Abstract
Viruses account for the most abundant biological entities in the biosphere and can be either symbiotic or pathogenic. While pathogenic viruses have developed strategies to evade immunity, the host immune system has evolved overlapping and redundant defenses to sense and fight viral infections. Nutrition and metabolic needs sculpt viral–host interactions and determine the course and outcomes of the infection. In this review, we focus on the hexosamine biosynthesis pathway (HBP), a nutrient-sensing pathway that controls immune responses and host–viral interactions. The HBP converges on O-GlcNAcylation, a dynamic post-translational modification of cellular proteins, that emerged as a critical effector of immune cell development, differentiation, and effector functions. We present a broad overview of uncovered O-GlcNAc substrates identified in the context of viral infections and with a functional impact on antiviral immunity and viral restriction, or conversely on exacerbating viral-induced pathologic inflammation or viral oncogenesis. We discuss the clinical implications of these findings, current limitations, and future perspectives to harness this pathway for therapeutic purposes.
1. Introduction
Viruses account for the most abundant biological entities in the biosphere [1]. They can be either symbiotic or pathogenic [1,2]. Viral pandemics have informed our understanding of viral emergence, transmission, and host–pathogen interactions [3]. Coevolutionary selective pressures have shaped both viral evasion and host defense mechanisms [4,5]. Viruses evade immune recognition through a plethora of sophisticated strategies, including modification of their molecular patterns to avoid recognition by the innate immune system, proteolytic cleavage of host innate sensors, inhibition of antigen presentation, subversion of apoptosis and autophagy, rapid antigenic variation, and cytokine mimicry [6,7,8]. The host immune system has also evolved overlapping and redundant defenses to fight viral infections. Cells of the innate immune system orchestrate immediate defenses through a plethora of constitutive and inducible mechanisms (Box 1). Multiple innate sensors detect the same or overlapping viral molecular patterns, providing backup if one is compromised (reviewed in [5]), and converge on the induction of type I, II, and III interferons, and of interferon-inducible genes, to induce an antiviral state [9,10]. The adaptive immune system bolsters the innate antiviral response with antibodies that neutralize extracellular viruses and cytotoxic T cells that eliminate infected cells, to establish specific long-term immune memory [11]. This reciprocal adaptation plays out across scales, from molecular interactions between viral epitopes and host receptors to population-level phenomena like antigenic drift. Recent theoretical and computational models illustrate how viral populations move through antigenic space in response to immune pressure [4]. Virus–immune coevolution is not merely a conceptual framework but a quantifiable process with implications for understanding viral persistence, vaccine design, and the limits of immune memory [12].
Box 1. Mechanisms of innate immune cells in anti-viral defense.
Innate immunity provides first-line defenses against viral infection. Through constitutive and inducible mechanisms, mediated by immune and barrier cells, it restricts viral replication and shapes anti-viral adaptive immunity [13].
- Epithelial and endothelial cells are barrier cells that detect viral patterns via specialized sensors and respond by secreting type I/III IFNs, chemokines, and antimicrobial peptides. They also coordinate immune cell recruitment through the upregulation of adhesion molecules and cytokines [14,15].
- Natural killer (NK) cells are cytotoxic innate lymphocytes that scan for virally infected cells lacking MHC I expression or displaying stress-induced ligands. Upon activation, they kill target cells via perforin and granzyme release and secrete IFN-γ to enhance macrophage and dendritic cell function [16].
- Plasmacytoid dendritic cells (pDCs) are key amplifiers of the antiviral state as they are principal producers of type I interferons (IFNs), a class of cytokines critical for antiviral defense [17].
- Conventional dendritic cells (cDCs), particularly cDC1, cross-present viral antigens to prime CD8+ T responses and secrete cytokines such as IL-12 that polarize downstream T cell responses and serve as link between innate and adaptive immunity [18,19].
- Macrophages and monocytes are phagocytic sentinels that engulf viral particles, secrete pro-inflammatory cytokines (e.g., TNF-α, IL-1β), and present antigens to adaptive cells. Depending on the virus, however, these cells may also serve as sites of replication or contributors to immunopathology [20,21].
- Granulocytes are increasingly recognized for their contributions to antiviral immunity. Their presence has been documented at respiratory mucosal barriers, particularly during early inflammatory responses to respiratory viral infections, including respiratory syncytial virus (RSV), influenza, and SARS-CoV-2 [21,22]. While these granulocytes can facilitate viral clearance through direct and indirect effector functions, their excessive or dysregulated activation has also been implicated in tissue damage and exacerbated inflammation, particularly in severe respiratory infections such as influenza and COVID-19 [23,24].
- ○
- Neutrophils, the most abundant circulating leukocytes, are rapidly recruited to infection sites, where, in addition to degranulation and production of reactive oxygen species (ROS), they form neutrophil extracellular traps (NETs), which immobilize and neutralize viral particles [24].
- ○
- Eosinophils degranulate to release cytotoxic mediators, secrete ribonucleases (RNases) capable of degrading viral RNA, and modulate the recruitment and activation of other immune cells [25].
- ○
- Mast cells release autocoids, proteases, and interferons, which increase vascular permeability, recruit immune cells, and restrict viral replication [26].
Nutrition and metabolic needs sculpt viral–host interactions and determine the course and outcomes of the infection [27,28]. Viruses, as obligate intracellular parasites, are entirely dependent on host-derived nutrients for energy and biosynthetic precursors, including nucleotides, amino acids, and lipids, to support replication, virion assembly, and spread. Similarly, the host immune system undergoes rapid and dynamic metabolic reprogramming during infection to support cellular proliferation, cytokine production, and effector functions. These opposing demands place cellular metabolism at the center of host–pathogen interactions [29]. Nutrient sensing has emerged as a critical regulatory mechanism by which immune cells detect fluctuations in nutrient availability and coordinate metabolic responses to maintain homeostasis and achieve effective responses to infection [30]. Undernutrition impairs innate and adaptive immunity, whereas overnutrition, particularly obesity, induces chronic low-grade inflammation and predisposes individuals to more severe viral disease outcomes [31,32]. Mechanistically, this relationship is controlled by the overlap of nutrient and pathogen-sensing pathways. Pattern recognition receptors (PRR), mammalian target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), the hexosamine biosynthesis pathway (HBP), and O-GlcNAcylation are not only responsive to nutrient fluxes and metabolic stress but also to infection and danger signals [33].
