Orthobiologics and Peptide Therapy for Central Nervous System Repair in Neurodegenerative Conditions
Highlights
- Orthobiologic derivatives and peptide therapeutics converge on shared neurodegenerative pathways, promoting mitochondrial resilience, glial reprogramming, and synaptic repair.
- Intranasal delivery emerges as a practical, minimally invasive route, capable of bypassing the blood–brain barrier while maintaining biological potency.
- Combining orthobiologics with peptide-based therapies offers a mechanistically complementary strategy that goes beyond symptomatic relief toward true circuit preservation.
- These insights provide a translational framework for future biomarker-guided and delivery-optimized clinical trials in Alzheimer’s and Parkinson’s disease.
Abstract
1. Introduction
2. Neurodegenerative Pathophysiology
3. Orthobiologics and Peptides as Interventions in Alzheimer’s and Parkinson’s Disease
3.1. Mesenchymal Stromal Cell Derivatives
3.2. Bone Marrow Products
3.3. Platelet-Derived Approaches
3.4. Adipose-Derived Products
3.5. Peptide Therapy
3.6. Delivery Strategies
4. Future Directions
Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sequeira, L.; Benfeito, S.; Fernandes, C.; Lima, I.; Peixoto, J.; Alves, C.; Machado, C.S.; Gaspar, A.; Borges, F.; Chavarria, D. Drug Development for Alzheimer’s and Parkinson’s Disease: Where Do We Go Now? Pharmaceutics 2024, 16, 708. [Google Scholar] [CrossRef] [PubMed]
- Pyka, P.; Garbo, S.; Fioravanti, R.; Jacob, C.; Hittinger, M.; Handzlik, J.; Zwergel, C.; Battistelli, C. Selenium-containing compounds: A new hope for innovative treatments in Alzheimer’s disease and Parkinson’s disease. Drug Discov. Today 2024, 29, 104062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, J.; Huang, S.; Wang, X.-Y.; Chen, X.; Liu, G.-H.; Ye, K.; Song, W.; Masters, C.L.; Wang, J.; et al. Antiageing strategy for neurodegenerative diseases: From mechanisms to clinical advances. Signal Transduct. Target. Ther. 2025, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Moreira, D.G.; Richner, M.; Mutsaers, H.A.M.; Ferreira, N.; Jan, A. GLP-1 Receptor Agonists in Neurodegeneration: Neurovascular Unit in the Spotlight. Cells 2022, 11, 2023. [Google Scholar] [CrossRef]
- Costa, F.R.; Pires, L.; Martins, R.A.; Santos, M.; Santos, G.S.; Lana, J.V.; Costa, B.R.; Santos, N.; de Macedo, A.P.; Kruel, A.; et al. Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Curr. Issues Mol. Biol. 2025, 47, 247. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Rajmohan, R.; Reddy, P.H. Amyloid Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimers Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef]
- Eroglu, E.; Harmanci, N. Emerging Molecular Targets in Neurodegenerative Disorders: New Avenues for Therapeutic Intervention. Basic Clin. Pharmacol. Toxicol. 2025, 137, e70107. [Google Scholar] [CrossRef]
- Lana, J.V.; Rios, A.; Takeyama, R.; Santos, N.; Pires, L.; Santos, G.S.; Rodrigues, I.J.; Jeyaraman, M.; Purita, J.; Lana, J.F. Nebulized Glutathione as a Key Antioxidant for the Treatment of Oxidative Stress in Neurodegenerative Conditions. Nutrients 2024, 16, 2476. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Alkhalifa, O.; Durdanovic, I.; Ibrahim, D.R.; Maragkou, S. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease: Insights into Pathophysiology and Treatment. J. Dement. Alzheimers Dis. 2025, 2, 17. [Google Scholar] [CrossRef]
- Mani, S.; Wasnik, S.; Shandilya, C.; Srivastava, V.; Khan, S.; Singh, K.K. Pathogenic synergy: Dysfunctional mitochondria and neuroinflammation in neurodegenerative diseases associated with aging. Front. Aging 2025, 6, 1615764. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, P.; Zhao, Z.; Li, J.; Fan, Z.; Li, X.; Cui, Z.; Fu, A. Improvement Effect of Mitotherapy on the Cognitive Ability of Alzheimer’s Disease through NAD+/SIRT1-Mediated Autophagy. Antioxidants 2023, 12, 2006. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Rao, A.; Devi, V.; Kumari, L.; Negi, A.; Kumar, M.; Bharti, R.; Medhi, B. Mitotherapy Restores mitochondrial function and improves cognitive deficits in Alzheimer’s disease. Mitochondrion 2025, 85, 102077. [Google Scholar] [CrossRef]
