Role of Calpains in Uremia-Related Functional and Structural Muscle Changes: Protective Effect of Calpastatin Overexpression
Highlights
- Adenine-induced CKD leads to a decline in muscle strength and deterioration of muscle quality, even in the absence of significant muscle mass loss.
- Uremic conditions upregulate CAPN2 expression and activity in skeletal muscle, suggesting a pathogenic role in muscle deterioration.
- Inhibition of calpain activity through CAST overexpression preserves muscle function and attenuates fibrosis, inflammation and adipogenic processes.
- The protective effects of CAPN inhibition occur independently of improvements in renal function.
- CAPN2 emerges as a potential therapeutic target to prevent sarcopenia in CKD.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Study Design
2.2. Plasma Determinations
2.3. Muscle Strength and Physical Performance
2.4. Nuclear Magnetic Resonance Imaging
2.5. Western Blot Analysis
2.6. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Calpain Activity Assay
2.8. Sirius Red Staining
2.9. Statistical Analysis
3. Results
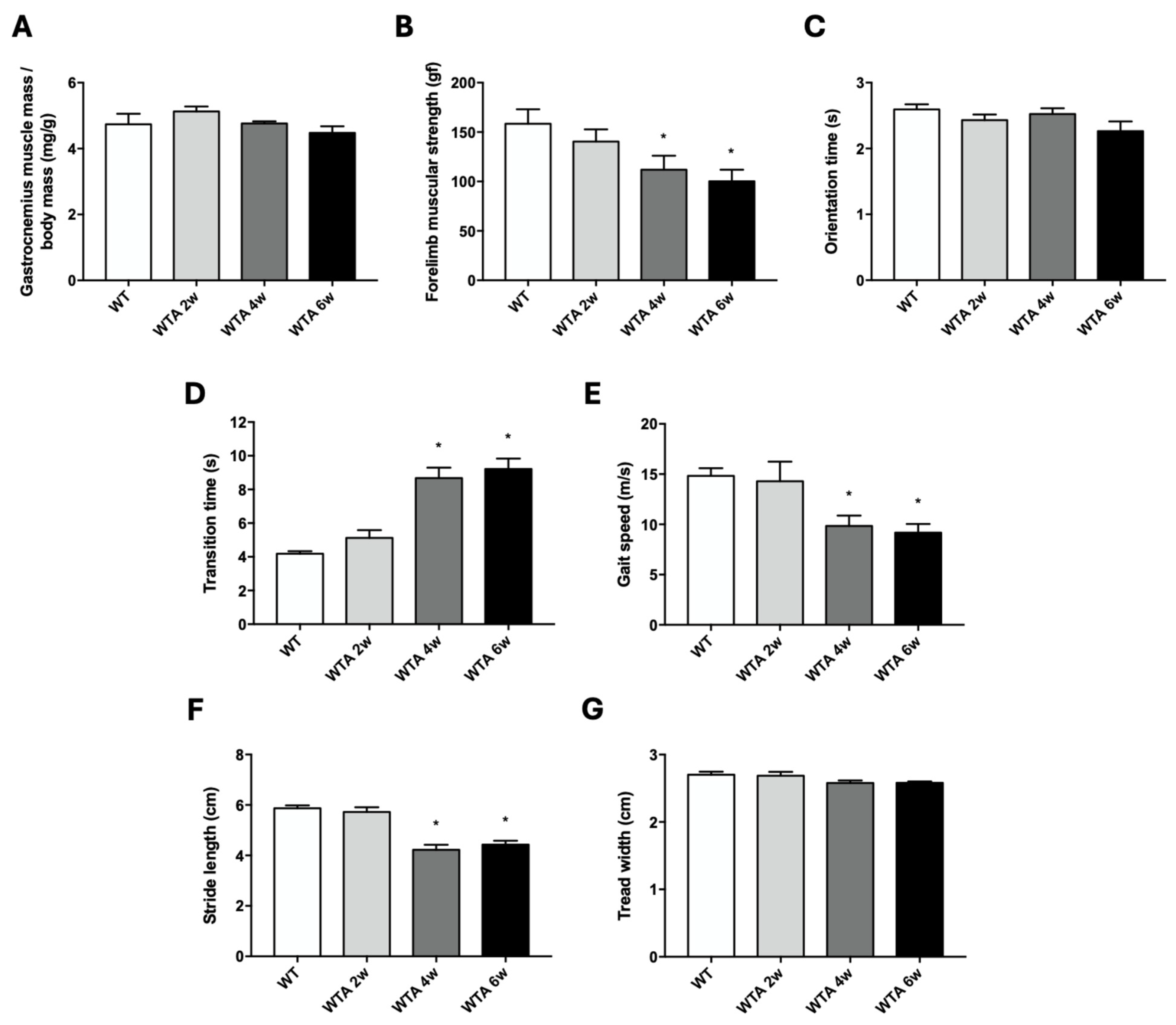
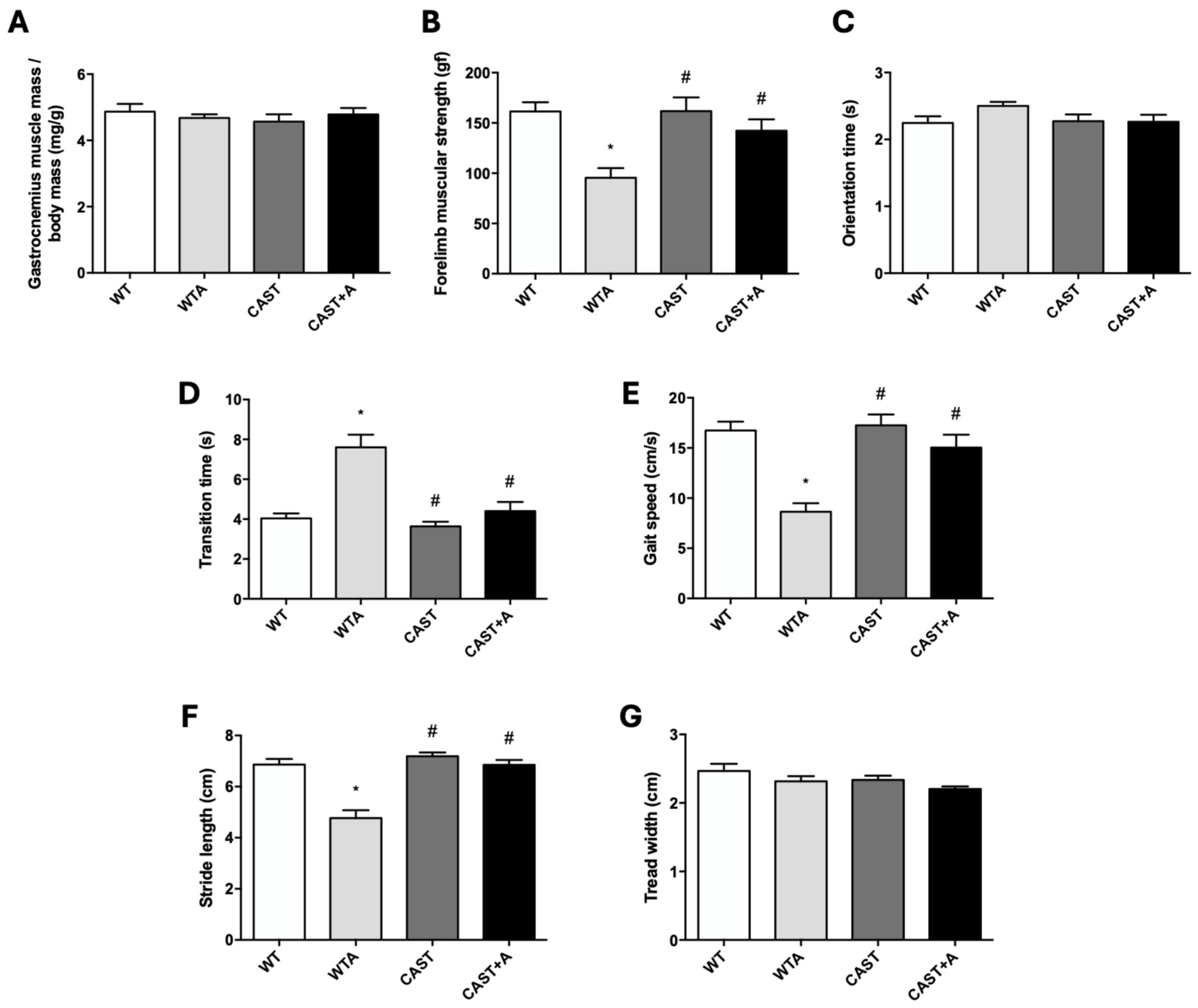
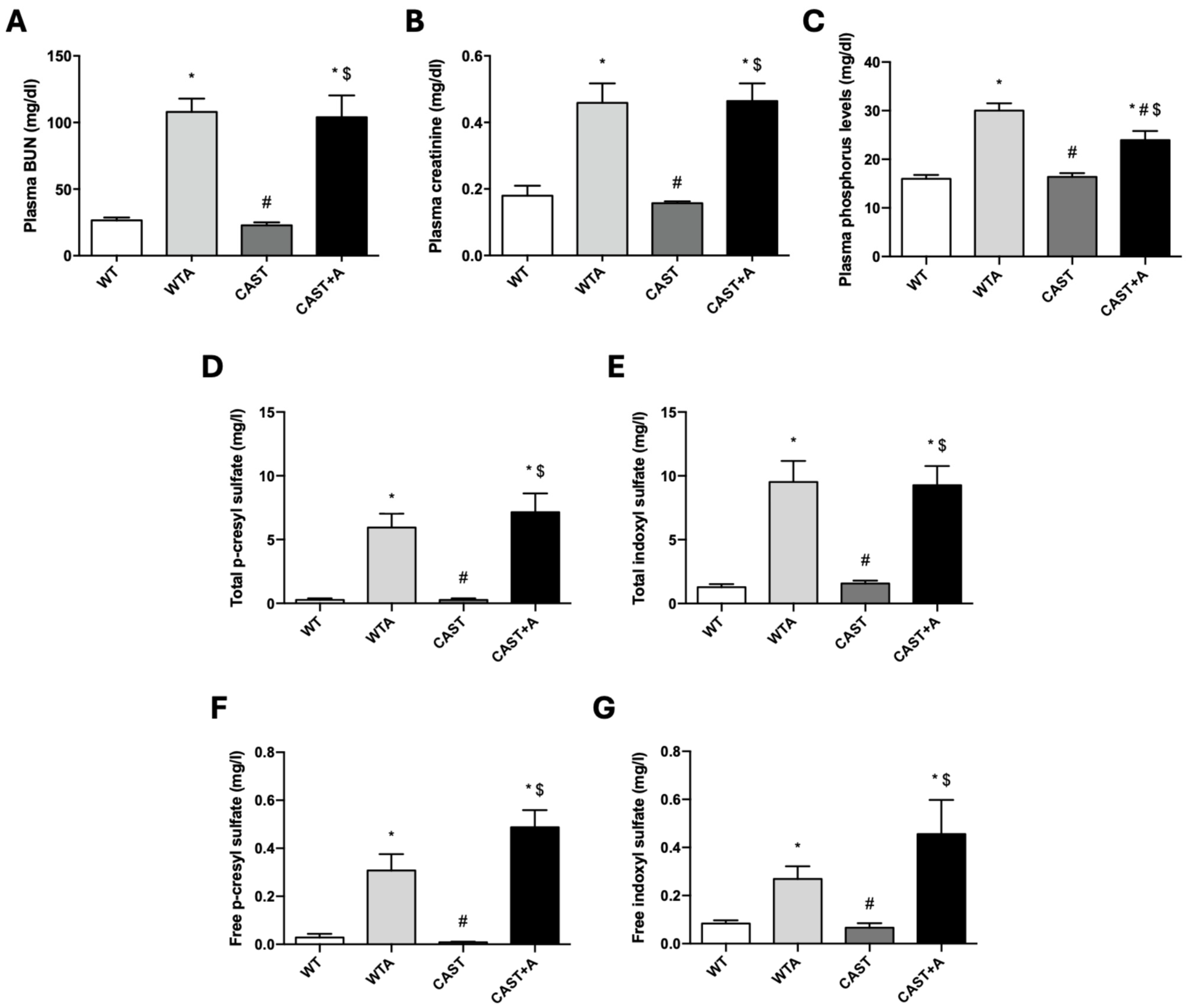
3.1. Functional and Structural Muscle Changes in Mice with Renal Damage Induced by Adenine Administration
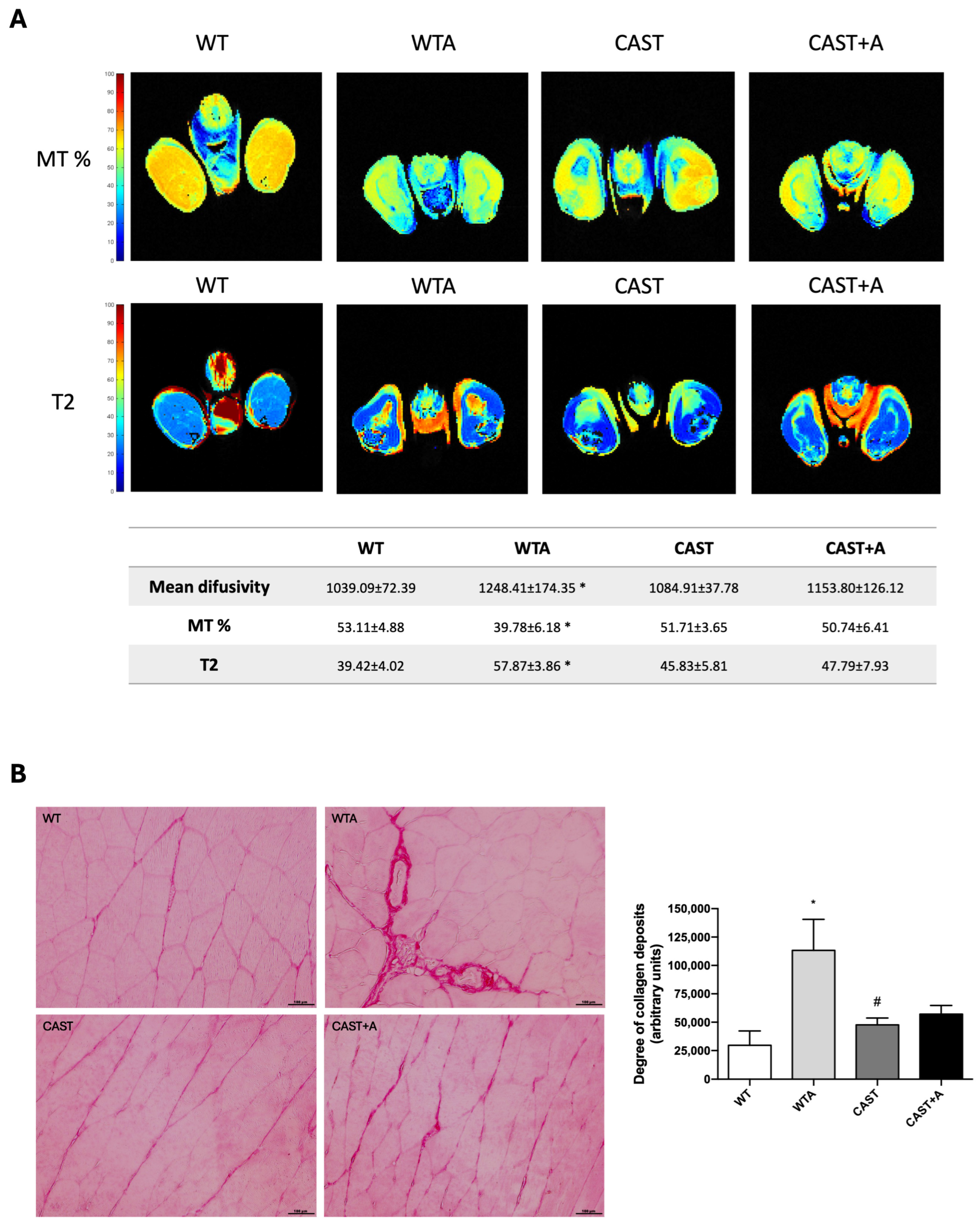
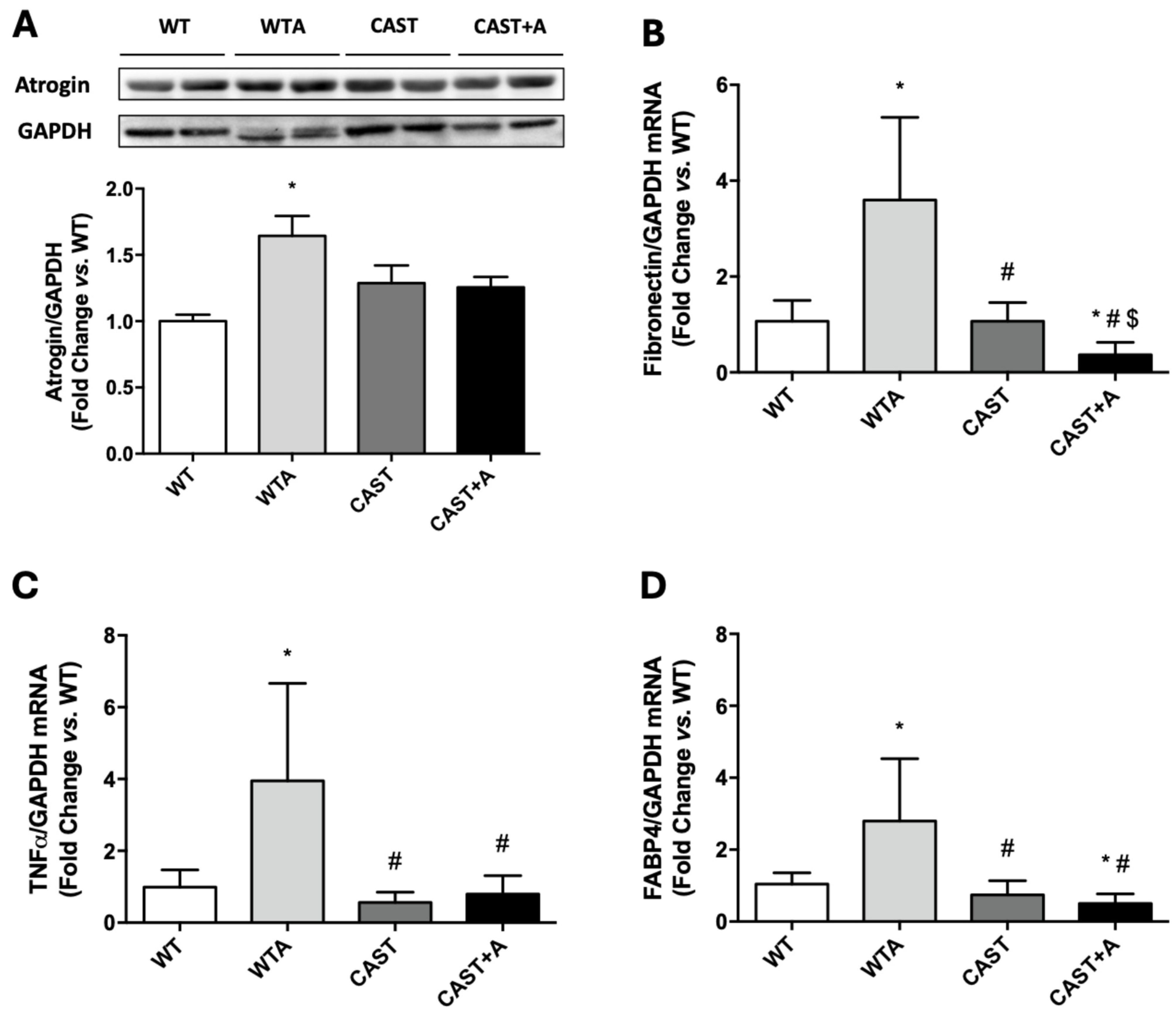
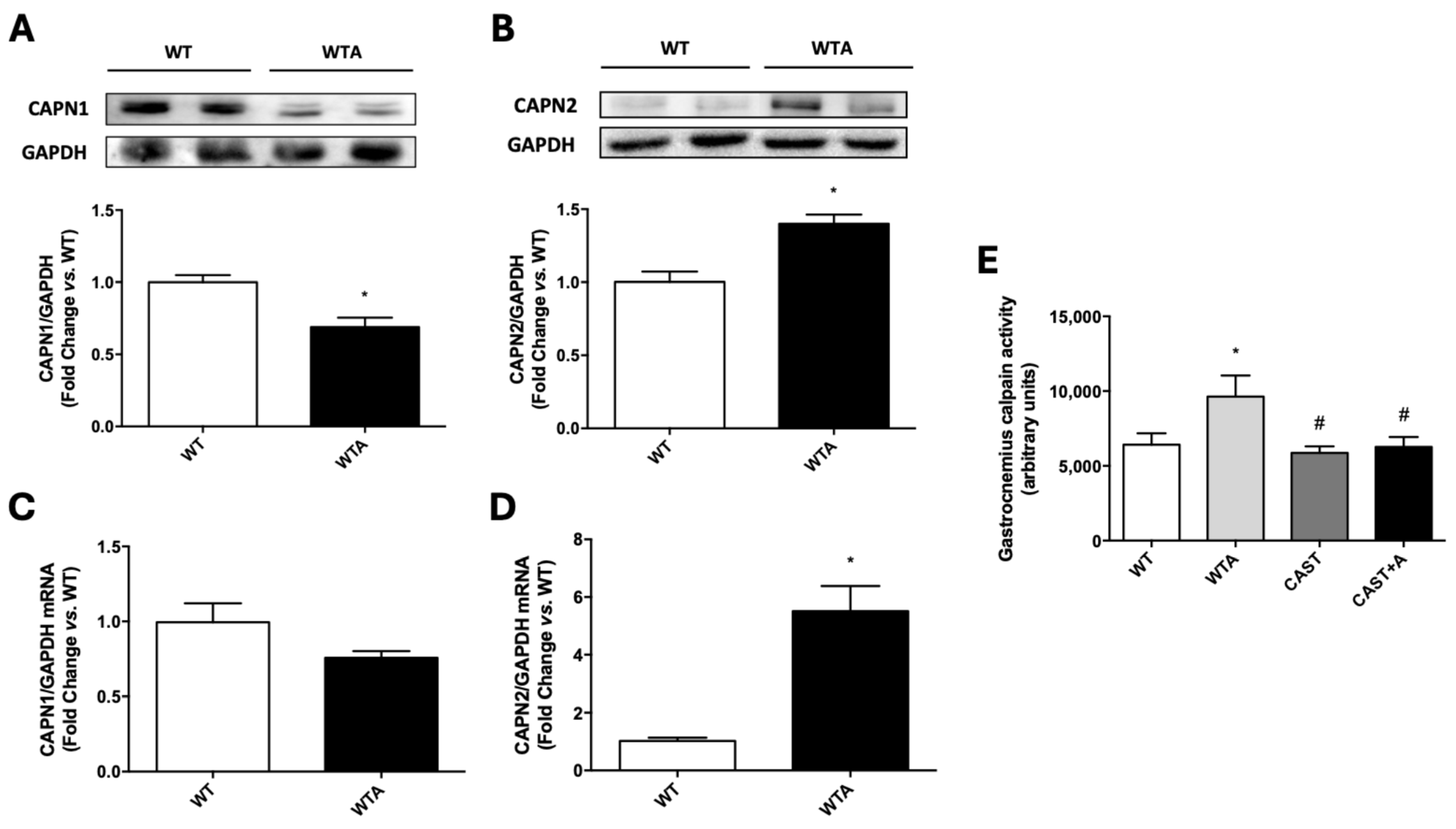
3.2. Role of Calpains in the Development of the Functional and Structural Muscle Changes Induced by Adenine Administration in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAPN | Calpain |
| CAST | Calpastatin |
| CKD | Chronic kidney disease |
| IS | Indoxyl sulfate |
| MD | Mean diffusivity |
| MT | Magnetization transfer |
| NMRI | Nuclear magnetic resonance imaging |
| pCS | p-cresyl sulfate |
| WT | Wild-type |
References
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2021, 34, 1347–1372. [Google Scholar] [CrossRef]
- Baehr, L.M.; Hughes, D.C.; Waddell, D.S.; Bodine, S.C. SnapShot: Skeletal muscle atrophy. Cell 2022, 185, 1618–1618.e1. [Google Scholar] [CrossRef]
- Sandri, M. Signaling in Muscle Atrophy and Hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef]
