Distinct Tumor-Associated Macrophage Signatures Shape the Immune Microenvironment and Patient Prognosis in Renal Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Utilization
2.2. Curation of Immune-Related Genes (IRGs)
2.3. Immune Cell Inference
2.4. Generation of TAM Signatures
2.5. LASSO Cox Regression
2.6. Survival Analysis
2.7. Statistical Analyses
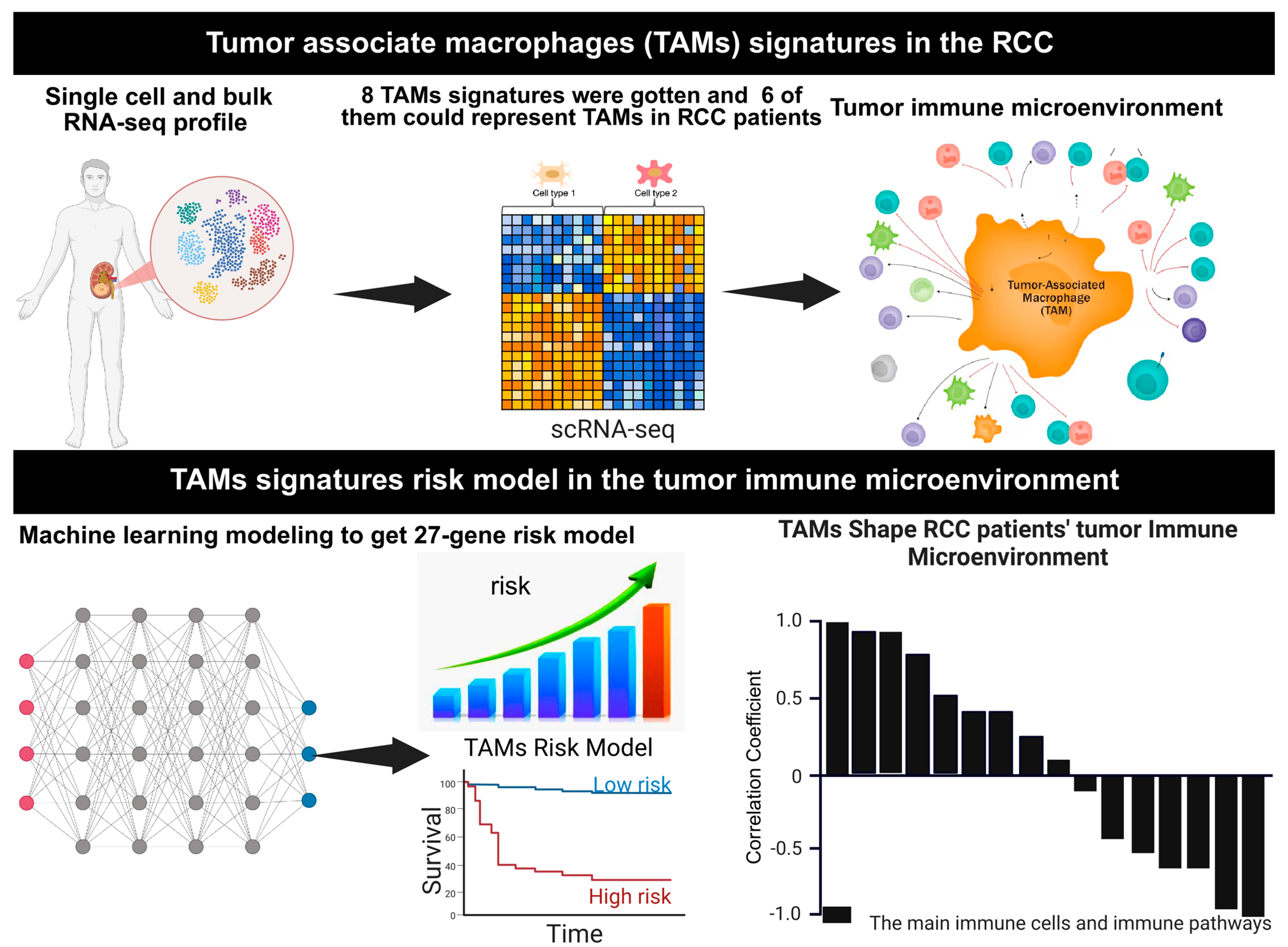
3. Results
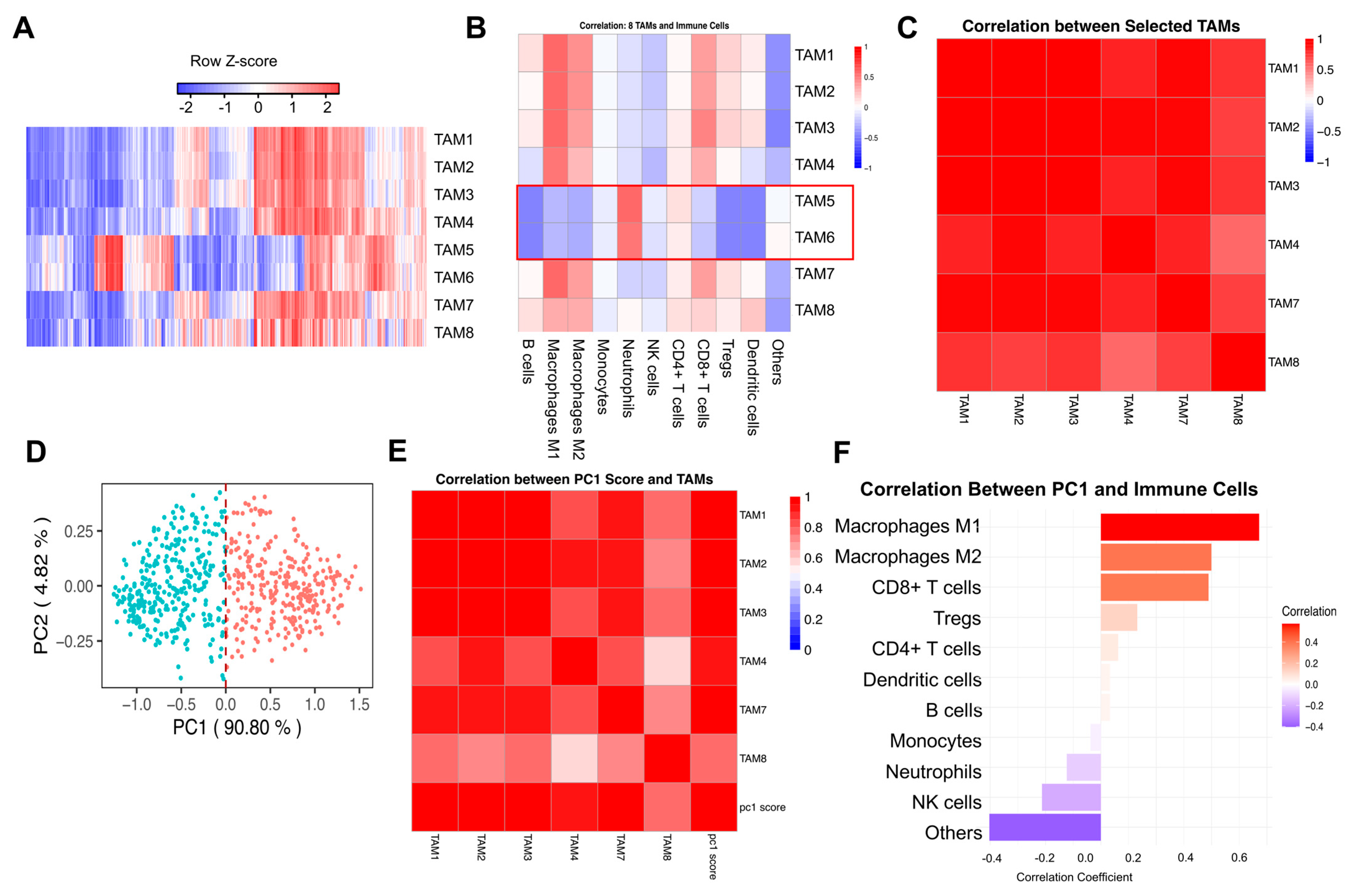
3.1. Characterization of Tumor-Associated Macrophage Subtypes and Their Immune Microenvironment in Renal Cell Carcinoma
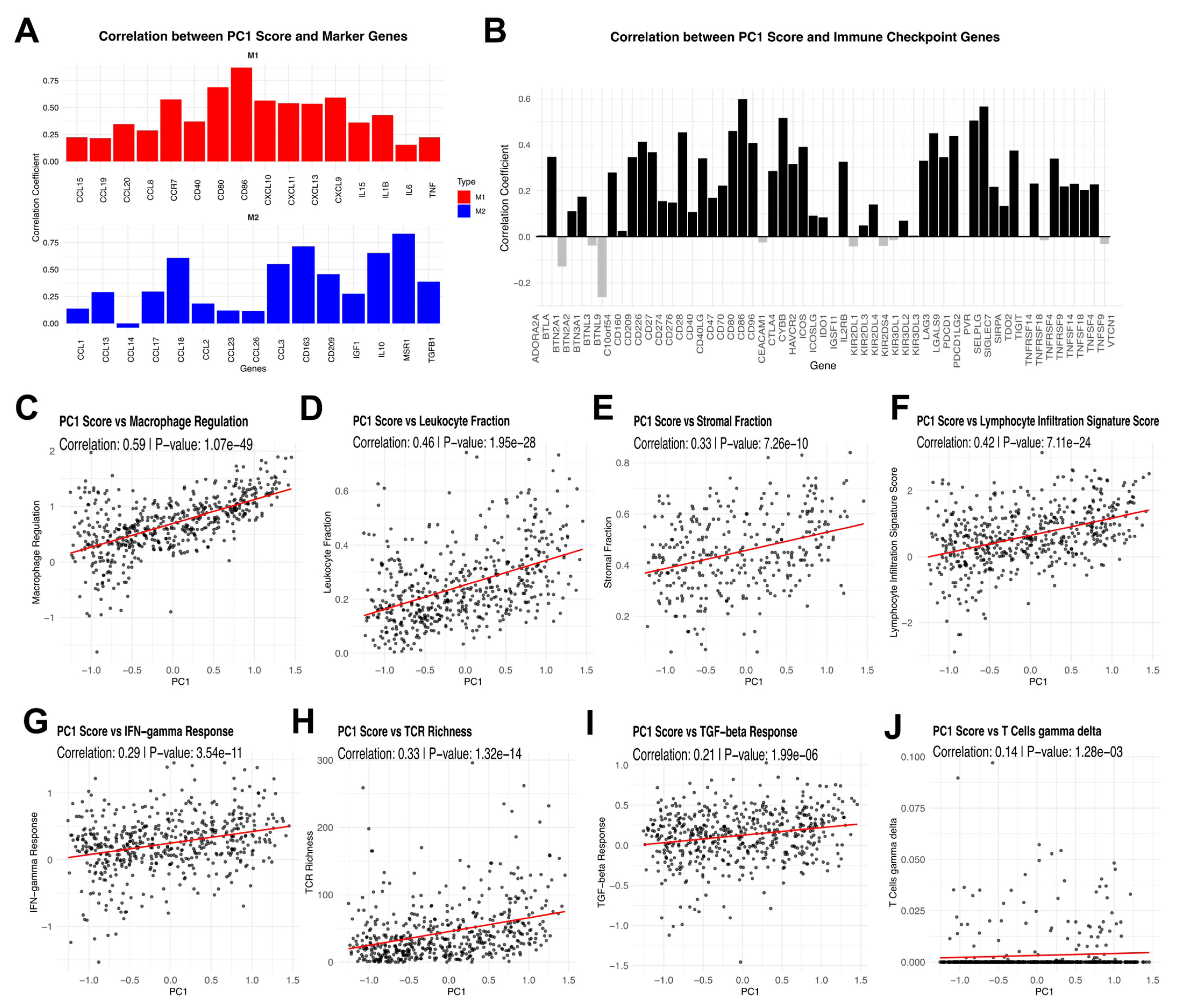
3.2. Principal Component Analysis of Tumor-Associated Macrophage Signatures and Their Immunological Associations in RCC
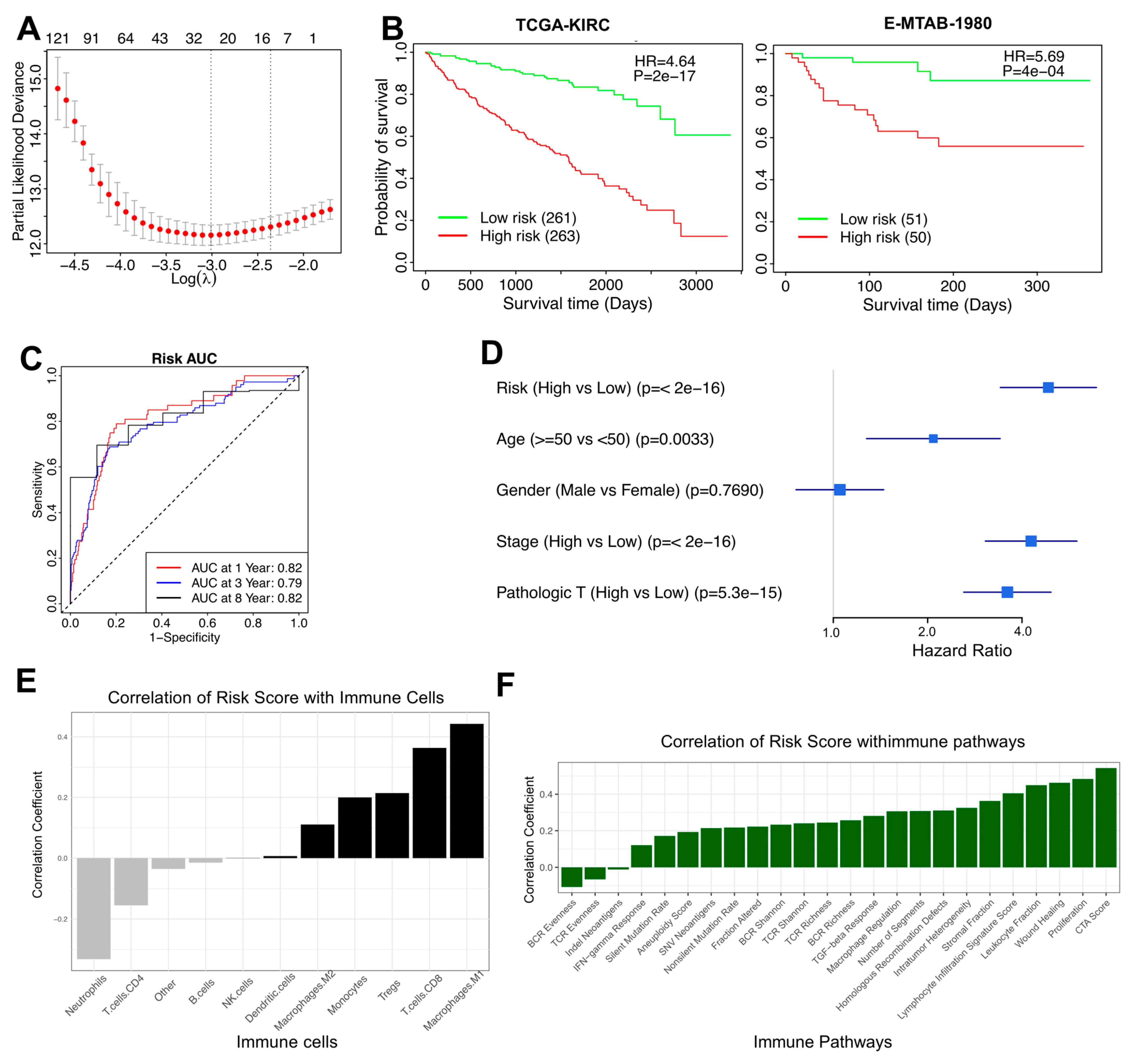
3.3. A 27-Gene Risk Score for Prognostic Prediction in RCC
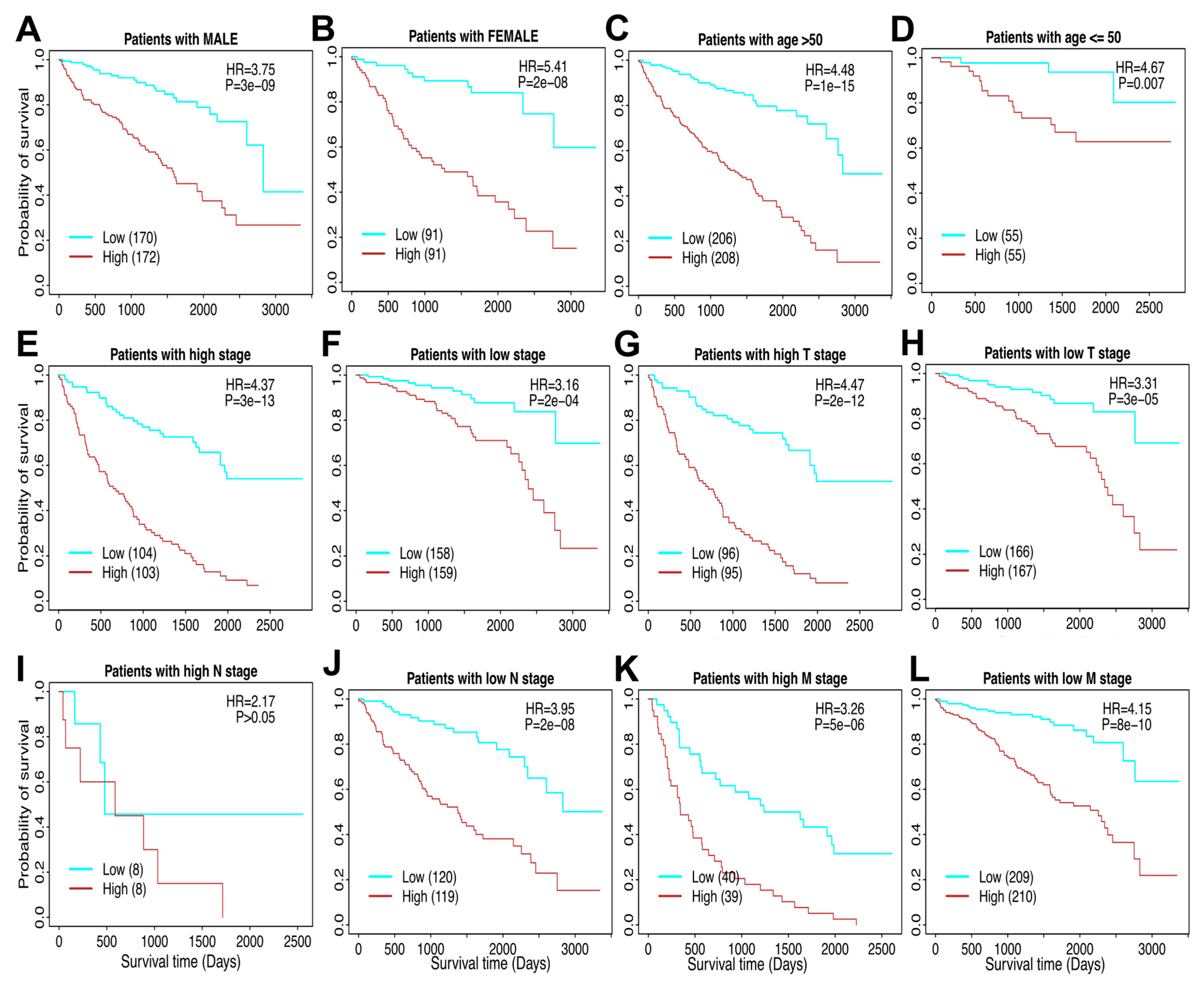
3.4. The TAM Risk Model Can Evaluate RCC Patients Across Different Clinicopathological Factors
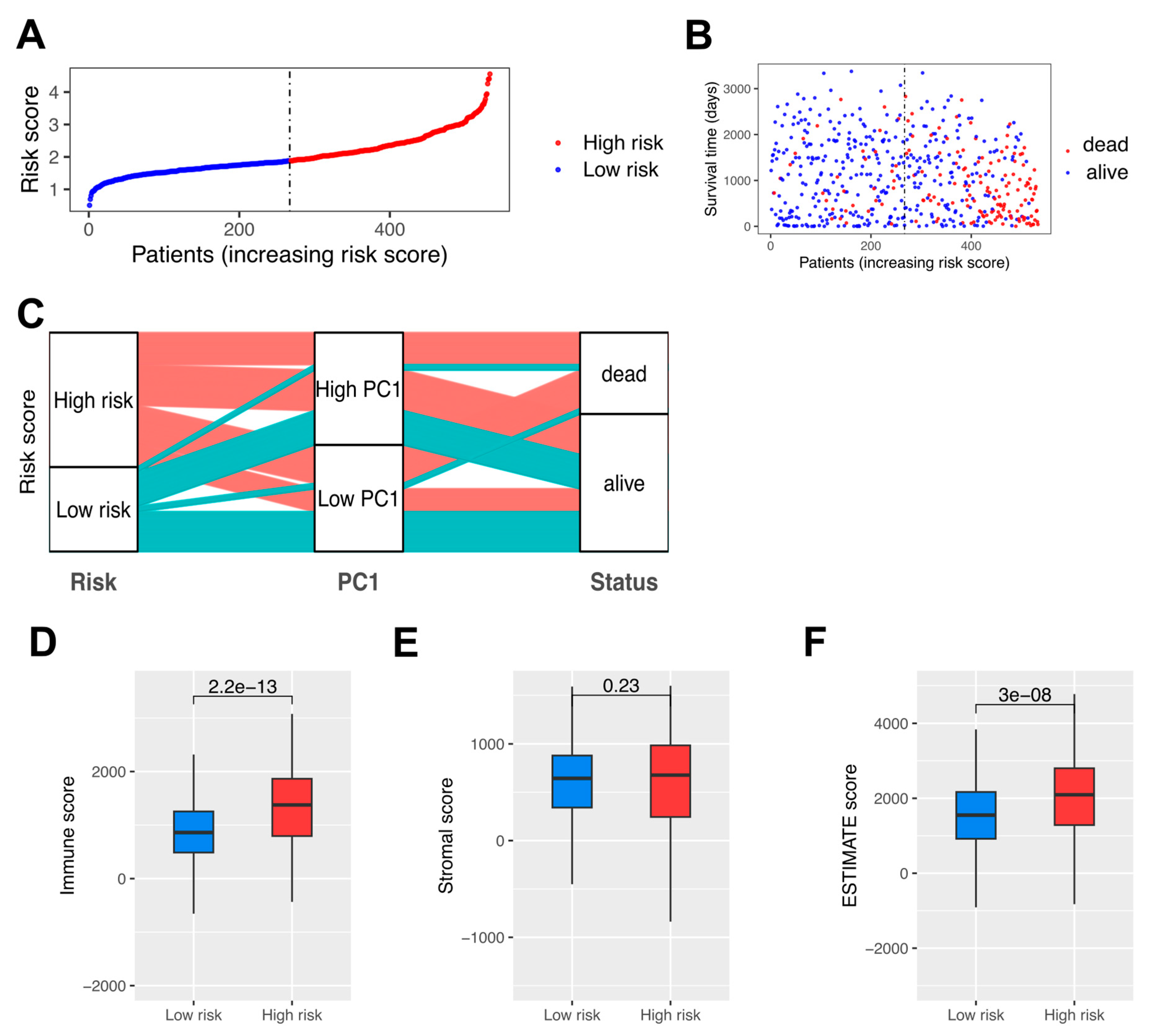
3.5. High-Risk Patients with Significantly Down-Regulated TAMs and Poor Prognosis in RCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Salmaninejad, A.; Valilou, S.F.; Soltani, A.; Ahmadi, S.; Abarghan, Y.J.; Rosengren, R.J.; Sahebkar, A. Tumor-Associated Macrophages: Role in Cancer Development and Therapeutic Implications. Cell. Oncol. 2019, 42, 591–608. [Google Scholar] [CrossRef]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Takeya, M. Tumor-Associated Macrophages: Potential Therapeutic Targets for Anti-Cancer Therapy. Adv. Drug Deliv. Rev. 2016, 99, 180–185. