WHITE MATTER MATTERS: New Approach to the Brain’s Hidden Half Using Circulating Oligodendrocyte-Derived Extracellular Vesicles
Highlights
- Using circulating oligodendrocyte-derived extracellular vesicles, we developed a novel blood test to assess the white matter integrity of the human brain.
- Preliminary clinical studies indicated that this test is applicable to a range of conditions in which brain health is a major concern.
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Assay Principle
2.3. Assessment of EVs
2.4. Plasma Samples
3. Results
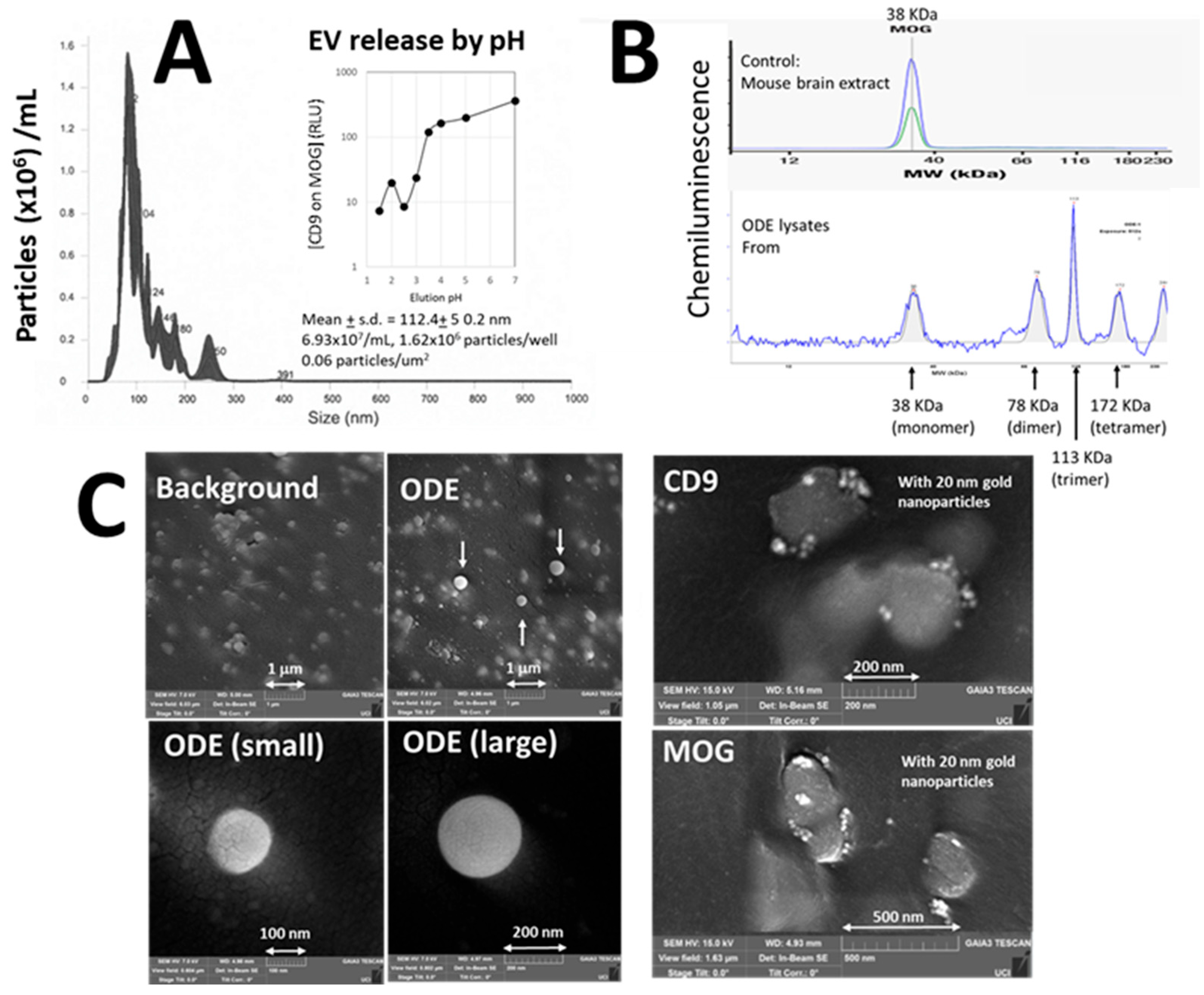
3.1. Validation of ODEs
3.1.1. Validation of EVs
3.1.2. Validation of MOG
3.1.3. Visualization of ODEs
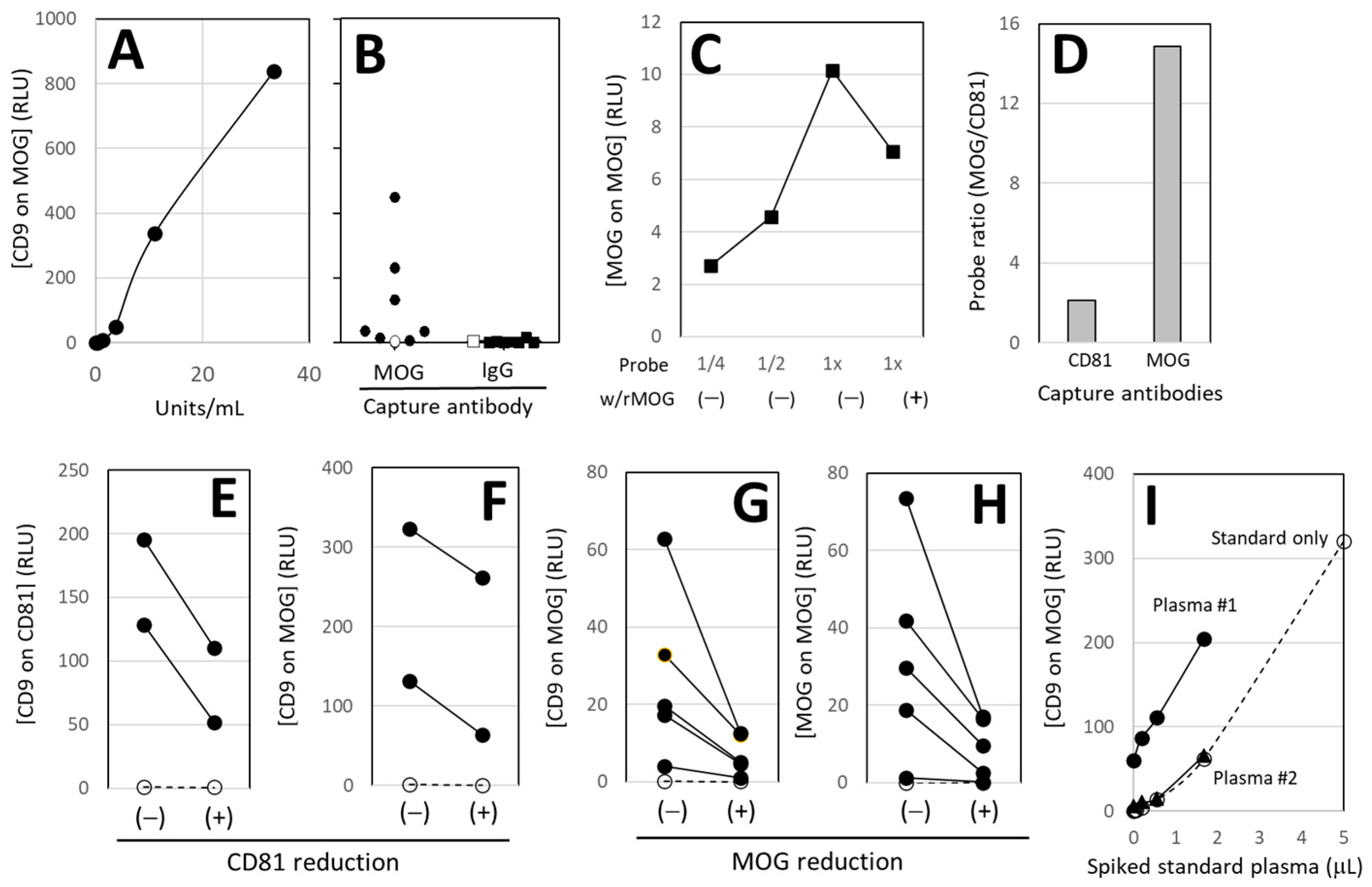
3.2. Validation of Sandwich Immunoassay
3.2.1. Standard and Specificity
3.2.2. MOG-Specificity
3.2.3. Enrichment of MOG+ EVs
3.2.4. EV Removal
3.2.5. MOG Removal
3.2.6. Gold Standard of the Assay
3.3. Results of Pilot Clinical Feasibility Studies
3.3.1. Soccer Heading Practice
3.3.2. Boxing and MMA
3.3.3. Hemorrhagic Stroke
3.3.4. Ischemic Stroke
3.3.5. Chemo Brain
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OL | oligodendrocytes |
| CPU | central processing unit |
| MRI | magnetic resonance imaging |
| fMRI | functional MRI |
| dMRI | diffusion MRI |
| MBP | myelin basic protein |
| NFL | neuron-specific neurofilament light chain |
| UCHL-1 | ubiquitin carboxyl-terminal hydrolase L1 |
| GFAP | glial fibrillary acidic protein |
| EV | extracellular vesicles |
| ODE | oligodendrocyte-derived extracellular vesicles |
| MOG | myelin oligodendrocyte glycoprotein |
| ELISA | enzyme-linked immunosorbent assay |
| NTA | nanoparticle tracking analysis |
| rMOG | Recombinant MOG |
| SEM | scanning electron microscopy |
| RBI | institutional review board |
| MMA | mixed martial arts |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| U/mL | units/mL |
| RLU | relative light units |
| PBS | phosphate-buffered saline |
| NDE | neuron-derived extracellular vesicles. |
References
- Grieb, P.; Świątkiewicz, M.; Kamińska, A.; Jünemann, A.; Rejdak, R.R.; Rejdak, K. Citicoline: A Candidate for Adjunct Treatment of Multiple Sclerosis. Pharmaceuticals 2021, 14, 326. [Google Scholar] [CrossRef]
