Integrated Bioinformatics and Experimental Analysis Revealed Crosstalk Between IL-6, Autophagy, Ubiquitination, and Key miRNAs in Female Infertility: Insights from Ovarian Endometriosis and Polycystic Ovary Syndrome
Abstract
1. Introduction
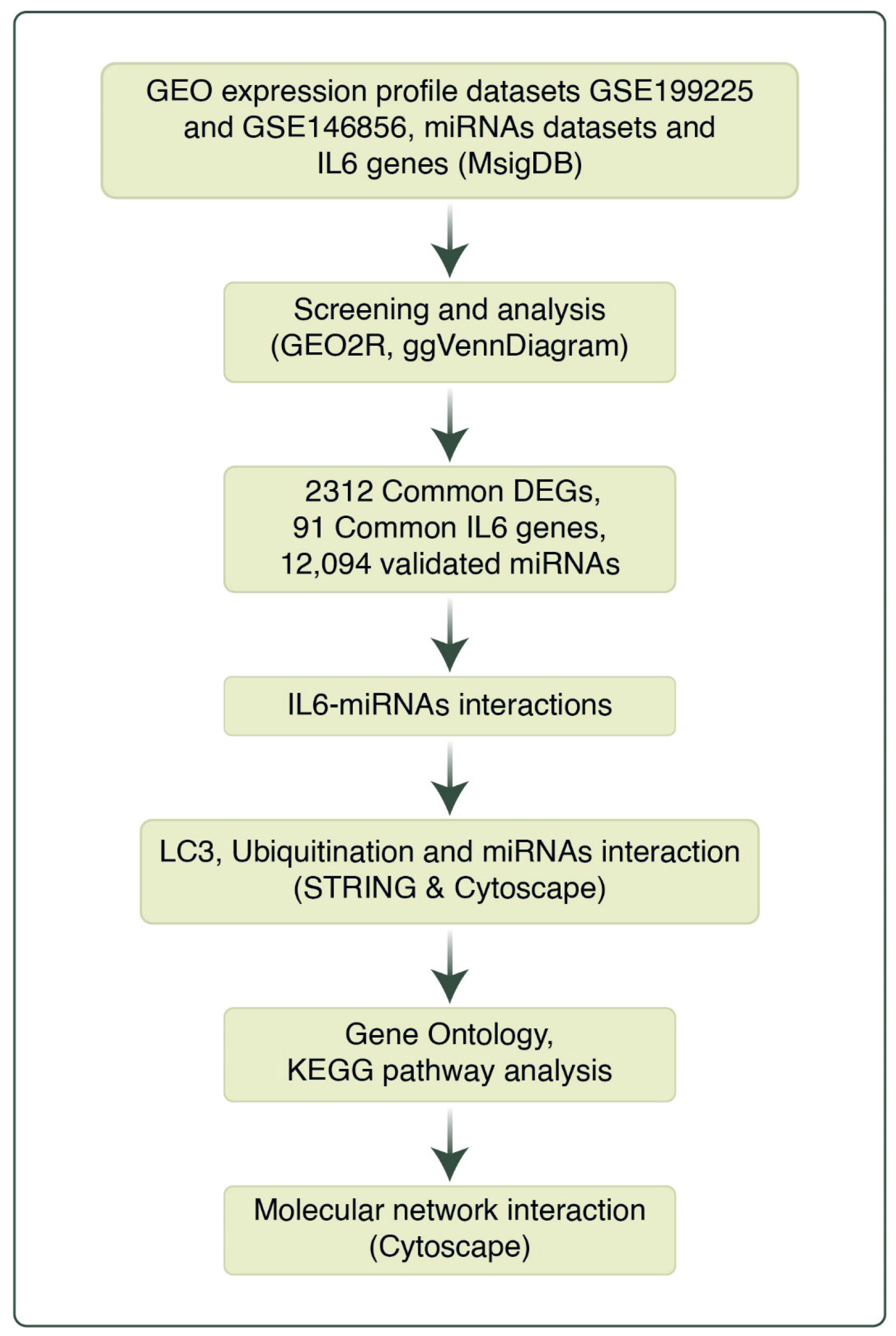
2. Materials and Methods
2.1. Data Processing
2.2. Analysis of Microarray Datasets
2.3. Identification of miRNA–Target Interactions
2.4. Analysis of IL6 and miRNA Interactions
2.5. Analysis of miRNA Interactions with LC3 and Ubiquitination
2.6. Construction of LC3, Ubiquitination, and miRNA Network
2.7. Pathway Analysis of LC3–Ubiquitination and miRNAs
2.8. qRT-PCR
2.9. Immunohistochemical Staining (IHC)
2.10. Statistical Analysis
2.11. Ethical Approval
3. Results
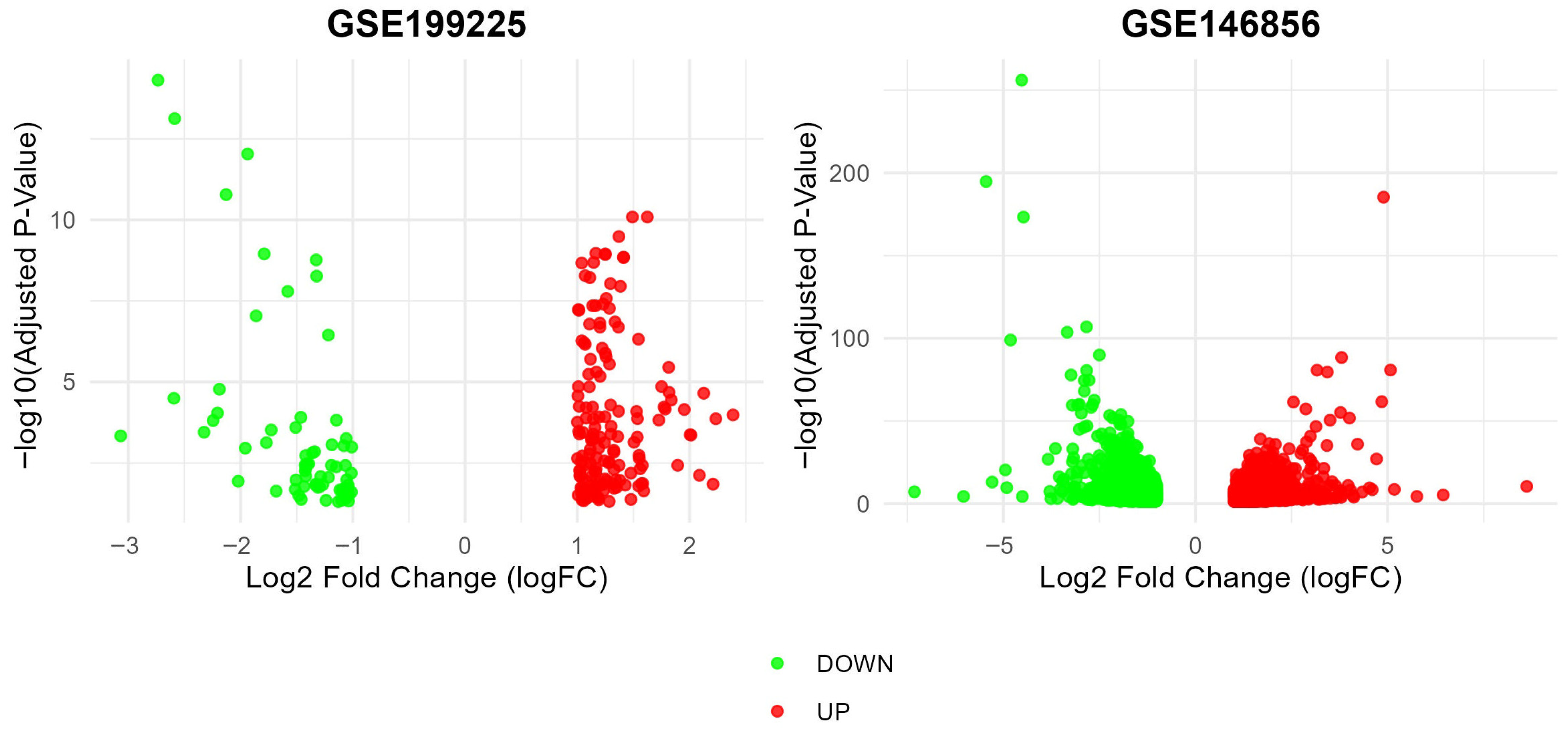
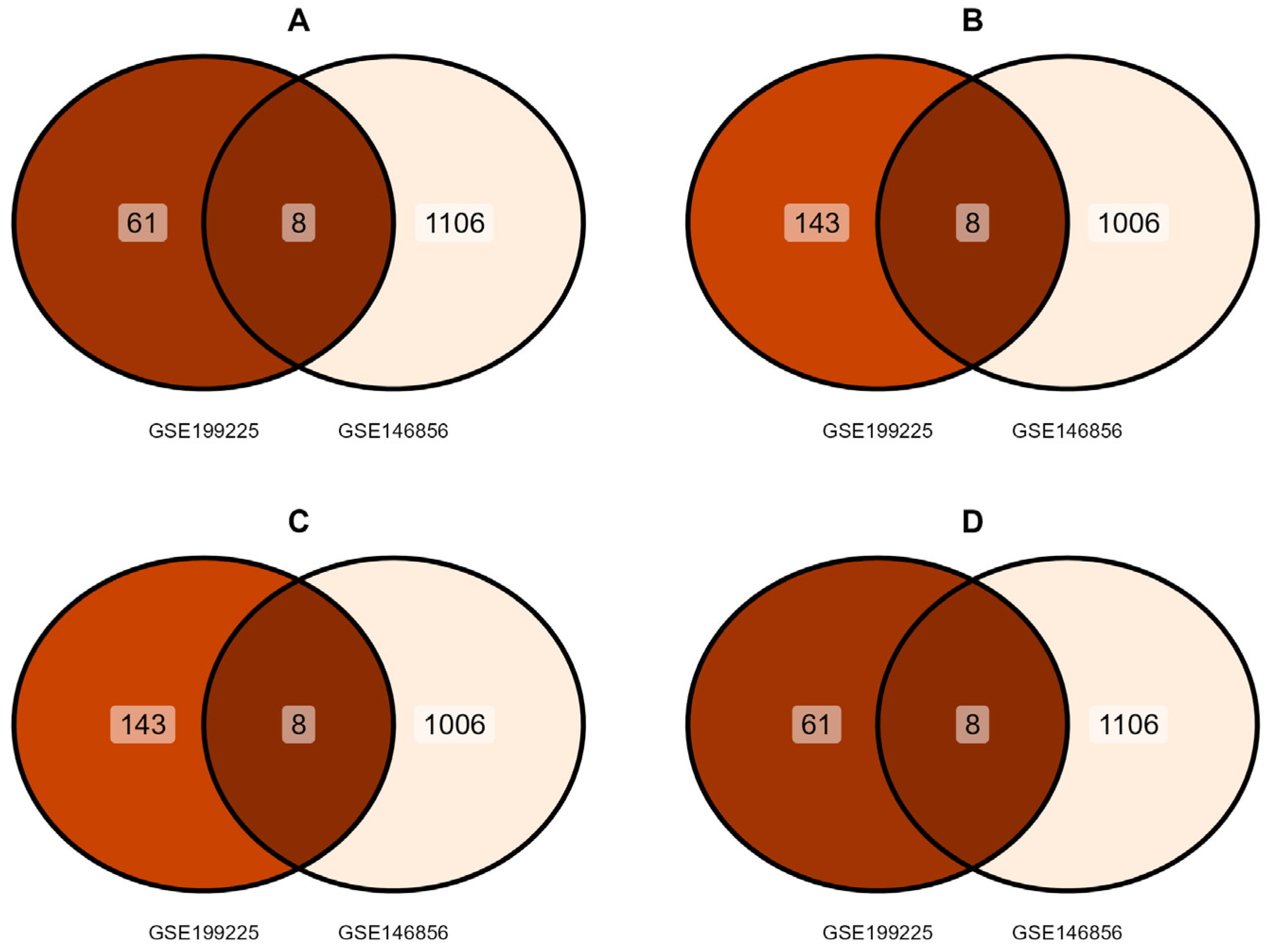
3.1. Identification of DEGs
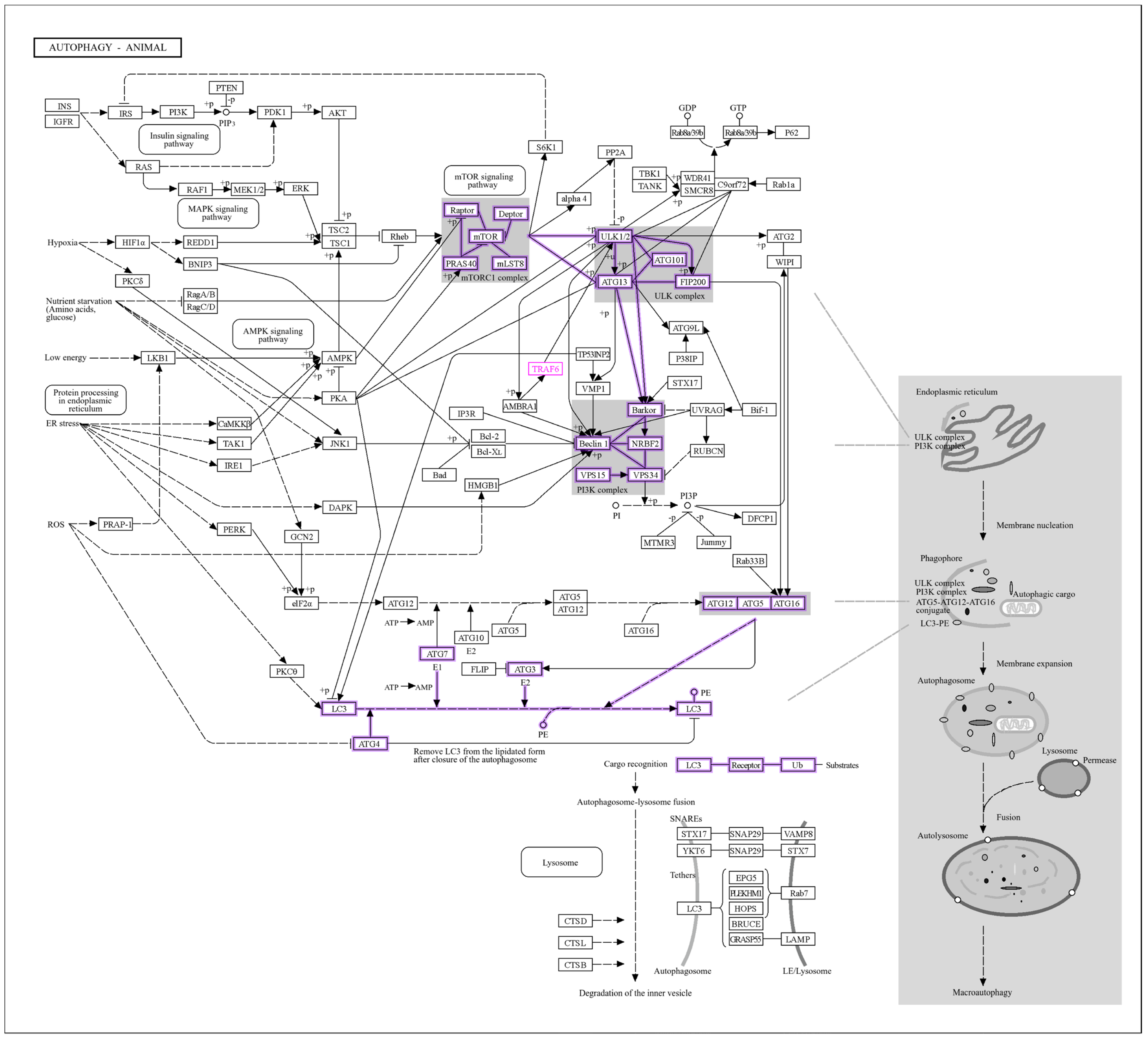
3.2. miR Gene Target Pathway Analysis
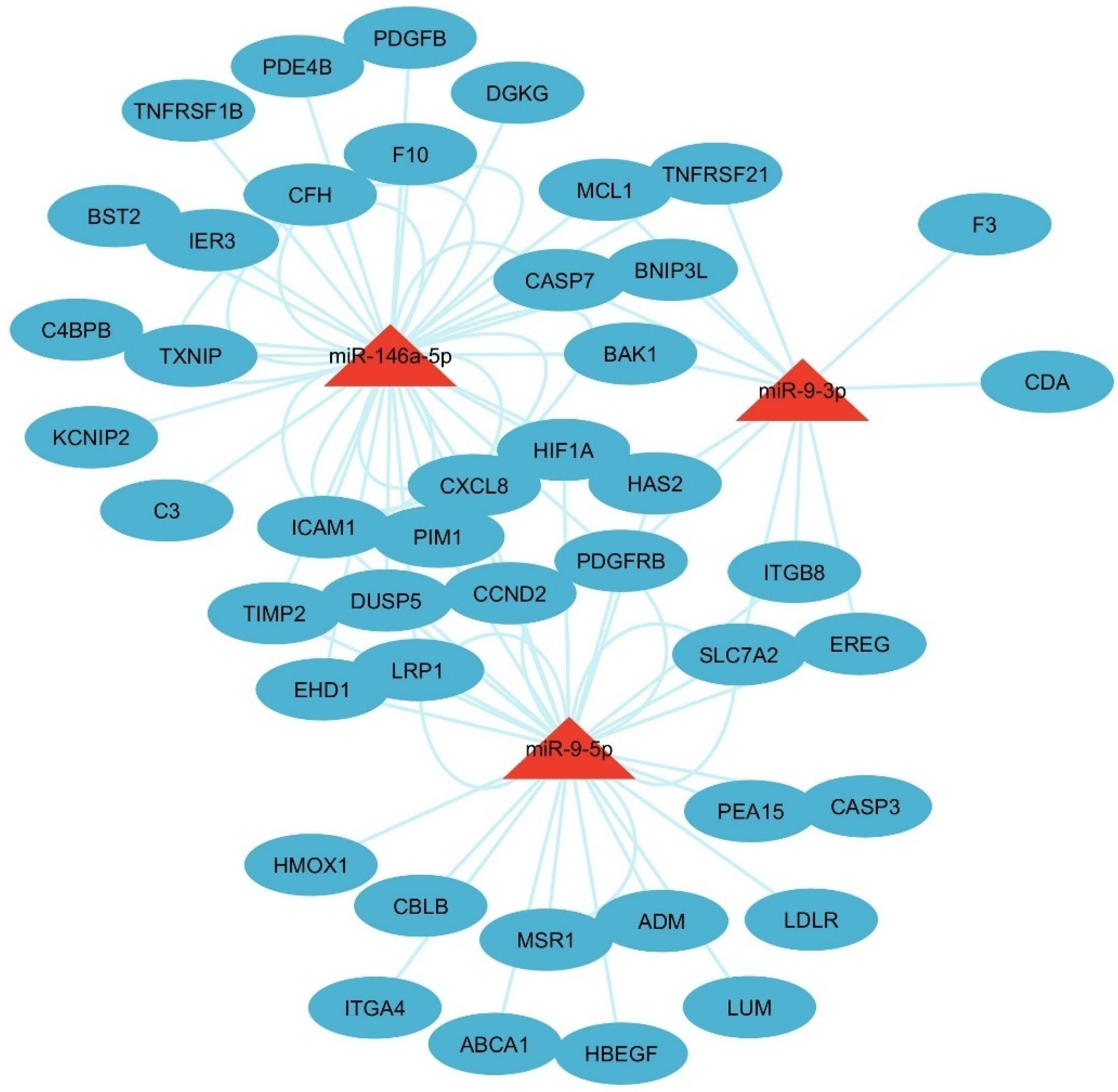
3.3. Identification of IL-6–miRNA Interactions
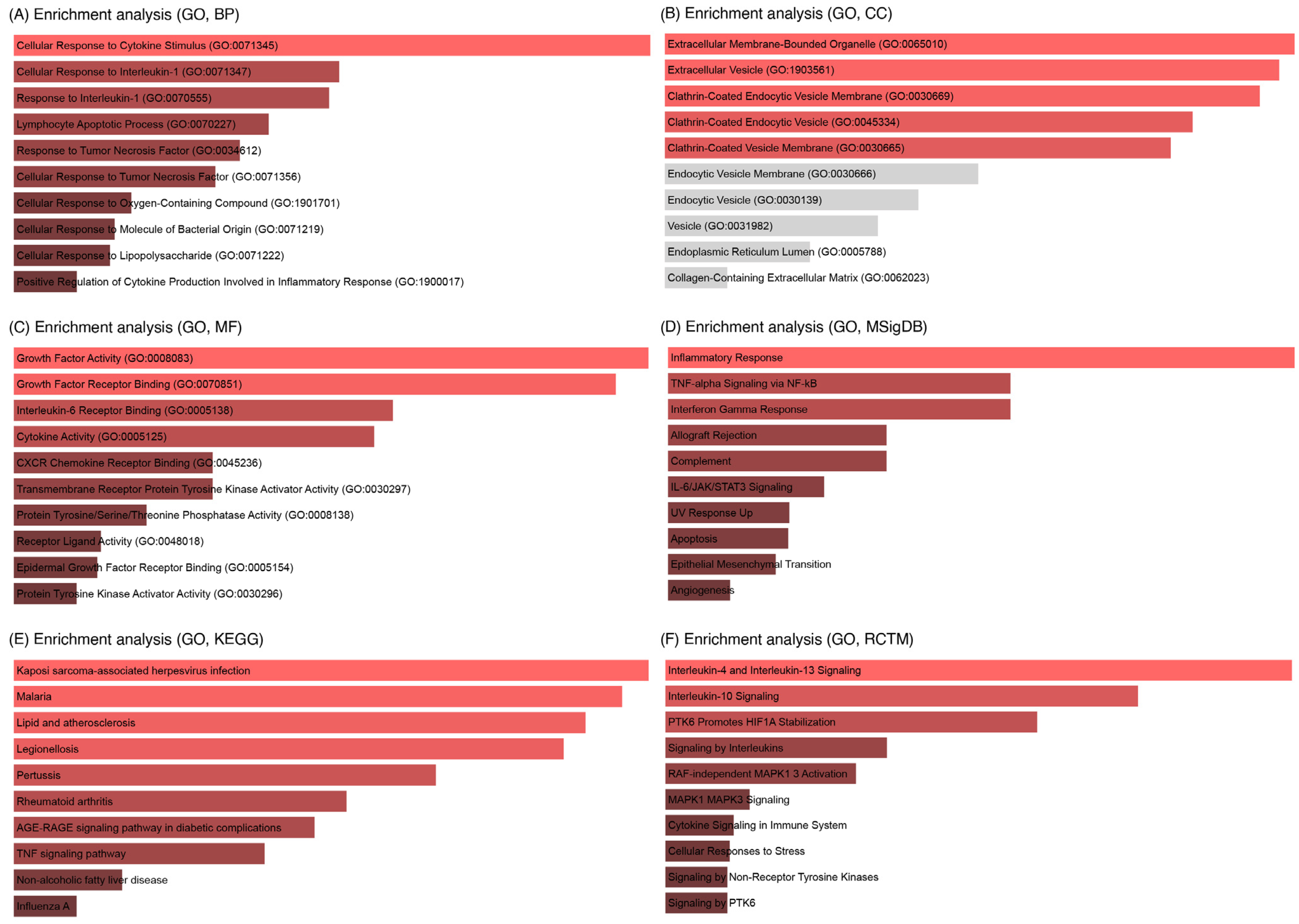
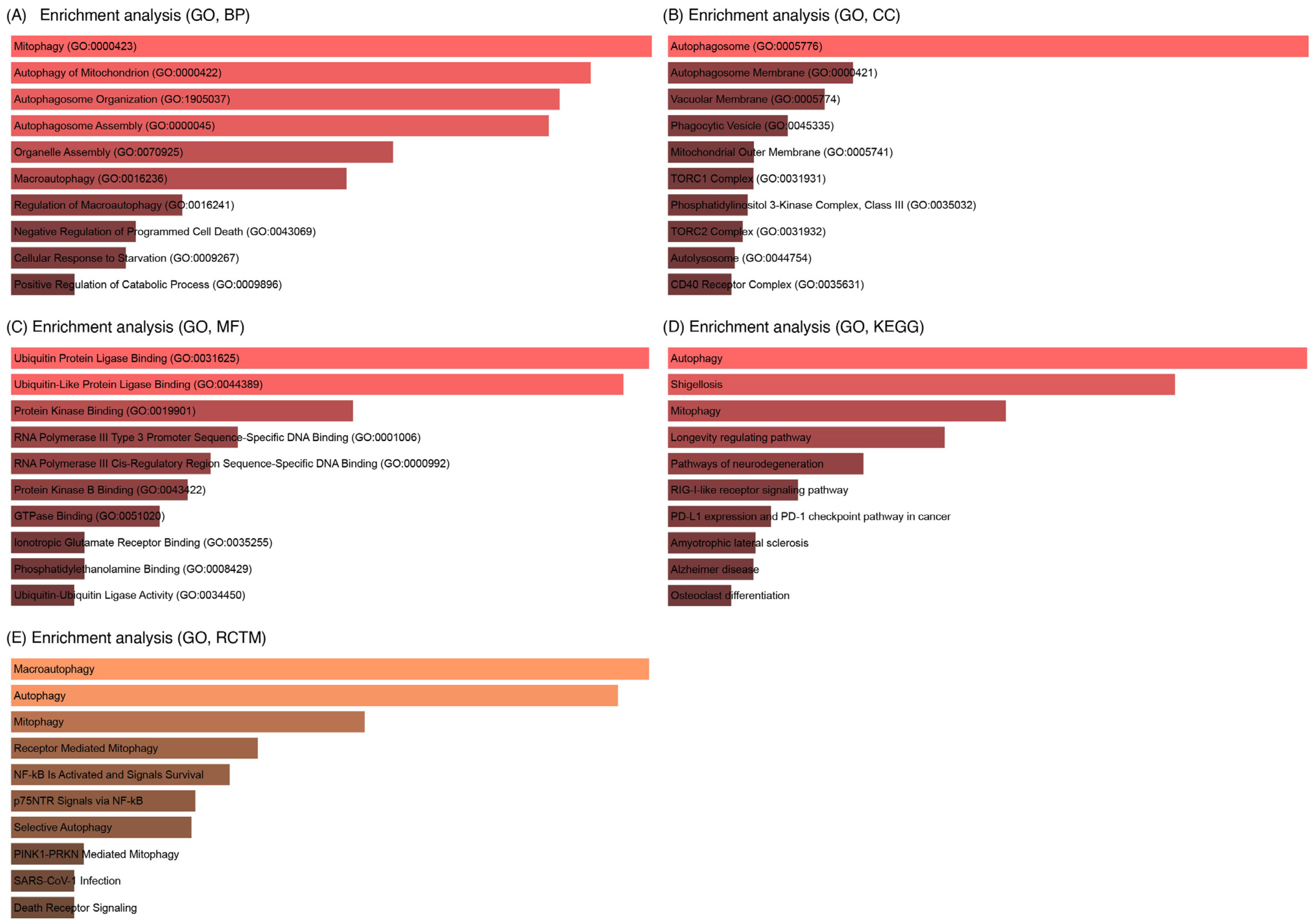
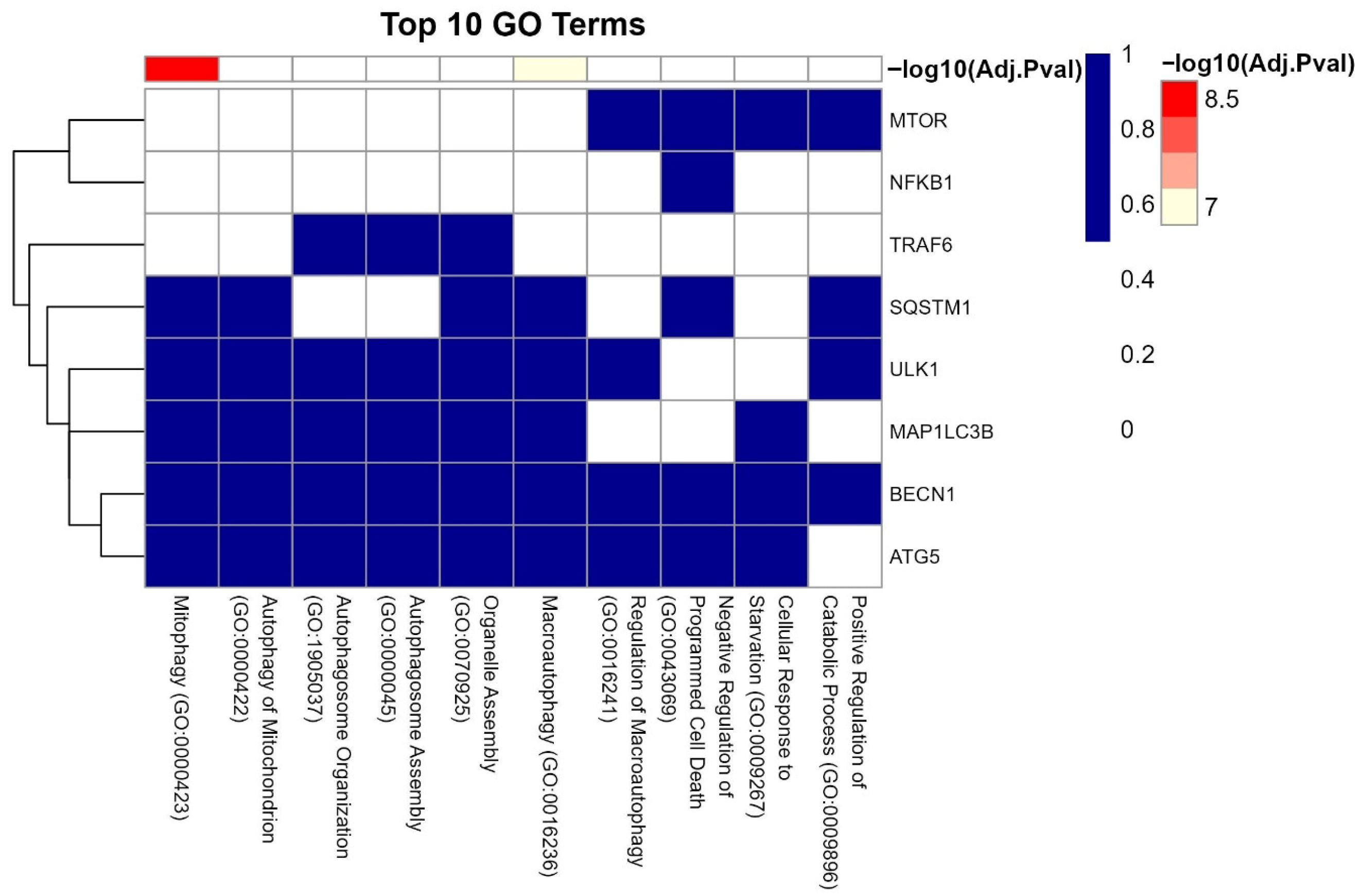
3.4. Enrichment Analysis of Key IL6 Genes
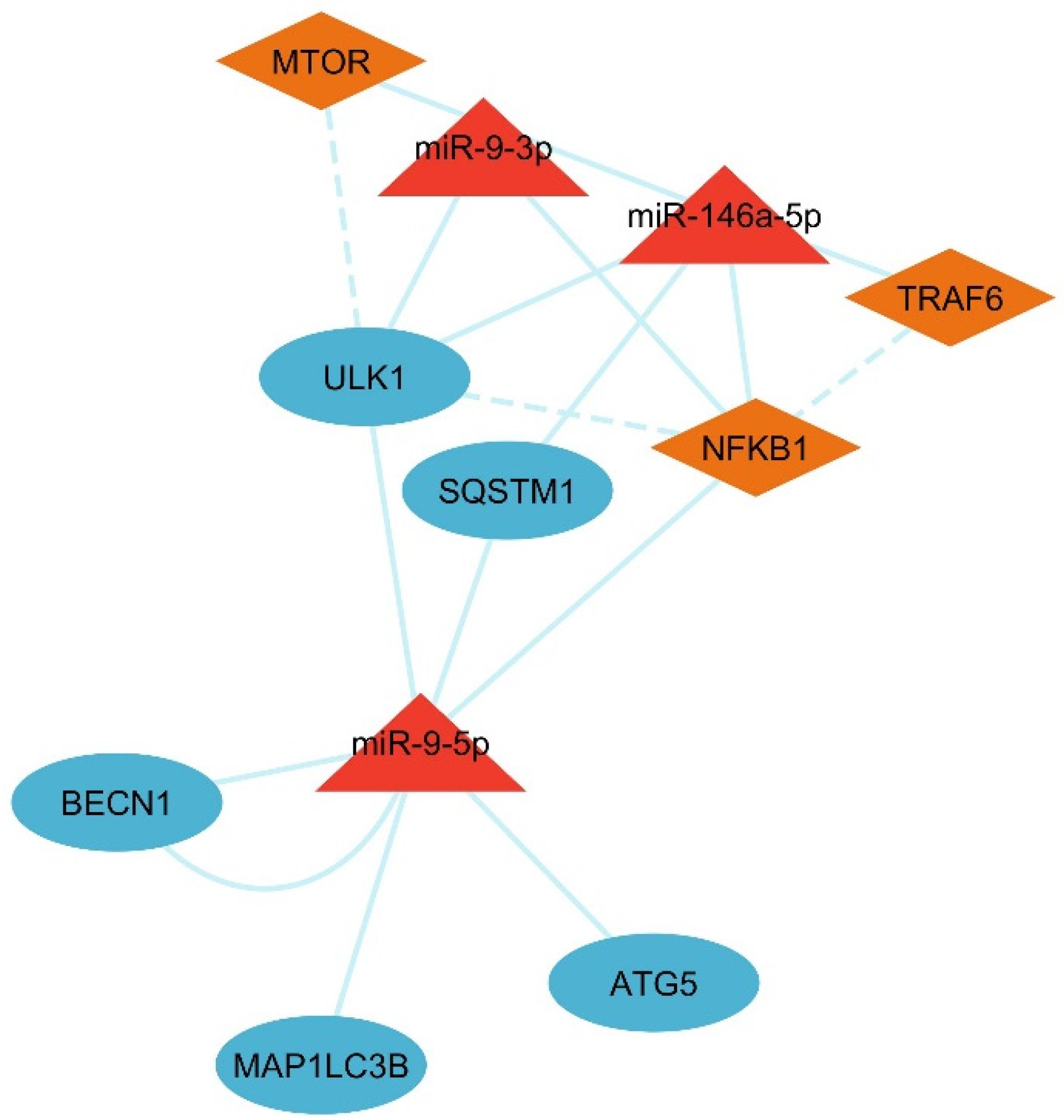
3.5. Identification of LC3 and Ubiquitination Interaction with miRNAs
3.6. Analysis of LC3–Ubiquitination and miRNA Network
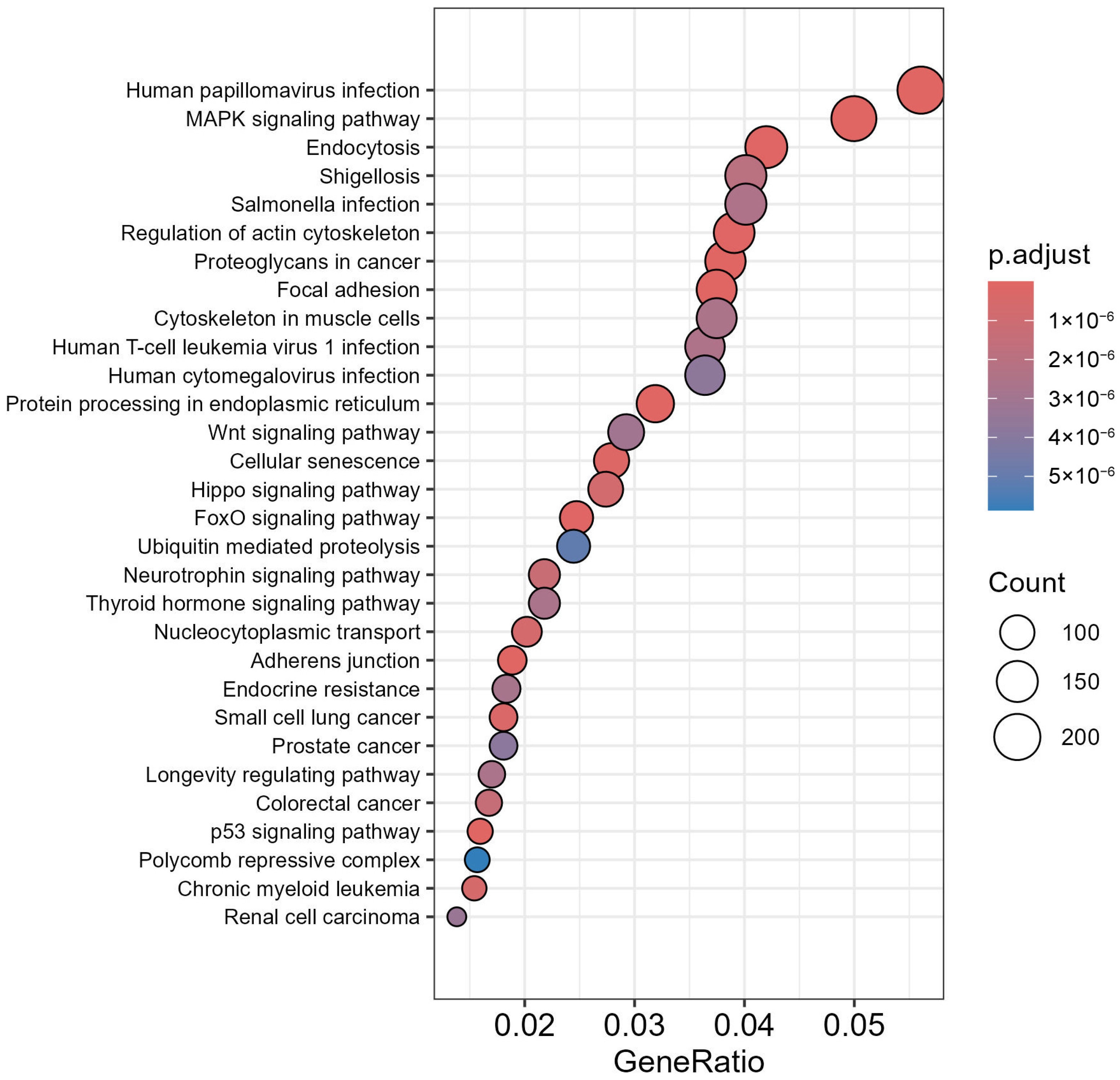
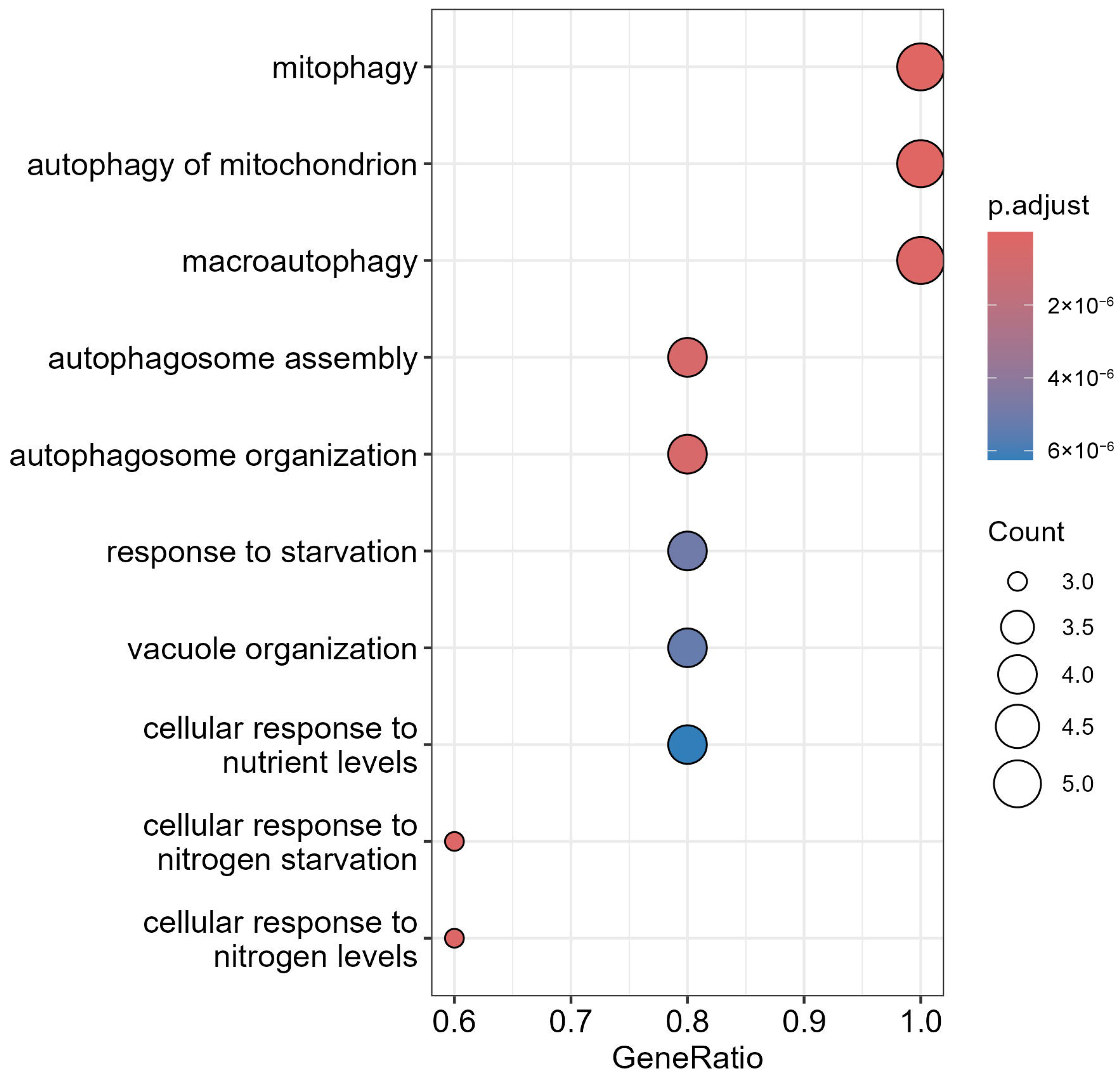
3.7. GO Analysis of LC3–Ubiquitination and miRNA Interactions
3.8. Interaction of LC3, IL6, and Ubiquitination Genes with Key Biological Processes
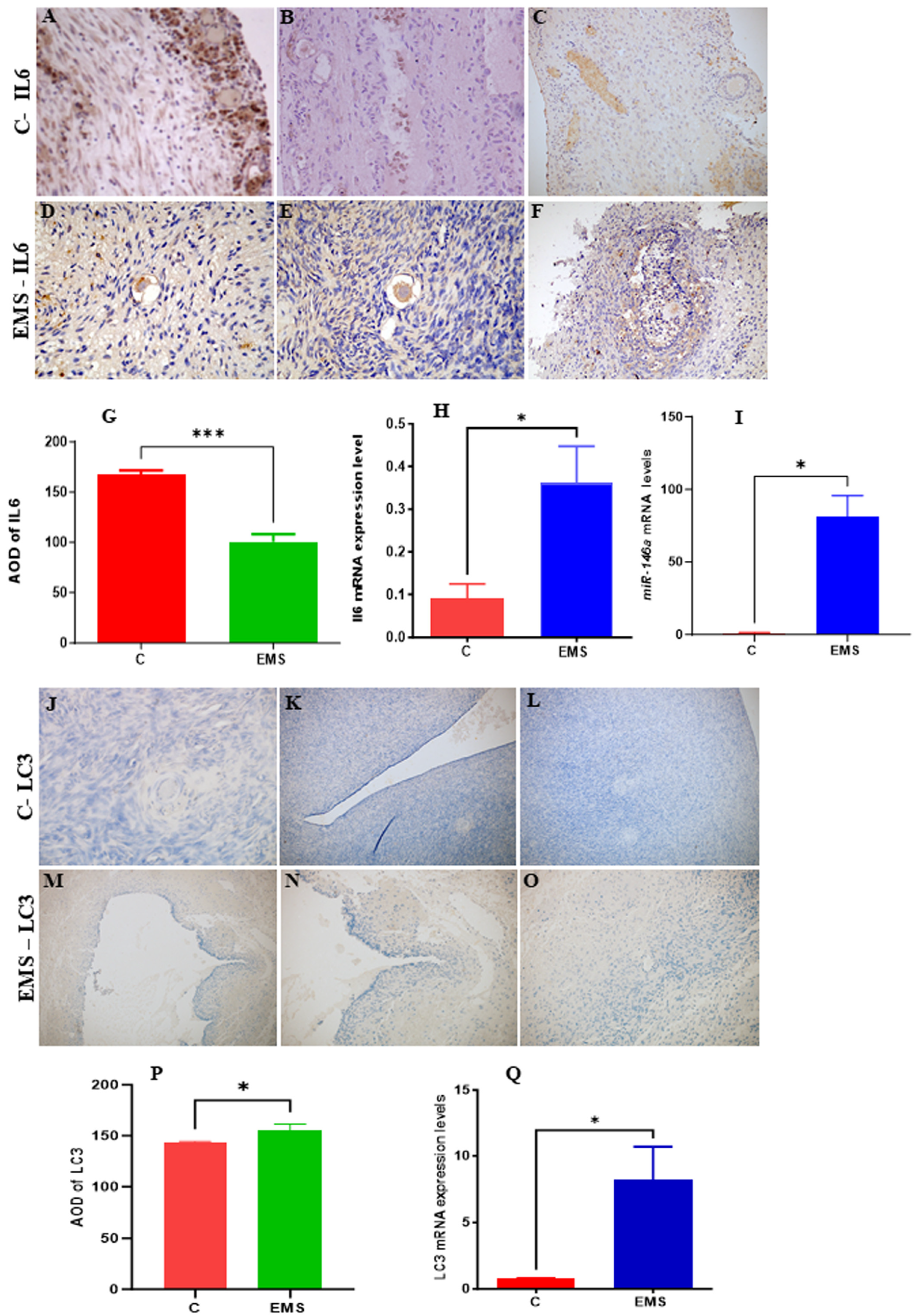
3.9. Experimental Analysis
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility—A clinician’s perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Kou, Z.; Huo, M.; An, J.; Zhang, X. GnRH agonist treatment regulates IL-6 and IL-11 expression in endometrial stromal cells for patients with HRT regiment in frozen embryo transfer cycles. Reprod. Biol. 2022, 22, 100608. [Google Scholar] [CrossRef]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Tan, B.; Wang, J. Role of IL-6 in Physiology and Pathology of the Ovary. Clin. Exp. Obstet. Gynecol. 2024, 51, 208. [Google Scholar] [CrossRef]
- Banerjee, S.; Cooney, L.G.; Stanic, A.K. Immune Dysfunction in Polycystic Ovary Syndrome. Immunohorizons 2023, 7, 323–332. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Gao, Y.; Wang, C.; Li, L.; Liao, Y.; Hu, K.; Liang, M. Roles of noncoding RNA in reproduction. Front. Genet. 2021, 12, 777510. [Google Scholar] [CrossRef]
