Neuroserpin: A Potential Neuroprotective Agent in Mild Neonatal Hypoxic–Ischaemic Encephalopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Model of Neonatal HIE
2.2. Cell Culture
2.3. Experimental Design
2.4. Immunohistochemistry
2.5. Immunoblotting
2.6. Statistical Analyses
3. Results
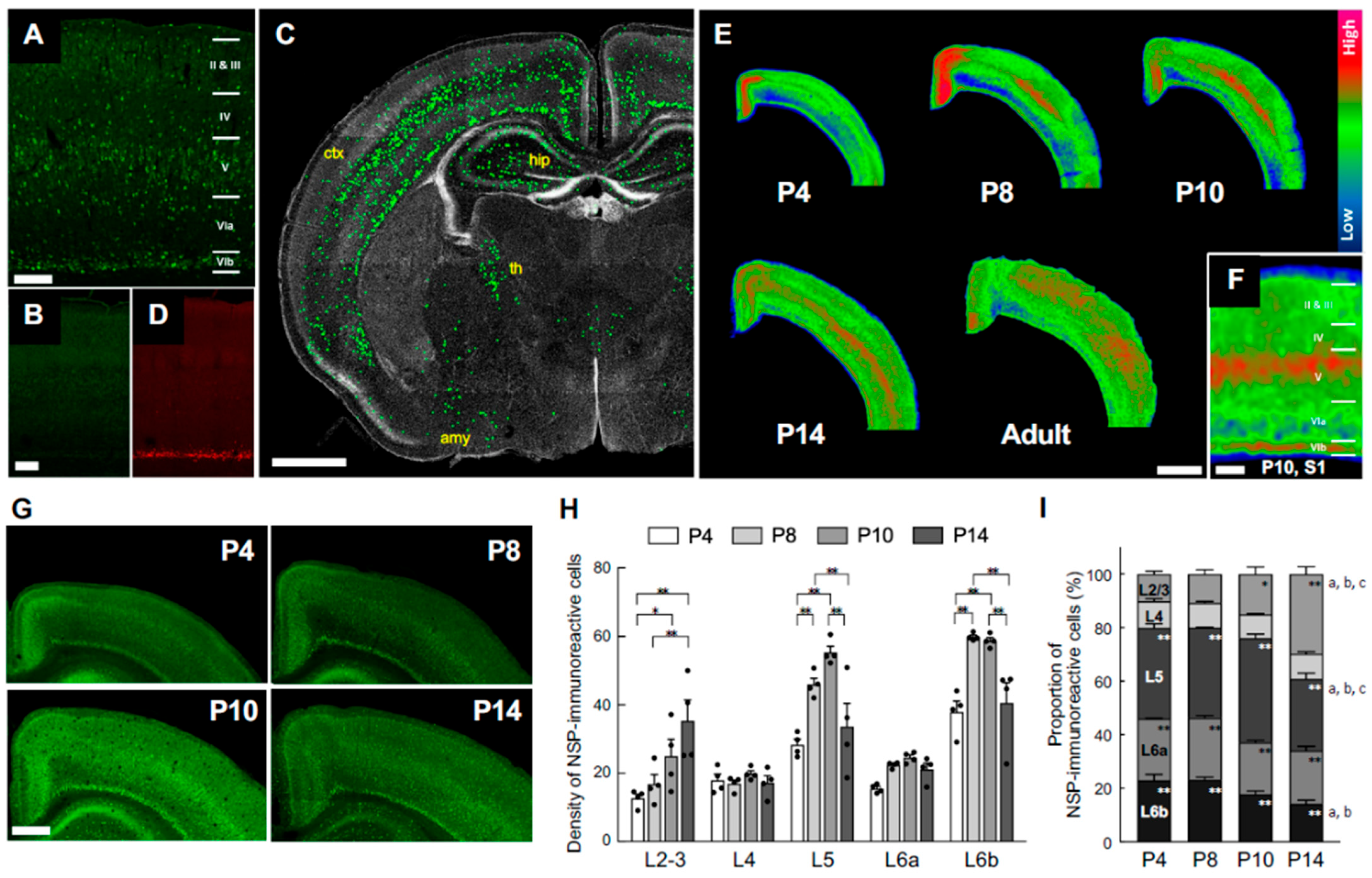
3.1. Transition of Neuroserpin Expression in the Cortex During Mouse Postnatal Development
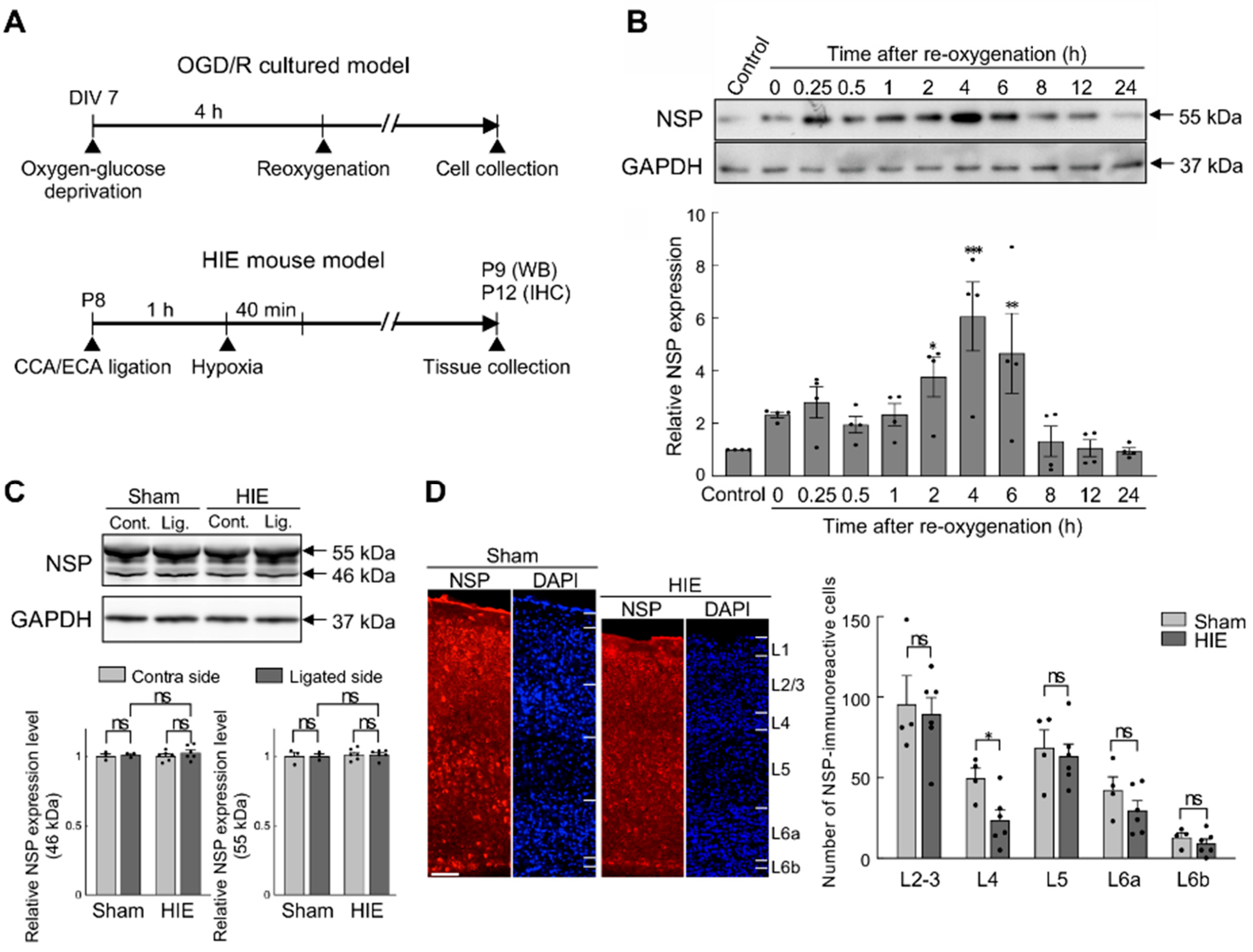
3.2. Effects of Hypoxia–Ischaemia on Neuroserpin Expression in Primary Cultured Cortical Neurons and Mouse Cortex
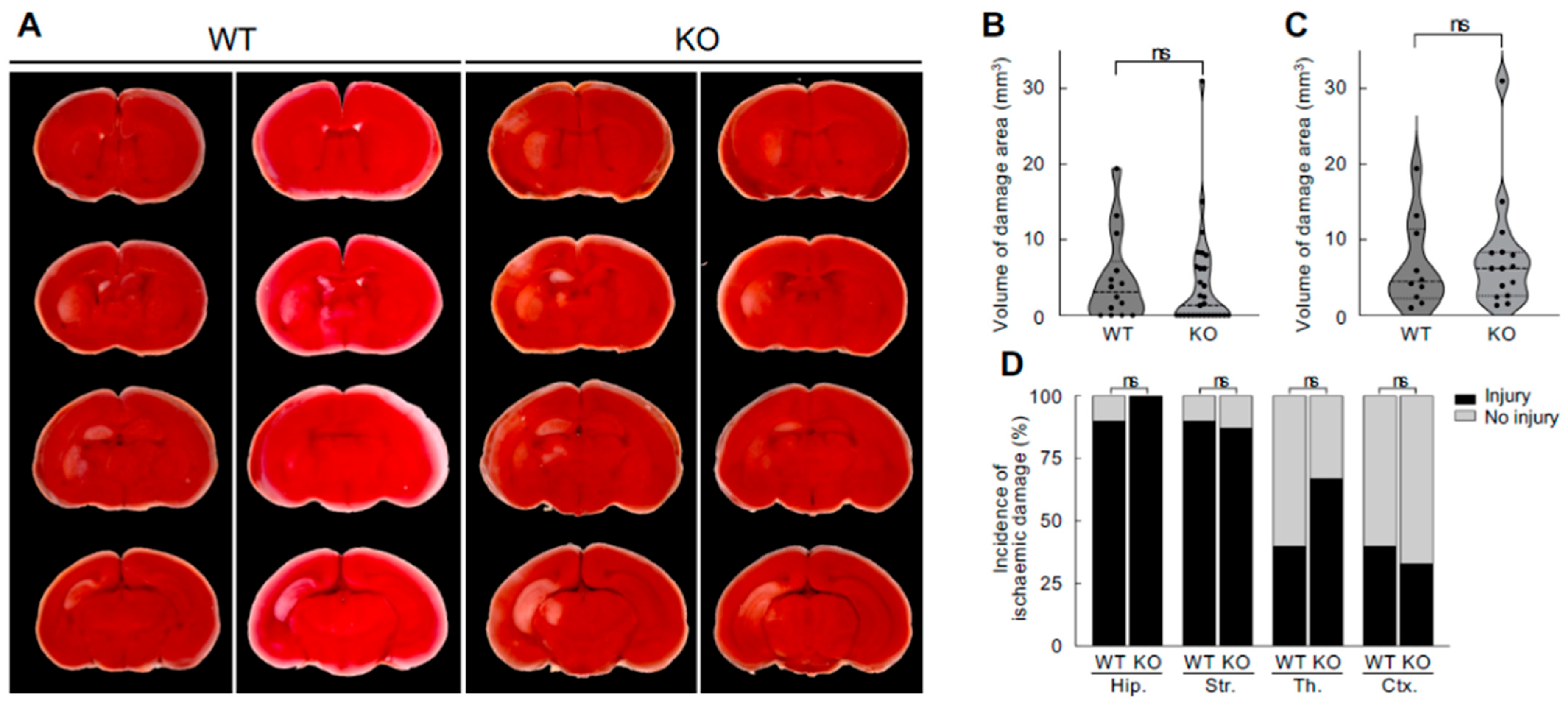
3.3. No Difference in Neonatal Hypoxic–Ischaemic Brain Damage Between Neuroserpin KO Mice and WT Mice
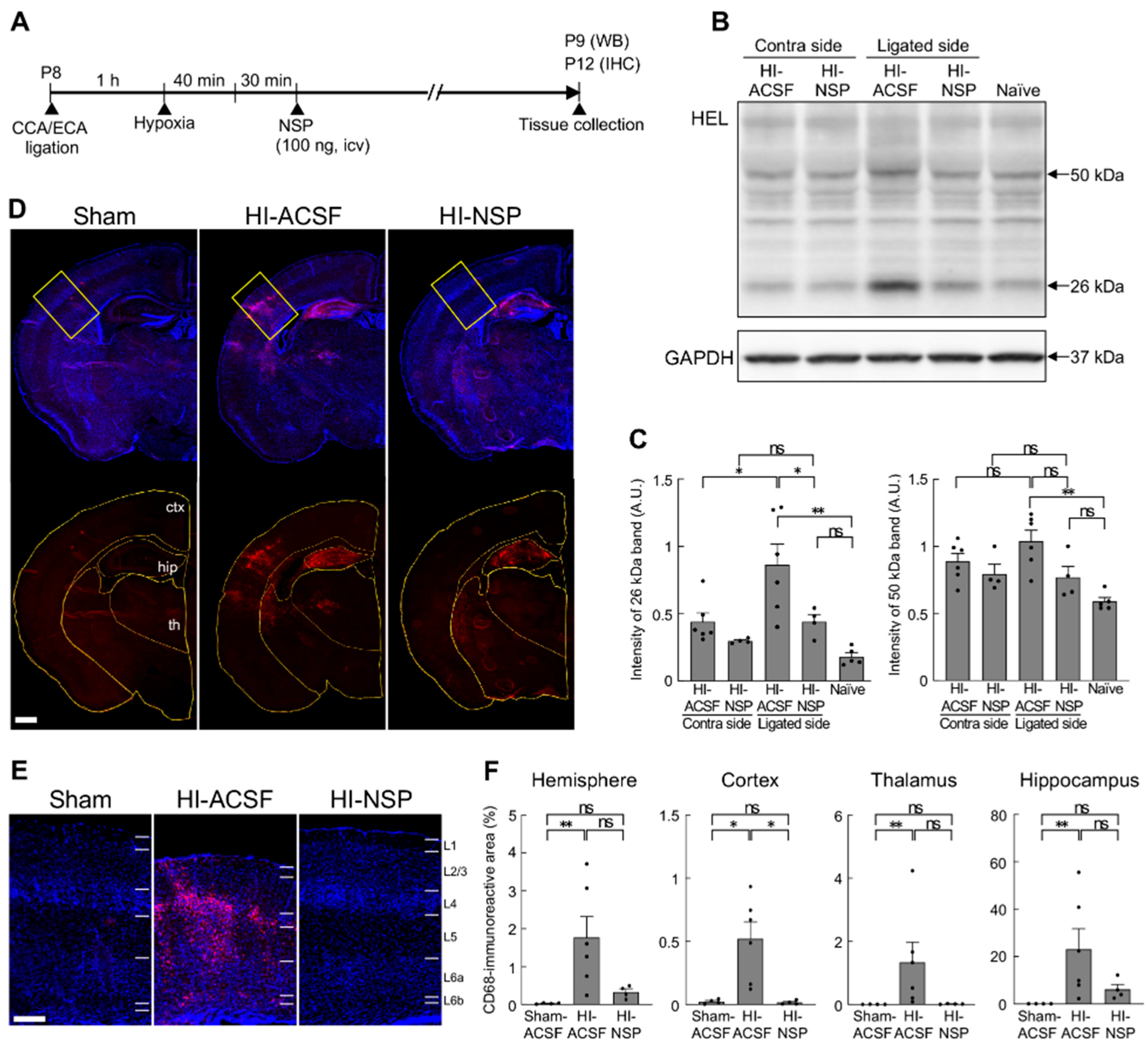
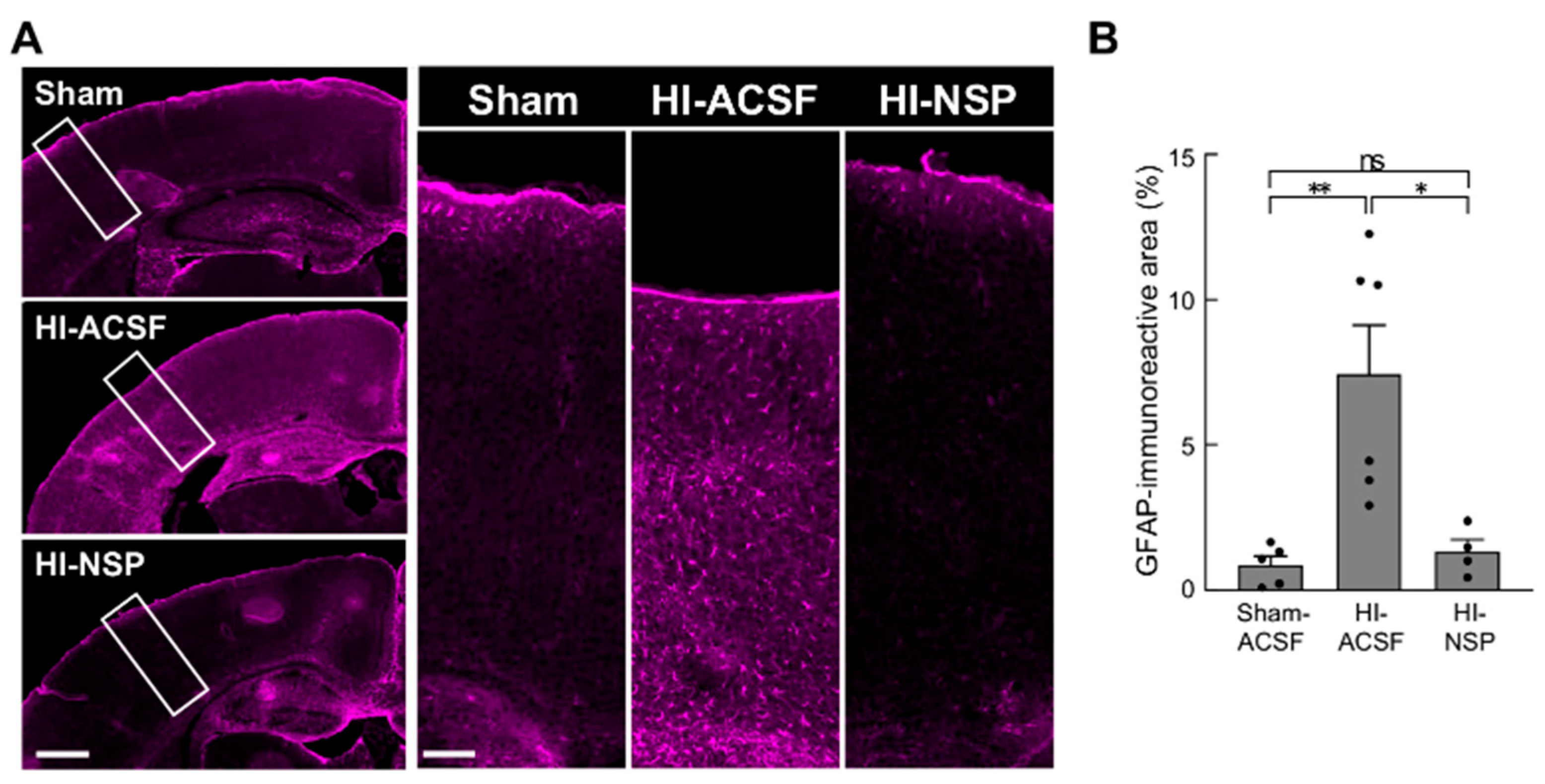
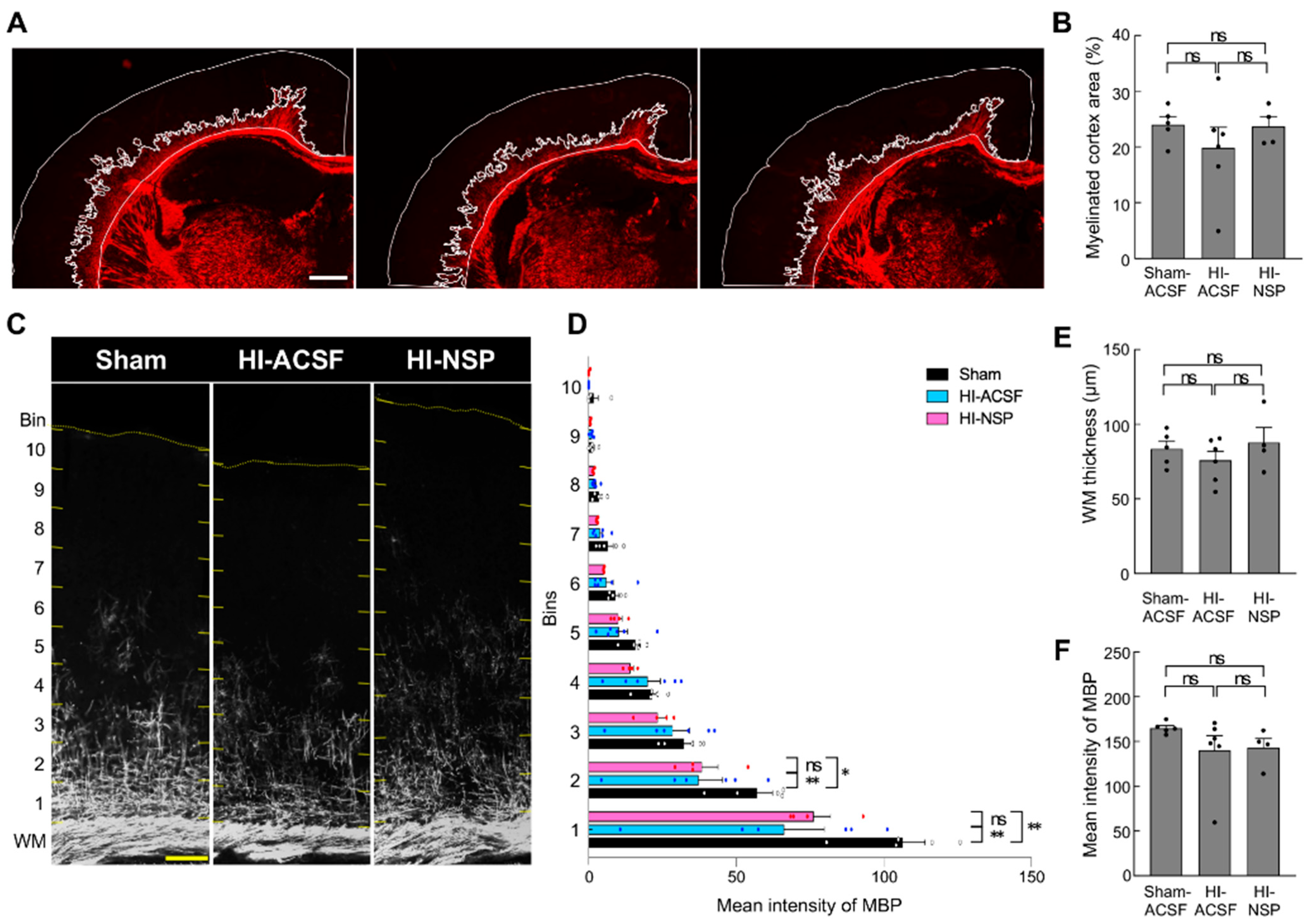
3.4. Neuroprotective Effect of Treatment with Neuroserpin on Brain Damage in Mild Neonatal HIE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volpe, J.J. Perinatal brain injury: From pathogenesis to neuroprotection. Ment. Retard. Dev. Disabil. Res. Rev. 2001, 7, 56–64. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.S.; Jongmans, M.J. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F220–F224. [Google Scholar] [CrossRef]
- Lee, A.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013, 74, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B.; Sarnat, M.S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 1976, 33, 696–705. [Google Scholar] [CrossRef]
- Thompson, C.M.; Puterman, A.S.; Linley, L.L.; Hann, F.M.; van der Elst, C.W.; Molteno, C.D.; Malan, A.F. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997, 86, 757–761. [Google Scholar] [CrossRef]
- Boutilier, R.G. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 2001, 204, 3171–3181. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef]
