Abstract
Pulmonary arterial hypertension (PAH) is a fatal disease with no cure. Until recently, most specific therapies for PAH had aimed at enhancing cyclic nucleotide (cAMP and cGMP) pathways, taking advantage of the vasorelaxant and antiproliferative properties of these key intracellular messengers. This process can be achieved by inhibiting phosphodiesterases (PDEs), which are intracellular enzymes responsible for cyclic nucleotide degradation. To date, only inhibitors of PDE type 5 (PDE5) have been approved for the treatment of PAH. Because the PDE superfamily comprises 11 families that encompass many variants, substantial experimental investigation has been conducted to assess the relevance of inhibiting other PDE families, aiming to offer therapeutic alternatives. This review synthesizes the main research work conducted on in vivo or ex vivo models, as well as on biological resources from patients. It helps provide evidence for the expression of PDE isoforms in the lung vasculature, as well as the efficacy and limitations of various pharmacological compounds tested for inhibiting pathological processes ongoing in the disease. Perspectives and suggestions for future research orientation are proposed.
1. Pulmonary Arterial Hypertension
1.1. Introduction
While pulmonary arterial hypertension (PAH) is a rare disease (48–55 cases/million), it is life-threatening and non-curable [1]. In PAH, progressive elevation of pulmonary vascular resistance leads to right heart failure and premature death. Over the last few decades, a few specific therapeutic options have improved survival rates and patient quality of life but have remained insufficient in reversing the progression of the disease. Therefore, research efforts have focused on better understanding the mechanisms of the disease and the diversification of therapeutic targets. Recently, sotatercept, a first-in-class activin signalling inhibitor, has yielded promising results in clinical trials [2,3]. Sotatercept can now be combined with classical PAH therapies [4], and future clinical investigations will aim at refining the strategies for optimal efficacy and safety. Drugs that promote the beneficial effects of cyclic nucleotide signalling in the lung vasculature, namely prostacyclin (PGI2) analogues and phosphodiesterase-type 5 inhibitors (PDE5i), remain important pillars of the disease management [4]. In the context of therapeutic research, since modulation of cyclic nucleotides can be achieved by various pharmacological means, it is crucial to investigate further options in order to optimize their potential. In particular, cyclic nucleotide phosphodiesterases (PDEs), as a very large and diverse family of druggable enzymes, may provide further insights for disease management [5]. This review synthesizes the research that sought to extend the field of PDEs as relevant pharmacological targets in PAH, beyond the approved class of PDE5i.
1.2. Definition, Classification, and Pathophysiology of PAH
Pulmonary hypertension (PH) is a heterogeneous cardiopulmonary disease, characterized hemodynamically by a mean pulmonary arterial pressure (mPAP) higher than 20 mmHg at rest, standardly measured by right heart catheterization [6]. PH results from a myriad of clinical conditions and thus is divided into five groups, which are: pulmonary arterial hypertension (PAH, group I), PH associated with left heart disease (group II), PH associated with lung diseases and/or hypoxia (group III), PH associated with pulmonary artery obstructions (group IV), and PH with unclear and/or multifactorial mechanisms (group V) [6] (Table 1). PAH is diagnosed by the concomitant presence of mPAP > 20 mmHg, pulmonary arterial wedge pressure ≤ 15 mmHg, and pulmonary vascular resistance > 2 WU at rest, and exclusion of groups III, IV, and V PH [6]. PAH is a primary disorder of the pulmonary vasculature and includes patients with similar pathophysiological, histological, and prognostic features, of variable causes. PAH is further classified into 6 subgroups, according to etiology or clinical manifestations (Table 1).

Table 1.
Clinical classification of pulmonary hypertension (PH) [6].
The pathological manifestations of pulmonary vasculopathy include hypercoagulation, vasoconstriction, remodelling of the pulmonary vessel wall caused by endothelial hyperproliferation or plexiform lesions, hyperplasia of pulmonary arterial smooth muscle cells (PASMCs), and hypertrophy. This is featured by inflammatory cell recruitment and fibrosis in the adventitia, as well as immune dysregulation [7].
1.3. Current Therapeutic Options
Prior to 1995, there were no specific treatments for PAH. Pharmacological management mainly used L-type calcium-channel blockers, anticoagulation therapy, digitalis, and diuretics to relieve symptoms of right ventricular failure. Consistently, the prognosis of PAH was very poor, with a 1-, 3-, and 5-year survival of 68%, 48% and 34%, respectively, and the median survival was 2.8 years [8]. The new pharmacological classes that emerged mainly aim to rescue the function of the pulmonary endothelium, which is impaired in PAH.
Endothelial dysfunction is typically characterized by chronically impaired bioavailability of paracrine, vasodilating mediators, such as nitric oxide (NO) and PGI2. Meanwhile, expression of some vasoconstrictors, such as endothelin-1 (ET-1), is increased. Treatment strategies were developed to target these specific pathways (reviewed in [9]): first, enhancing PGI2 pathway using pharmacological analogues (epoprostenol, treprostinil, and iloprost) or a synthetic agonist of the IP receptor, namely selexipag; second, enhancing NO-3′,5′ cyclic guanosine monophosphate (cGMP) pathway using PDE5i (sildenafil and tadalafil) or soluble guanylyl cyclase (sGC) stimulator (riociguat); third, repressing ET-1 signalling by using receptors antagonists (bosentan, ambrisentan, and macitentan). These pharmacotherapies have largely improved PAH survival [10,11], although the 5-year survival rate for these patients remains less than 60% [12,13]. Lung transplantation or heart–lung transplantation is regarded as the only way to cure this disease. Recently, sotatercept, a biological therapy approved by the FDA in 2024, has been developed to tackle the disease mechanism through a novel pathophysiological angle. This drug aims to impede the TGF-β–activin–nodal branch signalling by binding to ligands of deleterious downstream receptors. This would improve the balance toward the beneficial bone morphogenetic protein (BMP)–growth differentiation factor (GDF) branch signalling and therefore prevent the deleterious remodelling of the pulmonary arterial wall that underlies PAH [14]. The Phase 3 STELLAR clinical trial showed that sotatercept increases exercise capacity (6-min walk distance), improves WHO functional class and NT-proBNP levels, and reduces the risk of clinical worsening events in PAH patients [2]. Sotatercept is now part of the therapeutic arsenal that can be used in PAH [4]. The three classes previously described, administered initially or sequentially as part of a bi- or tri-therapy, are still recommended for managing moderate to severe PAH as shown in clinical guidelines [4,6]. In addition, the management of PAH may benefit from increased clinical experience, refinement of the treatment algorithm, and increased access to pharmaceuticals due to patent expirations and the advent of generic drugs.