- Cui, G.; Wu, J.; Mou, F.; Xie, W.; Wang, F.; Wang, Q.; Fang, J.; Xu, Y.; Dong, Y.; Liu, J.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef]
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177. [Google Scholar] [CrossRef]
- Upadhya, R.; Shetty, A.K. Extracellular Vesicles for the Diagnosis and Treatment of Parkinson’s Disease. Aging Dis. 2021, 12, 1438–1450. [Google Scholar] [CrossRef]
- Cueva, E.; Wiesheu, A.; Sordo, Z.; González, J.; Falconi, S.; Rodas, J.A.; Leon-Rojas, J.E. Tissue Stem Cell-Based Therapies in Parkinson’s Disease: A Scoping Review of Therapeutic Mechanisms and Translational Outcomes. Cells 2025, 14, 822. [Google Scholar] [CrossRef]
- Chen, H.-X.; Liang, F.-C.; Gu, P.; Xu, B.-L.; Xu, H.-J.; Wang, W.-T.; Hou, J.-Y.; Xie, D.-X.; Chai, X.-Q.; An, S.-J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Jiang, X.; Dong, Y.; Zheng, J.; Gao, J. New idea to promote the clinical applications of stem cells or their extracellular vesicles in central nervous system disorders: Combining with intranasal delivery. Acta Pharm. Sin. B 2022, 12, 3215–3232. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.; Fishel, I.; Offen, D. Intranasal Delivery of Mesenchymal Stem Cells-Derived Extracellular Vesicles for the Treatment of Neurological Diseases. Stem Cells 2021, 39, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Narbute, K.; Piļipenko, V.; Pupure, J.; Dzirkale, Z.; Jonavičė, U.; Tunaitis, V.; Kriaučiūnaitė, K.; Jarmalavičiūtė, A.; Jansone, B.; Kluša, V.; et al. Intranasal Administration of Extracellular Vesicles Derived from Human Teeth Stem Cells Improves Motor Symptoms and Normalizes Tyrosine Hydroxylase Expression in the Substantia Nigra and Striatum of the 6-Hydroxydopamine-Treated Rats. Stem Cells Transl. Med. 2019, 8, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.E.; Salameh, T.S.; Banks, W.A. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. AAPS J. 2015, 17, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Turano, E.; Scambi, I.; Virla, F.; Bonetti, B.; Mariotti, R. Extracellular Vesicles from Mesenchymal Stem Cells: Towards Novel Therapeutic Strategies for Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 2917. [Google Scholar] [CrossRef]
- Williams, A.; Branscome, H.; Kashanchi, F.; Batrakova, E.V. Targeting of Extracellular Vesicle-Based Therapeutics to the Brain. Cells 2025, 14, 548. [Google Scholar] [CrossRef]
- Sadekar, S.S.; Bowen, M.; Cai, H.; Jamalian, S.; Rafidi, H.; Shatz-Binder, W.; Lafrance-Vanasse, J.; Chan, P.; Meilandt, W.J.; Oldendorp, A.; et al. Translational Approaches for Brain Delivery of Biologics via Cerebrospinal Fluid. Clin. Pharmacol. Ther. 2022, 111, 826–834. [Google Scholar] [CrossRef]
- Wu, J.; Piao, Y.; Liu, Q.; Yang, X. Platelet-rich plasma-derived extracellular vesicles: A superior alternative in regenerative medicine? Cell Prolif. 2021, 54, e13123. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Delila, L.; Nebie, O.; Le, N.T.N.; Timmerman, K.; Lee, D.-Y.; Wu, Y.-W.; Chou, M.-L.; Buée, L.; Chou, S.-Y.; Blum, D.; et al. Neuroprotective effects of intranasal extracellular vesicles from human platelet concentrates supernatants in traumatic brain injury and Parkinson’s disease models. J. Biomed. Sci. 2024, 31, 87. [Google Scholar] [CrossRef]
- Schiess, M.; Suescun, J.; Doursout, M.; Adams, C.; Green, C.; Saltarrelli, J.G.; Savitz, S.; Ellmore, T.M. Allogeneic Bone Marrow–Derived Mesenchymal Stem Cell Safety in Idiopathic Parkinson’s Disease. Mov. Disord. 2021, 36, 1825–1834. [Google Scholar] [CrossRef]
- Schiess, M. A Randomized, Double-Blind, Placebo-Controlled Trial of Allogeneic Bone Marrow-derived Mesenchymal Stem Cells as a Disease-Modifying Therapy for Idiopathic Parkinson’s Disease. clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT04506073 (accessed on 10 September 2024).
