The Effect of Nutritional Supplementation in Ex Vivo Lung Perfusion Perfusate on Human Lung Endothelial Cell Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Mathematical Modeling
2.2. Experimental Perfusate Preparation
2.3. Cell Culture Screening Model
2.4. Cellular Apoptosis
2.5. Cellular Confluence
2.6. Cellular Migration
2.7. Cell Viability
2.8. Statistical Analysis
3. Results
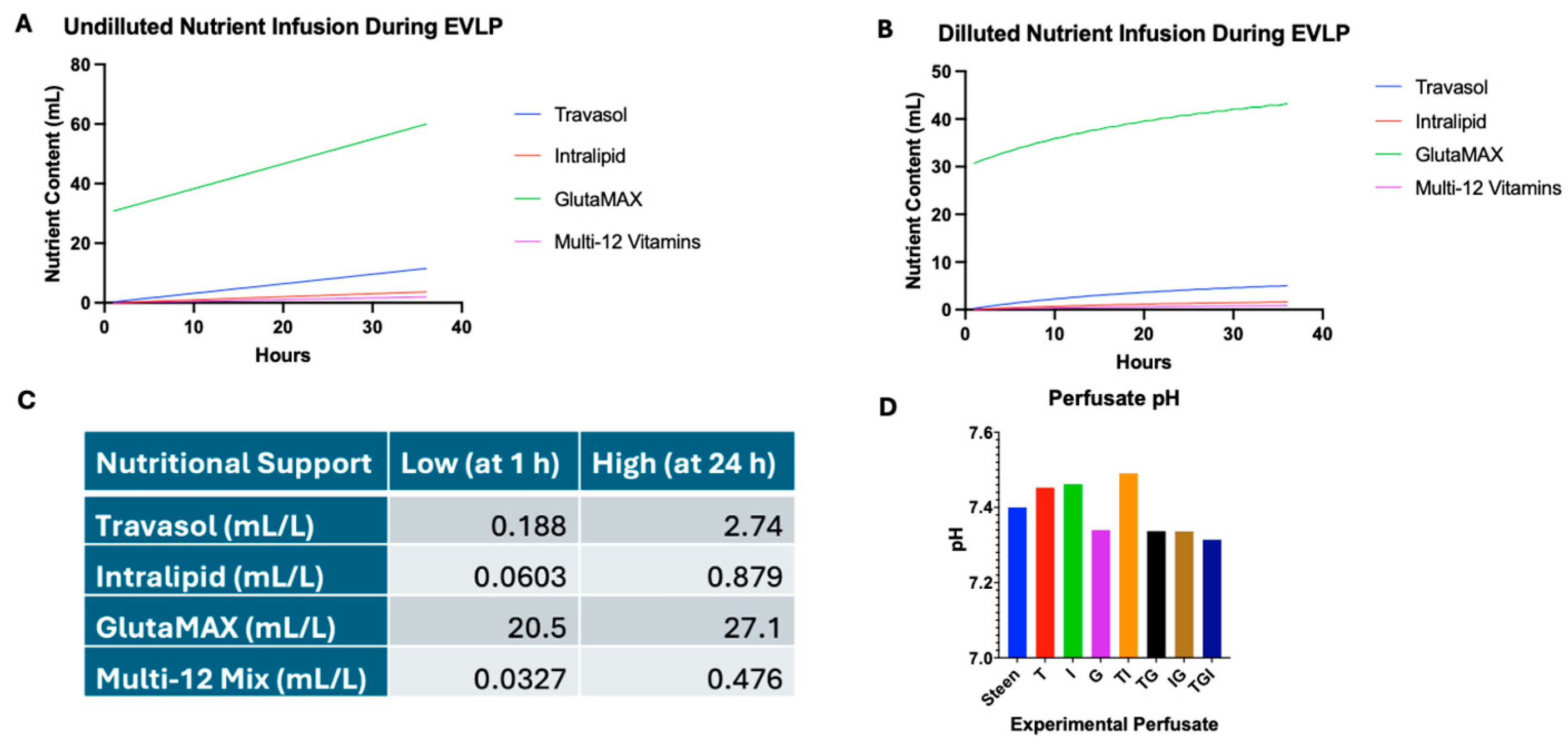
3.1. Identifying Nutrient Concentration for Experimental Perfusates
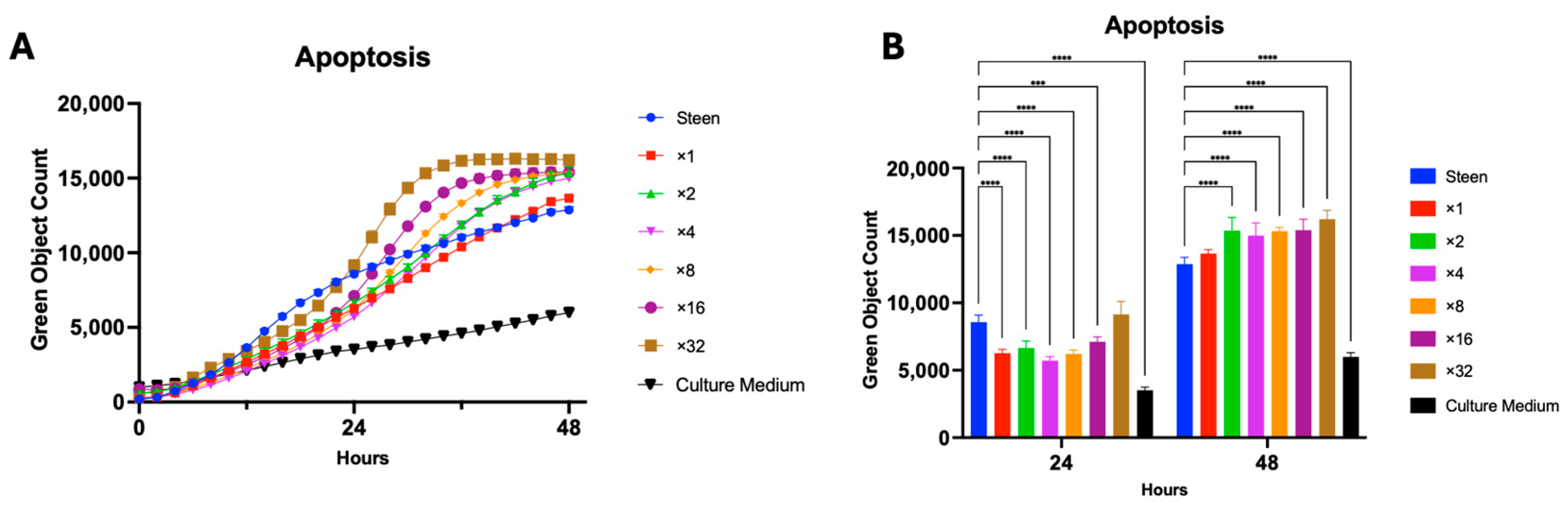
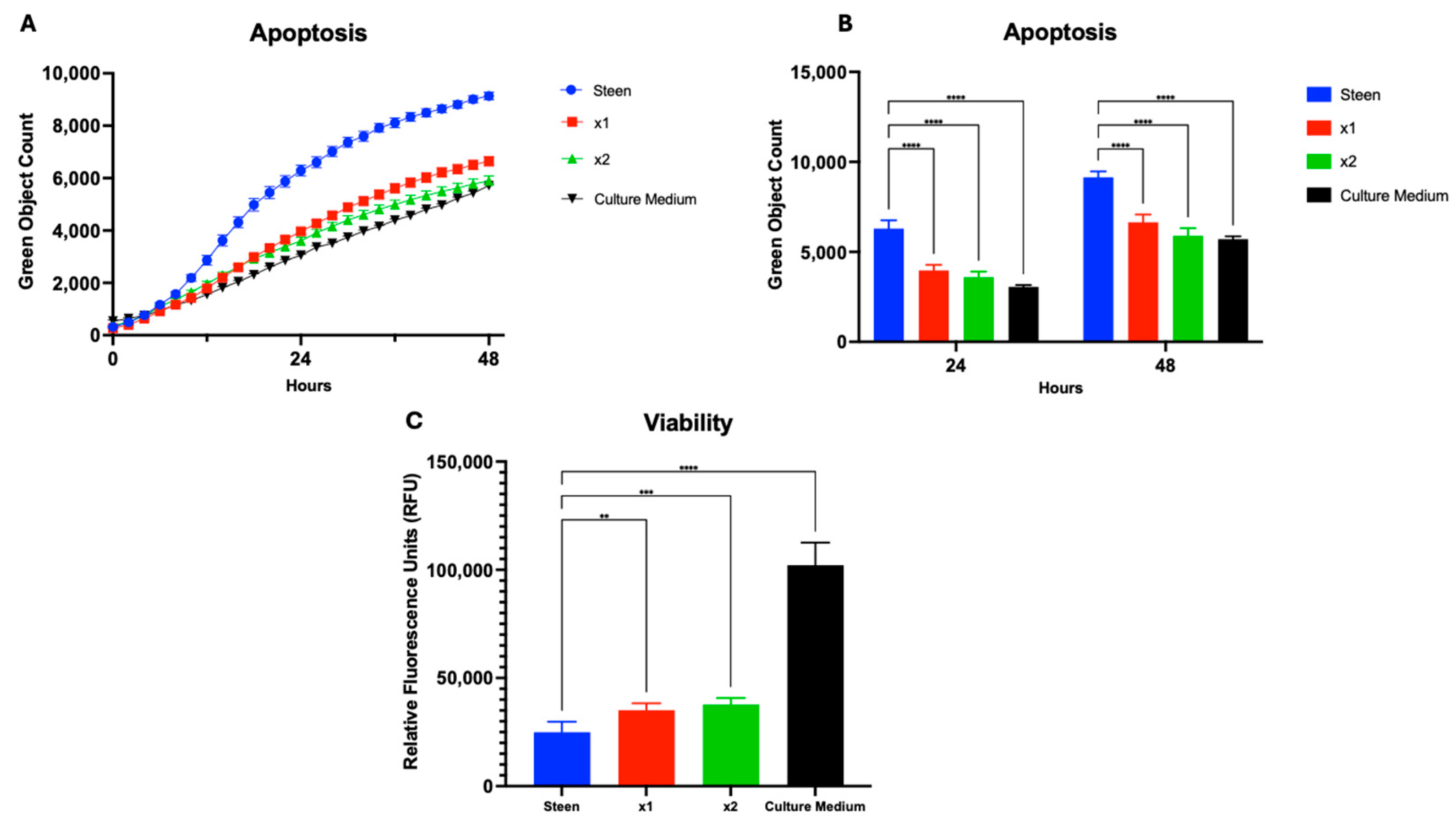
3.2. GlutaMAX Supplementation Protects Cells from Apoptosis Better than Individual TPN Components
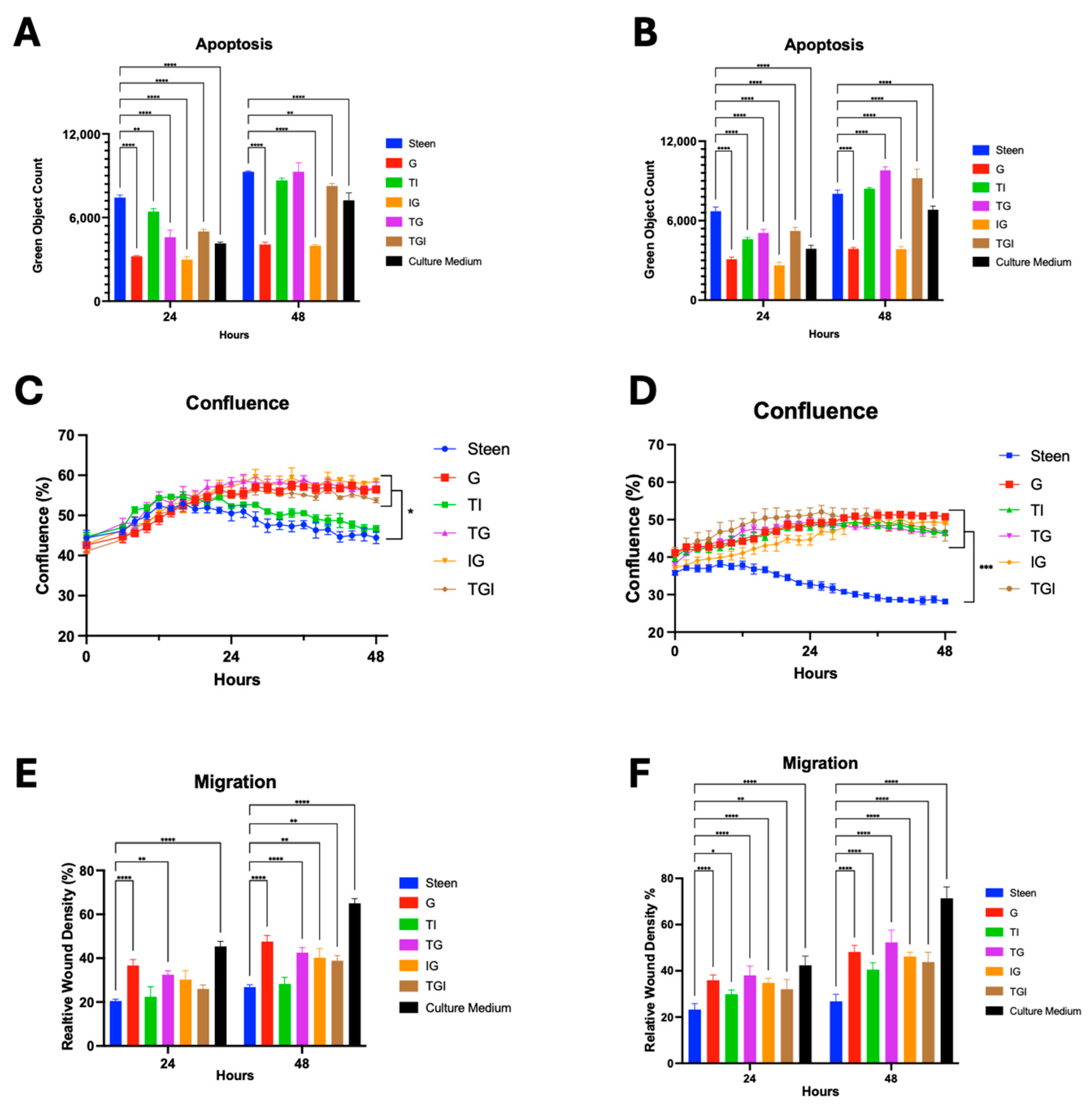
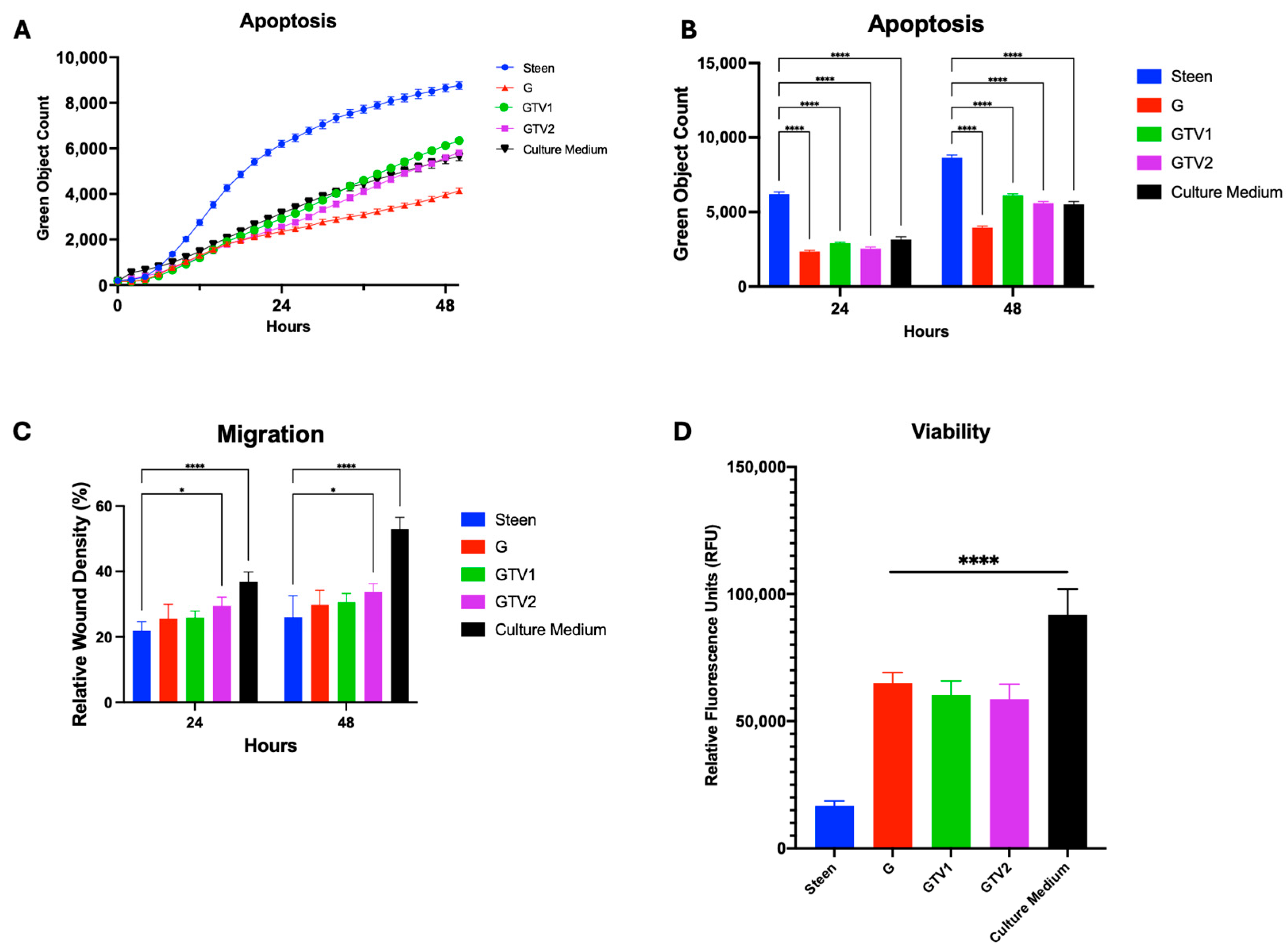
3.3. Combined Nutrient Experimental Perfusates Do Not Outperform GlutaMAX Supplemented Perfusate
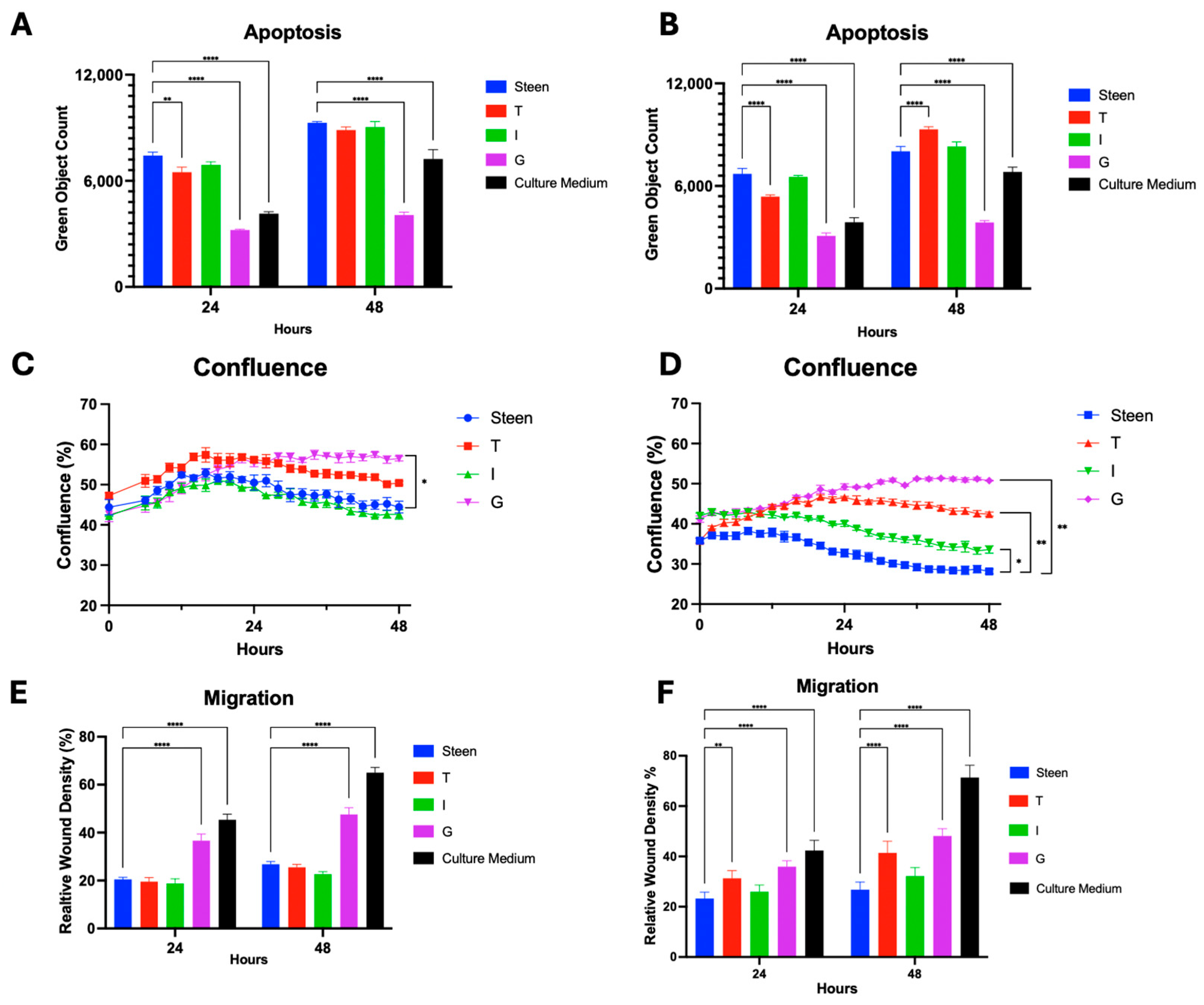
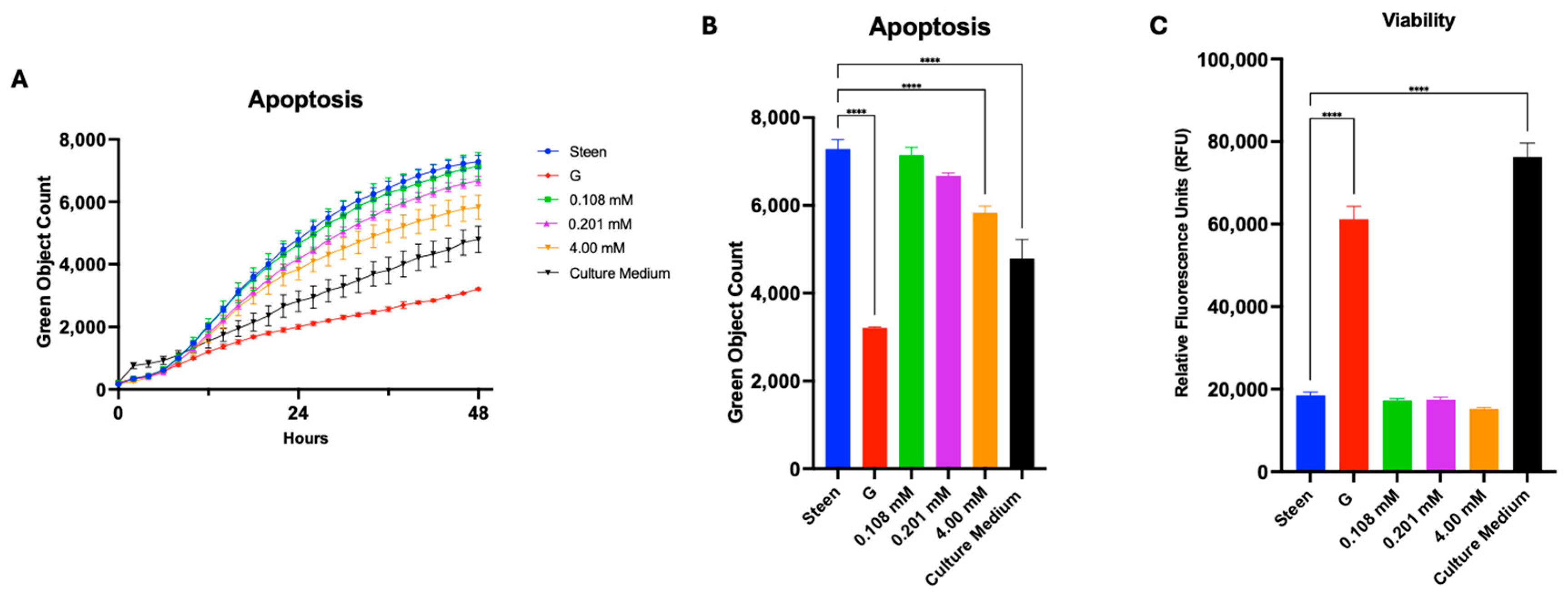
3.4. Vitamin Supplementation Improves Basic Cell Function for 48 h
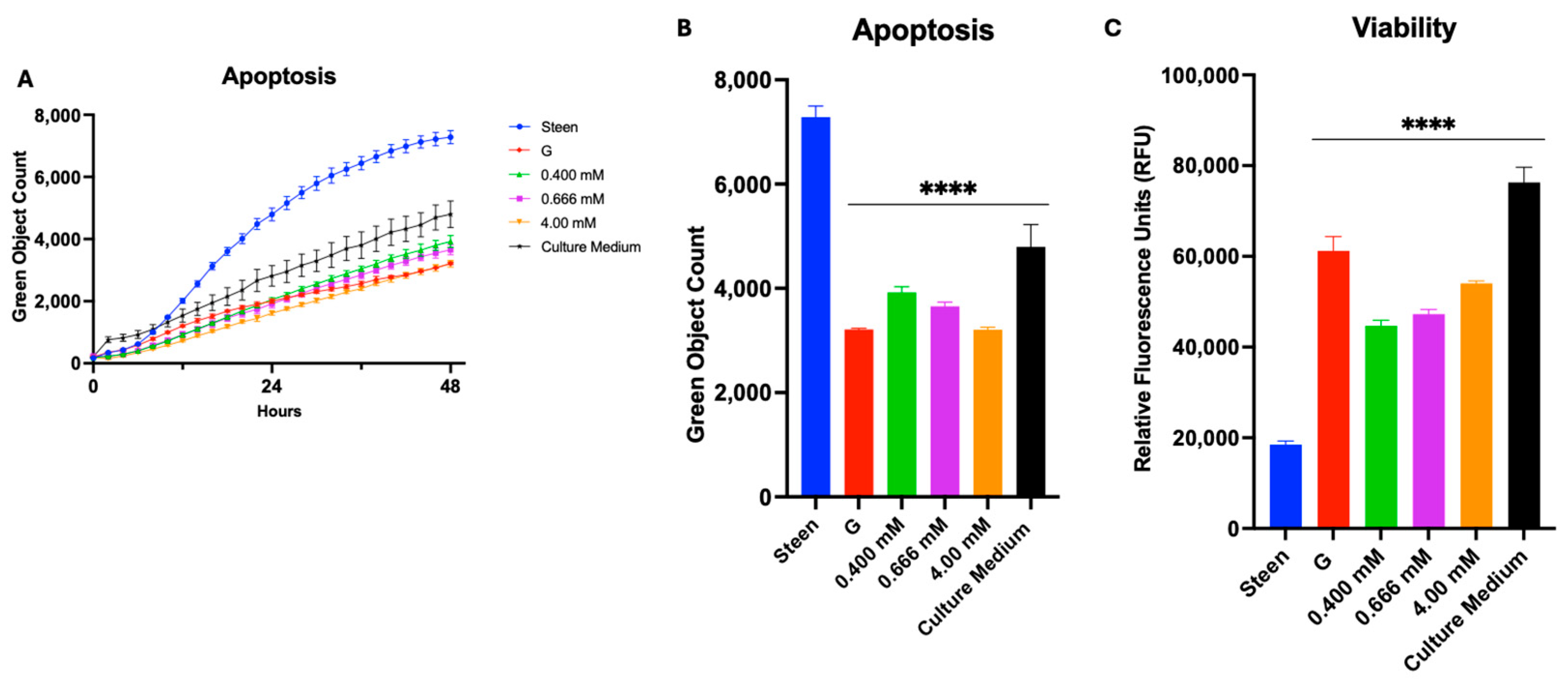
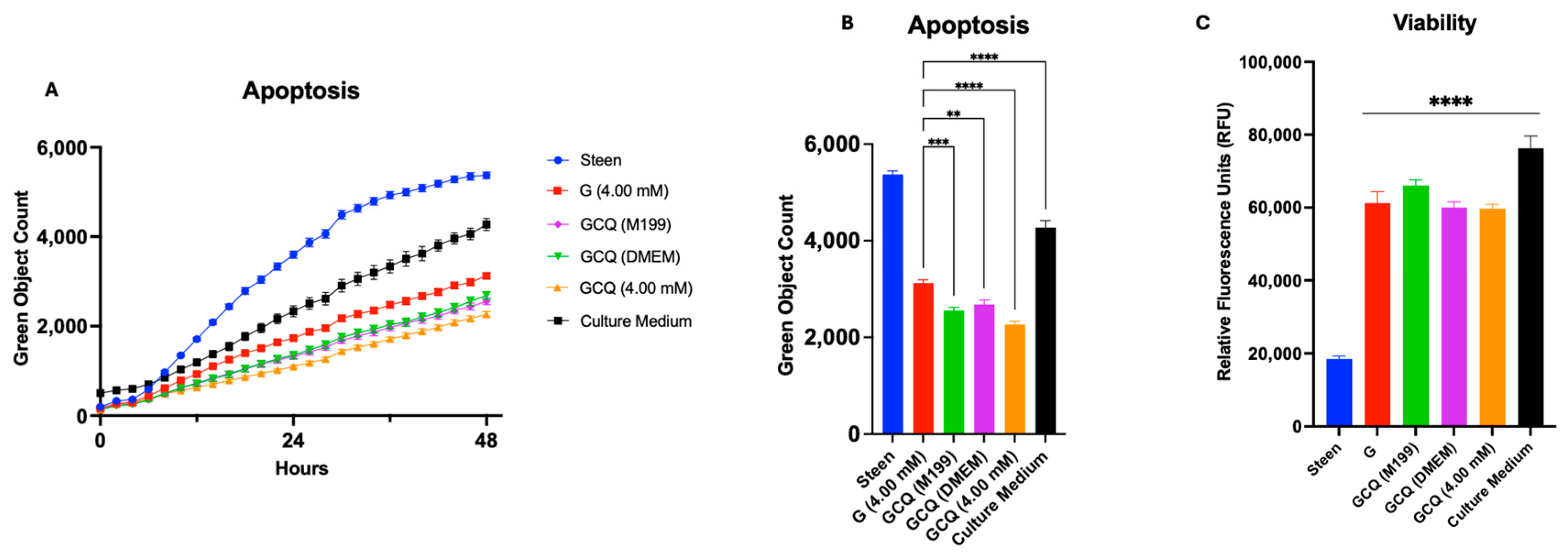
3.5. GlutaMAX, Cysteine, and Glycine Supplementation Synergistically Reduces Apoptosis and Improves Viability
4. Discussion
