Pulsatilla Saponin D Suppresses Proliferation and Induces Apoptosis in Human Prostatic Cells
Abstract
1. Introduction
2. Materials and Methods
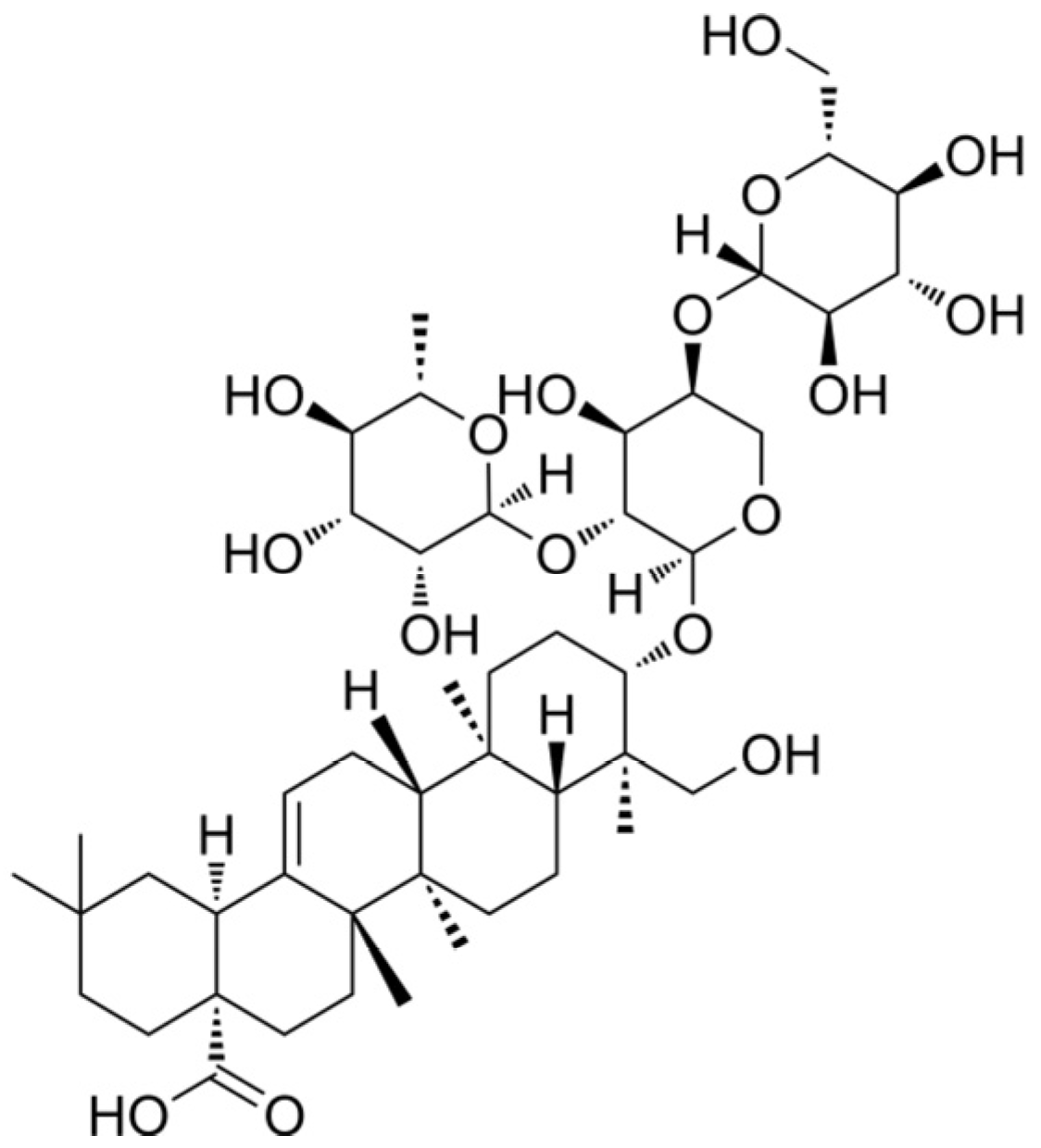
2.1. Chemicals
2.2. Cell Culture
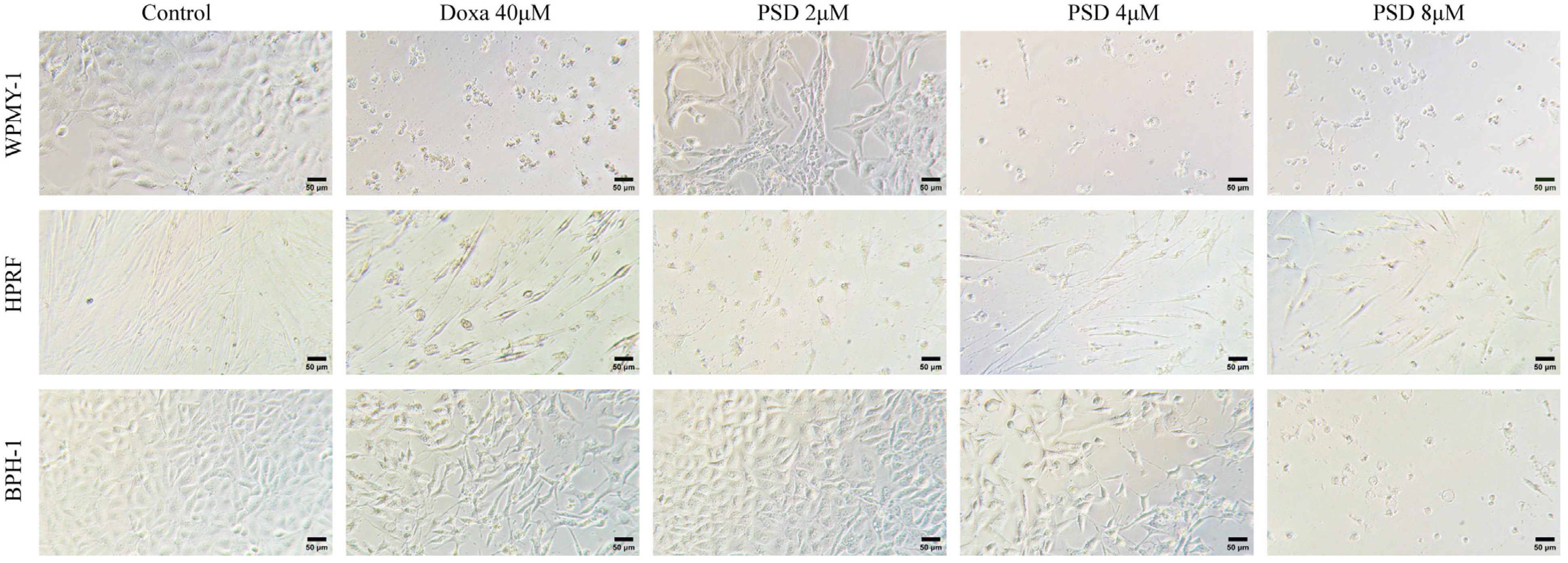
2.3. Cell Morphology
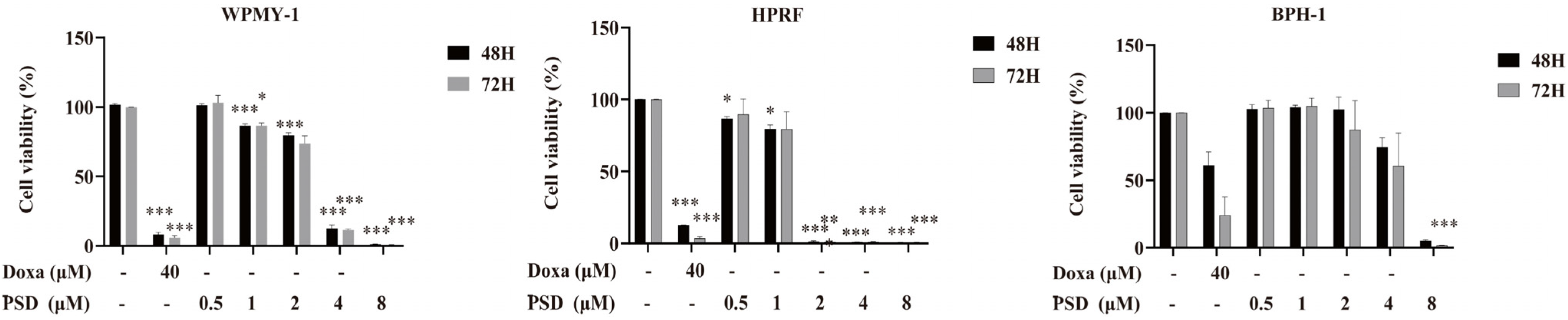
2.4. Cell Viability Assay
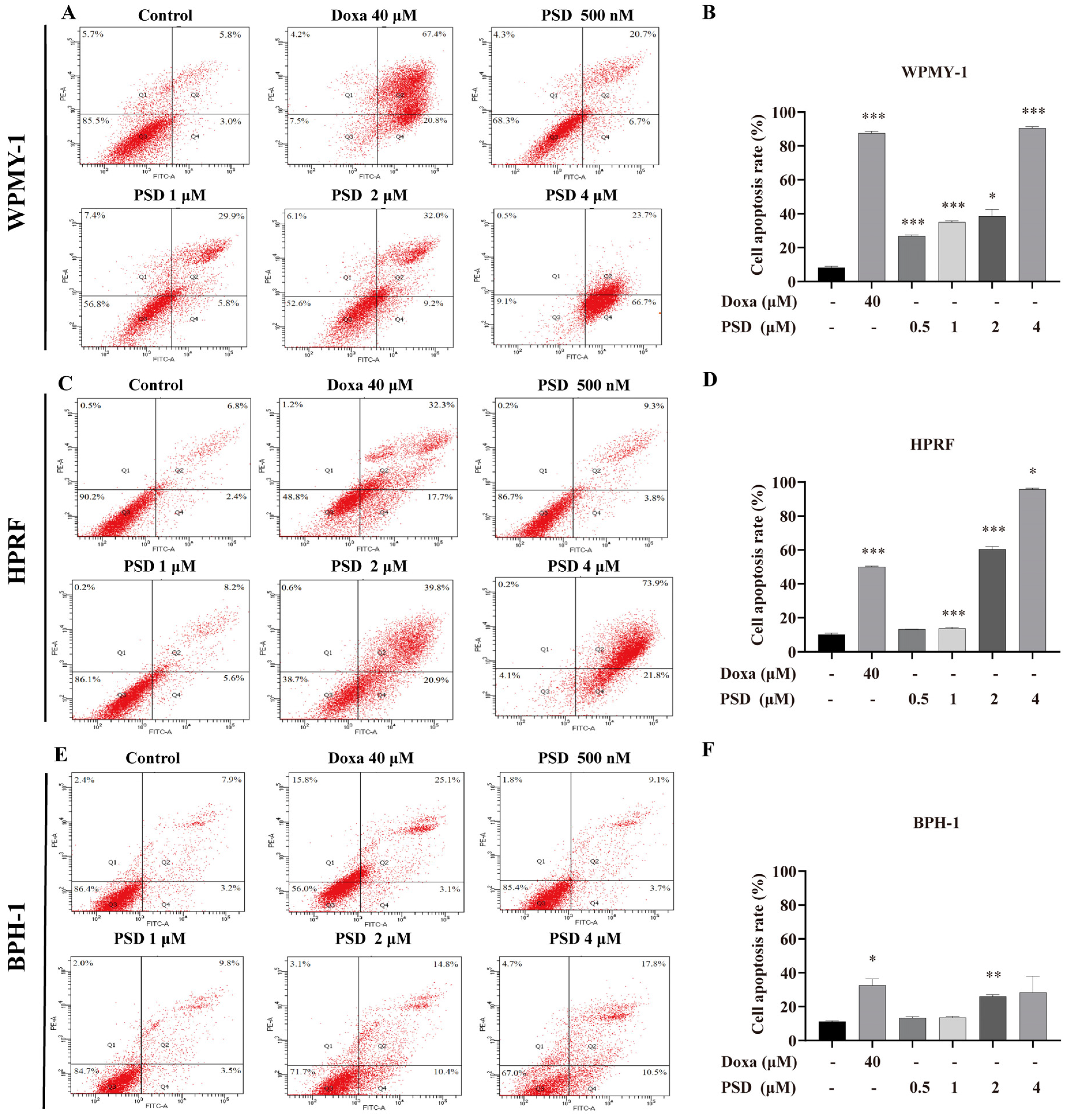
2.5. Annexin V-FITC/PI Double Staining Apoptosis Detection
2.6. Transcriptome Sequencing
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statistical Analysis
3. Results
3.1. Effect of PSD on the Morphology of WPMY-1, HPRF, and BPH-1 Cells
3.2. PSD Inhibits the Growth of WPMY-1, HPRF, and BPH-1 Cells
3.3. PSD Induces Apoptosis in WPMY-1, HPRF, and BPH-1 Cells
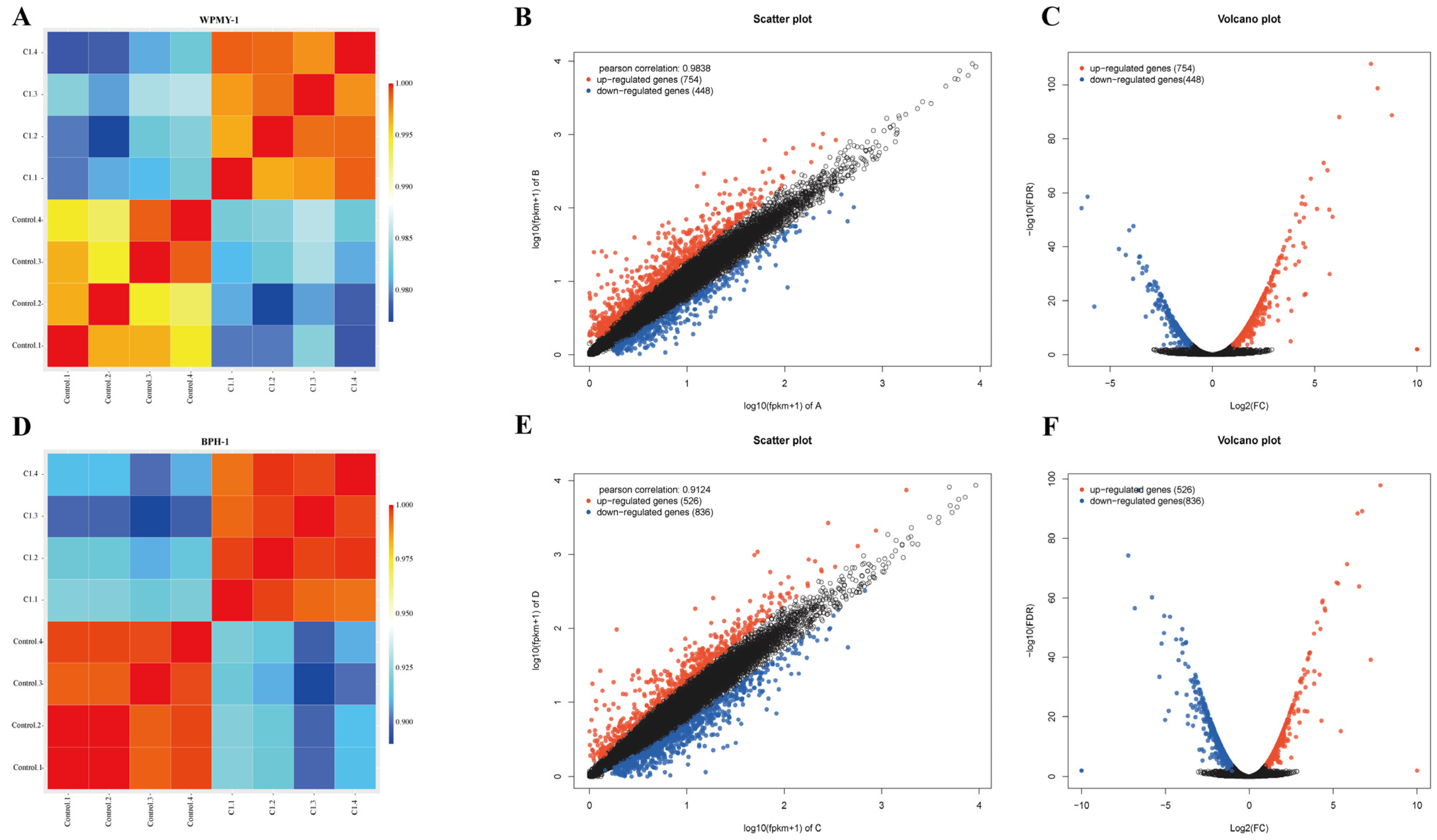
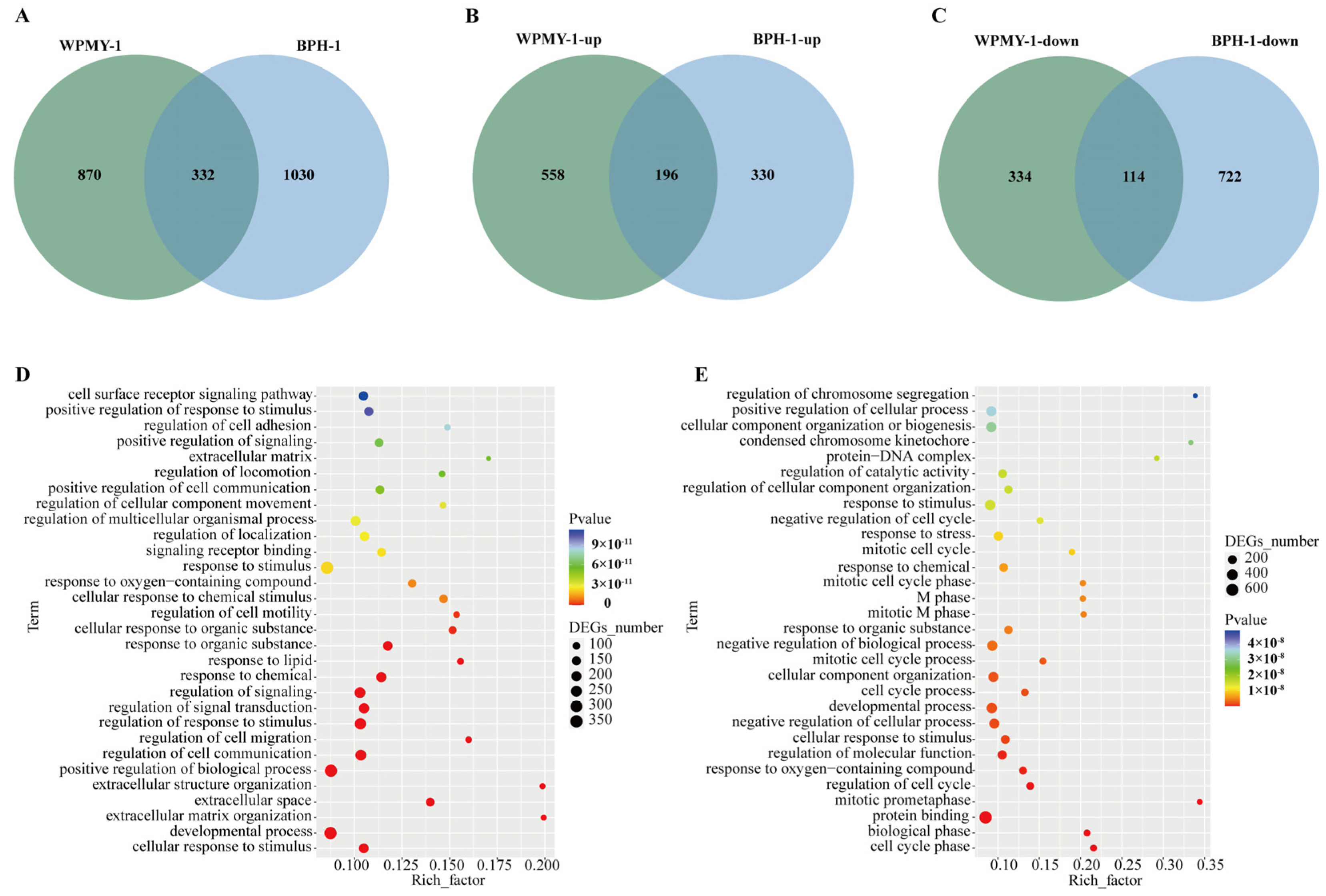
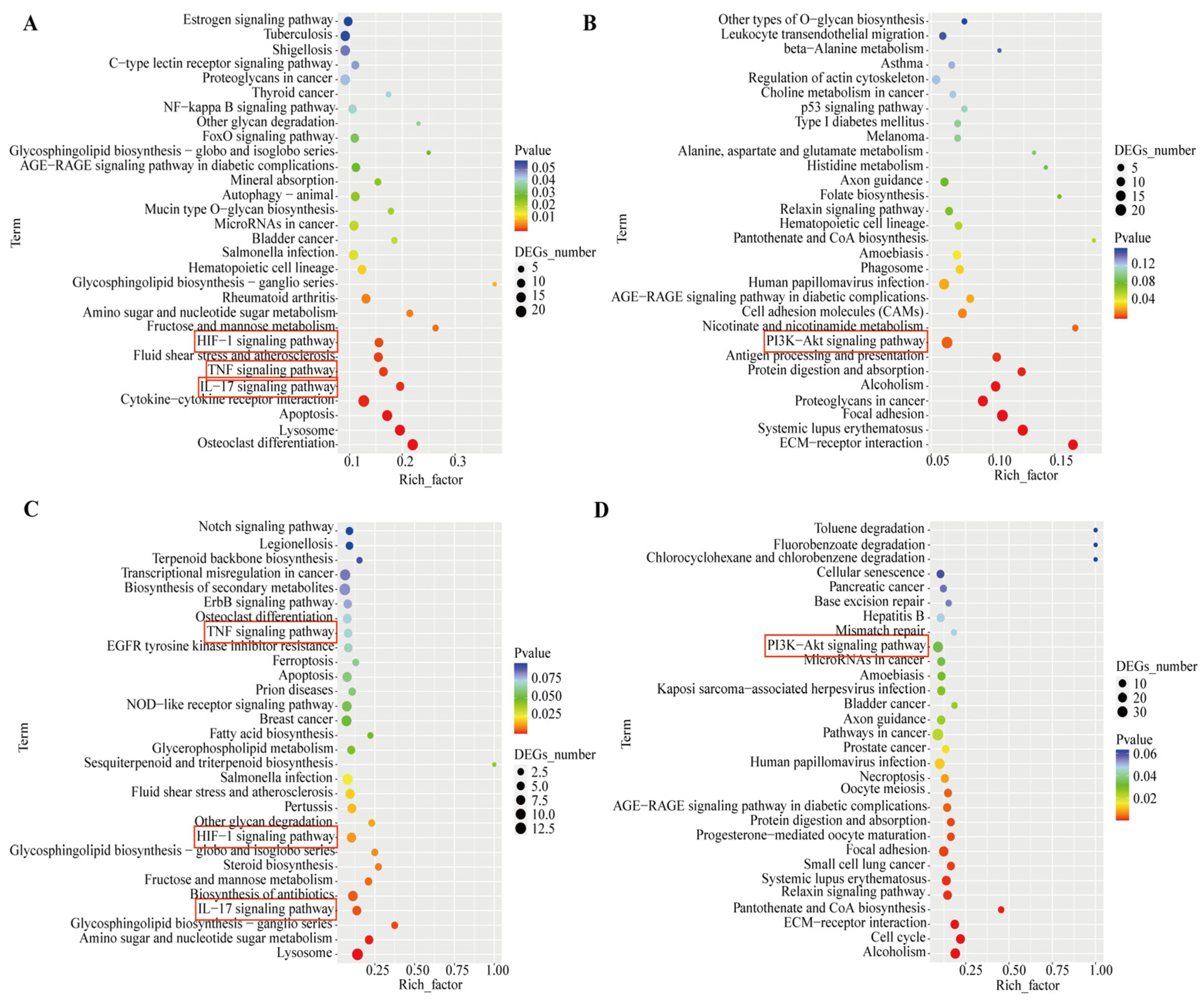
3.4. Transcriptomic Analysis Reveals PSD Modulation of Signaling Pathways in WPMY-1 and BPH-1 Cells
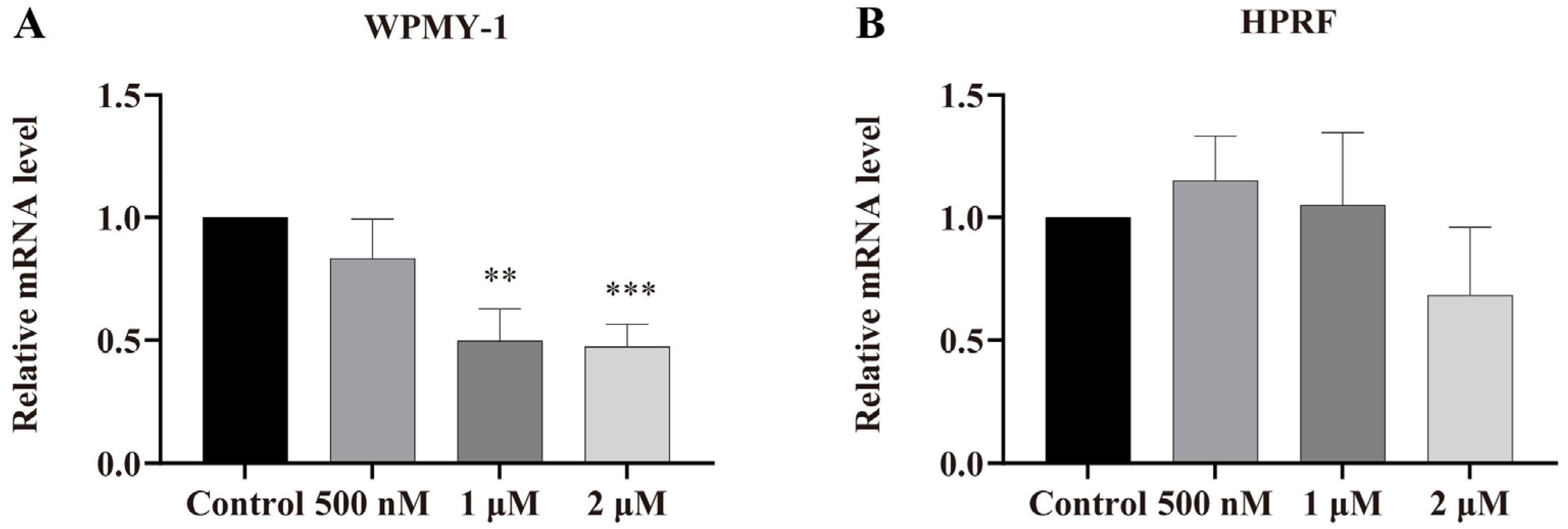
3.5. Regulation of AR Gene Expression by PSD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roehrborn, C.G. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005, 7 (Suppl. S9), S3–S14. [Google Scholar]