In this overview of the recent literature, we present a comprehensive discussion of identified O-GlcNAcylation substrates involved in host–viral interaction, precise their O-GlcNAcylation sites, overlapping PTMs, and the functional impact of their modification on viral control or pathogenesis. We further outline clinical studies targeting this pathway. Lastly, we discuss current limitations of O-GlcNAcylation research, outstanding questions, and future directions towards rational clinical application of metabolic inhibitors targeting this pathway to treat viral infections.
2. The Hexosamine Biosynthesis Pathway and O-GlcNAcylation: From Nutrient Sensing to the Control of Immunity
The HBP has emerged as a key pathway in immune regulation with the capacity to intercept signals from both extracellular nutrients and intracellular metabolic intermediates (Box 2).
Box 2. Immunometabolic pathways underpinning antiviral immunity.
Metabolic reprogramming is a defining feature of immune activation and viral defense. Upon sensing infection, immune cells remodel core metabolic pathways to meet the heightened energetic and biosynthetic demands of proliferation, cytokine production, and effector function [31].
- Glycolysis: Glycolysis converts glucose into pyruvate, generating ATP and metabolic intermediates needed for biosynthesis. It supports rapid energy production independent of oxygen through lactate formation. In immune cells, glycolysis fuels activation, proliferation, and effector cytokine synthesis during infection or inflammation [29,34].
- TCA Cycle: The tricarboxylic acid (TCA) cycle oxidizes acetyl-CoA derived from pyruvate or fatty acids to generate NADH and FADH2, which fuel oxidative phosphorylation for ATP production. It also metabolizes amino acids such as glutamate. The cycle yields intermediates including citrate, α-ketoglutarate, and succinate that serve as biosynthetic precursors and metabolic signals regulating gene expression and immune activation [35]. In immune cells, the TCA cycle supports oxidative metabolism that underlies quiescence, memory formation, and the resolution of inflammation [29].
- Pentose Phosphate Pathway (PPP): The PPP branches from glycolysis to generate NADPH and ribose-5-phosphate. NADPH supports lipid synthesis and antioxidant defense, while ribose-5-phosphate enables nucleotide production, including uridine triphosphate (UTP) [29]. This pathway sustains redox balance and biosynthetic processes during immune activation [36].
- Lipid Metabolism: Lipid metabolism includes anabolic fatty-acid synthesis and catabolic fatty-acid oxidation. Synthesis provides membrane lipids and signaling molecules, whereas oxidation generates ATP and acetyl-CoA [37]. The balance between these opposing arms determines inflammatory versus regulatory immune phenotypes [29].
- Hexosamine Biosynthesis Pathway (HBP): The HBP integrates glucose, amino acid, fatty acid, and nucleotide metabolism to produce UDP-GlcNAc. This metabolite serves as the donor substrate for protein O-GlcNAcylation, a nutrient-sensitive post-translational modification. Through O-GlcNAcylation, the HBP links metabolic flux to immune signaling and antiviral responses [29,38].
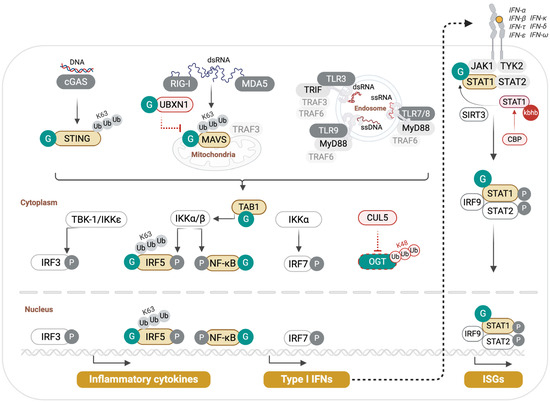
Key substrates driving the HBP include glucose, glucosamine, amino acids, fatty acids, and nucleotides (Figure 1). The HBP consumes 2–3% of glucose from the glycolytic pathway in the form of fructose-6-phosphate (F6P). Similarly, it derives acetyl-CoA (AcCoA) from the oxidation of pyruvate, fatty acids, and amino acids in the tricarboxylic acid (TCA) cycle, and obtains uridine triphosphate (UTP) from the de novo pyrimidine biosynthesis pathway. Exogenous glucosamine or GlcNAc can also be salvaged into the pathway by conversion to GlcNAc-6-phosphate (GlcNAc-6-p), bypassing the HBP rate-limiting step at Glutamine-fructose-6-phosphate amidotransferase (GFAT/GFPT) [39]. HBP links nutrient sensing to a dynamic and reversible post-translational modification (PTM) termed O-GlcNAcylation. This PTM of cytoplasmic, mitochondrial, and nuclear proteins regulates protein–protein interactions, protein stability, and DNA-binding affinity, often in synergy or competition with other PTMs, such as phosphorylation and ubiquitination. It is catalyzed by one writer, O-GlcNAc transferase (OGT), that couples UDP-GlcNAc to Serine (S) or Threonine (T) residues in target substrates and is reversed by an eraser termed O-GlcNAcase (OGA) [39,40]. Emerging research demonstrates the indispensable role of this PTM in immunity, from controlling immune cell development and differentiation to fine-tuning inducible innate and adaptive immune responses, through direct modification of central effectors (Table 1).
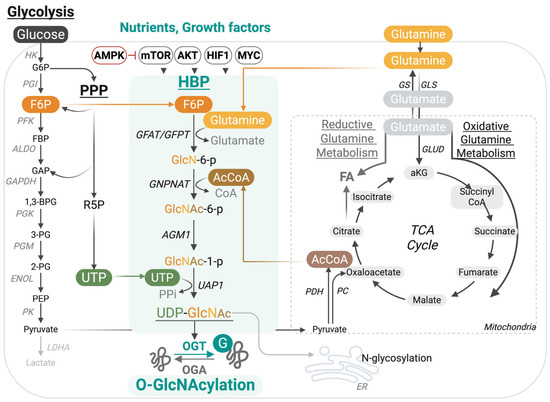
Figure 1.
The hexosamine biosynthesis pathway (HBP) links nutrient availability to dynamic protein O-GlcNAcylation. The HBP integrates inputs from multiple nutrient and metabolic sources to generate UDP-GlcNAc, the donor substrate for protein O-GlcNAcylation. A fraction of glucose entering glycolysis is diverted at fructose-6-phosphate (F6P), which, together with glutamine, fuels GFAT/GFPT, the rate-limiting step of the pathway. Additional inputs are provided by acetyl-CoA from the tricarboxylic acid (TCA) cycle, uridine triphosphate (UTP) from pyrimidine biosynthesis, and nutrient-sensitive regulators (AMPK, mTOR, AKT, HIF1, MYC). The resulting UDP-GlcNAc is utilized by O-GlcNAc transferase (OGT) to modify serine/threonine residues on nuclear, cytoplasmic, and mitochondrial proteins, while O-GlcNAcase (OGA) removes the modification.

