Ferroptosis in Oral Cancer: Mechanistic Insights and Clinical Prospects
Highlights
- Ferroptosis represents a targetable vulnerability in oral squamous cell carcinoma (OSCC).
- Genetic, epigenetic, and metabolic regulators, including GPX4, SLC7A11, NFE2L2, and ACSL4, shape ferroptosis sensitivity.
- Repurposed drugs, natural compounds, and nanomedicine formulations effectively induce ferroptosis in OSCC.
- 4.
- Ferroptosis activation enhances the therapeutic efficacy of cisplatin, radiotherapy, and immunotherapy in OSCC.
- 5.
- Ferroptosis-related biomarkers and non-cancer oral disease mechanisms broaden the clinical and translational relevance of ferroptosis.
Abstract
1. Introduction
2. Molecular Basis of Ferroptosis
2.1. Iron Metabolism and the Labile Iron Pool
2.2. Lipid Peroxidation: The Execution Step of Ferroptosis
2.3. Amino Acid Metabolism and the System Xc–/GSH/GPX4 Axis
2.4. Additional Ferroptosis Regulators
3. Ferroptosis in Oral Cancer: Preclinical Evidence
3.1. Suppression of Ferroptosis in Baseline OSCC Models
3.2. Tumor Suppressors and Oncogenes Regulating Ferroptosis in OSCC
3.3. Non-Coding RNAs and Epigenetic Modulation
3.4. Metabolic Enzymes and Ferroptosis Sensitivity
3.5. Chemoresistance and Ferroptosis in OSCC
3.6. Tumor Microenvironment and Ferroptosis
3.7. Dual Roles of Ferroptosis in Tumorigenesis
3.8. Natural Products and Nanomedicine as Ferroptosis Inducers
4. Ferroptosis and Therapy Resistance in Oral Cancer
4.1. Chemotherapy Resistance
4.2. Radiotherapy Resistance
4.3. Immunotherapy Resistance
4.4. Genomic and Transcriptomic Insights into Ferroptosis-Related Prognosis in OSCC
5. Therapeutic Strategies Targeting Ferroptosis in Oral Cancer
5.1. Small-Molecule Ferroptosis Inducers
5.2. Repurposed Drugs with Ferroptosis-Inducing Activity
5.3. Natural Products Targeting Ferroptosis
5.4. Nanomedicine-Based Ferroptosis Strategies
5.5. Combination Strategies Involving Ferroptosis
6. Ferroptosis in Oral Diseases Beyond Cancer
6.1. Pulpal and Endodontic Diseases
6.2. Periodontitis
6.3. Infection, Inflammation, and Immunity in the Oral Cavity
6.4. Implications for Oral Disease Therapy
7. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, P.; Dahlstrom, K.R.; Gross, N.; Li, G. Joint effect of human papillomavirus exposure, smoking and alcohol on risk of oral squamous cell carcinoma. BMC Cancer 2023, 23, 457. [Google Scholar] [CrossRef]
- Cunha, A.R.D.; Compton, K.; Xu, R.; Mishra, R.; Drangsholt, M.T.; Antunes, J.L.F.; Kerr, A.R.; Acheson, A.R.; Lu, D.; Wallace, L.E.; et al. The Global, Regional, and National Burden of Adult Lip, Oral, and Pharyngeal Cancer in 204 Countries and Territories: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2023, 9, 1401–1416. [Google Scholar] [CrossRef]
- Chinn, S.B.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276. [Google Scholar] [CrossRef]
- Ghanaati, S.; Udeabor, S.E.; Winter, A.; Sader, R.; Heselich, A. Cancer Recurrence in Operated Primary Oral Squamous Cell Carcinoma Patients Seems to Be Independent of the Currently Available Postoperative Therapeutic Approach: A Retrospective Clinical Study. Curr. Oncol. 2025, 32, 208. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Liu, M.; Jiang, H.; Wu, Y. Oral squamous cell carcinoma: Insights into cellular heterogeneity, drug resistance, and evolutionary trajectories. Cell Biol. Toxicol. 2025, 41, 101. [Google Scholar] [CrossRef]
- Nagarathna, P.J.; Patil, S.R.; Veeraraghavan, V.P.; Daniel, S.; Aileni, K.R.; Karobari, M.I. Oral cancer stem cells: A comprehensive review of key drivers of treatment resistance and tumor recurrence. Eur. J. Pharmacol. 2025, 989, 177222. [Google Scholar] [CrossRef]
- Hsu, P.C.; Tsai, C.C.; Lin, Y.H.; Kuo, C.Y. Therapeutic Targeting of Apoptosis, Autophagic Cell Death, Necroptosis, Pyroptosis, and Ferroptosis Pathways in Oral Squamous Cell Carcinoma: Molecular Mechanisms and Potential Strategies. Biomedicines 2025, 13, 1745. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. SLC7A11 as a Gateway of Metabolic Perturbation and Ferroptosis Vulnerability in Cancer. Antioxidants 2022, 11, 2444. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, ferroptosis, and diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Ding, X.; Cui, L.; Mi, Y.; Hu, J.; Cai, Z.; Tang, Q.; Yang, L.; Yang, Z.; Wang, Q.; Li, H.; et al. Ferroptosis in cancer: Revealing the multifaceted functions of mitochondria. Cell. Mol. Life Sci. 2025, 82, 277. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Antonelli, A.; Battaglia, A.M.; Sacco, A.; Petriaggi, L.; Giorgio, E.; Barone, S.; Biamonte, F.; Giudice, A. Ferroptosis and oral squamous cell carcinoma: Connecting the dots to move forward. Front. Oral Health 2024, 5, 1461022. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. Induction of ferroptosis in head and neck cancer: A novel bridgehead for fighting cancer resilience. Cancer Lett. 2022, 546, 215854. [Google Scholar] [CrossRef]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef]
- Vijayarangam, V.; Gopalakrishnan Deviparasakthi, M.K.; Balasubramanian, P.; Palaniyandi, T.; Ravindran, R.; Suliman, M.; Saeed, M.; Natarajan, S.; Sivaji, A.; Baskar, G. Ferroptosis as a hero against oral cancer. Pathol. Res. Pract. 2024, 263, 155637. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Wang, Z.; Jin, Y.; Gu, W. Ferroptosis as a new tool for tumor suppression through lipid peroxidation. Commun. Biol. 2024, 7, 1475. [Google Scholar] [CrossRef]
