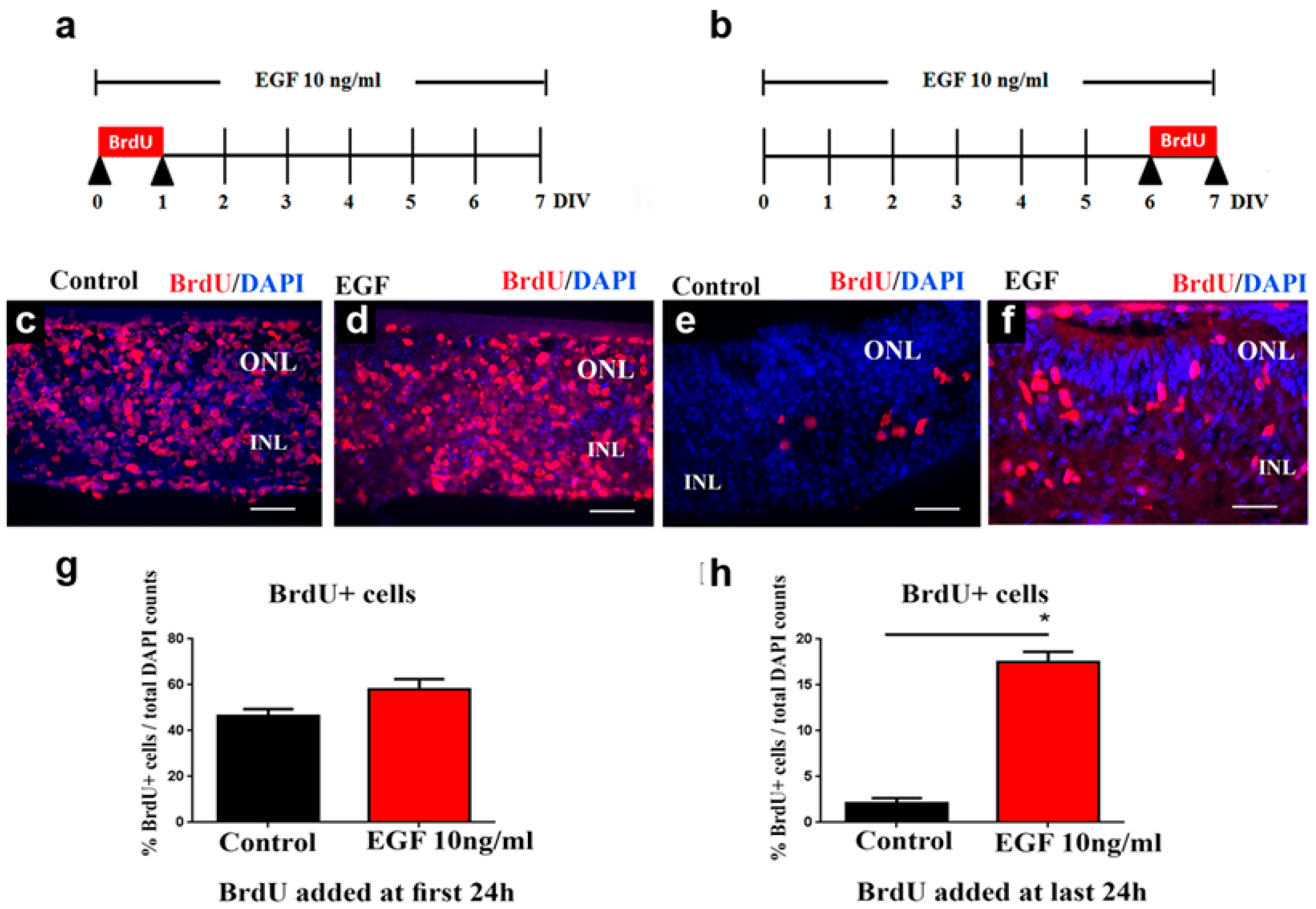
3.2. Sustained EGF Signaling Extends Progenitor Proliferation
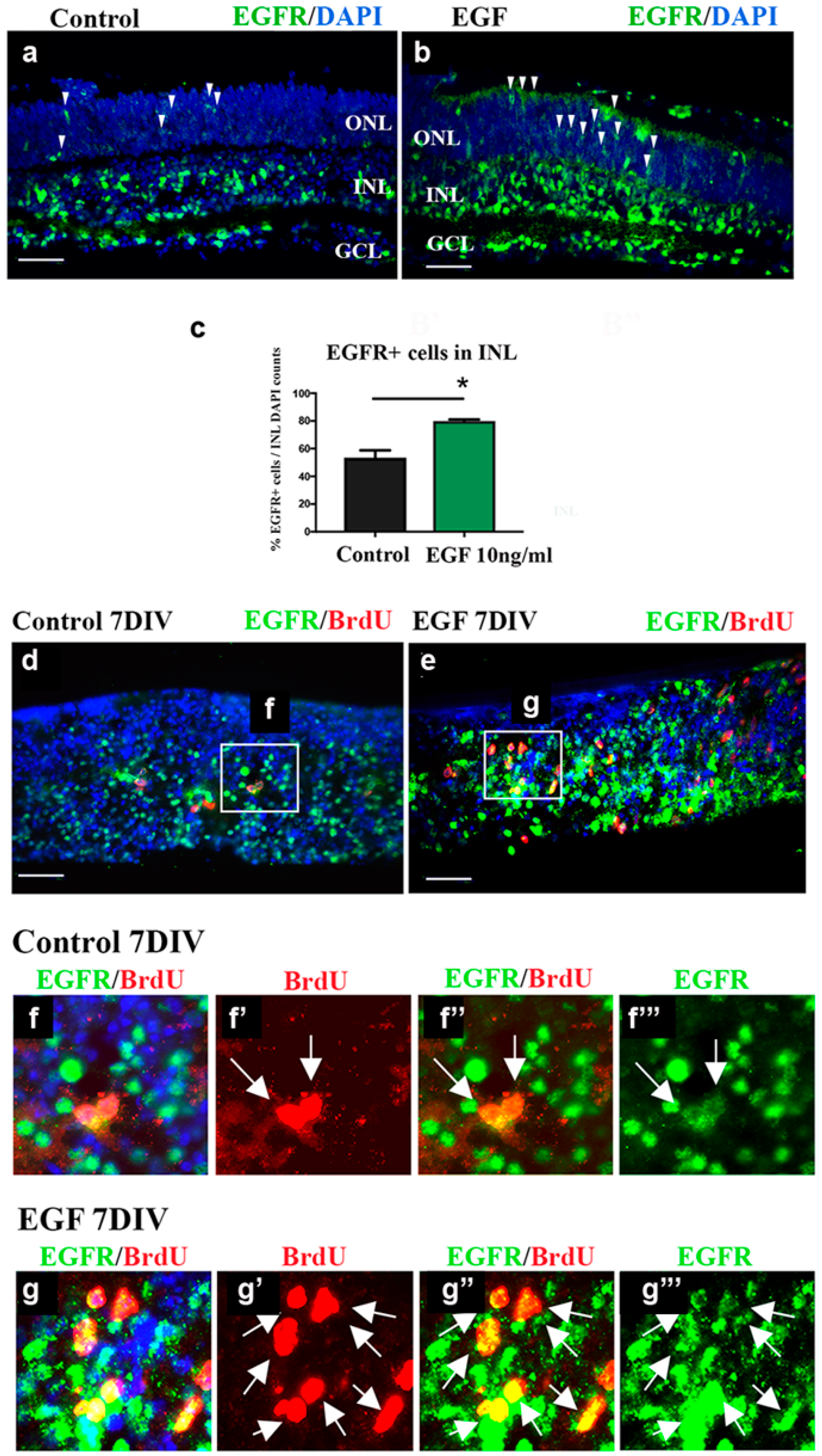
3.3. EGF Treatment Increases EGFR+ Cell Populations
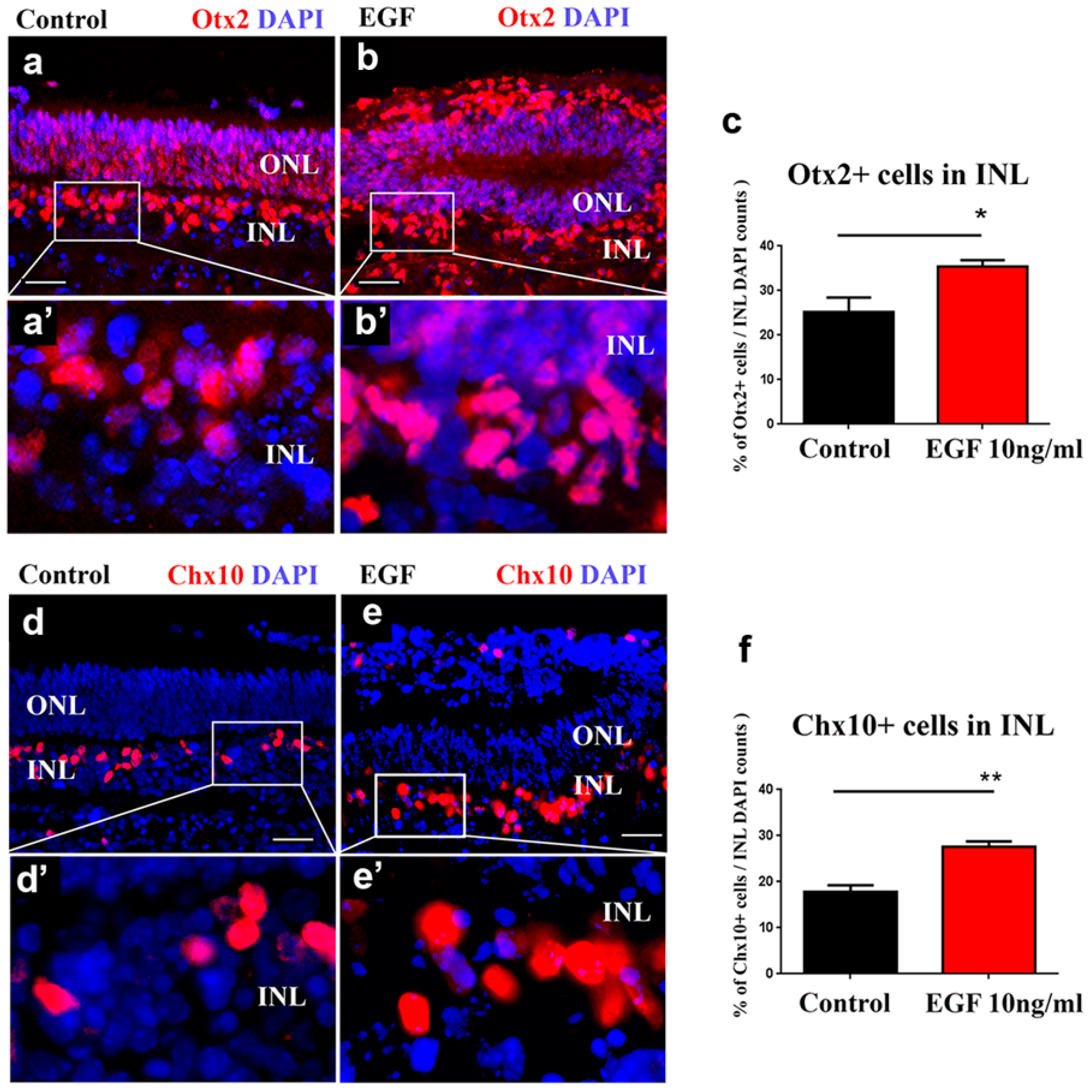
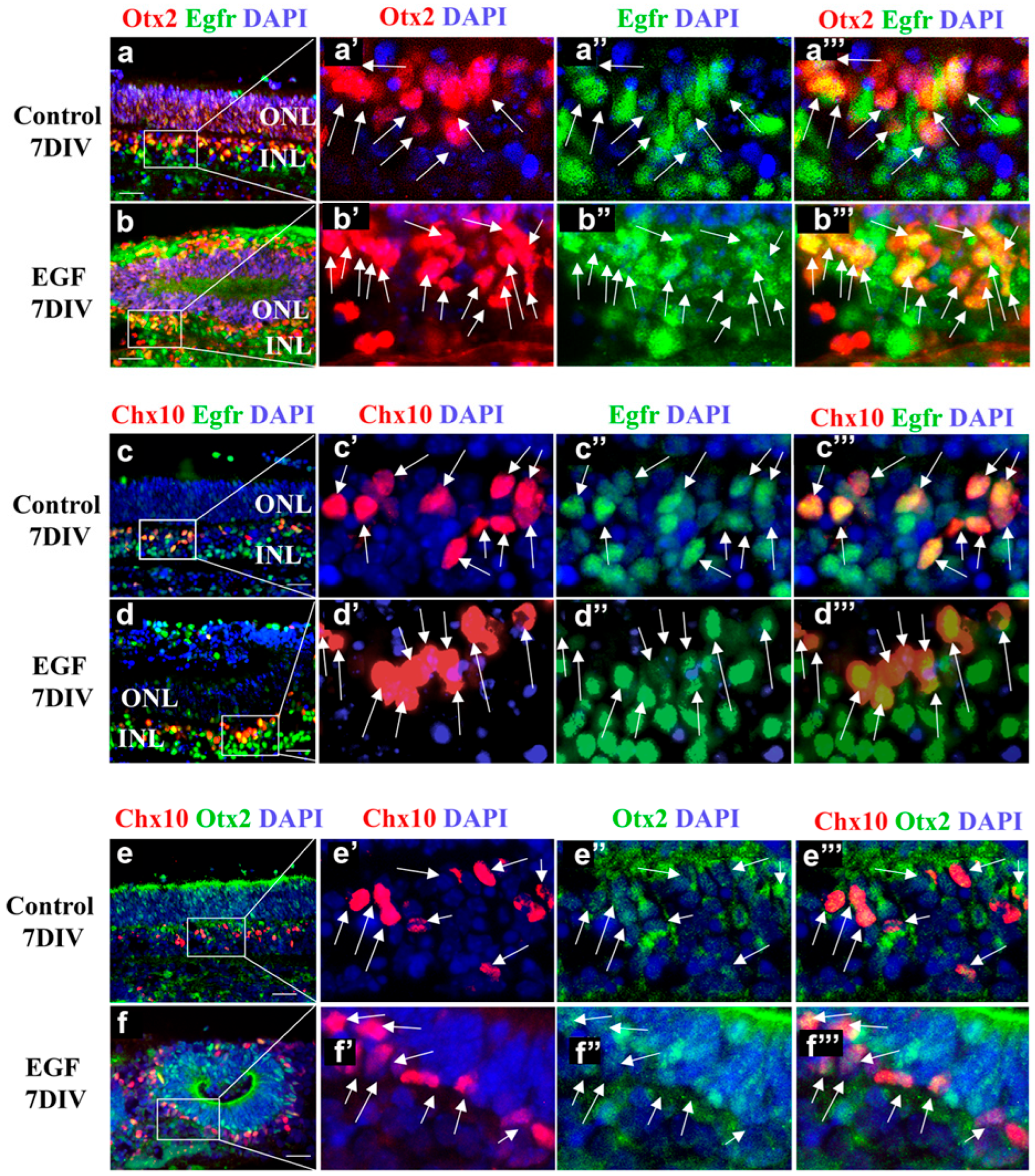
3.4. Sustained EGF Signaling Expands Otx2+ and Chx10+ Populations
3.5. The Subpopulation of EGFR+ Cells Comprises Otx2+ and Chx10+ Retinal Progenitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidman, R.L. The Structure of the Eye. In Seventh International Congress of Anatomists; Smelser, G.K., Ed.; Academic Press: New York, NY, USA, 1960; pp. 487–505. [Google Scholar]
- Young, R.W. Cell proliferation during postnatal development of the retina in the mouse. Brain Res. 1985, 353, 229–239. [Google Scholar] [CrossRef]
- Livesey, R.; Cepko, C. Vertebrate neural cell-fate determination: Lessons from the retina. Nat. Rev. Neurosci. 2001, 2, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lillien, L. Changes in retinal cell fate induced by overexpression of EGF receptor. Nature 1995, 377, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Lillien, L.; Wancio, D. Changes in Epidermal Growth Factor Receptor expression and competence to generate glia regulate timing and choice of differentiation in the Retina. Mol. Cell Neurosci. 1998, 10, 296–308. [Google Scholar] [CrossRef]
- Close, J.L.; Liu, J.; Gumuscu, B.; Reh, T.A. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia 2006, 54, 94–104. [Google Scholar] [CrossRef]
- Threadgill, D.W.; Dlugosz, A.A.; Hansen, L.A.; Tennenbaum, T.; Lichti, U.; Yee, D.; LaMantia, C.; Mourton, T.; Herrup, K.; Harris, R.C.; et al. Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science 1995, 269, 230–234. [Google Scholar] [CrossRef]