2. Cyclic Nucleotide Pathways as Therapeutic Targets in PAH
2.1. General Roles of Cyclic Nucleotide Pathways in Circulation
Cyclic nucleotides, namely 3′, 5′-cyclic adenosine monophosphate (cAMP) and 3′, 5′-cyclic guanosine monophosphate (cGMP), are ubiquitous second messengers involved in the intracellular signal transduction of specific extracellular stimuli (summarized in Figure 1). In the cardiovascular system, they are key regulators of multiple normal biological processes such as excitation-contraction coupling, blood vessel vasodilation, and natriuresis. In pulmonary circulation, cAMP and cGMP contribute to the highly dilated status of the pulmonary artery (PA) [9,15,16], thus ensuring low pulmonary vascular resistance and PA pressure. Importantly, cyclic nucleotides also interfere with some pathological processes, including tissue remodelling or inflammation. In the vasculature, cAMP- and cGMP-mediated pathways are generally regarded as protective because they mediate vasodilation, exert antiproliferative and pro-apoptotic effects on smooth muscle cells, inhibit platelet activity, and maintain the endothelial barrier [17,18,19,20,21]. Enhancing both cAMP and cGMP pathways results in promoting BMPR2 and Smad1/5 signalling, which are important for maintenance of the pulmonary vascular integrity [22,23]. Because sotatercept also aims to restore these pivotal mechanisms, cyclic nucleotide-based therapies may provide supplemental benefit in targeting the TGF-β axis.
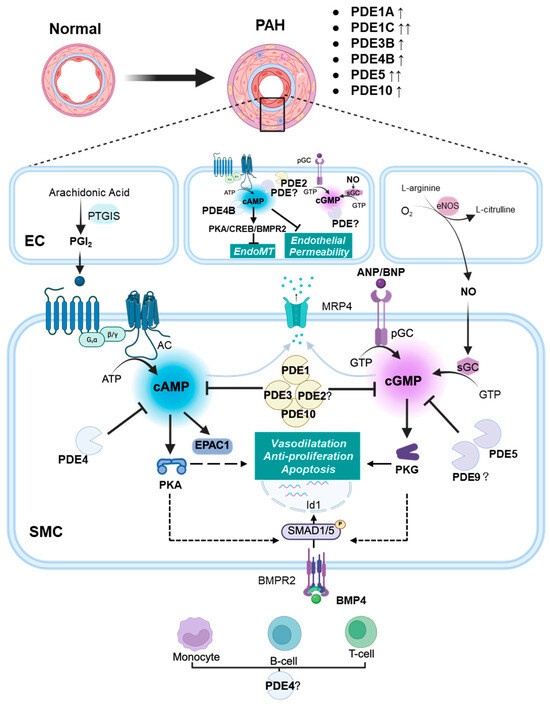
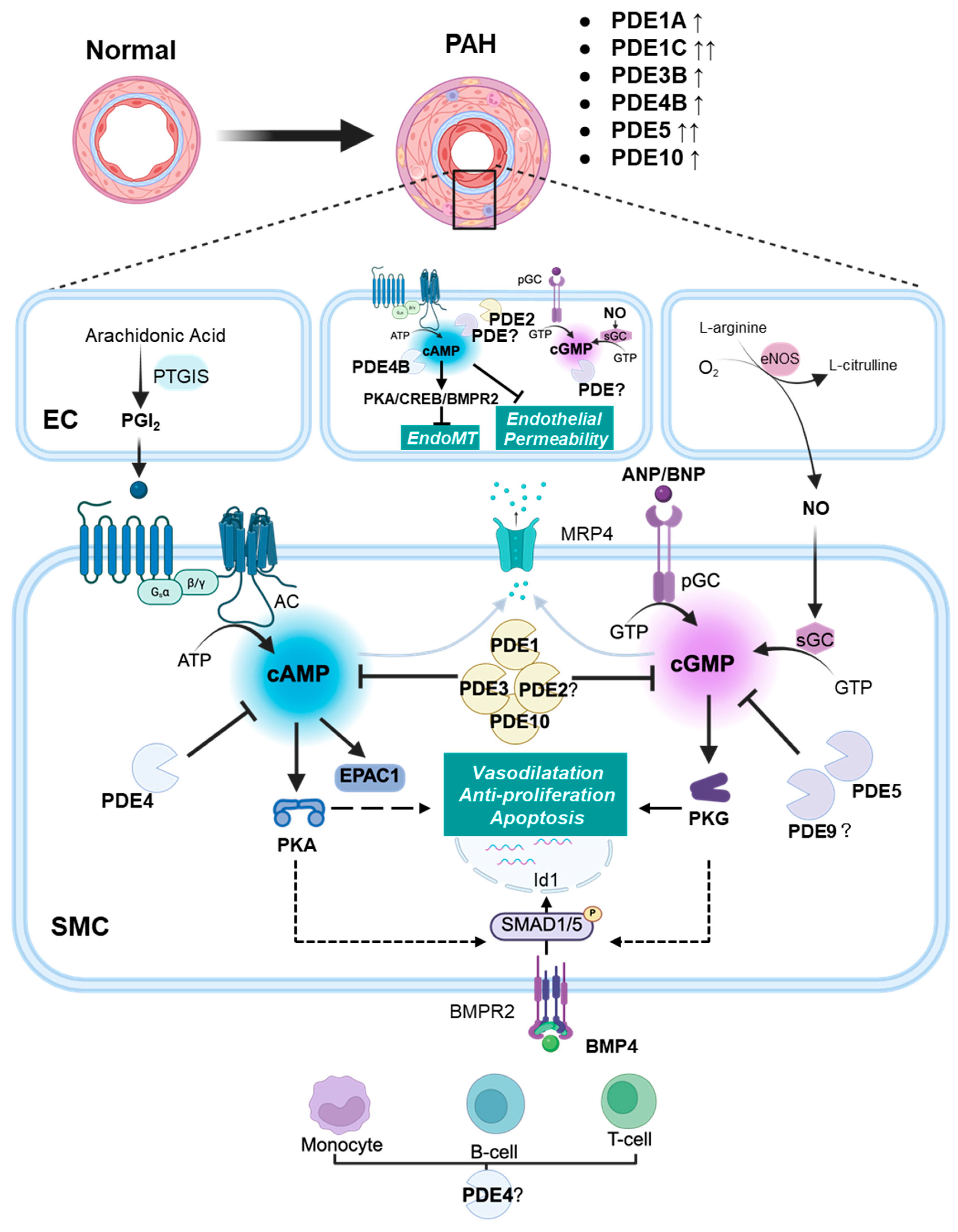
Figure 1.