- Gómez-Sancha, F. BPH in the next millennium:a glimpse to the future. Prostate Cancer Prostatic. Dis. 1999, 2, S21–S25. [Google Scholar] [CrossRef] [PubMed]
- Helman, T.A.; Browne, B.M. Advances in Outpatient Therapies and Treatment of Benign Prostatic Hyperplasia: A Comprehensive Review for Men’s Health. Med. Clin. North Am. 2024, 108, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Steers, W.D. 5alpha-reductase activity in the prostate. Urology 2001, 58, 17–24, discussion 24. [Google Scholar] [CrossRef]
- Ng, M.; Leslie, S.W.; Baradhi, K.M. Benign Prostatic Hyperplasia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kyprianou, N.; Litvak, J.P.; Borkowski, A.; Alexander, R.; Jacobs, S.C. Induction of prostate apoptosis by doxazosin in benign prostatic hyperplasia. J. Urol. 1998, 159, 1810–1815. [Google Scholar] [CrossRef]
- Kyprianou, N.; Jacobs, S.C. Induction of apoptosis in the prostate by alpha1-adrenoceptor antagonists: A novel effect of “old” drugs. Curr. Urol. Rep. 2000, 1, 89–96. [Google Scholar] [CrossRef]
- Gormley, G.J.; Stoner, E.; Bruskewitz, R.C.; Imperato-McGinley, J.; Walsh, P.C.; McConnell, J.D.; Andriole, G.L.; Geller, J.; Bracken, B.R.; Tenover, J.S.; et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N. Engl. J. Med. 1992, 327, 1185–1191. [Google Scholar] [CrossRef]
