Cellular and Molecular Mechanisms of Wound Repair: From Biology to Therapeutic Innovation
Abstract
1. Introduction
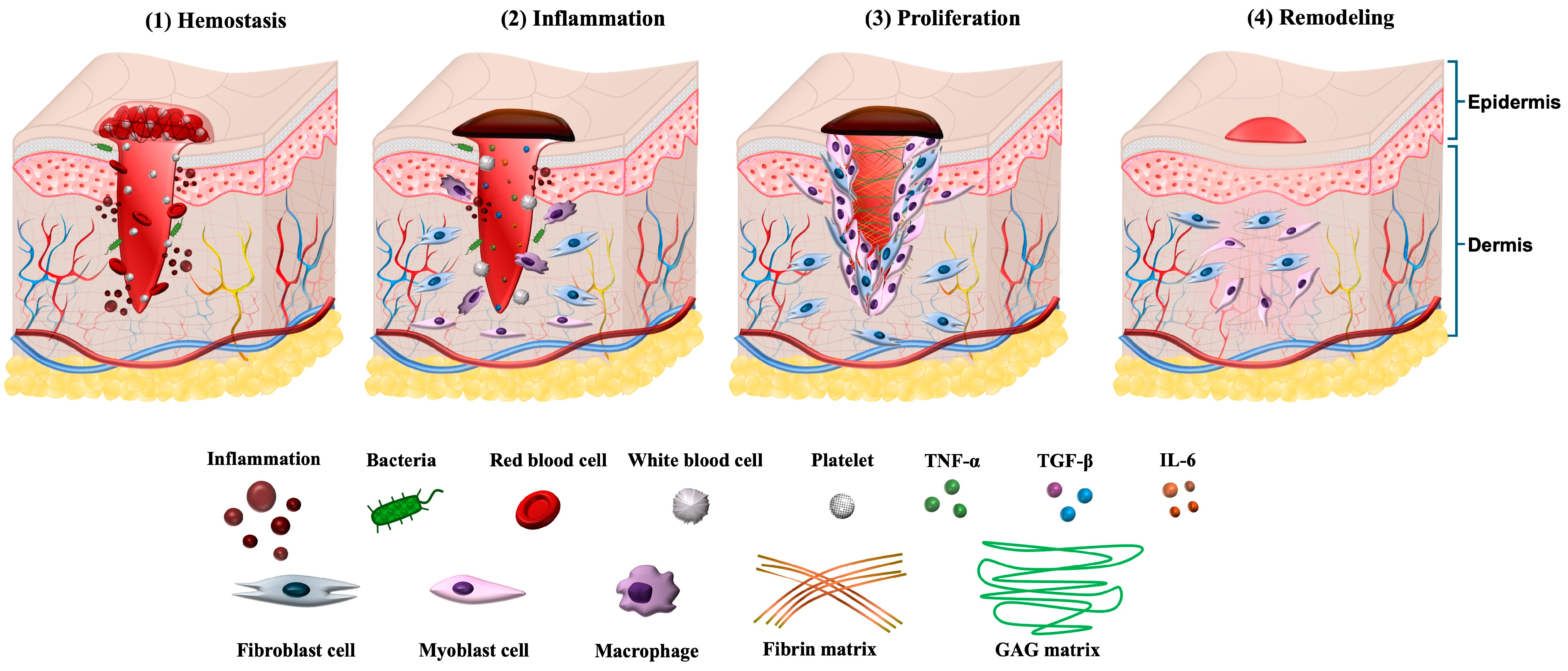
2. Phases of Wound Healing
2.1. Hemostasis
2.2. Inflammation
2.3. Proliferation
2.4. Remodeling (Maturation)
3. Cellular Players in Wound Repair
3.1. Platelets
3.2. Neutrophils
3.3. Macrophages
3.4. Fibroblasts and Myofibroblasts
3.5. Keratinocytes
3.6. Endothelial Cells
3.7. Stem and Progenitor Cells
| Cell Type | Primary Role in Wound Healing | Key Growth Factors/Cytokines Involved | Evidence (In Vitro/In Vivo/Ex Vivo) | Representative Signaling Pathways | Reference |
|---|---|---|---|---|---|
| Platelets | Initiate hemostasis and provide early pro-healing signals; release mediators that recruit immune and stromal cells. | PDGF, VEGF, TGF-β, EGF, PF4 | In vivo: mouse skin wound models; PRP clinical applications. | MAPK/ERK; PI3K/AKT; TGF-β/Smad | [107,108,109] |
| Neutrophils | Early antimicrobial defense, debris clearance, and inflammatory mediator release; excessive activity delays healing. | IL-1β, TNF-α, MPO, NETs-associated proteins | In vivo: acute and diabetic wound models; ex vivo: human wound exudates. | NF-κB; ROS-associated pathways; TLR signaling | [110,111,112] |
| Macrophages | Orchestrate transition from inflammation to repair; polarization from M1→M2 regulates ECM deposition and angiogenesis. | TNF-α, IL-6 (M1); IL-10, TGF-β, VEGF (M2) | In vivo: macrophage depletion and lineage tracing studies; ex vivo: human ulcer biopsies. | NF-κB (M1), STAT3/STAT6 (M2), PI3K/AKT | [113,114,115] |
| Fibroblasts/Myofibroblasts | Produce and remodel ECM; myofibroblasts contract wound tissue; lineage subtypes influence fibrosis vs. regeneration. | TGF-β1, CTGF, Collagen I/III, Fibronectin | In vivo: lineage tracing and scRNA-seq of wound fibroblasts; in vitro: fibroblast activation assays. | TGF-β/Smad; YAP/TAZ mechanotransduction; FAK–c-Jun axis | [79,116,117] |
| Keratinocytes | Re-epithelialization through migration and proliferation serves as an immune sentinel. | EGF, KGF, IL-1, IL-6, CCL20 | In vitro: scratch-wound migration assays; in vivo: re-epithelialization kinetics in murine wounds. | EGFR/MAPK; Integrin–FAK; cGAS–STING signaling | [118,119] |
| Endothelial Cells | Drive angiogenesis and restore perfusion; transient EndMT supports vascular remodeling. | VEGF, Ang-1/2, FGF-2 | In vivo: angiogenesis markers in wound beds; ex vivo: vascular sprouting assays. | VEGF/VEGFR2; Notch–DLL4; HIF-1α oxygen-sensing pathways | [120] |
| Stem/Progenitor Cells | Provide regenerative capacity, paracrine immunomodulation, and enhance angiogenesis and ECM remodeling. | VEGF, HGF, IL-10, SDF-1/CXCL12 | In vivo: MSC transplantation models; Clinical: cell-based wound therapies | PI3K/AKT; TGF-β/Smad3; CXCL12/CXCR4 axis | [121,122] |
4. Molecular Mediators and Pathways in Wound Repair
4.1. Growth Factors
4.2. Cytokines and Chemokines
4.3. Intracellular Signaling Pathways
4.4. Extracellular Matrix Remodeling
4.5. Immune-Metabolic Crosstalk
5. Pathological Wound Healing
5.1. Chronic Non-Healing Wounds
5.2. Fibrosis and Hypertrophic Scarring
5.3. Systemic and Environmental Influences
5.4. Clinical Implications
6. Emerging Concepts and Technologies in Wound Repair
6.1. The Microbiome in Wound Healing
6.2. Epigenetic and Transcriptomic Regulation
6.3. Single-Cell and Spatial Omics Approaches
6.4. Biomaterials and Bioengineered Scaffolds
7. Therapeutic Implications
7.1. Growth Factor–Based Therapies
7.2. Cytokine and Inflammation Modulation
7.3. Stem and Progenitor Cell–Based Approaches
7.4. Biomaterials and Tissue Engineering
7.5. Anti-Fibrotic Strategies
7.6. Challenges and Opportunities
8. Future Directions and Unanswered Questions
8.1. Integration of Local and Systemic Factors
8.2. Heterogeneity of Cellular Responses
8.3. Microbiome and Host–Microbe Interactions
8.4. Epigenetic and Non-Coding RNA Regulation
8.5. Translation of Regenerative Technologies
8.6. Toward Precision Wound Medicine
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet-derived growth factor |
| FGFs | Fibroblast growth factors |
| TGF-β | Transforming growth factor-β |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MMPs | Matrix metalloproteinases |
| IL-10 | Interleukin-10 |
| EGF | Epidermal growth factor |
| KGF | Keratinocyte growth factor |
| MSC | Mesenchymal stem cell |
| CXCL4/PF4 | Platelet factor 4 |
| CXCL7 | C-X-C motif chemokine ligand 7 |
| HGF | Hepatocyte growth factor |
| CXCL8/IL-8 | Interleukin-8 |
| LTB4 | Leukotriene B4 |
| CXCR 1 | CXC chemokine receptor 1 |
| CXCR 2 | CXC chemokine receptor |
| BLT1 | Leukotriene B4 Receptor 1 |
| ROS | Reactive oxygen species |
| NETs | Neutrophils extracellular traps |
| EndMT | Endothelial-to-mesenchymal transition |
| TLR-9 | Toll-like receptor 9 |
| PAK2 | p21-activated kinase 2 |
| NF2 | Neurofibromin 2 |
| YAP | Yes-associated protein |
| SMAD2 | Mothers against decapentaplegic homolog 2 |
| IL-27 | Interleukin-27 |
| STAT3 | Signal transducer and activator of transcription 3 |
| IL-25 | Interleukin-25 |
| DDR | DNA damage response |
| FAK | Focal adhesion kinase |
| TAZ | Transcriptional co-activator with PDZ-binding motif) |
| IL-33 | Interleukin-33 |
| IL-4 | Interleukin-4 |
| IL-13 | Interleukin-13 |
| Prg4 | Proteoglycan 4 |
| Col25a1 | Collagen XXVα1 |
| CRABP1 | Cellular retinoic acid-binding protein 1 |
| Pamr1 | Peptidase domain containing associated with muscle regeneration 1 |
| Ly6a | Lymphocyte antigen 6a |
| CCL2 | C–C motif chemokine ligand 2 |
| AMPK | AMP-activated protein kinase |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| ERK | Extracellular signal–regulated kinase |
| AP-1 | Activator protein-1 |
| cGAS | Cyclic GMP–AMP synthase |
| STING | Stimulator of interferon genes |
| MHC-II | Major histocompatibility complex class II |
| JMJD3 | Jumonji domain-containing protein D3 |
| CCL20 | C–C motif chemokine ligand 20 |
| LAMB3 | Laminin subunit beta 3 |
| EREG | Epiregulin |
| FOSL1 | FOS like 1 |
| CaMKII | Calcium-dependent protein kinase II |
| ATF3 | Activating transcription factor 3 |
| SLC7A11 | Solute carrier family 7 member 11 |
| A-SMA | α-smooth muscle actin |
| Zeb1 | Zinc finger E-box binding homeobox 1 |
| SMAD3 | Mothers against decapentaplegic homolog 3 |
| ELR | Tripeptide motif Glu–Leu–Arg |
| RTK | Receptor tyrosine kinase |
| TCF | T-cell factor |
| TIMPs | Tissue inhibitors of metalloproteinases |
| HIF-1α | Hypoxia-inducible factor-1α |
| ANGTP2 | Angiopoietin-2 |
| SDF-1 | Stromal cell–derived factor-1 |
| DNMT1 | DNA methyltransferase 1 |
| TET-2 | Ten-eleven translocation methylcytosine dioxygenase 2 |
| WAKMAR1 | Wound and keratinocyte migration associated lncRNA 1 |
| E2F1 | E2F transcription factor 1 |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| HDAC | Histone deacetylase |
| EZH2 | Enhancer of zeste homolog 2 |
| H3K4me3 | Trimethylation of histone H3 on lysine 4 |
| MLL1 | Mixed lineage leukemia 1 |
| SETDB2 | SET domain bifurcated 2 |
| SWI/SNF | SWItch/sucrose non-fermentable complex |
| PAD4 | Peptidylarginine deiminase 4 |
| HAT | Histone acetyltransferase |
| CRISPR/Cas | Clustered regularly interspaced short palindromic repeats/CRISPR-associated system |
References
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, L.; Zhang, X. Biological and Molecular Mechanisms of Wound Healing: Emerging Therapeutic Strategies and Future Directions. Am. Surg. 2025, 91, 1966–1973. [Google Scholar] [CrossRef]
- Redmond, M.C.; Gethin, G.; Finn, D.P. A Review of Chronic Wounds and Their Impact on Negative Affect, Cognition, and Quality of Life. Int. Wound J. 2025, 22, e70748. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shankar, R.; Yadav, A.K.; Pratap, A.; Ansari, M.A.; Srivastava, V. Burden of Chronic Nonhealing Wounds: An Overview of the Worldwide Humanistic and Economic Burden to the Healthcare System. Int. J. Low. Extrem. Wounds 2024, 15347346241246339. [Google Scholar] [CrossRef]
- Dowling, C.; Chu, L.; Etkin, Y.; Oropallo, A. Assessment and management of chronic venous, arterial, and diabetic wounds in older adults. Semin. Vasc. Surg. 2025, 38, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Kohlhauser, M.; Mayrhofer, M.; Kamolz, L.-P.; Smolle, C. An Update on Molecular Mechanisms of Scarring—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 11579. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Yang, L.; Shi, F.; Cao, F.; Wang, L.; She, J.; He, B.; Xu, X.; Kong, L.; Cai, B. Neutrophils in Tissue Injury and Repair: Molecular Mechanisms and Therapeutic Targets. MedComm 2025, 6, e70184. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- Velnar, T.; Gradisnik, L. Tissue Augmentation in Wound Healing: The Role of Endothelial and Epithelial Cells. Med. Arch. 2018, 72, 444–448. [Google Scholar] [CrossRef]