- Precious, S.V.; Smith, G.A.; Heuer, A.; Jaeger, I.; Lane, E.L.; Dunnett, S.B.; Li, M.; Kelly, C.M.; Rosser, A.E. Dopaminergic Progenitors Derived From Epiblast Stem Cells Function Similarly to Primary VM-Derived Progenitors When Transplanted Into a Parkinson’s Disease Model. Front. Neurosci. 2020, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Dawson, V.L.; Dawson, T.M. From metabolism to mind: The expanding role of the GLP-1 receptor in neurotherapeutics. Neurotherapeutics 2025, 22, e00712. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Macklin, E.A.; Schwarzschild, M.A. GLP-1 agonists to slow down Parkinson’s progression? The quest continues. Med 2025, 6, 100645. [Google Scholar] [CrossRef] [PubMed]
- Aillaud, I.; Kaniyappan, S.; Chandupatla, R.R.; Ramirez, L.M.; Alkhashrom, S.; Eichler, J.; Horn, A.H.C.; Zweckstetter, M.; Mandelkow, E.; Sticht, H.; et al. A novel D-amino acid peptide with therapeutic potential (ISAD1) inhibits aggregation of neurotoxic disease-relevant mutant Tau and prevents Tau toxicity in vitro. Alzheimers Res. Ther. 2022, 14, 15. [Google Scholar] [CrossRef]
- Aggidis, A.; Devitt, G.; Zhang, Y.; Chatterjee, S.; Townsend, D.; Fullwood, N.J.; Ortega, E.R.; Tarutani, A.; Hasegawa, M.; Cooper, A.; et al. A novel peptide-based tau aggregation inhibitor as a potential therapeutic for Alzheimer’s disease and other tauopathies. Alzheimers Dement. 2024, 20, 7788–7804. [Google Scholar] [CrossRef]
- Hu, D.; Sun, X.; Qi, X. Targeting the ClpP-αSynuclein Interaction with a Decoy Peptide to Mitigate Neuropathology in Parkinson’s Disease Models. bioRxiv 2025. [Google Scholar] [CrossRef]
- Popova, B.; Wang, D.; Rajavel, A.; Dhamotharan, K.; Lázaro, D.F.; Gerke, J.; Uhrig, J.F.; Hoppert, M.; Outeiro, T.F.; Braus, G.H. Identification of Two Novel Peptides That Inhibit α-Synuclein Toxicity and Aggregation. Front. Mol. Neurosci. 2021, 14, 659926. [Google Scholar] [CrossRef]
- Nhu, N.T.; Xiao, S.-Y.; Liu, Y.; Kumar, V.B.; Cui, Z.-Y.; Lee, S.-D. Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration. Front. Integr. Neurosci. 2022, 15, 747901. [Google Scholar] [CrossRef]
- Jia, Y.-L.; Wang, W.; Han, N.; Sun, H.-L.; Dong, F.-M.; Song, Y.-X.; Feng, R.-F.; Wang, J.-H. The mitochondria-targeted small molecule SS31 delays progression of behavioral deficits by attenuating β-amyloid plaque formation and mitochondrial/synaptic deterioration in APP/PS1 mice. Biochem. Biophys. Res. Commun. 2023, 658, 36–43. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Z.; Cao, J.; Fu, Q.; Wu, Y.; Zhang, X.; Long, Y.; Zhang, X.; Yang, Y.; Li, Y.; et al. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J. Neuroinflam. 2019, 16, 230. [Google Scholar] [CrossRef]
- Mengesha, Y.; Wondaya, M.; Workye, M.; Belete, L. Extracellular vesicles as carriers for protein and peptide therapeutics delivery: A review. Intell. Pharm. 2025, 3, 350–367. [Google Scholar] [CrossRef]
- Rytter, M.E.; Svane, C.A.B.; Størling, J.; Chen, W. Dysregulated insulin signaling and inflammation contribute to the pathogenesis of Alzheimer’s disease: From animal models to human cells. Neural Regen. Res. 2025, 21, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, Y.; Yang, T.; Liu, Q.; Guo, Q. Insulin resistance and Alzheimer’s disease: Exploring research hotspots and frontiers from a bibliometric and visual analysis (2005–2024). J. Alzheimers Dis. Rep. 2025, 9, 25424823251361056. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Chávez-Castillo, M.; Bautista, J.; Ortega, Á.; Nava, M.; Salazar, J.; Díaz-Camargo, E.; Medina, O.; Rojas-Quintero, J.; Bermúdez, V. Alzheimer’s disease and type 2 diabetes mellitus: Pathophysiologic and pharmacotherapeutics links. World J. Diabetes 2021, 12, 745–766. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, W.; Chen, Z.; Huang, Y.; Mao, J.; Zheng, W.; Hu, Y.; Shi, J. Advance in peptide-based drug development: Delivery platforms, therapeutics and vaccines. Signal Transduct. Target. Ther. 2025, 10, 74. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Thakor, A.S. Extracellular vesicles in Alzheimer’s disease: From pathology to therapeutic approaches. Neural Regen. Res. 2022, 18, 18–22. [Google Scholar] [CrossRef]