- Fabová, Z.; Loncová, B.; Harrath, A.H.; Sirotkin, A.V. Does the miR-105-1-Kisspeptin Axis Promote Ovarian Cell Functions? Reprod. Sci. 2024, 31, 2293–2308. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Alexa, R.; Kišová, G.; Harrath, A.H.; Alwasel, S.; Ovcharenko, D.; Mlynček, M. MicroRNAs control transcription factor NF-kB (p65) expression in human ovarian cells. Funct. Integr. Genom. 2015, 15, 271–275. [Google Scholar] [CrossRef]
- Carletti, M.Z.; Fiedler, S.D.; Christenson, L.K. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol. Reprod. 2010, 83, 286–295. [Google Scholar] [CrossRef]
- Ashrafnezhad, Z.; Naji, M.; Aleyasin, A.; Hedayatpour, A.; Mahdavinezhad, F.; Gharaei, R.; Qasemi, M.; Amidi, F. Evaluating the Differential Expression of miR-146a, miR-222, and miR-9 in Matched Serum and Follicular Fluid of Polycystic Ovary Syndrome Patients: Profiling and Predictive Value. Int. J. Mol. Cell. Med. 2022, 11, 320–333. [Google Scholar] [CrossRef]
- Heidarzadehpilehrood, R.; Pirhoushiaran, M. Biomarker potential of competing endogenous RNA networks in Polycystic Ovary Syndrome (PCOS). Noncod. RNA Res. 2024, 9, 624–640. [Google Scholar] [CrossRef]
- Hawkins, S.M.; Creighton, C.J.; Han, D.Y.; Zariff, A.; Anderson, M.L.; Gunaratne, P.H.; Matzuk, M.M. Functional microRNA involved in endometriosis. Mol. Endocrinol. 2011, 25, 821–832. [Google Scholar] [CrossRef]
- Faraji, M.; Roshanaei, K.; Afkhami, H.; Heidarieh, N. Elevated expression of microRNA-155, microRNA-383, and microRNA-9 in Iranian patients with polycystic ovary syndrome. Biochem. Biophys. Rep. 2025, 42, 101997. [Google Scholar] [CrossRef]
- Kong, F.; Du, C.; Wang, Y. MicroRNA-9 affects isolated ovarian granulosa cells proliferation and apoptosis via targeting vitamin D receptor. Mol. Cell. Endocrinol. 2019, 486, 18–24. [Google Scholar] [CrossRef]
- Choi, A.M.K.; Ryter, S.W.; Levine, B. Autophagy in Human Health and Disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Kumariya, S.; Ubba, V.; Jha, R.K.; Gayen, J.R. Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy 2021, 17, 2706–2733. [Google Scholar] [CrossRef]
- Ji, R.; Zhang, Z.; Yang, Z.; Chen, X.; Yin, T.; Yang, J. BOP1 contributes to the activation of autophagy in polycystic ovary syndrome via nucleolar stress response. Cell. Mol. Life Sci. 2024, 81, 101. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Tsakiri, E.N.; Trougakos, I.P. The amazing ubiquitin-proteasome system: Structural components and implication in aging. Int. Rev. Cell Mol. Biol. 2015, 314, 171–237. [Google Scholar] [CrossRef]