- Nork, T.M.; Wallow, I.H.; Sramek, S.J.; Anderson, G. Müller’s cell involvement in proliferative diabetic retinopathy. Arch. Ophthalmol. 1987, 105, 1424–1429. [Google Scholar] [CrossRef]
- Fariss, R.N.; Li, Z.Y.; Milam, A.H. Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Lipids 2000, 129, 215–223. [Google Scholar] [CrossRef]
- Anchan, R.M.; Reh, T.A.; Angello, J.; Balliet, A.; Walke, M. EGF and TGF-alpha stimulate retinal neuroepithelial cell proliferation in vitro. Neuron 1991, 6, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Baker, N.E. Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev. Cell. 2003, 4, 359–369. [Google Scholar] [CrossRef]
- Tio, M.; Moses, K. The Drosophila TGF alpha homolog Spitz acts in photoreceptor recruitment in the developing retina. Development 1997, 124, 343–351. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Yu, S.Y.; Katz, J.; Baker, N.E. Several levels of EGF receptor signaling during photoreceptor specification in wild-type, Ellipse, and null mutant Drosophila. Dev. Biol. 1999, 205, 129–144. [Google Scholar] [CrossRef]
- Li, X.; Carthew, R.W. A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell 2005, 123, 1267–1277. [Google Scholar] [CrossRef]
- Wan, J.; Ramachandran, R.; Goldman, D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev. Cell 2012, 22, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.O.; Hayes, S.; Nelson, B.R.; Tan, K.; Buckingham, B.; Reh, T.A. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 2008, 105, 19508–19513. [Google Scholar] [CrossRef] [PubMed]