- Lee, J.; Seo, Y.; Roh, J.L. Ferroptosis and Nrf2 Signaling in Head and Neck Cancer: Resistance Mechanisms and Therapeutic Prospects. Antioxidants 2025, 14, 993. [Google Scholar] [CrossRef]
- Roh, J.L. Targeting ferroptosis suppressor protein 1 in cancer therapy: Implications and perspectives, with emphasis on head and neck cancer. Crit. Rev. Oncol. Hematol. 2024, 202, 104440. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, H.; Zheng, J.; Yang, K. The PER1/HIF-1alpha negative feedback loop promotes ferroptosis and inhibits tumor progression in oral squamous cell carcinoma. Transl. Oncol. 2022, 18, 101360. [Google Scholar] [CrossRef]
- Wang, M.; Li, B.; Meng, W.; Chen, Y.; Liu, H.; Zhang, Z.; Li, L. System Xc(-) exacerbates metabolic stress under glucose depletion in oral squamous cell carcinoma. Oral Dis. 2024, 30, 2952–2964. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, S.; Zhao, R.; Liu, W.; Jin, L.; Ren, X.; He, H. Targeting ferroptosis as a potential strategy to overcome the resistance of cisplatin in oral squamous cell carcinoma. Front. Pharmacol. 2024, 15, 1402514. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Zhang, S.; Yang, X.; Yang, Y.; Han, T.; Luo, Y.; Shui, C.; Yang, M.; Lin, Y.; et al. Tetrahedral-DNA-Nanostructure-Modified Engineered Extracellular Vesicles Enhance Oral Squamous Cell Carcinomas Therapy by Targeting GPX4. ACS Nano 2025, 19, 9351–9366. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M.; Hashimoto, R.F. DNA Damage-Induced Ferroptosis: A Boolean Model Regulating p53 and Non-Coding RNAs in Drug Resistance. Proteomes 2025, 13, 6. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Z.; Li, Y.; Lu, X.; Shao, T.; Lv, X. Bioinformatic analysis indicated that STARD4-AS1 might be a novel ferroptosis-related biomarker of oral squamous cell carcinoma. Heliyon 2024, 10, e33193. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, J.; Guo, H. Circ_0000140 Alters miR-527/SLC7A11-Mediated Ferroptosis to Influence Oral Squamous Cell Carcinoma Cell Resistance to DDP. Pharmgenomics Pers. Med. 2023, 16, 1079–1089. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, H.; Chen, Y.; Hao, J.; Zhou, Y.; Li, N. Inhibition of TPI1 Sensitizes Cisplatin-Resistant Oral Cancer to Ferroptosis. Biomedicines 2025, 13, 1225. [Google Scholar] [CrossRef]
- Luo, Q.; Hu, S.; Tang, Y.; Yang, D.; Chen, Q. PPT1 Promotes Growth and Inhibits Ferroptosis of Oral Squamous Cell Carcinoma Cells. Curr. Cancer Drug Targets 2024, 24, 1047–1060. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Li, Y.Q.; Wang, D.Y.; Shen, Y.Q. Natural product piperlongumine inhibits proliferation of oral squamous carcinoma cells by inducing ferroptosis and inhibiting intracellular antioxidant capacity. Transl. Cancer Res. 2023, 12, 2911–2922. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, X.; Wang, X.; Weng, C.F. The Chemotherapy Medication of Evodia lepta (Spreng). Merr. on the Viability of Tongue Cancer Cells Through the PD-L1/MMP14/HSPA5 Pathway. Cancer Manag. Res. 2025, 17, 1613–1623. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Zhao, Y.; Yan, J.; Luo, K.; Li, F.; He, B.; Sun, Y.; Li, F.; Liang, Y. A Redox-Triggered Polymeric Nanoparticle for Disrupting Redox Homeostasis and Enhanced Ferroptosis. Small 2025, 21, e2404299. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Ma, X.; Yang, W.; Hao, Y.; Zhang, L.; Piao, S. Combination treatment with ferroptosis and autophagy inducers significantly inhibit the proliferation and migration of oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2024, 709, 149842. [Google Scholar] [CrossRef]
- Balachander, K.; Paramasivam, A. Ferroptosis: An emerging therapeutic target for oral cancer. Oral Oncol. 2022, 131, 105970. [Google Scholar] [CrossRef]
- Siquara da Rocha, L.O.; de Morais, E.F.; de Oliveira, L.Q.R.; Barbosa, A.V.; Lambert, D.W.; Gurgel Rocha, C.A.; Coletta, R.D. Exploring beyond Common Cell Death Pathways in Oral Cancer: A Systematic Review. Biology 2024, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T. Iron-Induced Oxidative Stress in Human Diseases. Cells 2022, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. Ferroptosis: Iron release mechanisms in the bioenergetic process. Cancer Metastasis Rev. 2025, 44, 36. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Roh, J.L. Altered iron metabolism as a target for ferroptosis induction in head and neck cancer. Cell. Oncol. 2023, 46, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Zhu, Y.; Fujimaki, M.; Rubinsztein, D.C. Autophagy-dependent versus autophagy-independent ferroptosis. Trends Cell Biol. 2025, 35, 745–760. [Google Scholar] [CrossRef]
- Petriaggi, L.; Giorgio, E.; Bulotta, S.; Antonelli, A.; Bonacci, S.; Frisina, M.; Procopio, A.; Prestagiacomo, L.E.; Giuliano, A.; Gaspari, M.; et al. Acute Exposure to Cadmium Triggers NCOA4-Mediated Ferritinophagy and Ferroptosis in Never-Smokers Oral Cancer Cells. Int. J. Biol. Sci. 2025, 21, 4131–4152. [Google Scholar] [CrossRef]
- Chen, F.; Cai, X.; Kang, R.; Liu, J.; Tang, D. Autophagy-Dependent Ferroptosis in Cancer. Antioxid. Redox Signal. 2023, 39, 79–101. [Google Scholar] [CrossRef]