- Silva-Spínola, A.; Baldeiras, I.; Santana, I.; Arrais, J.P.; Alzheimer’s Disease Neuroimaging Initiative. Predicting progression of mild cognitive impairment patients through four distinctive subgroups obtained by unsupervised learning algorithms. J. Alzheimers Dis. 2025, 108, S222–S232. [Google Scholar] [CrossRef]
- Chong Chie, J.A.K.; Persohn, S.A.; Pandey, R.S.; Carter, G.W.; Simcox, O.R.; Salama, P.; Territo, P.R.; For the Alzheimer’s Disease Neuroimaging Initiative. Neurometabolic and vascular dysfunction as an early diagnostic for Alzheimer’s disease and related dementias. Alzheimers Dement. 2025, 21, e70790. [Google Scholar] [CrossRef]
- Aquilani, R.; Cotta Ramusino, M.; Maestri, R.; Iadarola, P.; Boselli, M.; Perini, G.; Boschi, F.; Dossena, M.; Bellini, A.; Buonocore, D.; et al. Several dementia subtypes and mild cognitive impairment share brain reduction of neurotransmitter precursor amino acids, impaired energy metabolism, and lipid hyperoxidation. Front. Aging Neurosci. 2023, 15, 1237469. [Google Scholar] [CrossRef]
- Oporto-Colicoi, V.; Sepúlveda-Lara, A.; Marzuca-Nassr, G.N.; Sepúlveda-Figueroa, P. Mild Cognitive Impairment and Sarcopenia: Effects of Resistance Exercise Training on Neuroinflammation, Cognitive Performance, and Structural Brain Changes. Int. J. Mol. Sci. 2025, 26, 11036. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Gambardella, S.; Familiari, P.; Frati, A.; Fornai, F. Promiscuous Roles of Autophagy and Proteasome in Neurodegenerative Proteinopathies. Int. J. Mol. Sci. 2020, 21, 3028. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mannan, A.; Singh, T.G. Rethinking Parkinson’s: The role of proteostasis networks and autophagy in disease progression. Mol. Cell. Neurosci. 2025, 134, 104023. [Google Scholar] [CrossRef] [PubMed]
- Morrone, C.D.; Raghuraman, R.; Hussaini, S.A.; Yu, W.H. Proteostasis failure exacerbates neuronal circuit dysfunction and sleep impairments in Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Steffan, D.; Pezzini, C.; Esposito, M.; Franco-Romero, A. Mitochondrial Aging in the CNS: Unravelling Implications for Neurological Health and Disease. Biomolecules 2025, 15, 1252. [Google Scholar] [CrossRef]
- Trudler, D.; Nazor, K.L.; Eisele, Y.S.; Grabauskas, T.; Dolatabadi, N.; Parker, J.; Sultan, A.; Zhong, Z.; Goodwin, M.S.; Levites, Y.; et al. Soluble α-synuclein–antibody complexes activate the NLRP3 inflammasome in hiPSC-derived microglia. Proc. Natl. Acad. Sci. USA 2021, 118, e2025847118. [Google Scholar] [CrossRef]
- Choi, Y.; Chung, W.-S. Glial phagocytosis for synapse and toxic proteins in neurodegenerative diseases. Mol. Neurodegener. 2025, 20, 81. [Google Scholar] [CrossRef]
- Batista, A.F.; Khan, K.A.; Papavergi, M.-T.; Lemere, C.A. The Importance of Complement-Mediated Immune Signaling in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2024, 25, 817. [Google Scholar] [CrossRef]
- Castro-Gomez, S.; Heneka, M.T. Innate immune activation in neurodegenerative diseases. Immunity 2024, 57, 790–814. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of Oxidative Stress in Blood–Brain Barrier Disruption and Neurodegenerative Diseases. Antioxidants 2024, 13, 1462. [Google Scholar] [CrossRef] [PubMed]
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol. Res. 2022, 55, 39. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Prikhodko, O.; Freund, R.K.; Sullivan, E.; Kennedy, M.J.; Dell’Acqua, M.L. Amyloid-β Causes NMDA Receptor Dysfunction and Dendritic Spine Loss through mGluR1 and AKAP150-Anchored Calcineurin Signaling. J. Neurosci. 2024, 44, e0675242024. [Google Scholar] [CrossRef]
- Combs, B.; Mueller, R.L.; Morfini, G.; Brady, S.T.; Kanaan, N.M. Tau and Axonal Transport Misregulation in Tauopathies. Adv. Exp. Med. Biol. 2019, 1184, 81–95. [Google Scholar] [CrossRef]
