Ferroptosis in Anaplastic Thyroid Cancer: Molecular Mechanisms, Preclinical Evidence, and Therapeutic Prospects
Highlights
- ATC exhibits ferroptosis vulnerability due to dysregulation of iron and lipid metabolism.
- Genetic regulators, including SIRT6, EIF3H–β-catenin, and GPR34–USP8, shape ferroptosis sensitivity.
- RON signaling links glycolysis to ferroptosis resistance, offering a new therapeutic target.
- Natural compounds such as vitamin C, neferine, curcumin, and shikonin induce ferroptosis in ATC.
- Anlotinib triggers ferroptosis via ROS and ER stress, amplified by autophagy blockade.
- Combination regimens, including BRAF inhibitors with GPX4 blockade or isobavachalcone plus doxorubicin, enhance ATC suppression.
Abstract
1. Introduction
2. Molecular Background and Ferroptosis Vulnerabilities in ATC
2.1. Genomic Landscape and Ferroptosis Sensitivity
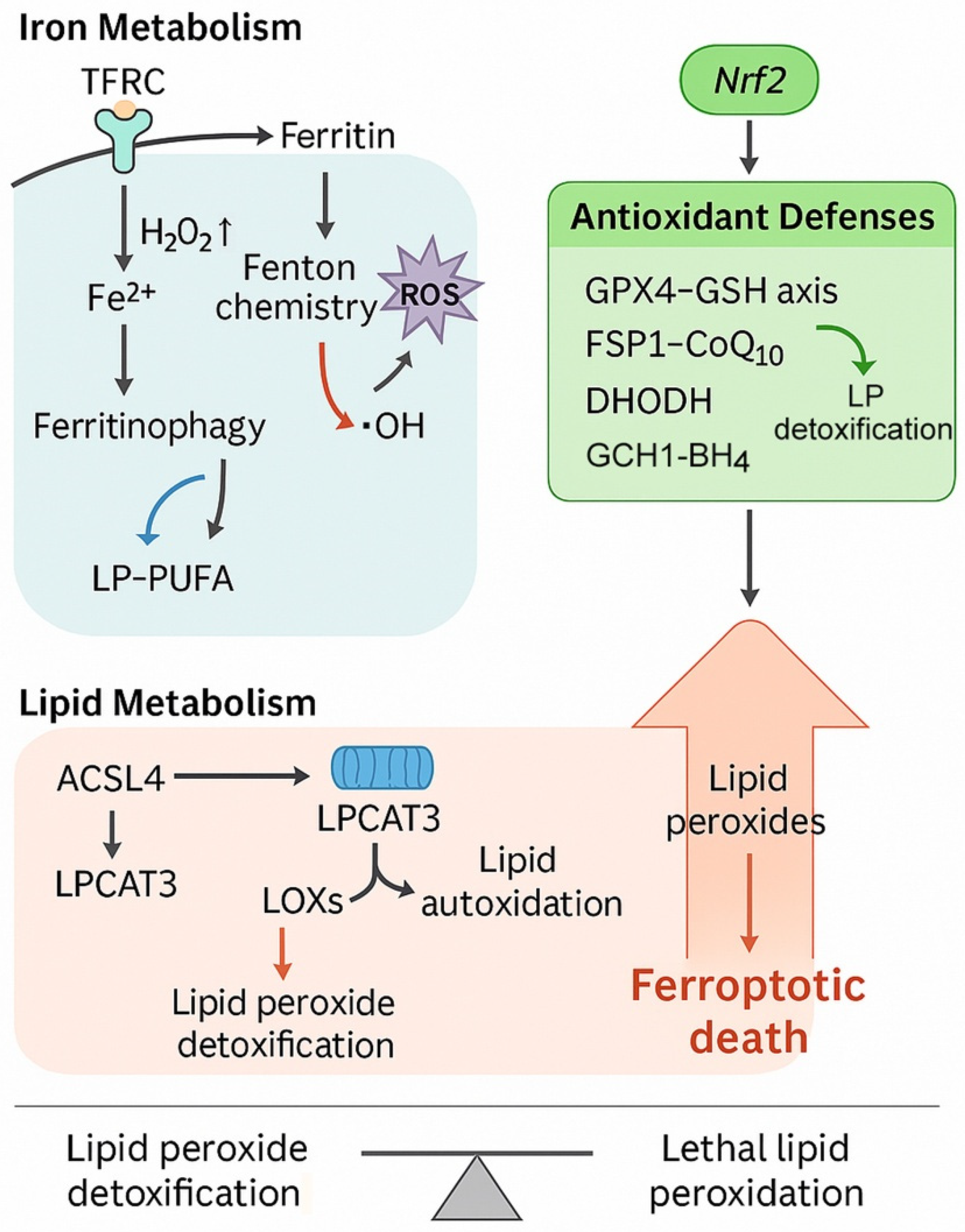
2.2. Iron Metabolism Dysregulation
2.3. Lipid Metabolism Remodeling
2.4. Antioxidant Defense Systems in ATC
2.5. Nrf2 Signaling and Redox Adaptation
2.6. EMT, Metabolic Rewiring, and Ferroptosis Sensitivity
3. Preclinical and Experimental Evidence of Ferroptosis in ATC
3.1. Pharmacological Inducers of Ferroptosis
3.2. Targeted Therapies Combined with Ferroptosis Inducers
3.3. Genetic Regulators of Ferroptosis in ATC
3.4. Nanotechnology-Driven Ferroptosis Strategies
3.5. Mechanisms of Resistance to Ferroptosis-Targeted Therapy in ATC
4. Therapeutic Strategies for Exploiting Ferroptosis in ATC
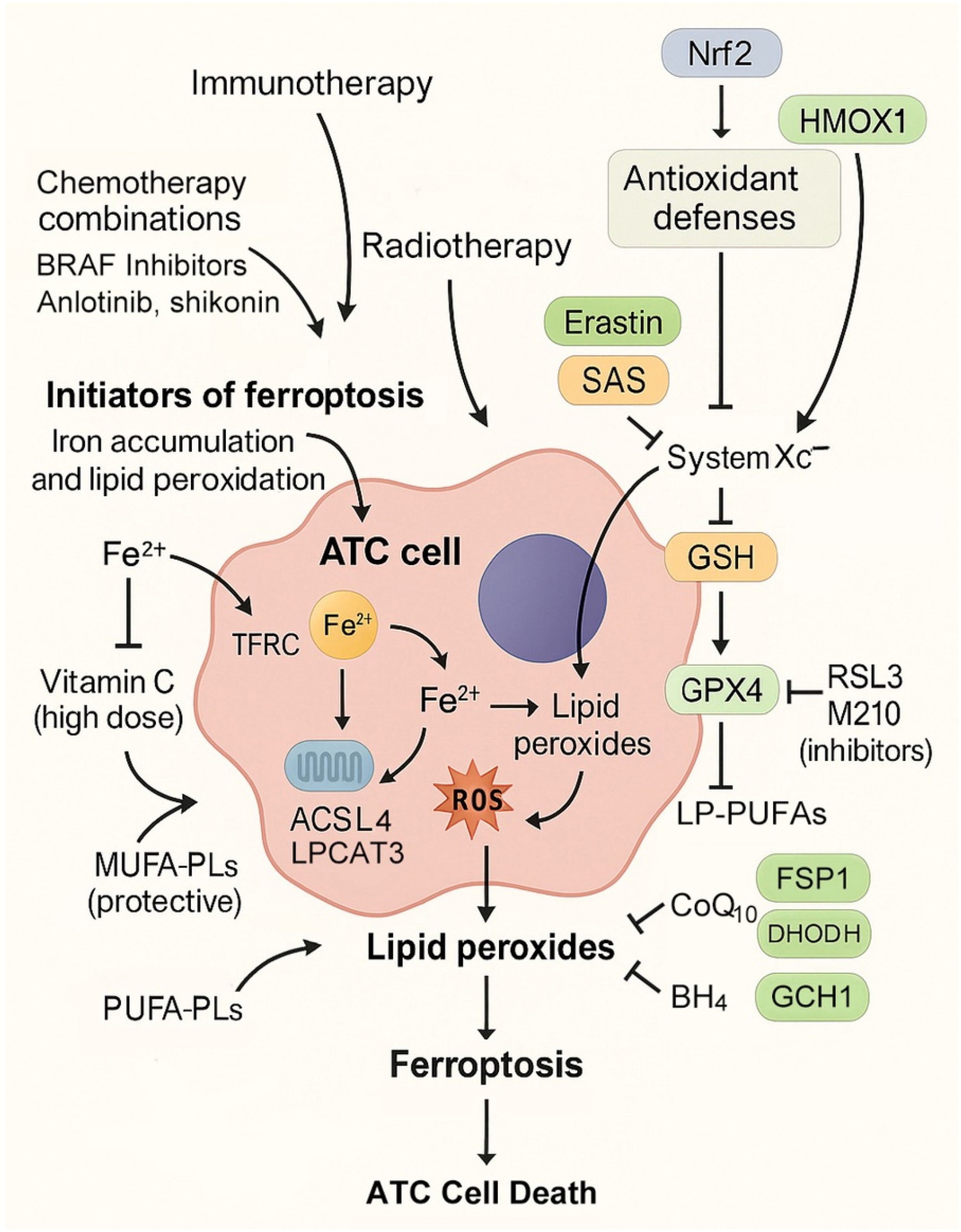
4.1. Initiators of Ferroptosis
4.2. Blockers of Antioxidant Defenses
4.3. Combination Regimens
4.4. Drug Repurposing and Natural Compounds
4.5. Challenges in Clinical Translation
5. Biomarkers, Prognostic Indicators, and Patient Selection for Ferroptosis-Based Therapy in ATC
5.1. Genetic Markers and Ferroptosis-Related Genes
5.2. Protein and Enzymatic Regulators as Biomarkers
5.3. Lipidomic and Metabolic Signatures
5.4. Immune Microenvironmental Correlations
5.5. Translational Challenges and Opportunities
6. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hamidi, S.; Maniakas, A.; Cabanillas, M.E. Advances in Anaplastic Thyroid Cancer Treatment. Endocrinol. Metab. Clin. N. Am. 2025, 54, 361–375. [Google Scholar] [CrossRef]
- Cleere, E.F.; Prunty, S.; O’Neill, J.P. Anaplastic thyroid cancer: Improved understanding of what remains a deadly disease. Surgeon 2024, 22, e48–e53. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Alves, F.; Lane, D.; Nguyen, T.P.M.; Bush, A.I.; Ayton, S. In defence of ferroptosis. Signal Transduct. Target. Ther. 2025, 10, 2. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Rose, M.M.; Bowles, D.W.; Schweppe, R.E. Molecular therapeutics for anaplastic thyroid cancer. Semin. Cancer Biol. 2020, 61, 23–29. [Google Scholar] [CrossRef]
- Silver Karcioglu, A.; Iwata, A.J.; Pusztaszeri, M.; Abdelhamid Ahmed, A.H.; Randolph, G.W. The American Thyroid Association (ATA) integrates molecular testing into its framework for managing patients with anaplastic thyroid carcinoma (ATC): Update on the 2021 ATA ATC guidelines. Cancer Cytopathol. 2022, 130, 174–180. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Xi, X.; Zhou, T.; Sun, Z.; Zhang, B. Targeting ferroptosis: A novel insight into thyroid cancer therapy. Front. Endocrinol. 2025, 16, 1527693. [Google Scholar] [CrossRef]
- Tian, W.; Su, X.; Hu, C.; Chen, D.; Li, P. Ferroptosis in thyroid cancer: Mechanisms, current status, and treatment. Front. Oncol. 2025, 15, 1495617. [Google Scholar] [CrossRef]