- Sun, L.; Ye, H.; Tian, H.; Xu, L.; Cai, J.; Zhang, C.; Wang, R.; Yang, H.; Zhao, S.; Zhang, J.; et al. The E3 Ubiquitin Ligase SYVN1 Plays an Antiapoptotic Role in Polycystic Ovary Syndrome by Regulating Mitochondrial Fission. Oxid. Med. Cell. Longev. 2022, 2022, 3639302. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- McIlvenna, L.C.; Altintas, A.; Patten, R.K.; McAinch, A.J.; Rodgers, R.J.; Stepto, N.K.; Barres, R.; Moreno-Asso, A. Transforming growth factor beta1 impairs the transcriptomic response to contraction in myotubes from women with polycystic ovary syndrome. J. Physiol. 2022, 600, 3313–3330. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Zou, K.; Li, C.; Chen, X.; Gu, H.; Zhou, Z.; Yang, Z.; Tu, Y.; Qin, N.; et al. Novel PGK1 determines SKP2-dependent AR stability and reprograms granular cell glucose metabolism facilitating ovulation dysfunction. EBioMedicine 2020, 61, 103058. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Kim, C.K.; Asimes, A.; Zhang, M.; Son, B.T.; Kirk, J.A.; Pak, T.R. Differential Stability of miR-9-5p and miR-9-3p in the Brain Is Determined by Their Unique Cis- and Trans-Acting Elements. eNeuro 2020, 7, ENEURO.0094-20.2020. [Google Scholar] [CrossRef]
- Iacona, J.R.; Lutz, C.S. miR-146a-5p: Expression, regulation, and functions in cancer. Wiley Interdiscip. Rev. RNA 2019, 10, e1533. [Google Scholar] [CrossRef]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Gao, C.-H.; Chen, C.; Yu, G.; Dusa, A.; Cao, B.; Cai, P.; Akyol, T.Y. ggVennDiagram: A “ggplot2” Implement of Venn Diagram; iMeta: Southampton, UK, 2024. [Google Scholar]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Ham, J.; Lim, W.; Song, G. Ethalfluralin impairs implantation by aggravation of mitochondrial viability and function during early pregnancy. Environ. Pollut. 2022, 307, 119495. [Google Scholar] [CrossRef]
- Tuncdemir, M.; Yenmis, G.; Tombulturk, K.; Arkan, H.; Soydas, T.; Burak Tek, R.; Altintas, O.; Ozkara, H.; Kanigur-Sultuybek, G. NFKB1 rs28362491 and pre-miRNA-146a rs2910164 SNPs on E-Cadherin expression in case of idiopathic oligospermia: A case-control study. Int. J. Reprod. Biomed. 2018, 16, 247–254. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sarker, A.; Ayaz, M.; Shatabdy, A.R.; Haque, N.; Jalouli, M.; Rahman, M.D.H.; Mou, T.J.; Dey, S.K.; Hoque Apu, E.; et al. An Update on the Study of the Molecular Mechanisms Involved in Autophagy during Bacterial Pathogenesis. Biomedicines 2024, 12, 1757. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.D.H.; Hossain, M.S.; Biswas, P.; Islam, R.; Uddin, M.J.; Rahman, M.H.; Rhim, H. Molecular Insights into the Multifunctional Role of Natural Compounds: Autophagy Modulation and Cancer Prevention. Biomedicines 2020, 8, 517. [Google Scholar] [CrossRef]
