A Comprehensive Review of Modern Cancer Therapies Utilizing Oncolytic Viruses
Abstract
1. Introduction
2. History of Using Oncolytic Viruses
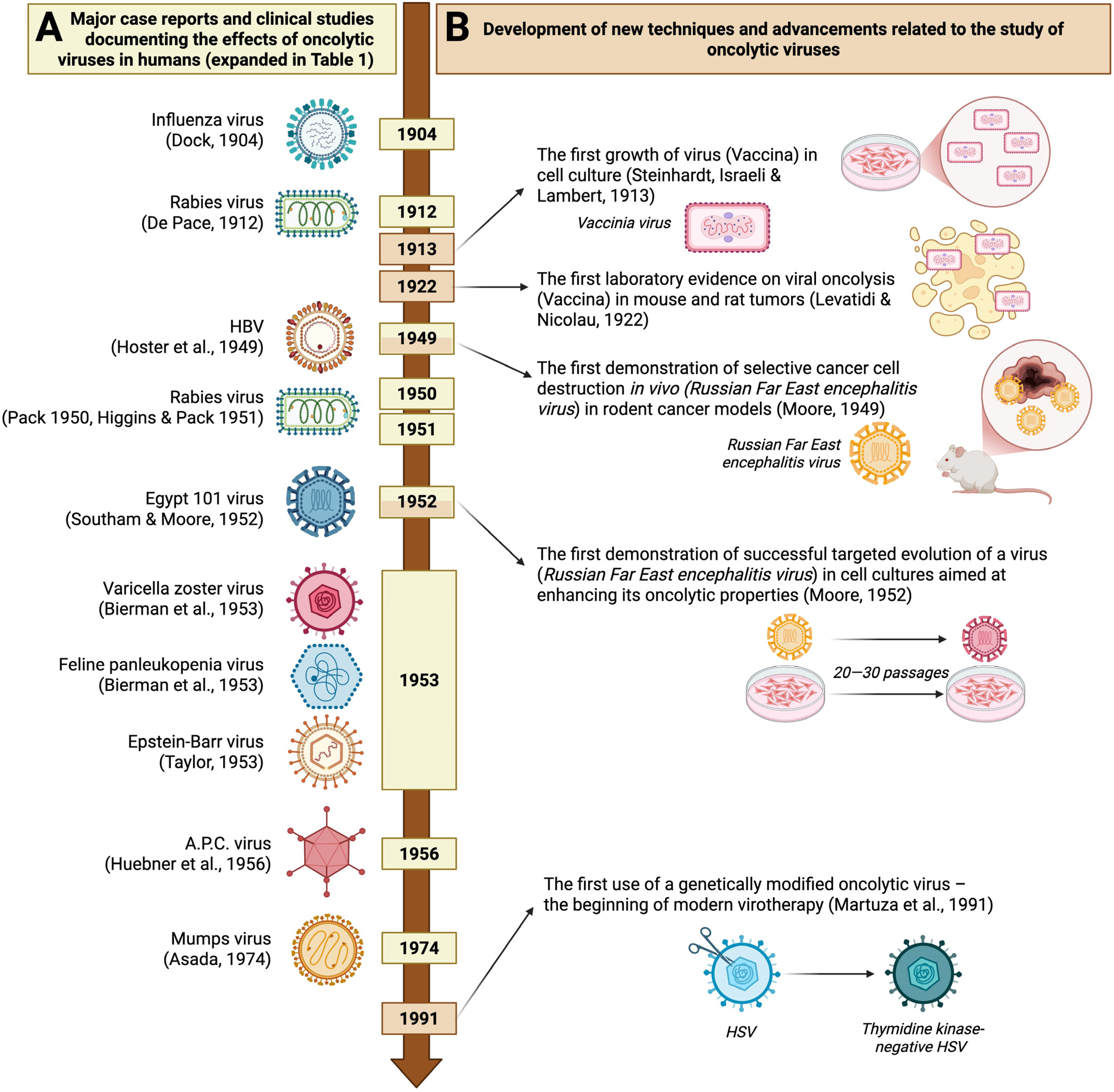
| Year | Authors | Virus (Family) | Disease | Study Detail | Outcome | Significance |
|---|---|---|---|---|---|---|
| 1904 | Dock, G. [4] | Influenza viruses (Orthomyxoviridae) | Leukemia | Case report describing a female patient who contracted influenza during her course of leukemia. | Several weeks of remission observed, with an overall improvement in well-being, followed by a reduction in the size of the liver and spleen and a decrease in leukocyte count. | The first significant study documenting the oncolytic effect of viral infection. |
| 1912 | De Pace, N. [9] | Attenuated Lyssavirus rabies (Rhabdoviridae) | Cervical cancer | Clinical study (8 patients) preceded by a case report demonstrating the oncolytic effect of an attenuated rabies vaccine. | Regression of cervical carcinoma in a patient who received Pasteur treatment after a dog bite, leading to the intentional use of an attenuated rabies virus in other patients. | The first recorded clinical trial of virotherapy. |
| 1949 | Hoster, H.A.; Zanes, R.P., Jr.; Von Haam, E. [13] | Hepatitis B virus (Hepadnaviridae) | Hodgkin lymphoma | Clinical study (21 patients), preceded by two case reports, showed that accidental hepatitis B virus infection had a beneficial effect on Hodgkin’s disease. | 13 of 21 patients developed viral symptoms, of whom 7 showed improvement (normalized leukocyte count, pain relief, tumor reduction). Patients without hepatitis symptoms showed no remission. | The first major clinical study using a hepatitis virus and showing a clear and significant therapeutic effect. |
| 1950 | Pack, G.T. [11] | Attenuated Lyssavirus rabies (Rhabdoviridae) | Melanoma | Clinical study of 12 private patients | Regressive changes were seen in 2 of 12 patients: loss of firmness in multiple existing cutaneous metastases (patient 1) and a reduction in the size of a liver previously affected by distant metastases (patient 2). | The first major series of clinical studies using attenuated live rabies virus (Harris rabies vaccine) for treating melanoma. |
| 1951 | Higgins, G.K.; Pack, G.T. [10] | Clinical study of 30 patients | Regressive changes observed in 8 of 30 patients with melanomatosis. | |||
| 1952 | Southam, C.M.; Moore, A. E. [16] | Egypt 101 virus [early passaged West Nile virus] (Flaviviridae) | Neoplasm diseases, with the majority being large bowel adenocarcinoma (26%), epidermoid carcinoma (21%), and breast cancer (9%). | Clinical study of 34 patients | 27 out of 34 patients were successfully infected. Among these, 4 out of 27 experienced transient regression of tumor growth; 5 out of 27 showed a probable viral effect on tumor growth inhibition; and 14 out of 27 demonstrated oncotropism, with the virus showing a preference for tumor tissue over healthy tissue in 5 out of 27 cases. | The first major clinical study using Flaviviridae viruses to target multiple neoplastic diseases. Demonstrates oncotropism without direct intratumoral viral administration. |
| 1953 | Bierman, H. R. et al. [8] | Varicella zoster virus (Orthoherpesviridae) | Leukemia | Clinical study involving 6 children, following a case report of a 1-month leukemia remission in a child who accidentally developed chickenpox. | 2 of the 6 children died during the incubation period, and the other four did not develop varicella (likely due to passive immunoglobulin transfer during blood transfusion). | First attempts at virotherapy using varicella and feline panleukopenia viruses against leukemia. |
| Feline panleukopenia virus (Parvoviridae) | Clinical study of 6 children | 2 out of 6 children died before showing any signs of viral infection; of the remaining four children who were injected with the virus, one experienced a one-month remission, characterized by a drop in leukocytes and clinical improvement. | ||||
| 1953 | Taylor, A. W. [12] | Epstein–Barr virus (Orthoherpesviridae) | Leukemia | Clinical study of 5 patients preceded by observation of spontaneous remission after accidental glandular fever infection. | 3 out of 5 patients showed a regression of leukemia symptoms, including a decrease in monoblast count and an increase in red blood cells and platelets, following the onset of glandular fever. In the remaining two patients, there was no evidence of viral infection. | First attempt at virotherapy using Epstein-Barr virus against leukemia. Documented exceptionally high effectiveness of the viral infection in inducing cancer remission. |
| 1956 | Huebner R.J. et al. [19] | Adenoidal-pharyngeal-conjunctival virus (Adenoviruses) | Epidermoid carcinoma of the cervix | Clinical study of 30 patients | 26 out of 40 virus injection cases caused local necrosis exclusively within the cancer tissue. The effect’s strength was inversely proportional to the amount of antibodies in the patients’ serum. | First attempt at virotherapy using a newly discovered family of viruses, first described in 1953. |
| 1974 | Asada, T. [22] | Mumps virus (Paramyxoviridae) | Terminal cancers of various types, mostly gastric (37% of cases), followed by lung (11%) and uterine cancers (10%) | Clinical study of 90 patients | Out of 90 cases, 37 showed tumor disappearance or more than 50% size reduction; 42 demonstrated tumor growth inhibition, regression, or overall health improvement; 11 exhibited no response, and 7 experienced adverse effects such as transient high fever and profuse bleeding. | The largest and most comprehensive clinical study on non-genetically engineered oncolytic viruses, including extensive visual and histological documentation of the observed changes. |
3. Types of OVs
3.1. Herpesvirus
3.2. Adenovirus
3.3. Vaccinia Virus
3.4. Reovirus
3.5. Coxsackievirus and Poliovirus
3.6. Measles Virus and Newcastle Disease Virus (NDV)
3.7. Vesicular Stomatitis Virus
4. OVs—Naturally Attenuated Viral Strains
5. OVs—Genetically Modified
6. Next-Generation OVs
7. Routes of Administration of Oncolytic Viruses in Anticancer Therapies
7.1. Local Administration (Intratumoral)
7.2. Systemic Administration (Intravenous)
8. Limitations of Oncolytic Virus Monotherapy
9. Combined OV Therapies
9.1. Combination of OVs with Radiotherapy or Chemotherapy
9.2. Combination of Oncolytic Viruses and Immune Checkpoint Inhibitors
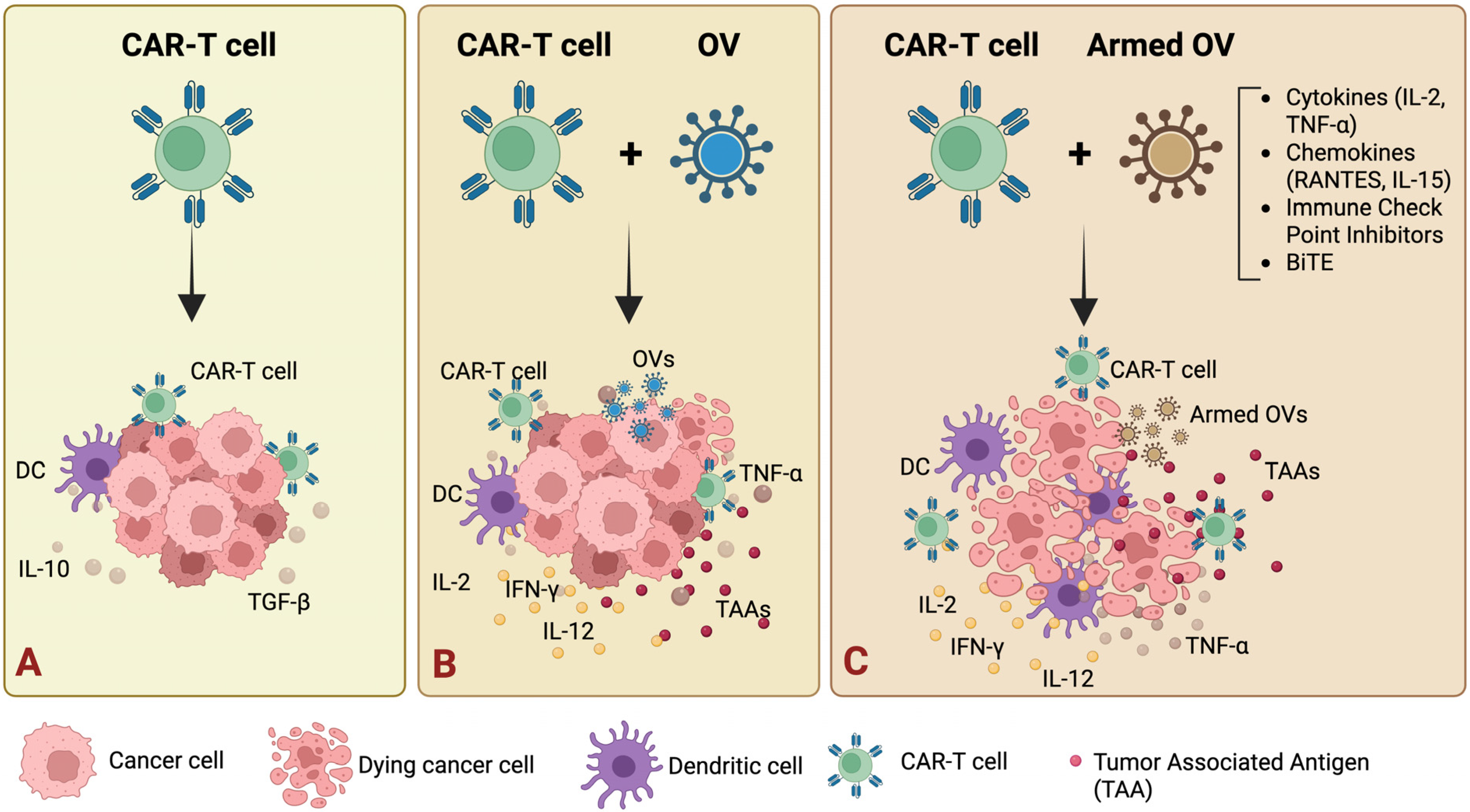