4.1. Cell Culture Model for Developing New EVLP Perfusates
4.2. TPN-Based Supplementation to EVLP Perfusates
4.3. GSH Nutritional Supplementation of Perfusates Offers Better Support
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valapour, M.; Lehr, C.J.; Schladt, D.P.; Smith, J.M.; Goff, R.; Mupfudze, T.G.; Swanner, K.; Gauntt, K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Lung. Am. J. Transplant. 2023, 23, S379–S442. [Google Scholar] [CrossRef]
- Kendzerska, T.; Nickerson, J.W.; Hsu, A.T.; Gershon, A.S.; Talarico, R.; Mulpuru, S.; Pakhale, S.; Tanuseputro, P. End-of-life care in individuals with respiratory diseases: A population study comparing the dying experience between those with chronic obstructive pulmonary disease and lung cancer. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1691–1701. [Google Scholar] [CrossRef]
- Hornby, K.; Ross, H.; Keshavjee, S.; Rao, V.; Shemie, S.D. Non-utilization of hearts and lungs after consent for donation: A Canadian multicentre study. Can. J. Anaesth. 2006, 53, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Ingemansson, R.; Eyjolfsson, A.; Mared, L.; Pierre, L.; Algotsson, L.; Ekmehag, B.; Gustafsson, R.; Johnsson, P.; Koul, B.; Lindstedt, S.; et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann. Thorac. Surg. 