Gap Junctional Communication Required for the Establishment of Long-Term Robust Ca2+ Oscillations Across Human Neuronal Spheroids and Extended 2D Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. LUHMES Cell Culture
2.2. Ca2+ Imaging
2.3. Patch Clamp Recordings
2.4. Dye-Transfer Experiments
2.5. Immunohistochemistry
2.6. Transcriptomics
2.7. Data Analysis
2.8. Data Handling and Statistics
3. Results
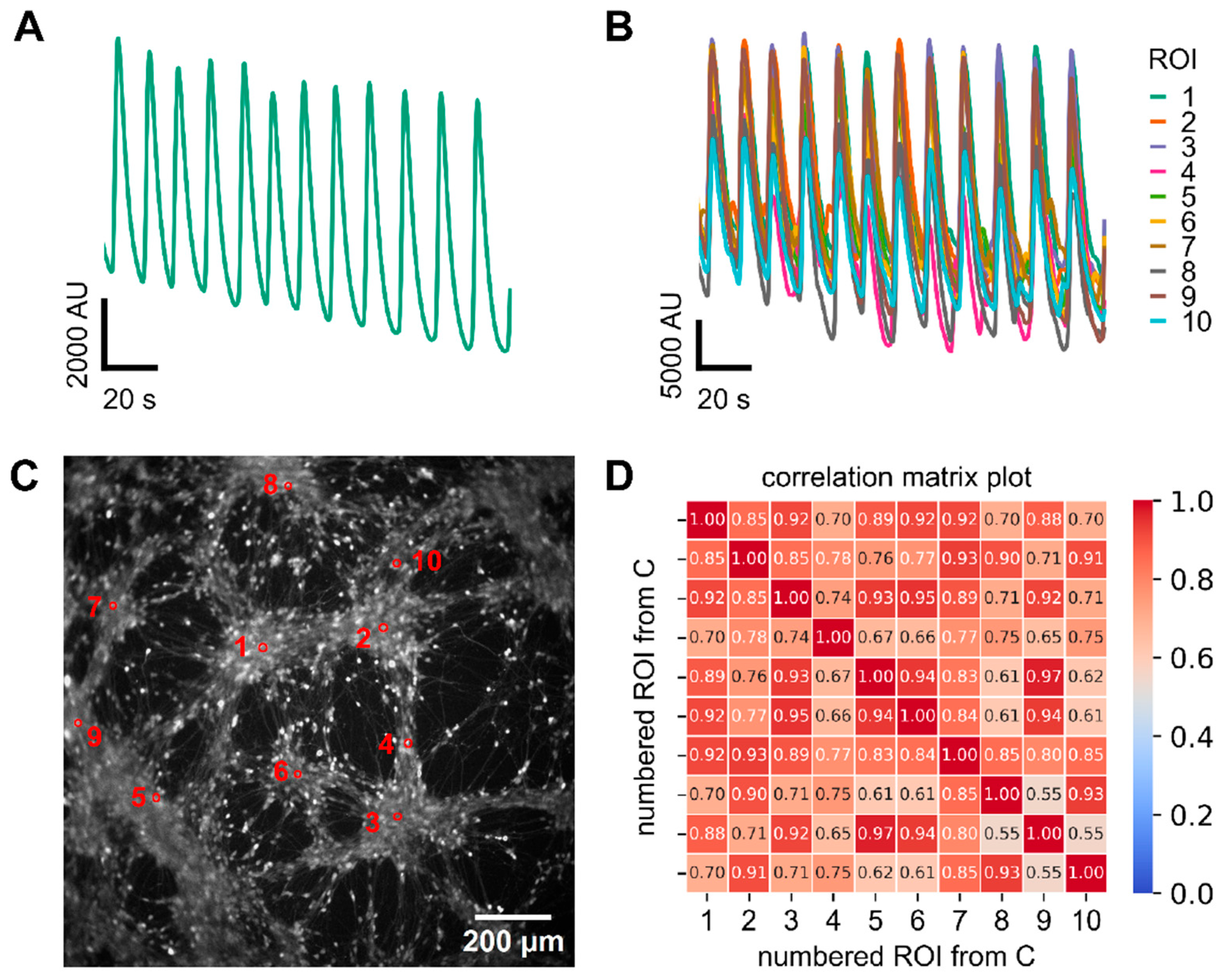
3.1. Synchronous Ca2+ Oscillations
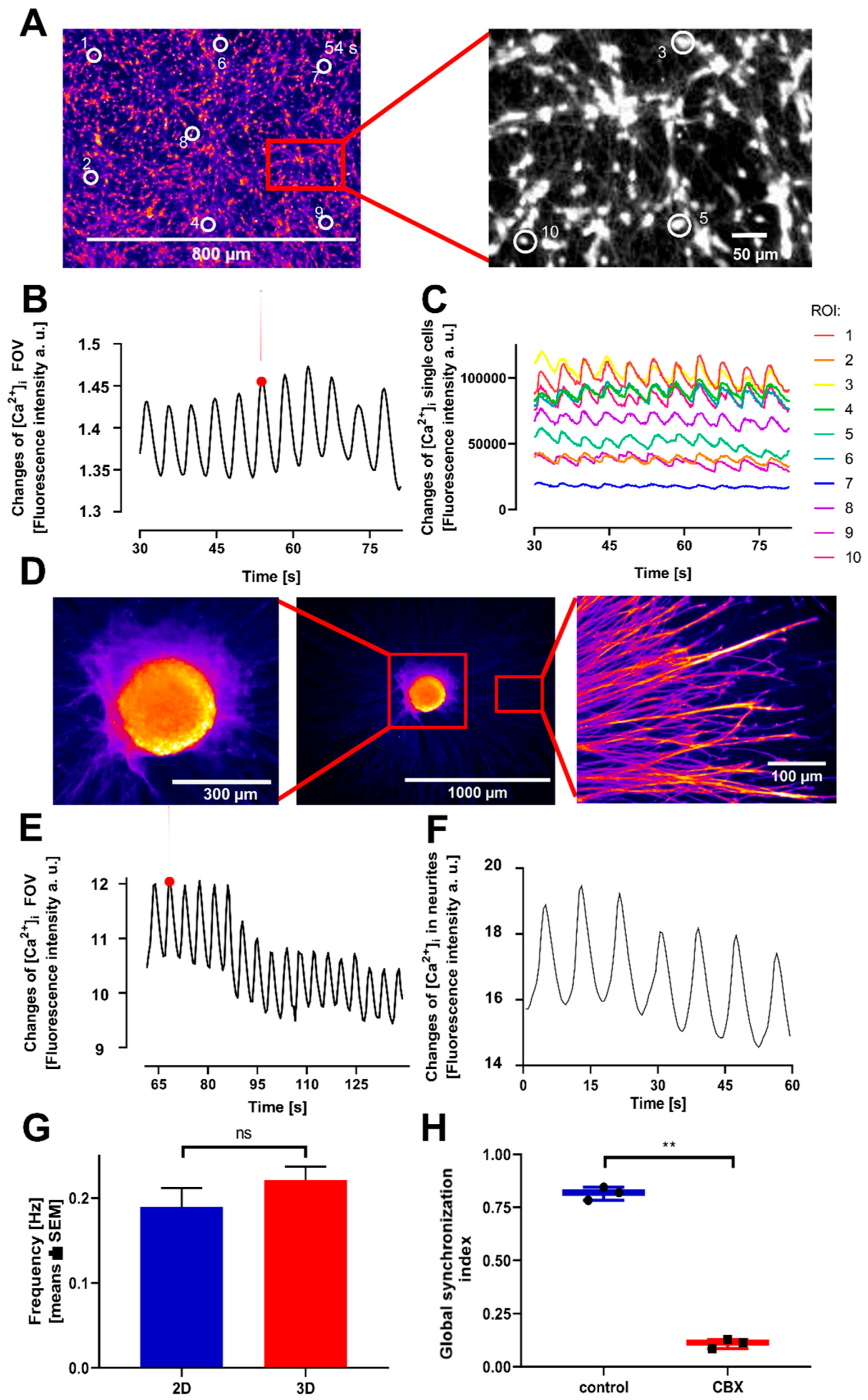
3.2. Oscillatory Activity in a 3D Model
3.3. Transferability and Viability Control of the Model System
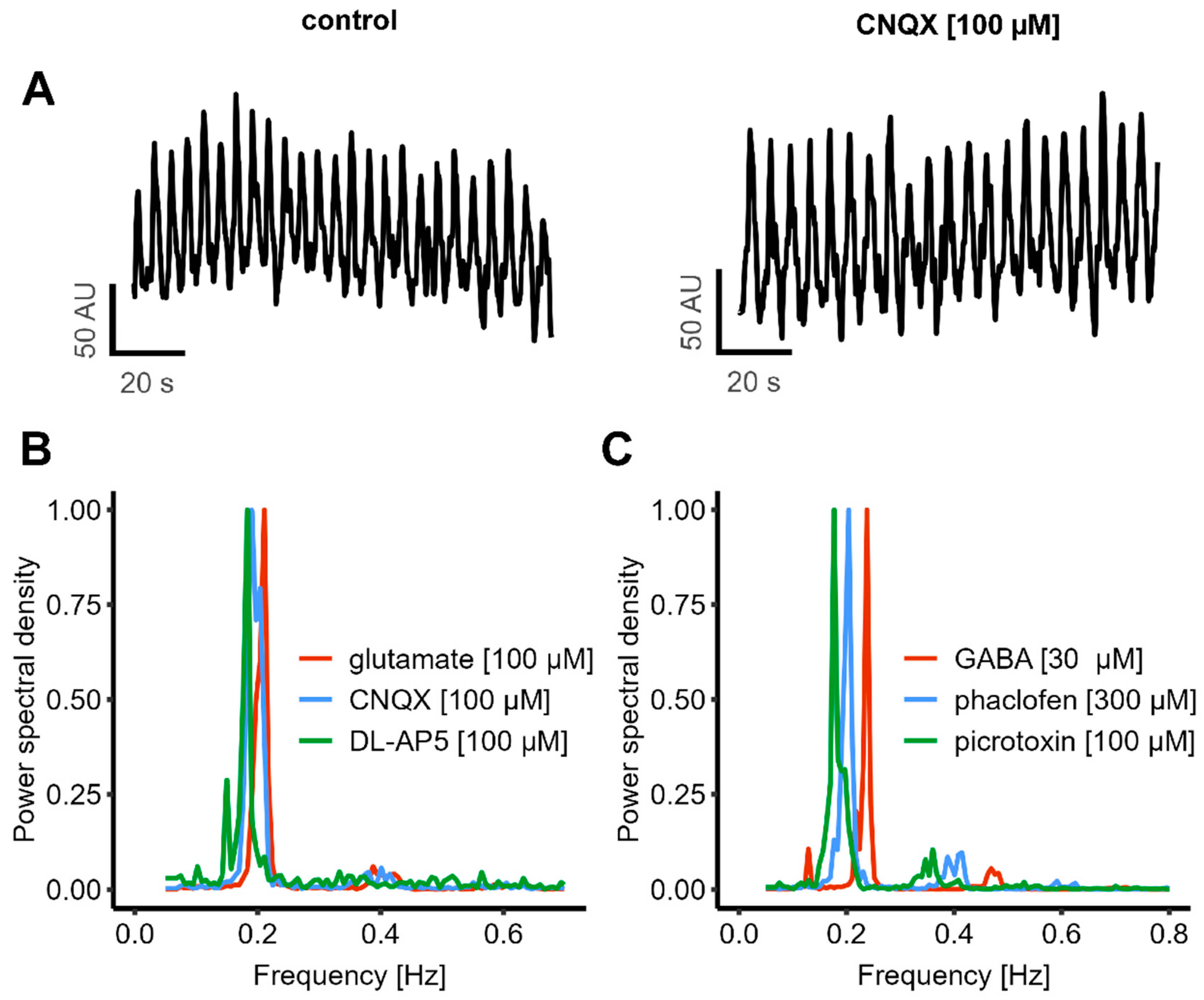
3.4. Continuation of Oscillatory Activity in the Presence of Strong Modulators of Chemical Synapses
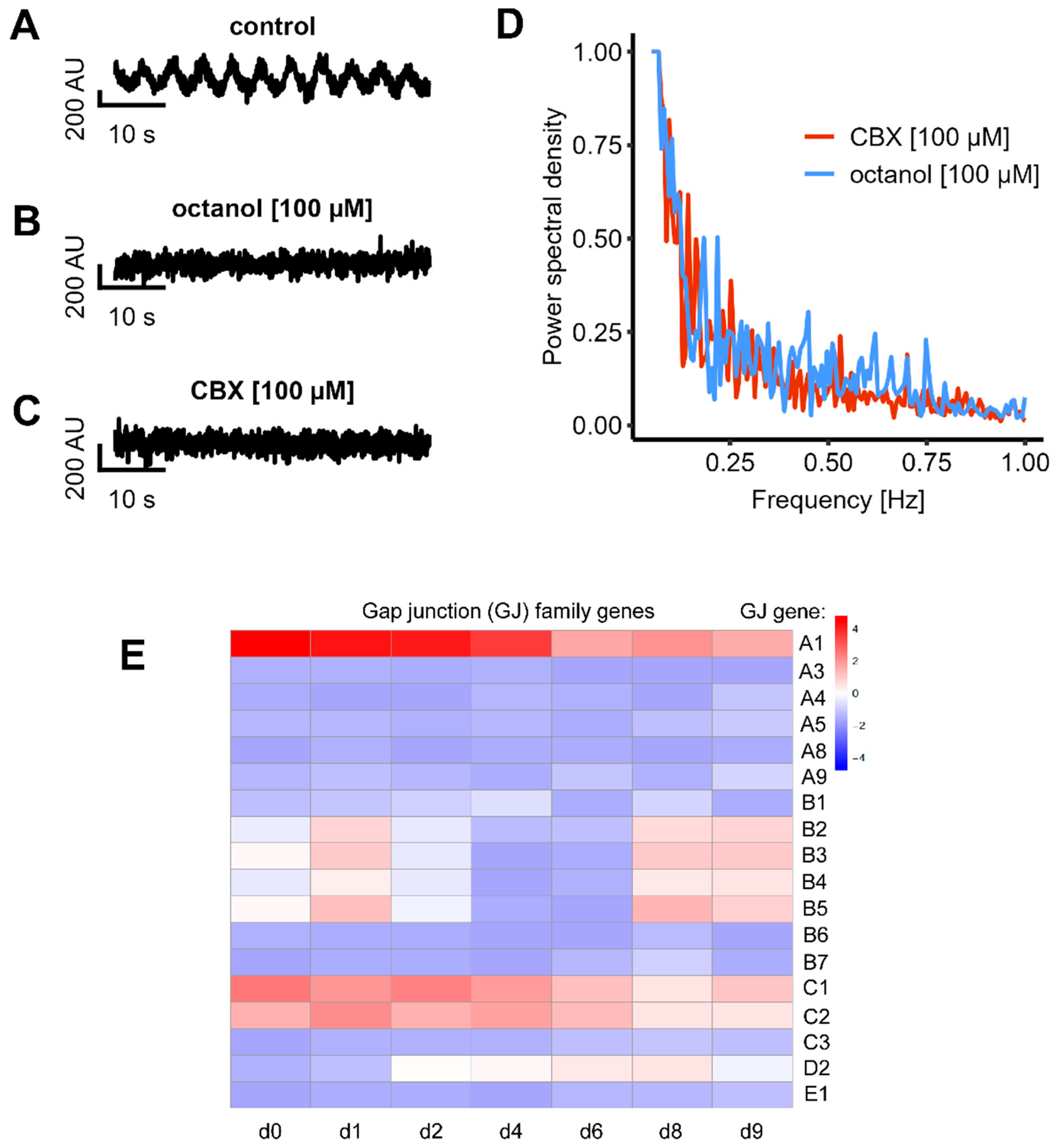
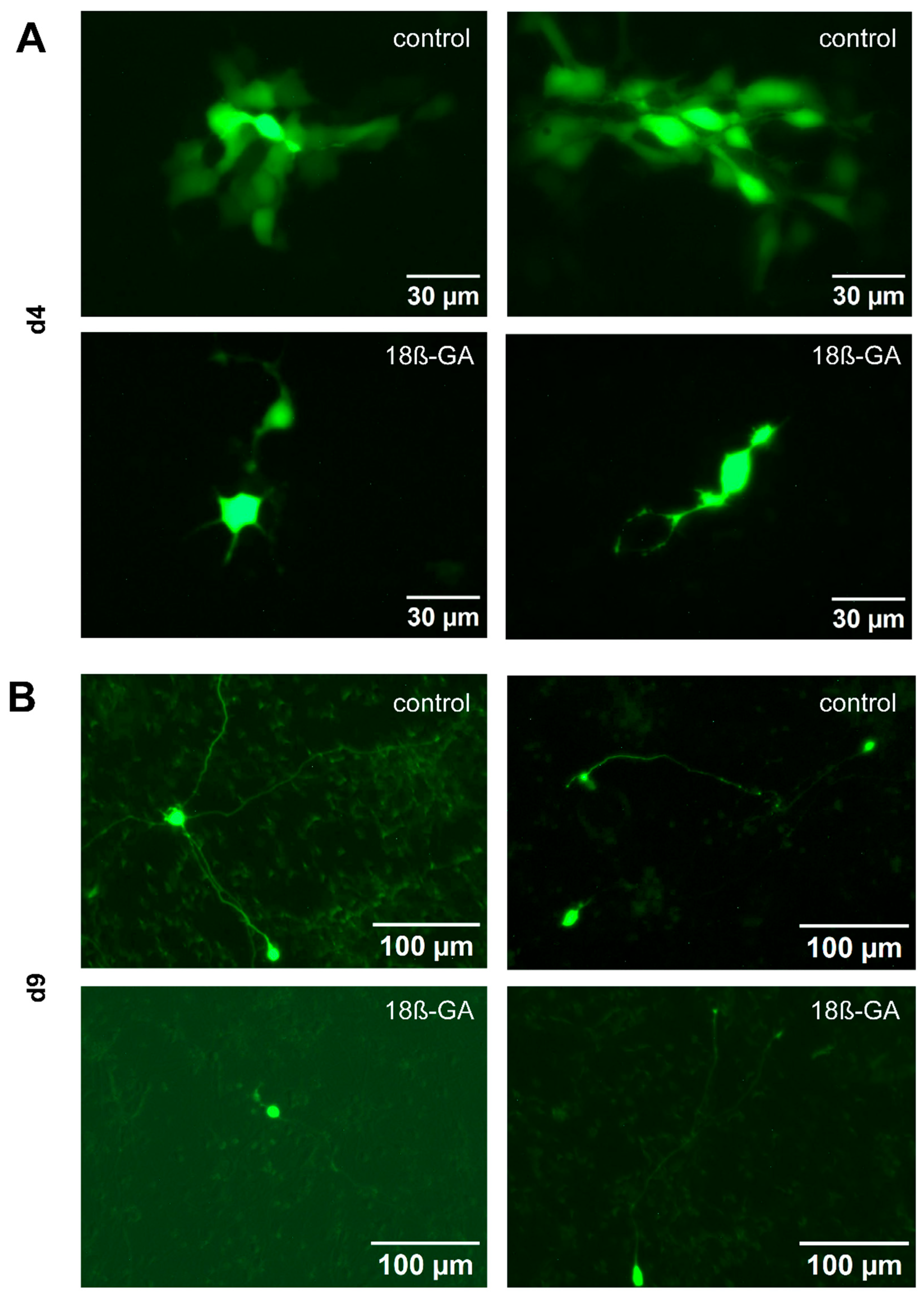
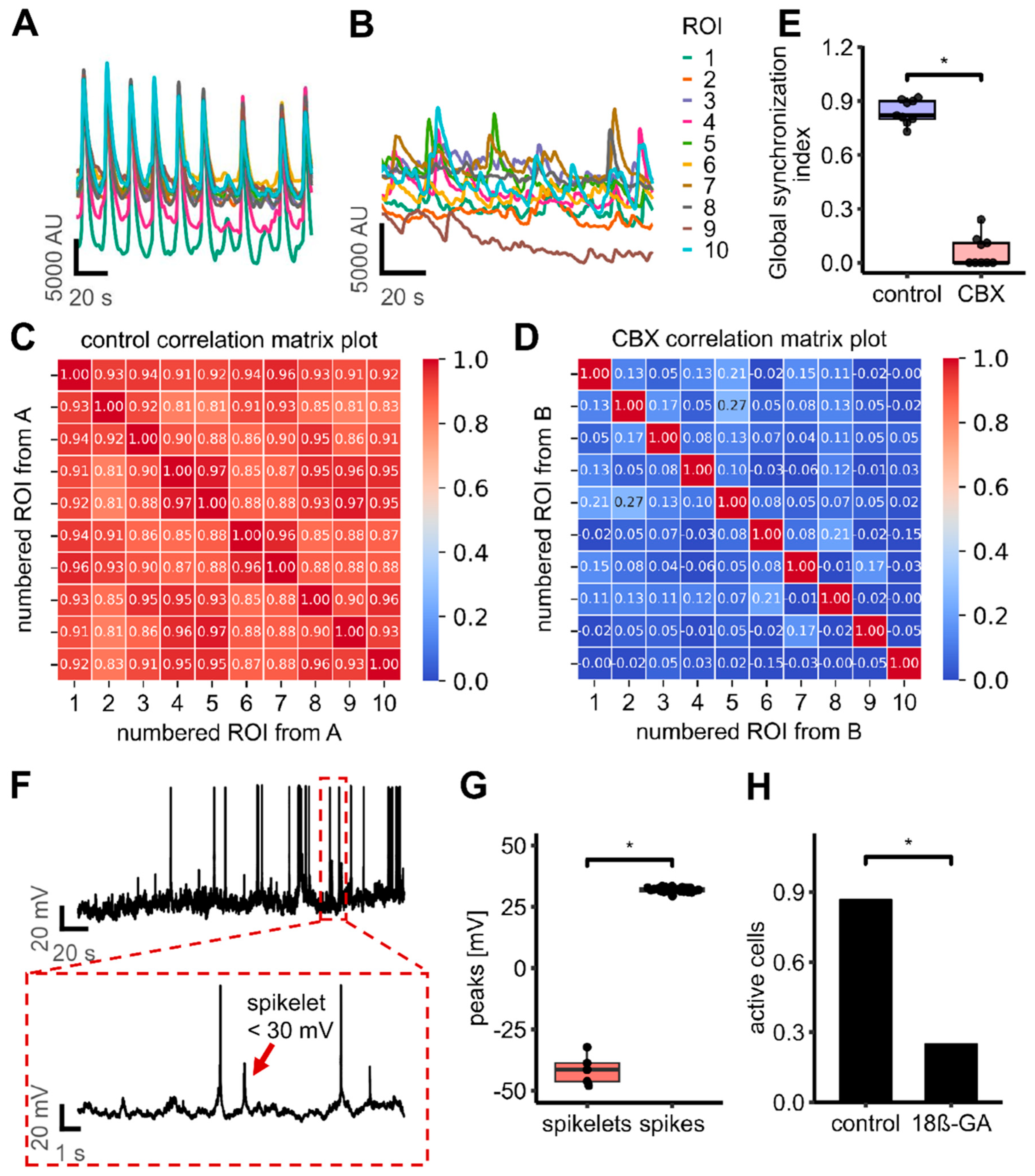
3.5. Expression and Role of Gap Junctions
3.6. Identification of Gap Junctions Between Adjacent LUHMES Cell Membranes
3.7. Inhibition of Gap Junctions Stops Network Connectivity, but Not Single Cell Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18β-GA | 18β-Glycyrrhetinic acid |
| AP | Action potential |
| CBX | Carbenoxolone |
| DNT | Developmental neurotoxicity |
| FCS | Fetal calf serum |
| FFT | Fast Fourier transformation |
| GSI | Global synchronization index |
| IHC | Immunohistochemistry |
| LUHMES | Lund human mesencephalic |
| NAM | New approach methodologies |
| NMI | Natural and Medical Sciences Institute |
| PBS | Phosphate-buffered saline |
| PEI | Polyethylenimine |
| PLO | Poly-L-ornithine |
| ROI | Region of interest |
| TEA | Tetraethylammonium |
References
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Müller, W.; Swandulla, D. Synaptic feedback excitation has hypothalamic neural networks generate quasirhythmic burst activity. J. Neurophysiol. 1995, 73, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Blatter, L.A.; Wier, W.G.; Baraban, J.M. Spontaneous synchronous synaptic calcium transients in cultured cortical neurons. J. Neurosci. 1992, 12, 4834–4845. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, A.M.; Graham, M.E.; Thorn, P.; Gallacher, D.V.; Burgoyne, R.D. Synchronous calcium oscillations in cerebellar granule cells in culture mediated by NMDA receptors. Neuroreport 1993, 4, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gruenstein, E.I. Mechanism of synchronized Ca2+ oscillations in cortical neurons. Brain Res. 1997, 767, 239–249. [Google Scholar] [CrossRef]