9.3. Combination of Oncolytic Viruses with CAR-T Cells
| Oncolytic Virus | Armed | Effect of OV | References |
|---|---|---|---|
| Adenovirus | Onc.—Ad RANTES IL-2 | Unblocking the tumor microenvironment for CAR-T (RANTES chemokine) and improving their survival/effector function and reducing systemic toxicity (local IL-2 effect) | [161] |
| Adenovirus | CAdvec-αPDL1 | Local, specific reversal of tumor microenvironment immunosuppression by blockade of the PD-1/PD-L1 axis without systemic effects. | [162] |
| Adenovirus | CAdvec-IL12 αPDL1 | Local, specific reversal of tumor microenvironment immunosuppression by blockade of the PD-1/PD-L1 axis without systemic effects, potent activation and proliferation of effector lymphocytes within the tumor via IL-12. | [163] |
| Adenovirus | oAD-IL7, B7H3-CAR-T | Significantly increased survival, proliferation, and tumor abundance of CAR-T lymphocytes, resulting in a more potent and long-lasting antitumor effect. | [164] |
| Adenovirus | CAd12_PDL1 | Local, specific reversal of tumor microenvironment immunosuppression by blockade of the PD-1/PD-L1 axis without systemic effects, potent activation and proliferation of effector lymphocytes within the tumor via IL-12. | [165] |
| Vaccinia virus | VSVmIFNβ EGFRvIII CAR T | Dual T cell activation—by tumor antigen (EGFRvIII) and by viral antigen VSV—and potent “reprogramming” of the tumor microenvironment—eliminating both the primary tumor and distant metastases. | [166] |
| Vaccinia virus | CAR/CXCL11 VV. CXCL11 | Significant increase in CAR-T influx and infiltration into the tumor due to CXCL11 secretion, transforming a “cold” tumor into a “hot” one. | [167] |
| Herpes virus | CAR-T/A56 antigen | Introduction of the A56 antigen into the tumor using an oncolytic virus enables highly selective and effective CAR-T therapy, even in areas where classic tumor target antigens are absent, and the side effects on healthy tissue are minimal. | [168] |
| Herpes virus | oHSV T7011 with CD19 or BCMA CAR T-cell | oHSV T7011 infects tumor cells and forces them to express “artificial” CAR antigens such as CD19 and BCMA, making them targets for conventional CAR-T. | [169] |
| Herpes virus | oHSV-1 CD70 CAR-T | The combination of oHSV-1 and CD70 CAR-T provides mutual enhancement of both therapies—enhancing CAR-T infiltration, activation, and survival, and “reprogramming” the tumor microenvironment from immunosuppressive to proinflammatory. | [170] |
| Pox virus | CD19-CAR-T | Introduction of the CD19 antigen into solid tumor cells that do not naturally express this marker, enabling the targeting of CD19-CAR-T cells (developed for the treatment of hematological malignancies) against solid tumors as well. | [171] |
10. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Garg, P.; Pareek, S.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Next-Generation Immunotherapy: Advancing Clinical Applications in Cancer Treatment. J. Clin. Med. 2024, 13, 6537. [Google Scholar] [CrossRef] [PubMed]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef]
- Dock, G. The influence of complicating diseases upon leukemia. Am. J. Med. Sci. 1904, 127, 563–592. [Google Scholar] [CrossRef]
- Rohdenburg, G.L. Fluctuations in the Growth Energy of Malignant Tumors in Man, with Especial Reference to Spontaneous Recession. J. Cancer Res. 1918, 3, 193–225. [Google Scholar]
- Kelly, E.; Russell, S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Wintrobe, M.M.; Cartwright, G.E.; Palmer, J.G.; Kuhns, W.J.; Samuels, L.T. Effect of corticotrophin and cortisone on the blood in various disorders in man. AMA Arch. Intern. Med. 1951, 88, 310–336. [Google Scholar] [CrossRef]
- Bierman, H.R.; Crile, D.M.; Dod, K.S.; Kelly, K.H.; Petrakis, N.L.; White, L.P.; Shimkin, M.B. Remissions in leukemia of childhood following acute infectious disease: Staphylococcus and streptococcus, varicella, and feline panleukopenia. Cancer 1953, 6, 591–605. [Google Scholar] [CrossRef]
- De Pace, N. Sulla scomparsa di un enorme cancro vegetante del collo dell’utero senza cura chirurgica. Ginecologia 1912, 9, 82–89. [Google Scholar]
- Higgins, G.K.; Pack, G.T. Virus therapy in the treatment of tumors. Bull. Hosp. Jt. Dis. 1951, 12, 379–382. [Google Scholar]
- Pack, G.T. Note on the experimental use of rabies vaccine for melanomatosis. AMA Arch. Derm. Syphilol. 1950, 62, 694–695. [Google Scholar] [CrossRef]
- Taylor, A.W. Effects of glandular fever infection in acute leukaemia. Br. Med. J. 1953, 1, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Hoster, H.A.; Zanes, R.P., Jr.; Von Haam, E. Studies in Hodgkin’s syndrome; the association of viral hepatitis and Hodgkin’s disease; a preliminary report. Cancer Res. 1949, 9, 473–480. [Google Scholar] [PubMed]
- Moore, A.E. The destructive effect of the virus of Russian Far East encephalitis on the transplantable mouse sarcoma 180. Cancer 1949, 2, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.E. Viruses with oncolytic properties and their adaptation to tumors. Ann. N. Y. Acad. Sci. 1952, 54, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Southam, C.M.; Moore, A.E. Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer 1952, 5, 1025–1034. [Google Scholar] [CrossRef]
- Rowe, W.P.; Huebner, R.J.; Gilmore, L.K.; Parrott, R.H.; Ward, T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1953, 84, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Huebner, R.J.; Rowe, W.P.; Ward, T.G.; Parrott, R.H.; Bell, J.A. Adenoidal-pharyngeal-conjunctival agents: A newly recognized group of common viruses of the respiratory system. N. Engl. J. Med. 1954, 251, 1077–1086. [Google Scholar] [CrossRef]
- Huebner, R.J.; Rowe, W.P.; Schatten, W.E.; Smith, R.R.; Thomas, L.B. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer 1956, 9, 1211–1218. [Google Scholar] [CrossRef]
- Pasquinucci, G. Possible effect of measles on leukaemia. Lancet 1971, 297, 136. [Google Scholar] [CrossRef]
- Zygiert, Z. Hodgkin’s disease: Remissions after measles. Lancet 1971, 297, 593. [Google Scholar] [CrossRef]
- Asada, T. Treatment of human cancer with mumps virus. Cancer 1974, 34, 1907–1928. [Google Scholar] [CrossRef]
- Southam, C.M. Division of Microbiology: Present Status of Oncolytic Virus Studies. Trans. N. Y. Acad. Sci. 1960, 22, 657–673. [Google Scholar] [CrossRef]
- Martuza, R.L.; Malick, A.; Markert, J.M.; Ruffner, K.L.; Coen, D.M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 1991, 252, 854–856. [Google Scholar] [CrossRef]