2009, 87, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Pluhacek, J.L.; Brown, A.W. Ex Vivo Lung Perfusion: A Review of Current and Future Application in Lung Transplantation. Pulm. Ther. 2022, 8, 149–165. [Google Scholar] [CrossRef]
- Watanabe, T.; Cypel, M.; Keshavjee, S. Ex vivo lung perfusion. J. Thorac. Dis. 2021, 13, 6602–6617. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.; Noda, K.; Chan, E.G.; Ryan, J.P.; Furukawa, M.; Hage, C.A.; Sanchez, P.G. The Relationship Between Ex Vivo Lung Perfusion Strategies and Transplantation Outcomes: Insights from the United Network for Organ Sharing Data. Transplantation 2025, 109, 1016–1025. [Google Scholar] [CrossRef]
- Boffini, M.; Ricci, D.; Barbero, C.; Bonato, R.; Ribezzo, M.; Mancuso, E.; Attisani, M.; Simonato, E.; Magistroni, P.; Mansouri, M.; et al. Ex vivo lung perfusion increases the pool of lung grafts: Analysis of its potential and real impact on a lung transplant program. Transplant. Proc. 2013, 45, 2624–2626. [Google Scholar] [CrossRef]
- Gao, Q.; Kahan, R.; Gonzalez, T.J.; Zhang, M.; Alderete, I.S.; DeLaura, I.; Kesseli, S.J.; Song, M.; Asokan, A.; Barbas, A.S.; et al. Gene delivery followed by ex vivo lung perfusion using an adeno-associated viral vector in a rodent lung transplant model. J. Thorac. Cardiovasc. Surg. 2024, 167, e131–e139. [Google Scholar] [CrossRef]