- Nunez, L.; Sanchez, A.; Fonteriz, R.I.; Garcia-Sancho, J. Mechanisms for synchronous calcium oscillations in cultured rat cerebellar neurons. Eur. J. Neurosci. 1996, 8, 192–201. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium regulation of neural rhythms, memory and Alzheimer’s disease. J. Physiol. 2014, 592, 281–293. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium oscillations. In Biochemical Society Symposia; Portland Press Ltd.: London, UK, 2007; pp. 1–7. [Google Scholar] [CrossRef]
- Berridge, M.J. Unlocking the secrets of cell signaling. Annu. Rev. Physiol. 2005, 67, 1–21. [Google Scholar] [CrossRef]
- Sasaki, T.; Hisada, S.; Kanki, H.; Nunomura, K.; Lin, B.; Nishiyama, K.; Kawano, T.; Matsumura, S.; Mochizuki, H. Modulation of Ca2+ oscillation following ischemia and nicotinic acetylcholine receptors in primary cortical neurons by high-throughput analysis. Sci. Rep. 2024, 14, 27667. [Google Scholar] [CrossRef]
- Anwar, H.; Khan, Q.U.; Nadeem, N.; Pervaiz, I.; Ali, M.; Cheema, F.F. Epileptic seizures. Discoveries 2020, 8, e110. [Google Scholar] [CrossRef]
- Leznik, E.; Llinás, R. Role of gap junctions in synchronized neuronal oscillations in the inferior olive. J. Neurophysiol. 2005, 94, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- LeBeau, F.E.N.; Traub, R.D.; Monyer, H.; Whittington, M.A.; Buhl, E.H. The role of electrical signaling via gap junctions in the generation of fast network oscillations. Brain Res. Bull. 2003, 62, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.A.; Chang, H.-C.; Spaeth, K.E.; Nitsche, J.M.; Nicholson, B.J. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 2004, 87, 958–973. [Google Scholar] [CrossRef]
- Connors, B.W.; Benardo, L.S.; Prince, D.A. Coupling between neurons of the developing rat neocortex. J. Neurosci. 1983, 3, 773–782. [Google Scholar] [CrossRef]