- Popli, P.; Kommagani, R. Autophagy is required for stem-cell-mediated endometrial programming and the establishment of pregnancy. Autophagy 2024, 20, 970–972. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ahmed, K.R.; Rahman, M.D.H.; Parvez, M.A.K.; Lee, I.S.; Kim, B. Therapeutic Aspects and Molecular Targets of Autophagy to Control Pancreatic Cancer Management. Biomedicines 2022, 10, 1459. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.D.H.; Mamun-Or-Rashid, A.N.M.; Hwang, H.; Chung, S.; Kim, B.; Rhim, H. Autophagy Modulation in Aggresome Formation: Emerging Implications and Treatments of Alzheimer’s Disease. Biomedicines 2022, 10, 1027. [Google Scholar] [CrossRef]
- Harrath, A.H.; Rahman, M.A.; Bhajan, S.K.; Bishwas, A.K.; Rahman, M.H.; Alwasel, S.; Jalouli, M.; Kang, S.; Park, M.N.; Kim, B. Autophagy and Female Fertility: Mechanisms, Clinical Implications, and Emerging Therapies. Cells 2024, 13, 1354. [Google Scholar] [CrossRef]
- Cheng, D.; Zheng, B.; Sheng, Y.; Zeng, Z.; Mo, Z. The Roles of Autophagy in the Genesis and Development of Polycystic Ovary Syndrome. Reprod. Sci. 2023, 30, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Virakul, S.; Heutz, J.W.; Dalm, V.A.; Peeters, R.P.; Paridaens, D.; van den Bosch, W.A.; Hirankarn, N.; van Hagen, P.M.; Dik, W.A. Basic FGF and PDGF-BB synergistically stimulate hyaluronan and IL-6 production by orbital fibroblasts. Mol. Cell. Endocrinol. 2016, 433, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, Y. MicroRNAs in Extracellular Vesicles of Alzheimer’s Disease. Cells 2023, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Youns, M.; Kollek, V.; Buchmuller, D.; Bollmann, F.; Seo, E.J.; Schupp, J.; Montermann, E.; Usanova, S.; Kleinert, H.; et al. Differentially Tolerized Mouse Antigen Presenting Cells Share a Common miRNA Signature Including Enhanced mmu-miR-223-3p Expression Which Is Sufficient to Imprint a Protolerogenic State. Front. Pharmacol. 2018, 9, 915. [Google Scholar] [CrossRef]
- Bonacini, M.; Rossi, A.; Ferrigno, I.; Muratore, F.; Boiardi, L.; Cavazza, A.; Bisagni, A.; Cimino, L.; De Simone, L.; Ghidini, A.; et al. miR-146a and miR-146b regulate the expression of ICAM-1 in giant cell arteritis. J. Autoimmun. 2024, 144, 103186. [Google Scholar] [CrossRef]
- Pakdaman Kolour, S.S.; Nematollahi, S.; Dehbozorgi, M.; Fattahi, F.; Movahed, F.; Esfandiari, N.; Kahrizi, M.S.; Ghavamikia, N.; Hajiagha, B.S. Extracecellulr vesicles (EVs) microRNAs (miRNAs) derived from mesenchymal stem cells (MSCs) in osteoarthritis (OA); detailed role in pathogenesis and possible therapeutics. Heliyon 2025, 11, e42258. [Google Scholar] [CrossRef]
- Jia, W.; Li, N.; Wang, J.; Gong, X.; Ouedraogo, S.Y.; Wang, Y.; Zhao, J.; Grech, G.; Chen, L.; Zhan, X. Immune-related gene methylation prognostic instrument for stratification and targeted treatment of ovarian cancer patients toward advanced 3PM approach. EPMA J. 2024, 15, 375–404. [Google Scholar] [CrossRef]
- Kraczkowska, W.; Jagodzinski, P.P. The Long Non-Coding RNA Landscape of Atherosclerotic Plaques. Mol. Diagn. Ther. 2019, 23, 735–749. [Google Scholar] [CrossRef]