- Loffler, K.; Schafer, P.; Volkner, M.; Holdt, T.; Karl, M.O. Age-dependent Muller glia neurogenic competence in the mouse retina. Glia 2015, 63, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, D.H.; Wong, L.L.; Wood, E.D.; Yasumura, D.; LaVail, M.M. Timing and topography of cell genesis in the rat retina. J. Comp. Neurol. 2004, 474, 304–324. [Google Scholar] [CrossRef]
- Brzezinski, J. A4th.; Lamba, D.A.; Reh, T.A. Blimp1 controls photoreceptor versus bipolar cell fate choice during retinal development. Development 2010, 137, 619–629. [Google Scholar] [CrossRef]
- Nishida, A.; Furukawa, A.; Koike, C.; Tano, Y.; Aizawa, S.; Matsuo, I. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 2003, 6, 1255–1263. [Google Scholar] [CrossRef]
- Koike, C.; Nishida, A.; Ueno, S.; Saito, H.; Sanuki, R.; Sato, S.; Furukawa, A.; Aizawa, S.; Matsuo, I.; Suzuki, N.; et al. Functional role of Otx2 transcription factor in postnatal mouse retinal development. Mol. Cell Biol. 2007, 27, 8318–8329. [Google Scholar] [CrossRef]
- Burmeister, M.; Novak, J.; Liang, M.-Y.; Basu, S.; Ploder, L.; Hawes, N.L.; Vidgen, D.; Hoover, F.; Goldman, D.; Kalnins, V.I.; et al. Ocular retardation mouse caused by Chx10 homeobox null allele: Impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 1996, 12, 376–384. [Google Scholar] [CrossRef]
- Livne-Bar, I.; Pacal, M.; Cheung, M.C.; Hankin, M.; Trogadis, J.; Chen, D.; Dorval, K.M.; Bremner, R. Chx10 is required to block photoreceptor differentiation but is dispensable for progenitor proliferation in the postnatal retina. Proc. Natl. Acad. Sci. USA 2006, 103, 4988–4993. [Google Scholar] [CrossRef]