- White, T.W.; Paul, D.L. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu. Rev. Physiol. 1999, 61, 283–310. [Google Scholar] [CrossRef]
- Kelsell, D.P.; Dunlop, J.; Stevens, H.P.; Lench, N.J.; Liang, J.N.; Parry, G.; Mueller, R.F.; Leigh, I.M. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997, 387, 80–83. [Google Scholar] [CrossRef]
- Zelante, L.; Gasparini, P.; Estivill, X.; Melchionda, S.; D’Agruma, L.; Govea, N.; Milá, M.; Monica, M.D.; Lutfi, J.; Shohat, M.; et al. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum. Mol. Genet. 1997, 6, 1605–1609. [Google Scholar] [CrossRef]
- Estivill, X.; Fortina, P.; Surrey, S.; Rabionet, R.; Melchionda, S.; D’Agruma, L.; Mansfield, E.; Rappaport, E.; Govea, N.; Milà, M.; et al. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet 1998, 351, 394–398. [Google Scholar] [CrossRef]
- Scholz, D.; Pöltl, D.; Genewsky, A.; Weng, M.; Waldmann, T.; Schildknecht, S.; Leist, M. Rapid, complete and large-scale generation of post-mitotic neurons from the human LUHMES cell line. J. Neurochem. 2011, 119, 957–971. [Google Scholar] [CrossRef]
- Keighron, C.N.; Avazzedeh, S.; Quinlan, L.R. Robust In Vitro Models for Studying Parkinson’s Disease? LUHMES Cells and SH-SH5Y Cells. Int. J. Mol. Sci. 2024, 25, 13122. [Google Scholar] [CrossRef]
- Loser, D.; Schaefer, J.; Danker, T.; Möller, C.; Brüll, M.; Suciu, I.; Ückert, A.-K.; Klima, S.; Leist, M.; Kraushaar, U. Human neuronal signaling and communication assays to assess functional neurotoxicity. Arch. Toxicol. 2021, 95, 229–252. [Google Scholar] [CrossRef] [PubMed]
- Neuhof, A.; Tian, Y.; Reska, A.; Falkenburger, B.H.; Grunder, S. Large Acid-Evoked Currents, Mediated by ASIC1a, Accompany Differentiation in Human Dopaminergic Neurons. Front. Cell Neurosci. 2021, 15, 668008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Yin, M.; Zhang, M.-H. Cell-based assays for Parkinson’s disease using differentiated human LUHMES cells. Acta Pharmacol. Sin. 2014, 35, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Prahl, J.D.; Pierce, S.E.; van der Schans, E.J.C.; Coetzee, G.A.; Tyson, T. The Parkinson’s disease variant rs356182 regulates neuronal differentiation independently from alpha-synuclein. Hum. Mol. Genet. 2023, 32, 1–14. [Google Scholar] [CrossRef]