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-Associated Macrophages: From Basic Research to Clinical Application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Coulton, A.; Murai, J.; Qian, D.; Thakkar, K.; Lewis, C.E.; Litchfield, K. Using a Pan-Cancer Atlas to Investigate Tumour Associated Macrophages as Regulators of Immunotherapy Response. Nat. Commun. 2024, 15, 5665. [Google Scholar] [CrossRef]
- Liu, H.; Lv, Z.; Zhang, G.; Yan, Z.; Bai, S.; Dong, D.; Wang, K. Molecular Understanding and Clinical Aspects of Tumor-Associated Macrophages in the Immunotherapy of Renal Cell Carcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 286. [Google Scholar] [CrossRef]
- Alchahin, A.M.; Mei, S.; Tsea, I.; Hirz, T.; Kfoury, Y.; Dahl, D.; Wu, C.-L.; Subtelny, A.O.; Wu, S.; Scadden, D.T.; et al. A Transcriptional Metastatic Signature Predicts Survival in Clear Cell Renal Cell Carcinoma. Nat. Commun. 2022, 13, 5747. [Google Scholar] [CrossRef]
- Zhang, D.; Ni, Y.; Wang, Y.; Feng, J.; Zhuang, N.; Li, J.; Liu, L.; Shen, W.; Zheng, J.; Zheng, W.; et al. Spatial Heterogeneity of Tumor Microenvironment Influences the Prognosis of Clear Cell Renal Cell Carcinoma. J. Transl. Med. 2023, 21, 489. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qian, S.; Zhang, L.; Pan, X.; Qu, F.; Yu, Y.; Cui, X.; Shen, H. Tumor-Associated Macrophages Promote Migration and Invasion via Modulating Il-6/STAT3 Signaling in Renal Cell Carcinoma. SSRN Electron. J. 2022, 111, 109139. [Google Scholar] [CrossRef]