- Faizal, A.M.; Elias, M.H.; Jin, N.M.; Abu, M.A.; Syafruddin, S.E.; Zainuddin, A.A.; Suzuki, N.; Karim, A.K.A. Unravelling the role of HAS2, GREM1, and PTGS2 gene expression in cumulus cells: Implications for human oocyte development competency—A systematic review and integrated bioinformatic analysis. Front. Endocrinol. 2024, 15, 1274376. [Google Scholar] [CrossRef]
- Broi, M.G.D.; Rocha, C.V.J.; Meola, J.; Martins, W.P.; Carvalho, F.M.; Ferriani, R.A.; Navarro, P.A. Expression of PGR, HBEGF, ITGAV, ITGB3 and SPP1 genes in eutopic endometrium of infertile women with endometriosis during the implantation window: A pilot study. JBRA Assist. Reprod. 2017, 21, 196–202. [Google Scholar] [CrossRef]
- Liao, C.; Wang, Z.; Li, H.; Zhao, F.; Ling, L. Effect of Chitosan-assisted Combination of Laparoscope and Hysteroscope on the Levels of IFN-gamma and ICAM-1 in Treatment of Infertility Caused by Obstruction of Fallopian Tubes. Cell. Mol. Biol. 2023, 69, 101–104. [Google Scholar] [CrossRef]
- Padmanabhan, R.A.; Johnson, B.S.; Dhyani, A.K.; Pillai, S.M.; Jayakrishnan, K.; Laloraya, M. Autoimmune regulator (AIRE): Takes a hypoxia-inducing factor 1A (HIF1A) route to regulate FOXP3 expression in PCOS. Am. J. Reprod. Immunol. 2023, 89, e13637. [Google Scholar] [CrossRef]
- Samare-Najaf, M.; Neisy, A.; Samareh, A.; Moghadam, D.; Jamali, N.; Zarei, R.; Zal, F. The constructive and destructive impact of autophagy on both genders’ reproducibility, a comprehensive review. Autophagy 2023, 19, 3033–3061. [Google Scholar] [CrossRef]
- Jenabi, M.; Khodarahmi, P.; Tafvizi, F.; Bostanabad, S.Z. Evaluation of expression CXCL8 chemokine and its relationship with oocyte maturation and embryo quality in the intracytoplasmic sperm injection method. Mol. Biol. Rep. 2022, 49, 8413–8427. [Google Scholar] [CrossRef]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in Female Fertility: A Role in Oxidative Stress and Aging. Antioxid. Redox Signal. 2020, 32, 550–568. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Li, R.; Wang, F.; Wang, N.; Zhang, M.; Bai, Y.; Wu, J.; Liu, L.; Han, D.; et al. miR-146a-5p Plays an Oncogenic Role in NSCLC via Suppression of TRAF6. Front. Cell Dev. Biol. 2020, 8, 847. [Google Scholar] [CrossRef]
- Vergani, E.; Dugo, M.; Cossa, M.; Frigerio, S.; Di Guardo, L.; Gallino, G.; Mattavelli, I.; Vergani, B.; Lalli, L.; Tamborini, E.; et al. miR-146a-5p impairs melanoma resistance to kinase inhibitors by targeting COX2 and regulating NFkB-mediated inflammatory mediators. Cell Commun. Signal 2020, 18, 156. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, Q.; Chen, R. miR-146a-5p regulates autophagy and NLRP3 inflammasome activation in epithelial barrier damage in the in vitro cell model of ulcerative colitis through the RNF8/Notch1/mTORC1 pathway. Immunobiology 2023, 228, 152386. [Google Scholar] [CrossRef]