- Chao CTer Lin, S.H. Uremic toxins and frailty in patients with chronic kidney disease: A molecular insight. Int. J. Mol. Sci. 2021, 22, 6270. [Google Scholar] [CrossRef]
- Rodrigues, G.G.C.; Dellê, H.; Brito, R.B.O.; Cardoso, V.O.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Cunha, R.S.; Stinghen, A.E.M.; Dalboni, M.A.; Barreto, F.C. Indoxyl Sulfate Contributes to Uremic Sarcopenia by Inducing Apoptosis in Myoblasts. Arch. Med. Res. 2020, 51, 21–29. [Google Scholar] [CrossRef]
- Alcalde-Estévez, E.; Sosa, P.; Asenjo-Bueno, A.; Plaza, P.; Olmos, G.; Naves-Díaz, M.; Rodríguez-Puyol, D.; López-Ongil, S.; Ruiz-Torres, M.P. Uraemic toxins impair skeletal muscle regeneration by inhibiting myoblast proliferation, reducing myogenic differentiation, and promoting muscular fibrosis. Sci. Rep. 2021, 11, 512. [Google Scholar]
- Hou, Y.C.; Liu, Y.M.; Liao, M.T.; Zheng, C.M.; Lu, C.L.; Liu, W.C.; Hung, K.C.; Lin, S.M.; Lu, K.C. Indoxyl sulfate mediates low handgrip strength and is predictive of high hospitalization rates in patients with end-stage renal disease. Front. Med. 2023, 10, 1023383. [Google Scholar] [CrossRef]
- Vervloet, M.G.; Sezer, S.; Massy, Z.A.; Johansson, L.; Cozzolino, M.; Fouque, D. The role of phosphate in kidney disease. Nat. Rev. Nephrol. 2017, 13, 27–38. [Google Scholar] [CrossRef]
- Sosa, P.; Alcalde-Estevez, E.; Plaza, P.; Troyano, N.; Alonso, C.; Martínez-Arias, L.; Evelem de Melo Aroeira, A.; Rodriguez-Puyol, D.; Olmos, G.; López-Ongil, S.; et al. Hyperphosphatemia Promotes Senescence of Myoblasts by Impairing Autophagy Through Ilk Overexpression, A Possible Mechanism Involved in Sarcopenia. Aging Dis. 2018, 9, 769. [Google Scholar] [CrossRef]
- Sosa, P.; Alcalde-Estévez, E.; Asenjo-Bueno, A.; Plaza, P.; Carrillo-López, N.; Olmos, G.; López-Ongil, S.; Ruiz-Torres, M.P. Aging-related hyperphosphatemia impairs myogenic differentiation and enhances fibrosis in skeletal muscle. J. Cachexia Sarcopenia Muscle 2021, 12, 1266–1279. [Google Scholar] [CrossRef]
- Chung, L.H.; Liu, S.T.; Huang, S.M.; Salter, D.M.; Lee, H.S.; Hsu, Y.J. High phosphate induces skeletal muscle atrophy and suppresses myogenic differentiation by increasing oxidative stress and activating Nrf2 signaling. Aging 2020, 12, 21446–21468. [Google Scholar] [CrossRef]