- Green, A.J.; Gelfand, J.M.; Cree, B.A.; Bevan, C.; Boscardin, W.J.; Mei, F.; Inman, J.; Arnow, S.; Devereux, M.; Abounasr, A.; et al. Clemastine Fumarate as a Remyelinating Therapy for Multiple Sclerosis (ReBUILD): A Randomised, Controlled, Double-Blind, Crossover Trial. Lancet 2017, 390, 2481–2489. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Hou, W.W.; Wu, X.H.; Liao, R.J.; Chen, Y.; Wang, Z.; Zhang, X.N.; Zhang, L.S.; Zhou, Y.D.; et al. Treatment of Minocycline Alleviates White Matter and Cognitive Impairments After Chronic Cerebral Hypoperfusion. Sci. Rep. 2015, 5, 12079. [Google Scholar] [CrossRef]
- Dadkhah, M.; Afshari, S.; Samizadegan, T.; Shirmard, L.R.; Barin, S. Pegylated Chitosan Nanoparticles of Fluoxetine Enhance Cognitive Performance and Hippocampal Brain Derived Neurotrophic Factor Levels in a Rat Model of Local Demyelination. Exp. Gerontol. 2024, 195, 112533. [Google Scholar] [CrossRef] [PubMed]
- Otero-Ortega, L.; Gutiérrez-Fernández, M.; Ramos-Cejudo, J.; Rodríguez-Frutos, B.; Fuentes, B.; Sobrino, T.; Hernanz, T.N.; Campos, F.; López, J.A.; Cerdán, S.; et al. White Matter Injury Restoration After Stem Cell Administration in Subcortical Ischemic Stroke. Stem Cell Res. Ther. 2015, 6, 121. [Google Scholar] [CrossRef]
- Zhang, J.; Buller, B.A.; Zhang, Z.G.; Zhang, Y.; Lu, M.; Rosene, D.L.; Medalla, M.; Moore, T.L.; Chopp, M. Exosomes Derived From Bone Marrow Mesenchymal Stromal Cells Promote Remyelination and Reduce Neuroinflammation in the Demyelinating Central Nervous System. Exp. Neurol. 2022, 347, 113895. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Villarraso, J.; Medina, F.J.; Escribano, B.M.; Agüera, E.; Santamaría, A.; Pascual-Leone, A.; Túnez, I.I. Mechanisms Involved in Neuroprotective Effects of Transcranial Magnetic Stimulation. CNS Neurol. Disord. Drug Targets 2022, 21, 557–573. [Google Scholar] [CrossRef]
- Leskinen, S.; Singha, A.; Mehta, N.H.; Quelle, M.; Shah, H.A.; D’Amico, R.S. Applications of Functional Magnetic Resonance Imaging to the Study of Functional Connectivity and Activation in Neurological Disease: A Scoping Review of the Literature. World Neurosurg. 2024, 189, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Rosenberg, G.; Caprihan, A. Review of Diffusion MRI Studies in Chronic White Matter Diseases. Neurosci. Lett. 2019, 694, 198–207. [Google Scholar] [CrossRef]
- Ohta, M.; Ohta, K.; Nishimura, M.; Saida, T. Detection of Myelin Basic Protein in Cerebrospinal Fluid and Serum From Patients with HTLV-1-associated Myelopathy/Tropical Spastic Paraparesis. Ann. Clin. Biochem. 2002, 39, 603–605. [Google Scholar] [CrossRef][Green Version]