- Steinhardt, E.; Israeli, C.; Lambert, R. Studies on the cultivation of the virus of vaccinia. J. Infect. Dis. 1913, 13, 204–300. [Google Scholar] [CrossRef]
- Levaditi, C.; Nicolau, S. Affinite du virus herpetique pour les neoplasmes epitheliaux. Comptes Rendus Soc. Biol. 1922, 87, 498–500. [Google Scholar]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, J.; Zhu, D.; Wang, N.; Gao, Q.; Chen, W.; Tang, H.; Wang, J.; Zhang, X.; Liu, H.; et al. Cryo-EM structure of a herpesvirus capsid at 3.1 A. Science 2018, 360, eaao7283. [Google Scholar] [CrossRef] [PubMed]
- Manservigi, R.; Argnani, R.; Marconi, P. HSV Recombinant Vectors for Gene Therapy. Open Virol. J. 2010, 4, 123–156. [Google Scholar] [CrossRef] [PubMed]
- Agelidis, A.M.; Shukla, D. Cell entry mechanisms of HSV: What we have learned in recent years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Weed, D.J.; Nicola, A.V. Herpes simplex virus Membrane Fusion. Adv. Anat. Embryol. Cell Biol. 2017, 223, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Maruzuru, Y.; Shindo, K.; Liu, Z.; Oyama, M.; Kozuka-Hata, H.; Arii, J.; Kato, A.; Kawaguchi, Y. Role of herpes simplex virus 1 immediate early protein ICP22 in viral nuclear egress. J. Virol. 2014, 88, 7445–7454. [Google Scholar] [CrossRef]
- Shen, Y.; Nemunaitis, J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006, 13, 975–992. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Fehm, T.; Wallwiener, D.; Lauer, U. Oncolytic virotherapy of gynecologic malignancies. Gynecol. Oncol. 2011, 120, 302–310. [Google Scholar] [CrossRef]
- Mozzi, A.; Cagliani, R.; Pontremoli, C.; Forni, D.; Saulle, I.; Saresella, M.; Pozzoli, U.; Cappelletti, G.; Vantaggiato, C.; Clerici, M.; et al. Simplexviruses Successfully Adapt to Their Host by Fine-Tuning Immune Responses. Mol. Biol. Evol. 2022, 39, msac142. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Pala, L.; Conforti, F.; Cocorocchio, E. Talimogene Laherparepvec (T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers 2021, 13, 1383. [Google Scholar] [CrossRef]
- Markert, J.M.; Razdan, S.N.; Kuo, H.C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Robinson, C.M.; Singh, G.; Lee, J.Y.; Dehghan, S.; Rajaiya, J.; Liu, E.B.; Yousuf, M.A.; Betensky, R.A.; Jones, M.S.; Dyer, D.W.; et al. Molecular evolution of human adenoviruses. Sci. Rep. 2013, 3, 1812. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.L.; Post, D.E.; Khuri, F.R.; Van Meir, E.G. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin. Cancer Res. 2004, 10, 5299–5312. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, N.; Burga, L.N.; Poirier, J.T.; Bostina, M. Virus-Receptor Interactions: Structural Insights For Oncolytic Virus Development. Oncolytic Virotherapy 2019, 8, 39–56. [Google Scholar] [CrossRef]
- Gaden, F.; Franqueville, L.; Magnusson, M.K.; Hong, S.S.; Merten, M.D.; Lindholm, L.; Boulanger, P. Gene transduction and cell entry pathway of fiber-modified adenovirus type 5 vectors carrying novel endocytic peptide ligands selected on human tracheal glandular cells. J. Virol. 2004, 78, 7227–7247. [Google Scholar] [CrossRef]
- Charman, M.; Herrmann, C.; Weitzman, M.D. Viral and cellular interactions during adenovirus DNA replication. FEBS Lett. 2019, 593, 3531–3550. [Google Scholar] [CrossRef] [PubMed]
- Schaack, J.; Bennett, M.L.; Colbert, J.D.; Torres, A.V.; Clayton, G.H.; Ornelles, D.; Moorhead, J. E1A and E1B proteins inhibit inflammation induced by adenovirus. Proc. Natl. Acad. Sci. USA 2004, 101, 3124–3129. [Google Scholar] [CrossRef]
- Abudoureyimu, M.; Lai, Y.; Tian, C.; Wang, T.; Wang, R.; Chu, X. Oncolytic Adenovirus—A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC. Front. Oncol. 2019, 9, 1182. [Google Scholar] [CrossRef]
- Hidalgo, P.; Ip, W.H.; Dobner, T.; Gonzalez, R.A. The biology of the adenovirus E1B 55K protein. FEBS Lett. 2019, 593, 3504–3517. [Google Scholar] [CrossRef]
- Liu, T.C.; Hallden, G.; Wang, Y.; Brooks, G.; Francis, J.; Lemoine, N.; Kirn, D. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol. Ther. 2004, 9, 786–803. [Google Scholar] [CrossRef] [PubMed]
- Niemann, J.; Kuhnel, F. Oncolytic viruses: Adenoviruses. Virus Genes 2017, 53, 700–706. [Google Scholar] [CrossRef]
- Gomez-Navarro, J.; Curiel, D.T. Conditionally replicative adenoviral vectors for cancer gene therapy. Lancet Oncol. 2000, 1, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zheng, S.; Li, X.F.; Huang, J.J.; Zheng, X.; Li, Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: A pilot phase II clinical trial. World J. Gastroenterol. 2004, 10, 3634–3638. [Google Scholar] [CrossRef]
- Makower, D.; Rozenblit, A.; Kaufman, H.; Edelman, M.; Lane, M.E.; Zwiebel, J.; Haynes, H.; Wadler, S. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin. Cancer Res. 2003, 9, 693–702. [Google Scholar] [PubMed]
- Nemunaitis, J.; Khuri, F.; Ganly, I.; Arseneau, J.; Posner, M.; Vokes, E.; Kuhn, J.; McCarty, T.; Landers, S.; Blackburn, A.; et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J. Clin. Oncol. 2001, 19, 289–298. [Google Scholar] [CrossRef]
- Burke, J.M.; Lamm, D.L.; Meng, M.V.; Nemunaitis, J.J.; Stephenson, J.J.; Arseneau, J.C.; Aimi, J.; Lerner, S.; Yeung, A.W.; Kazarian, T.; et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 2012, 188, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Millward, S.; Graham, A.F. Structural studies on reovirus: Discontinuities in the genome. Proc. Natl. Acad. Sci. USA 1970, 65, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Antar, A.A.; Konopka, J.L.; Campbell, J.A.; Henry, R.A.; Perdigoto, A.L.; Carter, B.D.; Pozzi, A.; Abel, T.W.; Dermody, T.S. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 2009, 5, 59–71. [Google Scholar] [CrossRef]
- McSherry, E.A.; McGee, S.F.; Jirstrom, K.; Doyle, E.M.; Brennan, D.J.; Landberg, G.; Dervan, P.A.; Hopkins, A.M.; Gallagher, W.M. JAM-A expression positively correlates with poor prognosis in breast cancer patients. Int. J. Cancer 2009, 125, 1343–1351. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, W.; Huang, B.; Liu, Z.; Sun, L.; Zhang, Q.; Qiu, X.; Xu, K.; Wang, E. Overexpression of JAM-A in non-small cell lung cancer correlates with tumor progression. PLoS ONE 2013, 8, e79173. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.P.; Sun, Y.F.; Fang, Y.; Song, Q.; Yan, Z.X.; Chen, Y.; Jiang, X.F.; Fei, X.C.; Zhao, Y.; Leboeuf, C.; et al. JAM-A overexpression is related to disease progression in diffuse large B-cell lymphoma and downregulated by lenalidomide. Sci. Rep. 2017, 7, 7433. [Google Scholar] [CrossRef]