- Gozuacik, D.; Akkoc, Y.; Ozturk, D.G.; Kocak, M. Autophagy-Regulating microRNAs and Cancer. Front. Oncol. 2017, 7, 65. [Google Scholar] [CrossRef]
- Guan, X.; Iyaswamy, A.; Sreenivasmurthy, S.G.; Su, C.; Zhu, Z.; Liu, J.; Kan, Y.; Cheung, K.H.; Lu, J.; Tan, J.; et al. Mechanistic Insights into Selective Autophagy Subtypes in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 3609. [Google Scholar] [CrossRef]
- Deng, T.; Hu, B.; Wang, X.; Ding, S.; Lin, L.; Yan, Y.; Peng, X.; Zheng, X.; Liao, M.; Jin, Y.; et al. TRAF6 autophagic degradation by avibirnavirus VP3 inhibits antiviral innate immunity via blocking NFKB/NF-kappaB activation. Autophagy 2022, 18, 2781–2798. [Google Scholar] [CrossRef]
- Yamada, T.; Dawson, T.M.; Yanagawa, T.; Iijima, M.; Sesaki, H. SQSTM1/p62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/parkin in mitophagy. Autophagy 2019, 15, 2012–2018. [Google Scholar] [CrossRef]
- Dinsdale, N.L.; Crespi, B.J. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol. Appl. 2021, 14, 1693–1715. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Harrath, A.H.; Alrezaki, A.; Jalouli, M.; Al-Dawood, N.; Dahmash, W.; Mansour, L.; Sirotkin, A.; Alwasel, S. Benzene exposure causes structural and functional damage in rat ovaries: Occurrence of apoptosis and autophagy. Environ. Sci. Pollut. Res. 2022, 29, 76275–76285. [Google Scholar] [CrossRef]
- Jalouli, M.; Mofti, A.; Elnakady, Y.A.; Nahdi, S.; Feriani, A.; Alrezaki, A.; Sebei, K.; Bizzarri, M.; Alwasel, S.; Harrath, A.H. Allethrin promotes apoptosis and autophagy associated with the oxidative stress-related PI3K/AKT/mTOR signaling pathway in developing rat ovaries. Int. J. Mol. Sci. 2022, 23, 6397. [Google Scholar] [CrossRef]
- Oală, I.E.; Mitranovici, M.-I.; Chiorean, D.M.; Irimia, T.; Crișan, A.I.; Melinte, I.M.; Cotruș, T.; Tudorache, V.; Moraru, L.; Moraru, R. Endometriosis and the role of pro-inflammatory and anti-inflammatory cytokines in pathophysiology: A narrative review of the literature. Diagnostics 2024, 14, 312. [Google Scholar] [CrossRef]
- Hannan, N.J.; Evans, J.; Salamonsen, L.A. Alternate roles for immune regulators: Establishing endometrial receptivity for implantation. Expert. Rev. Clin. Immunol. 2011, 7, 789–802. [Google Scholar] [CrossRef]