Table 1.
Select O-GlcNAcylated substrates in immunity or viral restriction.
In T cells, O-GlcNAcylation peaks shortly after T cell receptor (TCR) engagement and both NFAT and NF-κB interact with OGT and are O-GlcNAcylated, which promotes IL-2 production and T cell activation [52,53,54]. The precise role of O-GlcNAcylation in T cell development was investigated by Swamy et al., who demonstrated that O-GlcNAcylation is central in driving T cell self-renewal and transformation [58]. Using different T cell-specific Ogt knockout mouse strains, they showed that Ogt is required for the rapid self-renewal of T cell progenitors, malignant transformation of Pten-deficient thymocytes, and T cell positive selection and maturation. They demonstrated that c-Myc is O-GlcNAcylated, which enhanced its protein stability, and that conversely, c-Myc drives O-GlcNAcylation by upregulating nutrient uptake [58]. O-GlcNAcylation is equally essential for B cell development, survival, and antibody responses. Wu et al. revealed the role of Lyn S19 O-GlcNAcylation in promoting Syk recruitment for efficient B cell receptor (BCR) signaling [60]. Lee et al. further highlighted the importance of c-Myc O-GlcNAcylation in pre-B cell proliferation [59]. More recently, Jeon et al. showed that O-GlcNAcylation regulates chromatin architecture at the immunoglobulin locus, impacting V(D)J recombination during early B-cell development [61]. Using genetic and pharmacological approaches, they showed that caloric restriction, ketogenic diets, or OGT knockdown or inhibition led to a biased defect in V(D)J recombination and a selective reduction in distal VH gene usage. In contrast, Oga inhibition with Thiamet G, which boosts O-GlcNAcylation, rescued VH gene recombination. O-GlcNAcylation was shown to regulate the cohesin complex, essential for IgH loop extrusion, through direct modification of several of its components, including YY1, CTCF, SMC1, and SMC3. Notably, it promoted SMC1 and SMC3 interaction, as well as YY1 and CTCF DNA-binding to the IgH locus. Collectively, these results link nutritional status and O-GlcNAc metabolism to antibody repertoire diversity.
Besides lymphocyte development, O-GlcNAcylation regulates T cell lineage commitment and effector functions. The lineage stability and suppressive program of regulatory T cells (Treg) is governed by Ogt that controls both FoxP3 stability, by preventing its K48 ubiquitin-mediated degradation, and STAT5 activation [56]. The central role of Ogt in this compartment was demonstrated by the severe scurfy-like autoimmune disease observed in mice harboring Treg-specific loss of Ogt [56]. In Th17 cells, O-GlcNAcylation supports the RORγt transcriptional program. This is mediated by acetyl-CoA carboxylase 1 (ACC1) modification on S966 and S967 that boosts its enzymatic activity, which contributes to enhanced production of RORγt ligands [57]. It is thus posited that the chronic inflammation elicited in metabolic diseases might be driven by aberrant O-GlcNAcylation, leading to heightened ACC1 activity and pro-inflammatory Th17 responses.
While the role of O-GlcNAcylation in myeloid cell development has not been elucidated, key effectors of macrophage metabolism and inflammatory signaling, e.g., RIPK3 [41], S6K1 [42], and IRF5 [43], are O-GlcNAcylated with context-dependent functional outcomes on macrophage inflammatory responses. For instance, while the O-GlcNAcylation of RIPK3 [41] and S6K1 [42] attenuates their activity in inflammatory signaling, that of IRF5 [43] exerts the opposite effect and leads to heightened inflammation in viral infection, as we describe below.
4. O-GlcNAc Interference with the Viral Machinery
In addition to regulating antiviral innate immunity, O-GlcNAcylation can directly interfere with the viral machinery (Table 2).

Table 2.
Select O-GlcNAcylated viral substrates.
This has been demonstrated for human immunodeficiency virus (HIV-1), Kaposi’s sarcoma-associated herpesvirus (KSHV), hepatitis B virus (HBV), influenza A virus (IAV), and SARS-CoV-2. Jochmann et al. showed that glucosamine treatment or OGT overexpression inhibited HIV-1 transcription in infected primary CD4+ T cells. This was mediated by O-GlcNAcylation of the host transcription factor Sp1 and required functional Sp1-binding sites in the HIV-1 long terminal repeat (LTR) promoter [69]. While this evidence suggested a potential role of O-GlcNAcylation in restricting HIV-1 infection, the mechanism by which it occurred was not fully investigated, as the reported O-GlcNAcylation of Sp1 did not alter its nuclear levels or DNA-binding affinity to the LTR, suggesting that additional OGT substrates might be involved. In a different report, Jochmann et al. showed that OGT inhibits KSVH viral replication in a dose-dependent manner, potentially through direct modification of 18 viral proteins, demonstrated by mass spectrometry to be O-GlcNAcylated substrates [66] (Figure 3). Among these, ORF75 was confirmed to be O-GlcNAcylated in infected cells, and O-GlcNAc sites were identified in ORF3, ORF10, ORF8, ORF44, ORF21, and ORF29. Nearly half of the O-GlcNAcylated proteins are involved in DNA synthesis and replication, suggesting a central role for O-GlcNAc in regulating these processes, although this has not been formally demonstrated. Concordantly, Ko et al. showed that O-GlcNAcylation of KSVH replication and transcription activator (RTA [ORF50]) suppressed latent-lytic switch [67]. The HBP can additionally counteract viral replication through the O-GlcNAcylation of sterile alpha motif and histidine/aspartic acid domain-containing protein 1 (SAMHD1) [50], a nuclease and dNTP triphosphatase that restricts viral replication by cleaving viral genomes and depleting the dNTP pool. Hu et al. showed that inhibition or knockdown of GLUT1, GFPT1, or OGT increased HBV DNA replication. They identified that SAMHD1 is O-GlcNAcylated on S93, which protects it from K48 ubiquitin-mediated degradation and is required for its tetramerization and antiviral activity against HBV and HIV-1 [50] (Figure 3). In patients with chronic hepatitis B, UDP-GlcNAc levels, GLUT1 expression, and total O-GlcNAcylation are higher than in healthy controls, and SAMHD1 is O-GlcNAcylated in liver tissues. It is argued that enhancing O-GlcNAcylation might suppress HBV replication. In a recent report, Dong et al. identified a novel role of OGT in restricting IAV infection. Besides its effect on promoting MAVS antiviral immunity through MAVS O-GlcNAcylation, OGT exerted an additional antiviral function independently of its catalytic activity. The authors demonstrated that K908A catalytically inactive OGT dampened IAV infection both in vitro and in vivo. Mechanistically, OGT binds to IAV genomic RNA and limits lipid droplet accumulation, which is required for efficient IAV replication [70]. This was achieved by promoting perilipin-2 (PLIN2) K48-ubiquitination and proteosomal degradation (Figure 3). These findings place OGT at the junction of sugar and lipid metabolism pathways collectively engaged in the fight against IAV infection.