Schematic diagram illustrating the role of PDEs in pulmonary arterial hypertension (PAH). In the pulmonary artery, prostacyclin (PGI2) stimulates the production of cAMP, which subsequently activates downstream effectors such as PKA and EPAC1. Cyclic-GMP is generated either by NO-mediated stimulation of sGC or via the stimulation of pGC by natriuretic peptides ANP and BNP. Cyclic-GMP activates PKG. In endothelial cells (ECs), cAMP mitigates endothelial permeability and EndoMT. Within pulmonary artery smooth muscle cells (PASMCs), cyclic nucleotides exert their effects by activating PKG and PKA, targeting multiple downstream pathways involved in vasodilation, cell proliferation inhibition, and apoptosis. The pathway downstream of BMPR2 signalling is promoted by cAMP and cGMP. The reduction in cAMP and cGMP levels occurs either via degradation by PDEs or through export from PASMCs by MRP4. In PAH, upregulation of specific PDEs (e.g., PDE1A, PDE1C, PDE3B, PDE4B, PDE5A, and PDE10A) accelerates the degradation of cAMP and cGMP. This attenuates the beneficial effects of cyclic nucleotides in ECs and PASMCs. In the adventitia, upregulation of PDEs (possibly PDE4) may also contribute to immune cell activation and inflammation, further exacerbating PAH. Solid lines represent direct actions; dashed lines represent indirect actions. AC: adenylyl cyclase; BMPR2: bone morphogenetic protein receptor 2; EC: endothelial cell; EndoMT: endothelial-to-mesenchymal transition; EPAC1: exchange protein activated by cAMP 1; eNOS: endothelial nitric oxide synthase; Id1: inhibitor of DNA binding protein 1; NO: nitric oxide; MRP4: multidrug resistance-associated protein 4; PDE: phosphodiesterase; pGC: particulate guanylyl cyclase; PASMC: pulmonary artery smooth muscle cell; PKA: cAMP-activated protein kinase; PKG: cGMP-activated protein kinase; PGI2: prostacyclin; PTGIS: PGI2 synthase; sGC: soluble guanylyl cyclase; ↑: modest increase in expression; ↑↑: strong increase in expression.
Therefore, consensus in PAH is to favour both pathways in pulmonary circulation as an attempt to reduce the progressive increase in pulmonary vascular resistance (Figure 2).
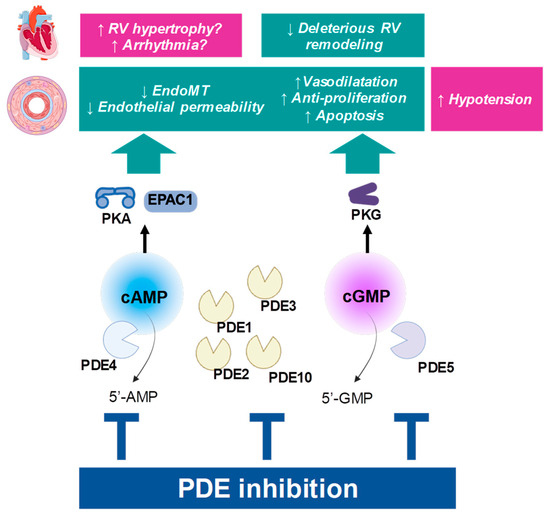
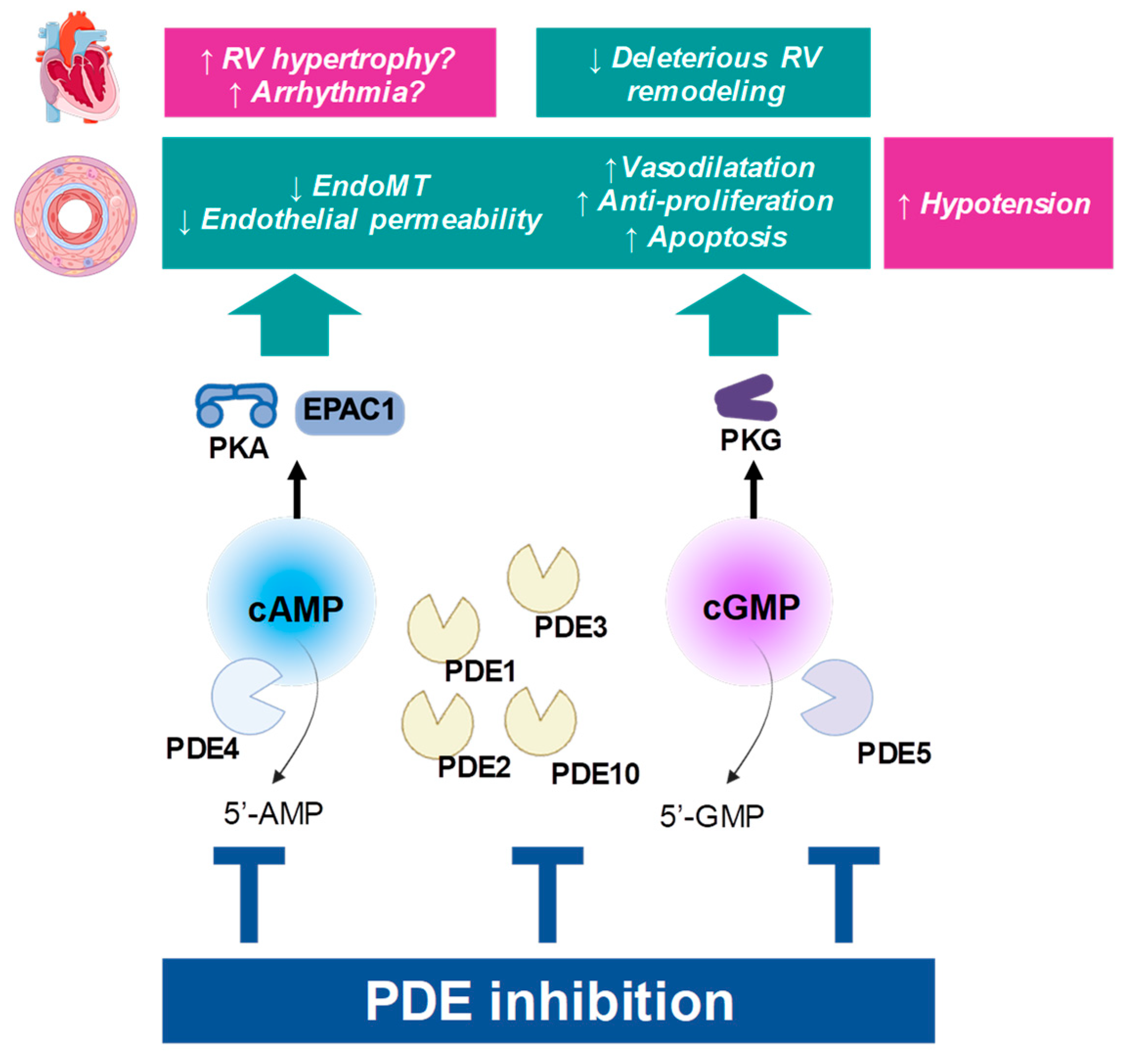
Figure 2.
Principle of PDE inhibition. Beneficial influence on PAH pathophysiology is depicted in green boxes, while possible cardiovascular adverse effects are presented in pink boxes. “↑”: the function is increased; “↓”: the function is decreased; RV: right ventricle.