- Nykänen, A.I.; Keshavjee, S.; Liu, M. Creating superior lungs for transplantation with next-generation gene therapy during ex vivo lung perfusion. J. Heart Lung Transplant. 2024, 43, 838–848. [Google Scholar] [CrossRef]
- Nakajima, D.; Watanabe, Y.; Ohsumi, A.; Pipkin, M.; Chen, M.; Mordant, P.; Kanou, T.; Saito, T.; Lam, R.; Coutinho, R.; et al. Mesenchymal stromal cell therapy during ex vivo lung perfusion ameliorates ischemia-reperfusion injury in lung transplantation. J. Heart Lung Transplant. 2019, 38, 1214–1223. [Google Scholar] [CrossRef]
- Lin, H.; Chen, M.; Tian, F.; Tikkanen, J.; Ding, L.; Cheung, H.Y.A.; Nakajima, D.; Wang, Z.; Mariscal, A.; Hwang, D.; et al. α1-Anti-trypsin improves function of porcine donor lungs during ex-vivo lung perfusion. J. Heart Lung Transplant. 2018, 37, 656–666. [Google Scholar] [CrossRef]
- Mariscal, A.; Nykanen, A.; Tikkanen, J.; Ali, A.; Soltanieh, S.; Duong, A.; Galasso, M.; Juvet, S.; Martinu, T.; Cypel, M.; et al. Alpha 1 Antitrypsin Treatment during Human Ex Vivo Lung Perfusion Improves Lung Function by Protecting Lung Endothelium. J. Heart Lung Transplant. 