- Azzopardi, D.V.; Strohm, B.; Edwards, A.D.; Dyet, L.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; Levene, M.; Marlow, N.; Porter, E.; et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 2009, 361, 1349–1358. [Google Scholar] [CrossRef]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal hypoxia ischaemia: Mechanisms, models, and uherapeutic challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Gulati, A. Advances in therapies to treat neonatal hypoxic-ischemic encephalopathy. J. Clin. Med. 2023, 12, 6653. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.O.; Wassink, G.; van den Heuij, L.G.; Bennet, L.; Gunn, A.J. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy - where to from here? Front. Neurol. 2015, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Thayyil, S.; Pant, S.; Montaldo, P.; Shukla, D.; Oliveira, V.; Ivain, P.; Bassett, P.; Swamy, R.; Mendoza, J.; Moreno-Morales, M.; et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): A randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health 2021, 9, e1273–e1285. [Google Scholar] [CrossRef]
- Robertson, C.; Finer, N. Term infants with hypoxic-ischemic encephalopathy: Outcome at 3.5 years. Dev. Med. Child Neurol. 1985, 27, 473–484. [Google Scholar] [CrossRef]
- Robertson, C.M.; Finer, N.N.; Grace, M.G. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J. Pediatr. 1989, 114, 753–760. [Google Scholar] [CrossRef]
- Chalak, L.F.; Nguyen, K.A.; Prempunpong, C.; Heyne, R.; Thayyil, S.; Shankaran, S.; Laptook, A.R.; Rollins, N.; Pappas, A.; Koclas, L.; et al. Prospective research in infants with mild encephalopathy identified in the first six hours of life: Neurodevelopmental outcomes at 18–22 months. Pediatr. Res. 2018, 84, 861–868. [Google Scholar] [CrossRef]
- Halpin, S.; McCusker, C.; Fogarty, L.; White, J.; Cavalière, E.; Boylan, G.; Murray, D. Long-term neuropsychological and behavioral outcome of mild and moderate hypoxic ischemic encephalopathy. Early Hum. Dev. 2022, 165, 105541. [Google Scholar] [CrossRef]