- Farabi, B.; Roster, K.; Hirani, R.; Tepper, K.; Atak, M.F.; Safai, B. The Efficacy of Stem Cells in Wound Healing: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3006. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef] [PubMed]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.E.; Leeming, D.J.; Henriksen, K.; Mortensen, J.H.; Nielsen, S.H.; Anstee, Q.M.; Sanyal, A.J.; Karsdal, M.A.; Schuppan, D. ECM formation and degradation during fibrosis, repair, and regeneration. NPJ Metab. Health Dis. 2025, 3, 25. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Vandereyken, K.; Sifrim, A.; Thienpont, B.; Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 2023, 24, 494–515. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Zeng, A.; Long, X.; Yu, N.; Wang, X. The role of the skin microbiome in wound healing. Burn. Trauma 2024, 12, tkad059. [Google Scholar] [CrossRef]
- Pastar, I.; Marjanovic, J.; Stone, R.C.; Chen, V.; Burgess, J.L.; Mervis, J.S.; Tomic-Canic, M. Epigenetic regulation of cellular functions in wound healing. Exp. Dermatol. 2021, 30, 1073–1089. [Google Scholar] [CrossRef]
- Qin, J.; Chen, F.; Wu, P.; Sun, G. Recent Advances in Bioengineered Scaffolds for Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 841583. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating platelet and coagulation activation in fibrin clot formation. Res. Pr. Thromb. Haemost. 2018, 2, 450–460. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Y.; Ren, Y.; Xu, L.; Wang, H.; Ling, X.; Jin, L.; Hu, Y.; Zhang, H.; Miao, C.; et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. 2021, 12, 984. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef]
- Chitturi, R.T.; Balasubramaniam, A.M.; Parameswar, R.A.; Kesavan, G.; Haris, K.T.; Mohideen, K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J. Int. Oral. Health 2015, 7, 75–80. [Google Scholar]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Shin, D.; Minn, K.W. The effect of myofibroblast on contracture of hypertrophic scar. Plast. Reconstr. Surg. 2004, 113, 633–640. [Google Scholar] [CrossRef]
- Kremastiotis, G.; Handa, I.; Jackson, C.; George, S.; Johnson, J. Disparate effects of MMP and TIMP modulation on coronary atherosclerosis and associated myocardial fibrosis. Sci. Rep. 2021, 11, 23081. [Google Scholar] [CrossRef]
- Korntner, S.; Lehner, C.; Gehwolf, R.; Wagner, A.; Grütz, M.; Kunkel, N.; Tempfer, H.; Traweger, A. Limiting angiogenesis to modulate scar formation. Adv. Drug Deliv. Rev. 2019, 146, 170–189. [Google Scholar] [CrossRef]
- Locatelli, L.; Colciago, A.; Castiglioni, S.; Maier, J.A. Platelets in Wound Healing: What Happens in Space? Front. Bioeng. Biotechnol. 2021, 9, 716184. [Google Scholar] [CrossRef]
- Scopelliti, F.; Cattani, C.; Dimartino, V.; Mirisola, C.; Cavani, A. Platelet Derivatives and the Immunomodulation of Wound Healing. Int. J. Mol. Sci. 2022, 23, 8370. [Google Scholar] [CrossRef]
- Johnson, J.; Law, S.Q.K.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.L.; Silva, A.; Kong, K.J.W.; Schoppet, M.; Blyth, C.; et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J. Extracell. Vesicles 2023, 12, e12332. [Google Scholar] [CrossRef]
- Levoux, J.; Prola, A.; Lafuste, P.; Gervais, M.; Chevallier, N.; Koumaiha, Z.; Kefi, K.; Braud, L.; Schmitt, A.; Yacia, A.; et al. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021, 33, 283–299.e289. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Kumar, S.; Garg, P.; Verma, Y.K. Platelet-rich plasma: A comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank. 2023, 24, 285–306. [Google Scholar] [CrossRef]
- Everts, P.A.; Lana, J.F.; Alexander, R.W.; Dallo, I.; Kon, E.; Ambach, M.A.; van Zundert, A.; Podesta, L. Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing. Int. J. Mol. Sci. 2024, 25, 7914. [Google Scholar] [CrossRef]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- Simpson, D.M.; Ross, R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J. Clin. Investig. 1972, 51, 2009–2023. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- McCourt, M.; Wang, J.H.; Sookhai, S.; Redmond, H.P. Proinflammatory Mediators Stimulate Neutrophil-Directed Angiogenesis. Arch. Surg. 1999, 134, 1325–1331. [Google Scholar] [CrossRef]
- McCourt, M.; Wang, J.H.; Sookhai, S.; Redmond, H.P. Activated human neutrophils release hepatocyte growth factor/scatter factor. Eur. J. Surg. Oncol. 2001, 27, 396–403. [Google Scholar] [CrossRef]
- Feiken, E.; Rømer, J.; Eriksen, J.; Lund, L.R. Neutrophils express tumor necrosis factor-alpha during mouse skin wound healing. J. Investig. Dermatol. 1995, 105, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Theilgaard-Mönch, K.; Knudsen, S.; Follin, P.; Borregaard, N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J. Immunol. 2004, 172, 7684–7693. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Fu, L.; Tian, C.; Yang, J.; He, F.; et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Nourshargh, S.; Renshaw, S.A.; Imhof, B.A. Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol. 2016, 37, 273–286. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, S.; Li, S.; Gong, S.; Zhang, Q. Neutrophil extracellular traps in wound healing. Trends Pharmacol. Sci. 2024, 45, 1033–1045. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, X.; He, Q.; Xiao, Q.A.; Wang, D.; Huang, M.; Zhang, X. Diabetes-associated neutrophil NETosis: Pathogenesis and interventional target of diabetic complications. Front. Endocrinol. 2023, 14, 1202463. [Google Scholar] [CrossRef]
- Xiao, Y.; Ding, T.; Fang, H.; Lin, J.; Chen, L.; Ma, D.; Zhang, T.; Cui, W.; Ma, J. Innovative Bio-based Hydrogel Microspheres Micro-Cage for Neutrophil Extracellular Traps Scavenging in Diabetic Wound Healing. Adv. Sci. 2024, 11, e2401195. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Chen, L.; Wang, Z.; Chen, J.; Ni, Q.; Guo, X.; Zhang, L.; Xue, G. Neutrophil Extracellular Traps Delay Diabetic Wound Healing by Inducing Endothelial-to-Mesenchymal Transition via the Hippo pathway. Int. J. Biol. Sci. 2023, 19, 347–361. [Google Scholar] [CrossRef]
- Willenborg, S.; Injarabian, L.; Eming, S.A. Role of Macrophages in Wound Healing. Cold Spring Harb. Perspect. Biol. 2022, 14, a041216. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef]
- Tao, L.; Wu, S.; Wang, Q.; Xi, Z.; Zou, Y.; Cao, M.; Liang, K.; Xu, W.; Hu, Q.; Ge, Y.; et al. IL-27 accelerates diabetic wound healing by modulating macrophage polarization. Int. Immunopharmacol. 2025, 155, 114575. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Zhang, H.; Ding, Y.; Tan, Q. IL-25 improves diabetic wound healing through stimulating M2 macrophage polarization and fibroblast activation. Int. Immunopharmacol. 2022, 106, 108605. [Google Scholar] [CrossRef]
- Thompson, S.M.; Phan, Q.M.; Winuthayanon, S.; Driskell, I.M.; Driskell, R.R. Parallel Single-Cell Multiomics Analysis of Neonatal Skin Reveals the Transitional Fibroblast States that Restrict Differentiation into Distinct Fates. J. Investig. Dermatol. 2022, 142, 1812–1823.e3. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, aaa2151. [Google Scholar] [CrossRef]
- Mascharak, S.; desJardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.S.; et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef]
- Cui, L.; Chen, S.Y.; Lerbs, T.; Lee, J.W.; Domizi, P.; Gordon, S.; Kim, Y.H.; Nolan, G.; Betancur, P.; Wernig, G. Activation of JUN in fibroblasts promotes pro-fibrotic programme and modulates protective immunity. Nat. Commun. 2020, 11, 2795. [Google Scholar] [CrossRef]
- Jiang, D.; Singh, K.; Muschhammer, J.; Schatz, S.; Sindrilaru, A.; Makrantonaki, E.; Qi, Y.; Wlaschek, M.; Scharffetter-Kochanek, K. MSCs rescue impaired wound healing in a murine LAD1 model by adaptive responses to low TGF-β1 levels. EMBO Rep. 2020, 21, e49115. [Google Scholar] [CrossRef]
- Griffin, M.F.; Borrelli, M.R.; Garcia, J.T.; Januszyk, M.; King, M.; Lerbs, T.; Cui, L.; Moore, A.L.; Shen, A.H.; Mascharak, S.; et al. JUN promotes hypertrophic skin scarring via CD36 in preclinical in vitro and in vivo models. Sci. Transl. Med. 2021, 13, eabb3312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Christ, S.; Correa-Gallegos, D.; Ramesh, P.; Kalgudde Gopal, S.; Wannemacher, J.; Mayr, C.H.; Lupperger, V.; Yu, Q.; Ye, H.; et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 2020, 11, 5653. [Google Scholar] [CrossRef]