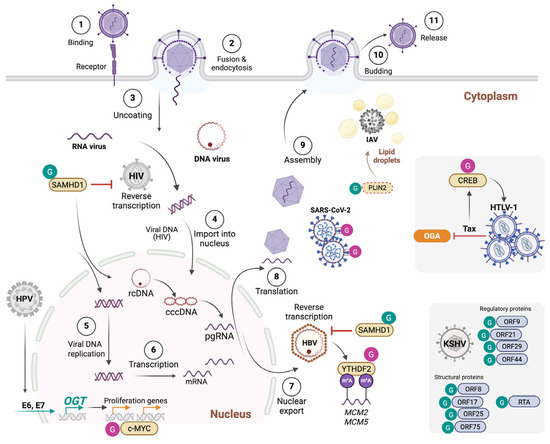
Figure 3.
O-GlcNAcylation modulates multiple steps of the viral life cycle and viral oncogenesis. O-GlcNAcylation stabilizes the antiviral factor SAMHD1, thereby enhancing restriction of HIV-1 and HBV. In HBV infection, O-GlcNAcylation of the m6A reader YTHDF2 promotes stabilization and binding to MCM2/5 transcripts, supporting viral oncogenesis. In influenza A virus (IAV), OGT restricts replication by targeting lipid droplet metabolism via PLIN2 degradation, while in SARS-CoV-2, O-GlcNAcylation of the spike protein enhances stability and particle packaging. In HTLV-1, the viral oncoprotein Tax inhibits OGA, elevating CREB O-GlcNAcylation and driving proviral transcription. HPV E6/E7 stimulates OGT expression, leading to c-MYC O-GlcNAcylation and stabilization, which sustains cellular transformation. In KSHV, O-GlcNAcylation of multiple proteins inhibits viral replication and the latent-lytic cycle.
6. Harnessing the HBP-O-GlcNAc Pathway for Clinical Applications
(i) The HBP and O-GlcNAcylation as potential biomarkers of disease progression in viral infections. Severe influenza infection provides clinical evidence that O-GlcNAcylation correlates with disease severity [43]. Wang et al. noted that patients with acute influenza had significantly higher global O-GlcNAc levels and OGT expression in their PBMCs compared to healthy individuals. Heightened O-GlcNAc was linked to elevated serum cytokine levels in patients, linking O-GlcNAcylation to a cytokine storm phenotype in severe flu. Patients with chronic HBV infection also show higher UDP-GlcNAc levels and greater protein O-GlcNAcylation in their liver tissue compared to healthy controls [50]. In HBV-related HCC, the HBP-O-GlcNAc axis is often upregulated [51]. As mentioned before, YTHDF2 is heavily O-GlcNAcylated, and its levels correlate with worse HBV-HCC patient survival, implicating O-GlcNAcylated YTHDF2 as a potential biomarker of aggressive disease. Similarly, elevated O-GlcNAcylation is a marker of HPV-driven oncogenesis, e.g., in cervical cancer [73]. In clinical cervical tumor samples, HPV-positive lesions exhibit higher OGT and O-GlcNAc levels than HPV-negative tissue. The HPV16 E6–driven increase in total cellular O-GlcNAcylation is not only associated with tumorigenesis but also with disease severity, as higher OGT and O-GlcNAc levels correlate with advanced grade or malignant progression. Hence, O-GlcNAcylation can be measured as a clinically relevant biomarker of HPV-associated malignant transformation and progression.
(ii) HBP and O-GlcNAcylation as therapeutic targets in viral Infections: Several studies have underscored immunometabolic modulation as a feasible adjunct treatment in counteracting virus-induced hyperinflammatory states. For instance, in a retrospective study of hospitalized COVID-19 patients, Sukkar et al. reported that a eucaloric ketogenic diet was associated with reduced mortality, fewer ICU admissions, and a favorable trend toward lower IL-6 levels [75]. N-acetyl glucosamine (GlcNAc) that drives the HBP has been tested as a low-cost anti-inflammatory immunomodulatory agent for inflammatory joint illnesses, inflammatory bowel disease, and autoimmunity [76,77,78,79,80]. It has also been investigated as a potential anti-inflammatory treatment in respiratory viral infections. In a prospective observational study of COVID-19 patients, GlcNAc was associated with improved clinical outcome [81]. In addition, it has shown antiviral activity in vitro [82]. Beyond metabolic modulation, direct pharmacological enhancement of the O-GlcNAc pathway is now being evaluated clinically using OGA inhibitors. Ceperognastat (LY3372689) is the most clinically advanced OGA inhibitor [83]. Phase I trials demonstrated oral bioavailability, robust target engagement across dose ranges, >95% OGA enzyme occupancy, and an acceptable safety profile, supporting its progression to Phase II trials. Other OGA inhibitors have also advanced into clinical evaluation. ASN90 (ASN120290) is an orally bioavailable compound in Phase II development with early human studies demonstrating a dose-dependent increase in protein O-GlcNAcylation, reliable target engagement confirmed by positron emission tomography, and a favorable safety profile [84]. MK-8719 is another potent, selective OGA inhibitor that completed first-in-human testing [85]. Pharmacodynamic assays confirmed sustained elevation of O-GlcNAc in brain tissue, establishing the feasibility of systemic OGA inhibition in humans. Although these compounds were initially developed for tauopathies such as Alzheimer’s disease, their pharmacological properties establish OGA inhibition as clinically viable and highlight the potential for its repurposing in viral infections, where O-GlcNAcylation shapes host–viral interactions.