- Caffe, A.R.; Ahuja, P.; Holmqvist, B.; Azadi, S.; Forsell, J.; Holmqvist, I.; Söderpalm, A.; van Veen, T. Mouse retina explants after long-term culture in serum free medium. J. Chem. Neuroanat. 2001, 22, 263–273. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Kageyama, R. Retrovirus-mediated gene transfer to retinal explants. Methods 2002, 28, 387–395. [Google Scholar] [CrossRef]
- Rhee, K.D.; Goureau, O.; Chen, S.; Yang, X.-J. Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J. Neurosci. 2004, 24, 9779–9788. [Google Scholar] [CrossRef] [PubMed]
- Ivkovic, S.; Jovanovic Macura, I.; Antonijevic, T.; Kanazir, S.; Domingos, H. Different levels of epidermal growth factor signaling modifies the differentiation of specific cell types in mouse postnatal retina. Arch. Biol. Sci. 2019, 71, 711–719. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Makino, K.; Xia, W.; Matin, A.; Wen, Y.; Kwong, K.Y.; Bourguignon, L.; Hung, M.-C. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001, 3, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Fossat, N.; Le Greneur, C.; Beby, F.; Vincent, S.; Godement, P. A new GFP-tagged line reveals unexpected Otx2 protein localization in retinal photoreceptors. BMC Dev. Biol. 2007, 7, 122–133. [Google Scholar] [CrossRef]
- Glubrecht, D.D.; Kim, J.-H.; Russell, L.; Bamforth, J.S.; Godbout, R. Differential CRX and OTX2 expression in human retina and retinoblastoma. J. Neurochem. 2009, 111, 250–263. [Google Scholar] [CrossRef]
- Ivkovic, S.; Canoll, P.; Goldman, J.E. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J. Neurosci. 2008, 28, 914–922. [Google Scholar] [CrossRef]