- Kelly, K.R.; Espitia, C.M.; Zhao, W.; Wendlandt, E.; Tricot, G.; Zhan, F.; Carew, J.S.; Nawrocki, S.T. Junctional adhesion molecule-A is overexpressed in advanced multiple myeloma and determines response to oncolytic reovirus. Oncotarget 2015, 6, 41275–41289. [Google Scholar] [CrossRef]
- Marcato, P.; Shmulevitz, M.; Pan, D.; Stoltz, D.; Lee, P.W. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol. Ther. 2007, 15, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Errington, F.; White, C.L.; Twigger, K.R.; Rose, A.; Scott, K.; Steele, L.; Ilett, L.J.; Prestwich, R.; Pandha, H.S.; Coffey, M.; et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008, 15, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Berkeley, R.; Barr, T.; Ilett, E.; Errington-Mais, F. Past, Present and Future of Oncolytic Reovirus. Cancers 2020, 12, 3219. [Google Scholar] [CrossRef]
- Mahalingam, D.; Fountzilas, C.; Moseley, J.; Noronha, N.; Tran, H.; Chakrabarty, R.; Selvaggi, G.; Coffey, M.; Thompson, B.; Sarantopoulos, J. A phase II study of REOLYSIN((R)) (pelareorep) in combination with carboplatin and paclitaxel for patients with advanced malignant melanoma. Cancer Chemother. Pharmacol. 2017, 79, 697–703. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Fong, Y.; Warner, S.G. Oncolytic Virotherapy for Cancer: Clinical Experience. Biomedicines 2021, 9, 419. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Shafren, D.R.; Au, G.G.; Nguyen, T.; Newcombe, N.G.; Haley, E.S.; Beagley, L.; Johansson, E.S.; Hersey, P.; Barry, R.D. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus a21. Clin. Cancer Res. 2004, 10, 53–60. [Google Scholar] [CrossRef]
- Burke, M.J. Oncolytic Seneca Valley Virus: Past perspectives and future directions. Oncolytic Virotherapy 2016, 5, 81–89. [Google Scholar] [CrossRef]
- Gromeier, M.; Lachmann, S.; Rosenfeld, M.R.; Gutin, P.H.; Wimmer, E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl. Acad. Sci. USA 2000, 97, 6803–6808. [Google Scholar] [CrossRef]
- Cox, R.M.; Plemper, R.K. Structure and organization of paramyxovirus particles. Curr. Opin. Virol. 2017, 24, 105–114. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Yadava, P.K. Measles virus: Background and oncolytic virotherapy. Biochem. Biophys. Rep. 2018, 13, 58–62. [Google Scholar] [CrossRef]
- Leber, M.F.; Neault, S.; Jirovec, E.; Barkley, R.; Said, A.; Bell, J.C.; Ungerechts, G. Engineering and combining oncolytic measles virus for cancer therapy. Cytokine Growth Factor Rev. 2020, 56, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, M.D. Measles virus in cancer therapy. Curr. Opin. Virol. 2020, 41, 85–97. [Google Scholar] [CrossRef]
- Meng, Q.; He, J.; Zhong, L.; Zhao, Y. Advances in the Study of Antitumour Immunotherapy for Newcastle Disease Virus. Int. J. Med. Sci. 2021, 18, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Palese, P. Oncolytic Newcastle disease virus for cancer therapy: Old challenges and new directions. Future Microbiol. 2012, 7, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Martinez-Sobrido, L.; Kelly, K.; Mansour, M.; Sheng, G.; Vigil, A.; Garcia-Sastre, A.; Palese, P.; Fong, Y. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol. Ther. 2009, 17, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Bai, F.; Sun, T.; Tian, H.; Yu, D.; Yin, J.; Li, S.; Li, T.; Cao, H.; Yu, Q.; et al. Recombinant Newcastle Disease virus Expressing IL15 Demonstrates Promising Antitumor Efficiency in Melanoma Model. Technol. Cancer Res. Treat. 2015, 14, 607–615. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, Y.; Hong, X.; Liu, X.; Su, X.; Li, S.; Dong, X.; Zhao, G.; Li, Y. Newcastle disease virus enhances the growth-inhibiting and proapoptotic effects of temozolomide on glioblastoma cells in vitro and in vivo. Sci. Rep. 2018, 8, 11470. [Google Scholar] [CrossRef]
- Lam, H.Y.; Yeap, S.K.; Pirozyan, M.R.; Omar, A.R.; Yusoff, K.; Suraini, A.A.; Abd-Aziz, S.; Alitheen, N.B. Safety and clinical usage of newcastle disease virus in cancer therapy. J. Biomed. Biotechnol. 2011, 2011, 718710. [Google Scholar] [CrossRef]
- Melzer, M.K.; Lopez-Martinez, A.; Altomonte, J. Oncolytic Vesicular Stomatitis Virus as a Viro-Immunotherapy: Defeating Cancer with a “Hammer” and “Anvil”. Biomedicines 2017, 5, 8. [Google Scholar] [CrossRef]
- Felt, S.A.; Grdzelishvili, V.Z. Recent advances in vesicular stomatitis virus-based oncolytic virotherapy: A 5-year update. J. Gen. Virol. 2017, 98, 2895–2911. [Google Scholar] [CrossRef] [PubMed]
- Kohlhapp, F.J.; Zloza, A.; Kaufman, H.L. Talimogene laherparepvec (T-VEC) as cancer immunotherapy. Drugs Today 2015, 51, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Dorig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 1993, 75, 295–305. [Google Scholar] [CrossRef]
- Au, G.G.; Lincz, L.F.; Enno, A.; Shafren, D.R. Oncolytic Coxsackievirus A21 as a novel therapy for multiple myeloma. Br. J. Haematol. 2007, 137, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Norell, H.; Bryceson, Y.T.; Poschke, I.; Schedvins, K.; Ljunggren, H.G.; Kiessling, R.; Malmberg, K.J. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol. 2009, 183, 4921–4930. [Google Scholar] [CrossRef]
- Di Piazza, M.; Mader, C.; Geletneky, K.; Herrero, Y.C.M.; Weber, E.; Schlehofer, J.; Deleu, L.; Rommelaere, J. Cytosolic activation of cathepsins mediates parvovirus H-1-induced killing of cisplatin and TRAIL-resistant glioma cells. J. Virol. 2007, 81, 4186–4198. [Google Scholar] [CrossRef]
- Angelova, A.L.; Grekova, S.P.; Heller, A.; Kuhlmann, O.; Soyka, E.; Giese, T.; Aprahamian, M.; Bour, G.; Ruffer, S.; Cziepluch, C.; et al. Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer. J. Virol. 2014, 88, 5263–5276. [Google Scholar] [CrossRef]
- Grekova, S.; Aprahamian, M.; Giese, N.; Schmitt, S.; Giese, T.; Falk, C.S.; Daeffler, L.; Cziepluch, C.; Rommelaere, J.; Raykov, Z. Immune cells participate in the oncosuppressive activity of parvovirus H-1PV and are activated as a result of their abortive infection with this agent. Cancer Biol. Ther. 2010, 10, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Kombe Kombe, A.J.; Fotoohabadi, L.; Gerasimova, Y.; Nanduri, R.; Lama Tamang, P.; Kandala, M.; Kelesidis, T. The Role of Inflammation in the Pathogenesis of Viral Respiratory Infections. Microorganisms 2024, 12, 2526. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; et al. The interaction of innate immune and adaptive immune system. MedComm 2024, 5, e714. [Google Scholar] [CrossRef] [PubMed]