- Yang, M.; Wang, K.; Liu, B.; Shen, Y.; Liu, G. Hypoxic-ischemic encephalopathy: Pathogenesis and promising therapies. Mol. Neurobiol. 2024, 62, 2105–2122. [Google Scholar] [CrossRef]
- Disdier, C.; Stonestreet, B.S. Hypoxic-ischemic-related cerebrovascular changes and potential therapeutic strategies in the neonatal brain. J. Neurosci. Res. 2020, 98, 1468–1484. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Osterwalder, T.; Contartese, J.; Stoeckli, E.T.; Kuhn, T.B.; Sonderegger, P. Neuroserpin, an axonally secreted serine protease inhibitor. EMBO J. 1996, 15, 2944–2953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ragg, H. Ancestry and evolution of a secretory pathway serpin. BMC Evol. Biol. 2008, 8, 250. [Google Scholar] [CrossRef]
- Kondo, S.; Al-Hasani, H.; Hoerder-Suabedissen, A.; Wang, W.Z.; Molnár, Z. Secretory function in subplate neurons during cortical development. Front. Neurosci. 2015, 9, 100. [Google Scholar] [CrossRef]
- Adorjan, I.; Tyler, T.; Bhaduri, A.; Demharter, S.; Finszter, C.K.; Bako, M.; Sebok, O.M.; Nowakowski, T.J.; Khodosevich, K.; Møllgård, K.; et al. Neuroserpin expression during human brain development and in adult brain revealed by immunohistochemistry and single cell RNA sequencing. J. Anat. 2019, 235, 543–554. [Google Scholar] [CrossRef]
- Yepes, M.; Sandkvist, M.; Wong, M.K.; Coleman, T.A.; Smith, E.; Cohan, S.L.; Lawrence, D.A. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood 2000, 96, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, P.; Madani, R.; Tsuzuki, N.; Vallet, P.; Arras, M.; Zhao, C.N.; Osterwalder, T.; Rülicke, T.; Sonderegger, P. Neuroserpin, a neuroprotective factor in focal ischemic stroke. Mol. Cell. Neurosci. 2001, 18, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhang, Q.; Shi, Q.; Liu, P.; Zhong, P.; Guo, L.; Huang, Z.; Peng, Y.; Liu, W.; Zhang, S.; et al. Neuroserpin alleviates cerebral ischemia-reperfusion injury by suppressing ischemia-induced endoplasmic reticulum stress. Neural Regen. Res. 2026, 21, 333–345. [Google Scholar] [CrossRef]