- Wu, J.; Liang, J.; Liu, R.; Lv, T.; Fu, K.; Jiang, L.; Ma, W.; Pan, Y.; Tan, Z.; Liu, Q.; et al. Autophagic blockade potentiates anlotinib-mediated ferroptosis in anaplastic thyroid cancer. Endocr. Relat. Cancer 2023, 30, e230036, Erratum in Endocr. Relat. Cancer 2023, 32, 2. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhang, L.; Cheng, X.; Yu, H.; Bao, J.; Lu, R. Vitamin C induces ferroptosis in anaplastic thyroid cancer cells by ferritinophagy activation. Biochem. Biophys. Res. Commun. 2021, 551, 46–53. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhang, J.; Yu, B.; Wang, W.; Jia, B.; Chang, J.; Liu, J. Neferine Exerts Ferroptosis-Inducing Effect and Antitumor Effect on Thyroid Cancer through Nrf2/HO-1/NQO1 Inhibition. J. Oncol. 2022, 2022, 7933775. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Chi, Y.B.; Teng, D.K.; Lin, Y.Q.; Zhu, L.Y.; Li, H.Q.; Yang, J.Y.; Du, J.R.; Zhang, Z.T.; Ran, H.T.; et al. Cascade-penetrating domino-ferroptosis nano inducer synergizes with sonodynamic therapy for anaplastic thyroid cancer. Mater. Today Bio 2025, 34, 102206. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Mao, C.; Yan, Y.; Zhuang, L.; Gan, B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell 2021, 12, 836–857. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Diao, J.; Jia, Y.; Dai, E.; Liu, J.; Kang, R.; Tang, D.; Han, L.; Zhong, Y.; Meng, L. Ferroptotic therapy in cancer: Benefits, side effects, and risks. Mol. Cancer 2024, 23, 89. [Google Scholar] [CrossRef]
- Karimi, A.H.; Zeng, P.Y.; Cecchini, M.; Barrett, J.W.; Pan, H.; Ying, S.; Le, N.; Mymryk, J.S.; Ailles, L.E.; Nichols, A.C. NOVEL INSIGHTS IN ADVANCED THYROID CARCINOMA: FROM MECHANISMS TO TREATMENTS: Molecular insights into the origin, biology, and treatment of anaplastic thyroid carcinoma. Eur. Thyroid. J. 2025, 14, e250057. [Google Scholar] [CrossRef]
- Zou, Z.; Zhong, L. Anaplastic thyroid cancer: Genetic roles, targeted therapy, and immunotherapy. Genes Dis. 2025, 12, 101403. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Ren, T.; Wang, X.; Liu, X.; Fei, Y.; Meng, S.; Han, X.; Sun, C.; Shen, H.; Li, L.; et al. APR-246 triggers ferritinophagy and ferroptosis of diffuse large B-cell lymphoma cells with distinct TP53 mutations. Leukemia 2022, 36, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, W. p53 in ferroptosis regulation: The new weapon for the old guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Luo, X.; Zhang, X.; Cheng, B. The evolving process of ferroptosis in thyroid cancer: Novel mechanisms and opportunities. J. Cell. Mol. Med. 2024, 28, e18587. [Google Scholar] [CrossRef]
- Tarangelo, A.; Magtanong, L.; Bieging-Rolett, K.T.; Li, Y.; Ye, J.; Attardi, L.D.; Dixon, S.J. p53 Suppresses Metabolic Stress-Induced Ferroptosis in Cancer Cells. Cell Rep. 2018, 22, 569–575. [Google Scholar] [CrossRef]
- Andreani, C.; Bartolacci, C.; Scaglioni, P.P. Ferroptosis: A Specific Vulnerability of RAS-Driven Cancers? Front. Oncol. 2022, 12, 923915. [Google Scholar] [CrossRef]
- Hu, J.; Ghosh, C.; Khaket, T.P.; Yang, Z.; Tabdili, Y.; Alamaw, E.D.; Boufraqech, M.; Dixon, S.J.; Kebebew, E. Dual Targeting of BRAF (V600E) and Ferroptosis Results in Synergistic Anticancer Activity via Iron Overload and Enhanced Oxidative Stress. bioRxiv 2025. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, J.; Wu, J.; Thompson, C.B.; Jiang, X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 31189–31197. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, S.; Xie, R.; Zhang, J.; Chen, X.; Xu, S. Epigenetic control in thyroid cancer: Mechanisms and clinical perspective. Cell Death Discov. 2025, 11, 387. [Google Scholar] [CrossRef]