- Qiu, B.; Zandkarimi, F.; Bezjian, C.T.; Reznik, E.; Soni, R.K.; Gu, W.; Jiang, X.; Stockwell, B.R. Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell 2024, 187, 1177–1190.e1118. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Sun, D.; Wang, L.; Wu, Y.; Yu, Y.; Yao, Y.; Yang, H.; Hao, C. Lipid metabolism in ferroptosis: Mechanistic insights and therapeutic potential. Front. Immunol. 2025, 16, 1545339. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, H.; Graham, E.T.; Deik, A.A.; Eaton, J.K.; Wang, W.; Sandoval-Gomez, G.; Clish, C.B.; Doench, J.G.; Schreiber, S.L. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol. 2020, 16, 302–309. [Google Scholar] [CrossRef]
- Zhao, J.; Dar, H.H.; Deng, Y.; St Croix, C.M.; Li, Z.; Minami, Y.; Shrivastava, I.H.; Tyurina, Y.Y.; Etling, E.; Rosenbaum, J.C.; et al. PEBP1 acts as a rheostat between prosurvival autophagy and ferroptotic death in asthmatic epithelial cells. Proc. Natl. Acad. Sci. USA 2020, 117, 14376–14385. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; Liu, W.; Li, B.W.; Tian, Z.; Zhao, Y.Y.; Yu, G.T. Manganese drives ferroptosis of cancer cells via YAP/TAZ phase separation activated ACSL4 in OSCC. Oral Dis. 2024, 30, 4898–4908. [Google Scholar] [CrossRef]
- Rao, Y.; Li, J.; Shi, L.; Chen, X.; Hu, Y.; Mao, Y.; Zhang, X.; Liu, X. Silencing CK19 regulates ferroptosis by affecting the expression of GPX4 and ACSL4 in oral squamous cell carcinoma in vivo and in vitro. Sci. Rep. 2024, 14, 15968. [Google Scholar] [CrossRef]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular basis for redox control by the human cystine/glutamate antiporter system xc−. Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. Targeting GPX4 in human cancer: Implications of ferroptosis induction for tackling cancer resilience. Cancer Lett. 2023, 559, 216119. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; MohamedAl-Sharani, H.; Zhang, B. EZH2-mediated SLC7A11 upregulation via miR-125b-5p represses ferroptosis of TSCC. Oral Dis. 2023, 29, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Kang, J.; Wei, S.; Wang, Y.; Liu, Y.; Yuan, B.; Lu, Q.; Li, H.; Yan, J.; Yang, X.; et al. A pH responsive nanocomposite for combination sonodynamic-immunotherapy with ferroptosis and calcium ion overload via SLC7A11/ACSL4/LPCAT3 pathway. Exploration 2025, 5, 20240002. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Wu, Y. Hsa-miR-26a-5p improves OSCC sensitivity to ferroptosis by inhibiting SLC7A11. Arch. Oral Biol. 2023, 156, 105807. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, Q.; Pang, N.; Xue, J. TCF12 induces ferroptosis by suppressing OTUB1-mediated SLC7A11 deubiquitination to promote cisplatin sensitivity in oral squamous cell carcinoma. Cell Biol. Int. 2024, 48, 1649–1663. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, S. Nrf2/HO-1 Alleviates Disulfiram/Copper-Induced Ferroptosis in Oral Squamous Cell Carcinoma. Biochem. Genet. 2024, 62, 144–155. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, Y.; Yuan, F.; Zhang, L.; Cai, Y.; Liao, L. A ferroptosis-related prognostic model with excellent clinical performance based on the exploration of the mechanism of oral squamous cell carcinoma progression. Sci. Rep. 2023, 13, 1461. [Google Scholar] [CrossRef]
- Shimura, T.; Yin, C.; Ma, R.; Zhang, A.; Nagai, Y.; Shiratori, A.; Ozaki, H.; Yamashita, S.; Higashi, K.; Sato, Y.; et al. The prognostic importance of the negative regulators of ferroptosis, GPX4 and HSPB1, in patients with colorectal cancer. Oncol. Lett. 2025, 29, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hua, L.; Ma, P.; Song, Y.; Min, J.; Guo, Y.; Yang, C.; Li, J.; Su, H. HSPA5 repressed ferroptosis to promote colorectal cancer development by maintaining GPX4 stability. Neoplasma 2022, 69, 1054–1069. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Hüther, J.A.; Wank, B.; Rath, A.; Tykwe, R.; Aldrovandi, M.; Henkelmann, B.; Mergner, J.; Nakamura, T.; Laschat, S.; et al. Interplay of ferroptotic and apoptotic cell death and its modulation by BH3-mimetics. Cell Death Differ. 2025, 1–16. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Liu, K.; Yu, J.; Huang, X.; Gao, H.; Wang, J. WGCNA and ferroptosis genes in OSCC: Unraveling prognostic biomarkers and therapeutic targets. Discov. Oncol. 2025, 16, 379. [Google Scholar] [CrossRef]
- Fukuda, M.; Ogasawara, Y.; Hayashi, H.; Okuyama, A.; Shiono, J.; Inoue, K.; Sakashita, H. Down-regulation of Glutathione Peroxidase 4 in Oral Cancer Inhibits Tumor Growth Through SREBP1 Signaling. Anticancer. Res. 2021, 41, 1785–1792. [Google Scholar] [CrossRef]
- Wu, J.E.; Li, Y.; Hou, J. Downregulation of SLC3A2 mediates immune evasion and accelerates metastasis in oral squamous cell carcinoma. J. Cell. Mol. Med. 2024, 28, e18010. [Google Scholar] [CrossRef]
- Wu, Y.C.; Huang, C.S.; Hsieh, M.S.; Huang, C.M.; Setiawan, S.A.; Yeh, C.T.; Kuo, K.T.; Liu, S.C. Targeting of FSP1 regulates iron homeostasis in drug-tolerant persister head and neck cancer cells via lipid-metabolism-driven ferroptosis. Aging 2024, 16, 627–647. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Yang, G.; Han, L.; Wang, X. NFE2L1 restrains ferroptosis by transcriptionally regulating HJURP and participates in the progress of oral squamous cell carcinoma. J. Bioenerg. Biomembr. 2023, 55, 467–478. [Google Scholar] [CrossRef]
- Mao, C.; Gong, L.; Kang, W. Effect and mechanism of resveratrol on ferroptosis mediated by p53/SLC7A11 in oral squamous cell carcinoma. BMC Oral Health 2024, 24, 773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, X.; Zhang, Y.; Wang, L.; Chen, Z. Inhibition of AEBP1 predisposes cisplatin-resistant oral cancer cells to ferroptosis. BMC Oral Health 2022, 22, 478. [Google Scholar] [CrossRef]