- Ma, J.; Yu, D.; Tong, Y.; Mao, M. Effect of neuroserpin in a neonatal hypoxic-ischemic injury model ex vivo. Biol. Res. 2012, 45, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Asakawa, T.; Li, W.; Han, S.; Li, Q.; Xiao, B.; Namba, H.; Lu, C.; Dong, Q. Neuroprotective effect of neuroserpin in oxygen-glucose deprivation- and reoxygenation-treated rat astrocytes in vitro. PLoS ONE 2015, 10, e0123932. [Google Scholar] [CrossRef]
- Yang, X.; Asakawa, T.; Han, S.; Liu, L.; Li, W.; Wu, W.; Luo, Y.; Cao, W.; Cheng, X.; Xiao, B.; et al. Neuroserpin protects rat neurons and microglia-mediated inflammatory response sgainst oxygen-glucose deprivation- and reoxygenation treatments in an in vitro study. Cell. Physiol. Biochem. 2016, 38, 1472–1482. [Google Scholar] [CrossRef]
- Hastings, G.A.; Coleman, T.A.; Haudenschild, C.C.; Stefansson, S.; Smith, E.P.; Barthlow, R.; Cherry, S.; Sandkvist, M.; Lawrence, D.A. Neuroserpin, a brain-associated inhibitor of tissue plasminogen activator is localized primarily in neurons. Implications for the regulation of motor learning and neuronal survival. J. Biol. Chem. 1997, 272, 33062–33067. [Google Scholar] [CrossRef]
- Dupré, N.; Arabo, A.; Orset, C.; Maucotel, J.; Detroussel, Y.; Hauchecorne, M.; Gonzalez, B.J.; Marret, S.; Vivien, D.; Leroux, P. Neonatal cerebral hypoxia-ischemia in mice triggers age-dependent vascular effects and disabilities in adults; implication of tissue plasminogen activator (tPA). Exp. Neurol. 2020, 323, 113087. [Google Scholar] [CrossRef]
- Kilicdag, H.; Akillioglu, K.; Kilic Bagır, E.; Kose, S.; Erdogan, S. Neuroserpinas an adjuvant therapy for hypothermia on brain injury in neonatal hypoxic-ischemic rats. Am. J. Perinatol. 2024, 41, 1538–1543. [Google Scholar] [CrossRef]