7. Conclusions
O-GlcNAcylation has emerged as a pivotal post-translational modification linking nutrient flux to immune and viral processes. By integrating signals from glucose, amino acids, lipids, and nucleotides through the hexosamine biosynthetic pathway, it functions as a nutrient-sensitive rheostat that regulates cellular metabolism and immune signaling. In the antiviral context, O-GlcNAcylation exerts multifaceted control of host defense, fine-tuning innate viral sensing pathways by directly targeting central effectors such as MAVS, STING, and IRF family members, while regulating adaptive responses through modulation of master transcription factors in T and B cell subpopulations [Table 1]. This balanced cycling of O-GlcNAc, regulated by the actions of OGT and OGA, ensures that immune activation remains metabolically sustained while preventing excessive inflammation and tissue injury. Through years of coevolution, viruses have evolved to exploit or subvert this modification to enhance replication, latency, and immune evasion [Figure 3]. The bidirectional relationship between O-GlcNAcylation and viral pathogenesis underscores its dualistic nature, being protective during acute infection but permissive to persistence or transformation under chronic metabolic stress.
Despite the growing literature, several fundamental questions remain unresolved. Firstly, how do OGT and OGA distinguish among thousands of potential substrates to generate context-dependent outcomes? The lack of a strict O-GlcNAcylation consensus sequence and the influence of subcellular localization together contribute to the complexity of deciphering this question. Secondly, from a spatiotemporal perspective, how do fluctuations in nutrient availability or infection stage reprogram the O-GlcNAc proteome across distinct immune cell compartments? Addressing this question will require the integration of advanced glycoproteomics, single-cell metabolomics, and high-resolution imaging approaches, particularly when coupled with emerging artificial intelligence tools capable of resolving these complex dynamics. Regarding pathway crosstalk, O-GlcNAcylation often interacts with phosphorylation, acetylation, and ubiquitination on shared residues, but whether these interactions represent antagonism, synergy, or sequential modulation during viral infection remains unclear. Furthermore, chronic metabolic disorders such as obesity and diabetes alter systemic HBP flux and global O-GlcNAc levels, yet their precise influence on antiviral immunity and viral persistence requires further exploration.
From a translational standpoint, the reversibility of O-GlcNAcylation offers an attractive therapeutic strategy. Pharmacological inhibitors or activators of OGA and OGT, such as Thiamet-G, MK-8719, or Ceperognastat, can modulate global O-GlcNAc levels, but their systemic application carries the risk of off-target effects due to the ubiquity of this modification. An open question is whether O-GlcNAcylation should be increased to strengthen antiviral signaling or decreased to stop viruses from exploiting host metabolism. Another major challenge is achieving cell-type-specific and temporally controlled modulation that enhances antiviral immunity without triggering hyperinflammatory responses or metabolic toxicity. Answering this will require integrative in vivo models that map O-GlcNAc fluxes across the immune system, infection stages, and tissue microenvironments, thereby paving the way for new possibilities in the emerging field of metabolic therapies.
Ultimately, O-GlcNAcylation serves as a central link between nutrient sensing and host–virus interaction. As analytical tools advance, answering the outstanding questions will bring the field closer to leveraging O-GlcNAcylation as both a biomarker and a therapeutic target for immune restoration in viral, inflammatory, and oncogenic diseases.
Author Contributions
T.I.O. and M.S. synthesized the published literature and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the Saleh laboratory on this topic is funded by the Natural Sciences and Engineering Research Council of Canada (NSERC-RGPIN-2025-07017). Other funding to the lab is from the Institut National de la Recherche Scientifique (INRS), the Cancer Research Society (CRS), the Canadian Institutes for Health Research (CIHR), and the Canada Foundation for Innovation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koonin, E.V.; Krupovic, M.; Dolja, V.V. The global virome: How much diversity and how many independent origins? Environ. Microbiol. 2023, 25, 40–44. [Google Scholar] [CrossRef]
- Carroll, D.; Daszak, P.; Wolfe, N.D.; Gao, G.F.; Morel, C.M.; Morzaria, S.; Pablos-Mendez, A.; Tomori, O.; Mazet, J.A.K. The Global Virome Project. Science 2018, 359, 872–874. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Ahmed, A.B.F.; Bhattacharjee, S.; Slama, P. Viral Pandemics of the Last Four Decades: Pathophysiology, Health Impacts and Perspectives. Int. J. Environ. Res. Public Health 2020, 17, 9411. [Google Scholar] [CrossRef]
- Marchi, J.; Lassig, M.; Walczak, A.M.; Mora, T. Antigenic waves of virus-immune coevolution. Proc. Natl. Acad. Sci. USA 2021, 118, e2103398118. [Google Scholar] [CrossRef]
- Muhammad, I.; Contes, K.; Bility, M.T.; Tang, Q. Chasing Virus Replication and Infection: PAMP-PRR Interaction Drives Type I Interferon Production, Which in Turn Activates ISG Expression and ISGylation. Viruses 2025, 17, 528. [Google Scholar] [CrossRef] [PubMed]
- Beachboard, D.C.; Horner, S.M. Innate immune evasion strategies of DNA and RNA viruses. Curr. Opin. Microbiol. 2016, 32, 113–119. [Google Scholar] [CrossRef]