- Magro, G.; Cataldo, I.; Amico, P.; Torrisi, A.; Vecchio, G.M.; Parenti, R.; Asioli, S.; Recupero, D.; D’Agata, V.; Mucignat, M.T.; et al. Aberrant expression of TfR1/CD71 in thyroid carcinomas identifies a novel potential diagnostic marker and therapeutic target. Thyroid 2011, 21, 267–277. [Google Scholar] [CrossRef]
- Campisi, A.; Bonfanti, R.; Raciti, G.; Bonaventura, G.; Legnani, L.; Magro, G.; Pennisi, M.; Russo, G.; Chiacchio, M.A.; Pappalardo, F.; et al. Gene Silencing of Transferrin-1 Receptor as a Potential Therapeutic Target for Human Follicular and Anaplastic Thyroid Cancer. Mol. Ther. Oncolytics 2020, 16, 197–206. [Google Scholar] [CrossRef]
- He, J.; Abikoye, A.M.; McLaughlin, B.P.; Middleton, R.S.; Sheldon, R.; Jones, R.G.; Schafer, Z.T. Reprogramming of iron metabolism confers ferroptosis resistance in ECM-detached cells. iScience 2023, 26, 106827. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, R.; Wang, Y.; Guan, Q.; Li, D.; Wu, Y.; Liao, T.; Wang, Y.; Xiang, J. SIRT6 drives sensitivity to ferroptosis in anaplastic thyroid cancer through NCOA4-dependent autophagy. Am. J. Cancer Res. 2023, 13, 464–474. [Google Scholar]
- Alonso, E.G.; Mascaró, M.; Schweitzer, K.; Giorgi, G.; Carballido, J.A.; Ibarra, A.; Clemente, V.; Pichel, P.; Recio, S.; Fernández Chávez, L.; et al. Heme-oxygenase-1: A key player in thyroid carcinoma development. Endocr. Relat. Cancer 2025, 32, e250177. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.; Xu, J.; Zhang, N.; Chen, J.; Wang, G.; Zhao, Y. Curcumin Induces Ferroptosis in Follicular Thyroid Cancer by Upregulating HO-1 Expression. Oxid. Med. Cell. Longev. 2023, 2023, 6896790. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Sokol, K.H.; Lee, C.J.; Rogers, T.J.; Waldhart, A.; Ellis, A.E.; Madireddy, S.; Daniels, S.R.; House, R.R.J.; Ye, X.; Olesnavich, M.; et al. Lipid availability influences ferroptosis sensitivity in cancer cells by regulating polyunsaturated fatty acid trafficking. Cell Chem. Biol. 2025, 32, 408–422.e406. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, H.; Chen, X.; Yang, Y.; Song, D. RON receptor tyrosine kinase regulates glycolysis through MAPK/CREB signaling to affect ferroptosis and chemotherapy sensitivity of thyroid cancer cells. Mol. Med. Rep. 2024, 30, 234. [Google Scholar] [CrossRef] [PubMed]
- Magtanong, L.; Ko, P.J.; To, M.; Cao, J.Y.; Forcina, G.C.; Tarangelo, A.; Ward, C.C.; Cho, K.; Patti, G.J.; Nomura, D.K.; et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26, 420–432.e429. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, J.; Chen, Y.; Wang, Y.; Liu, Z.; Huang, L.; Liu, B.; Feng, Y.; Yao, S.; Zhou, L.; et al. Monounsaturated fatty acids promote cancer radioresistance by inhibiting ferroptosis through ACSL3. Cell Death Dis. 2025, 16, 184, Erratum in Cell Death Dis. 2025, 16, 1430. [Google Scholar] [CrossRef]
- Cui, W.; Liu, D.; Gu, W.; Chu, B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021, 28, 2536–2551. [Google Scholar] [CrossRef]
- von Roemeling, C.A.; Copland, J.A. Targeting lipid metabolism for the treatment of anaplastic thyroid carcinoma. Expert Opin. Ther. Targets 2016, 20, 159–166. [Google Scholar] [CrossRef]