Stimulation of cGMP may also favour the condition of the right ventricle (RV), by limiting cardiac deleterious remodelling of the myocardium [24,25]. By contrast, stimulating cAMP in the cardiac myocytes is considered deleterious, as it promotes hypertrophy, cell death, and cardiac fibrosis [24]. Importantly, strategies aiming at preserving the cAMP pathway in PAH may be limited and may have to be restricted to the vascular territory.
The biological activity of cyclic nucleotides directly depends on their respective intracellular levels. Thus, therapeutic interventions modulate these pathways by altering the balance between the rates of synthesis or elimination of cAMP and cGMP.
2.2. Molecular Determinants of the cAMP and cGMP Pathway
2.2.1. Synthesis of cGMP and cAMP
The modalities of cAMP and cGMP synthesis have been extensively described in dedicated reviews [15,16,17,26,27], and will only be summarized here and in Figure 1.
cGMP
Synthesis of cGMP in vascular smooth muscle cells is catalyzed by two classes of enzymes, which convert GTP into cGMP: (i) sGC, a cytosolic heme-containing heterodimer, typically produces cGMP upon binding to nitric oxide (NO); (ii) particulate guanylate cyclases (pGC), which comprise 2 membrane receptors for natriuretic peptides (NPs), namely GC-A and GC-B (also called NPR-A, -B). These membrane proteins produce cGMP by an intracellular catalytic activity. GC-A and GC-B can be stimulated by different NPs, including ANP, BNP or CNP, with various potencies [17,26]. All these pathways were reported to be active in PA [15,16,28]. The cGMP-activated protein kinase (PKG) is one important effector of cGMP.
cAMP
Synthesis of cAMP from ATP is achieved by adenylyl cyclases (ACs). There are nine membrane-bound AC isoforms and one soluble (sAC) isoenzyme, each with distinct expression and regulatory profiles [29]. Several isoforms were described in PASMCs, with variations among species [30,31]. ACs are classically stimulated following stimulation of G(α)s protein-coupled receptors by their respective agonists. In vascular cells, such receptors include β-adrenoreceptors, IP receptors (stimulated by PGI2), or A2 adenosine receptors, among others [19,32]. In the vasculature, cAMP primarily exerts its effects via the activation of cAMP-activated protein kinase (PKA) and exchange protein activated by cAMP-1 (EPAC1) [19].
2.2.2. Hydrolysis of cGMP and cAMP by PDEs
Intracellular levels of cAMP and cGMP are mainly mitigated by the PDEs. These enzymes hydrolyze cyclic nucleotides into non-cyclic 5′-GMP or 5′-AMP, which are unable to activate cyclic nucleotide effectors. PDEs comprise a superfamily of enzymes classified into 11 families (Table 2), altogether encoded by more than 20 genes [5,33,34,35]. PDE families notably differ by their affinity (Km) to either cyclic nucleotide: some PDEs (types 5, 6, and 9) are cGMP specific, some are cAMP-specific (PDE4, PDE7, PDE8), while others are dual specific (Table 2). PDE families also distinguish themselves through various regulatory mechanisms, including possibilities for post-translational modifications, and feedback regulation by cGMP or cAMP through binding to “GAF” domains (Table 2). Additionally, as cGMP interacts with some PDEs that mitigate cAMP, crosstalk between pathways can be established (see Section 3.2 and Section 3.3).
In addition to PDEs, export of cyclic nucleotides by multidrug resistance-associated protein 4 (MRP4, also known as Abcc4) was demonstrated in arterial smooth muscle cells [36]. This mechanism is of pathophysiological relevance because MRP4 expression has been found to increase in PAH, and the inhibition or deletion of MRP4 has been shown to prevent the development of hypoxia-induced PH in mice [37].

Table 2.
Properties of PDEs and their distribution in the normal pulmonary artery.
Table 2.
Properties of PDEs and their distribution in the normal pulmonary artery.
| PDE | Gene | Km (µM) [38] | Substrate Selectivity and Salient Features | Pulmonary Artery Activity/Expression | Inhibitors (IC50, nM) |
|---|---|---|---|---|---|
| PDE1 | PDE1A | cAMP: 73–120 cGMP: 2.6–5 | dual substrate Ca2+/calmodulin-regulated | Vessel: (+) [39,40,41,42] VSMC: (+) [43,44,45] (PDE1C highly expressed in proliferative phenotype) [43] EC: ND | 8MM-IBMX (NS) vinpocetine: 14,000 |
| PDE1B | cAMP: 10–24 cGMP: 1.2–5.9 | ||||
| PDE1C | cAMP: 0.3–1.2 cGMP: 0.6–2.2 | ||||
| PDE2 | PDE2A | cAMP: 30–50 cGMP: 10–30 | dual substrate cGMP-stimulated | Vessel: (+) [39,40,42,46,47] VSMC: (+) [44] EC: (+) [48] | EHNA: 800 Bay 60-7550: 4.7 |
| PDE3 | PDE3A PDE3B | cAMP: 0.02–0.15 cGMP: 0.18 | dual substrate cGMP-inhibited | Vessel: (++) [39,40,42] VSMC: (+) [44,49] EC: (+) [48] | cilostamide: 25–50 cilostazol: 200 milrinone: 150 |
| PDE4 | PDE4A PDE4B PDE4C PDE4D | cAMP: 2.9–10 | cAMP-selective | Vessel: (++) [39,40,42] VSMC: (++) [44] EC: (+) [48] | rolipram:1000 cilomilast: 70–120 roflumilast: 0.4–0.6 |
| PDE5 | PDE5A | cGMP: 1–6.2 | cGMP-binding, cGMP-selective | Vessel: (+++) [39,40,42] VSMC: (+++) [43,49,50] EC: ND | zaprinast: 500–700 sildenafil: 5 vardenafil: 1 tadalafil: 5 |
| PDE6 | PDE6A | cGMP: 15–17 | cGMP-selective, photoreceptor | not expressed [34] | ND |
| PDE7 | PDE7A PDE7B | cAMP: 0.1–0.2 | cAMP-selective | VSMC: (+) (mRNA) [51,52] EC: ND | rolipram-insensitive |
| PDE8 | PDE8A PDE8B | cAMP: 0.04–0.06 | cAMP-selective | VSMC: (+) (human mRNA) [52]; (−) (rat mRNA) [51] EC: ND | ND |
| PDE9 | PDE9A | cGMP: 0.17–0.39 | cGMP-selective | VSMC: (±) (mRNA) [51,52] EC: ND | Bay 73-6691: 55 PF-04447943: 2.8 |
| PDE10 | PDE10A | cAMP: 0.26 cGMP: 7.2 | cAMP-inhibited, dual substrate | VSMC: (+) (mRNA) [52]; (−) (rat mRNA) [51]; (immunoreactivity) [53] EC: ND | papaverine: 36 |
| PDE11 | PDE11A | cAMP: 1.04–5.7 cGMP: 0.52–4.2 | dual substrate | VSMC: (+) (mRNA) [52] EC: ND | ND |
VSMC: vascular smooth muscle cell; EC: endothelial cell; NS: non-selective; ND: not documented; (−): no expression detected; (±): no change in expression; (+), (++), to (+++): moderate, fair or high expression.