2020, 39, S71–S72. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Hirayama, S.; Rubacha, M.; Fischer, S.; Anraku, M.; Sato, M.; Harwood, S.; Pierre, A.; Waddell, T.K.; et al. Technique for Prolonged Normothermic Ex Vivo Lung Perfusion. J. Heart Lung Transplant. 2008, 27, 1319–1325. [Google Scholar] [CrossRef]
- Takahashi, M.; Andrew Cheung, H.Y.; Watanabe, T.; Zamel, R.; Cypel, M.; Liu, M.; Keshavjee, S. Extended Pig EVLP Research Group Strategies to prolong homeostasis of ex vivo perfused lungs. J. Thorac. Cardiovasc. Surg. 2021, 161, 1963–1973. [Google Scholar] [CrossRef]
- Ali, A.; Nykanen, A.I.; Beroncal, E.; Brambate, E.; Mariscal, A.; Michaelsen, V.; Wang, A.; Kawashima, M.; Ribeiro, R.V.P.; Zhang, Y.; et al. Successful 3-day lung preservation using a cyclic normothermic ex vivo lung perfusion strategy. EBioMedicine 2022, 83, 104210. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic Ex Vivo Lung Perfusion in Clinical Lung Transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef]
- Shin, J.; Hsin, M.K.; Baciu, C.; Chen, Y.; Zamel, R.; Machuca, T.; Yeung, J.; Cypel, M.; Keshavjee, S.; Liu, M. Use of metabolomics to identify strategies to improve and prolong ex vivo lung perfusion for lung transplants. J. Heart Lung Transplant. 2021, 40, 525–535. [Google Scholar] [CrossRef]