- Delmas, D.; Mialhe, A.; Cotte, A.K.; Connat, J.L.; Bouyer, F.; Hermetet, F.; Aires, V. Lipid metabolism in cancer: Exploring phospholipids as potential biomarkers. Biomed. Pharmacother. 2025, 187, 118095. [Google Scholar] [CrossRef]
- Guo, W.; Duan, Z.; Wu, J.; Zhou, B.P. Epithelial-mesenchymal transition promotes metabolic reprogramming to suppress ferroptosis. Semin. Cancer Biol. 2025, 112, 20–35. [Google Scholar] [CrossRef]
- Dang, T.; Yu, J.; Yu, Y.; Jiang, J.; Shi, Y.; Yu, S.; Peng, C.; Min, X.; Xiong, Y.; Long, P.; et al. GPX4 inhibits apoptosis of thyroid cancer cells through regulating the FKBP8/Bcl-2 axis. Cancer Biomark. 2024, 39, 349–360. [Google Scholar] [CrossRef]
- Sekhar, K.R.; Hanna, D.N.; Cyr, S.; Baechle, J.J.; Kuravi, S.; Balusu, R.; Rathmell, K.; Baregamian, N. Glutathione peroxidase 4 inhibition induces ferroptosis and mTOR pathway suppression in thyroid cancer. Sci. Rep. 2022, 12, 19396. [Google Scholar] [CrossRef]
- Lin, S.; Cai, H.; Song, X. Synergy between isobavachalcone and doxorubicin suppressed the progression of anaplastic thyroid cancer through ferroptosis activation. Braz. J. Med. Biol. Res. 2024, 57, e13679. [Google Scholar] [CrossRef] [PubMed]
- D’Aprile, S.; Denaro, S.; Pavone, A.M.; Giallongo, S.; Giallongo, C.; Distefano, A.; Salvatorelli, L.; Torrisi, F.; Giuffrida, R.; Forte, S.; et al. Anaplastic thyroid cancer cells reduce CD71 levels to increase iron overload tolerance. J. Transl. Med. 2023, 21, 780. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590, Erratum in Nature 2021, 596, 7873. [Google Scholar] [CrossRef]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020, 6, 41–53. [Google Scholar] [CrossRef]
- Trotter, E.W.; Grant, C.M. Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep. 2003, 4, 184–188. [Google Scholar] [CrossRef]
- de Cubas, L.; Boronat, S.; Vega, M.; Domènech, A.; Gómez-Armengol, F.; Artemov, A.; Lyublinskaya, O.; Ayté, J.; Hidalgo, E. The glutathione system maintains the thiol redox balance in the mitochondria of fission yeast. Free Radic. Biol. Med. 2025, 234, 100–112. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Li, D.; Tan, J.; Jia, Q.; Sun, H.; Meng, Z.; Wang, Y. Shikonin inhibits the growth of anaplastic thyroid carcinoma cells by promoting ferroptosis and inhibiting glycolysis. Heliyon 2024, 10, e34291. [Google Scholar] [CrossRef]
- Anandhan, A.; Dodson, M.; Shakya, A.; Chen, J.; Liu, P.; Wei, Y.; Tan, H.; Wang, Q.; Jiang, Z.; Yang, K.; et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023, 9, eade9585. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R. NFE2L2 and ferroptosis resistance in cancer therapy. Cancer Drug Resist. 2024, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Dodson, M.; Schmidlin, C.J.; Liu, P.; Zhang, D.D. Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell Chem. Biol. 2020, 27, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Seo, Y.; Roh, J.L. Ferroptosis and Nrf2 Signaling in Head and Neck Cancer: Resistance Mechanisms and Therapeutic Prospects. Antioxidants 2025, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Renaud, C.O.; Ziros, P.G.; Chartoumpekis, D.V.; Bongiovanni, M.; Sykiotis, G.P. Keap1/Nrf2 Signaling: A New Player in Thyroid Pathophysiology and Thyroid Cancer. Front. Endocrinol. 2019, 10, 510. [Google Scholar] [CrossRef]