PDEs have been considered for a long time as druggable targets, and some PDE inhibitors are used in various therapeutic areas (Table 3; reviewed in [5,35]).

Table 3.
General PDE expression and therapeutic relevance in humans.
2.3. General Modulation of cAMP and cGMP Pathways in PAH
2.3.1. Alterations of cAMP and cGMP Levels in PAH
Under normal conditions, cGMP concentration is around 2 times higher than cAMP in rat PA [54], although the cAMP levels are reported around 5 times higher than cGMP in systemic artery [19]. PAs isolated from hypoxic rats showed decreased cAMP in large vessels [54]. Likewise, stimulation of PASMCs from PAH patients with forskolin, a direct activator of AC, was reduced [52]. Evidence suggests that expression or activity of some AC isoforms can be depressed by various pathological stimuli (cytokines, hypoxia) [31,55].
Similar to the changes observed in cAMP, the level of cGMP content in PA from hypoxic rats was decreased [54]. By contrast, cGMP in lung perfusate was found to be higher in a PH model [56]. In the latter study, because cGMP-PDE activity was unchanged, the authors proposed that production of cGMP in the lungs should be enhanced following chronic hypoxia (CHx) [56]. One cannot rule out the possibility that high circulating levels of NPs resulting from cardiac overload lead to increased cGMP release in vivo [56]. Expressions of some sGC subunits were found to increase in PAH donors and models, although the identity of modulated genes or proteins varies among detection methods and preparation [57]. The activity of sGC may also be altered by redox state, the oxidized form being unresponsive to NO [25]. Thus, the intensity of cyclic nucleotide signalling in pathological tissue remains unclear. While cGMP levels likely result from various states of the synthesis machinery, PDE activity is another crucial determinant of the cyclic nucleotide responses (see Section 3).
2.3.2. Therapies to Stimulate the cGMP Pathway in PAH
In addition, alterations in pathways upstream of these systems are also important features in PAH. Several strategies have been employed to stimulate these pathways in an attempt to boost cyclic cAMP or cGMP in pulmonary circulation, yielding important progress in the management of PAH [9].
Promoting the NO–sGC Axis
The release of NO by the pulmonary endothelium is an important stimulator of cGMP synthesis in the PASMCs. Studies in mice demonstrated that the enzyme endothelial nitric oxide synthase (eNOS) is crucial in attenuating PA pressure and resistance [58]. The perturbation of NO bioavailability is a hallmark of PAH, but it is also present in other cardiovascular disorders as part of the endothelial dysfunction affecting various blood vessels [25,59]. Decreased eNOS expression [60] and low NO levels [61,62] have been observed in patients with PAH. Inhalation of NO at low doses has been approved to dilate PA and lower pulmonary vascular resistance in PAH patients [63]. However, long-term NO therapy is limited by the short half-life (15–30 s) of NO, which requires continuous inhalation and special delivery devices [64]. Currently, inhaled NO remains a standard method in evaluating acute vasoactive response during right heart catheterization testing as part of the diagnosis of PAH patients. NO is also used in pediatric medicine to treat PH associated with congenital heart disease [65,66] or in persistent PH of the newborn. More recently, the sGC stimulator, riociguat (Bay 63-2521), was developed as an alternative to stimulate cGMP [57,67]. This pharmacological class not only directly stimulates sGC but also sensitizes it to NO [68]. Riociguat showed curative effects in PAH animal models by partially reversing right heart hypertrophy and structural remodelling of the lung vasculature in PH models induced by CHx in mice or by monocrotaline (MCT) in rats [57]. Riociguat demonstrated efficacy in PAH patients and was approved to treat PAH and chronic thromboembolic pulmonary hypertension [9,25,67].
Promoting the NP System
Unlike NO, circulating BNP is elevated in PAH patients due to RV overload and dysfunction [69]. Genetic disruption of ANP or NPR-A receptor in mice demonstrated increased susceptibility to PH [70,71]. Infusion of BNP was well tolerated in PAH patients, but it did not significantly improve pulmonary hemodynamics. However, BNP potentiated the acute pulmonary vasodilator effect of the PDE5i sildenafil [72]. Because the circulatory half-lives of ANP (2–4 min) and BNP (~20 min) are short, both agents require continuous delivery and are thus difficult to use clinically. Intravenous administration of nesiritide, a recombinant BNP analogue, improved hemodynamic status in patients with post-capillary PH, but not with pre-capillary PH [73]. Drugs that include inhibitors of the breakdown of NPs by neutral endopeptidase neprilysin, such as ecadotril or sacubitril, have yielded promising results in animal models when combined with another pharmacological intervention, such as PDE5i or angiotensin receptor antagonist [74,75]. Such combinations would need further translational investigations. Another neprilysin inhibitor, racecadotril, was tested in patients with PAH and exhibited favourable short-term hemodynamic and tolerability [76]. These results warrant further investigation into the use of neprilysin inhibitors, possibly in combination with other therapies, including cGMP-elevating agents or PDE5i.
2.3.3. Therapies to Stimulate the cAMP Pathway in PAH
The impairment of cAMP generation is consistent with the depression of the PGI2 pathway. PGI2 is a paracrine mediator produced in the endothelium by the PGI2 synthase, following processing of arachidonic acid by cyclooxygenases, COX. PGI2 and thromboxane A2 (TxA2), another arachidonic derivative, are important regulators of vascular tone in PA, although they have opposite effects [77]. PGI2 can bind to the prostaglandin I, IP receptor, which is coupled to G(α)s and to the cAMP pathway. The associated signalling in smooth muscle cells evokes vasodilation, while it inhibits PASMCs proliferation and platelet aggregation. TxA2 evokes vasoconstriction and platelet aggregation. Increased pulmonary vascular resistance is thought to result from an imbalance in biosynthesis of PGI2 and TxA2 in the pulmonary vascular bed, where the influence of TxA2 is stronger [78,79]. In fact, the expression of PGI2 synthase is reduced in small and medium-sized PAs in PAH patients [80]. Additionally, the response of AC to PGI2 is reduced in hypoxia-induced rat PA [81]. Alternatively, enhanced cAMP hydrolysis by PDEs, as detailed in the following sections, may also be an underlying cause of the deteriorated response to PGI2 in pathological pulmonary vasculature. Supplementation of PGI2 has been a strategy to improve the deteriorated IP receptor signalling in PAH patients. Infusion of epoprostenol, a synthetic PGI2–sodium salt, was the first therapy specifically approved for PAH. It significantly improves hemodynamics and long-term survival [82,83,84]. Continuous administration of PGI2 or epoprostenol is challenging due to its short half-life (2–3 min). Several PGI2 analogues (called “prostanoids”) have been developed with extended half-life and increased bioavailability: treprostinil, beraprost, and iloprost [9,85]. However, the constraints caused by their mode of administration (i.v. or s.c. infusion) and adverse effects complicate their use in routine. Side effects include pain at the site of injection and typical prostanoid-related symptoms such as headache, flushing, diarrhea, and jaw pain. As an alternative, selexipag was developed as an orally available, selective IP receptor agonist [9,86]. The active metabolite ACT-333679 (previously known as MRE-269) shows further selectivity for the IP receptor [86]. Selexipag was approved to treat PAH based on the beneficial hemodynamic effects [87] and the significant reduction in morbidity/mortality events in PAH [88].