- Tay, D.J.W.; Lew, Z.Z.R.; Chu, J.J.H.; Tan, K.S. Uncovering Novel Viral Innate Immune Evasion Strategies: What Has SARS-CoV-2 Taught Us? Front. Microbiol. 2022, 13, 844447. [Google Scholar] [CrossRef]
- Chan, Y.K.; Gack, M.U. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Microbiol. 2016, 14, 360–373. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Acchioni, M.; Acchioni, C.; Hiscott, J.; Sgarbanti, M. Origin and function of anti-interferon type I viral proteins. Virology 2025, 605, 110456. [Google Scholar] [CrossRef]
- Chi, H.; Pepper, M.; Thomas, P.G. Principles and therapeutic applications of adaptive immunity. Cell 2024, 187, 2052–2078. [Google Scholar] [CrossRef]
- Mora, T.; Walczak, A.M. Quantitative Theory of Viral-Immune Coevolution May Be within Reach. PRX Life 2023, 1, 011001. [Google Scholar] [CrossRef]
- Pradeu, T.; Thomma, B.; Girardin, S.E.; Lemaitre, B. The conceptual foundations of innate immunity: Taking stock 30 years later. Immunity 2024, 57, 613–631. [Google Scholar] [CrossRef]
- Wells, A.I.; Coyne, C.B. Type III Interferons in Antiviral Defenses at Barrier Surfaces. Trends Immunol. 2018, 39, 848–858. [Google Scholar] [CrossRef]
- Tomalka, J.A.; Suthar, M.S.; Diamond, M.S.; Sekaly, R.P. Innate antiviral immunity: How prior exposures can guide future responses. Trends Immunol. 2022, 43, 696–705. [Google Scholar] [CrossRef]
- Vivier, E.; Rebuffet, L.; Narni-Mancinelli, E.; Cornen, S.; Igarashi, R.Y.; Fantin, V.R. Natural killer cell therapies. Nature 2024, 626, 727–736. [Google Scholar] [CrossRef]
- Ngo, C.; Garrec, C.; Tomasello, E.; Dalod, M. The role of plasmacytoid dendritic cells (pDCs) in immunity during viral infections and beyond. Cell Mol. Immunol. 2024, 21, 1008–1035. [Google Scholar] [CrossRef]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef]
- Carroll, S.L.; Pasare, C.; Barton, G.M. Control of adaptive immunity by pattern recognition receptors. Immunity 2024, 57, 632–648. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, X.; Ho, W.Z. Roles of Macrophages in Viral Infections. Viruses 2024, 16, 1643. [Google Scholar] [CrossRef]
- Brancewicz, J.; Wojcik, N.; Sarnowska, Z.; Robak, J.; Krol, M. The Multifaceted Role of Macrophages in Biology and Diseases. Int. J. Mol. Sci. 2025, 26, 2107. [Google Scholar] [CrossRef]
- Rodrigo-Munoz, J.M.; Sastre, B.; Canas, J.A.; Gil-Martinez, M.; Redondo, N.; Del Pozo, V. Eosinophil Response Against Classical and Emerging Respiratory Viruses: COVID-19. J. Investig. Allergol. Clin. Immunol. 2021, 31, 94–107. [Google Scholar] [CrossRef]
- Rupani, H.; Busse, W.W.; Howarth, P.H.; Bardin, P.G.; Adcock, I.M.; Konno, S.; Jackson, D.J. Therapeutic relevance of eosinophilic inflammation and airway viral interactions in severe asthma. Allergy 2024, 79, 2589–2604. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Zhu, L. Role of neutrophils in acute viral infection. Immun. Inflamm. Dis. 2021, 9, 1186–1196. [Google Scholar] [CrossRef]
- Sasaki, H.; Miyata, J.; Kawana, A.; Fukunaga, K. Antiviral roles of eosinophils in asthma and respiratory viral infection. Front. Allergy 2025, 6, 1548338. [Google Scholar] [CrossRef]
- Gebremeskel, S.; Schanin, J.; Coyle, K.M.; Butuci, M.; Luu, T.; Brock, E.C.; Xu, A.; Wong, A.; Leung, J.; Korver, W.; et al. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021, 12, 650331. [Google Scholar] [CrossRef]
- Palmer, C.S. Innate metabolic responses against viral infections. Nat. Metab. 2022, 4, 1245–1259. [Google Scholar] [CrossRef]
- Perakakis, N.; Harb, H.; Hale, B.G.; Varga, Z.; Steenblock, C.; Kanczkowski, W.; Alexaki, V.I.; Ludwig, B.; Mirtschink, P.; Solimena, M.; et al. Mechanisms and clinical relevance of the bidirectional relationship of viral infections with metabolic diseases. Lancet Diabetes Endocrinol. 2023, 11, 675–693. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Baker, S.A.; Rutter, J. Metabolites as signalling molecules. Nat. Rev. Mol. Cell Biol. 2023, 24, 355–374. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef]
- El Safadi, D.; Paulo-Ramos, A.; Hoareau, M.; Roche, M.; Krejbich-Trotot, P.; Viranaicken, W.; Lebeau, G. The Influence of Metabolism on Immune Response: A Journey to Understand Immunometabolism in the Context of Viral Infection. Viruses 2023, 15, 2399. [Google Scholar] [CrossRef]
- Gong, J.; Gao, X.; Ge, S.; Li, H.; Wang, R.; Zhao, L. The Role of cGAS-STING Signalling in Metabolic Diseases: From Signalling Networks to Targeted Intervention. Int. J. Biol. Sci. 2024, 20, 152–174. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gomez de Las Heras, M.M.; Gabande-Rodriguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef]
- Chandel, N.S. Lipid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040576. [Google Scholar] [CrossRef]
- Mannino, M.P.; Hart, G.W. The Beginner’s Guide to O-GlcNAc: From Nutrient Sensitive Pathway Regulation to Its Impact on the Immune System. Front. Immunol. 2022, 13, 828648. [Google Scholar] [CrossRef]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef]
- Issad, T.; Al-Mukh, H.; Bouaboud, A.; Pagesy, P. Protein O-GlcNAcylation and the regulation of energy homeostasis: Lessons from knock-out mouse models. J. Biomed. Sci. 2022, 29, 64. [Google Scholar] [CrossRef]