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Madani, R.; Kozlov, S.; Akhmedov, A.; Cinelli, P.; Kinter, J.; Lipp, H.P.; Sonderegger, P.; Wolfer, D.P. Impaired explorative behavior and neophobia in genetically modified mice lacking or overexpressing the extracellular serine protease inhibitor neuroserpin. Mol. Cell. Neurosci. 2003, 23, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.B.; Feindel, K.W.; Cross, J.L.; Anderton, R.S.; Clark, V.W.; Knuckey, N.W.; Meloni, B.P. Modification to the Rice-Vannucci perinatal hypoxic-ischaemic encephalopathy model in the P7 rat improves the reliability of cerebral infarct development after 48 hours. J. Neurosci. Methods 2017, 288, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Grunke, S.D.; Levites, Y.; Golde, T.E.; Jankowsky, J.L. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J. Vis. Exp. 2014, 19, 51863. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.Y.; Pan, Y.; Zou, X.; Wang, C.; Peng, Y.; Ao, Y.L.; Lam, M.F.; Zhang, X.; Zhang, X.Q.; et al. The protective effect of (-)-Tetrahydroalstonine against OGD/R-induced neuronal injury via autophagy regulation. Molecules 2023, 28, 2370. [Google Scholar] [CrossRef]
- Liang, W.; Chuan-Zhen, L.; Qiang, D.; Jian, Q.; Hui-Min, R.; Bao-Guo, X. Reductions in mRNA of the neuroprotective agent, neuroserpin, after cerebral ischemia/reperfusion in diabetic rats. Brain Res. 2004, 1015, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Amano, K.; Takaki, E.; Ebrahim, A.S.; Shimohata, A.; Shibazaki, N.; Inoue, I.; Takaki, M.; Ueda, Y.; Sago, H.; et al. Increased lipid peroxidation in Down’s syndrome mouse models. J. Neurochem. 2009, 110, 1965–1976. [Google Scholar] [CrossRef]
- McRae, A.; Gilland, E.; Bona, E.; Hagberg, H. Microglia activation after neonatal hypoxic-ischemia. Brain Res. Dev. Brain Res. 1995, 84, 245–252. [Google Scholar] [CrossRef]
- Brégère, C.; Schwendele, B.; Radanovic, B.; Guzman, R. Microglia and stem-cell mediated neuroprotection after neonatal hypoxia-ischemia. Stem Cell Rev. Rep. 2022, 18, 474–522. [Google Scholar] [CrossRef]