3. Exploring PDE Families in Pulmonary Arteries and Relevance in PAH
3.1. Overview of PDE Families
Myriads of PDE isoforms are expressed in PA tissue (Table 2). In general, the total cGMP-PDE activity is much higher than the cAMP-PDE activity [42,52,89]. In PASMCs, PDE1, PDE3, and PDE4 are major enzymes that hydrolyze cAMP. In control patients, cAMP-PDEs activity ranked as follows: PDE4 > PDE3 > PDE1 > PDE2 [52]. As to cGMP-hydrolysis in PASMCs, most activity is ascribed to PDE5, and, to a lesser extent, to PDE1 [40,42,89]. More recently discovered PDEs, such as PDE7, PDE8, PDE9, PDE10, have been less characterized due to the lack of pharmacological or biochemical tools.
PDE activities for cAMP were found to increase in PASMCs from patients with PAH [52]. In PASMCs from PAH patients, PDE1 and PDE3 activities were higher, ranking as follows: PDE3 > PDE1 ≥ PDE4 > PDE2 [52,89,90]. In particular, the protein amounts of PDE1A, PDE1C, and PDE3B exhibited the most striking changes (Table 4) [39,52]. In animal models, cAMP-PDE activity measured in isolated PA was also higher in PH compared to control groups [39]. Likewise, cGMP-PDE activity appeared higher in PASMCs from PAH donors [52]. Nevertheless, tissues from rats exposed to CHx yielded inconsistent results: activity was higher in isolated PA, whereas it remained unchanged when measuring in the whole lung [39,56]. However, individual changes in specific cGMP-PDEs, PDE5 in particular, were reported (Table 4).

Table 4.
Summary of non-clinical explorations on PDEs as therapeutic targets for the treatment of PAH.
Pharmacological inhibition of PDEs is an efficient way to rescue cAMP responses, or to enhance cGMP levels [52,89,97] (Figure 2).
The following sections include a review of the basic research and clinical data about the respective contributions of PDE families in PAH. A summary of available data is provided in Table 4.
3.2. Phosphodiesterase 1 (PDE1)
3.2.1. Enzymatic Properties
PDE1 is the unique PDE family that is activated by the Ca2+-calmodulin (CaM, a 16 kDa Ca2+-binding protein) complex, and is therefore often referred to as the “Ca2+/CaM-stimulated PDE” [102,103] (Table 2). Thus, this family potentially represents a unique regulatory link between cyclic nucleotides and intracellular Ca2+ signalling/handling, especially with respect to vascular tone. The PDE1 family is encoded by 3 genes: PDE1A, PDE1B, and PDE1C. The resulting isoforms, PDE1A and PDE1B, exhibit high affinity for cGMP, the latter being more selective for cGMP than the former. High affinity for both cAMP and cGMP is observed with PDE1C [102,103].
3.2.2. Expression Pattern
Data from human and animal PA tissue and PASMCs show expression of PDE1A and PDE1C, while PDE1B is less abundant [41-43,51,52]. PDE1-related cAMP hydrolysis can be detected in PA in various species and is particularly prominent in human proliferative PASMCs [39,42,52]. This is consistent with the notion that PDE1C is highly expressed when proliferating smooth muscle cells have switched to a synthetic phenotype, as opposed to the contractile phenotype [103]. Nevertheless, only slight Ca2+-CaM-stimulated, cGMP- and cAMP hydrolyzing activities were detected in cytosolic and particulate fractions of human PA [40] and were ascribed to PDE1. Contribution of PDE1 to cGMP-hydrolysis in PA is limited compared to that of PDE5 in the absence of Ca2+-CaM [40,104].
In a CHx-induced rat model, it was observed that PDE1 contributed to increased cGMP-PDE and cAMP-PDE activities in the main PA [39]. PDE1A and PDE1C expressions were higher in PASMCs from idiopathic PAH (iPAH) patients compared with healthy donors, both at the mRNA and protein levels [43,52]. In addition, the immunolabelling signal for PDE1C was particularly prominent in PA from iPAH patients. In animal models, however, upregulation of PDE1 isoforms in tissue was less striking, with only the expression of PDE1A mRNA in PASMCs being significantly increased [43]. Interestingly, another study showed that in a cold-induced PAH model in rats, protein expression of PDE1C in PA was increased, whereas PDE1A expression was unaffected [41]. Although the quantification of protein expression in the lung vasculature was not reported, this may indicate that the regulation of PDE1 isoforms may vary among species and models. On the whole, these data suggest that PDE1 activity increases in pathological PA; however, the identities of underlying isoforms may vary between species and models, with PDE1C as likely the most relevant candidate in human PAH.
3.2.3. Functional Role and Therapeutic Potential
It was reported that treatment with PDE1C-targeted siRNA both enhanced cAMP accumulation and inhibited cellular proliferation to a greater extent in PASMC from PAH patients than in controls [52]. This is consistent with the role played by PDE1C in promoting vascular smooth muscle cell proliferation in other diseases, where vessel wall remodelling was involved [5,103,105,106]. Accordingly, PDE1 inhibitors (PI79, vinpocetine) slowed proliferation in human and rat PASMCs [43,51]. PDE1 was shown to promote proliferation and inhibit apoptosis through various mechanisms (reviewed in [5]). In addition, PDE1 inhibitors have been reported to exert a vasorelaxant effect on mouse PA, rat lung, and lamb PA [43,51,107].
Preclinical therapeutic assessment of another PDE1 inhibitor (8-methoxymethyl 3-isobutyl-1-methylxanthine, 8MM-IBMX) was performed in three animal models of PAH, namely MCT-treated rats, cold-induced PAH rats, and CHx mice [41,43]. Pharmacological treatments were administered for 1 to 2 weeks once the disease was established, following a curative-like strategy. PDE1 inhibitor resulted in reduced PAP, reversed remodelling of the lung vasculature, and a reduction in RV hypertrophy. In addition, 8MM-IBMX was reported to suppress macrophage infiltration and superoxide production [41]. Taken together, these findings indicate that upregulation of PDE1, especially PDE1C in humans, plays a role in the structural remodelling process underlying PAH and, thus, offers a novel therapeutic target.