- Harris, G.; Hogberg, H.; Hartung, T.; Smirnova, L. 3D Differentiation of LUHMES Cell Line to Study Recovery and Delayed Neurotoxic Effects. Curr. Protoc. Toxicol. 2017, 73, 11–23. [Google Scholar] [CrossRef]
- Witt, B.; Friese, S.; Walther, V.; Ebert, F.; Bornhorst, J.; Schwerdtle, T. Cellular mechanisms of copper neurotoxicity in human, differentiated neurons. Arch. Toxicol. 2025, 99, 689–699. [Google Scholar] [CrossRef]
- Tong, Z.B.; Kim, H.; El Touny, L.; Simeonov, A.; Gerhold, D. LUHMES Dopaminergic Neurons Are Uniquely Susceptible to Ferroptosis. Neurotox. Res. 2022, 40, 1526–1536. [Google Scholar] [CrossRef]
- Tong, Z.B.; Sakamuru, S.; Travers, J.; Xu, T.; Yang, S.; Xia, M.; Simeonov, A.; Huang, R.; Gerhold, D. MT1G activation in dopaminergic neurons identifies chelators and their relationships to cytotoxicity. SLAS Discov. 2025, 35, 100244. [Google Scholar] [CrossRef]
- Harischandra, D.S.; Rokad, D.; Neal, M.L.; Ghaisas, S.; Manne, S.; Sarkar, S.; Panicker, N.; Zenitsky, G.; Jin, H.; Lewis, M.; et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci. Signal. 2019, 12, eaau4543. [Google Scholar] [CrossRef]
- Matelski, L.; Morgan, R.K.; Grodzki, A.C.; Van de Water, J.; Lein, P.J. Effects of cytokines on nuclear factor-kappa B, cell viability, and synaptic connectivity in a human neuronal cell line. Mol. Psychiatry 2021, 26, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Lotharius, J.; Barg, S.; Wiekop, P.; Lundberg, C.; Raymon, H.K.; Brundin, P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J. Biol. Chem. 2002, 277, 38884–38894. [Google Scholar] [CrossRef] [PubMed]
- Gutbier, S.; May, P.; Berthelot, S.; Krishna, A.; Trefzer, T.; Behbehani, M.; Efremova, L.; Delp, J.; Gstraunthaler, G.; Waldmann, T.; et al. Major changes of cell function and toxicant sensitivity in cultured cells undergoing mild, quasi-natural genetic drift. Arch. Toxicol. 2018, 92, 3487–3503. [Google Scholar] [CrossRef] [PubMed]
- Schildknecht, S.; Karreman, C.; Pöltl, D.; Efrémova, L.; Kullmann, C.; Gutbier, S.; Krug, A.; Scholz, D.; Gerding, H.R.; Leist, M. Generation of genetically-modified human differentiated cells for toxicological tests and the study of neurodegenerative diseases. ALTEX 2013, 30, 427–444. [Google Scholar] [CrossRef]