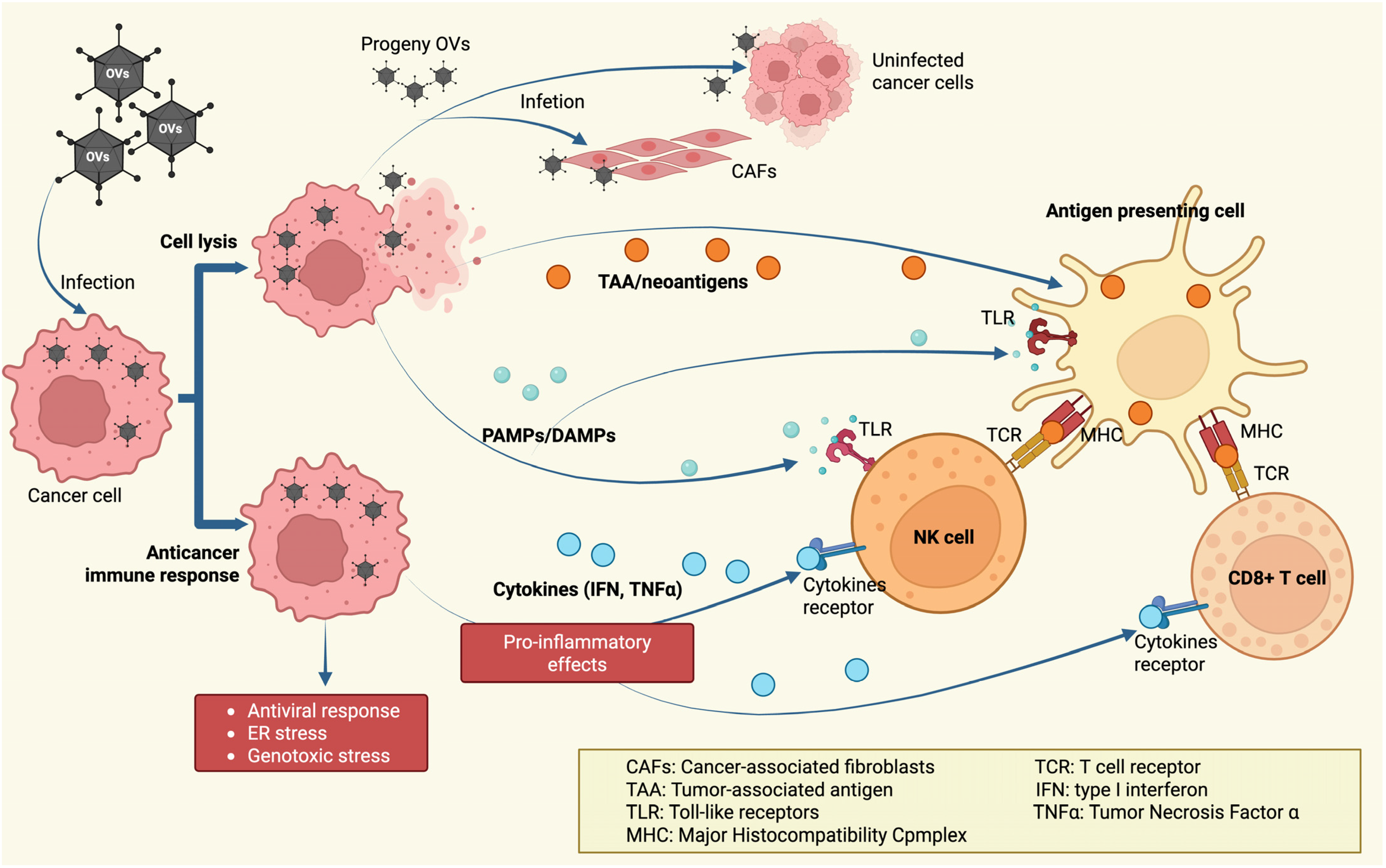
- Jhawar, S.R.; Thandoni, A.; Bommareddy, P.K.; Hassan, S.; Kohlhapp, F.J.; Goyal, S.; Schenkel, J.M.; Silk, A.W.; Zloza, A. Oncolytic Viruses-Natural and Genetically Engineered Cancer Immunotherapies. Front. Oncol. 2017, 7, 202. [Google Scholar] [CrossRef]
- Mechanisms of Action of Oncolytic Virus Destroying Tumor Cells. Available online: https://www.creative-biolabs.com/oncolytic-virus/mechanisms-of-action-of-oncolytic-virus-destroying-tumor-cells.htm (accessed on 25 September 2025).
- Dart, A. Tumour evolution: Metastasis takes a different route. Nat. Rev. Cancer 2017, 17, 509. [Google Scholar] [CrossRef]
- Rivera-Orellana, S.; Bautista, J.; Palacios-Zavala, D.; Ojeda-Mosquera, S.; Altamirano-Colina, A.; Alcocer-Veintimilla, M.; Parrales-Rosales, G.; Izquierdo-Condoy, J.S.; Vasconez-Gonzalez, J.; Ortiz-Prado, E.; et al. Oncolytic virotherapy and tumor microenvironment modulation. Clin. Exp. Med. 2025, 25, 256. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Fang, H. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets 2007, 7, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Grigg, C.; Blake, Z.; Gartrell, R.; Sacher, A.; Taback, B.; Saenger, Y. Talimogene laherparepvec (T-Vec) for the treatment of melanoma and other cancers. Semin. Oncol. 2016, 43, 638–646. [Google Scholar] [CrossRef]
- DeWeese, T.L.; van der Poel, H.; Li, S.; Mikhak, B.; Drew, R.; Goemann, M.; Hamper, U.; DeJong, R.; Detorie, N.; Rodriguez, R.; et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001, 61, 7464–7472. [Google Scholar]
- Cristi, F.; Gutierrez, T.; Hitt, M.M.; Shmulevitz, M. Genetic Modifications That Expand Oncolytic Virus Potency. Front. Mol. Biosci. 2022, 9, 831091. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhao, X.; Wu, X.; Guo, Y.; Guo, H.; Cao, J.; Guo, Y.; Lou, D.; Yu, D.; Li, J. A Phase I study of KH901, a conditionally replicating granulocyte-macrophage colony-stimulating factor: Armed oncolytic adenovirus for the treatment of head and neck cancers. Cancer Biol. Ther. 2009, 8, 676–682. [Google Scholar] [CrossRef]
- Neuman, E.; Flemington, E.K.; Sellers, W.R.; Kaelin, W.G., Jr. Transcription of the E2F-1 gene is rendered cell cycle dependent by E2F DNA-binding sites within its promoter. Mol. Cell. Biol. 1994, 14, 6607–6615. [Google Scholar] [CrossRef]
- Ramesh, N.; Ge, Y.; Ennist, D.L.; Zhu, M.; Mina, M.; Ganesh, S.; Reddy, P.S.; Yu, D.C. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor--armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. 2006, 12, 305–313. [Google Scholar] [CrossRef]
- Post, D.E.; Sandberg, E.M.; Kyle, M.M.; Devi, N.S.; Brat, D.J.; Xu, Z.; Tighiouart, M.; Van Meir, E.G. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 2007, 67, 6872–6881. [Google Scholar] [CrossRef]
- Kokoris, M.S.; Black, M.E. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci. 2002, 11, 2267–2272. [Google Scholar] [CrossRef]
- Doronin, K.; Toth, K.; Kuppuswamy, M.; Ward, P.; Tollefson, A.E.; Wold, W.S. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J. Virol. 2000, 74, 6147–6155. [Google Scholar] [CrossRef]
- Ghasemi Darestani, N.; Gilmanova, A.I.; Al-Gazally, M.E.; Zekiy, A.O.; Ansari, M.J.; Zabibah, R.S.; Jawad, M.A.; Al-Shalah, S.A.J.; Rizaev, J.A.; Alnassar, Y.S.; et al. Mesenchymal stem cell-released oncolytic virus: An innovative strategy for cancer treatment. Cell Commun. Signal. 2023, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Hirvinen, M.; Rajecki, M.; Kapanen, M.; Parviainen, S.; Rouvinen-Lagerstrom, N.; Diaconu, I.; Nokisalmi, P.; Tenhunen, M.; Hemminki, A.; Cerullo, V. Immunological effects of a tumor necrosis factor alpha-armed oncolytic adenovirus. Hum. Gene Ther. 2015, 26, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Sova, P.; Ren, X.W.; Ni, S.; Bernt, K.M.; Mi, J.; Kiviat, N.; Lieber, A. A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol. Ther. 2004, 9, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Jouan-Lanhouet, S.; Arshad, M.I.; Piquet-Pellorce, C.; Martin-Chouly, C.; Le Moigne-Muller, G.; Van Herreweghe, F.; Takahashi, N.; Sergent, O.; Lagadic-Gossmann, D.; Vandenabeele, P.; et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012, 19, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Sosna, J.; Philipp, S.; Fuchslocher Chico, J.; Saggau, C.; Fritsch, J.; Foll, A.; Plenge, J.; Arenz, C.; Pinkert, T.; Kalthoff, H.; et al. Differences and Similarities in TRAIL- and Tumor Necrosis Factor-Mediated Necroptotic Signaling in Cancer Cells. Mol. Cell. Biol. 2016, 36, 2626–2644. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, D.; Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 117. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Patel, A.; Hossain, S.; Kaufman, H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, P.K.; Silk, A.W.; Kaufman, H.L. Intratumoral Approaches for the Treatment of Melanoma. Cancer J. 2017, 23, 40–47. [Google Scholar] [CrossRef]
- Mastrangelo, M.J.; Maguire, H.C., Jr.; Eisenlohr, L.C.; Laughlin, C.E.; Monken, C.E.; McCue, P.A.; Kovatich, A.J.; Lattime, E.C. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999, 6, 409–422. [Google Scholar] [CrossRef]
- Yan, W.; Xuan, Y.; Wang, R.; Huan, Z.; Guo, Y.; Dun, H.; Xu, L.; Han, R.; Sun, X.; Si, L.; et al. Oncolytic Vaccinia Virus Armed with GM-CSF and IL-7 Enhances Antitumor Immunity in Pancreatic Cancer. Biomedicines 2025, 13, 882. [Google Scholar] [CrossRef]
- Deng, L.; Yang, X.; Fan, J.; Ding, Y.; Peng, Y.; Xu, D.; Huang, B.; Hu, Z. An Oncolytic Vaccinia Virus Armed with GM-CSF and IL-24 Double Genes for Cancer Targeted Therapy. Onco Targets Ther. 2020, 13, 3535–3544. [Google Scholar] [CrossRef]