- Sandhu, J.S. Therapeutic options in the treatment of benign prostatic hyperplasia. Patient Prefer. Adherence 2009, 3, 213–223. [Google Scholar] [CrossRef][Green Version]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Koczurkiewicz, P.; Klaś, K.; Grabowska, K.; Piska, K.; Rogowska, K.; Wójcik-Pszczoła, K.; Podolak, I.; Galanty, A.; Michalik, M.; Pękala, E. Saponins as chemosensitizing substances that improve effectiveness and selectivity of anticancer drug-Minireview of in vitro studies. Phytother. Res. 2019, 33, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hong, B.; Wu, S.; Niu, T. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumour Biol. 2015, 36, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.L.; Zhang, C.L.; Yuan, D.D.; Tong, X.H.; Tao, L. Panax notoginseng saponins enhances the cytotoxicity of cisplatin via increasing gap junction intercellular communication. Biol. Pharm. Bull. 2012, 35, 1230–1237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, G.J.; Wang, C.Z.; Zhang, Z.Y.; Wen, X.D.; Somogyi, J.; Calway, T.; He, T.C.; Du, W.; Yuan, C.S. Caspase-mediated pro-apoptotic interaction of panaxadiol and irinotecan in human colorectal cancer cells. J. Pharm. Pharmacol. 2012, 64, 727–734. [Google Scholar] [CrossRef]