- Krug, A.K.; Balmer, N.V.; Matt, F.; Schönenberger, F.; Merhof, D.; Leist, M. Evaluation of a human neurite growth assay as specific screen for developmental neurotoxicants. Arch. Toxicol. 2013, 87, 2215–2231. [Google Scholar] [CrossRef]
- Brüll, M.; Geese, N.; Celardo, I.; Laumann, M.; Leist, M. Preparation of Viable Human Neurites for Neurobiological and Neurodegeneration Studies. Cells 2024, 13, 242. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Rossum, G.v. Python Tutorial; Technical Report CS-R9526; Centrum Voor Wiskunde en Informatica (CWI): Amsterdam, The Netherlands, 1995. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Li, X.; Cui, D.; Jiruska, P.; Fox, J.E.; Yao, X.; Jefferys, J.G.R. Synchronization measurement of multiple neuronal populations. J. Neurophysiol. 2007, 98, 3341–3348. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference; SciPy: Tacoma, WA, USA, 2010; pp. 56–61. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Wichert, S.; Fokianos, K.; Strimmer, K. Identifying periodically expressed transcripts in microarray time series data. Bioinformatics 2004, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Ahdesmaki, M.; Fokianos, K.; Strimmer, K. GeneCycle: Identification of Periodically Expressed Genes. R Package Version 1.1.6. 2025. Available online: https://CRAN.R-project.org/package=GeneCycle (accessed on 31 August 2025).
- Ahdesmäki, M.; Lähdesmäki, H.; Gracey, A.; Shmulevich, L.; Yli-Harja, O. Robust regression for periodicity detection in non-uniformly sampled time-course gene expression data. BMC Bioinform. 2007, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Ahdesmäki, M.; Lähdesmäki, H.; Pearson, R.; Huttunen, H.; Yli-Harja, O. Robust detection of periodic time series measured from biological systems. BMC Bioinform. 2005, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. (Ed.) Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hadley Wickham, R.F.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2022. Available online: https://dplyr.tidyverse.org (accessed on 31 August 2025).