- Xie, J.; Lan, T.; Zheng, D.L.; Ding, L.C.; Lu, Y.G. CDH4 inhibits ferroptosis in oral squamous cell carcinoma cells. BMC Oral Health 2023, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, J.; Yang, B.; Kong, L.; Zhang, D.; Guo, J.; Zhou, L.; Bai, G.; Zhao, H.; Meng, Z. METTL3-mediated m(6)A methylation of lncRNA PVT1 promotes the progression of oral squamous cell carcinoma via the miR-185-5p/SERPINB4 axis. Am. J. Cancer Res. 2025, 15, 2872–2889. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Ren, Z.; Han, Y.; Jiang, X.; Wu, Z.; Tan, S.; Yang, W.; Oyang, L.; Luo, X.; et al. CircRNF13 enhances IGF2BP1 phase separation-mediated ITGB1 mRNA stabilization in an m6A-dependent manner to promote oral cancer cisplatin chemoresistance. Mol. Cancer 2025, 24, 36. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Cai, H.; Liang, J.; Jiang, Y.; Song, F.; Hou, C.; Hou, J. FTO Sensitizes Oral Squamous Cell Carcinoma to Ferroptosis via Suppressing ACSL3 and GPX4. Int. J. Mol. Sci. 2023, 24, 16339. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Li, Y.C.; Liu, H.M.; Xu, R.; Liu, Y.T.; Zhang, W.; Yang, H.Y.; Chen, G. Synthetic lethal CRISPR screen identifies a cancer cell-intrinsic role of PD-L1 in regulation of vulnerability to ferroptosis. Cell Rep. 2024, 43, 114477. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Z.; Li, W.; Xu, X.; Shen, P.; Zhang, S.E.; Cheng, B.; Xia, J. PDPN(+) CAFs facilitate the motility of OSCC cells by inhibiting ferroptosis via transferring exosomal lncRNA FTX. Cell Death Dis. 2023, 14, 759. [Google Scholar] [CrossRef]
- Xia, L.; Wang, H.; Du, G.; Cheng, X.; Zhang, R.; Yu, H.; Cheng, M.; Chen, Y.; Qin, S.; Leng, W. Receptor accessory protein 6, a novel ferroptosis suppressor, drives oral squamous cell carcinoma by maintaining endoplasmic reticulum hemostasis. Int. J. Biol. Macromol. 2024, 283, 137565. [Google Scholar] [CrossRef]
- Liu, S.; Yan, S.; Zhu, J.; Lu, R.; Kang, C.; Tang, K.; Zeng, J.; Ding, M.; Guo, Z.; Lai, X.; et al. Combination RSL3 Treatment Sensitizes Ferroptosis- and EGFR-Inhibition-Resistant HNSCCs to Cetuximab. Int. J. Mol. Sci. 2022, 23, 9014. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Yi, C.; He, Y.; Chen, X.; Zhao, W.; Yu, D. Ferroptosis-related gene signature predicts the prognosis in Oral squamous cell carcinoma patients. BMC Cancer 2021, 21, 835. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Y.; Ying, Y.; Ding, Y. Current knowledge of ferroptosis in the pathogenesis and prognosis of oral squamous cell carcinoma. Cell. Signal. 2024, 119, 111176. [Google Scholar] [CrossRef]
- Gu, W.; Kim, M.; Wang, L.; Yang, Z.; Nakajima, T.; Tsushima, Y. Multi-omics Analysis of Ferroptosis Regulation Patterns and Characterization of Tumor Microenvironment in Patients with Oral Squamous Cell Carcinoma. Int. J. Biol. Sci. 2021, 17, 3476–3492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Tao, Y.; Huang, Q.; Chen, Z.; Jiang, L.; Yan, H.; Zhong, J.; Liang, L. Identification of ferroptosis-related genes as potential biomarkers of tongue squamous cell carcinoma using an integrated bioinformatics approach. FEBS Open Bio 2022, 12, 412–429. [Google Scholar] [CrossRef]
- Sun, K.; Ren, W.; Li, S.; Zheng, J.; Huang, Y.; Zhi, K.; Gao, L. MiR-34c-3p upregulates erastin-induced ferroptosis to inhibit proliferation in oral squamous cell carcinomas by targeting SLC7A11. Pathol. Res. Pract. 2022, 231, 153778. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, X.H.; Luan, K.F.; Huang, Y.D. Circular RNA FNDC3B Protects Oral Squamous Cell Carcinoma Cells From Ferroptosis and Contributes to the Malignant Progression by Regulating miR-520d-5p/SLC7A11 Axis. Front. Oncol. 2021, 11, 672724. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; Qin, H.; Lin, X.; Liu, X.; Zhang, H. The emerging significance of the METTL family as m6A-modified RNA methyltransferases in head and neck cancer. Cell. Signal. 2025, 132, 111798. [Google Scholar] [CrossRef]
- Sun, W.Y.; Tyurin, V.A.; Mikulska-Ruminska, K.; Shrivastava, I.H.; Anthonymuthu, T.S.; Zhai, Y.J.; Pan, M.H.; Gong, H.B.; Lu, D.H.; Sun, J.; et al. Phospholipase iPLA(2)β averts ferroptosis by eliminating a redox lipid death signal. Nat. Chem. Biol. 2021, 17, 465–476. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, S.; Cai, L.; Wang, Q.; Pan, D.; Dong, Y.; Zhou, H.; Li, J.; Ji, N.; Zeng, X.; et al. DRP1 inhibition-mediated mitochondrial elongation abolishes cancer stemness, enhances glutaminolysis, and drives ferroptosis in oral squamous cell carcinoma. Br. J. Cancer 2024, 130, 1744–1757. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, Y.; Xing, X.; Xiao, J.; Chen, H.; Zhang, H.; Wang, D.; Zhang, Y.; Zhang, G.; Wu, Z.; et al. Manganese-deposited iron oxide promotes tumor-responsive ferroptosis that synergizes the apoptosis of cisplatin. Theranostics 2021, 11, 5418–5429. [Google Scholar] [CrossRef]
- Han, L.; Li, L.; Wu, G. Induction of ferroptosis by carnosic acid-mediated inactivation of Nrf2/HO-1 potentiates cisplatin responsiveness in OSCC cells. Mol. Cell. Probes 2022, 64, 101821. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Lin, W.; Yi, Y.; Wu, H.; Yang, J.; Long, H.; Zou, G.; Wu, Y. Caveolin-1 modulates cisplatin sensitivity in oral squamous cell carcinoma through ferroptosis. Clin. Transl. Oncol. 2025, 27, 2160–2173. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, H.; Wang, X. Current advances on the role of ferroptosis in tumor immune evasion. Discov. Oncol. 2024, 15, 736. [Google Scholar] [CrossRef]