- Berlana, D. Parenteral Nutrition Overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef]
- Buchko, M.T.; Stewart, C.J.; Hatami, S.; Himmat, S.; Freed, D.H.; Nagendran, J. Total parenteral nutrition in ex vivo lung perfusion: Addressing metabolism improves both inflammation and oxygenation. Am. J. Transplant. 2019, 19, 3390–3397. [Google Scholar] [CrossRef]
- Huang, L.; Hough, O.; Vellanki, R.N.; Takahashi, M.; Zhu, Z.; Xiang, Y.-Y.; Chen, M.; Gokhale, H.; Shan, H.; Soltanieh, S.; et al. L-alanyl-L-glutamine modified perfusate improves human lung cell functions and extend porcine ex vivo lung perfusion. J. Heart Lung Transplant. 2023, 42, 183–195. [Google Scholar] [CrossRef]
- Hough, O.; Mariscal, A.; Yamamoto, H.; Mangat, H.; Taniguchi, D.; Gokhale, H.; Chen, M.; Shan, H.; Bojic, D.; Aulja, T.; et al. Improved ex Vivo Lung Perfusion (EVLP) with Dialysis and Nutrition to Achieve Successful 36h EVLP and Lung Transplantation. J. Heart Lung Transplant. 2023, 42, S60. [Google Scholar] [CrossRef]