- Santoni, M.; Massari, F.; Amantini, C.; Nabissi, M.; Maines, F.; Burattini, L.; Berardi, R.; Santoni, G.; Montironi, R.; Tortora, G.; et al. Emerging Role of Tumor-Associated Macrophages as Therapeutic Targets in Patients with Metastatic Renal Cell Carcinoma. Cancer Immunol. Immunother. 2013, 62, 1757–1768. [Google Scholar] [CrossRef]
- Daurkin, I.; Eruslanov, E.; Stoffs, T.; Perrin, G.Q.; Algood, C.; Gilbert, S.M.; Rosser, C.J.; Su, L.-M.; Vieweg, J.; Kusmartsev, S. Tumor-Associated Macrophages Mediate Immunosuppression in the Renal Cancer Microenvironment by Activating the 15-Lipoxygenase-2 Pathway. Cancer Res. 2011, 71, 6400–6409. [Google Scholar] [CrossRef]
- Núñez, S.Y.; Trotta, A.; Regge, M.V.; Amarilla, M.S.; Secchiari, F.; Sierra, J.M.; Santilli, M.C.; Gantov, M.; Rovegno, A.; Richards, N.; et al. Tumor-associated Macrophages Impair NK Cell IFN-γ Production and Contribute to Tumor Progression in Clear Cell Renal Cell Carcinoma. Eur. J. Immunol. 2024, 54, 2350878. [Google Scholar] [CrossRef]
- Shen, H.; Liu, J.; Chen, S.; Ma, X.; Ying, Y.; Li, J.; Wang, W.; Wang, X.; Xie, L. Prognostic Value of Tumor-Associated Macrophages in Clear Cell Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 657318. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, A.; Salgia, N.J.; Li, H.; Castro, D.V.; Mirzapoiazova, T.; Armstrong, B.; Zhao, D.; Mercier, B.D.; Dizman, N.; Chawla, N.; et al. Characterization of Papillary and Clear Cell Renal Cell Carcinoma through Imaging Mass Cytometry Reveals Distinct Immunologic Profiles. Front. Immunol. 2023, 14, 1182581. [Google Scholar] [CrossRef]
- Wei, C.; Ma, Y.; Wang, M.; Wang, S.; Yu, W.; Dong, S.; Deng, W.; Bie, L.; Zhang, C.; Shen, W.; et al. Tumor-Associated Macrophage Clusters Linked to Immunotherapy in a Pan-Cancer Census. npj Precis. Oncol. 2024, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Raghubar, A.M.; Matigian, N.A.; Crawford, J.; Francis, L.; Ellis, R.; Healy, H.G.; Kassianos, A.J.; Ng, M.S.Y.; Roberts, M.J.; Wood, S.; et al. High-Risk Clear Cell Renal Cell Carcinoma Microenvironments Are Enriched for pro-Tumor Immune Phenotypes. npj Precis. Oncol. 2023, 7, 53. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, G.; Xie, P.; Zhao, X.; Chen, C.; Li, X.; Zhang, Y.; Wang, B.; Luo, Y. High CD204+ Tumor-Associated Macrophage Density Predicts a Poor Prognosis in Patients with Clear Cell Renal Cell Carcinoma. J. Cancer 2024, 15, 1511–1522. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, D.; Hu, Z.; Zhang, C.; Chang, L.; Li, Y.; Li, Z.; Hu, W.; Li, H.; Li, S.; et al. Macrophage-Derived Nanosponges Adsorb Cytokines and Modulate Macrophage Polarization for Renal Cell Carcinoma Immunotherapy. Adv. Healthc. Mater. 2024, 13, e2400303. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Daugan, M.V.; Noé, R.; Petitprez, F.; Vano, Y.A.; Sanchez-Salas, R.; Becht, E.; Meilleroux, J.; Clec’h, B.L.; Giraldo, N.A.; et al. Tumor Cells Hijack Macrophage-Produced Complement C1q to Promote Tumor Growth. Cancer Immunol. Res. 2019, 7, 1091–1105. [Google Scholar] [CrossRef]
- Kourtis, N.; Wang, Q.; Wang, B.; Oswald, E.; Adler, C.; Cherravuru, S.; Malahias, E.; Zhang, L.; Golubov, J.; Wei, Q.; et al. A Single-Cell Map of Dynamic Chromatin Landscapes of Immune Cells in Renal Cell Carcinoma. Nat. Cancer 2022, 3, 1164–1180. [Google Scholar] [CrossRef]
- Chakiryan, N.H.; Kim, Y.; Berglund, A.; Chang, A.; Kimmel, G.J.; Hajiran, A.; Nguyen, J.; Moran-Segura, C.; Saeed-Vafa, D.; Katende, E.N.; et al. Geospatial Characterization of Immune Cell Distributions and Dynamics across the Microenvironment in Clear Cell Renal Cell Carcinoma. J. Immunother. Cancer 2023, 11, e006195. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lv, Y.; Lu, W.; Yu, Z.; Ye, Y.; Guo, B.; Liu, D.; Yan, H.; Li, T.; Zhang, Q.; et al. Single-Cell RNA Sequencing in Multiple Pathologic Types of Renal Cell Carcinoma Revealed Novel Potential Tumor-Specific Markers. Front. Oncol. 2021, 11, 719564. [Google Scholar] [CrossRef]
- Obradovic, A.; Chowdhury, N.; Haake, S.M.; Ager, C.; Wang, V.; Vlahos, L.; Guo, X.V.; Aggen, D.H.; Rathmell, W.K.; Jonasch, E.; et al. Single-Cell Protein Activity Analysis Identifies Recurrence-Associated Renal Tumor Macrophages. Cell 2021, 184, 2988–3005.e16. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated Molecular Analysis of Clear-Cell Renal Cell Carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Charoentong, P.; Angelova, M.; Charoentong, P.; Finotello, F.; Angelova, M.; Mayer, C.; Efremova, M. Pan-Cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep. 2017, 18, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Xu, L.; Deng, C.; Pang, B.; Zhang, X.; Liu, W.; Liao, G.; Yuan, H.; Cheng, P.; Li, F.; Long, Z.; et al. Tip: A Web Server for Resolving Tumor Immunophenotype Profiling. Cancer Res. 2018, 78, 6575–6580. [Google Scholar] [CrossRef]
- Finotello, F.; Mayer, C.; Plattner, C.; Laschober, G.; Rieder, D.; Hackl, H.; Krogsdam, A.; Loncova, Z.; Posch, W.; Wilflingseder, D.; et al. Molecular and Pharmacological Modulators of the Tumor Immune Contexture Revealed by Deconvolution of RNA-Seq Data. Genome Med. 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yan, X.; Sun, F.; Li, L.M. Inferring Activity Changes of Transcription Factors by Binding Association with Sorted Expression Profiles. BMC Bioinform. 2007, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Chao, C.-C.; Li, J.; Ge, X.; Shen, A.; Jucaud, V.; Cheng, C.; Shen, X. Tissue-Resident Memory T Cell Signatures from Single-Cell Analysis Associated with Better Melanoma Prognosis. iScience 2024, 27, 109277. [Google Scholar] [CrossRef]