- Gong, Z.; Xue, L.; Wei, M.; Liu, Z.; Vlantis, A.C.; van Hasselt, C.A.; Chan, J.Y.K.; Li, D.; Zeng, X.; Tong, M.C.F.; et al. The Knockdown of Nrf2 Suppressed Tumor Growth and Increased the Sensitivity to Lenvatinib in Anaplastic Thyroid Cancer. Oxid. Med. Cell. Longev. 2021, 2021, 3900330. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, S.; Chen, R.; Xu, S.; Yu, G.; Ji, L. Interplay between Nrf2 and ROS in regulating epithelial-mesenchymal transition: Implications for cancer metastasis and therapy. Mol. Biol. Rep. 2025, 52, 628. [Google Scholar] [CrossRef]
- Luo, H.; Xia, X.; Kim, G.D.; Liu, Y.; Xue, Z.; Zhang, L.; Shu, Y.; Yang, T.; Chen, Y.; Zhang, S.; et al. Characterizing dedifferentiation of thyroid cancer by integrated analysis. Sci. Adv. 2021, 7, eabf3657. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.R.; Henderson, Y.C.; Bai, S.; Yang, J.; Hu, M.; Shiau, C.K.; Pan, T.; Yan, Y.; Tran, T.M.; et al. Anaplastic transformation in thyroid cancer revealed by single-cell transcriptomics. J. Clin. Investig. 2023, 133, e169653. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Zhao, L.; Wang, C.; Zhang, Z. Anti-Anaplastic Thyroid Cancer (ATC) Effects and Mechanisms of PLX3397 (Pexidartinib), a Multi-Targeted Tyrosine Kinase Inhibitor (TKI). Cancers 2022, 15, 172. [Google Scholar] [CrossRef]
- Liao, T.; Zeng, Y.; Xu, W.; Shi, X.; Shen, C.; Du, Y.; Zhang, M.; Zhang, Y.; Li, L.; Ding, P.; et al. A spatially resolved transcriptome landscape during thyroid cancer progression. Cell Rep. Med. 2025, 6, 102043. [Google Scholar] [CrossRef]
- Wan, Y.; Li, G.; Cui, G.; Duan, S.; Chang, S. Reprogramming of Thyroid Cancer Metabolism: From Mechanism to Therapeutic Strategy. Mol. Cancer 2025, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, G.; Wu, F.; Zhang, Y.; Li, Y.; Luo, D. Ferroptosis in thyroid cancer: Potential mechanisms, effective therapeutic targets and predictive biomarker. Biomed. Pharmacother. 2024, 177, 116971, Erratum in Biomed. Pharmacother. 2024, 184, 117867. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 2019, 572, 402–406, Erratum in Nature 2019, 572, 7770. [Google Scholar] [CrossRef]
- He, W.; Hu, X.; Ge, M.; Meng, K. The central role of ferroptosis-induced therapy mediated by tenacissoside H in anaplastic thyroid cancer. J. Ethnopharmacol. 2025, 348, 119908. [Google Scholar] [CrossRef]
- Noronha, S.; Liu, Y.; Geneti, G.; Li, H.; Wu, X.; Sun, D.; Gujar, V.; Furusawa, T.; Lobanov, A.; Cam, M.; et al. CRISPR-Based Gene Dependency Screens reveal Mechanism of BRAF Inhibitor Resistance in Anaplastic Thyroid Cancer. bioRxiv 2025. [Google Scholar] [CrossRef]
- Guo, Y.; Liang, J.; Ding, L.; Wu, J.; Teng, W.; Wang, J.; Jiang, L.; Tan, Z. The endoplasmic reticulum stress-ferroptosis reciprocal signaling orchestrates anti-tumor effect of anlotinib in anaplastic thyroid cancer. Cancer Cell Int. 2025, 25, 310. [Google Scholar] [CrossRef]
- Yan, B.; Guo, J.; Huang, M.; Li, Z.; Sun, J.; Tan, H.; Lai, W.; Chang, S. GPR34 Stabilized by Deubiquitinase USP8 Suppresses Ferroptosis of ATC. Mediat. Inflamm. 2025, 2025, 5576056. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, D.; Qiu, X.; Xia, F.; Li, X. N6-methyladenosine-mediated EIF3H promotes anaplastic thyroid cancer progression and ferroptosis resistance by stabilizing β-catenin. Free Radic. Biol. Med. 2025, 231, 38–47. [Google Scholar] [CrossRef]