3.2.4. Perspectives and Limitations
Although vinpocetine [108] and 8-MM-IBMX [106,109] are commonly used PDE1 inhibitors, the selectivity of these compounds for PDE1 is moderate. For example, vinpocetine also inhibits several sodium channels [110] and inhibits NF-κB-dependent inflammation via an IκB-dependent but PDE-independent mechanism [111] and is more selective for PDE1A and PDE1B in comparison with PDE1C [112]. 8-MM-IBMX has increased selectivity for PDE1 over other PDEs by 30–50 fold [109]. Therefore, pharmacological effects should be interpreted cautiously. However, some promising PDE1 selective inhibitors have emerged. For example, Lu AF41228 and Lu AF58027 can induce vasodilation of the mesenteric artery and lower blood pressure [113]. Furthermore, dioclein and ITI-214 can enhance cardiac function acutely (reviewed in [5]). As previously discussed, because expression patterns in animal models may not accurately mimic those in patients, the translational value of in vivo studies remains uncertain. Data from human tissue models or clinical studies are clearly needed to ascertain the therapeutic potential of recent PDE1 inhibitors in PAH.
To date, no clinical trial has been conducted to test the efficacy of PDE1 inhibitors in PAH [114]. ITI-214 was recently entered into clinical trials (phase 1/2, NCT03387215) for the treatment of heart failure, providing an interesting opportunity to evaluate the safety and efficacy of PDE1 inhibitors in cardiovascular disorders [115]. Considering that PDE1A and C are expressed in the RV [116] and that their expression rises in the failing ventricle [5], PDE1 inhibition may promote cardiac effects in the hypertrophied RV of PAH patients. Whether these cardiac and vascular effects would translate into beneficial (improved RV function) or deleterious (arrhythmias) outcomes remains uncertain and needs to be investigated.
3.3. Phosphodiesterase 2 (PDE2)
3.3.1. Enzymatic Properties
PDE2 hydrolyzes both cGMP and cAMP with similar maximal velocity rates and relatively high Km values [117,118]. PDE2 contains two homologous GAF domains tandemly arranged in its regulatory regions. The GAF-A domain of PDE2 is involved in dimerization, while the GAF-B domain binds cGMP. The latter triggers conformational changes, increasing cAMP hydrolysis up to 30-fold [117,118,119]. Thus, PDE2 is unique in that it is the only cGMP-activated, cAMP-hydrolyzing PDE. PDE2A GAF-B is moderately selective with around 20-fold preference for cGMP (KD < 10 nM) over cAMP [119,120]. PDE2 isozymes are encoded by a single gene, PDE2A, containing three known variations (PDE2A1, PDE2A2, PDE2A3). The resulting isoforms differ from one another in their N-terminal domain, which likely influences the subcellular localization of the proteins. Specifically, PDE2A1 is more soluble while PDE2A2/A3 are preferentially membrane-associated [102].
3.3.2. Expression Pattern
PDE2 mRNA and activity can be detected in PA from various species, but they exhibit lower levels compared to PDE3, PDE4, and PDE5 [39,42,47,52]. A clear cGMP-stimulated cAMP hydrolyzing activity was detected in rat PA [46]. This result did not differ from that obtained in “denuded” PA (with the endothelial layer disrupted), indicating the expression of PDE2 in the media layer. Overall, substantial data support the existence of PDE2 protein and its activity in PASMCs. By contrast, PDE2 expression in the media of other vascular beds remains elusive (further discussed in a study by Wang et al. [121]).
Studies on the expression of PDE2A in PASMCs from PAH patients yielded variable results, reporting either up-regulation [52] or down-regulation [47] compared to control samples. PDE2-related activity was, however, not significantly altered [52].
In addition, PDE2 expression in ECs, including in PA, was also documented [48,122].
3.3.3. Functional Role and Therapeutic Potential
Pharmacological inhibitors of PDE2, such as erythro-9-(2-hydroxyl-3-nonyl) adenine (ENHA, IC50 = 0.8 μM) and Bay 60-7550 (IC50 = 4.7 nM), have been used to explore the contribution of the enzyme to physiological and pathological processes [123,124,125]. EHNA can increase forskolin-induced cAMP accumulation in PASMCs [52], demonstrating PDE2 activity. In the study by Bubb et al. [47], the effect of Bay 60-7550 was investigated alone or in combination with cyclic nucleotide stimuli in various experimental systems. As summarized earlier in this review (Section 2.2), responses to ANP and NO are typically ascribed to cGMP, while treprostinil is mediated by the cAMP pathway. The proliferation of PASMCs from PAH patients was reduced by Bay 60-7550, an effect potentiated in the presence of ANP, NO, or treprostinil. As to vascular reactivity, EHNA can reverse hypoxic vasoconstriction ex vivo in perfused rat lungs [46] and exhibits moderate vasorelaxant effects [42]. In PA isolated from rats exposed to CHx, Bay 60-7550 produced a dose-dependent relaxation at the micromolar range [47]. Furthermore, Bay 60-7550 (0.1 µM) potentiated vasorelaxant responses to ANP or treprostinil. In vessels from normoxic rats, responses to a NO donor were also potentiated by Bay 60-7550. However, responses to treprostinil remained unchanged. Paradoxically, the authors found that PDE2A expression was down-regulated in isolated PA tissue from CHx-induced rat models [47]. Overall, these data show that PDE2 is likely involved in PA contractile reactivity and suggest that PDE2 differentially mitigate various cyclic nucleotide pathways in health or disease. Nevertheless, the specific contributions of either endothelial or smooth muscle PDE2 remain unclear and need to be further defined. Genetic ablation of PDE2A, restricted in a cell-type-specific manner, will possibly address this question. PDE2 plays a role in endothelial cells (ECs) as a regulator of barrier integrity and angiogenesis [5,48]. Inhibition of PDE2 is beneficial in reducing endothelial permeability triggered by tumour necrosis factor-α or H2O2 [48,126]. These findings are consistent with the general roles of PDE2 demonstrated in ECs, hindering endothelial barrier function (reviewed in [118]).
Most importantly, Bay 60-7550 prevented the development of both hypoxia- and bleomycin-induced PH in mice without influencing the systemic arterial pressure [47]. Interestingly, this protective effect on RV systolic pressure and on RV hypertrophy was decreased in NPR-A (GC-A) knockout animals, but not in mice receiving the inhibitor of nitric oxide synthases L-NAME. These results suggest that PDE2 inhibition exerts a salutary effect in PH by preferentially promoting cGMP derived from the NP pathway, rather than the NO pathway, a mechanism similar to that reported in cardiac myocytes [127]. Nevertheless, the PDE2 inhibitor alone, administered from day 14 once PH was established, was not effective in curing PH. When administered in combination with the neutral endopeptidase inhibitor ecadotril (to enhance endogenous NPs levels), treprostinil, inorganic nitrate (NO donor), or the PDE5i sildenafil, Bay 60-7550 produced a significantly greater reduction in the salient features of the model compared with monotherapy with each compound alone. Therefore, one may hypothesize that PDE2 inhibition could serve as an effective adjuvant therapy to bolster both cGMP and cAMP signalling in pulmonary circulation, synergizing with pharmaceuticals such as NPs, PGI2 analogues, or even inorganic nitrates.