- Son, M.K.; Jung, K.H.; Lee, H.S.; Lee, H.; Kim, S.J.; Yan, H.H.; Ryu, Y.L.; Hong, S.S. SB365, Pulsatilla saponin D suppresses proliferation and induces apoptosis of pancreatic cancer cells. Oncol. Rep. 2013, 30, 801–808. [Google Scholar] [CrossRef]
- Ye, W.C.; Ou, B.X.; Ji, N.N.; Zhao, S.X.; Ye, T.; McKervey, M.A.; Stevenson, P. Patensin, a saponin from Pulsatilla patens var. multifida. Phytochemistry 1995, 39, 937–939. [Google Scholar] [CrossRef]
- Jin, K.; Shen, C.; Yu, W.; Lin, J.; Zhu, J.; Tao, H.; Liu, B. Pulsatilla saponin D inhibited the growth of osteosarcoma by regulating the JNK/ATF3 signaling pathway. Chem. Biol. Interact. 2025, 410, 111420. [Google Scholar] [CrossRef]
- Jang, W.J.; Park, B.; Jeong, G.S.; Hong, S.S.; Jeong, C.H. SB365, Pulsatilla saponin D, suppresses the growth of gefitinib-resistant NSCLC cells with Met amplification. Oncol. Rep. 2014, 32, 2612–2618. [Google Scholar] [CrossRef]
- Lin, S.; Sun, B.; Zhu, Y.; Huang, Y.; Qin, Y.; Yao, N.; Liu, Y.; Chen, G. Natural product Pulsatilla saponin D sensitizes BRCA-proficient ovarian cancers to PARP inhibitors through inhibiting homologous recombination repair. J. Pharm. Pharmacol. 2025, 77, 511–523. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Dey, N.; De, P.; Leyland-Jones, B. PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell signaling to clinical trials. Pharmacol. Ther. 2017, 175, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, S.; Kuroda, H.; Takahashi, R.; Sasano, T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015, 137, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, X.; Feng, Y.; Li, X.; Xuan, Y. Tenascin C regulates cancer cell glycolysis and tumor progression in prostate cancer. Int. J. Urol. 2022, 29, 578–585. [Google Scholar] [CrossRef]
- Cheng, X.; Li, F.; Tao, Z. Tenascin-C promotes epithelial-to-mesenchymal transition and the mTOR signaling pathway in nasopharyngeal carcinoma. Oncol. Lett. 2021, 22, 570. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, S.H.; Seo, W.Y.; Jeong, Y.; Shin, M.C.; Ryu, D.; Lee, S.B.; Choi, Y.J.; Kim, K. Effects of human collagen α-1 type I-derived proteins on collagen synthesis and elastin production in human dermal fibroblasts. BMB Rep. 2021, 54, 329–334. [Google Scholar] [CrossRef]
- Yoon, H.; Tang, C.M.; Banerjee, S.; Yebra, M.; Noh, S.; Burgoyne, A.M.; Torre, J.; Siena, M.; Liu, M.; Klug, L.R.; et al. Cancer-associated fibroblast secretion of PDGFC promotes gastrointestinal stromal tumor growth and metastasis. Oncogene 2021, 40, 1957–1973. [Google Scholar] [CrossRef] [PubMed]