- Robinson, D.; Hayes, A.; Couch, S. Broom: Convert Statistical Objects into Tidy Tibbles. R Package Version 1.0.10. 2025. Available online: https://broom.tidymodels.org/ (accessed on 31 August 2025).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Wilke, C.O. cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. R Package Version 1.2.0.9000. 2025. Available online: https://github.com/wilkelab/cowplot (accessed on 31 August 2025).
- Murrell, P. The Grid Graphics Package. R J. 2013, 7/1, 148–160. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Bache, S.M.; Wickham, H. Magrittr: A Forward-Pipe Operator for R. R Package Version 2.0.4.9000. 2025. Available online: https://github.com/tidyverse/magrittr (accessed on 31 August 2025).
- Lemon, B.B.J.; Oom, S.; Klein, E.; Rowlingson, B.; Wickham, H.; Tyagi, A.; Eterradossi, O.; Grothendieck, G.; Toews, M.; Kane, J.; et al. Plotrix: A Package in the Red Light District of R. R-News 2006, 6, 8–12. [Google Scholar]
- Pedersen, T.L. Patchwork: The Composer of Plots. R Package Version 1.3.2.9000. 2025. Available online: https://patchwork.data-imaginist.com (accessed on 31 August 2025).
- Clarke, S.S.-M.E.; Dawson, C. ggbeeswarm: Categorical Scatter (Violin Point) Plots. R Package Version 0.7.2. 2024. Available online: https://github.com/eclarke/ggbeeswarm (accessed on 31 August 2025).
- Hadley Wickham, J.H.; Bryan, J. Readr: Read Rectangular Text Data. R Package Version 2.1.5. 2024. Available online: https://github.com/tidyverse/readr (accessed on 31 August 2025).
- Hadley Wickham, J.B. Readxl: Read Excel Files. R Package Version 1.4.5.9000. 2025. Available online: https://github.com/tidyverse/readxl (accessed on 31 August 2025).
- Ahlmann-Eltze, C.; Patil, I. Ggsignif: R Package for Displaying Significance Brackets for ‘ggplot2’. PsyArxiv. 2021. Available online: https://psyarxiv.com/7awm6 (accessed on 31 August 2025).
- Hadley Wickham, L.H. Purrr: Functional Programming Tools. R Package Version 1.1.0. 2025. Available online: https://purrr.tidyverse.org/ (accessed on 31 August 2025).
- Lenaeus, M.J.; Vamvouka, M.; Focia, P.J.; Gross, A. Structural basis of TEA blockade in a model potassium channel. Nat. Struct. Mol. Biol. 2005, 12, 454–459. [Google Scholar] [CrossRef]
- Kirsch, G.E.; Taglialatela, M.; Brown, A.M. Internal and external TEA block in single cloned K+ channels. Am. J. Physiol. 1991, 261, C583–C590. [Google Scholar] [CrossRef]
- Kutluay, E.; Roux, B.; Heginbotham, L. Rapid intracellular TEA block of the KcsA potassium channel. Biophys. J. 2005, 88, 1018–1029. [Google Scholar] [CrossRef][Green Version]
- Armstrong, C.M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 1971, 58, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. The validation of regulatory test methods—Conceptual, ethical, and philosophical foundations. ALTEX-Altern. Anim. Exp. 2024, 41, 525–544. [Google Scholar] [CrossRef]
- Weiner, A.M.J.; Irijalba, I.; Gallego, M.P.; Ibarburu, I.; Sainz, L.; Goni-de-Cerio, F.; Quevedo, C.; Muriana, A. Validation of a zebrafish developmental defects assay as a qualified alternative test for its regulatory use following the ICH S5(R3) guideline. Reprod. Toxicol. 2024, 123, 108513. [Google Scholar] [CrossRef] [PubMed]
- van der Zalm, A.J.; Barroso, J.; Browne, P.; Casey, W.; Gordon, J.; Henry, T.R.; Kleinstreuer, N.C.; Lowit, A.B.; Perron, M.; Clippinger, A.J. A framework for establishing scientific confidence in new approach methodologies. Arch. Toxicol. 2022, 96, 2865–2879. [Google Scholar] [CrossRef] [PubMed]
- Bal-Price, A.; Hogberg, H.T.; Crofton, K.M.; Daneshian, M.; FitzGerald, R.E.; Fritsche, E.; Heinonen, T.; Hougaard Bennekou, S.; Klima, S.; Piersma, A.H.; et al. Recommendation on test readiness criteria for new approach methods in toxicology: Exemplified for developmental neurotoxicity. ALTEX 2018, 35, 306–352. [Google Scholar] [CrossRef]
- Hendriks, G.; Adriaens, E.; Allemang, A.; Clements, J.; Cole, G.; Derr, R.; Engel, M.; Hamel, A.; Kidd, D.; Kellum, S.; et al. Interlaboratory validation of the ToxTracker assay: An in vitro reporter assay for mechanistic genotoxicity assessment. Environ. Mol. Mutagen. 2024, 65, 4–24. [Google Scholar] [CrossRef]
- Bacci, A.; Verderio, C.; Pravettoni, E.; Matteoli, M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur. J. Neurosci. 1999, 11, 389–397. [Google Scholar] [CrossRef]
- Traub, R.D.; Kopell, N.; Bibbig, A.; Buhl, E.H.; LeBeau, F.E.; Whittington, M.A. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J. Neurosci. 2001, 21, 9478–9486. [Google Scholar] [CrossRef]
- Tchumatchenko, T.; Clopath, C. Oscillations emerging from noise-driven steady state in networks with electrical synapses and subthreshold resonance. Nat. Commun. 2014, 5, 5512. [Google Scholar] [CrossRef]
- Galarreta, M.; Hestrin, S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 1999, 402, 72–75. [Google Scholar] [CrossRef]
- Iversen, L.L. The chemistry of the brain. Sci. Am. 1979, 241, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.O.; Alonso, G.; Guerineau, N.C. Agrin mediates a rapid switch from electrical coupling to chemical neurotransmission during synaptogenesis. J. Cell Biol. 2005, 169, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Loewenstein, W.R. Low-resistance coupling between gland cells. Some observations on intercellular contact mem- 837 branes and intercellular space. Nature 1964, 201, 194–195. [Google Scholar]
- Fischbach, G.D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev. Biol. 1972, 28, 407–429. [Google Scholar] [CrossRef]
- Peinado, A.; Yuste, R.; Katz, L.C. Gap junctional communication and the development of local circuits in neocortex. Cereb. Cortex. 1993, 3, 488–498. [Google Scholar] [CrossRef]
- Sanderson, M.J.; Charles, A.C.; Boitano, S.; Dirksen, E.R. Mechanisms and function of intercellular calcium signaling. Mol. Cell Endocrinol. 1994, 98, 173–187. [Google Scholar] [CrossRef]
- Giaume, C.; Venance, L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia 1998, 24, 50–64. [Google Scholar] [CrossRef]