- Guroff, G. A neutral, calcium-activated proteinase from the soluble fraction of rat brain. J. Biol. Chem. 1964, 239, 149–155. [Google Scholar] [CrossRef]
- Sorimachi, H.; Hata, S.; Ono, Y. Impact of genetic insights into calpain biology. J. Biochem. 2011, 150, 23–37. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The Calpain System. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Dirks, A.J.; Leeuwenburgh, C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic. Biol. Med. 2004, 36, 27–39. [Google Scholar] [CrossRef]
- Arrington, D.D.; Van Vleet, T.R.; Schnellmann, R.G. Calpain 10: A mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am. J. Physiol.-Cell Physiol. 2006, 291, C1159–C1171. [Google Scholar] [CrossRef]
- Dupont-Versteegden, E.E. Apoptosis in muscle atrophy: Relevance to sarcopenia. Exp. Gerontol. 2005, 40, 473–481. [Google Scholar] [CrossRef]
- Fulle, S.; Didonna, S.; Puglielli, C.; Pietrangelo, T.; Beccafico, S.; Bellomo, R.; Protasi, F.; Fanò, G. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp. Gerontol. 2005, 40, 189–197. [Google Scholar] [CrossRef]
- Peltier, J.; Bellocq, A.; Perez, J.; Doublier, S.; Dubois, Y.C.X.; Haymann, J.P.; Camussi, G.; Baud, L. Calpain Activation and Secretion Promote Glomerular Injury in Experimental Glomerulonephritis. J. Am. Soc. Nephrol. 2006, 17, 3415–3423. [Google Scholar] [CrossRef]
- De Frutos, S.; Luengo, A.; García-Jérez, A.; Hatem-Vaquero, M.; Griera, M.; O’Valle, F.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D.; Calleros, L. Chronic kidney disease induced by an adenine rich diet upregulates integrin linked kinase (ILK) and its depletion prevents the disease progression. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 1284–1297. [Google Scholar] [CrossRef]
- Lin, C.N.; Wu, I.W.; Huang, Y.F.; Peng, S.Y.; Huang, Y.C.; Ning, H.C. Measuring serum total and free indoxyl sulfate and p-cresyl sulfate in chronic kidney disease using UPLC-MS/MS. J. Food Drug Anal. 2019, 27, 502–509. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef]
- Lamb, G.D. Calpains in muscle: Selective and protective? J. Physiol. 2007, 582, 897. [Google Scholar] [CrossRef]