- Deng, L.; Yang, X.; Fan, J.; Ding, Y.; Peng, Y.; Xu, D.; Huang, B.; Hu, Z. IL-24-Armed Oncolytic Vaccinia Virus Exerts Potent Antitumor Effects via Multiple Pathways in Colorectal Cancer. Oncol. Res. 2021, 28, 579–590. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, H.L.; Zhang, X.R.; Xu, J.P.; Hu, W.K.; Liang, M.; Chen, S.Y.; Hu, F.; Chu, D.T. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009, 16, 376–382. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Fischietti, M.; Zazzeroni, F.; Alesse, E. Targeting costimulatory molecules to improve antitumor immunity. J. Biomed. Biotechnol. 2012, 2012, 926321. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Milenova, I.; Wenthe, J.; Stahle, M.; Leja-Jarblad, J.; Ullenhag, G.; Dimberg, A.; Moreno, R.; Alemany, R.; Loskog, A. Shaping the Tumor Stroma and Sparking Immune Activation by CD40 and 4-1BB Signaling Induced by an Armed Oncolytic Virus. Clin. Cancer Res. 2017, 23, 5846–5857. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Deraffele, G.; Mitcham, J.; Moroziewicz, D.; Cohen, S.M.; Hurst-Wicker, K.S.; Cheung, K.; Lee, D.S.; Divito, J.; Voulo, M.; et al. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J. Clin. Investig. 2005, 115, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Autio, K.; Knuuttila, A.; Kipar, A.; Pesonen, S.; Guse, K.; Parviainen, S.; Rajamaki, M.; Laitinen-Vapaavuori, O.; Vaha-Koskela, M.; Kanerva, A.; et al. Safety and biodistribution of a double-deleted oncolytic vaccinia virus encoding CD40 ligand in laboratory Beagles. Mol. Ther. Oncolytics 2014, 1, 14002. [Google Scholar] [CrossRef]
- Ylosmaki, E.; Ylosmaki, L.; Fusciello, M.; Martins, B.; Ahokas, P.; Cojoc, H.; Uoti, A.; Feola, S.; Kreutzman, A.; Ranki, T.; et al. Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform. Mol. Ther. Oncolytics 2021, 20, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Wenthe, J.; Naseri, S.; Hellstrom, A.C.; Wiklund, H.J.; Eriksson, E.; Loskog, A. Immunostimulatory oncolytic virotherapy for multiple myeloma targeting 4-1BB and/or CD40. Cancer Gene Ther. 2020, 27, 948–959. [Google Scholar] [CrossRef]
- Wenthe, J.; Naseri, S.; Labani-Motlagh, A.; Enblad, G.; Wikstrom, K.I.; Eriksson, E.; Loskog, A.; Lovgren, T. Boosting CAR T-cell responses in lymphoma by simultaneous targeting of CD40/4-1BB using oncolytic viral gene therapy. Cancer Immunol. Immunother. 2021, 70, 2851–2865. [Google Scholar] [CrossRef]
- Ylosmaki, E.; Cerullo, V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 25–36. [Google Scholar] [CrossRef]
- Chen, L.; Wang, P.; Di Gioia, C.; Yuan, M.; Zhang, Z.; Miao, J.; Yan, W.; Zhao, G.; Jia, Y.; Wang, N.; et al. A novel oncolytic Vaccinia virus armed with IL-12 augments antitumor immune responses leading to durable regression in murine models of lung cancer. Front. Immunol. 2024, 15, 1492464. [Google Scholar] [CrossRef] [PubMed]
- Hatami, J.; Das, K.; Wolf, L.; Aufschnaiter, A.; Kimpel, J.; Nolden, T.; Schreiber, L.M.; Mullauer, B.; Podgorschek, E.; Schwaiger, T.; et al. Interleukin-12 encoded by the oncolytic virus VSV-GP enhances therapeutic antitumor efficacy by inducing CD8+ T-cell responses with a long-lived effector cell phenotype. J. Immunother. Cancer 2025, 13, e010675. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, N.; Jin, J.; Zhang, Z.; Lu, H.; Xu, L.; Chen, Y.; Jin, L.; Zhou, L.; Yang, H.; et al. Preclinical and clinical evaluation of intratumoral injection of an IL-12 expressing SKV-012 oncolytic virus for advanced solid tumors. J. Immunother. Cancer 2025, 13, e011642. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Shen, Y.; Dong, X.; He, R.; Chen, G.; Zhang, Y.; Tan, H.; Zhang, K. Nanoengineering-armed oncolytic viruses drive antitumor response: Progress and challenges. MedComm 2024, 5, e755. [Google Scholar] [CrossRef]
- Bazan-Peregrino, M.; Garcia-Carbonero, R.; Laquente, B.; Alvarez, R.; Mato-Berciano, A.; Gimenez-Alejandre, M.; Morgado, S.; Rodriguez-Garcia, A.; Maliandi, M.V.; Riesco, M.C.; et al. VCN-01 disrupts pancreatic cancer stroma and exerts antitumor effects. J. Immunother. Cancer 2021, 9, e003254. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Bazan-Peregrino, M.; Gil-Martin, M.; Alvarez, R.; Macarulla, T.; Riesco-Martinez, M.C.; Verdaguer, H.; Guillen-Ponce, C.; Farrera-Sal, M.; Moreno, R.; et al. Phase I, multicenter, open-label study of intravenous VCN-01 oncolytic adenovirus with or without nab-paclitaxel plus gemcitabine in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e003255. [Google Scholar] [CrossRef]
- Moreno, V.; Barretina-Ginesta, M.P.; Garcia-Donas, J.; Jayson, G.C.; Roxburgh, P.; Vazquez, R.M.; Michael, A.; Anton-Torres, A.; Brown, R.; Krige, D.; et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev with or without paclitaxel in platinum-resistant ovarian cancer: A phase 1 clinical trial. J. Immunother. Cancer 2021, 9, e003645. [Google Scholar] [CrossRef]
- Fakih, M.; Harb, W.; Mahadevan, D.; Babiker, H.; Berlin, J.; Lillie, T.; Krige, D.; Carter, J.; Cox, C.; Patel, M.; et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev, in combination with nivolumab, in patients with advanced/metastatic epithelial cancer: A phase I clinical trial (SPICE). J. Immunother. Cancer 2023, 11, e006561. [Google Scholar] [CrossRef]
- Dyer, A.; Di, Y.; Calderon, H.; Illingworth, S.; Kueberuwa, G.; Tedcastle, A.; Jakeman, P.; Chia, S.L.; Brown, A.; Silva, M.A.; et al. Oncolytic Group B Adenovirus Enadenotucirev Mediates Non-apoptotic Cell Death with Membrane Disruption and Release of Inflammatory Mediators. Mol. Ther. Oncolytics 2017, 4, 18–30. [Google Scholar] [CrossRef]
- Machiels, J.P.; Salazar, R.; Rottey, S.; Duran, I.; Dirix, L.; Geboes, K.; Wilkinson-Blanc, C.; Pover, G.; Alvis, S.; Champion, B.; et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE). J. Immunother. Cancer 2019, 7, 20. [Google Scholar] [CrossRef]
- Shoaf, M.L.; Desjardins, A. Oncolytic Viral Therapy for Malignant Glioma and Their Application in Clinical Practice. Neurotherapeutics 2022, 19, 1818–1831. [Google Scholar] [CrossRef]
- Suryawanshi, Y.R.; Schulze, A.J. Oncolytic Viruses for Malignant Glioma: On the Verge of Success? Viruses 2021, 13, 1294. [Google Scholar] [CrossRef] [PubMed]
- Perez-Larraya, J.G.; de la Nava, D.; González-Huárriz, M.; García-Moure, M.; Labiano, S.; Zalacaín, M.; Marrodán, L.; Ausejo, I.; Laspidea, V.; Hervás, I.; et al. P17.15.A Thank Phase I Trial of DNX-2440 Oncolytic Adenovirus in Patients with First or Second Recurrence of Glioblastoma: Preliminary Results. Neuro-Oncol. 2023, 25 (Suppl. S2), ii120. [Google Scholar] [CrossRef]
- Maruyama, Y.; Sakurai, A.; Noda, S.; Fujiwara, Y.; Okura, N.; Takagi, T.; Asano, J.; Honda, F. Regulatory Issues: PMDA—Review of Sakigake Designation Products: Oncolytic Virus Therapy with Delytact Injection (Teserpaturev) for Malignant Glioma. Oncologist 2023, 28, 664–670. [Google Scholar] [CrossRef]
- Wu, X.; Alvarez-Breckenridge, C. Moving the Pendulum for Glioblastoma Treatment: One Injection at a Time. Oncologist 2023, 28, 651–653. [Google Scholar] [CrossRef]
- Asija, S.; Chatterjee, A.; Goda, J.S.; Yadav, S.; Chekuri, G.; Purwar, R. Oncolytic immunovirotherapy for high-grade gliomas: A novel and an evolving therapeutic option. Front. Immunol. 2023, 14, 1118246. [Google Scholar] [CrossRef]