- Patel, B.; Hiscott, P.; Charteris, D.; Mather, J.; McLeod, D.; Boulton, M. Retinal and preretinal localisation of epidermal growth factor, transforming growth factor alpha, and their receptor in proliferative diabetic retinopathy. Br. J. Ophthalmol. 1994, 78, 714–718. [Google Scholar] [CrossRef]
- Chen, H.; Liu, B.; Neufeld, A.H. Epidermal growth factor receptor in adult retinal neurons of rat, mouse, and human. J. Comp. Neurol. 2007, 500, 299–310. [Google Scholar] [CrossRef]
- Ueki, Y.; Reh, T.A. EGF stimulates Müller glial proliferation via a BMP-dependent mechanism. Glia 2013, 61, 778–789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sardar Pasha, S.P.B.; Münch, R.; Schäfer, P.; Oertel, P.; Sykes, A.M.; Zhu, Y.; Karl, M.O. Retinal cell death dependent reactive proliferative gliosis in the mouse retina. Sci. Rep. 2017, 7, 9517. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Zhou, S.R.; Zhang, H.H.; Wang, T.; Chen, X.D. Inhibition of EGFR attenuates EGF-induced activation of retinal pigment epithelium cell via EGFR/AKT signaling pathway. Int. J. Ophthalmol. 2024, 17, 1018–1027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, J.; Wu, Z.; Shan, G.; Huang, Y.; Liang, J.; Zhan, C. Nuclear epidermal growth factor receptor (nEGFR) in clinical treatment. Heliyon 2024, 10, e40150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, W.; Xing, Y.-Q.; Yang, A.-H. Epidermal growth factor promotes the differentiation of stem cells derived from human umbilical cord blood into neuron-like cells via taurine induction in vitro. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 321–327. [Google Scholar] [CrossRef]