- Jiao, R.; Long, H. Ferroptosis: A New Challenge and Target in Oral Diseases. Oral Dis. 2025, 31, 1626–1636. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, L.; Guo, J.; Ma, J. The crosstalk between ferroptosis and anti-tumor immunity in the tumor microenvironment: Molecular mechanisms and therapeutic controversy. Cancer Commun. 2023, 43, 1071–1096. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Liu, C.L.; Li, X.M.; Shang, Y. Quercetin induces ferroptosis by inactivating mTOR/S6KP70 pathway in oral squamous cell carcinoma. Toxicol. Mech. Methods 2024, 34, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Chang, P.C.; Lin, Y.T.; Huang, P.T.; Chen, J.Y.; Lin, C.S.; Wu, B.N.; Chang, H.M.; Wu, W.J.; Chang, C.I.; et al. Repurposing of the Antipsychotic Trifluoperazine Induces SLC7A11/GPX4- Mediated Ferroptosis of Oral Cancer via the ROS/Autophagy Pathway. Int. J. Biol. Sci. 2024, 20, 6090–6113. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hua, C.; Chu, C.; Jiang, M.; Zhang, Q.; Zhang, Y.; Wu, L.; Liu, J.; Yang, H.; Yu, X.F.; et al. A photothermal-responsive multi-enzyme nanoprobe for ROS amplification and glutathione depletion to enhance ferroptosis. Biosens. Bioelectron. 2025, 278, 117384. [Google Scholar] [CrossRef]
- Wen, J.; Wang, J.; Wang, S.; Zhou, X.; Fu, Y. Characterization and application of fluorescent hydrogel films with superior mechanical properties in detecting iron(Ⅲ) ions and ferroptosis in oral cancer. Front. Bioeng. Biotechnol. 2024, 12, 1526877. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Xiao, S.; Lei, Y.; Zhou, Y.; Tan, J.; Chen, X.; Ma, D.; Liang, C.; Liu, Q.; Liu, W.; et al. The advancement of ubiquitination regulation in apoptosis, ferroptosis, autophagy, drug resistance and treatment of cancer. Arch. Biochem. Biophys. 2025, 771, 110497. [Google Scholar] [CrossRef]
- Kerkhove, L.; Geirnaert, F.; Dufait, I.; De Ridder, M. Ferroptosis: Frenemy of Radiotherapy. Int. J. Mol. Sci. 2024, 25, 3641. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.F.; Chaudhary, K.R.; Zandkarimi, F.; Harken, A.D.; Kinslow, C.J.; Upadhyayula, P.S.; Dovas, A.; Higgins, D.M.; Tan, H.; Zhang, Y.; et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem. Biol. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Noh, J.K.; Lee, M.K.; Lee, Y.; Bae, M.; Min, S.; Kong, M.; Lee, J.W.; Kim, S.I.; Lee, Y.C.; Ko, S.G.; et al. Targeting ferroptosis for improved radiotherapy outcomes in HPV-negative head and neck squamous cell carcinoma. Mol. Oncol. 2025, 19, 540–557. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef]
- Sant’Angelo, D.; Descamps, G.; Lecomte, V.; Stanicki, D.; Penninckx, S.; Dragan, T.; Van Gestel, D.; Laurent, S.; Journe, F. Therapeutic Approaches with Iron Oxide Nanoparticles to Induce Ferroptosis and Overcome Radioresistance in Cancers. Pharmaceuticals 2025, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shuai, Y.; Liu, Z.; Li, H.; Yin, Y. Astaxanthin Synergizes with Ionizing Radiation (IR) in Oral Squamous Cell Carcinoma (OSCC). Mol. Biotechnol. 2024, 66, 1220–1228. [Google Scholar] [CrossRef]
- Wang, C.W.; Biswas, P.K.; Islam, A.; Chen, M.K.; Chueh, P.J. The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC). Cells 2024, 13, 413. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, J.; Xiong, Y.; Su, K.; Liu, C.; Cheng, B.; Wu, T. Sulfasalazine combined with anti-IL-1β mAb induces ferroptosis and immune modulation in oral squamous cell carcinoma. Cell. Mol. Life Sci. 2025, 82, 216. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; Wang, C.; Sun, W.; Zheng, Y.; Ou, B.; Wu, L.; Shi, L.; Lin, X.; Chen, W. Ferroptosis boosted oral cancer photodynamic therapy by carrier-free Sorafenib-Ce6 self-assembly nanoparticles. J. Control. Release 2024, 366, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, K.; Gong, H.; Li, D.; Chu, W.; Zhao, D.; Wang, X.; Xu, D. Death by histone deacetylase inhibitor quisinostat in tongue squamous cell carcinoma via apoptosis, pyroptosis, and ferroptosis. Toxicol. Appl. Pharmacol. 2021, 410, 115363. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Li, X.; Tao, Z.; Zhu, W.; Su, Y.; Choi, W.S. Melatonin and erastin emerge synergistic anti-tumor effects on oral squamous cell carcinoma by inducing apoptosis, ferroptosis, and inhibiting autophagy through promoting ROS. Cell. Mol. Biol. Lett. 2023, 28, 36. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Li, D.; Deng, F.; Fu, S.; Chen, S.; Zou, H.; Wen, L.; Tang, Q. Brusatol modulates the Nrf2/GCLC pathway to enhance ferroptosis in the treatment of oral squamous cell carcinoma. Eur. J. Pharmacol. 2025, 1003, 177935. [Google Scholar] [CrossRef]