- Wan, L.; Jiang, D.; Correa-Gallegos, D.; Ramesh, P.; Zhao, J.; Ye, H.; Zhu, S.; Wannemacher, J.; Volz, T.; Rinkevich, Y. Connexin43 gap junction drives fascia mobilization and repair of deep skin wounds. Matrix Biol. 2021, 97, 58–71. [Google Scholar] [CrossRef]
- Hinz, B.; Pittet, P.; Smith-Clerc, J.; Chaponnier, C.; Meister, J.J. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol. Biol. Cell 2004, 15, 4310–4320. [Google Scholar] [CrossRef]
- Hinz, B.; McCulloch, C.A.; Coelho, N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp. Cell Res. 2019, 379, 119–128. [Google Scholar] [CrossRef]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef]
- Chen, K.; Kwon, S.H.; Henn, D.; Kuehlmann, B.A.; Tevlin, R.; Bonham, C.A.; Griffin, M.; Trotsyuk, A.A.; Borrelli, M.R.; Noishiki, C.; et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat. Commun. 2021, 12, 5256. [Google Scholar] [CrossRef] [PubMed]
- Boothby, I.C.; Kinet, M.J.; Boda, D.P.; Kwan, E.Y.; Clancy, S.; Cohen, J.N.; Habrylo, I.; Lowe, M.M.; Pauli, M.; Yates, A.E.; et al. Early-life inflammation primes a T helper 2 cell-fibroblast niche in skin. Nature 2021, 599, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Koester, J.; Miroshnikova, Y.A.; Ghatak, S.; Chacón-Martínez, C.A.; Morgner, J.; Li, X.; Atanassov, I.; Altmüller, J.; Birk, D.E.; Koch, M.; et al. Niche stiffening compromises hair follicle stem cell potential during ageing by reducing bivalent promoter accessibility. Nat. Cell Biol. 2021, 23, 771–781. [Google Scholar] [CrossRef]
- Almet, A.A.; Liu, Y.; Nie, Q.; Plikus, M.V. Integrated Single-Cell Analysis Reveals Spatially and Temporally Dynamic Heterogeneity in Fibroblast States during Wound Healing. J. Investig. Dermatol. 2025, 145, 645–659.e625. [Google Scholar] [CrossRef]
- Amiri, N.; Golin, A.P.; Jalili, R.B.; Ghahary, A. Roles of cutaneous cell-cell communication in wound healing outcome: An emphasis on keratinocyte-fibroblast crosstalk. Exp. Dermatol. 2022, 31, 475–484. [Google Scholar] [CrossRef]
- Bártolo, I.; Reis, R.L.; Marques, A.P.; Cerqueira, M.T. Keratinocyte Growth Factor-Based Strategies for Wound Re-Epithelialization. Tissue Eng. Part B Rev. 2022, 28, 665–676. [Google Scholar] [CrossRef]
- Holt, J.R.; Zeng, W.Z.; Evans, E.L.; Woo, S.H.; Ma, S.; Abuwarda, H.; Loud, M.; Patapoutian, A.; Pathak, M.M. Spatiotemporal dynamics of PIEZO1 localization controls keratinocyte migration during wound healing. eLife 2021, 10, e65415. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Yang, S.; Cui, Y.H.; He, Y.Y. Keratinocyte autophagy enables the activation of keratinocytes and fibroblastsand facilitates wound healing. Autophagy 2021, 17, 2128–2143. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Li, D.; Peng, H.; Qu, L.; Sommar, P.; Wang, A.; Chu, T.; Li, X.; Bi, X.; Liu, Q.; Gallais Sérézal, I.; et al. miR-19a/b and miR-20a Promote Wound Healing by Regulating the Inflammatory Response of Keratinocytes. J. Investig. Dermatol. 2021, 141, 659–671. [Google Scholar] [CrossRef]
- Li, D.; Wang, A.; Liu, X.; Meisgen, F.; Grünler, J.; Botusan, I.R.; Narayanan, S.; Erikci, E.; Li, X.; Blomqvist, L.; et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J. Clin. Investig. 2015, 125, 3008–3026. [Google Scholar] [CrossRef]
- Na, J.; Lee, K.; Na, W.; Shin, J.Y.; Lee, M.J.; Yune, T.Y.; Lee, H.K.; Jung, H.S.; Kim, W.S.; Ju, B.G. Histone H3K27 Demethylase JMJD3 in Cooperation with NF-κB Regulates Keratinocyte Wound Healing. J. Investig. Dermatol. 2016, 136, 847–858. [Google Scholar] [CrossRef]
- Tamoutounour, S.; Han, S.J.; Deckers, J.; Constantinides, M.G.; Hurabielle, C.; Harrison, O.J.; Bouladoux, N.; Linehan, J.L.; Link, V.M.; Vujkovic-Cvijin, I.; et al. Keratinocyte-intrinsic MHCII expression controls microbiota-induced Th1 cell responses. Proc. Natl. Acad. Sci. USA 2019, 116, 23643–23652. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Zhang, L.; Bian, X.; Wu, J.; Li, L.; Chen, Y.; Luo, L.; Pan, L.; Kong, L.; et al. The lncRNA SNHG26 drives the inflammatory-to-proliferative state transition of keratinocyte progenitor cells during wound healing. Nat. Commun. 2024, 15, 8637. [Google Scholar] [CrossRef]
- Liu, Z.; Bian, X.; Luo, L.; Björklund, Å.K.; Li, L.; Zhang, L.; Chen, Y.; Guo, L.; Gao, J.; Cao, C.; et al. Spatiotemporal single-cell roadmap of human skin wound healing. Cell Stem Cell 2025, 32, 479–498.e478. [Google Scholar] [CrossRef]
- Byrne, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J. Cell. Mol. Med. 2005, 9, 777–794. [Google Scholar] [CrossRef]
- Nami, N.; Feci, L.; Napoliello, L.; Giordano, A.; Lorenzini, S.; Galeazzi, M.; Rubegni, P.; Fimiani, M. Crosstalk between platelets and PBMC: New evidence in wound healing. Platelets 2016, 27, 143–148. [Google Scholar] [CrossRef]
- Mitchell, K.; Szekeres, C.; Milano, V.; Svenson, K.B.; Nilsen-Hamilton, M.; Kreidberg, J.A.; DiPersio, C.M. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J. Cell Sci. 2009, 122, 1778–1787. [Google Scholar] [CrossRef]
- Dulmovits, B.M.; Herman, I.M. Microvascular remodeling and wound healing: A role for pericytes. Int. J. Biochem. Cell Biol. 2012, 44, 1800–1812. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, D.P.; Lin, Z.; Lin, Y.Z.; Shi, Y.F.; Dong, X.Y.; Jin, M.Q.; Song, F.Q.; Du, S.T.; Feng, Y.Z.; et al. Piezo1-Mediated Ferroptosis Delays Wound Healing in Aging Mice by Regulating the Transcriptional Activity of SLC7A11 through Activating Transcription Factor 3. Research 2025, 8, 0718. [Google Scholar] [CrossRef]
- Vu, R.; Dragan, M.; Sun, P.; Werner, S.; Dai, X. Epithelial-Mesenchymal Plasticity and Endothelial-Mesenchymal Transition in Cutaneous Wound Healing. Cold Spring Harb. Perspect. Biol. 2023, 15, a041237. [Google Scholar] [CrossRef]
- Sireesha, K.; Samundeshwari, E.L.; Surekha, K.; Chandrasekhar, C.; Sarma, P. In vitro generation of epidermal keratinocytes from human CD34-positive hematopoietic stem cells. Vitr. Cell. Dev. Biol. Anim. 2024, 60, 236–248. [Google Scholar] [CrossRef]
- Dos Santos, J.F.; Borçari, N.R.; da Silva Araújo, M.; Nunes, V.A. Mesenchymal stem cells differentiate into keratinocytes and express epidermal kallikreins: Towards an in vitro model of human epidermis. J. Cell. Biochem. 2019, 120, 13141–13155. [Google Scholar] [CrossRef]
- Azari, Z.; Nazarnezhad, S.; Webster, T.J.; Hoseini, S.J.; Brouki Milan, P.; Baino, F.; Kargozar, S. Stem cell-mediated angiogenesis in skin tissue engineering and wound healing. Wound Repair. Regen. 2022, 30, 421–435. [Google Scholar] [CrossRef]
- Paganelli, A.; Benassi, L.; Rossi, E.; Tarentini, E.; Magnoni, C. Mesenchymal stromal cells promote the proliferation of basal stem cells and efficient epithelization in organotypic models of wound healing. Microsc. Res. Tech. 2022, 85, 2752–2756. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Short, W.D.; Steen, E.; Kaul, A.; Wang, X.; Olutoye, O.O., 2nd; Vangapandu, H.V.; Templeman, N.; Blum, A.J.; Moles, C.M.; Narmoneva, D.A.; et al. IL-10 promotes endothelial progenitor cell infiltration and wound healing via STAT3. FASEB J. 2022, 36, e22298. [Google Scholar] [CrossRef]
- Joost, S.; Jacob, T.; Sun, X.; Annusver, K.; La Manno, G.; Sur, I.; Kasper, M. Single-Cell Transcriptomics of Traced Epidermal and Hair Follicle Stem Cells Reveals Rapid Adaptations during Wound Healing. Cell Rep. 2018, 25, 585–597.e7. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-H.; Wang, A.Y.L.; Loh, C.Y.Y.; Pai, A.A.; Kao, H.-K. Therapeutic Potential of Exosomes Derived from Diabetic Adipose Stem Cells in Cutaneous Wound Healing of db/db Mice. Pharmaceutics 2022, 14, 1206. [Google Scholar] [CrossRef]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, Z.; Bu, P.; Yu, T.; Endo, Y.; Zhou, W.; Sun, Y.; Cao, F.; Dai, G.; Hu, Y.; et al. A Whole-Course-Repair System Based on Neurogenesis-Angiogenesis Crosstalk and Macrophage Reprogramming Promotes Diabetic Wound Healing. Adv. Mater. 2023, 35, e2212300. [Google Scholar] [CrossRef]
- Ng, S.-L.; Azhar, N.A.; Budin, S.B.; Ibrahim, N.; Ghani, N.A.A.; Ghafar, N.A.; Law, J.-X. Effects of Platelet Lysate Gels Derived from Different Blood Sources on Oral Mucosal Wound Healing: An In Vitro Study. Gels 2023, 9, 343. [Google Scholar] [CrossRef]