- Li, X.; Gong, W.; Wang, H.; Li, T.; Attri, K.S.; Lewis, R.E.; Kalil, A.C.; Bhinderwala, F.; Powers, R.; Yin, G.; et al. O-GlcNAc Transferase Suppresses Inflammation and Necroptosis by Targeting Receptor-Interacting Serine/Threonine-Protein Kinase 3. Immunity 2019, 50, 576–590.e6. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Luan, H.H.; Zhang, B.; Zhang, K.; Nam, J.H.; Li, Z.; Fu, M.; Munk, A.; Zhang, D.; et al. OGT suppresses S6K1-mediated macrophage inflammation and metabolic disturbance. Proc. Natl. Acad. Sci. USA 2020, 117, 16616–16625. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, P.; He, R.; Li, M.; Yu, H.; Zhou, L.; Yi, Y.; Wang, F.; Rong, Y.; Zhang, Y.; et al. O-GlcNAc transferase promotes influenza A virus-induced cytokine storm by targeting interferon regulatory factor-5. Sci. Adv. 2020, 6, eaaz7086. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Attri, K.S.; Liu, C.; Li, L.; Herring, L.E.; Asara, J.M.; Lei, Y.L.; Singh, P.K.; Gao, C.; et al. O-GlcNAc Transferase Links Glucose Metabolism to MAVS-Mediated Antiviral Innate Immunity. Cell Host Microbe 2018, 24, 791–803.e6. [Google Scholar] [CrossRef]
- Song, N.; Qi, Q.; Cao, R.; Qin, B.; Wang, B.; Wang, Y.; Zhao, L.; Li, W.; Du, X.; Liu, F.; et al. MAVS O-GlcNAcylation Is Essential for Host Antiviral Immunity against Lethal RNA Viruses. Cell Rep. 2019, 28, 2386–2396.e5. [Google Scholar] [CrossRef]
- Seo, J.; Park, Y.S.; Kweon, T.H.; Kang, J.; Son, S.; Kim, H.B.; Seo, Y.R.; Kang, M.J.; Yi, E.C.; Lee, Y.H.; et al. O-Linked N-Acetylglucosamine Modification of Mitochondrial Antiviral Signaling Protein Regulates Antiviral Signaling by Modulating Its Activity. Front. Immunol. 2020, 11, 589259. [Google Scholar] [CrossRef]
- Xia, W.; Jiang, P. p53 promotes antiviral innate immunity by driving hexosamine metabolism. Cell Rep. 2024, 43, 113724. [Google Scholar] [CrossRef]
- Li, Y.; An, W.; Lu, L.; Yuan, J.; Wu, D.; Yang, Q.; Guo, J.; Yang, J.; Liu, M.; He, K.; et al. O-GlcNAc of STING mediates antiviral innate immunity. Cell Commun. Signal 2024, 22, 157. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, Q.; Tian, W.; Zheng, Z.; He, W.; Zhang, R.; Zhao, Q.; Miao, Y.; Yuan, Y.; Wang, J.; et al. beta-hydroxybutyrylation and O-GlcNAc modifications of STAT1 modulate antiviral defense in aging. Cell Mol. Immunol. 2025, 22, 403–417. [Google Scholar] [CrossRef]
- Hu, J.; Gao, Q.; Yang, Y.; Xia, J.; Zhang, W.; Chen, Y.; Zhou, Z.; Chang, L.; Hu, Y.; Zhou, H.; et al. Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation. Theranostics 2021, 11, 805–823. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Y.; Yin, J.; Tang, N.; Wang, K.; Huang, L.; Hu, J.; Feng, Z.; Gao, Q.; Huang, A. O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N(6)-methyladenosine-dependent manner. Signal Transduct. Target. Ther. 2023, 8, 63. [Google Scholar] [CrossRef]
- Golks, A.; Tran, T.T.; Goetschy, J.F.; Guerini, D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 2007, 26, 4368–4379. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Clark, P.M.; Mason, D.E.; Peters, E.C.; Hsieh-Wilson, L.C.; Baltimore, D. Activation of the transcriptional function of the NF-kappaB protein c-Rel by O-GlcNAc glycosylation. Sci. Signal 2013, 6, ra75. [Google Scholar] [CrossRef]
- de Jesus, T.J.; Tomalka, J.A.; Centore, J.T.; Staback Rodriguez, F.D.; Agarwal, R.A.; Liu, A.R.; Kern, T.S.; Ramakrishnan, P. Negative regulation of FOXP3 expression by c-Rel O-GlcNAcylation. Glycobiology 2021, 31, 812–826. [Google Scholar] [CrossRef]
- Groussaud, D.; Khair, M.; Tollenaere, A.I.; Waast, L.; Kuo, M.S.; Mangeney, M.; Martella, C.; Fardini, Y.; Coste, S.; Souidi, M.; et al. Hijacking of the O-GlcNAcZYME complex by the HTLV-1 Tax oncoprotein facilitates viral transcription. PLoS Pathog. 2017, 13, e1006518. [Google Scholar] [CrossRef]
- Liu, B.; Salgado, O.C.; Singh, S.; Hippen, K.L.; Maynard, J.C.; Burlingame, A.L.; Ball, L.E.; Blazar, B.R.; Farrar, M.A.; Hogquist, K.A.; et al. The lineage stability and suppressive program of regulatory T cells require protein O-GlcNAcylation. Nat. Commun. 2019, 10, 354. [Google Scholar] [CrossRef]
- Machacek, M.; Saunders, H.; Zhang, Z.; Tan, E.P.; Li, J.; Li, T.; Villar, M.T.; Artigues, A.; Lydic, T.; Cork, G.; et al. Elevated O-GlcNAcylation enhances pro-inflammatory Th17 function by altering the intracellular lipid microenvironment. J. Biol. Chem. 2019, 294, 8973–8990. [Google Scholar] [CrossRef]
- Swamy, M.; Pathak, S.; Grzes, K.M.; Damerow, S.; Sinclair, L.V.; van Aalten, D.M.; Cantrell, D.A. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 2016, 17, 712–720. [Google Scholar] [CrossRef]
- Lee, D.H.; Kwon, N.E.; Lee, W.J.; Lee, M.S.; Kim, D.J.; Kim, J.H.; Park, S.K. Increased O-GlcNAcylation of c-Myc Promotes Pre-B Cell Proliferation. Cells 2020, 9, 158. [Google Scholar] [CrossRef]
- Wu, J.L.; Chiang, M.F.; Hsu, P.H.; Tsai, D.Y.; Hung, K.H.; Wang, Y.H.; Angata, T.; Lin, K.I. O-GlcNAcylation is required for B cell homeostasis and antibody responses. Nat. Commun. 2017, 8, 1854. [Google Scholar] [CrossRef]
- Jeon, B.C.; Kim, Y.J.; Park, A.K.; Song, M.R.; Na, K.M.; Lee, J.; An, D.; Park, Y.; Hwang, H.; Kim, T.D.; et al. Dynamic O-GlcNAcylation governs long-range chromatin interactions in V(D)J recombination during early B-cell development. Cell Mol. Immunol. 2025, 22, 68–82. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Chu, H.; Zhang, H.; Wu, H.; Song, G.; Wang, P.; Zhao, K.; Hou, J.; Wang, X.; et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination. Nat. Immunol. 2017, 18, 214–224. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, K.; Li, W.; Yang, X.; Gou, Y.; Su, X.; Qian, F.; Sun, L. Cullin5 drives experimental asthma exacerbations by modulating alveolar macrophage antiviral immunity. Nat. Commun. 2024, 15, 252. [Google Scholar] [CrossRef]
- Williams, T.C.; Loo, S.L.; Nichol, K.S.; Reid, A.T.; Veerati, P.C.; Esneau, C.; Wark, P.A.B.; Grainge, C.L.; Knight, D.A.; Vincent, T.; et al. IL-25 blockade augments antiviral immunity during respiratory virus infection. Commun. Biol. 2022, 5, 415. [Google Scholar] [CrossRef]