- Gonda, Y.; Andrews, W.D.; Tabata, H.; Namba, T.; Parnavelas, J.G.; Nakajima, K.; Kohsaka, S.; Hanashima, C.; Uchino, S. Robo1 regulates the migration and laminar distribution of upper-layer pyramidal neurons of the cerebral cortex. Cereb. Cortex 2013, 23, 1495–1508. [Google Scholar] [CrossRef]
- Hoerder-Suabedissen, A.; Molnár, Z. Development, evolution and pathology of neocortical subplate neurons. Nat. Rev. Neurosci. 2015, 16, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, S.; Shin, M.; Kitazawa, A.; Ishii, K.; Tanuma, M.; Kasai, A.; Hashimoto, H.; Kubo, K.I.; Nakajima, K. Comprehensive characterization of migration profiles of murine cerebral cortical neurons during development using FlashTag labeling. iScience 2021, 24, 102277. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, E.; van Kammen, C.M.; de Theije, C.G.M.; van Meer, M.P.A.; Dijkhuizen, R.M.; Nijboer, C.H. A quantitative method for microstructural analysis of myelinated axons in the injured rodent brain. Sci. Rep. 2017, 7, 16492. [Google Scholar] [CrossRef]
- Garvey, A.A.; Pavel, A.M.; O’Toole, J.M.; Walsh, B.H.; Korotchikova, I.; Livingstone, V.; Dempsey, E.M.; Murray, D.M.; Boylan, G.B. Multichannel EEG abnormalities during the first 6 hours in infants with mild hypoxic-ischaemic encephalopathy. Pediatr. Res. 2021, 90, 117–124. [Google Scholar] [CrossRef]
- Natarajan, N.; Benedetti, G.; Perez, F.A.; Wood, T.R.; German, K.R.; Lockrow, J.P.; Puia-Dumitrescu, M.; Myers, E.; Mietzsch, U. Association between early EEG background and outcomes in infants with mild HIE undergoing therapeutic hypothermia. Pediatr. Neurol. 2022, 134, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Z.; Kwan, K.Y. Development and evolution of thalamocortical connectivity. Cold Spring Harb. Perspect. Biol. 2024, 16, a041503. [Google Scholar] [CrossRef]
- Erzurumlu, R.S.; Gaspar, P. Development and critical period plasticity of the barrel cortex. Eur. J. Neurosci. 2012, 35, 1540–1553. [Google Scholar] [CrossRef]