- Kuessel, L.; Wenzl, R.; Proestling, K.; Balendran, S.; Pateisky, P.; Yotova, I.; Yerlikaya, G.; Streubel, B.; Husslein, H. Soluble VCAM-1/soluble ICAM-1 ratio is a promising biomarker for diagnosing endometriosis. Hum. Reprod. 2017, 32, 770–779. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Ohlsson Teague, E.M.C.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Filigheddu, N.; Gregnanin, I.; Porporato, P.E.; Surico, D.; Perego, B.; Galli, L.; Patrignani, C.; Graziani, A.; Surico, N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. BioMed Res. Int. 2010, 2010, 369549. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, Y.; Gao, G.; Zhang, Y. Long noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate LPS-induced WI-38 cell apoptosis and inflammation in acute pneumonia. Life Sci. 2019, 228, 189–197. [Google Scholar] [CrossRef]
- Yan, F.; Wufuer, D.; Ding, J.; Wang, J. MicroRNA miR-146a-5p inhibits the inflammatory response and injury of airway epithelial cells via targeting TNF receptor-associated factor 6. Bioengineered 2021, 12, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Chen, B.; Fan, X.; Li, G.; Dong, P.; Zheng, J. Epigenetically-Regulated MicroRNA-9-5p Suppresses the Activation of Hepatic Stellate Cells via TGFBR1 and TGFBR2. Cell. Physiol. Biochem. 2017, 43, 2242–2252. [Google Scholar] [CrossRef]
- Morita, Y.; Maravei, D.V.; Bergeron, L.; Wang, S.; Perez, G.I.; Tsutsumi, O.; Taketani, Y.; Asano, M.; Horai, R.; Korsmeyer, S.J.; et al. Caspase-2 deficiency prevents programmed germ cell death resulting from cytokine insufficiency but not meiotic defects caused by loss of ataxia telangiectasia-mutated (Atm) gene function. Cell Death Differ. 2001, 8, 614–620. [Google Scholar] [CrossRef]
- Zheng, J.; Luo, X.; Bao, J.; Huang, X.; Jin, Y.; Chen, L.; Zheng, F. Decreased expression of HOXA10 may activate the autophagic process in ovarian endometriosis. Reprod. Sci. 2018, 25, 1446–1454. [Google Scholar] [CrossRef]
- Allavena, G.; Carrarelli, P.; Del Bello, B.; Luisi, S.; Petraglia, F.; Maellaro, E. Autophagy is upregulated in ovarian endometriosis: A possible interplay with p53 and heme oxygenase-1. Fertil. Steril. 2015, 103, 1244–1251.e1241. [Google Scholar] [CrossRef]
- Yang, H.-L.; Mei, J.; Chang, K.-K.; Zhou, W.-J.; Huang, L.-Q.; Li, M.-Q. Autophagy in endometriosis. Am. J. Transl. Res. 2017, 9, 4707. [Google Scholar]
- Genovese, M.C.; McKay, J.D.; Nasonov, E.L.; Mysler, E.F.; da Silva, N.A.; Alecock, E.; Woodworth, T.; Gomez-Reino, J.J. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: The tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008, 58, 2968–2980. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, V.; Naar, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]


| Gene | Functional Role | Associated Reproductive Process | Reference |
|---|---|---|---|
| MAP1LC3B | Autophagosome membrane formation | Impaired oocyte maturation, embryo quality | [39] |
| ATG5 | Autophagosome elongation (ATG5-ATG12 complex) | Follicular atresia, granulosa cell apoptosis | [40] |