- Liang, Y.; Ge, Y.; Sun, J. IL-33 in COVID-19: Friend or foe? Cell Mol. Immunol. 2021, 18, 1602–1604. [Google Scholar] [CrossRef]
- Jochmann, R.; Pfannstiel, J.; Chudasama, P.; Kuhn, E.; Konrad, A.; Sturzl, M. O-GlcNAc transferase inhibits KSHV propagation and modifies replication relevant viral proteins as detected by systematic O-GlcNAcylation analysis. Glycobiology 2013, 23, 1114–1130. [Google Scholar] [CrossRef]
- Ko, Y.C.; Tsai, W.H.; Wang, P.W.; Wu, I.L.; Lin, S.Y.; Chen, Y.L.; Chen, J.Y.; Lin, S.F. Suppressive regulation of KSHV RTA with O-GlcNAcylation. J. Biomed. Sci. 2012, 19, 12. [Google Scholar] [CrossRef]
- Xu, T.; Li, J.; Lu, X.; Song, S.; Wang, S.; Li, J.; Zhang, L. O-GlcNAcylation at S659 enhances SARS-CoV-2 spike protein stability and pseudoparticle packaging efficiency. Microbiol. Spectr. 2025, 13, e0052725. [Google Scholar] [CrossRef]
- Jochmann, R.; Thurau, M.; Jung, S.; Hofmann, C.; Naschberger, E.; Kremmer, E.; Harrer, T.; Miller, M.; Schaft, N.; Sturzl, M. O-linked N-acetylglucosaminylation of Sp1 inhibits the human immunodeficiency virus type 1 promoter. J. Virol. 2009, 83, 3704–3718. [Google Scholar] [CrossRef]
- Dong, H.; Liang, C.; Zhang, J.; Wu, W.; Kumar, N.; Liu, Z.; Sun, Y.; Liao, Z.; Cheng, X.; Yu, Y.; et al. O-GlcNAc transferase plays dual antiviral roles by integrating innate immunity and lipid metabolism. Nat. Commun. 2025, 16, 7721. [Google Scholar] [CrossRef]
- Fricke, J.; Koo, L.Y.; Brown, C.R.; Collins, P.L. p38 and OGT sequestration into viral inclusion bodies in cells infected with human respiratory syncytial virus suppresses MK2 activities and stress granule assembly. J. Virol. 2013, 87, 1333–1347. [Google Scholar] [CrossRef]
- Ciraku, L.; Esquea, E.M.; Reginato, M.J. O-GlcNAcylation regulation of cellular signaling in cancer. Cell Signal 2022, 90, 110201. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhao, R.X.; Chen, J.; Li, Y.; Li, X.D.; Liu, X.L.; Zhang, W.M.; Quan, C.S.; Wang, Y.S.; Zhai, Y.X.; et al. O-linked GlcNAcylation elevated by HPV E6 mediates viral oncogenesis. Proc. Natl. Acad. Sci. USA 2016, 113, 9333–9338. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S.; Choi, M.Y.; Kim, M.; Yang, J.H.; Park, H.O.; Jang, I.S.; Moon, S.H.; Kim, H.O.; Song, D.H.; et al. O-linked-N-acetylglucosamine transferase is associated with metastatic spread of human papillomavirus E6 and E7 oncoproteins to the lungs of mice. Biochem. Biophys. Res. Commun. 2017, 483, 793–802. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Cogorno, L.; Pisciotta, L.; Pasta, A.; Vena, A.; Gradaschi, R.; Dentone, C.; Guiddo, E.; Martino, E.; Beltramini, S.; et al. Clinical efficacy of eucaloric ketogenic nutrition in the COVID-19 cytokine storm: A retrospective analysis of mortality and intensive care unit admission. Nutrition 2021, 89, 111236. [Google Scholar] [CrossRef]
- Salvatore, S.; Heuschkel, R.; Tomlin, S.; Davies, S.E.; Edwards, S.; Walker-Smith, J.A.; French, I.; Murch, S.H. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment. Pharmacol. Ther. 2000, 14, 1567–1579. [Google Scholar] [CrossRef]
- Grigorian, A.; Araujo, L.; Naidu, N.N.; Place, D.J.; Choudhury, B.; Demetriou, M. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J. Biol. Chem. 2011, 286, 40133–40141. [Google Scholar] [CrossRef]
- Richter, J.; Capkova, K.; Hribalova, V.; Vannucci, L.; Danyi, I.; Maly, M.; Fiserova, A. Collagen-induced arthritis: Severity and immune response attenuation using multivalent N-acetyl glucosamine. Clin. Exp. Immunol. 2014, 177, 121–133. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Wakuda, T.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Suppressive effects of N-acetyl-D-glucosamine on rheumatoid arthritis mouse models. Inflammation 2012, 35, 1462–1465. [Google Scholar] [CrossRef]
- Lee, S.U.; Li, C.F.; Mortales, C.L.; Pawling, J.; Dennis, J.W.; Grigorian, A.; Demetriou, M. Increasing cell permeability of N-acetylglucosamine via 6-acetylation enhances capacity to suppress T-helper 1 (TH1)/TH17 responses and autoimmunity. PLoS ONE 2019, 14, e0214253. [Google Scholar] [CrossRef]
- Hassan, A.E. An observational cohort study to assess N-acetylglucosamine for COVID-19 treatment in the inpatient setting. Ann. Med. Surg. 2021, 68, 102574. [Google Scholar] [CrossRef]
- Marchetti, M.; De Berardis, B.; Bigioni, I.; Mariano, A.; Superti, F.; Scotto d’Abusco, A. In Vitro Antiviral and Anti-Inflammatory Activities of N-Acetylglucosamine: Development of an Alternative and Safe Approach to Fight Viral Respiratory Infections. Int. J. Mol. Sci. 2023, 24, 5129. [Google Scholar] [CrossRef]
- Kielbasa, W.; Goldsmith, P.; Donnelly, K.B.; Nuthall, H.N.; Shcherbinin, S.; Fleisher, A.S.; Hendle, J.; DuBois, S.L.; Lowe, S.L.; Zhang, F.F.; et al. Discovery and clinical translation of ceperognastat, an O-GlcNAcase (OGA) inhibitor, for the treatment of Alzheimer’s disease. Alzheimer’s Dement. 2024, 10, e70020. [Google Scholar] [CrossRef]
- Permanne, B.; Sand, A.; Ousson, S.; Neny, M.; Hantson, J.; Schubert, R.; Wiessner, C.; Quattropani, A.; Beher, D. O-GlcNAcase Inhibitor ASN90 is a Multimodal Drug Candidate for Tau and alpha-Synuclein Proteinopathies. ACS Chem. Neurosci. 2022, 13, 1296–1314. [Google Scholar] [CrossRef]
- Selnick, H.G.; Hess, J.F.; Tang, C.; Liu, K.; Schachter, J.B.; Ballard, J.E.; Marcus, J.; Klein, D.J.; Wang, X.; Pearson, M.; et al. Discovery of MK-8719, a Potent O-GlcNAcase Inhibitor as a Potential Treatment for Tauopathies. J. Med. Chem. 2019, 62, 10062–10097. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).