- Brzozowski, C.F.; Challa, H.; Gcwensa, N.Z.; Hall, D.; Nabert, D.; Chambers, N.; Gallardo, I.; Millet, M.; Volpicelli-Daley, L.; Moehle, M.S. Early α-synuclein aggregation decreases corticostriatal glutamate drive and synapse density. Neurobiol. Dis. 2025, 210, 106918. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, Z.; Sagare, A.P.; Wu, Y.; Wang, M.; Owens, N.C.; Verghese, P.B.; Herz, J.; Holtzman, D.M.; Zlokovic, B.V. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol. Neurodegener. 2018, 13, 57. [Google Scholar] [CrossRef]
- Lau, K.; Kotzur, R.; Richter, F. Blood–brain barrier alterations and their impact on Parkinson’s disease pathogenesis and therapy. Transl. Neurodegener. 2024, 13, 37. [Google Scholar] [CrossRef]
- Paul, G.; Elabi, O.F. Microvascular Changes in Parkinson’s Disease- Focus on the Neurovascular Unit. Front. Aging Neurosci. 2022, 14, 853372. [Google Scholar] [CrossRef]
- Li, W.; Fu, Y.; Halliday, G.M.; Sue, C.M. PARK Genes Link Mitochondrial Dysfunction and Alpha-Synuclein Pathology in Sporadic Parkinson’s Disease. Front. Cell Dev. Biol. 2021, 9, 612476. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Chen, J.; Li, J.; Găman, M.-A.; Zou, Z. The multifaceted roles of apolipoprotein E4 in Alzheimer’s disease pathology and potential therapeutic strategies. Cell Death Discov. 2025, 11, 312. [Google Scholar] [CrossRef]
- Williamson, M.G.; Madureira, M.; McGuinness, W.; Heon-Roberts, R.; Mock, E.D.; Naidoo, K.; Cramb, K.M.L.; Caiazza, M.-C.; Malpartida, A.B.; Lavelle, M.; et al. Mitochondrial dysfunction and mitophagy defects in LRRK2-R1441C Parkinson’s disease models. Hum. Mol. Genet. 2023, 32, 2808–2821. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. An Overview of the Epigenetic Modifications in the Brain under Normal and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 3881. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.E.; Kim, S.M.; Han, K.; Kim, N.H.; Chung, H.S.; Kim, J.W.; Han, B.; Cho, S.J.; Yu, J.H.; Park, Y.G.; et al. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med. 2018, 15, e1002640. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Hwang, J.; Son, S.U.; Choi, J.; You, S.-W.; Park, H.; Cha, S.-Y.; Maeng, S. How Can Insulin Resistance Cause Alzheimer’s Disease? Int. J. Mol. Sci. 2023, 24, 3506. [Google Scholar] [CrossRef] [PubMed]
- Pfaffinger, J.M.; Hays, K.E.; Seeley, J.; Ramesh Babu, P.; Ryznar, R. Gut dysbiosis as a potential driver of Parkinson’s and Alzheimer’s disease pathogenesis. Front. Neurosci. 2025, 19, 1600148. [Google Scholar] [CrossRef]
- Hermann, D.M.; Wang, C.; Mohamud Yusuf, A.; Herz, J.; Doeppner, T.R.; Giebel, B. Extracellular vesicles lay the ground for neuronal plasticity by restoring mitochondrial function, cell metabolism and immune balance. J. Cereb. Blood Flow. Metab. 2025. ahead of print. [Google Scholar] [CrossRef]
- Regmi, S.; Liu, D.D.; Shen, M.; Kevadiya, B.D.; Ganguly, A.; Primavera, R.; Chetty, S.; Yarani, R.; Thakor, A.S. Mesenchymal stromal cells for the treatment of Alzheimer’s disease: Strategies and limitations. Front. Mol. Neurosci. 2022, 15, 1011225. [Google Scholar] [CrossRef]
- Wang, X.-S.; Wang, Y.; Xu, Y.; Zhang, S.-R.; Zhang, Y.; Peng, L.-L.; Wu, N.; Ye, J.-S. Effectiveness of mesenchymal stem cell-derived extracellular vesicles therapy for Parkinson’s disease: A systematic review of preclinical studies. World J. Stem Cells 2025, 17, 102421. [Google Scholar] [CrossRef]
- Lana, J.F.; Purita, J.; Jeyaraman, M.; de Souza, B.F.; Rodrigues, B.L.; Huber, S.C.; Caliari, C.; Santos, G.S.; da Fonseca, L.F.; Dallo, I.; et al. Innovative Approaches in Knee Osteoarthritis Treatment: A Comprehensive Review of Bone Marrow-Derived Products. Biomedicines 2024, 12, 2812. [Google Scholar] [CrossRef]
- Zohora, F.T.; Aliyu, M.; Saboor-Yaraghi, A.A. Secretome-based acellular therapy of bone marrow-derived mesenchymal stem cells in degenerative and immunological disorders: A narrative review. Heliyon 2023, 9, e18120. [Google Scholar] [CrossRef]