- Okusa, C.; Oeschger, F.; Ginet, V.; Wang, W.Z.; Hoerder-Suabedissen, A.; Matsuyama, T.; Truttmann, A.C.; Molnár, Z. Subplate in a rat model of preterm hypoxia-ischemia. Ann. Clin. Transl. Neurol. 2014, 1, 679–691. [Google Scholar] [CrossRef]
- Sheikh, A.; Meng, X.; Kao, J.P.Y.; Kanold, P.O. Neonatal hypoxia-ischemia causes persistent intracortical circuit changes in layer 4 of rat auditory cortex. Cereb. Cortex 2022, 32, 2575–2589. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yepes, M.; Jiang, Q.; Li, Q.; Arniego, P.; Coleman, T.A.; Lawrence, D.A.; Chopp, M. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation 2002, 106, 740–745. [Google Scholar] [CrossRef]
- Gelderblom, M.; Neumann, M.; Ludewig, P.; Bernreuther, C.; Krasemann, S.; Arunachalam, P.; Gerloff, C.; Glatzel, M.; Magnus, T. Deficiency in serine protease inhibitor neuroserpin exacerbates ischemic brain injury by increased postischemic inflammation. PLoS ONE 2013, 8, e63118. [Google Scholar] [CrossRef]
- Lebeurrier, N.; Liot, G.; Lopez-Atalaya, J.P.; Orset, C.; Fernandez-Monreal, M.; Sonderegger, P.; Ali, C.; Vivien, D. The brain-specific tissue-type plasminogen activator inhibitor, neuroserpin, protects neurons against excitotoxicity both in vitro and in vivo. Mol. Cell. Neurosci. 2005, 30, 552–558. [Google Scholar] [CrossRef]
- Cheng, Y.; Loh, Y.P.; Birch, N.P. Neuroserpin attenuates H2O2-induced oxidative stress in hippocampal neurons via AKT and BCL-2 signaling pathways. J. Mol. Neurosci. 2017, 61, 123–131. [Google Scholar] [CrossRef]
- Reumann, R.; Vierk, R.; Zhou, L.; Gries, F.; Kraus, V.; Mienert, J.; Romswinkel, E.; Morellini, F.; Ferrer, I.; Nicolini, C.; et al. The serine protease inhibitor neuroserpin is required for normal synaptic plasticity and regulates learning and social behavior. Learn. Mem. 2017, 24, 650–659. [Google Scholar] [CrossRef]