- Du, H.F.; Wu, J.W.; Zhu, Y.S.; Hua, Z.H.; Jin, S.Z.; Ji, J.C.; Wang, C.S.; Qian, G.Y.; Jin, X.D.; Ding, H.M. Fucoxanthin Induces Ferroptosis in Cancer Cells via Downregulation of the Nrf2/HO-1/GPX4 Pathway. Molecules 2024, 29, 2832. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Gao, B.; Chen, X. Baicalin induces ferroptosis in oral squamous cell carcinoma by suppressing the activity of FTH1. J. Gene Med. 2024, 26, e3669. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Q.; Wang, Y.; Liu, Y.; Li, Z.; Liu, Q.; Huang, Z.; Li, M.; Zhang, B.; Zhan, Q. Aqueous-soluble components of sporoderm-removed Ganoderma lucidum spore powder promote ferroptosis in oral squamous cell carcinoma. Chin. J. Cancer Res. 2023, 35, 176–190. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Pang, X.; Wang, W.L.; Wang, X.C.; Shen, Z.L.; Shi, R.J.; Tang, Y.L.; Liang, X.H. An injectable hydrogel with photothermal and chemodynamic therapies for targeted promotion of ferroptosis in oral squamous cell carcinoma. Nanoscale 2025, 17, 10277–10291. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, Z.; Wang, D.; Li, Y.; Hao, M.; Tao, B.; Feng, Q.; Wu, H.; Li, Q.; Wu, J.; et al. Iron Knights with Nanosword Induced Ferroptosis in the Battle Against Oral Carcinoma. Nano Lett. 2025, 25, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Chen, Y.; Chen, C.; Liu, M. CD44-targeted therapy using mP6/Rg3 micelles inhibits oral cancer stem cell proliferation and migration. Cell Biol. Toxicol. 2025, 41, 122. [Google Scholar] [CrossRef]
- Takami, Y.; Tomita, K.; Igarashi, K.; Kuwahara, Y.; Kitanaka, J.; Kitanaka, N.; Roudkenar, M.H.; Roushandeh, A.M.; Saijo, H.; Kurimasa, A.; et al. Amoxicillin, a β-lactam antibiotic, enhances cisplatin sensitivity in cancer cells affecting mitochondria. Biochem. Biophys. Res. Commun. 2025, 766, 151888. [Google Scholar] [CrossRef]
- Liu, J.; An, W.; Zhao, Q.; Liu, Z.; Jiang, Y.; Li, H.; Wang, D. Hyperbaric oxygen enhances X-ray induced ferroptosis in oral squamous cell carcinoma cells. Oral Dis. 2024, 30, 116–127. [Google Scholar] [CrossRef]
- Zhu, T.; Shi, L.; Yu, C.; Dong, Y.; Qiu, F.; Shen, L.; Qian, Q.; Zhou, G.; Zhu, X. Ferroptosis Promotes Photodynamic Therapy: Supramolecular Photosensitizer-Inducer Nanodrug for Enhanced Cancer Treatment. Theranostics 2019, 9, 3293–3307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Cai, Y.; Yuan, H.; Chen, Q.; Zhao, S.W.; Yang, J.; Liu, M.; Arreola, A.X.; Ren, Y.; Xu, C.; et al. Evaluation of Ferroptosis as a Biomarker to Predict Treatment Outcomes of Cancer Immunotherapy. Cancer Res. Commun. 2025, 5, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Long, H.Z.; Zhou, Z.W.; Luo, H.Y.; Xu, S.G.; Gao, L.C. System X(c) (-)/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front. Pharmacol. 2022, 13, 910292. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct. Target. Ther. 2023, 8, 372. [Google Scholar] [CrossRef]
- Lee, J.; Seo, Y.; Roh, J.L. Emerging Therapeutic Strategies Targeting GPX4-Mediated Ferroptosis in Head and Neck Cancer. Int. J. Mol. Sci. 2025, 26, 6452. [Google Scholar] [CrossRef]
- Dharini, S.; Ramani, P.; Doble, M.; Ramasubramanian, A. Ferroptosis Mediated Novel Drug Design Approach in the Treatment of Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2023, 24, 2321–2327. [Google Scholar] [CrossRef]
- Huang, K.J.; Wei, Y.H.; Chiu, Y.C.; Wu, S.R.; Shieh, D.B. Assessment of zero-valent iron-based nanotherapeutics for ferroptosis induction and resensitization strategy in cancer cells. Biomater. Sci. 2019, 7, 1311–1322. [Google Scholar] [CrossRef]
- Wang, S.; Bai, X.; Wang, X.; Wang, J.; Tao, W.; Gao, Y.; Ning, J.; Hao, J.; Gao, M. Metal Polyphenol Nanoparticle-Based Chemo/Ferroptosis Synergistic Therapy for the Treatment of Oral Squamous Cell Carcinoma. Bioconjug Chem. 2024, 35, 1835–1842. [Google Scholar] [CrossRef]
- Chang, Z.; Liang, Z.; Lan, Y.; Huang, J.; Feng, L.; Xu, J. Strategy of “Controllable Ions Interference” for Boosting MRI-Guided Ferroptosis Therapy of Tumors. ACS Appl. Mater. Interfaces 2025, 17, 11688–11703. [Google Scholar] [CrossRef]
- Huang, C.; Zhan, L. Network Pharmacology Identifies Therapeutic Targets and the Mechanisms of Glutathione Action in Ferroptosis Occurring in Oral Cancer. Front. Pharmacol. 2022, 13, 851540. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Hong, W.; Chen, X.; Pan, Y.; Weng, Y.; Liu, W.; Wang, L.; Qiu, S. Identification of a ferroptosis-associated gene signature and the related therapeutic targets in head and neck squamous carcinoma. Int. Immunopharmacol. 2022, 102, 108431. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, W.; You, H.; Zhang, M.; Ma, Y.; Liu, L.; Lin, M.; He, S.; Huang, Y. EZH2 knockout in mice activates STAT3 signalling via STAT3 methylation and modulates ferroptosis in pulpitis-affected dental pulp vascular endothelial cells: A laboratory investigation. Int. Endod. J. 2025, 58, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Chen, Z.; Zhang, M.; Luo, C.; Yang, B.; Lv, Y.; Li, Y.; Zeng, L.; Lin, W. Melatonin mitigates the lipopolysaccharide-induced myocardial injury in rats by blocking the p53/xCT pathway-mediated ferroptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Yu, H.; Liu, Z.; Zhou, B.; Fang, F.; Qiu, W.; Wu, H. Identification and characterization of the ferroptosis-related ceRNA network in irreversible pulpitis. Front. Immunol. 2023, 14, 1198053. [Google Scholar] [CrossRef]
- Xing, L.; Dong, W.; Chen, Y.; Dai, W.; Xiao, X.; Liu, Z.; Zhang, X.; Bai, D.; Xu, H. Fibroblast ferroptosis is involved in periodontitis-induced tissue damage and bone loss. Int. Immunopharmacol. 2023, 114, 109607. [Google Scholar] [CrossRef]
- Guan, P.; Ruan, Q.; Li, J.; Xi, M.; Qi, W.; Ko, K.I.; Ni, J. Ferroptosis in periodontitis: Mechanisms, impacts, and systemic connections. Cell Death Discov. 2025, 11, 283. [Google Scholar] [CrossRef]
- Torres, A.; Michea, M.A.; Végvári, Á.; Arce, M.; Pérez, V.; Alcota, M.; Morales, A.; Vernal, R.; Budini, M.; Zubarev, R.A.; et al. A multi-platform analysis of human gingival crevicular fluid reveals ferroptosis as a relevant regulated cell death mechanism during the clinical progression of periodontitis. Int. J. Oral Sci. 2024, 16, 43. [Google Scholar] [CrossRef]