- Clinkenbeard, E.L.; Noonan, M.L.; Thomas, J.C.; Ni, P.; Hum, J.M.; Aref, M.; Swallow, E.A.; Moe, S.M.; Allen, M.R.; White, K.E. Increased FGF23 protects against detrimental cardio-renal consequences during elevated blood phosphate in CKD. JCI Insight 2019, 4, e123817. [Google Scholar] [CrossRef]
- Kim, K.; Anderson, E.M.; Thome, T.; Lu, G.; Salyers, Z.R.; Cort, T.A.; O’Malley, K.A.; Scali, S.T.; Ryan, T.E. Skeletal myopathy in CKD: A comparison of adenine-induced nephropathy and 5/6 nephrectomy models in mice. Am. J. Physiol.-Ren. Physiol. 2021, 321, F106–F119. [Google Scholar] [CrossRef]
- Berru, F.N.; Gray, S.E.; Thome, T.; Kumar, R.A.; Salyers, Z.R.; Coleman, M.; Le, D.; O’Malley, K.; Ferreira, L.F.; Berceli, S.A.; et al. Chronic kidney disease exacerbates ischemic limb myopathy in mice via altered mitochondrial energetics. Sci. Rep. 2019, 9, 15547. [Google Scholar] [CrossRef]
- Uchiyama, K.; Wakino, S.; Irie, J.; Miyamoto, J.; Matsui, A.; Tajima, T.; Itoh, T.; Oshima, Y.; Yoshifuji, A.; Kimura, I.; et al. Contribution of uremic dysbiosis to insulin resistance and sarcopenia. Nephrol. Dial. Transplant. 2020, 35, 1501–1517. [Google Scholar] [CrossRef]
- Czaya, B.; Heitman, K.; Campos, I.; Yanucil, C.; Kentrup, D.; Westbrook, D.; Gutierrez, O.; Babitt, J.L.; Jung, G.; Salusky, I.B.; et al. Hyperphosphatemia increases inflammation to exacerbate anemia and skeletal muscle wasting independently of FGF23-FGFR4 signaling. eLife 2022, 11, e74782. [Google Scholar] [CrossRef]
- Thome, T.; Kumar, R.A.; Burke, S.K.; Khattri, R.B.; Salyers, Z.R.; Kelley, R.C.; Coleman, M.D.; Christou, D.D.; Hepple, R.T.; Scali, S.T.; et al. Impaired muscle mitochondrial energetics is associated with uremic metabolite accumulation in chronic kidney disease. JCI Insight 2021, 6, e139826. [Google Scholar] [CrossRef]
- Organ, J.M.; Srisuwananukorn, A.; Price, P.; Joll, J.E.; Biro, K.C.; Rupert, J.E.; Chen, N.X.; Avin, K.G.; Moe, S.M.; Allen, M.R. Reduced skeletal muscle function is associated with decreased fiber cross-sectional area in the Cy/+ rat model of progressive kidney disease. Nephrol. Dial. Transplant. 2015, 31, 223–230. [Google Scholar] [CrossRef]