- Bazarian, J.J.; Zetterberg, H.; Buki, A.; Dengler, B.A.; Diaz-Arrastia, R.; Korley, F.K.; Lazarus, R.; Meier, T.B.; Mondello, S.; Moritz, K.; et al. Blood-Based Biomarkers for Improved Characterization of Traumatic Brain Injury: Recommendations From the 2024 National Institute for Neurological Disorders and Stroke Traumatic Brain Injury Classification and Nomenclature Initiative Blood-Based Biomarkers Working Group. J. Neurotrauma 2025, 42, 1065–1085. [Google Scholar][Green Version]
- Ohmichi, T.; Mitsuhashi, M.; Tatebe, H.; Kasai, T.; El-Agnaf, O.M.A.; Tokuda, T. Quantification of brain-derived extracellular vesicles in plasma as a biomarker to diagnose Parkinson’s and related diseases. Park. Relat. Disord. 2019, 61, 82–87. [Google Scholar] [CrossRef]
- Edwardson, M.A.; Mitsuhashi, M.; Epps, D.V. Elevation of astrocyte-derived extracellular vesicles over the first month post-stroke in humans. Sci. Rep. 2024, 14, 5272. [Google Scholar] [CrossRef]
- Kawata, K.; Mitsuhashi, M.; Aldret, R. A preliminary report on brain-derived extracellular vesicle as novel blood biomarkers for sport-related concussions. Front. Neurol. 2018, 9, 239. [Google Scholar] [CrossRef]
- Hotta, N.; Tadokoro, T.; Henry, J.; Koga, D.; Kawata, K.; Ishida, H.; Oguma, Y.; Hirata, A.; Mitsuhashi, M.; Yoshitani, K. Monitoring of Post-Brain Injuries By Measuring Plasma Levels of Neuron-Derived Extracellular Vesicles. Biomark. Insights 2022, 17, 11772719221128145. [Google Scholar] [CrossRef] [PubMed]
- Huibregtse, M.E.; Nowak, M.K.; Kim, J.E.; Kalbfell, R.M.; Koppineni, A.; Ejima, K.; Kawata, K. Does acute soccer heading cause an increase in plasma S100B? A randomized controlled trial. PLoS ONE 2020, 15, e0239507. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12451. [Google Scholar] [CrossRef] [PubMed]
- Clements, C.S.; Reid, H.H.; Beddoe, T.; Tynan, F.E.; Perugini, M.A.; Johns, T.G.; Bernard, C.C.A.; Rossjohn, J. The Crystal Structure of Myelin Oligodendrocyte Glycoprotein, a Key Autoantigen in Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 11059–11064. [Google Scholar] [CrossRef]
- Matsos, A.; Loomes, M.; Zhou, L.; Macmillan, E.; Sabel, I.; Rotziokos, E.; Beckwith, W.; Johnston, I.N. Chemotherapy-Induced Cognitive Impairments: White Matter Pathologies. Cancer Treat. Rev. 2017, 61, 6–14. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015, 11, 600–607. [Google Scholar] [CrossRef]