- Goda, N.; Dozier, S.J.; Johnson, R.S. HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxid. Redox Signal. 2003, 5, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.J.; Haubert, D.; Krönke, M.; Leptin, M. Looking beyond death: A morphogenetic role for the TNF signalling pathway. J. Cell Sci. 2009, 122, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef]
- Son, M.K.; Jung, K.H.; Hong, S.W.; Lee, H.S.; Zheng, H.M.; Choi, M.J.; Seo, J.H.; Suh, J.K.; Hong, S.S. SB365, Pulsatilla saponin D suppresses the proliferation of human colon cancer cells and induces apoptosis by modulating the AKT/mTOR signalling pathway. Food Chem. 2013, 136, 26–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, J.; Wang, K.; Jia, X.; Zhang, C.; Huang, B.; Chen, M.; Wan, J.B.; Su, H.; Wang, Y.; et al. Pulsatilla Saponin D Inhibits Autophagic Flux and Synergistically Enhances the Anticancer Activity of Chemotherapeutic Agents Against HeLa Cells. Am. J. Chin. Med. 2015, 43, 1657–1670. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, L.; Xiao, H.; Xiao, C.; Wang, Y.; Liu, X. Momordin Ic induces HepG2 cell apoptosis through MAPK and PI3K/Akt-mediated mitochondrial pathways. Apoptosis 2013, 18, 751–765. [Google Scholar] [CrossRef]