- Anderson, E.M.; Kim, K.; Fazzone, B.J.; Harland, K.C.; Hu, Q.; Salyers, Z.; Palzkill, V.R.; Cort, T.A.; Kunz, E.M.; Martin, A.J.; et al. Influences of renal insufficiency and ischemia on mitochondrial bioenergetics and limb dysfunction in a novel murine iliac arteriovenous fistula model. JVS Vasc. Sci. 2022, 3, 345–362. [Google Scholar] [CrossRef]
- Acevedo, L.M.; Vidal, Á.; Aguilera-Tejero, E.; Rivero, J.L.L. Muscle plasticity is influenced by renal function and caloric intake through the FGF23-vitamin D axis. Am. J. Physiol.-Cell Physiol. 2023, 324, C14–C28. [Google Scholar] [CrossRef]
- Kemp, C.M.; Oliver, W.T.; Wheeler, T.L.; Chishti, A.H.; Koohmaraie, M. The effects of Capn1 gene inactivation on skeletal muscle growth, development, and atrophy, and the compensatory role of other proteolytic systems1. J. Anim. Sci. 2013, 91, 3155–3167. [Google Scholar] [CrossRef]
- Piper, A.K.; Sophocleous, R.A.; Ross, S.E.; Evesson, F.J.; Saleh, O.; Bournazos, A.; Yasa, J.; Reed, C.; Woolger, N.; Sluyter, R.; et al. Loss of calpains-1 and -2 prevents repair of plasma membrane scrape injuries, but not small pores, and induces a severe muscular dystrophy. Am. J. Physiol.-Cell Physiol. 2020, 318, C1226–C1237. [Google Scholar] [CrossRef]
- Zeng, X.; Zhao, L.; Chen, S.; Li, X. Inhibition of mitochondrial and cytosolic calpain attenuates atrophy in myotubes co-cultured with colon carcinoma cells. Oncol. Lett. 2020, 21, 124. [Google Scholar] [CrossRef]
- Whitmore, C.; Pratt, E.P.S.; Anderson, L.; Bradley, K.; Latour, S.M.; Hashmi, M.N.; Urazaev, A.K.; Weilbaecher, R.; Davie, J.K.; Wang, W.H.; et al. The ERG1a potassium channel increases basal intracellular calcium concentration and calpain activity in skeletal muscle cells. Skelet. Muscle 2020, 10, 1. [Google Scholar] [CrossRef]
- Gurevich, M.; Iocolano, K.; Martin, I.N.; Singh, G.; Khan, S.U.; Bui, D.T.; Dagum, A.B.; Komatsu, D.E. Efficacy of leupeptin in treating ischemia in a rat hind limb model. Physiol. Rep. 2022, 10, e15411. [Google Scholar] [CrossRef]
- Yajima, Y.; Sato, M.; Sorimachi, H.; Inomata, M.; Maki, M.; Kawashima, S. Calpain System Regulates the Differentiation of Adult Primitive Mesenchymal ST-13 Adipocytes. Endocrinology 2006, 147, 4811–4819. [Google Scholar] [CrossRef]