- Kumar, A.; Nader, M.A.; Deep, G. Emergence of Extracellular Vesicles as “Liquid Biopsy” for Neurological Disorders: Boom or Bust. Pharmacol. Rev. 2024, 76, 199–227. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Liu, C.; Liu, K.K. A New Diagnostic Tool for Brain Disorders: Extracellular Vesicles Derived from Neuron, Astrocyte, and Oligodendrocyte. Front. Mol. Neurosci. 2023, 16, 1194210. [Google Scholar] [CrossRef]
- Manolopoulos, A.; Yao, P.I.; Kapogiannis, D. Extracellular Vesicles: Translational Research and Applications in Neurology. Nat. Rev. Neurol. 2025, 21, 265–282. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bolognesi, E.; Picciolini, S.; Caputo, D.; Rovaris, M.; Pasanisi, M.B.; Clerici, M. Myelin Basic Protein in Oligodendrocyte-Derived Extracellular Vesicles as a Diagnostic and Prognostic Biomarker in Multiple Sclerosis: A Pilot Study. Int. J. Mol. Sci. 2023, 24, 894. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, M.; Stewart, T.; Fernagut, P.O.; Huang, Y.; Tian, C.; Dehay, B.; Atik, A.; Yang, D.; De Giorgi, F.; et al. Reduced Oligodendrocyte Exosome Secretion in Multiple System Atrophy Involves SNARE Dysfunction. Brain J. Neurol. 2020, 143, 1780–1797. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Srinivasan, S.; Vannberg, F.O.; Dixon, J.B. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 2016, 6, 24436. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Characteristics of Exosomes and the Vascular Landscape Regulate Exosome Sequestration by Peripheral Tissues and Brain. Int. J. Mol. Sci. 2022, 23, 12513. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Takahashi, Y.; Chang, H.Y.; Wu, Y.W.; Yamamoto, A.; Ishihama, Y.; Takakura, Y. Blood Concentrations of Small Extracellular Vesicles Are Determined by a Balance Between Abundant Secretion and Rapid Clearance. J. Extracell. Vesicles 2020, 9, 1696517. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Varga, Z.; Lenzinger, D.; Visnovitz, T.; Koncz, A.; Hegedűs, N.; Kittel, Á.; Máthé, D.; Szigeti, K.; Lőrincz, P.; et al. Extracellular Vesicle Release and Uptake by the Liver Under Normo-And Hyperlipidemia. Cell. Mol. Life Sci. 2021, 78, 7589–7604. [Google Scholar] [CrossRef]
- Linari, I.; Juantorena, G.E.; Ibáñez, A.; Petroni, A.; Kamienkowski, J.E. Unveiling Trail Making Test: Visual and Manual Trajectories Indexing Multiple Executive Processes. Sci. Rep. 2022, 12, 14265. [Google Scholar] [CrossRef] [PubMed]
- Allum, J.H.; Tang, K.S.; Carpenter, M.G.; Nijhuis, L.B.O.; Bloem, B.R. Review of First Trial Responses in Balance Control: Influence of Vestibular Loss and Parkinson’s Disease. Hum. Mov. Sci. 2011, 30, 279–295. [Google Scholar] [CrossRef]