- Lana, J.F.; Navani, A.; Jeyaraman, M.; Santos, N.; Pires, L.; Santos, G.S.; Rodrigues, I.J.; Santos, D.; Mosaner, T.; Azzini, G.; et al. Sacral Bioneuromodulation: The Role of Bone Marrow Aspirate in Spinal Cord Injuries. Bioengineering 2024, 11, 461. [Google Scholar] [CrossRef]
- Burnouf, T.; Walker, T.L. The multifaceted role of platelets in mediating brain function. Blood 2022, 140, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.C.; Gupta, K.; Lu, Y.-H.; Srinivasan, A.; Delila, L.; Yen, N.T.H.; Nyam-Erdene, A.; Burnouf, T. Platelet Extracellular Vesicles as Natural Delivery Vehicles for Mitochondrial Dysfunction Therapy? ACS Biomater. Sci. Eng. 2025, 11, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Spakova, T.; Janockova, J.; Rosocha, J. Characterization and Therapeutic Use of Extracellular Vesicles Derived from Platelets. Int. J. Mol. Sci. 2021, 22, 9701. [Google Scholar] [CrossRef]
- Macedo, R.d.R.; Fonseca, L.F.d.; Lana, J.F.S.D.; Mosaner, T.; Purita, J.; de Andrade, M.; Rodrigues, L.M.; Centurion, P. Biofat grafts as an orthobiologic tool in osteoarthritis: An update and classification proposal. World J. Meta-Anal. 2021, 9, 29–39. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Lana, A.V.S.D.; da Fonseca, L.F.; Coelho, M.A.; Marques, G.G.; Mosaner, T.; Ribeiro, L.L.; Azzini, G.O.M.; Santos, G.S.; Fonseca, E.; et al. Stromal Vascular Fraction for Knee Osteoarthritis—An Update. J. Stem Cells Regen. Med. 2022, 18, 11–20. [Google Scholar] [CrossRef]
- Feng, H.; Gong, S.; Liu, J.; Aghayants, S.; Liu, Y.; Wu, M.; Wu, Y.; Song, J. Adipose-derived stem cell exosomes: Mechanisms and therapeutic potentials in wound healing. Biomark. Res. 2025, 13, 88. [Google Scholar] [CrossRef]
- Urkon, M.; Ferencz, E.; Szász, J.A.; Szabo, M.I.M.; Orbán-Kis, K.; Szatmári, S.; Nagy, E.E. Antidiabetic GLP-1 Receptor Agonists Have Neuroprotective Properties in Experimental Animal Models of Alzheimer’s Disease. Pharmaceuticals 2025, 18, 614. [Google Scholar] [CrossRef]
- Vijiaratnam, N.; Girges, C.; Auld, G.; McComish, R.; King, A.; Skene, S.S.; Hibbert, S.; Wong, A.; Melander, S.; Gibson, R.; et al. Exenatide once a week versus placebo as a potential disease-modifying treatment for people with Parkinson’s disease in the UK: A phase 3, multicentre, double-blind, parallel-group, randomised, placebo-controlled trial. Lancet 2025, 405, 627–636. [Google Scholar] [CrossRef]
- Liao, J.; He, W.; Li, L.; Wang, J.; Gong, L.; Zhang, Q.; Lin, Z. Mitochondria in brain diseases: Bridging structural-mechanistic insights into precision-targeted therapies. Cell Biomater. 2025, 1, 100016. [Google Scholar] [CrossRef]
- Lu, J.; Cao, Q.; Wang, C.; Zheng, J.; Luo, F.; Xie, J.; Li, Y.; Ma, X.; He, L.; Eisenberg, D.; et al. Structure-Based Peptide Inhibitor Design of Amyloid-β Aggregation. Front. Mol. Neurosci. 2019, 12, 54. [Google Scholar] [CrossRef]
- Murakami, K.; Nguyen, T.H.V.; Nagao, C.; Mizuguchi, K.; Bitan, G. Lysine-Targeting Inhibitors of Amyloidogenic Protein Aggregation: A Promise for Neurodegenerative Proteinopathies. JACS Au 2025, 5, 3680–3700. [Google Scholar] [CrossRef]
- Liang, Z.; Chan, H.Y.E.; Lee, M.M.; Chan, M.K. A SUMO1-Derived Peptide Targeting SUMO-Interacting Motif Inhibits α-Synuclein Aggregation. Cell Chem. Biol. 2021, 28, 180–190.e6. [Google Scholar] [CrossRef]
- Ikeda, T.; Kawabori, M.; Zheng, Y.; Yamaguchi, S.; Gotoh, S.; Nakahara, Y.; Yoshie, E.; Fujimura, M. Intranasal Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Hypoxic-Ischemic Brain Injury. Pharmaceutics 2024, 16, 446. [Google Scholar] [CrossRef]
- Wu, J.; Li, A.; Shi, Y.; Wang, Y.; Luo, J.; Zhuang, W.; Ma, X.; Qiao, Z.; Xiu, X.; Lang, X.; et al. Intranasal delivery of mesenchymal stem cell-derived exosomes ameliorates experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2025, 146, 113853. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sun, S.; Zhu, X.; Liu, X.; Yin, R.; Chen, Y.; Chang, J.; Ye, L.; Gao, J.; Zhao, X.; et al. Intranasal delivery of engineered extracellular vesicles promotes neurofunctional recovery in traumatic brain injury. J. Nanobiotechnol. 2025, 23, 229. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Menéndez-González, M.; Schreiner, O.D.; Ciobanu, R.C. Intrathecal Therapies for Neurodegenerative Diseases: A Review of Current Approaches and the Urgent Need for Advanced Delivery Systems. Biomedicines 2025, 13, 2167. [Google Scholar] [CrossRef] [PubMed]