- Krump-Konvalinkova, V.; Bittinger, F.; Unger, R.E.; Peters, K.; Lehr, H.A.; Kirkpatrick, C.J. Generation of human pulmonary microvascular endothelial cell lines. Lab. Investig. 2001, 81, 1717–1727. [Google Scholar] [CrossRef]
- Abunnaja, S.; Cuviello, A.; Sanchez, J.A. Enteral and Parenteral Nutri-tion in the Perioperative Period: State of the Art. Nutrients 2013, 5, 608–623. [Google Scholar] [CrossRef]
- Petrat, F.; Boengler, K.; Schulz, R.; de Groot, H. Glycine, a simple physi-ological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: Current knowledge. Br. J. Pharmacol. 2012, 165, 2059–2072. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Ami-no Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef]
- Raichani, N.E.; Guiraut, C.; Morin, G.; Mohamed, I.; Lavoie, J.-C. Stability of glutathione added as a supplement to parenteral nutrition. J. Parenter. Enter. Nutr. 2022, 46, 1080–1087. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Vellanki, R.N.; Zhu, Z.; Wouters, B.G.; Keshavjee, S.; Liu, M. De Novo Design and Development of a Nutrient-Rich Perfusate for Ex Vivo Lung Perfusion with Cell Culture Models. Int. J. Mol. Sci. 2023, 24, 13117. [Google Scholar] [CrossRef]