| BECN1 | Autophagy initiation (PI3K complex) | Dysregulated autophagy in endometrial cells | [41,42] |
| SQSTM1 | Selective autophagy of ubiquitinated proteins | Protein aggregate clearance in ovarian cells | [39,43] |
| ULK1 | Master kinase regulating autophagy initiation | Stress response in cumulus cells | [44,45] |
| Term | p-Value | q-Value | Overlap Genes |
|---|---|---|---|
| Macroautophagy | 3.325892 × 10−12 | 5.372732 × 10−10 | BECN1, MAP1LC3B, ULK1, SQSTM1, MTOR, ATG5 |
| Autophagy | 6.105378 × 10−12 | 5.372732 × 10−10 | BECN1, MAP1LC3B, ULK1, SQSTM1, MTOR, ATG5 |
| Mitophagy | 8.588874 × 10−10 | 5.038806 × 10−8 | MAP1LC3B, ULK1, SQSTM1, ATG5 |
| Receptor-Mediated Mitophagy | 6.919482 × 10−9 | 3.044572 × 10−7 | MAP1LC3B, ULK1, ATG5 |
| NF-kB Is Activated and Signals Survival | 1.198939 × 10−8 | 4.220264 × 10−7 | TRAF6, SQSTM1, NFKB1 |
| p75NTR Signals via NF-kB | 2.346278 × 10−8 | 6.359648 × 10−7 | TRAF6, SQSTM1, NFKB1 |
| Selective Autophagy | 2.529405 × 10−8 | 6.359648 × 10−7 | MAP1LC3B, ULK1, SQSTM1, ATG5 |
| PINK1-PRKN-Mediated Mitophagy | 2.071973 × 10−7 | 4.393258 × 10−6 | MAP1LC3B, SQSTM1, ATG5 |
| SARS-CoV-1 Infection | 2.496169 × 10−7 | 4.393258 × 10−6 | BECN1, MAP1LC3B, TRAF6, NFKB1 |
| Death Receptor Signaling | 2.496169 × 10−7 | 4.393258 × 10−6 | TRAF6, ULK1, SQSTM1, NFKB1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahdi, S.; Arafah, M.; Harrath, A.H. Integrated Bioinformatics and Experimental Analysis Revealed Crosstalk Between IL-6, Autophagy, Ubiquitination, and Key miRNAs in Female Infertility: Insights from Ovarian Endometriosis and Polycystic Ovary Syndrome. Cells 2025, 14, 1693. https://doi.org/10.3390/cells14211693
Nahdi S, Arafah M, Harrath AH. Integrated Bioinformatics and Experimental Analysis Revealed Crosstalk Between IL-6, Autophagy, Ubiquitination, and Key miRNAs in Female Infertility: Insights from Ovarian Endometriosis and Polycystic Ovary Syndrome. Cells. 2025; 14(21):1693. https://doi.org/10.3390/cells14211693
Chicago/Turabian StyleNahdi, Saber, Maria Arafah, and Abdel Halim Harrath. 2025. "Integrated Bioinformatics and Experimental Analysis Revealed Crosstalk Between IL-6, Autophagy, Ubiquitination, and Key miRNAs in Female Infertility: Insights from Ovarian Endometriosis and Polycystic Ovary Syndrome" Cells 14, no. 21: 1693. https://doi.org/10.3390/cells14211693
APA StyleNahdi, S., Arafah, M., & Harrath, A. H. (2025). Integrated Bioinformatics and Experimental Analysis Revealed Crosstalk Between IL-6, Autophagy, Ubiquitination, and Key miRNAs in Female Infertility: Insights from Ovarian Endometriosis and Polycystic Ovary Syndrome. Cells, 14(21), 1693. https://doi.org/10.3390/cells14211693