- Sachan, R.; Kundu, A.; Jeon, Y.; Choi, W.S.; Yoon, K.; Kim, I.S.; Kwak, J.H.; Kim, H.S. Afrocyclamin A, a triterpene saponin, induces apoptosis and autophagic cell death via the PI3K/Akt/mTOR pathway in human prostate cancer cells. Phytomedicine 2018, 51, 139–150. [Google Scholar] [CrossRef]
- Xia, H.; Hu, C.; Bai, S.; Lyu, J.; Zhang, B.Y.; Yu, X.; Zhan, Y.; Zhao, L.; Dong, Y. Raddeanin A down-regulates androgen receptor and its splice variants in prostate cancer. J. Cell Mol. Med. 2019, 23, 3656–3664. [Google Scholar] [CrossRef]
- Liu, W.K.; Xu, S.X.; Che, C.T. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000, 67, 1297–1306. [Google Scholar] [CrossRef]
- Park, J.; Bui, P.T.C.; Song, H.; Kim, S.K.; Rhee, D.K.; Kim, E.Y.; Rhyu, M.R.; Lee, M.S.; Lee, Y.J. Ginseng on Nuclear Hormone Receptors. Am. J. Chin. Med. 2017, 45, 1147–1156. [Google Scholar] [CrossRef]
- Pranweerapaiboon, K.; Garon, A.; Seidel, T.; Janta, S.; Plubrukarn, A.; Chaithirayanon, K.; Langer, T. In vitro and in silico studies of holothurin A on androgen receptor in prostate cancer. J. Biomol. Struct. Dyn. 2022, 40, 12674–12682. [Google Scholar] [CrossRef]

| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| AR | GTGGACGACCAGATGGCTGTCATTC | GGCGAAGTAGAGCATCCTGGAGTTG |