- Du, X.; Zhao, J.; Ren, Q.; Ma, Y.; Duan, P.; Huang, Y.; Wang, S. Clinical application of platelet rich plasma to promote healing of open hand injury with skin defect. Regen. Ther. 2024, 26, 308–314. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, B.; Tian, J. Platelet-rich plasma may accelerate diabetic wound healing by modulating epithelial/endothelial-mesenchymal transition through inhibiting reactive oxygen species-mediated oxidative stress. Front. Bioeng. Biotechnol. 2025, 13, 1623780. [Google Scholar] [CrossRef]
- OuYang, L.; Lin, Z.; He, X.; Sun, J.; Liao, J.; Liao, Y.; Xie, X.; Hu, W.; Zeng, R.; Tao, R.; et al. Conductive Hydrogel Inspires Neutrophil Extracellular Traps to Combat Bacterial Infections in Wounds. ACS Nano 2025, 19, 9868–9884. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Chen, J.; Huang, H.; Liang, F.; Li, S.; Huang, F.; Guo, J. Neutrophil Extracellular Trap Formation Suppressed by Ro 106-9920 Enhances Diabetic Wound Healing by Blocking NLRP3 Inflammasome Activation. FBL 2025, 30, 37393. [Google Scholar] [CrossRef]
- Chu, Z.; Huang, Q.; Ma, K.; Liu, X.; Zhang, W.; Cui, S.; Wei, Q.; Gao, H.; Hu, W.; Wang, Z.; et al. Novel neutrophil extracellular trap-related mechanisms in diabetic wounds inspire a promising treatment strategy with hypoxia-challenged small extracellular vesicles. Bioact. Mater. 2023, 27, 257–270. [Google Scholar] [CrossRef]
- Zhu, M.; Ou, J.; Chen, Y.; Tian, Y.; Song, W.; Hu, X.; Ju, X.; Jiang, S.; Huang, S.; Niu, Z. Programming of macrophage polarization in different stages for accelerating wound healing. Chem. Eng. J. 2024, 491, 152131. [Google Scholar] [CrossRef]
- Chen, C.; Liu, T.; Tang, Y.; Luo, G.; Liang, G.; He, W. Epigenetic regulation of macrophage polarization in wound healing. Burn. Trauma 2023, 11, tkac057. [Google Scholar] [CrossRef] [PubMed]
- Kuninaka, Y.; Ishida, Y.; Ishigami, A.; Nosaka, M.; Matsuki, J.; Yasuda, H.; Kofuna, A.; Kimura, A.; Furukawa, F.; Kondo, T. Macrophage polarity and wound age determination. Sci. Rep. 2022, 12, 20327. [Google Scholar] [CrossRef]
- Wang, H.H.; Korah, M.; Jing, S.L.; Berry, C.E.; Griffin, M.F.; Longaker, M.T.; Januszyk, M. Characterizing Fibroblast Heterogeneity in Diabetic Wounds Through Single-Cell RNA-Sequencing. Biomedicines 2024, 12, 2538. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Olabi, B.; Roberts, K.; Mazin, P.V.; Koplev, S.; Tudor, C.; Rumney, B.; Admane, C.; Jiang, T.; Correa-Gallegos, D.; et al. A single-cell and spatial genomics atlas of human skin fibroblasts reveals shared disease-related fibroblast subtypes across tissues. Nat. Immunol. 2025, 26, 1807–1820. [Google Scholar] [CrossRef]
- Mathioudaki, E.; Rallis, M.; Politopoulos, K.; Alexandratou, E. Photobiomodulation and Wound Healing: Low-Level Laser Therapy at 661 nm in a Scratch Assay Keratinocyte Model. Ann. Biomed. Eng. 2024, 52, 376–385. [Google Scholar] [CrossRef]
- Feng, X.; Feng, W.; Ji, Y.; Jin, T.; Li, J.; Guo, J. Transforming growth factor-β1 negatively regulates SOCS7 via EGR1 during wound healing. Cell Commun. Signal. 2022, 20, 86. [Google Scholar] [CrossRef]
- Du, H.; Li, S.; Lu, J.; Tang, L.; Jiang, X.; He, X.; Liang, J.; Liao, X.; Cui, T.; Huang, Y.; et al. Single-cell RNA-seq and bulk-seq identify RAB17 as a potential regulator of angiogenesis by human dermal microvascular endothelial cells in diabetic foot ulcers. Burn. Trauma 2023, 11, tkad020. [Google Scholar] [CrossRef]
- Farahat, M.; Brosset, S.; Chen, Y.; Aijaz, A.; Rix, G.; Challagundla, B.; Elloso, M.; Hutter, M.F.; Rogers, I.M.; Jeschke, M.G. Human iPSCs-derived mesenchymal stem cells promote skin regeneration and burn wound healing. npj Regen. Med. 2025, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Iwasaki, K.; Peng, Y.; Honda, Y. Mesenchymal Stem Cell Extract Promotes Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 13745. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair. Regen 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Petrie, N.C.; Vranckx, J.J.; Hoeller, D.; Yao, F.; Eriksson, E. Gene delivery of PDGF for wound healing therapy. J. Tissue Viability 2005, 15, 16–21. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Huda, F.; Pant, J.; Ghosh Kar, A.; Banerjee, T.; Shukla, V.K. An appraisal of vascular endothelial growth factor (VEGF): The dynamic molecule of wound healing and its current clinical applications. Growth Factors 2022, 40, 73–88. [Google Scholar] [CrossRef]
- Sim, M.; Ohnuki, H.; Durell, S.; Bulut, H.; Wang, Y.; Dyba, M.; Tarasov, S.G.; Jenkins, L.M.; Tosato, G. Angiopoietin-2 binds to FGFR2, inhibits FGF-FGFR2 signaling, and delays cutaneous wound healing by inhibiting wound angiogenesis. Angiogenesis 2025, 28, 43. [Google Scholar] [CrossRef]
- Brew, E.C.; Mitchell, M.B.; Harken, A.H. Fibroblast growth factors in operative wound healing. J. Am. Coll. Surg. 1995, 180, 499–504. [Google Scholar]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef]
- Hardwicke, J.; Schmaljohann, D.; Boyce, D.; Thomas, D. Epidermal growth factor therapy and wound healing—Past, present and future perspectives. Surgeon 2008, 6, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Chen, B.; Kao, H.K.; Murphy, G.; Orgill, D.P.; Guo, L. Lack of FGF-7 further delays cutaneous wound healing in diabetic mice. Plast. Reconstr. Surg. 2011, 128, 673e–684e. [Google Scholar] [CrossRef] [PubMed]
- Legrand, J.M.; Martino, M.M. Growth Factor and Cytokine Delivery Systems for Wound Healing. Cold Spring Harb. Perspect. Biol. 2022, 14, a041234. [Google Scholar] [CrossRef]
- Niu, Y.; Li, Q.; Ding, Y.; Dong, L.; Wang, C. Engineered delivery strategies for enhanced control of growth factor activities in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 190–208. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, C.; Lei, K.; Xiao, C.; Jiang, X.; Zhang, W.; Wu, L.; Huang, J.; Li, W. Multifunctional MOF-Based Microneedle Patch With Synergistic Chemo-Photodynamic Antibacterial Effect and Sustained Release of Growth Factor for Chronic Wound Healing. Adv. Heal. Mater. 2023, 12, e2300250. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, C.; Li, F.; Li, B.; McCarthy, A.; Zhang, Y.; Chen, S.; Zhang, L. Hierarchically Assembled Nanofiber Scaffolds with Dual Growth Factor Gradients Promote Skin Wound Healing Through Rapid Cell Recruitment. Adv. Sci. 2024, 11, e2309993. [Google Scholar] [CrossRef]
- Du, H.C.; Jiang, L.; Geng, W.X.; Li, J.; Zhang, R.; Dang, J.G.; Shu, M.G.; Li, L.W. Growth Factor-Reinforced ECM Fabricated from Chemically Hypoxic MSC Sheet with Improved In Vivo Wound Repair Activity. Biomed Res. Int. 2017, 2017, 2578017. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2019, 7, 469. [Google Scholar] [CrossRef]
- Sun, J.; Wu, J.; Jin, H.; Ying, T.; Jin, W.; Fan, M.; Zhou, J.; Chen, H.; Jin, L.; Zhou, J. Structure-guided design, generation, and biofunction of PEGylated fibroblast growth factor 2 variants for wound healing. Nanoscale 2020, 12, 18200–18213. [Google Scholar] [CrossRef] [PubMed]
- Sarangthem, V.; Sharma, H.; Goel, R.; Ghose, S.; Park, R.W.; Mohanty, S.; Chaudhuri, T.K.; Dinda, A.K.; Singh, T.D. Application of elastin-like polypeptide (ELP) containing extra-cellular matrix (ECM) binding ligands in regenerative medicine. Int. J. Biol. Macromol. 2022, 207, 443–453. [Google Scholar] [CrossRef]
- Boeringer, T.; Gould, L.J.; Koria, P. Protease-Resistant Growth Factor Formulations for the Healing of Chronic Wounds. Adv. Wound Care 2020, 9, 612–622. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Zelisko, N.; Lesyk, R.; Stoika, R. Structure, unique biological properties, and mechanisms of action of transforming growth factor β. Bioorganic Chem. 2024, 150, 107611. [Google Scholar] [CrossRef]
- Ramirez, H.; Patel, S.B.; Pastar, I. The Role of TGFβ Signaling in Wound Epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Hasegawa, M.; Higashi, K.; Matsushita, T.; Hamaguchi, Y.; Saito, K.; Fujimoto, M.; Takehara, K. Dermokine inhibits ELR(+)CXC chemokine expression and delays early skin wound healing. J. Dermatol. Sci. 2013, 70, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, K.L.; Boink, M.A.; Sampat-Sardjoepersad, S.C.; Waaijman, T.; Scheper, R.J.; Gibbs, S. Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J. Investig. Dermatol. 2012, 132, 216–225. [Google Scholar] [CrossRef]
- Devalaraja, R.M.; Nanney, L.B.; Du, J.; Qian, Q.; Yu, Y.; Devalaraja, M.N.; Richmond, A. Delayed wound healing in CXCR2 knockout mice. J. Investig. Dermatol. 2000, 115, 234–244. [Google Scholar] [CrossRef]