- Hamidi, S.; Dadu, R.; Zafereo, M.E.; Ferrarotto, R.; Wang, J.R.; Maniakas, A.; Gunn, G.B.; Lee, A.; Spiotto, M.T.; Iyer, P.C.; et al. Initial Management of BRAF V600E-Variant Anaplastic Thyroid Cancer: The FAST Multidisciplinary Group Consensus Statement. JAMA Oncol. 2024, 10, 1264–1271. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: Updated analysis from the phase II ROAR basket study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.; Peng, M.; Oyang, L.; Jiang, X.; Peng, Q.; Zhou, Y.; He, Z.; Liao, Q. Ferritinophagy: Research advance and clinical significance in cancers. Cell Death Discov. 2023, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, H.; Hara, S. Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins Other Lipid Mediat. 2019, 144, 106363. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, J.Y.; Oh, M.; Lee, E.W. An integrated view of lipid metabolism in ferroptosis revisited via lipidomic analysis. Exp. Mol. Med. 2023, 55, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Ping, P.; Ma, Y.; Xu, X.; Li, J. Reprogramming of fatty acid metabolism in thyroid cancer: Potential targets and mechanisms. Chin. J. Cancer Res. 2025, 37, 227–249. [Google Scholar] [CrossRef]
- Wood, W.M.; Sharma, V.; Bauerle, K.T.; Pike, L.A.; Zhou, Q.; Fretwell, D.L.; Schweppe, R.E.; Haugen, B.R. PPARγ Promotes Growth and Invasion of Thyroid Cancer Cells. PPAR Res. 2011, 2011, 171765. [Google Scholar] [CrossRef]
- Pratama, A.M.; Sharma, M.; Naidu, S.; Bömmel, H.; Prabhuswamimath, S.C.; Madhusudhan, T.; Wihadmadyatami, H.; Bachhuka, A.; Karnati, S. Peroxisomes and PPARs: Emerging role as master regulators of cancer metabolism. Mol. Metab. 2024, 90, 102044. [Google Scholar] [CrossRef]
- Hendricks, J.M.; Doubravsky, C.E.; Wehri, E.; Li, Z.; Roberts, M.A.; Deol, K.K.; Lange, M.; Lasheras-Otero, I.; Momper, J.D.; Dixon, S.J.; et al. Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem. Biol. 2023, 30, 1090–1103.e1097. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Chen, L.; Lu, Y.; Wu, Y.; Deng, A.; Pan, F.; Huang, H.; Liu, Y.; Li, Y.; et al. DHODH-mediated mitochondrial redox homeostasis: A novel ferroptosis regulator and promising therapeutic target. Redox Biol. 2025, 85, 103788. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Bagheri-Yarmand, R.; Busaidy, N.L.; McBeath, E.; Danysh, B.P.; Evans, K.W.; Moss, T.J.; Akcakanat, A.; Ng, P.K.S.; Knippler, C.M.; Golden, J.A.; et al. RAC1 Alterations Induce Acquired Dabrafenib Resistance in Association with Anaplastic Transformation in a Papillary Thyroid Cancer Patient. Cancers 2021, 13, 4950. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, N.; Koyama, T.; Sugitani, I. Anticancer drug therapy for anaplastic thyroid cancer. Eur. Thyroid J. 2025, 14, e240287. [Google Scholar] [CrossRef]
- Sekihara, K.; Himuro, H.; Toda, S.; Saito, N.; Hirayama, R.; Suganuma, N.; Sasada, T.; Hoshino, D. Recent Trends and Potential of Radiotherapy in the Treatment of Anaplastic Thyroid Cancer. Biomedicines 2024, 12, 1286. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Ebrahimnezhad, M.; Valizadeh, A.; Yousefi, B. Ferroptosis and immunotherapy: Breaking barriers in cancer treatment resistance. Crit. Rev. Oncol. Hematol. 2025, 214, 104907. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Han, P.Z.; Ye, W.D.; Yu, P.C.; Tan, L.C.; Shi, X.; Chen, X.F.; He, C.; Hu, J.Q.; Wei, W.J.; Lu, Z.W.; et al. A distinct tumor microenvironment makes anaplastic thyroid cancer more lethal but immunotherapy sensitive than papillary thyroid cancer. JCI Insight 2024, 9, e173712. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Huang, Q.F.; Li, Y.H.; Huang, Z.J.; Jun, M.; Wang, W.; Chen, X.L.; Wang, G.H. Artesunate carriers induced ferroptosis to overcome biological barriers for anti-cancer. Eur. J. Pharm. Biopharm. 