- Alcalde-Estévez, E.; Sosa, P.; Asenjo-Bueno, A.; Plaza, P.; Valenzuela, P.L.; Naves-Díaz, M.; Olmos, G.; López-Ongil, S.; Ruiz-Torres, M.P. Dietary phosphate restriction prevents the appearance of sarcopenia signs in old mice. J. Cachexia Sarcopenia Muscle 2023, 14, 1060–1074. [Google Scholar] [CrossRef]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of sarcopenia: Old evidence and new insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef]
- Farrow, M.; Biglands, J.; Tanner, S.F.; Clegg, A.; Brown, L.; Hensor, E.M.A.; O’Connor, P.; Emery, P.; Tan, A.L. The effect of ageing on skeletal muscle as assessed by quantitative MR imaging: An association with frailty and muscle strength. Aging Clin. Exp. Res. 2021, 33, 291–301. [Google Scholar] [CrossRef]
- Power, G.A.; Allen, M.D.; Booth, W.J.; Thompson, R.T.; Marsh, G.D.; Rice, C.L. The influence on sarcopenia of muscle quality and quantity derived from magnetic resonance imaging and neuromuscular properties. Age 2014, 36, 9642. [Google Scholar] [CrossRef]
- Hiba, B.; Richard, N.; Hébert, L.J.; Coté, C.; Nejjari, M.; Vial, C.; Bouhour, F.; Puymirat, J.; Janier, M. Quantitative assessment of skeletal muscle degeneration in patients with myotonic dystrophy type 1 using MRI. J. Magn. Reson. Imaging 2012, 35, 678–685. [Google Scholar] [CrossRef]
- Abramowitz, M.K.; Paredes, W.; Zhang, K.; Brightwell, C.R.; Newsom, J.N.; Kwon, H.J.; Custodio, M.; Buttar, R.S.; Farooq, H.; Zaidi, B.; et al. Skeletal muscle fibrosis is associated with decreased muscle inflammation and weakness in patients with chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2018, 315, F1658–F1669. [Google Scholar] [CrossRef]
- Peng, H.; Cao, J.; Yu, R.; Danesh, F.; Wang, Y.; Mitch, W.E.; Xu, J.; Hu, Z. CKD Stimulates Muscle Protein Loss Via Rho-associated Protein Kinase 1 Activation. J. Am. Soc. Nephrol. 2016, 27, 509–519. [Google Scholar] [CrossRef]
- Enoki, Y.; Nagai, T.; Hamamura, Y.; Osa, S.; Nakamura, H.; Taguchi, K.; Watanabe, H.; Maruyama, T.; Matsumoto, K. The G protein-coupled receptor ligand apelin-13 ameliorates skeletal muscle atrophy induced by chronic kidney disease. J. Cachexia Sarcopenia Muscle 2023, 14, 553–564. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Huang, J.; Hu, R.; You, H.; Liu, L.; Wang, D.; Wei, L. Paeoniflorin Ameliorates Skeletal Muscle Atrophy in Chronic Kidney Disease via AMPK/SIRT1/PGC-1α-Mediated Oxidative Stress and Mitochondrial Dysfunction. Front. Pharmacol. 2022, 13, 859723. [Google Scholar] [CrossRef]