- Amuso, V.M.; Haas, M.R.; Cooper, P.O.; Chatterjee, R.; Hafiz, S.; Salameh, S.; Gohel, C.; Mazumder, M.F.; Josephson, V.; Kleb, S.S.; et al. Fibroblast-Mediated Macrophage Recruitment Supports Acute Wound Healing. J. Investig. Dermatol. 2025, 145, 1781–1797.e8. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.; Wang, Z.; Li, C.; Wang, D.; Li, C. CCL3 Promotes Cutaneous Wound Healing Through Recruiting Macrophages in Mice. Cell Transplant. 2024, 33, 9636897241264912. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, L.; Du, C.; Wang, J.; Zhang, C.; Hu, L.; Wang, S. Dental pulp stem cells accelerate wound healing through CCL2-induced M2 macrophages polarization. iScience 2023, 26, 108043. [Google Scholar] [CrossRef] [PubMed]
- Whelan, D.S.; Caplice, N.M.; Clover, A.J.P. Mesenchymal stromal cell derived CCL2 is required for accelerated wound healing. Sci. Rep. 2020, 10, 2642. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. TLR4, rather than TLR2, regulates wound healing through TGF-β and CCL5 expression. J. Dermatol. Sci. 2014, 73, 117–124. [Google Scholar] [CrossRef]
- Xie, Y.; Ni, X.; Wan, X.; Xu, N.; Chen, L.; Lin, C.; Zheng, X.; Cai, B.; Lin, Q.; Ke, R.; et al. KLF5 enhances CXCL12 transcription in adipose-derived stem cells to promote endothelial progenitor cells neovascularization and accelerate diabetic wound healing. Cell. Mol. Biol. Lett. 2025, 30, 24. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.C.; Whaley, D.; Kulasekeran, P.; Hancock, W.W.; Lu, B.; Bodnar, R.; Newsome, J.; Hebda, P.A.; Wells, A. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am. J. Pathol. 2007, 171, 484–495. [Google Scholar] [CrossRef]
- Huen, A.C.; Wells, A. The Beginning of the End: CXCR3 Signaling in Late-Stage Wound Healing. Adv. Wound Care 2012, 1, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.C.; Krishna, P.; Whaley, D.; Bodnar, R.; Turner, T.; Wells, A. Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am. J. Pathol. 2010, 176, 1743–1755. [Google Scholar] [CrossRef] [PubMed]
- Shallo, H.; Plackett, T.P.; Heinrich, S.A.; Kovacs, E.J. Monocyte chemoattractant protein-1 (MCP-1) and macrophage infiltration into the skin after burn injury in aged mice. Burns 2003, 29, 641–647. [Google Scholar] [CrossRef]
- Dipietro, L.A.; Reintjes, M.G.; Low, Q.E.; Levi, B.; Gamelli, R.L. Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound Repair Regen. 2001, 9, 28–33. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Hangoc, G.; Cooper, S.; Campbell, T.; Ito, S.; Mantel, C. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann. N. Y Acad. Sci. 2007, 1106, 1–19. [Google Scholar] [CrossRef]
- Chen, H.; Li, G.; Liu, Y.; Ji, S.; Li, Y.; Xiang, J.; Zhou, L.; Gao, H.; Zhang, W.; Sun, X.; et al. Pleiotropic Roles of CXCR4 in Wound Repair and Regeneration. Front. Immunol. 2021, 12, 668758. [Google Scholar] [CrossRef]
- Escuin-Ordinas, H.; Li, S.; Xie, M.W.; Sun, L.; Hugo, W.; Huang, R.R.; Jiao, J.; de-Faria, F.M.; Realegeno, S.; Krystofinski, P.; et al. Cutaneous wound healing through paradoxical MAPK activation by BRAF inhibitors. Nat. Commun. 2016, 7, 12348. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.S.; Jung, S.J.; Kim, D.; Park, H.J.; Cho, D. ERK activating peptide, AES16-2M promotes wound healing through accelerating migration of keratinocytes. Sci. Rep. 2018, 8, 14398. [Google Scholar] [CrossRef]
- Kim, J.; Shin, Y.K.; Kim, K.Y. Promotion of Keratinocyte Proliferation by Tracheloside through ERK1/2 Stimulation. Evid. Based Complement. Altern. Med. 2018, 2018, 4580627. [Google Scholar] [CrossRef]
- Yang, H.L.; Tsai, Y.C.; Korivi, M.; Chang, C.T.; Hseu, Y.C. Lucidone Promotes the Cutaneous Wound Healing Process via Activation of the PI(3)K/AKT, Wnt/β-catenin and NF-κB Signaling Pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Squarize, C.H.; Castilho, R.M.; Bugge, T.H.; Gutkind, J.S. Accelerated Wound Healing by mTOR Activation in Genetically Defined Mouse Models. PLoS ONE 2010, 5, e10643. [Google Scholar] [CrossRef]
- Huang, C.; Sheng, S.; Li, R.; Sun, X.; Liu, J.; Huang, G. Lactate promotes resistance to glucose starvation via upregulation of Bcl-2 mediated by mTOR activation. Oncol. Rep. 2015, 33, 875–884. [Google Scholar] [CrossRef]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2017, 161, 1–13. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Li, G.; Xu, C.; Yang, L.; Zhang, J.; Wu, Y.; Liu, Y.; Liu, Z.; Wang, M.; et al. N-acetyltransferase 10 promotes cutaneous wound repair via the NF-κB-IL-6 axis. Cell Death Discov. 2023, 9, 324. [Google Scholar] [CrossRef]
- Huang, P.; Yan, R.; Zhang, X.; Wang, L.; Ke, X.; Qu, Y. Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol. Ther. 2019, 196, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bastakoty, D.; Young, P.P. Wnt/β-catenin pathway in tissue injury: Roles in pathology and therapeutic opportunities for regeneration. FASEB J. 2016, 30, 3271–3284. [Google Scholar] [CrossRef]
- Dorey, K.; Amaya, E. FGF signalling: Diverse roles during early vertebrate embryogenesis. Development 2010, 137, 3731–3742. [Google Scholar] [CrossRef]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P.; et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res. Ther. 2015, 6, 120. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, M.; Zheng, Y.; Wang, C.; Ding, X.; Wu, H.; Wu, Y.; Zhang, W.; Song, X. Preliminary Study on Human Adipose Stem Cells Promoting Skin Wound Healing through Notch Signaling Pathway. Curr. Stem Cell Res. Ther. 2023, 18, 699–711. [Google Scholar] [CrossRef]
- Abbas, O.L.; Özatik, O.; Terzi, Y.K.; Özatik, F.Y.; Nar, R.; Turna, G. The Notch Signaling System Is Involved in the Regulation of Reparative Angiogenesis in the Zone of Stasis. J. Burn. Care Res. 2017, 38, e923–e933. [Google Scholar] [CrossRef] [PubMed]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef] [PubMed]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Chen, K.; Xu, M.; Lu, F.; He, Y. Development of Matrix Metalloproteinases-Mediated Extracellular Matrix Remodeling in Regenerative Medicine: A Mini Review. Tissue Eng. Regen. Med. 2023, 20, 661–670. [Google Scholar] [CrossRef]
- Durr, H.A.; Abri, S.; Salinas, S.D.; Adkins-Travis, K.; Amini, R.; Shriver, L.P.; Leipzig, N.D. Extracellular matrix repair and organization of chronic infected diabetic wounds treated with methacrylated chitosan-based hydrogels. Acta Biomater. 2025, 199, 166–177. [Google Scholar] [CrossRef]
- de Castro Bras, L.E.; Frangogiannis, N.G. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.Y.; Pan, Z.W.; Li, Q.Q.; Sun, L.H.; Li, X.; Gong, M.Y.; Yang, X.W.; Wang, Y.Y.; Li, H.D.; et al. GDF11 promotes wound healing in diabetic mice via stimulating HIF-1α-VEGF/SDF-1α-mediated endothelial progenitor cell mobilization and neovascularization. Acta Pharmacol. Sin. 2023, 44, 999–1013. [Google Scholar] [CrossRef]
- Li, G.; Ko, C.N.; Li, D.; Yang, C.; Wang, W.; Yang, G.J.; Di Primo, C.; Wong, V.K.W.; Xiang, Y.; Lin, L.; et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat. Commun. 2021, 12, 3363. [Google Scholar] [CrossRef]
- Ruthenborg, R.J.; Ban, J.J.; Wazir, A.; Takeda, N.; Kim, J.W. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol. Cells 2014, 37, 637–643. [Google Scholar] [CrossRef]
- Sun, J.X.; Xu, X.H.; Jin, L. Effects of Metabolism on Macrophage Polarization Under Different Disease Backgrounds. Front. Immunol. 2022, 13, 880286. [Google Scholar] [CrossRef]
- Li, Q.; Song, H.; Li, S.; Hu, P.; Zhang, C.; Zhang, J.; Feng, Z.; Kong, D.; Wang, W.; Huang, P. Macrophage metabolism reprogramming EGCG-Cu coordination capsules delivered in polyzwitterionic hydrogel for burn wound healing and regeneration. Bioact. Mater. 2023, 29, 251–264. [Google Scholar] [CrossRef]
- Fabre, P.; Molina, T.; Larose, J.; Greffard, K.; Généreux-Gamache, G.; Deprez, A.; Mokhtari, I.; Pellerito, O.; Duchesne, E.; Dort, J.; et al. Bioactive lipid mediator class switching regulates myogenic cell progression and muscle regeneration. Nat. Commun. 2025, 16, 5578. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Tang, Y.; Spite, M. Proresolving lipid mediators and diabetic wound healing. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic orchestration of the wound healing response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef]
- Werdin, F.; Tenenhaus, M.; Rennekampff, H.O. Chronic wound care. Lancet 2008, 372, 1860–1862. [Google Scholar] [CrossRef]