2023, 190, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Hu, N.; Xu, W.; Zhao, L.; Tian, C.; Kamei, K.I. Ferroptosis inducers: A new frontier in cancer therapy. Bioorganic Chem. 2024, 146, 107331. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bai, X.Y.; Zhang, L.; Hu, Q.Q.; Zhang, N.; Cheng, J.Z.; Hou, M.Z.; Liu, X.L. Ferroptosis in Cancer Therapy: Mechanisms, Small Molecule Inducers, and Novel Approaches. Drug Des. Dev. Ther. 2024, 18, 2485–2529. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant p53 in cancer: From molecular mechanism to therapeutic modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef]
- Pakkianathan, J.; Yamauchi, C.R.; Barseghyan, L.; Cruz, J.; Simental, A.A.; Khan, S. Mutational Landmarks in Anaplastic Thyroid Cancer: A Perspective of a New Treatment Strategy. J. Clin. Med. 2025, 14, 2898. [Google Scholar] [CrossRef]
- Chen, H.; Peng, F.; Xu, J.; Wang, G.; Zhao, Y. Increased expression of GPX4 promotes the tumorigenesis of thyroid cancer by inhibiting ferroptosis and predicts poor clinical outcomes. Aging 2023, 15, 230–245. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. SLC7A11 as a Gateway of Metabolic Perturbation and Ferroptosis Vulnerability in Cancer. Antioxidants 2022, 11, 2444. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Guo, L.; Gao, W.; Tang, T.L.; Yan, M. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022, 13, 355. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Y.; Li, M.; Luo, Z. Emerging roles of ferroptosis in the tumor immune landscape: From danger signals to anti-tumor immunity. Febs J. 2022, 289, 3655–3665. [Google Scholar] [CrossRef]
- Wächter, S.; Bartsch, D.K.; Knorrenschild, J.R.; Pehl, A.; Eilsberger, F.; Pfestroff, A.; Luster, M.; Holzer, K.; Neubauer, A.; Maurer, E. Mutation-based, neoadjuvant treatment for advanced anaplastic thyroid carcinoma. Front. Endocrinol. 2025, 16, 1619875. [Google Scholar] [CrossRef]

| Authors (Year) | Model | Intervention | Mechanism | Key Findings | References |
|---|---|---|---|---|---|
| Wang et al. (2021) | 8505C cells | Vitamin C | Ferritinophagy, Fe2+ release, lipid ROS | Ferroptotic death, rescued by ferrostatin-1 | [13] |
| He et al. (2025) | ATC cells, xenograft | Tenacissoside H | ↓ GPX4, ↓ SLC7A11, ↑ ROS | Reduced proliferation, invasion, tumor growth | [76] |
| Li et al. (2023) | ATC cells | Neferine | Inhibition of Nrf2/HO-1/NQO1 | Enhanced lipid peroxidation, ferroptosis | [14] |
| Chen et al. (2024) | ATC cells | Curcumin | HO-1 activation, ↓ GPX4 | Ferroptotic sensitivity, reduced growth | [39] |
| Noronha et al. (2025) | ATC cells, orthotopic model | BRAF inhibitor + GPX4 inhibitor | ↑ Lipid ROS, ↓ FPN1 | Overcome dabrafenib resistance, tumor regression | [77] |
| Guo et al. (2025); Wu et al. (2023) | ATC cells, xenograft | Anlotinib | ↑ ROS, ↓ GPX4, PERK–CHOP ER stress | Ferroptosis induction, amplified by autophagy inhibition | [12,78] |
| Yang et al. (2023) | ATC cells, xenograft | SIRT6 + sulfasalazine | ↑ Ferritinophagy (NCOA4), ↓ system Xc− | Sensitized to ferroptosis, tumor suppression | [36] |
| Yan et al. (2025) | ATC cells, xenograft | USP8 inhibitor (DUB-IN-3) | ↓ GPR34 stabilization | Restored ferroptosis, suppressed tumor growth | [79] |
| Dong et al. (2025) | ATC xenograft | FCIPL nanoplatform | Fe2+ release + curcumin, mitochondrial lipid ROS | Domino-ferroptosis, sonodynamic synergy | [15] |