- Ricordel, M.; Foloppe, J.; Pichon, C.; Sfrontato, N.; Antoine, D.; Tosch, C.; Cochin, S.; Cordier, P.; Quemeneur, E.; Camus-Bouclainville, C.; et al. Cowpox Virus: A New and Armed Oncolytic Poxvirus. Mol. Ther. Oncolytics 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mirbahari, S.N.; Da Silva, M.; Zuniga, A.I.M.; Kooshki Zamani, N.; St-Laurent, G.; Totonchi, M.; Azad, T. Recent progress in combination therapy of oncolytic vaccinia virus. Front. Immunol. 2024, 15, 1272351. [Google Scholar] [CrossRef]
- Gao, P.; Ding, G.; Wang, L. The efficacy and safety of oncolytic viruses in the treatment of intermediate to advanced solid tumors: A systematic review and meta-analysis. Transl. Cancer Res. 2021, 10, 4290–4302. [Google Scholar] [CrossRef]
- Kadowaki, N. Intratumoral cancer immunotherapy exploiting anti-viral immunity. J. Clin. Exp. Hematop. 2022, 62, 1–8. [Google Scholar] [CrossRef]
- Atasheva, S.; Shayakhmetov, D.M. Oncolytic Viruses for Systemic Administration: Engineering a Whole Different Animal. Mol. Ther. 2021, 29, 904–907. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, J.; Liu, W.; Lin, Y.; Guo, L.; Liu, X.; Qin, Z.; Xu, C.; Zhang, Y.; Su, X.; et al. Intravenous injection of the oncolytic virus M1 awakens antitumor T cells and overcomes resistance to checkpoint blockade. Cell Death Dis. 2020, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, C.; Kreppel, F. Capsid Engineering of Adenovirus Vectors: Overcoming Early Vector-Host Interactions for Therapy. Hum. Gene Ther. 2017, 28, 820–832. [Google Scholar] [CrossRef]
- Guedan, S.; Alemany, R. CAR-T Cells and Oncolytic Viruses: Joining Forces to Overcome the Solid Tumor Challenge. Front. Immunol. 2018, 9, 2460. [Google Scholar] [CrossRef]
- Sharp, D.W.; Lattime, E.C. Recombinant Poxvirus and the Tumor Microenvironment: Oncolysis, Immune Regulation and Immunization. Biomedicines 2016, 4, 19. [Google Scholar] [CrossRef]
- Nayyar, G.; Chu, Y.; Cairo, M.S. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front. Oncol. 2019, 9, 51. [Google Scholar] [CrossRef]
- Vaupel, P. The role of hypoxia-induced factors in tumor progression. Oncologist 2004, 9 (Suppl. S5), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Na, J.; Zhong, L.; Zhang, P. Advances in preclinical and clinical studies of oncolytic virus combination therapy. Front. Oncol. 2025, 15, 1545542. [Google Scholar] [CrossRef]
- Chowaniec, H.; Slubowska, A.; Mroczek, M.; Borowczyk, M.; Braszka, M.; Dworacki, G.; Dobosz, P.; Wichtowski, M. New hopes for the breast cancer treatment: Perspectives on the oncolytic virus therapy. Front. Immunol. 2024, 15, 1375433. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.R.; Relph, K.; Harrington, K.; Melcher, A.; Pandha, H. Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: Recent advances. Oncolytic Virotherapy 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- McHayleh, W.; Bedi, P.; Sehgal, R.; Solh, M. Chimeric Antigen Receptor T-Cells: The Future is Now. J. Clin. Med. 2019, 8, 207. [Google Scholar] [CrossRef]
- Ponterio, E.; Haas, T.L.; De Maria, R. Oncolytic virus and CAR-T cell therapy in solid tumors. Front. Immunol. 2024, 15, 1455163. [Google Scholar] [CrossRef]
- Nishio, N.; Diaconu, I.; Liu, H.; Cerullo, V.; Caruana, I.; Hoyos, V.; Bouchier-Hayes, L.; Savoldo, B.; Dotti, G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014, 74, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, K.; Rosewell Shaw, A.; Watanabe, N.; Porter, C.; Rana, B.; Gottschalk, S.; Brenner, M.; Suzuki, M. Armed Oncolytic Adenovirus-Expressing PD-L1 Mini-Body Enhances Antitumor Effects of Chimeric Antigen Receptor T Cells in Solid Tumors. Cancer Res. 2017, 77, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Rosewell Shaw, A.; Porter, C.E.; Watanabe, N.; Tanoue, K.; Sikora, A.; Gottschalk, S.; Brenner, M.K.; Suzuki, M. Adenovirotherapy Delivering Cytokine and Checkpoint Inhibitor Augments CAR T Cells against Metastatic Head and Neck Cancer. Mol. Ther. 2017, 25, 2440–2451. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465. [Google Scholar] [CrossRef]
- McKenna, M.K.; Englisch, A.; Brenner, B.; Smith, T.; Hoyos, V.; Suzuki, M.; Brenner, M.K. Mesenchymal stromal cell delivery of oncolytic immunotherapy improves CAR-T cell antitumor activity. Mol. Ther. 2021, 29, 3529–3533. [Google Scholar] [CrossRef]
- Evgin, L.; Huff, A.L.; Wongthida, P.; Thompson, J.; Kottke, T.; Tonne, J.; Schuelke, M.; Ayasoufi, K.; Driscoll, C.B.; Shim, K.G.; et al. Oncolytic virus-derived type I interferon restricts CAR T cell therapy. Nat. Commun. 2020, 11, 3187. [Google Scholar] [CrossRef]
- Moon, E.K.; Wang, L.S.; Bekdache, K.; Lynn, R.C.; Lo, A.; Thorne, S.H.; Albelda, S.M. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology 2018, 7, e1395997. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, N.; Xu, L.; Lu, H.; Chen, Y.; Wang, Z.; Lu, Q.; Zhong, K.; Zhu, Z.; Wang, G.; et al. Systemic delivery of oncolytic herpes virus using CAR-T cells enhances targeting of antitumor immuno-virotherapy. Cancer Immunol. Immunother. 2024, 73, 173. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Deng, T.; Huang, Y.; Liu, Z.; Zhan, B.; Zhou, X.; Yan, R.; Ren, J.; Xing, Y.; et al. Oncolytic herpes simplex virus delivery of dual CAR targets of CD19 and BCMA as well as immunomodulators to enhance therapeutic efficacy in solid tumors combined with CAR T cell therapy. Front. Oncol. 2022, 12, 1037934. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, J.; Zhang, Q.; Jin, G.; Su, X.; Liu, S.; Liu, F. Enhancement of CD70-specific CAR T treatment by IFN-gamma released from oHSV-1-infected glioblastoma. Cancer Immunol. Immunother. 2022, 71, 2433–2448. [Google Scholar] [CrossRef]
- Park, A.K.; Fong, Y.; Kim, S.I.; Yang, J.; Murad, J.P.; Lu, J.; Jeang, B.; Chang, W.C.; Chen, N.G.; Thomas, S.H.; et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020, 12, eaaz1863. [Google Scholar] [CrossRef] [PubMed]
- Evgin, L.; Vile, R.G. Parking CAR T Cells in Tumours: Oncolytic Viruses as Valets or Vandals? Cancers 2021, 13, 1106. [Google Scholar] [CrossRef]