- Cheung, W.W.; Zheng, R.; Hao, S.; Wang, Z.; Gonzalez, A.; Zhou, P.; Hoffman, H.M.; Mak, R.H. The role of IL-1 in adipose browning and muscle wasting in CKD-associated cachexia. Sci. Rep. 2021, 11, 15141. [Google Scholar] [CrossRef]
- Zhang, L.; Rajan, V.; Lin, E.; Hu, Z.; Han, H.Q.; Zhou, X.; Song, Y.; Min, H.; Wang, X.; Du, J.; et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011, 25, 1653–1663. [Google Scholar] [CrossRef]
- Kawao, N.; Kawaguchi, M.; Ohira, T.; Ehara, H.; Mizukami, Y.; Takafuji, Y.; Kaji, H. Renal failure suppresses muscle irisin expression, and irisin blunts cortical bone loss in mice. J. Cachexia Sarcopenia Muscle 2022, 13, 758–771. [Google Scholar] [CrossRef]
- Saadane, A.; Du, Y.; Thoreson, W.B.; Miyagi, M.; Lessieur, E.M.; Kiser, J.; Wen, X.; Berkowitz, B.A.; Kern, T.S. Photoreceptor Cell Calcium Dysregulation and Calpain Activation Promote Pathogenic Photoreceptor Oxidative Stress and Inflammation in Prodromal Diabetic Retinopathy. Am. J. Pathol. 2021, 191, 1805–1821. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Chen, J.; Yu, Y.; Yu, Y.; Shi, H.; Liu, X.; Chen, Z.; Chen, R.; Ge, J. Macrophage CAPN4 regulates CVB3-induced cardiac inflammation and injury by promoting NLRP3 inflammasome activation and phenotypic transformation to the inflammatory subtype. Free Radic. Biol. Med. 2023, 208, 430–444. [Google Scholar] [CrossRef]
- Yan, W.; Wang, C.; Zhao, Y.; Jiang, Y.; Sun, M. Involvement of Calpain in Neurovascular Unit Damage through Up-regulating PARP-NF-κB Signaling during Experimental Ischemic Stroke. Mol. Neurobiol. 2024, 61, 8104–8122. [Google Scholar] [CrossRef]
- Gamboa, J.L.; Roshanravan, B.; Towse, T.; Keller, C.A.; Falck, A.M.; Yu, C.; Frontera, W.R.; Brown, N.J.; Ikizler, T.A. Skeletal Muscle Mitochondrial Dysfunction Is Present in Patients with CKD before Initiation of Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 926–936. [Google Scholar] [CrossRef]