| Cell Lines | IC50 (48 h) | IC50 (72 h) |
|---|---|---|
| WPMY-1 | 2.649 | 2.511 |
| HPRF | 1.201 | 1.192 |
| BPH-1 | 4.816 | 4.315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhou, P.; Jin, Y.; Huang, D.; Su, X.; Shao, C.; Jiang, J.; Yang, R.; Wu, J. Pulsatilla Saponin D Suppresses Proliferation and Induces Apoptosis in Human Prostatic Cells. Cells 2025, 14, 1706. https://doi.org/10.3390/cells14211706
Chen Y, Zhou P, Jin Y, Huang D, Su X, Shao C, Jiang J, Yang R, Wu J. Pulsatilla Saponin D Suppresses Proliferation and Induces Apoptosis in Human Prostatic Cells. Cells. 2025; 14(21):1706. https://doi.org/10.3390/cells14211706
Chicago/Turabian StyleChen, Yuzhong, Ping Zhou, Yangtao Jin, Dongyan Huang, Xin Su, Congcong Shao, Juan Jiang, Rongfu Yang, and Jianhui Wu. 2025. "Pulsatilla Saponin D Suppresses Proliferation and Induces Apoptosis in Human Prostatic Cells" Cells 14, no. 21: 1706. https://doi.org/10.3390/cells14211706
APA StyleChen, Y., Zhou, P., Jin, Y., Huang, D., Su, X., Shao, C., Jiang, J., Yang, R., & Wu, J. (2025). Pulsatilla Saponin D Suppresses Proliferation and Induces Apoptosis in Human Prostatic Cells. Cells, 14(21), 1706. https://doi.org/10.3390/cells14211706