- Nishio, N.; Dotti, G. Oncolytic virus expressing RANTES and IL-15 enhances function of CAR-modified T cells in solid tumors. Oncoimmunology 2015, 4, e988098. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.; Fajardo, C.A.; Posey, A.D., Jr.; Shaw, C.; Da, T.; Young, R.M.; Alemany, R.; June, C.H.; Guedan, S. Improving CART-Cell Therapy of Solid Tumors with Oncolytic Virus-Driven Production of a Bispecific T-cell Engager. Cancer Immunol. Res. 2018, 6, 605–616. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.; Dotti, G. Combining Oncolytic Viruses with Chimeric Antigen Receptor T Cell Therapy. Hum. Gene Ther. 2021, 32, 150–157. [Google Scholar] [CrossRef]
- Conte, M.; Xella, A.; Woodall, R.T.; Cassady, K.A.; Branciamore, S.; Brown, C.E.; Rockne, R.C. CAR T-cell and oncolytic virus dynamics and determinants of combination therapy success for glioblastoma. Math. Biosci. 2025, 389, 109531. [Google Scholar] [CrossRef]
- Tanyi, J.L.; O’Hara, M.H.; Hexner, E.; Marshall, A.; Jadlowsky, J.; Ferrara, M.; Farrelly, O.; Runkle, A.; Chew, A.; Dowd, E.; et al. 671 Phase 1 trial of human chimeric antigen receptor modified T cells (huCART-meso) administered in combination with oncolytic virus VCN-01 in patients with pancreatic and ovarian cancer. J. Immunother. Cancer 2023, 11 (Suppl. S1), A760–A761. [Google Scholar]

| Oncolytic Virus | Cancer | Clinical Phase/Status | Therapy | Trail No. |
|---|---|---|---|---|
| AdV (Enadenotucirev) | Rectal Cancer | Phase 1/completed | radiotherapy | NCT03916510 |
| Herpes simplex virus (GM-CSF) | Melanoma Stage IV | Phase 1/completed | radiotherapy | NCT05068453 |
| Ad (ADV/HSV-tk) | Metastatic Non-small Cell Lung Cancer/Metastatic Triple-negative Breast Cancer | Phase 2/completed | radiotherapy | NCT03004183 |
| Ad (NSC-CRAd-S-p7) | Glioma | Phase 1/completed | radiotherapy/chemotherapy | NCT03072134 |
| Vaccinia virus (GL-ONC1) | Head and Neck Cancer | Phase 1/completed | radiotherapy/chemotherapy | NCT01584284 |
| Ad (Ad-39yCD/mutTKSR1rep-ADP) | Non-small Cell Lung Cancer Stage I | Phase 1/completed | radiotherapy | NCT03029871 |
| HSV (G207) | Recurrent/progressive pediatric high-grade gliomas | Phase 2/ongoing | radiotherapy | NCT04482933 |
| Ad (LOAd703) | Pancreatic Cancer | Phase 1/2/ongoing | chemotherapy | NCT02705196 |
| Ad 5 VCN-01 | Pancreatic Cancer | Phase 1/completed | chemotherapy | NCT02045602 NCT02045589 |
| Vaccinia virus (KM1) | Ovarian Cancer | Phase 1/ongoing | chemotherapy | NCT05684731 |
| Vaccinia virus (TG6002) | Glioblastoma | Phase 1/2/completed | chemotherapy | NCT03294486 |
| Vaccinia virus (GL-ONC1) | Ovarian Cancer | Phase 1/2/completed | chemotherapy | NCT02759588 |
| HSV-2 (OH2) | Melanoma | Phase 3/ongoing | chemotherapy | NCT05868707 |
| Measles virus (MV-NIS) | Ovarian/ Peritoneal Cancer | Phase 2/ongoing | chemotherapy | NCT02364713 |
| HSV-1 (HF10) | Pancreatic Cancer | Phase 1/ongoing | chemotherapy | NCT03252808 |
| Vaccinia virus (GL-ONC1) | Ovarian Cancer | Phase 3/ongoing | chemotherapy | NCT05281471 |
| Herpes simplex 1 virus (Talimogene laherparepve) | Triple Negative Breast Cancer | Phase 1/2/completed | chemotherapy | NCT02779855 |
| Vaccinia virus (Pexa-Vec) | Hepatocellular Carcinoma | Phase 3/completed | chemotherapy | NCT02562755 |
| Reovirus (REOLYSIN) | Metastatic Colorectal Cancer | Phase 1/completed | chemotherapy | NCT01274624 |
| Oncolytic virus VRT106 | Pancreatic cancer | Phase 1/ongoing | chemotherapy | NCT06866977 |
| Oncolytic Virus | Cancer | Clinical Phase/Status | Combination Drug Effect | Trail No. |
|---|---|---|---|---|
| RT-01 | Advanced Solid Tumor | Phase 1/completed | Nivolumab & ANTI-PD-1 | NCT05228119 NCT05122572 |
| HSV-1 (RP1) | Solid tumors | Phase 1/2/completed | Nivolumab & ANTI-PD-1 | NCT03767348 |
| HSV-1 (RP3) | Squamous Cell Carcinoma of Head and Neck Hepatocellular Carcinoma | Phase 2/ongoing Phase 2/ongoing | Nivolumab & ANTI-PD-1 Atezolizumab & ANTI-PD-L1 | NCT05743270 NCT05733598 |
| HSV-1 (HF10) | Melanoma Stage III/IV Malignant Melanoma | Phase 2/completed Phase 2/completed | Ipilimumab & ANTI-CTLA4 Ipilimumab &ANTI-CTLA4 | NCT03153085 NCT02272855 |
| HSV-2 (OH2) | Melanoma | Phase 1/2/completed | Pembrolizumab & ANTI-PD-1 | NCT04386967 |
| Ad (DNX-2401) | Glioblastoma/Gliosarcoma | Phase 2/completed | Pembrolizumab & ANTI-PD-1 | NCT02798406 |
| Ad (TILT-123) | Ovarian Cancer Solid Tumor | Phase 1/ongoing Phase 1/ongoing | Pembrolizumab & ANTI-PD-1 Avelumab & ANTI-PD-1 | NCT05271318 NCT05222932 |
| EnAd (Ad11/Ad3) | Epithelial tumor | Phase 1/completed | Enadenotucirev & nivolumab | NCT02636036 |
| Vaccinia virus (TBio-1) | Solid Tumor | Phase 1/2/completed | Pembrolizumab & ANTI-PD-1 | NCT04301011 |
| HSV (M032) | Glioblastoma Multiforme | Phase 1/2/ongoing | Pembrolizumab & ANTI-PD-1 | NCT05084430 |
| Reovirus (REOLYSIN) | Pancreatic Adenocarcinoma | Phase 1/completed | Pembrolizumab & ANTI-PD-1 | NCT02620423 |
| Ad (LOAd703) | Pancreatic Cancer Malignant Melanoma | Phase 1/2/ongoing Phase 1/2/completed | Atezolizumab & ANTI-PD-L1 Atezolizumab & ANTI-PD-L1 | NCT02705196 NCT04123470 |
| Ad (H101) | Recurrent Cervical Cancer Bladder Cancer | Phase 2/ongoing Phase 2/ongoing | Camrelizumab & ANTI-PD-1 Camrelizumab & ANTI-PD-1 | NCT05234905 NCT05564897 |
| Reovirus (PeLareorEp) | Breast Cancer Metastatic | Phase 2/completed | Avelumab & ANTI-PD-L1 | NCT04215146 |
| Vaccinia virus (BT-001) | Metastatic/Advanced Solid Tumors | Phase 1/2/ongoing | Pembrolizumab & ANTI-PD-1 | NCT04725331 |
| Coxsackie virus (A21) | Uveal Melanoma | Phase 1/completed | Ipilimumab & ANTI-CTLA4 | NCT03408587 |
| MG1-MAGEA3 | Non-Small Cell Lung Cancer | Phase 1/2/completed | Pembrolizumab & ANTI-PD-1 | NCT02879760 |
| MEDI5395 | Advanced Solid Tumors | Phase 1/completed | Durvalumab & ANTI-PD-L1 | NCT03889275 |
| Vaccinia virus (Pexa-Vec) | Refractory Colorectal Cancer | Phase 1/2/completed | Durvalumab & ANTI-PD-L1 Tremelimumab & ANTI-CTLA4 | NCT03206073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułek, M.; Szuster-Ciesielska, A. A Comprehensive Review of Modern Cancer Therapies Utilizing Oncolytic Viruses. Cells 2025, 14, 1825. https://doi.org/10.3390/cells14221825
Sułek M, Szuster-Ciesielska A. A Comprehensive Review of Modern Cancer Therapies Utilizing Oncolytic Viruses. Cells. 2025; 14(22):1825. https://doi.org/10.3390/cells14221825
Chicago/Turabian StyleSułek, Michał, and Agnieszka Szuster-Ciesielska. 2025. "A Comprehensive Review of Modern Cancer Therapies Utilizing Oncolytic Viruses" Cells 14, no. 22: 1825. https://doi.org/10.3390/cells14221825
APA StyleSułek, M., & Szuster-Ciesielska, A. (2025). A Comprehensive Review of Modern Cancer Therapies Utilizing Oncolytic Viruses. Cells, 14(22), 1825. https://doi.org/10.3390/cells14221825