- Spray, D.C.; Dermietzel, R. Gap Junctions in the Nervous System; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Wang, M.; Chen, J.J.; Huang, Q.; Su, X.; Yu, Y.C.; Liu, L.Y. Connexin43 in neonatal excitatory neurons is important for short-term motor learning. Brain Res. 2019, 1720, 146287. [Google Scholar] [CrossRef]
- Homkajorn, B.; Sims, N.R.; Muyderman, H. Connexin 43 regulates astrocytic migration and proliferation in response to injury. Neurosci. Lett. 2010, 486, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.W.; Blethyn, K.L.; Cope, D.W.; Crunelli, V. Properties and origin of spikelets in thalamocortical neurones in vitro. Neuroscience 2002, 110, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, P.; Crochet, S.; Timofeev, I.; Bazhenov, M.; Sejnowski, T.J.; Steriade, M. Experimental evidence and modeling studies support a synchronizing role for electrical coupling in the cat thalamic reticular neurons in vivo. Eur. J. Neurosci. 2004, 20, 111–119. [Google Scholar] [CrossRef]
- Michalikova, M.; Remme, M.W.H.; Schmitz, D.; Schreiber, S.; Kempter, R. Spikelets in pyramidal neurons: Generating mechanisms, distinguishing properties, and functional implications. Rev. Neurosci. 2019, 31, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.V.; Zukin, R.S. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron 2004, 41, 495–511. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Zhang, L.; Carlen, P.L. Electrotonic coupling between stratum oriens interneurones in the intact in vitro mouse juvenile hippocampus. J. Physiol. 2004, 558, 825–839. [Google Scholar] [CrossRef]
- Mercer, A.; Bannister, A.P.; Thomson, A.M. Electrical coupling between pyramidal cells in adult cortical regions. Brain Cell Biol. 2006, 35, 13–27. [Google Scholar] [CrossRef]
- Moore, A.R.; Zhou, W.L.; Sirois, C.L.; Belinsky, G.S.; Zecevic, N.; Antic, S.D. Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc. Natl. Acad. Sci. USA 2014, 111, E3919–E3928. [Google Scholar] [CrossRef]
- Jang, J.; Zhu, M.H.; Jogdand, A.H.; Antic, S.D. Studying Synaptically Evoked Cortical Responses ex vivo With Combination of a Single Neuron Recording (Whole-Cell) and Population Voltage Imaging (Genetically Encoded Voltage Indicator). Front. Neurosci. 2021, 15, 773883. [Google Scholar] [CrossRef]
- Patel, A.A.; Zhu, M.H.; Yan, R.; Antic, S.D. Ex vivo propagation of synaptically-evoked cortical depolarizations in a mouse model of Alzheimer’s disease at 20 Hz, 40 Hz, or 83 Hz. Sci. Rep. 2024, 14, 23365. [Google Scholar] [CrossRef]
- Milicevic, K.D.; Ivanova, V.O.; Lovic, D.D.; Platisa, J.; Andjus, P.R.; Antic, S.D. Plateau depolarizations in spontaneously active neurons detected by calcium or voltage imaging. Sci. Rep. 2024, 14, 22787. [Google Scholar] [CrossRef] [PubMed]
- Labbaf, A.; Krauth, V.; Rychlik, N.; Narayanan Naik, V.; Vinnenberg, L.; Karabatak, E.; Teasley, A.; Perissinotti, P.P.; White, J.A.; Meuth, S.G.; et al. TREK1 channels shape spindle-like oscillations, neuronal activity, and short-term synaptic plasticity in thalamocortical circuits. J. Neurosci. 2025, e0432242025. [Google Scholar] [CrossRef]
- Blankenship, A.G.; Hamby, A.M.; Firl, A.; Vyas, S.; Maxeiner, S.; Willecke, K.; Feller, M.B. The role of neuronal connexins 36 and 45 in shaping spontaneous firing patterns in the developing retina. J. Neurosci. 2011, 31, 9998–10008. [Google Scholar] [CrossRef]
- Bertram, R.; Sherman, A.; Satin, L.S. Metabolic and electrical oscillations: Partners in controlling pulsatile insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E890–E900. [Google Scholar] [CrossRef]
- Magnus, G.; Keizer, J. Minimal model of beta-cell mitochondrial Ca2+ handling. Am. J. Physiol. 1997, 273, C717–C733. [Google Scholar] [CrossRef]
- Chan, C.S.; Glajch, K.E.; Gertler, T.S.; Guzman, J.N.; Mercer, J.N.; Lewis, A.S.; Goldberg, A.B.; Tkatch, T.; Shigemoto, R.; Fleming, S.M.; et al. HCN channelopathy in external globus pallidus neurons in models of Parkinson’s disease. Nat. Neurosci. 2011, 14, 85–92. [Google Scholar] [CrossRef]
- Guzman, J.N.; Sanchez-Padilla, J.; Wokosin, D.; Kondapalli, J.; Ilijic, E.; Schumacker, P.T.; Surmeier, D.J. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 2010, 468, 696–700. [Google Scholar] [CrossRef]
- Chan, C.S.; Guzman, J.N.; Ilijic, E.; Mercer, J.N.; Rick, C.; Tkatch, T.; Meredith, G.E.; Surmeier, D.J. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 2007, 447, 1081–1086. [Google Scholar] [CrossRef]
- Belinsky, G.S.; Rich, M.T.; Sirois, C.L.; Short, S.M.; Pedrosa, E.; Lachman, H.M.; Antic, S.D. Patch-clamp recordings and calcium imaging followed by single-cell PCR reveal the developmental profile of 13 genes in iPSC-derived human neurons. Stem Cell Res. 2014, 12, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Naus, C.C.; Sáez, J.C.; Leybaert, L. Glial Connexins and Pannexins in the Healthy and Diseased Brain. Physiol. Rev. 2021, 101, 93–145. [Google Scholar] [CrossRef]
- Talukdar, S.; Emdad, L.; Das, S.K.; Fisher, P.B. GAP junctions: Multifaceted regulators of neuronal differentiation. Tissue Barriers 2022, 10, 1982349. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.; Eitelmann, S.; Zhou, M. Approaches to Study Gap Junctional Coupling. Front. Cell Neurosci. 2021, 15, 640406. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Fromaget, C.; el Aoumari, A.; Cordier, J.; Glowinski, J.; Gros, D. Gap junctions in cultured astrocytes: Single-channel currents and characterization of channel-forming protein. Neuron 1991, 6, 133–143. [Google Scholar] [CrossRef]
- Nadarajah, B.; Jones, A.M.; Evans, W.H.; Parnavelas, J.G. Differential expression of connexins during neocortical development and neuronal circuit formation. J. Neurosci. 1997, 17, 3096–3111. [Google Scholar] [CrossRef] [PubMed]
- Pina-Benabou, M.H.d.; Szostak, V.; Kyrozis, A.; Rempe, D.; Uziel, D.; Urban-Maldonado, M.; Benabou, S.; Spray, D.C.; Federoff, H.J.; Stanton, P.K.; et al. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke 2005, 36, 2232–2237. [Google Scholar] [CrossRef]