- Naseri Kouzehgarani, G.; Feldsien, T.; Engelhard, H.H.; Mirakhur, K.K.; Phipps, C.; Nimmrich, V.; Clausznitzer, D.; Lefebvre, D.R. Harnessing cerebrospinal fluid circulation for drug delivery to brain tissues. Adv. Drug Deliv. Rev. 2021, 173, 20–59. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezabakhsh, A.; Rezaie, J.; Nouri, M.; Rahbarghazi, R. Exosomal cargos modulate autophagy in recipient cells via different signaling pathways. Cell Biosci. 2020, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Zhang, D.; Dai, X.; Cai, J.; Zhou, H.; Song, T.; Wang, X.; Kong, Q.; Tang, Z.; Tan, J.; et al. Mesenchymal stem cells and exosomes: A novel therapeutic approach for aging. Exp. Gerontol. 2025, 206, 112781. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Lucci, C.; Vandenbroucke, R.E. Mesenchymal stromal cell extracellular vesicles as immune modulators and drug carriers in neurodegenerative disorders. Trends Neurosci. 2025, 48, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Fan, L.; Zeng, X.; Zheng, D.; Wang, H.; Li, M.; Jiang, Y.; Wang, H.; Liu, H.; Liang, S.; et al. Mesenchymal stem cell-derived extracellular vesicles alleviate autism by regulating microglial glucose metabolism reprogramming and neuroinflammation through PD-1/PD-L1 interaction. J. Nanobiotechnol. 2025, 23, 201. [Google Scholar] [CrossRef]
- Miyazawa, B.; Trivedi, A.; Togarrati, P.P.; Potter, D.; Baimukanova, G.; Vivona, L.; Lin, M.; Lopez, E.; Callcut, R.; Srivastava, A.K.; et al. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J. Trauma. Acute Care Surg. 2019, 86, 931–942. [Google Scholar] [CrossRef]
- Bordin, A.; Chirivì, M.; Pagano, F.; Milan, M.; Iuliano, M.; Scaccia, E.; Fortunato, O.; Mangino, G.; Dhori, X.; De Marinis, E.; et al. Human platelet lysate-derived extracellular vesicles enhance angiogenesis through miR-126. Cell Prolif. 2022, 55, e13312. [Google Scholar] [CrossRef]
- Song, S.J.; Nam, Y.; Rim, Y.A.; Ju, J.H.; Sohn, Y. Comparative analysis of regulations and studies on stem cell therapies: Focusing on induced pluripotent stem cell (iPSC)-based treatments. Stem Cell Res. Ther. 2024, 15, 447. [Google Scholar] [CrossRef]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef]
- Tey, R.V.; Haldankar, P.; Joshi, V.R.; Raj, R.; Maradi, R. Variability in Platelet-Rich Plasma Preparations Used in Regenerative Medicine: A Comparative Analysis. Stem Cells Int. 2022, 2022, 3852898. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Bingi, S.K.; Muthu, S.; Jeyaraman, N.; Packkyarathinam, R.P.; Ranjan, R.; Sharma, S.; Jha, S.K.; Khanna, M.; Rajendran, S.N.S.; et al. Impact of the Process Variables on the Yield of Mesenchymal Stromal Cells from Bone Marrow Aspirate Concentrate. Bioengineering 2022, 9, 57. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef]
- Galli, M.; Benenati, S.; Laudani, C.; Simeone, B.; Sarto, G.; Ortega-Paz, L.; Rocco, E.; Bernardi, M.; Spadafora, L.; D’Amario, D.; et al. Cardiovascular Effects and Tolerability of GLP-1 Receptor Agonists. JACC 2025, 86, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Prasad-Reddy, L.; Isaacs, D. A clinical review of GLP-1 receptor agonists: Efficacy and safety in diabetes and beyond. Drugs Context 2015, 4, 212283. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Terrazas, J.A.; Buford, D.A. Bone marrow concentrate and platelet-rich plasma acquisition and preparation: Why technique matters. Tech. Reg. Anesth. Pain Manag. 2015, 19, 19–25. [Google Scholar] [CrossRef]