- D’Acunto, E.; Fra, A.; Visentin, C.; Manno, M.; Ricagno, S.; Galliciotti, G.; Miranda, E. Neuroserpin: Structure, function, physiology and pathology. Cell. Mol. Life Sci. 2021, 78, 6409–6430. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Z.; Luhmann, H.J.; Kanold, P.O. Transient cortical circuits match spontaneous and sensory-driven activity during development. Science 2020, 370, eabb2153. [Google Scholar] [CrossRef] [PubMed]
- Kement, D.; Reumann, R.; Schostak, K.; Voß, H.; Douceau, S.; Dottermusch, M.; Schweizer, M.; Schlüter, H.; Vivien, D.; Glatzel, M.; et al. Neuroserpin is strongly expressed in the developing and adult mouse neocortex but its absence does not perturb cortical lamination and synaptic proteome. Front. Neuroanat. 2021, 15, 627896. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawashita, E.; Fukuzaki, Y.; Fischer, J.; Shi, L.; Liao, Y.; Millar, L.J.; Zhong, P.; Hoerder-Suabedissen, A.; Soares, L.C.; Molnár, Z. Neuroserpin: A Potential Neuroprotective Agent in Mild Neonatal Hypoxic–Ischaemic Encephalopathy. Cells 2025, 14, 1840. https://doi.org/10.3390/cells14231840
Kawashita E, Fukuzaki Y, Fischer J, Shi L, Liao Y, Millar LJ, Zhong P, Hoerder-Suabedissen A, Soares LC, Molnár Z. Neuroserpin: A Potential Neuroprotective Agent in Mild Neonatal Hypoxic–Ischaemic Encephalopathy. Cells. 2025; 14(23):1840. https://doi.org/10.3390/cells14231840
Chicago/Turabian StyleKawashita, Eri, Yumi Fukuzaki, Jan Fischer, Lei Shi, Yumei Liao, Lancelot Jamie Millar, Peiyun Zhong, Anna Hoerder-Suabedissen, Luana Campos Soares, and Zoltán Molnár. 2025. "Neuroserpin: A Potential Neuroprotective Agent in Mild Neonatal Hypoxic–Ischaemic Encephalopathy" Cells 14, no. 23: 1840. https://doi.org/10.3390/cells14231840
APA StyleKawashita, E., Fukuzaki, Y., Fischer, J., Shi, L., Liao, Y., Millar, L. J., Zhong, P., Hoerder-Suabedissen, A., Soares, L. C., & Molnár, Z. (2025). Neuroserpin: A Potential Neuroprotective Agent in Mild Neonatal Hypoxic–Ischaemic Encephalopathy. Cells, 14(23), 1840. https://doi.org/10.3390/cells14231840