- Shen, A.; Garrett, A.; Chao, C.-C.; Liu, D.; Cheng, C.; Wang, Z.; Qian, C.; Zhu, Y.; Mai, J.; Jiang, C. A Comprehensive Meta-Analysis of Tissue Resident Memory T Cells and Their Roles in Shaping Immune Microenvironment and Patient Prognosis in Non-Small Cell Lung Cancer. Front. Immunol. 2024, 15, 1416751. [Google Scholar] [CrossRef]
- Cheng, C.; Nguyen, T.T.; Tang, M.; Wang, X.; Jiang, C.; Liu, Y.; Gorlov, I.; Gorlova, O.; Iafrate, J.; Lanuti, M.; et al. Immune Infiltration in Tumor and Adjacent Non-Neoplastic Regions Codetermines Patient Clinical Outcomes in Early-Stage Lung Cancer. J. Thorac. Oncol. 2023, 18, 1184–1198. [Google Scholar] [CrossRef]
- Schaafsma, E.; Jiang, C.; Cheng, C. B Cell Infiltration Is Highly Associated with Prognosis and an Immune-Infiltrated Tumor Microenvironment in Neuroblastoma. J. Cancer Metastasis Treat. 2021, 7, 10–20517. [Google Scholar] [CrossRef]
- Varn, F.S.; Wang, Y.; Mullins, D.W.; Fiering, S.; Cheng, C. Systematic Pan-Cancer Analysis Reveals Immune Cell Interactions in the Tumor Microenvironment. Cancer Res. 2017, 77, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, J.; Helman, E.; Shen, X.; Cheng, C. Abstract 3119: A Novel Tissue Resident Memory T Cell (TRM) Signature Predicts Prognosis and Tumor Microenvironment of Patients with Melanoma. Cancer Res. 2023, 83, 3119. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. Cox Proportional-Hazards Regression for Survival Data in R. Available online: https://www.john-fox.ca/Companion/appendices/Appendix-Cox-Regression.pdf (accessed on 19 October 2025).