- Pastar, I.; Balukoff, N.C.; Marjanovic, J.; Chen, V.Y.; Stone, R.C.; Tomic-Canic, M. Molecular Pathophysiology of Chronic Wounds: Current State and Future Directions. Cold Spring Harb. Perspect. Biol. 2023, 15, a041243. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117, 35s–41s. [Google Scholar] [CrossRef]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- Kolahreez, D.; Ghasemi-Mobarakeh, L.; Liebner, F.; Alihosseini, F.; Quartinello, F.; Guebitz, G.M.; Ribitsch, D. Approaches to Control and Monitor Protease Levels in Chronic Wounds. Adv. Ther. 2024, 7, 2300396. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Zuo, A.; Zhang, J.; Wen, W.; Jiang, W.; Chen, H.; Liang, D.; Sun, J.; Wang, M. HIF-1α/JMJD1A signaling regulates inflammation and oxidative stress following hyperglycemia and hypoxia-induced vascular cell injury. Cell. Mol. Biol. Lett. 2021, 26, 40. [Google Scholar] [CrossRef]
- Lal, B.K.; Saito, S.; Pappas, P.J.; Padberg, F.T., Jr.; Cerveira, J.J.; Hobson, R.W., 2nd; Duran, W.N. Altered proliferative responses of dermal fibroblasts to TGF-beta1 may contribute to chronic venous stasis ulcer. J. Vasc. Surg. 2003, 37, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Dawi, J.; Tumanyan, K.; Tomas, K.; Misakyan, Y.; Gargaloyan, A.; Gonzalez, E.; Hammi, M.; Tomas, S.; Venketaraman, V. Diabetic Foot Ulcers: Pathophysiology, Immune Dysregulation, and Emerging Therapeutic Strategies. Biomedicines 2025, 13, 1076. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Mi, B.; Xiong, Y.; Fu, Z.; Zhou, W.; Liu, W.; Liu, G.; Dai, G. Angiogenesis during diabetic wound repair: From mechanism to therapy opportunity. Burn. Trauma 2025, 13, tkae052. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, M.; Wang, Q.; Cao, X.; Chen, P.; Gong, Z. Single-cell RNA-seq in diabetic foot ulcer wound healing. Wound Repair. Regen. 2024, 32, 880–889. [Google Scholar] [CrossRef]
- Jiang, N.; Xu, C.; Xu, Y.; Zhuo, Y.; Chen, P.; Deng, S.; Zhao, Z.; Long, Y.; Bai, X.; Wang, Q.; et al. Comprehensive transcriptomic analysis of immune-related genes in diabetic foot ulcers: New insights into mechanisms and therapeutic targets. Int. Immunopharmacol. 2024, 139, 112638. [Google Scholar] [CrossRef]
- Bosanquet, D.C.; Sanders, A.J.; Ruge, F.; Lane, J.; Morris, C.A.; Jiang, W.G.; Harding, K.G. Development and validation of a gene expression test to identify hard-to-heal chronic venous leg ulcers. Br. J. Surg. 2019, 106, 1035–1042. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef]
- Su, Y.; Yrastorza, J.T.; Matis, M.; Cusick, J.; Zhao, S.; Wang, G.; Xie, J. Biofilms: Formation, Research Models, Potential Targets, and Methods for Prevention and Treatment. Adv. Sci. 2022, 9, e2203291. [Google Scholar] [CrossRef]
- Hong, Y.K.; Chang, Y.H.; Lin, Y.C.; Chen, B.; Guevara, B.E.K.; Hsu, C.K. Inflammation in Wound Healing and Pathological Scarring. Adv. Wound Care 2023, 12, 288–300. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-beta signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zhao, W.Y.; Cao, Y.; Liu, Y.Q.; Sun, Q.; Shi, P.; Cai, J.Q.; Shen, X.Z.; Tan, W.Q. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front. Immunol. 2020, 11, 603187. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Li, S.; Yu, Z.; Liu, B.; Song, B.; Su, Y. The clinical dynamic changes of macrophage phenotype and function in different stages of human wound healing and hypertrophic scar formation. Int. Wound J. 2019, 16, 360–369. [Google Scholar] [CrossRef]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, S.; Yang, C.; Wang, Y.; Shi, B.; Zheng, Q.; Zeng, N.; Huang, H. Mechanotransduction in skin wound healing and scar formation: Potential therapeutic targets for controlling hypertrophic scarring. Front. Immunol. 2022, 13, 1028410. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Q.; Zhang, Y.; Geng, L.; Wang, W.; Zhang, H.; He, X.; Li, Q. Multimodal roles of transient receptor potential channel activation in inducing pathological tissue scarification. Front. Immunol. 2023, 14, 1237992. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair. Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Seth, I.; Lim, B.; Cevik, J.; Gracias, D.; Chua, M.; Kenney, P.S.; Rozen, W.M.; Cuomo, R. Impact of nutrition on skin wound healing and aesthetic outcomes: A comprehensive narrative review. JPRAS Open 2024, 39, 291–302. [Google Scholar] [CrossRef]
- Dierckx, S.; Patrizi, M.; Merino, M.; Gonzalez, S.; Mullor, J.L.; Nergiz-Unal, R. Collagen peptides affect collagen synthesis and the expression of collagen, elastin, and versican genes in cultured human dermal fibroblasts. Front. Med. 2024, 11, 1397517. [Google Scholar] [CrossRef]
- Giacolone, J.; Kulkarni, D.; Pace, C.; Matheson, B.; Kanagy, N.; Clark, R.M. Impaired Diabetic Myocutaneous Wound Revascularization Is Associated With Reduced Transdermal H2S. J. Surg. Res. 2025, 313, 457–464. [Google Scholar] [CrossRef]
- Wang, Y.; Vizely, K.; Li, C.Y.; Shen, K.; Shakeri, A.; Khosravi, R.; Smith, J.R.; Alteza, E.A.I.I.; Zhao, Y.; Radisic, M. Biomaterials for immunomodulation in wound healing. Regen. Biomater. 2024, 11, rbae032. [Google Scholar] [CrossRef]
- Zhou, G.; Tao, M.; Zhu, J.; Li, S.; Zhang, L. Hypoxia preconditioned plasma increases the expression of growth factors to accelerate diabetic wound healing by promoting angiogenesis. Sci. Rep. 2025, 15, 23154. [Google Scholar] [CrossRef]
- Boleti, A.P.A.; Jacobowski, A.C.; Frihling, B.E.F.; Cruz, M.V.; Santos, K.; Migliolo, L.; de Andrade, L.R.M.; Macedo, M.L.R. Wound Healing: Molecular Mechanisms, Antimicrobial Peptides, and Emerging Technologies in Regenerative Medicine. Pharmaceuticals 2025, 18, 1525. [Google Scholar] [CrossRef]
- Razdan, K.; Garcia-Lara, J.; Sinha, V.R.; Singh, K.K. Pharmaceutical strategies for the treatment of bacterial biofilms in chronic wounds. Drug Discov. Today 2022, 27, 2137–2150. [Google Scholar] [CrossRef]
- Zhu, D.; Wei, W.; Zhang, J.; Zhao, B.; Li, Q.; Jin, P. Mechanism of damage of HIF-1 signaling in chronic diabetic foot ulcers and its related therapeutic perspectives. Heliyon 2024, 10, e24656. [Google Scholar] [CrossRef]
- Kazemeini, S.; Nadeem-Tariq, A.; Hajian, P.; Anil, B.; Easterly, J.; Sraa, K.; Pokharel, S.; Metellus, R.; Kazemeini, M. Hypertrophic and Keloid Scar Management: Advances in Diagnosis, Perioperative Care, and Anesthetic Modulation. Cureus 2025, 17, e88810. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ranallo, R.; Rios, C.; Grice, E.A.; Moon, K.; Gallo, R.L. Crosstalk between skin microbiota and immune system in health and disease. Nat. Immunol. 2023, 24, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.; Gallo, R.L. The Central Roles of Keratinocytes in Coordinating Skin Immunity. J. Investig. Dermatol. 2024, 144, 2377–2398. [Google Scholar] [CrossRef] [PubMed]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.M. Wound healing and microbiome, an unexpected relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37 Suppl 3, 7–15. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Bădăluță, V.A.; Curuțiu, C.; Dițu, L.M.; Holban, A.M.; Lazăr, V. Probiotics in Wound Healing. Int. J. Mol. Sci. 2024, 25, 5723. [Google Scholar] [CrossRef]
- Kadwaikar, M.; Shinde, V. Healing with microbial Allies: Exploration of probiotics in wound management. Microb. Pathog. 2025, 207, 107906. [Google Scholar] [CrossRef]
- Ling, L.; Ren, M.; Yang, C.; Lao, G.; Chen, L.; Luo, H.; Feng, Z.; Yan, L. Role of site-specific DNA demethylation in TNFα-induced MMP9 expression in keratinocytes. J. Mol. Endocrinol. 2013, 50, 279–290. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Wang, C.; Liu, D.; Lao, G.; Liang, Y.; Sun, K.; Luo, H.; Tan, Q.; Ren, M.; et al. AGE-induced keratinocyte MMP-9 expression is linked to TET2-mediated CpG demethylation. Wound Repair. Regen. 2016, 24, 489–500. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, S.; Shu, B.; Chen, L.; Yang, R.; Xu, Y.; Xie, J.; Liu, X.; Qi, S. Transient High Glucose Causes Persistent Vascular Dysfunction and Delayed Wound Healing by the DNMT1-Mediated Ang-1/NF-κB Pathway. J. Investig. Dermatol. 2021, 141, 1573–1584. [Google Scholar] [CrossRef]
- Li, D.; Kular, L.; Vij, M.; Herter, E.K.; Li, X.; Wang, A.; Chu, T.; Toma, M.A.; Zhang, L.; Liapi, E.; et al. Human skin long noncoding RNA WAKMAR1 regulates wound healing by enhancing keratinocyte migration. Proc. Natl. Acad. Sci. USA 2019, 116, 9443–9452. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Vicuña, Y.; Ruiz-Ojeda, D.; González-Ramírez, J.; Flores-Balderas, X.; Springall, R.; Sánchez-Muñoz, F.; Guzmán-Martín, C.A. LncRNA MALAT1 in Keratinocyte function: A review of recent advances. Noncoding RNA Res. 2024, 9, 594–601. [Google Scholar] [CrossRef]