- Youssef, L.A.; Rebbaa, A.; Pampou, S.; Weisberg, S.P.; Stockwell, B.R.; Hod, E.A.; Spitalnik, S.L. Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood 2018, 131, 2581–2593. [Google Scholar] [CrossRef]
- Luo, X.; Gong, H.B.; Gao, H.Y.; Wu, Y.P.; Sun, W.Y.; Li, Z.Q.; Wang, G.; Liu, B.; Liang, L.; Kurihara, H.; et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021, 28, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Lee, Y.B.; Shin, H.W.; Shrestha, S.; Kim, J.K.; Jung, S.J.; Shin, M.S.; Hong, C.W. Ferroptosis in neutrophils. J. Leukoc. Biol. 2025, 117, qiaf039. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Xiang, X.; Chen, C. Development and Validation of a Ferroptosis-Related lncRNAs Prognosis Model in Oral Squamous Cell Carcinoma. Front. Genet. 2022, 13, 847940. [Google Scholar] [CrossRef] [PubMed]

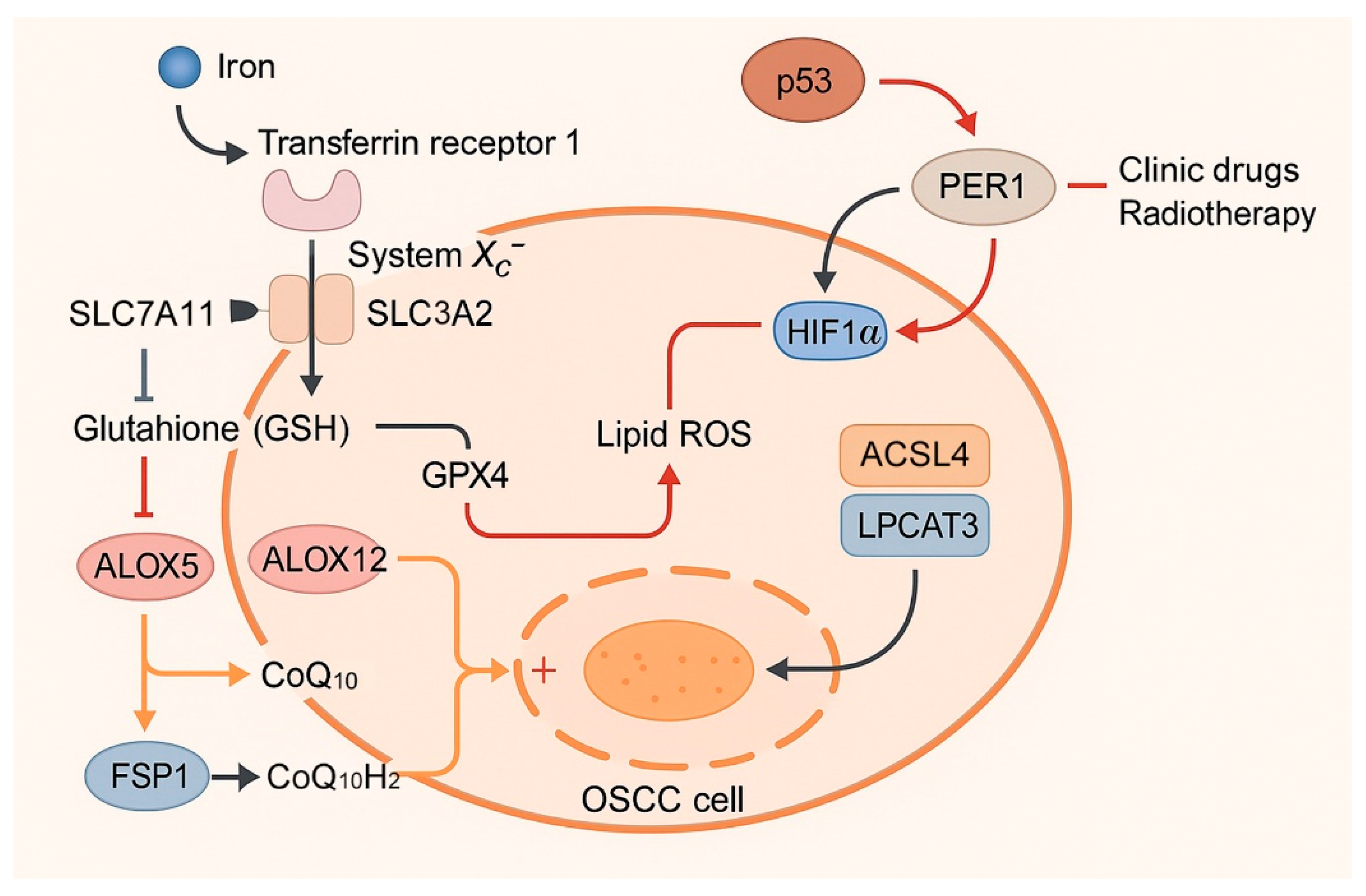

| Category | Molecule/ Pathway | Function in OSCC | Effect on Ferroptosis | Expression Pattern in Cell Line(S) | References |
|---|---|---|---|---|---|
| Antioxidant defenses | GPX4 | Detoxifies lipid hydroperoxides | Inhibits ferroptosis | Upregulated (confers resistance) | [77] |
| SLC7A11 (system Xc−) | Imports cystine for GSH synthesis | Inhibits ferroptosis | Upregulated | [25] | |
| SLC3A2 (system Xc− partner) | Forms heterodimer with SLC7A11; regulates amino acid transport, immune evasion, and metastasis in OSCC | Inhibits ferroptosis | Downregulated in immune evasion | [78] | |
| FSP1 | Regenerates CoQ10H2 via NADPH | Inhibits ferroptosis | Upregulated in drug-tolerant cells | [23,79] | |
| Nrf2 (NFE2L2) | Activates GPX4/SLC7A11, HO-1 | Mostly inhibits ferroptosis | Upregulated/hyperactivated | [66,80] | |
| Transcription factors | Nrf1 (NFE2L1) → HJURP | Binds HJURP promoter, upregulates GPX4/SLC7A11, reduces lipid ROS, promotes OSCC proliferation and resistance | Inhibits ferroptosis | Upregulated | [81] |
| Tumor suppressors | p53 | Represses SLC7A11 | Promotes ferroptosis | Mutated or inactivated (↓ functional) | [82] |
| PER1 → HIF-1α | Reduces HIF-1α, ROS/LPO | Promotes ferroptosis | Downregulated | [24] | |
| TCF12 | Represses OTUB1, destabilizes SLC7A11, restores cisplatin sensitivity | Promotes ferroptosis | Downregulated | [61] | |
| Oncogenes | AEBP1 | Activates JNK/p38/ERK | Inhibits ferroptosis | Upregulated | [83] |
| PPT1 | Stabilizes GPX4 | Inhibits ferroptosis | Upregulated | [32] | |
| CK19 | Suppresses ACSL4 | Inhibits ferroptosis | Upregulated | [54] | |
| CDH4 (R-cadherin) | Upregulated in OSCC; enhances proliferation, migration, EMT; elevates GPX4 and GSH, reduces lipid peroxidation; poor survival correlation | Inhibits ferroptosis | Upregulated | [84] | |
| Metabolic enzymes | ACSL4, LPCAT3 | Incorporate PUFAs into membranes | Promote ferroptosis | Downregulated | [26] |
| TPI1 | Reduces ROS/LPO, cisplatin resistance | Inhibits ferroptosis | Upregulated | [31] | |
| Epigenetic factors | circ_0000140/miR-527/SLC7A11 | Upregulates SLC7A11, cisplatin resistance | Inhibits ferroptosis | Upregulated | [30] |
| miR-26a-5p | Targets SLC7A11 3′UTR, decreases cystine uptake and GSH, sensitizes OSCC to ferroptosis | Promotes ferroptosis | Downregulated | [60] | |
| STARD4-AS1 (lncRNA) | Enhances proliferation, LPO | Inhibits ferroptosis | Upregulated | [29] | |
| METTL3 (m6A methyltransferase) | Stabilizes SLC7A11 mRNA via m6A modification, enhancing ferroptosis resistance | Inhibits ferroptosis | Upregulated | [85,86] | |
| FTO (m6A demethylase) | Destabilizes ACSL3 and GPX4 mRNA via m6A demethylation, enhancing ferroptosis | Promotes ferroptosis | Downregulated | [87] | |
| Protein quality control | HSPA5 (GRP78) | ER chaperone; its downregulation accompanies ferroptosis in OTSCC | Inhibits ferroptosis (loss → ↑ferroptosis) | Downregulated during RSL3 or erastin treatment | [34] |
| Immune checkpoint | PD-L1 (intrinsic function) | Activates SOD2, maintains redox homeostasis; loss increases ferroptosis and immunogenic death | Inhibits ferroptosis | Upregulated | [88] |
| TME-derived regulators | PDPN+ CAF–exosomal lncRNA FTX | Transfers to OSCC cells, forms FTX/FEN1 complex, suppresses ACSL4-mediated ferroptosis, enhances motility and invasiveness | Inhibits ferroptosis | Upregulated in CAF-rich OSCC | [89] |
| ER stress regulators | REEP6 | Maintains ER homeostasis, downregulates ACSL4, confers resistance to RSL3 | Inhibits ferroptosis | Upregulated | [90] |
| Transcriptional co-activators | YAP/TAZ–ACSL4 axis | Activated by manganese; phase separation promotes ACSL4 activation; correlates with survival | Promotes ferroptosis | Upregulated under Mn exposure | [53] |
| Strategy | Agent/Example | Mechanism | Preclinical OSCC Evidence | References |
|---|---|---|---|---|
| System Xc− inhibitors | Erastin, sulfasalazine | Block cystine uptake → ↓GSH/GPX4 | Induce ferroptosis, restore cisplatin sensitivity | [97,119] |
| GPX4 inhibitors | RSL3, FIN56 | Direct GPX4 inhibition → ↑LPO | Ferroptotic death in CAL27, SCC9, HSC3 | [87] |
| Iron modulators | Ferritinophagy activators | ↑Labile Fe2+, lipid ROS | Enhance ferroptosis | [45] |
| Repurposed drugs | Artesunate | Iron-dependent ROS, ↑LIP | Induces ferroptosis, synergy with cisplatin | [32,66] |
| Sorafenib | System Xc− inhibitor | Enhances cisplatin sensitivity | [120] | |
| Trifluoperazine (TFP) | GPX4 inhibition, autophagy | Induces ferroptosis, poor prognosis with GPX4high | [108] | |