- Ohno, S.; Inagawa, H.; Dhar, D.K.; Fujii, T.; Ueda, S.; Tachibana, M.; Suzuki, N.; Inoue, M.; Soma, G.-I.; Nagasue, N. The Degree of Macrophage Infiltration into the Cancer Cell Nest Is a Significant Predictor of Survival in Gastric Cancer Patients. Anticancer. Res. 2003, 23, 5015–5022. [Google Scholar]
- Takeya, M.; Komohara, Y. Role of Tumor-associated Macrophages in Human Malignancies: Friend or Foe? Pathol. Int. 2016, 66, 491–505. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Gong, C.; Shi, H.; Zeng, Y.; Wang, X.; Zhao, Y.; Wei, Y. Prognostic Significance of Tumor-Associated Macrophages in Solid Tumor: A Meta-Analysis of the Literature. PLoS ONE 2012, 7, e50946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Prognostic Significance of Tumor-Associated Macrophages in Breast Cancer: A Meta-Analysis of the Literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-Associated Neutrophils (TAN) Develop pro-Tumorigenic Properties during Tumor Progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Gubin, M.M.; Esaulova, E.; Ward, J.P.; Malkova, O.N.; Runci, D.; Wong, P.; Noguchi, T.; Arthur, C.D.; Meng, W.; Alspach, E.; et al. High-Dimensional Analysis Delineates Myeloid and Lymphoid Compartment Remodeling during Successful Immune-Checkpoint Cancer Therapy. Cell 2018, 175, 1014–1030.e19. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, T.; Zhang, R.; Chen, C.; Li, J. New Insights into the Role of Macrophages in Cancer Immunotherapy. Front. Immunol. 2024, 15, 1381225. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A Natural Killer-Dendritic Cell Axis Defines Checkpoint Therapy-Responsive Tumor Microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- Kruk, L.; Mamtimin, M.; Braun, A.; Anders, H.-J.; Andrassy, J.; Gudermann, T.; Mammadova-Bach, E. Inflammatory Networks in Renal Cell Carcinoma. Cancers 2023, 15, 2212. [Google Scholar] [CrossRef]
- Siddiqui, I.; Schaeuble, K.; Chennupati, V.; Fuertes Marraco, S.A.; Calderon-Copete, S.; Pais Ferreira, D.; Carmona, S.J.; Scarpellino, L.; Gfeller, D.; Pradervand, S.; et al. Intratumoral Tcf1+PD-1+CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 2019, 50, 195–211.e10. [Google Scholar] [CrossRef]
- Menjivar, R.E.; Nwosu, Z.C.; Du, W.; Donahue, K.L.; Hong, H.S.; Espinoza, C.; Brown, K.; Velez-Delgado, A.; Yan, W.; Lima, F.; et al. Arginase 1 Is a Key Driver of Immune Suppression in Pancreatic Cancer. eLife 2023, 12, e80721. [Google Scholar] [CrossRef]
- Spranger, S.; Gajewski, T.F. A New Paradigm for Tumor Immune Escape: β-Catenin-Driven Immune Exclusion. J. Immunother. Cancer 2015, 3, 43. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as Regulators of Tumour Immunity and Immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The Complex Role of Tumor-Infiltrating Macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, S.; Hong, B.-J.; Lee, C.-J.; Kim, Y.-E.; Bok, S.; Oh, J.-M.; Gwak, S.-H.; Yoo, M.Y.; Lee, M.S.; et al. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019, 79, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Shen, A.; Chao, C.-c.; Yeung, L.; Garrett, A.; Zeng, J.; Kawakita, S.; Wang, J.; Wang, Z.; Hassani, A.; et al. Distinct Tumor-Associated Macrophage Signatures Shape the Immune Microenvironment and Patient Prognosis in Renal Cell Carcinoma. Cells 2025, 14, 1740. https://doi.org/10.3390/cells14211740
Han Y, Shen A, Chao C-c, Yeung L, Garrett A, Zeng J, Kawakita S, Wang J, Wang Z, Hassani A, et al. Distinct Tumor-Associated Macrophage Signatures Shape the Immune Microenvironment and Patient Prognosis in Renal Cell Carcinoma. Cells. 2025; 14(21):1740. https://doi.org/10.3390/cells14211740
Chicago/Turabian StyleHan, Youngsoo, Aidan Shen, Cheng-chi Chao, Lucas Yeung, Aliesha Garrett, Jianming Zeng, Satoru Kawakita, Jesse Wang, Zhaohui Wang, Alireza Hassani, and et al. 2025. "Distinct Tumor-Associated Macrophage Signatures Shape the Immune Microenvironment and Patient Prognosis in Renal Cell Carcinoma" Cells 14, no. 21: 1740. https://doi.org/10.3390/cells14211740
APA StyleHan, Y., Shen, A., Chao, C.-c., Yeung, L., Garrett, A., Zeng, J., Kawakita, S., Wang, J., Wang, Z., Hassani, A., Shen, X., & Jiang, C. (2025). Distinct Tumor-Associated Macrophage Signatures Shape the Immune Microenvironment and Patient Prognosis in Renal Cell Carcinoma. Cells, 14(21), 1740. https://doi.org/10.3390/cells14211740