- Nemenoff, R. Wound healing: A role for HDACs in inhibition of fibroblast proliferation through repression of PDGF receptor-α. Focus on "Repression of PDGF-R-α after cellular injury involves TNF-α, formation of a c-Fos-YY1 complex, and negative regulation by HDAC". Am. J. Physiol. Cell Physiol. 2012, 302, C1588–C1589. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, H.G.; Zhang, B.; Jiang, L.L.; Nie, K.Y.; Deng, C.L.; Liu, Y. Potential roles of histone deacetylases in diabetic wound healing. World J. Diabetes 2025, 16, 108346. [Google Scholar] [CrossRef]
- Guo, J.R.; Yin, L.; Chen, Y.Q.; Jin, X.J.; Zhou, X.; Zhu, N.N.; Liu, X.Q.; Wei, H.W.; Duan, L.S. Autologous blood transfusion augments impaired wound healing in diabetic mice by enhancing lncRNA H19 expression via the HIF-1α signaling pathway. Cell Commun. Signal. 2018, 16, 84. [Google Scholar] [CrossRef]
- Kimball, A.S.; Joshi, A.; Carson, W.F.t.; Boniakowski, A.E.; Schaller, M.; Allen, R.; Bermick, J.; Davis, F.M.; Henke, P.K.; Burant, C.F.; et al. The Histone Methyltransferase MLL1 Directs Macrophage-Mediated Inflammation in Wound Healing and Is Altered in a Murine Model of Obesity and Type 2 Diabetes. Diabetes 2017, 66, 2459–2471. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Y.; Chen, Y.; Sun, X.; Zhang, Z. Epigenetic regulatory mechanism of macrophage polarization in diabetic wound healing (Review). Mol. Med. Rep. 2025, 31, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Arfman, T.; Wichapong, K.; Reutelingsperger, C.P.M.; Voorberg, J.; Nicolaes, G.A.F. PAD4 takes charge during neutrophil activation: Impact of PAD4 mediated NET formation on immune-mediated disease. J. Thromb. Haemost. 2021, 19, 1607–1617. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, X.; Xiao, L.; Wang, L.; Qiang, S. The Role of microRNA in the Inflammatory Response of Wound Healing. Front. Immunol. 2022, 13, 852419. [Google Scholar] [CrossRef] [PubMed]
- Ross, K. MiR equal than others: MicroRNA enhancement for cutaneous wound healing. J. Cell. Physiol. 2021, 236, 8050–8059. [Google Scholar] [CrossRef]
- Cheng, J.; Qian, W.; Chen, F.; Liu, X.; Fu, M.; Cao, W.; Zhou, Y. Function of epigenetic modifications in wound healing and potential therapies (Review). Int. J. Mol. Med. 2025, 56, 190. [Google Scholar] [CrossRef]
- Arechederra, M.; Recalde, M.; Gárate-Rascón, M.; Fernández-Barrena, M.G.; Ávila, M.A.; Berasain, C. Epigenetic Biomarkers for the Diagnosis and Treatment of Liver Disease. Cancers 2021, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.C.; Tong, W.Z.; Rui, W.; Feng, Z.; Shuai, H.; Zhe, W. Single-cell sequencing technology in skin wound healing. Burn. Trauma 2024, 12, tkae043. [Google Scholar] [CrossRef]
- Foster, D.S.; Januszyk, M.; Yost, K.E.; Chinta, M.S.; Gulati, G.S.; Nguyen, A.T.; Burcham, A.R.; Salhotra, A.; Ransom, R.C.; Henn, D.; et al. Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc. Natl. Acad. Sci. USA 2021, 118, e2110025118. [Google Scholar] [CrossRef]
- Chen, P.; Tang, S.; Li, M.; Wang, D.; Chen, C.; Qiu, Y.; Fang, Z.; Zhang, H.; Gao, H.; Weng, H.; et al. Single-Cell and Spatial Transcriptomics Decodes Wharton’s Jelly-Derived Mesenchymal Stem Cells Heterogeneity and a Subpopulation with Wound Repair Signatures. Adv. Sci. 2023, 10, e2204786. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, C.; Liu, L.; Wang, C.; Hu, C.; Rung, S.; Man, Y.; Qu, Y. Tracing immune cells around biomaterials with spatial anchors during large-scale wound regeneration. Nat. Commun. 2023, 14, 5995. [Google Scholar] [CrossRef]
- Luo, H.; Yu, X.; Sun, T.; Sun, Z.; Zhao, T.; Li, C.; Angelova Volponi, A.; Flores-Borja, F.; Sun, H.; An, Z. Macrophage-derived IL-1beta directs fibroblast progenitor cell fate via metabolic reprogramming in wound healing. Commun. Biol. 2025, 8, 1291. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Castro-Alpízar, J.; Lopretti-Correa, M.; Vega-Baudrit, J. Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing. Int. J. Mol. Sci. 2021, 22, 1408. [Google Scholar] [CrossRef]
- Smiell, J.M.; Wieman, T.J.; Steed, D.L.; Perry, B.H.; Sampson, A.R.; Schwab, B.H. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: A combined analysis of four randomized studies. Wound Repair Regen. 1999, 7, 335–346. [Google Scholar] [CrossRef]
- Thanigaimani, S.; Jin, H.; Ahmad, U.; Anbalagan, R.; Golledge, J. Comparative efficacy of growth factor therapy in healing diabetes-related foot ulcers: A network meta-analysis of randomized controlled trials. Diabetes Metab. Res. Rev. 2023, 39, e3670. [Google Scholar] [CrossRef]
- Tejedor, S.; Wågberg, M.; Correia, C.; Åvall, K.; Hölttä, M.; Hultin, L.; Lerche, M.; Davies, N.; Bergenhem, N.; Snijder, A.; et al. The Combination of Vascular Endothelial Growth Factor A (VEGF-A) and Fibroblast Growth Factor 1 (FGF1) Modified mRNA Improves Wound Healing in Diabetic Mice: An Ex Vivo and In Vivo Investigation. Cells 2024, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, J.; Jurczuk, V.; Tose, L.V.; Cintron Diaz, Y.; Fernandez Lima, F.; Abdo Abujamra, B.; Danker, S.; Jabori, S.; Singh, D.; Burgess, J.L.; et al. Scaffolds with spatiotemporally controlled growth factor delivery and cyclodextrin-enabled antagonism of growth factor receptor sequestration promote cutaneous wound healing. NPJ Regen. Med. 2025, 10, 42. [Google Scholar] [CrossRef]
- Bian, D.; Wu, Y.; Song, G. Basic fibroblast growth factor combined with extracellular matrix-inspired mimetic systems for effective skin regeneration and wound healing. Mater. Today Commun. 2023, 35, 105876. [Google Scholar] [CrossRef]
- Nirenjen, S.; Narayanan, J.; Tamilanban, T.; Subramaniyan, V.; Chitra, V.; Fuloria, N.K.; Wong, L.S.; Ramachawolran, G.; Sekar, M.; Gupta, G.; et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 2023, 14, 1216321. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.-H.; Chang, L.; Ho, C.-Y.; Tsai, C.-H.; Liu, Y.-C.; Huang, W.-Y.; Wang, Y.-N.; Wang, W.-H.; Wang, T.-W. Immunomodulatory hydrogel orchestrates pro-regenerative response of macrophages and angiogenesis for chronic wound healing. Biomaterials 2025, 314, 122848. [Google Scholar] [CrossRef]
- Cao, Y.; Harvey, B.P.; Jin, L.; Westmoreland, S.; Wang, J.; Puri, M.; Yang, Y.; Robb, H.M.; Tanriverdi, S.; Hu, C.; et al. Therapeutic TNF Inhibitors Exhibit Differential Levels of Efficacy in Accelerating Cutaneous Wound Healing. JID Innov. 2024, 4, 100250. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, B.; Rao, F.; Hu, H.; Tian, F.; Lu, Y.; Zhang, L.; Xia, Y.; Xue, J. Spatiotemporally controlled delivery of biological effectors from nanofiber scaffolds accelerates skin wound healing in porcine models. Sci. Adv. 2025, 11, eadz5302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Liu, B.; Shao, C.; Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- Markandeywar, T.S.; Singh, D.; Singh, G.; Kurmi, B.D.; Narang, R.K. Endothelial Progenitor Cell (EPC) is a Prime Target in Diabetic Wound Healing: Mechanisms and Target Therapies. Curr. Mol. Med. 2024, 24, 1073–1076. [Google Scholar] [CrossRef]
- Henn, D.; Zhao, D.; Sivaraj, D.; Trotsyuk, A.; Bonham, C.A.; Fischer, K.S.; Kehl, T.; Fehlmann, T.; Greco, A.H.; Kussie, H.C.; et al. Cas9-mediated knockout of Ndrg2 enhances the regenerative potential of dendritic cells for wound healing. Nat. Commun. 2023, 14, 4729. [Google Scholar] [CrossRef]
- Farag, V.E.; Devey, E.A.; Leong, K.W. The Interface of Gene Editing with Regenerative Medicine. Engineering 2025, 46, 73–100. [Google Scholar] [CrossRef]
- Shaabani, E.; Sharifiaghdam, M.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Gene therapy to enhance angiogenesis in chronic wounds. Mol. Ther. Nucleic Acids 2022, 29, 871–899. [Google Scholar] [CrossRef]
- Palomeque Chavez, J.C.; McGrath, M.; O’Connor, C.; Dervan, A.; Dixon, J.E.; Kearney, C.J.; Browne, S.; O’Brien, F.J. Development of a VEGF-activated scaffold with enhanced angiogenic and neurogenic properties for chronic wound healing applications. Biomater. Sci. 2025, 13, 1993–2011. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Jin, Y.; Chien, P.N.; Heo, C.Y. Acellular Extracellular Matrix Scaffolds in Regenerative Medicine: Advances in Decellularization and Clinical Applications. J. Funct. Biomater. 2025, 16, 383. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Shan, M.; Hao, Z.; Zhang, X.; Meng, L.; Zhai, Z.; Zhang, L.; Liu, X.; Wang, X. Convergence of 3D Bioprinting and Nanotechnology in Tissue Engineering Scaffolds. Biomimetics 2023, 8, 94. [Google Scholar] [CrossRef]