| Quisinostat (HDACi) | ROS stress, lipid peroxidation | Sensitizes OSCC to ferroptosis | [121] | |
| Disulfiram (±Cu) | Nrf2/HO-1 modulation | Promotes ferroptosis in OSCC | [67] | |
| Melatonin | ROS amplification, mitochondrial stress | Potentiates erastin-induced ferroptosis | [122] | |
| Natural compounds | Piperlongumine | ↓GPX4/SLC7A11, ↑ROS | Suppresses growth, synergizes with CB-839 | [33] |
| Evodia lepta extract | ↓GPX4/HSPA5, ↓PD-L1 | Cytotoxic and immunomodulatory | [34] | |
| Quercetin | Inactivates mTOR/S6K | Induces ferroptosis, enhances cisplatin | [107] | |
| Brusatol | Inhibits Nrf2/GCLC, ↓SLC7A11, GSH depletion | Promotes ferroptosis, suppresses OSCC growth in vitro and in vivo | [123] | |
| Resveratrol | Activates p53, represses SLC7A11, ↓GSH, ↑Fe2+/ROS | Induces ferroptosis, inhibits OSCC proliferation and invasion | [82] | |
| Fucoxanthin | Downregulates Nrf2/HO-1/GPX4, ↑ROS, ↑Fe2+, ↑p53 | Induces ferroptosis in SCC-25 tongue carcinoma cells | [124] | |
| Baicalin | Suppresses FTH1, ↓EMT, ↑ferroptosis | Inhibits proliferation and invasion in OSCC cells | [125] | |
| Ganoderma lucidum spore powder (A-GSP) | ↑Fe2+ influx, GSH depletion, ↑ACSL4, ↓GPX4; induces mitochondrial dysfunction | Induces ferroptosis, suppresses OSCC growth in vivo | [126] | |
| Nanomedicine | MnO2-RSL3 NPs | ROS amplification, GSH depletion | “Explosive” ferroptosis in vivo | [109] |
| Carbon dot–hydrogel films | Fe3+ detection + ferroptosis | Theranostic system in OSCC | [110] | |
| Fe-dopamine composites | Fenton-like ROS generation | Induce lipid peroxidation | [127] | |
| Sorafenib–Ce6 nanoparticles | Photodynamic + ferroptosis | Overcome hypoxia resistance | [120] | |
| Exo-AuMn nanoclusters | ROS generation, immune targeting | Selective ferroptosis + imaging | [128] | |
| CD44-targeted mP6/Rg3 micelles | Inhibit ABCB1, promote ferroptosis in cancer stem cells | Suppress CSC proliferation, migration, and OSCC growth in vitro and in vivo | [129] | |
| Combination therapies | Cisplatin + RSL3/erastin | Chemo-ferroptosis synergy | Overcome cisplatin resistance | [26,30] |
| Evodia lepta + cisplatin | GPX4/HSPA5 suppression + cytotoxicity | Enhance chemosensitivity | [34] | |
| Carnosic acid + cisplatin | Inactivation of Nrf2/HO-1 | Reverse cisplatin resistance | [102] | |
| Amoxicillin + cisplatin | Mitochondrial dysfunction, ferroptosis | Enhance cisplatin efficacy | [130] | |
| RSL3 + LYN-1604 | Autophagy + ferroptosis induction | Synergistic tumor suppression | [36] | |
| Radiotherapy + ferroptosis inducers | Radiation-induced ROS + ferroptosis | Radiosensitization in OSCC | [113,114] | |
| Hyperbaric oxygen + ionizing radiation | Suppresses GPX4, enhances ferroptosis | Re-sensitizes radio-resistant OSCC cells, improves tumor control | [131] | |
| Photodynamic therapy: Ce6–erastin nanodrug | Relieves hypoxia, inhibits SLC7A11, sustained ROS via Fenton reaction | Enhances PDT efficacy in CAL-27 and xenograft models | [132] | |
| Astaxanthin + ionizing radiation | Inhibits GPX4/SLC7A11, ↑ACSL4, ↑ferroptosis | Enhances radiosensitivity in OSCC cells and xenografts | [117] | |
| Immunotherapy + ferroptosis | PD-L1 downregulation, immune activation | Enhance ICI efficacy | [34,133] |
| Domain | Current Evidence | Potential Clinical Impact | Future Directions | References |
|---|---|---|---|---|
| OSCC prognosis | FRG signatures; circRNAs/lncRNAs (e.g., circ_0000140, STARD4-AS1); 8 ferroptosis-related lncRNAs model; FGS validated in HNSCC/OSCC, linked to CD276+ fibroblasts and ATG5-mediated immune exclusion | Biomarker-driven risk stratification; prediction of immunotherapy responsiveness | Large prospective validation | [76,92,142,154] |
| Bioinformatics/systems biology | Network pharmacology identified GSH-related ferroptosis targets (EGFR, PTGS2, HIF1A, SLC3A2, etc.) | Provides candidate targets for therapy and biomarkers | Integrate in silico predictions with experimental validation | [141] |
| Chemoresistance | Ferroptosis suppressed in cisplatin-resistant OSCC; FSP1 upregulated in drug-tolerant persister cells | Restore cisplatin sensitivity via FINs, ncRNA targeting, FSP1 inhibition in persister cells | Clinical trials combining cisplatin + FINs | [26,30,79] |
| Radiotherapy | Radiation induces lipid ROS, ferroptosis enhances radiosensitivity | Radiosensitization | Nanoparticle-mediated ROS amplification | [113,114] |
| Immunotherapy | Ferroptosis boosts immunogenicity, but may impair T cells | Guide ICI combinations | Balance tumor vs. immune ferroptosis | [34,93] |
| Non-malignant diseases | Pulpitis, periodontitis linked to ferroptosis-driven injury | Ferroptosis inhibitors may protect tissues | Disease-specific studies | [6,148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Roh, J.-L. Ferroptosis in Oral Cancer: Mechanistic Insights and Clinical Prospects. Cells 2025, 14, 1685. https://doi.org/10.3390/cells14211685
Lee J, Roh J-L. Ferroptosis in Oral Cancer: Mechanistic Insights and Clinical Prospects. Cells. 2025; 14(21):1685. https://doi.org/10.3390/cells14211685
Chicago/Turabian StyleLee, Jaewang, and Jong-Lyel Roh. 2025. "Ferroptosis in Oral Cancer: Mechanistic Insights and Clinical Prospects" Cells 14, no. 21: 1685. https://doi.org/10.3390/cells14211685
APA StyleLee, J., & Roh, J.-L. (2025). Ferroptosis in Oral Cancer: Mechanistic Insights and Clinical Prospects. Cells, 14(21), 1685. https://doi.org/10.3390/cells14211685