- Yajima, T. Skeletal muscle density measured by computed tomography as a predictor of mortality in patients receiving hemodialysis. J. Nephrol. 2022, 35, 1535–1537. [Google Scholar] [CrossRef]
- Muniappan, L.; Javidan, A.; Jiang, W.; Mohammadmoradi, S.; Moorleghen, J.J.; Katz, W.S.; Balakrishnan, A.; Howatt, D.A.; Subramanian, V. Calpain Inhibition Attenuates Adipose Tissue Inflammation and Fibrosis in Diet-induced Obese Mice. Sci. Rep. 2017, 7, 14398. [Google Scholar] [CrossRef]
- Li, G.; Iyengar, R. Calpain as an effector of the Gq signaling pathway for inhibition of Wnt/β-catenin-regulated cell proliferation. Proc. Natl. Acad. Sci. USA 2002, 99, 13254–13259. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Calabrés, E.; Campillo, S.; Alcalde-Estévez, E.; Cuevas-Delgado, P.; Barbas, C.; García-Villoria, S.; Silvestre-Vargas, A.; Griera, M.; de Frutos, S.; Ruiz-Torres, M.P.; et al. Role of Calpains in Uremia-Related Functional and Structural Muscle Changes: Protective Effect of Calpastatin Overexpression. Cells 2025, 14, 1846. https://doi.org/10.3390/cells14231846
Gutiérrez-Calabrés E, Campillo S, Alcalde-Estévez E, Cuevas-Delgado P, Barbas C, García-Villoria S, Silvestre-Vargas A, Griera M, de Frutos S, Ruiz-Torres MP, et al. Role of Calpains in Uremia-Related Functional and Structural Muscle Changes: Protective Effect of Calpastatin Overexpression. Cells. 2025; 14(23):1846. https://doi.org/10.3390/cells14231846
Chicago/Turabian StyleGutiérrez-Calabrés, Elena, Sofía Campillo, Elena Alcalde-Estévez, Paula Cuevas-Delgado, Coral Barbas, Sergio García-Villoria, Alba Silvestre-Vargas, Mercedes Griera, Sergio de Frutos, María P. Ruiz-Torres, and et al. 2025. "Role of Calpains in Uremia-Related Functional and Structural Muscle Changes: Protective Effect of Calpastatin Overexpression" Cells 14, no. 23: 1846. https://doi.org/10.3390/cells14231846
APA StyleGutiérrez-Calabrés, E., Campillo, S., Alcalde-Estévez, E., Cuevas-Delgado, P., Barbas, C., García-Villoria, S., Silvestre-Vargas, A., Griera, M., de Frutos, S., Ruiz-Torres, M. P., Rodríguez-Puyol, D., & Calleros, L. (2025). Role of Calpains in Uremia-Related Functional and Structural Muscle Changes: Protective Effect of Calpastatin Overexpression. Cells, 14(23), 1846. https://doi.org/10.3390/cells14231846