- Enkvist, M.O.; McCarthy, K.D. Astroglial gap junction communication is increased by treatment with either glutamate or high K+ concentration. J. Neurochem. 1994, 62, 489–495. [Google Scholar] [CrossRef]
- Rose, B.; Loewenstein, W.R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature 1975, 254, 250–252. [Google Scholar] [CrossRef]
- Xu, Q.; Kopp, R.F.; Chen, Y.; Yang, J.J.; Roe, M.W.; Veenstra, R.D. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am. J. Physiol. Cell Physiol. 2012, 302, C1548–C1556. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J. Neurosci. 1998, 18, 5374–5388. [Google Scholar] [CrossRef]
- Celardo, I.; Aschner, M.; Ashton, R.S.; Carstens, K.E.; Cediel-Ulloa, A.; Collen, E.; Crofton, K.M.; Debad, S.J.; Dreser, N.; Fitzpatrick, S.; et al. Developmental neurotoxicity (DNT): A call for implementation of new approach methodologies for regulatory purposes: Summary of the 5th International Conference on DNT Testing. ALTEX 2025, 42, 323–349. [Google Scholar] [CrossRef] [PubMed]
- Collen, E.; Bartmann, K.; Blum, J.; Carstens, K.; Celardo, I.; Chatterjee, N.; Corvaro, M.; Dreser, N.; Fritsche, E.; Hartung, T.; et al. Mapping out strategies to further develop human-relevant, new approach methodology (NAM)-based developmental neurotoxicity (DNT) testing. ALTEX-Altern. Anim. Exp. 2025, 42, 308–322. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.W.; Guo, S.; Chaunsali, L.; O’Rourke, L.; Dahlka, J.; Deaver, S.; Lunski, M.; Nurmemmedov, E.; Sontheimer, H.; Sheng, Z.; et al. Cytoplasmic connexin43-microtubule interactions promote glioblastoma stem-like cell maintenance and tumorigenicity. Cell Death Dis. 2025, 16, 388. [Google Scholar] [CrossRef]
- Bonacquisti, E.E.; Nguyen, J. Connexin 43 (Cx43) in cancer: Implications for therapeutic approaches via gap junctions. Cancer Lett. 2019, 442, 439–444. [Google Scholar] [CrossRef]
- Oguro, K.; Jover, T.; Tanaka, H.; Lin, Y.; Kojima, T.; Oguro, N.; Grooms, S.Y.; Bennett, M.V.; Zukin, R.S. Global ischemia-induced increases in the gap junctional proteins connexin 32 (Cx32) and Cx36 in hippocampus and enhanced vulnerability of Cx32 knock-out mice. J. Neurosci. 2001, 21, 7534–7542. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.-H.; Denisova, J.V.; Park, W.-M.; Fontes, J.D.; Belousov, A.B. Neuronal gap junction coupling is regulated by glutamate and plays critical role in cell death during neuronal injury. J. Neurosci. 2012, 32, 713–725. [Google Scholar] [CrossRef]
- Frantseva, M.V.; Kokarovtseva, L.; Naus, C.G.; Carlen, P.L.; MacFabe, D.; Perez Velazquez, J.L. Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J. Neurosci. 2002, 22, 644–653. [Google Scholar] [CrossRef]
- Reaume, A.G.; Sousa, P.A.d.; Kulkarni, S.; Langille, B.L.; Zhu, D.; Davies, T.C.; Juneja, S.C.; Kidder, G.M.; Rossant, J. Cardiac malformation in neonatal mice lacking connexin43. Science 1995, 267, 1831–1834. [Google Scholar] [CrossRef]
- Pérez-Atencio, L.F.; Casarrubios, A.M.; Ibarz, J.M.; Barios, J.A.; Medrano, C.; Pestaña, D.; Paul, D.L.; Barrio, L.C. Respiratory disturbances and high risk of sudden death in the neonatal connexin-36 knockout mouse. Physiol. Rep. 2021, 9, e15109. [Google Scholar] [CrossRef]
- King, T.J.; Lampe, P.D. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis 2004, 25, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Ouvrier, R.; Geevasingha, N.; Ryan, M.M. Autosomal-recessive and X-linked forms of hereditary motor and sensory neuropathy in childhood. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2007, 36, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Abrams, C.; Oh, S.; Ri, Y.; Bargiello, T. Mutations in Connexin32: The molecular and biophysical bases for the X-linked form of 918 Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 2000, 5, 246–247. [Google Scholar] [CrossRef]
- Zwart-Storm, E.A.d.; Martin, P.E.; van Steensel, M.a. Gap junction diseases of the skin: Novel insights from new mutations. Expert. Rev. Dermatol. 2009, 4, 455–468. [Google Scholar] [CrossRef]
- Lilly, E.; Sellitto, C.; Milstone, L.M.; White, T.W. Connexin channels in congenital skin disorders. Semin. Cell Dev. Biol. 2016, 50, 4–12. [Google Scholar] [CrossRef]
- Ponnam, S.P.G.; Ramesha, K.; Tejwani, S.; Ramamurthy, B.; Kannabiran, C. Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. J. Med. Genet. 2007, 44, e85. [Google Scholar] [CrossRef]
- Szarka, G.; Balogh, M.; Tengölics, Á.J.; Ganczer, A.; Völgyi, B.; Kovács-Öller, T. The role of gap junctions in cell death and neuromodulation in the retina. Neural Regen. Res. 2021, 16, 1911–1920. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kormann, J.; Cöllen, E.; Aksoy-Aksel, A.; Schneider, J.; Tanaskov, Y.; Wulkesch, K.; Leist, M.; Kraushaar, U. Gap Junctional Communication Required for the Establishment of Long-Term Robust Ca2+ Oscillations Across Human Neuronal Spheroids and Extended 2D Cultures. Cells 2025, 14, 1744. https://doi.org/10.3390/cells14211744
Kormann J, Cöllen E, Aksoy-Aksel A, Schneider J, Tanaskov Y, Wulkesch K, Leist M, Kraushaar U. Gap Junctional Communication Required for the Establishment of Long-Term Robust Ca2+ Oscillations Across Human Neuronal Spheroids and Extended 2D Cultures. Cells. 2025; 14(21):1744. https://doi.org/10.3390/cells14211744
Chicago/Turabian StyleKormann, Jasmin, Eike Cöllen, Ayla Aksoy-Aksel, Jana Schneider, Yaroslav Tanaskov, Kevin Wulkesch, Marcel Leist, and Udo Kraushaar. 2025. "Gap Junctional Communication Required for the Establishment of Long-Term Robust Ca2+ Oscillations Across Human Neuronal Spheroids and Extended 2D Cultures" Cells 14, no. 21: 1744. https://doi.org/10.3390/cells14211744
APA StyleKormann, J., Cöllen, E., Aksoy-Aksel, A., Schneider, J., Tanaskov, Y., Wulkesch, K., Leist, M., & Kraushaar, U. (2025). Gap Junctional Communication Required for the Establishment of Long-Term Robust Ca2+ Oscillations Across Human Neuronal Spheroids and Extended 2D Cultures. Cells, 14(21), 1744. https://doi.org/10.3390/cells14211744