- Batts, K.W.; Whitney, S.L.; Heiderscheit, B.C.; Grove, C.R. Gait Disorientation as a Proxy for Impaired Spatial Navigation: Associations Between the Gait Disorientation Test and Vestibular-Mediated Functions. Neuropsychologia 2025, 217, 109212. [Google Scholar] [CrossRef] [PubMed]
- Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, 23, 6001. [Google Scholar] [CrossRef] [PubMed]

| Conditions | Sample Sources | Blood Collection | Gender | Age (y.o.) |
|---|---|---|---|---|
| Soccer heading practice | Indiana Univ. | 0, 2, 24, 72 h | 15 M | 27 ± 7.5 |
| Boxing and MMA | NanoSomiX | 0, 1, 7, 14, 35 days | 10 M | 18–26 [16] |
| Hemorrhagic stroke | Duke Univ. | 1, 3, 5, 7 days | 2 M, 5 F | 57 ± 7.7 |
| Ischemic stroke | Georgetown Univ. | 1, 15, 30 days | 8 M, 6 F | 67 ± 10.6 |
| Cancer chemotherapy | Kawasaki Med. School | 0, upto 196 days | 11 F | 64 ± 14 |
| 0: pre-event |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsuhashi, M.; Van Epps, D.; Sun, H.; Xing, L.; Kawata, K.; Jimenez, V.; Williams, V.B.; Sasannejad, C.; James, M.L.; Edwardson, M.A.; et al. WHITE MATTER MATTERS: New Approach to the Brain’s Hidden Half Using Circulating Oligodendrocyte-Derived Extracellular Vesicles. Cells 2025, 14, 1771. https://doi.org/10.3390/cells14221771
Mitsuhashi M, Van Epps D, Sun H, Xing L, Kawata K, Jimenez V, Williams VB, Sasannejad C, James ML, Edwardson MA, et al. WHITE MATTER MATTERS: New Approach to the Brain’s Hidden Half Using Circulating Oligodendrocyte-Derived Extracellular Vesicles. Cells. 2025; 14(22):1771. https://doi.org/10.3390/cells14221771
Chicago/Turabian StyleMitsuhashi, Masato, Dennis Van Epps, Haiping Sun, Li Xing, Keisuke Kawata, Viviana Jimenez, Vernon B. Williams, Cina Sasannejad, Michael L. James, Matthew A. Edwardson, and et al. 2025. "WHITE MATTER MATTERS: New Approach to the Brain’s Hidden Half Using Circulating Oligodendrocyte-Derived Extracellular Vesicles" Cells 14, no. 22: 1771. https://doi.org/10.3390/cells14221771
APA StyleMitsuhashi, M., Van Epps, D., Sun, H., Xing, L., Kawata, K., Jimenez, V., Williams, V. B., Sasannejad, C., James, M. L., Edwardson, M. A., & Murata, T. (2025). WHITE MATTER MATTERS: New Approach to the Brain’s Hidden Half Using Circulating Oligodendrocyte-Derived Extracellular Vesicles. Cells, 14(22), 1771. https://doi.org/10.3390/cells14221771