- Angenieux, B.; Schorderet, D.F.; Arsenijevic, Y. Epidermal growth factor is a neuronal differentiation factor for retinal stem cells in vitro. Stem Cell 2006, 24, 696–706. [Google Scholar] [CrossRef]
- Pereira de Melo Guimaraes, R.; Soares Landeira, B.; Coelho, D.M.; Ferreira Golbert, D.C.; Silveira, M.S.; Linden, R.; de Melo Reis, R.A.; Costa, M.R. Evidence of Muller glia conversion into retina ganglion cells using Neurogenin2. Front. Cell Sci. 2018, 12, 410–425. [Google Scholar] [CrossRef]
- Lee, J.H.; Choy, M.L.; Marks, P.A. Mechanisms of resistance to histone deacetylase inhibitors. Adv. Cancer Res. 2012, 116, 39–86. [Google Scholar] [CrossRef]
- Karl, M.O.; Reh, T.A. Regenerative medicine for retinal diseases: Activating endogenous repair mechanisms. Trends Mol. Med. 2010, 16, 193–202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivkovic, S.; Major, T.; Adzic, M. Sustained EGFR Signaling Expands Otx2+ and Chx10+ Retinal Progenitors in the Postnatal Mouse Retina. Cells 2025, 14, 1854. https://doi.org/10.3390/cells14231854
Ivkovic S, Major T, Adzic M. Sustained EGFR Signaling Expands Otx2+ and Chx10+ Retinal Progenitors in the Postnatal Mouse Retina. Cells. 2025; 14(23):1854. https://doi.org/10.3390/cells14231854
Chicago/Turabian StyleIvkovic, Sanja, Tamara Major, and Miroslav Adzic. 2025. "Sustained EGFR Signaling Expands Otx2+ and Chx10+ Retinal Progenitors in the Postnatal Mouse Retina" Cells 14, no. 23: 1854. https://doi.org/10.3390/cells14231854
APA StyleIvkovic, S., Major, T., & Adzic, M. (2025). Sustained EGFR Signaling Expands Otx2+ and Chx10+ Retinal Progenitors in the Postnatal Mouse Retina. Cells, 14(23), 1854. https://doi.org/10.3390/cells14231854