| Yang et al. (2024) | ATC cells, xenograft | Shikonin | ↓ GPX4, ↓ TXNRD1, ↓ PKM2, ↓ GLUT1, ↑ ROS | Dual inhibition of glycolysis and ferroptosis induction, tumor growth inhibition | [60] |
| Zhang et al. (2025) | ATC cells | EIF3H knockdown | β-catenin destabilization, ↓ Wnt/β-catenin signaling | Reduced proliferation, invasion, and ferroptosis resistance | [80] |
| Jin et al. (2024) | ATC cells | RON inhibition | MAPK/CREB blockade, ↓ GLUT1, ↓ HK2, ↓ PKM2, ↑ ferroptosis | Suppressed glycolysis, increased chemosensitivity | [43] |
| Lin et al. (2024) | ATC cells, xenograft | Isobavachalcone + doxorubicin | ↑ ROS, ↑ MDA, ↑ iron, ↓ GSH, ↓ GPX4, ↓ SLC7A11 | Synergistic ferroptosis activation, enhanced tumor suppression | [52] |
| Strategy | Mechanism | Representative Agents | Translational Implications |
|---|---|---|---|
| Initiators of ferroptosis | Promote iron overload, PUFA lipid peroxidation | Vitamin C, TDH, neferine, curcumin, shikonin | Direct tumor suppression; redox and metabolic dual targeting |
| GPX4 inhibition | Block lipid peroxide detoxification | RSL3, ML210 | Strong ferroptosis induction, but toxicity risk |
| System Xc− inhibition | Deplete cystine and GSH | Erastin, sulfasalazine | Synergistic with SIRT6, drug repurposing option |
| FSP1–CoQ10 inhibition | Block radical-trapping antioxidant system | iFSP1 (preclinical) | Synergy with GPX4 inhibitors, not tested in ATC |
| DHODH inhibition | Block mitochondrial lipid antioxidant defense | Brequinar (preclinical) | Potential in high mitochondrial activity ATC |
| Nrf2/HO-1 inhibition | Reduce transcriptional antioxidant defense | ML385, ZnPP (preclinical) | Overcome ferroptosis resistance in ATC |
| Wnt/β-catenin axis inhibition | Destabilize β-catenin, reduce ferroptosis resistance | EIF3H knockdown (preclinical) | Epitranscriptomic regulation of ferroptosis, novel biomarker potential |
| RTK/glycolysis inhibition | ↓ MAPK/CREB signaling, suppress glycolysis, promote ferroptosis | RON inhibition (preclinical) | Cross-talk between metabolic rewiring and ferroptosis; enhances chemosensitivity |
| Combination regimens | Target oncogenic drivers + ferroptosis | Dabrafenib + RSL3, anlotinib + autophagy inhibitors, IBC + doxorubicin | Overcome kinase inhibitor or chemotherapy resistance, enhanced tumor suppression |
| Drug repurposing | Leverage approved drugs | Sulfasalazine, statins, artesunate | Accelerate translation into clinical testing |
| Nanoplatforms | Targeted delivery, multimodal therapy | FCIPL | Tumor-selective ferroptosis with imaging capacity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Roh, J.-L. Ferroptosis in Anaplastic Thyroid Cancer: Molecular Mechanisms, Preclinical Evidence, and Therapeutic Prospects. Cells 2025, 14, 1800. https://doi.org/10.3390/cells14221800
Lee J, Roh J-L. Ferroptosis in Anaplastic Thyroid Cancer: Molecular Mechanisms, Preclinical Evidence, and Therapeutic Prospects. Cells. 2025; 14(22):1800. https://doi.org/10.3390/cells14221800
Chicago/Turabian StyleLee, Jaewang, and Jong-Lyel Roh. 2025. "Ferroptosis in Anaplastic Thyroid Cancer: Molecular Mechanisms, Preclinical Evidence, and Therapeutic Prospects" Cells 14, no. 22: 1800. https://doi.org/10.3390/cells14221800
APA StyleLee, J., & Roh, J.-L. (2025). Ferroptosis in Anaplastic Thyroid Cancer: Molecular Mechanisms, Preclinical Evidence, and Therapeutic Prospects. Cells, 14(22), 1800. https://doi.org/10.3390/cells14221800