- Karunakar, K.K.; Cheriyan, B.V.; Anandakumar, R.; Murugathirumal, A.; Senthilkumar, A.; Nandhini, J.; Kataria, K.; Yabase, L. Stimuli-responsive smart materials: Bridging the gap between biotechnology and regenerative medicine. Bioprinting 2025, 48, e00415. [Google Scholar] [CrossRef]
- Ding, K.; Liao, M.; Wang, Y.; Lu, J.R. Advances in Composite Stimuli-Responsive Hydrogels for Wound Healing: Mechanisms and Applications. Gels 2025, 11, 420. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Murakami, T.; Shigeki, S. Pharmacotherapy for Keloids and Hypertrophic Scars. Int. J. Mol. Sci. 2024, 25, 4674. [Google Scholar] [CrossRef]
- Fang, Q.Q.; Wang, X.F.; Zhao, W.Y.; Ding, S.L.; Shi, B.H.; Xia, Y.; Yang, H.; Wu, L.H.; Li, C.Y.; Tan, W.Q. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical TGF-beta1 pathways. Sci. Rep. 2018, 8, 3332. [Google Scholar] [CrossRef]
- Monika, P.; Waiker, P.V.; Chandraprabha, M.N.; Rangarajan, A.; Murthy, K.N.C. Myofibroblast progeny in wound biology and wound healing studies. Wound Repair Regen. 2021, 29, 531–547. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, D.; Ho, C.; Yu, L.; Zheng, D.; O’Reilly, S.; Gao, Y.; Li, Q.; Zhang, Y. Epigenetics as a versatile regulator of fibrosis. J. Transl. Med. 2023, 21, 164. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, M.; Su, J.; Cai, B.; Li, J.; Zhang, K. New insights into balancing wound healing and scarless skin repair. J. Tissue Eng. 2023, 14, 20417314231185848. [Google Scholar] [CrossRef]
- Bai, H.; Arnedo, A.S.; Liu, Y.; Segura, T.; Muddiman, D. Unraveling the molecular dynamics of wound healing: Integrating spatially resolved lipidomics and temporally resolved proteomics. Anal. Bioanal. Chem. 2025, 417, 3299–3314. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, E.; Watt, F.M. Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol. 2018, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, M.A.; Lev-Tov, H.A.; Tomic-Canic, M.; Lee, W.D.; Williams, R.; Strasfeld, D.; Kirsner, R.S.; Herman, I.M. Advanced Wound Diagnostics: Toward Transforming Wound Care into Precision Medicine. Adv. Wound Care 2022, 11, 330–359. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.; Lombardi, G.; Gianfrancesco, S.; Piccolo, R.; Chirico, G.; Pellegrino, M.; Santella, L.; Tecce, N.; Volpicelli, A.; Sollo, E.; et al. Triticum vulgare Extract and Polyhexanide (Fitostimoline(®) Hydrogel/Fitostimoline(®) Plus Gauze) versus Saline Gauze Dressing in Patients with Diabetic Foot Ulcers: Results of a Randomized Controlled Trial. J. Clin. Med. 2023, 12, 3596. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, T.; Zhao, Q.; Ma, J.; Jiang, J.; Shi, H. Adipose Tissue-Derived Extracellular Vesicles: A Promising Biomarker and Therapeutic Strategy for Metabolic Disorders. Stem Cells Int. 2023, 2023, 9517826. [Google Scholar] [CrossRef]
- Perin, E.; Loveland, L.; Caporusso, J.; Dove, C.; Motley, T.; Sigal, F.; Vartivarian, M.; Oliva, F.; Armstrong, D.G. Gene therapy for diabetic foot ulcers: Interim analysis of a randomised, placebo-controlled phase 3 study of VM202 (ENGENSIS), a plasmid DNA expressing two isoforms of human hepatocyte growth factor. Int. Wound J. 2023, 20, 3531–3539. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Markiewicz, E.; Idowu, O.C. Aging, senescence, and cutaneous wound healing-a complex relationship. Front. Immunol. 2024, 15, 1429716. [Google Scholar] [CrossRef]
- Theocharidis, G.; Thomas, B.E.; Sarkar, D.; Mumme, H.L.; Pilcher, W.J.R.; Dwivedi, B.; Sandoval-Schaefer, T.; Sîrbulescu, R.F.; Kafanas, A.; Mezghani, I.; et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nat. Commun. 2022, 13, 181. [Google Scholar] [CrossRef]
- Zielińska, M.; Pawłowska, A.; Orzeł, A.; Sulej, L.; Muzyka-Placzyńska, K.; Baran, A.; Filipecka-Tyczka, D.; Pawłowska, P.; Nowińska, A.; Bogusławska, J.; et al. Wound Microbiota and Its Impact on Wound Healing. Int. J. Mol. Sci. 2023, 24, 17318. [Google Scholar] [CrossRef]
- Gan, Y.; Zhang, J.; Qi, F.; Hu, Z.; Sweren, E.; Reddy, S.K.; Chen, L.; Feng, X.; Grice, E.A.; Garza, L.A.; et al. Commensal microbe regulation of skin cells in disease. Cell Host Microbe 2024, 32, 1264–1279. [Google Scholar] [CrossRef]
- Molla, G.; Bitew, M. Revolutionizing Personalized Medicine: Synergy with Multi-Omics Data Generation, Main Hurdles, and Future Perspectives. Biomedicines 2024, 12, 2750. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef]
- Reifs Jiménez, D.; Casanova-Lozano, L.; Grau-Carrión, S.; Reig-Bolaño, R. Artificial Intelligence Methods for Diagnostic and Decision-Making Assistance in Chronic Wounds: A Systematic Review. J. Med. Syst. 2025, 49, 29. [Google Scholar] [CrossRef] [PubMed]




| Intervention/Strategy | Phase | Target Condition | Study Design | Outcome Measures | Clinical Trial Numbers | Status/Year | Reference |
|---|---|---|---|---|---|---|---|
| Botanical hydrogel (Lavior®)—a natural extract-based hydrogel for DFU | Phase 2 | Diabetic foot ulcers | Randomized non-inferiority trial vs. standard hydrogel | Primary: Ulcer healing rate. Secondary: time to closure, wound size reduction | NCT05607979 | Completed (2023) | - |
| Triticum vulgare (Fitostimoline®) hydrogel—polyhexanide and wheat extract for DFU | Phase 4 | Diabetic foot ulcers | Randomized controlled trial vs. saline gauze dressing | Primary: Proportion of wounds achieving full healing. Secondary: infection rate, adverse events. | NCT05661474 | Completed (2022) | [282] |
| Autologous adipose-derived stem cells (ASCs) platelet-rich plasma (PRP) | Phase 1 | Diabetic foot ulcers | 3-arm randomized trial: cultured ASCs + PRP, stromal vascular fraction + PRP, vs. standard care | Primary: Wound closure rate and time to healing; Secondary: safety (adverse events), wound re-epithelialization quality | NCT05610865 | Recruiting (2020–2025) | - |
| Adipose-derived exosome therapy—topical application of adipose exosome product | Observational (Pilot) | Refractory full-thickness skin ulcers | One-arm open-label pilot study | Primary: Percentage area reduction, time to closure. Infection and inflammation | NCT05475418 | Completed (2022) | [283] |
| Adipose derived extracellular vesicles therapy—topical product | Interventional (RCT) | Chronic full-thickness skin ulcers | Multicenter randomized controlled trial | Primary: Incidence of complete wound closure at predefined time point. Secondary: Healing rate, time to closure, adverse events. | NCT06253975 | Ongoing (Initiated 2024) | - |
| Purified exosome product + fibrin sealant (PEP + Tisseel)—topical exosomal biologic combined with fibrin gel | Phase 2a | Diabetic foot ulcers | Multicenter randomized controlled trial | Primary: Wound closure rate at 12 weeks. Secondary: ulcer area reduction, time to 50% healing, incidence of adverse events | NCT04326959 | Recruiting (2024) | - |
| Engineered probiotic (ILP100-Topical)—Limosilactobacillus reuteri modified to secrete CXCL12 for wound repair | Phase 2a | Diabetic foot ulcers | Randomized, double-blind, placebo-controlled trial | Primary: Safety and incidence of target ulcer healing at 5 weeks. Secondary: Percent wound area reduction over 5 weeks, long-term wound recurrence | NCT05608187 | Terminated early (2024) | - |
| hepatocyte growth factor (HGF) gene therapy | Phase 3 | Neuroischemic diabetic foot ulcers | Multicenter placebo-controlled Phase III | Primary: Complete ulcer closure rate at 3–5 months. Secondary: Time to wound closure, change in ulcer area, safety, and tolerability. | NCT02563522 | Completed (2023) | [284] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Jin, Y.; Ding, Z.; Nuch, K.S.; Han, M.; Shim, J.; Chien, P.N.; Heo, C.Y. Cellular and Molecular Mechanisms of Wound Repair: From Biology to Therapeutic Innovation. Cells 2025, 14, 1850. https://doi.org/10.3390/cells14231850
Jin C, Jin Y, Ding Z, Nuch KS, Han M, Shim J, Chien PN, Heo CY. Cellular and Molecular Mechanisms of Wound Repair: From Biology to Therapeutic Innovation. Cells. 2025; 14(23):1850. https://doi.org/10.3390/cells14231850
Chicago/Turabian StyleJin, Caijun, Yongxun Jin, Zhiyuan Ding, Kong Srey Nuch, Mira Han, JungHee Shim, Pham Ngoc Chien, and Chan Yeong Heo. 2025. "Cellular and Molecular Mechanisms of Wound Repair: From Biology to Therapeutic Innovation" Cells 14, no. 23: 1850. https://doi.org/10.3390/cells14231850
APA StyleJin, C., Jin, Y., Ding, Z., Nuch, K. S., Han, M., Shim, J., Chien, P. N., & Heo, C. Y. (2025). Cellular and Molecular Mechanisms of Wound Repair: From Biology to Therapeutic Innovation. Cells, 14(23), 1850. https://doi.org/10.3390/cells14231850