- Liebig, B.E.; Kisiday, J.D.; Bahney, C.S.; Ehrhart, N.P.; Goodrich, L.R. The platelet-rich plasma and mesenchymal stem cell milieu: A review of therapeutic effects on bone healing. J. Orthop. Res. 2020, 38, 2539–2550. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Shao, J.; Sun, J.; Hu, L.; Yun, X.; Liuqing, C.; Gong, L.; Wu, S. Exploring Regulatory Frameworks for Exosome Therapy: Insights and Perspectives. Health Care Sci. 2025, 4, 299–309. [Google Scholar] [CrossRef]
- Bell, S.M.; Barnes, K.; De Marco, M.; Shaw, P.J.; Ferraiuolo, L.; Blackburn, D.J.; Venneri, A.; Mortiboys, H. Mitochondrial Dysfunction in Alzheimer’s Disease: A Biomarker of the Future? Biomedicines 2021, 9, 63. [Google Scholar] [CrossRef]
- Rudolph, M.D.; Sutphen, C.L.; Register, T.C.; Whitlow, C.T.; Solingapuram Sai, K.K.; Hughes, T.M.; Bateman, J.R.; Dage, J.L.; Russ, K.A.; Mielke, M.M.; et al. Associations among plasma, MRI, and amyloid PET biomarkers of Alzheimer’s disease and related dementias and the impact of health-related comorbidities in a community-dwelling cohort. Alzheimers Dement. 2024, 20, 4159–4173. [Google Scholar] [CrossRef]
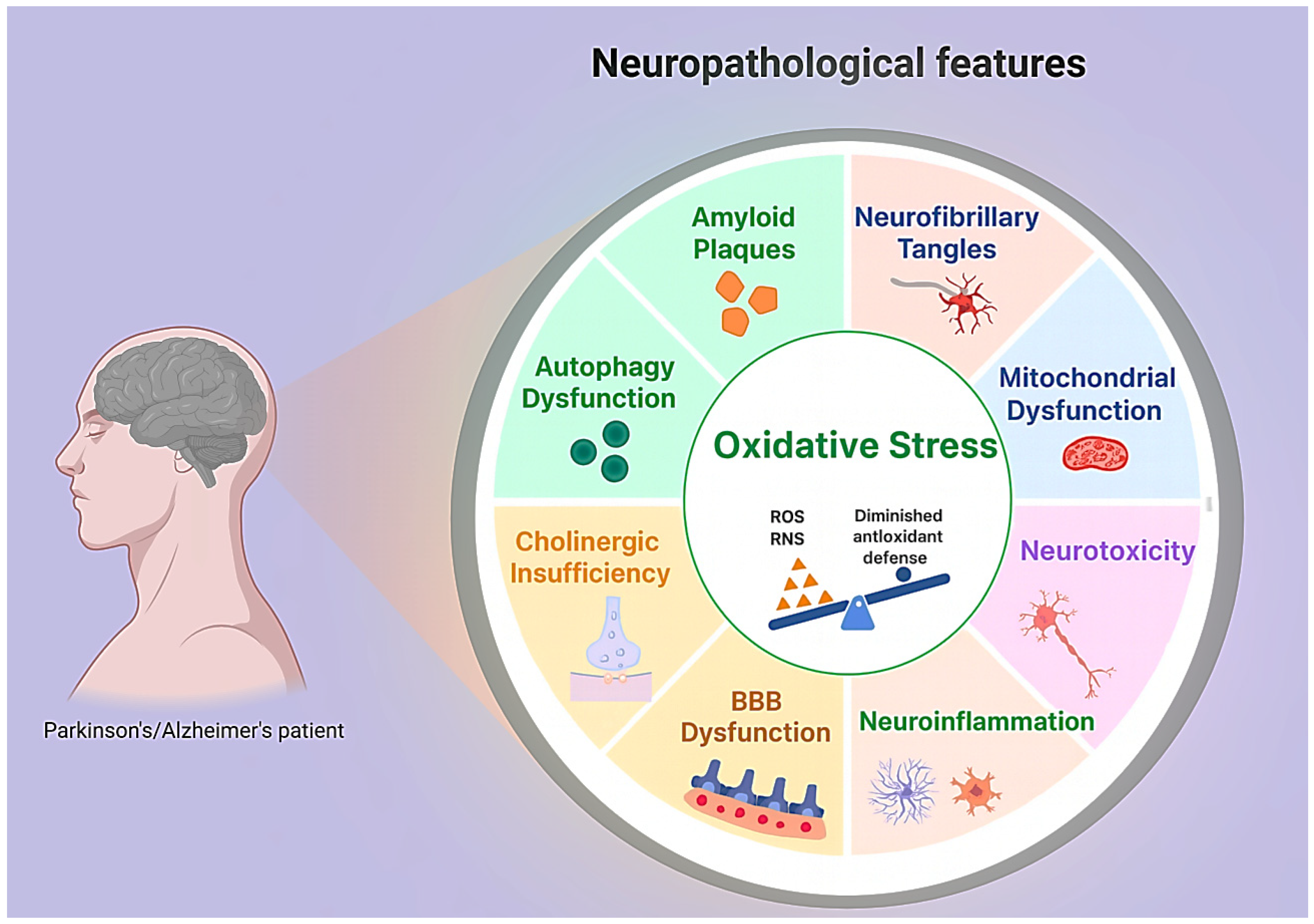
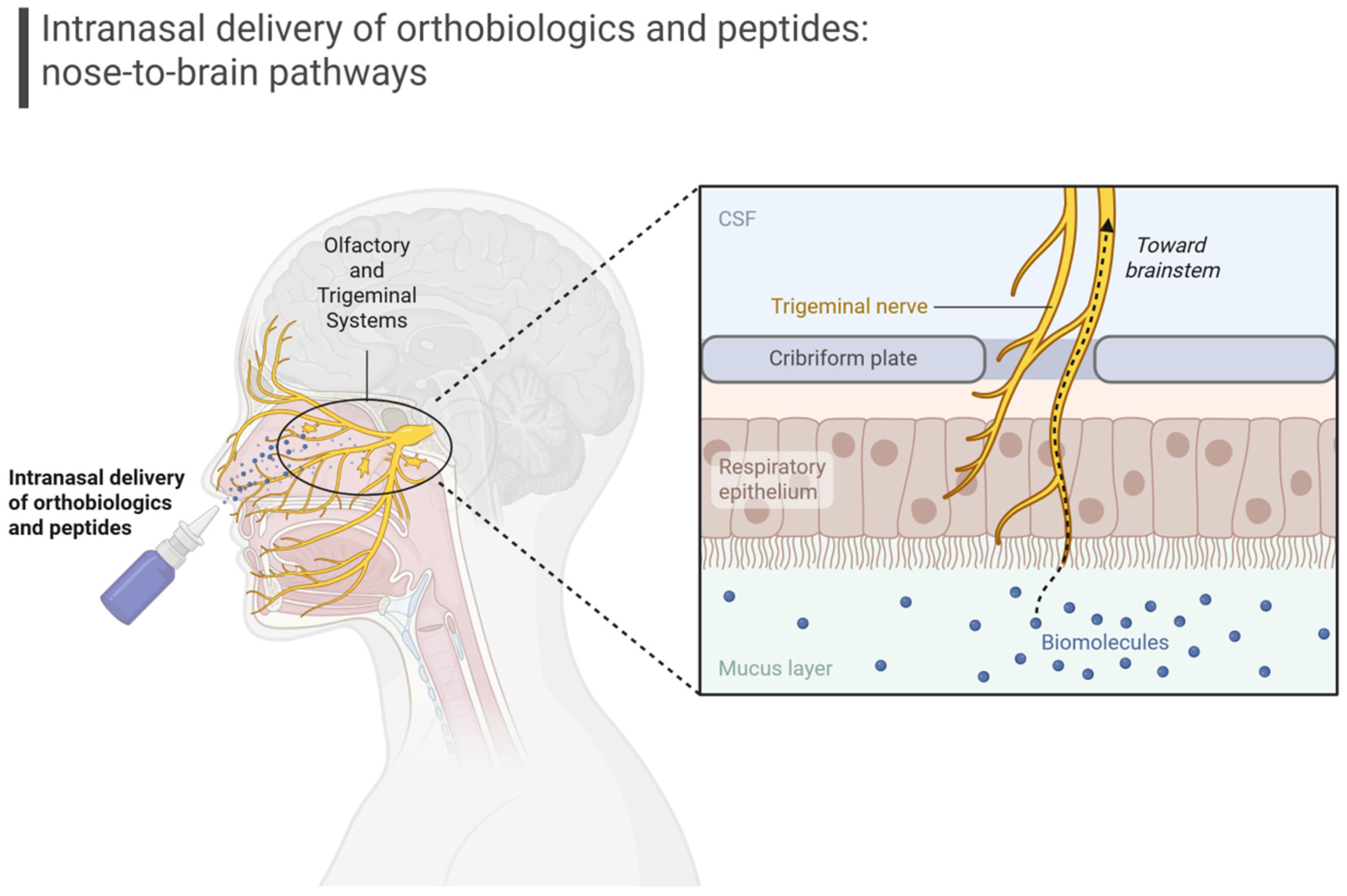
| Pathophysiological Axis | Orthobiologic Contributions | Peptide Contributions | Delivery Options | Strategic Notes | References |
|---|---|---|---|---|---|
| Protein aggregation and proteostasis | MSC exosomes modulating autophagy and proteostasis; platelet vesicles providing protease regulators | Tau- and α-synuclein–modulating sequences; peptides blocking aggregation | Intranasal, engineered vesicle carriers | Orthobiologics act broadly, peptides add specificity to aggregation pathways | [27,96,102,103] |
| Mitochondrial dysfunction | Vesicle transfer of antioxidant enzymes, mitochondrial fragments; BMAC trophic factors | GLP-1 receptor agonists restoring bioenergetics; mitochondria-targeting peptides | Intranasal, IV, intrathecal | Orthobiologics stabilize redox environment, peptides fine-tune metabolic signaling | [4,40,73,82] |
| Neuroinflammation and glial activation | MSC vesicles reprogramming microglia; PRP exosomes reducing cytokines | GLP-1 receptor agonists attenuating inflammatory cascades | Intranasal, IV | Shared target: glial tone; synergy plausible if combined | [4,27,29,104,105] |
| Synaptic degeneration and network disconnection | Vesicles restoring synaptic proteins and neurotransmitter release | Peptides enhancing synaptic plasticity and receptor function | Intranasal, intrathecal | Circuit preservation is the final readout; orthobiologics supply broad cues, peptides sharpen synaptic response | [4,40,78] |
| Vascular and BBB compromise | Platelet-derived vesicles stabilizing endothelium, enhancing perfusion | Indirect vascular benefits from metabolic peptides | Intranasal, IV | BBB stabilization critical for delivery of both classes | [19,33,106,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, C.A.A.; Oliveira, B.S.; Oliveira, A.S.; de Souza Loduca, R.D.; Junior, C.R.M.; Santos, G.S. Orthobiologics and Peptide Therapy for Central Nervous System Repair in Neurodegenerative Conditions. Cells 2025, 14, 1853. https://doi.org/10.3390/cells14231853
de Oliveira CAA, Oliveira BS, Oliveira AS, de Souza Loduca RD, Junior CRM, Santos GS. Orthobiologics and Peptide Therapy for Central Nervous System Repair in Neurodegenerative Conditions. Cells. 2025; 14(23):1853. https://doi.org/10.3390/cells14231853
Chicago/Turabian Stylede Oliveira, Cézar Augusto Alves, Bernardo Scaldini Oliveira, Amanda Scaldini Oliveira, Rafael Duarte de Souza Loduca, Carlos Roberto Massella Junior, and Gabriel Silva Santos. 2025. "Orthobiologics and Peptide Therapy for Central Nervous System Repair in Neurodegenerative Conditions" Cells 14, no. 23: 1853. https://doi.org/10.3390/cells14231853
APA Stylede Oliveira, C. A. A., Oliveira, B. S., Oliveira, A. S., de Souza Loduca, R. D., Junior, C. R. M., & Santos, G. S. (2025). Orthobiologics and Peptide Therapy for Central Nervous System Repair in Neurodegenerative Conditions. Cells, 14(23), 1853. https://doi.org/10.3390/cells14231853