- Chilvers, N.J.S.; Gilmour, J.; Brown, M.L.; Bates, L.; Pang, C.Y.; Pauli, H.; Dark, J.; Fisher, A.J. A Split-Lung Ex Vivo Perfusion Model for Time- and Cost-Effective Evaluation of Therapeutic Interventions to the Human Donor Lung. Transpl. Int. 2024, 37, 12573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojic, D.; Main, K.; Aujla, T.; Hough, O.; Keshavjee, S.; Liu, M. The Effect of Nutritional Supplementation in Ex Vivo Lung Perfusion Perfusate on Human Lung Endothelial Cell Function. Cells 2025, 14, 1668. https://doi.org/10.3390/cells14211668
Bojic D, Main K, Aujla T, Hough O, Keshavjee S, Liu M. The Effect of Nutritional Supplementation in Ex Vivo Lung Perfusion Perfusate on Human Lung Endothelial Cell Function. Cells. 2025; 14(21):1668. https://doi.org/10.3390/cells14211668
Chicago/Turabian StyleBojic, Dejan, Kimberly Main, Tanroop Aujla, Olivia Hough, Shaf Keshavjee, and Mingyao Liu. 2025. "The Effect of Nutritional Supplementation in Ex Vivo Lung Perfusion Perfusate on Human Lung Endothelial Cell Function" Cells 14, no. 21: 1668. https://doi.org/10.3390/cells14211668
APA StyleBojic, D., Main, K., Aujla, T., Hough, O., Keshavjee, S., & Liu, M. (2025). The Effect of Nutritional Supplementation in Ex Vivo Lung Perfusion Perfusate on Human Lung Endothelial Cell Function. Cells, 14(21), 1668. https://doi.org/10.3390/cells14211668