3.3.4. Perspectives and Limitations
The unique molecular properties of PDE2 enable crosstalk between both cAMP and cGMP pathways, with potential pathophysiological and therapeutic consequences [118]. Interestingly, an additive effect of PDE2 inhibition on top of PDE5i was observed against PASMCs proliferation or in preventing in vivo development of PAH [47]. The reason for this may be that the inhibition of PDE5 exacerbates PDE2 activity by promoting a rise in cGMP and binding to the GAF-B domain. This may potentially explain how the inhibition of both enzymes may bolster cyclic nucleotide pathways. The safety of such a combination would need to be carefully monitored, as it may increase the risk of hypotension; however, no change in mean arterial pressure was observed in the mouse CHx model used in the study by Bubb et al. [47]. Considering the various roles that PDE2 is thought to play in ECs (promoting angiogenesis and barrier leakage) and in the hypertrophied cardiomyocyte (reviewed in [118]), the efficacy and safety of PDE2 inhibition in pulmonary circulation require further exploration using different models. Because global genetic knockout of PDE2A is lethal at the embryonic stage, cell-type-specific models would be valuable tools for further investigations. In conclusion, PDE2 may be an interesting target to consider in PAH, although mechanisms impacted by enzyme inhibition are still uncertain and may impact various functions. Furthermore, a combination with other PDE inhibitors or therapies that enhance cyclic nucleotides would be more likely to reach efficacy endpoints.
Several PDE2 or mixed PDE2/5 inhibitors developed to treat migraine and cancer have failed to reach a significant stage in clinical trials, primarily due to safety concerns (reviewed in Baillie et al. 2019) [35]. Recent small molecules have been investigated: PF-05180999 (Pfizer) was terminated in a Phase I clinical trial due to safety concerns in patients with migraines [35]. TAK-915 (NCT02461160), which recently completed a Phase I trial.
3.4. Phosphodiesterase 3 (PDE3)
3.4.1. Enzymatic Properties
The PDE3 family is coded by two genes, namely PDE3A and PDE3B, which show high affinity for both cAMP and cGMP [117] (Table 2). A lower Vmax for cGMP compared to cAMP (approximately 4 to 10-fold) makes cGMP a competitive inhibitor for cAMP hydrolysis [128]. Therefore, PDE3 is often referred to as the “cGMP-inhibited, cAMP-hydrolyzing PDE”.
A variety of compounds demonstrate PDE3 inhibiting properties [129], and some of these were used at selective concentrations to assess PDE3 activity in PA tissue and cells (e.g., motapizone, milrinone, cilostamide, cilostazol, etc.) [35,39,40,42,117]. PDE3 activity contributes to the largest portion of cAMP hydrolysis in PA and PASMCs, either in control samples (30–60%) or in patients as well as PH models (around 40–50%) [39,40,42,52]. PDE3 also contributes to cGMP hydrolysis [40].
3.4.2. Expression Pattern
Data obtained in PASMCs from human patients showed that both PDE3A and PDE3B are expressed in human PASMCs. Both mRNA levels are upregulated in idiopathic PAH patients, but only PDE3B protein expression is increased [52].
PDE3 activity increases in PASMCs from iPAH patients and in large, intralobar PA from rats exposed to CHx, which can partly explain the total increase in cAMP-related PDE activity in these samples and dovetails well with the changes in PDE3 expression [39,49,52]. PDE3 activity is also prominent in PAECs, where PDE3 inhibition with motapizone can protect against the H2O2-induced increase in endothelial permeability [48].
3.4.3. Functional Role and Therapeutic Potential
The vasorelaxant effects of PDE3 inhibitors have been extensively explored in the pulmonary vasculature from various species, including humans. PDE3 inhibitors effectively relax ex vivo precontracted PA [40,42,130] and potentiate cAMP-mediated vasorelaxant responses [131]. For example, milrinone rescued the cAMP responses in the CHx context, where cAMP signalling was otherwise blunted [131]. This result is consistent with increased PDE3 expression limiting the cAMP activity in pathological PA. Similar effects were reported as to the capacity to drop pulmonary vascular resistance in perfused lungs or in vivo [132,133,134]. These properties potentiate or synergize with responses to approved PAH therapies, such as PDE5i or inhaled PGI2 [132]. PDE3 inhibition also enhances cAMP levels and produces an anti-proliferative effect in PASMCs, particularly in cells from patients with iPAH [52]. Taken together, data show that PDE3 is a master repressor of cAMP levels in PA under both physiological and pathological conditions. In vivo, PDE3 inhibitors (cilostazol, cilostamide) attenuate RV systolic pressure and RV hypertrophy in the MCT-induced PH model, but not in CHx-induced models [51,91,92], unless combined with another cAMP-elevating agent [51]. Likewise, nebulization of a dual PDE3-PDE4 inhibitor improved the MCT-induced pulmonary vascular damage and RV hypertrophy [135].
3.4.4. Perspectives and Limitations
Consistent with ubiquitous PDE3 expression through smooth muscle cells [27], the vasodilatory effects of PDE3 inhibitors, or other mixed and non-selective inhibitors, extend to systemic vasculature and promote hypotension. This is a limitation to straightforward translation of these compounds for use in clinics [133,136].
In addition, PDE3 inhibitors such as milrinone, like other inotropic agents, are only used for the treatment of patients with acute heart failure, while chronic use is limited due to safety concerns [137]. Likewise, milrinone produces inotropic effects on the RV in infants with persistent PH of the newborn, where it may be useful as an adjuvant therapy in addition to NO [138,139]. Nevertheless, PDE3 inhibition in the RV may potentially turn arrhythmogenic, as suggested by non-clinical data obtained in a porcine model [140]. Such systemic effect of PDE inhibition may be circumvented by titrating the lowest effective dose according to the pulmonary versus systemic selectivity and by using the respiratory route of administration of nebulized inhibitors, as investigated by Schermuly et al. [141].
In summary, PDE3 inhibitors administered alone showed modest advantages in experimental PAH treatment and present potential safety issues due to cardiac and systemic pharmacodynamics. To date, there has been no clinical trial testing PDE3 inhibitors for PH in chronic settings. In practice, such pharmacological approaches are currently relevant in an acute setting to address PH and RV dysfunction during cardiac surgery [142] and pediatric intensive care units, such as persistent PH of the newborn [138,139,142].
3.5. Phosphodiesterase 4 (PDE4)
3.5.1. Enzymatic Properties
PDE4 is well characterized by its substrate selectivity for cAMP and its sensitivity to inhibitors such as rolipram or roflumilast [5,35]. PDE4 isozymes comprise four subfamilies (4A, 4B, 4C, and 4D), each encoded by a distinct gene, resulting in more than 25 PDE4 isoforms in humans [143]. Based on the presence of upstream conserved regions domains 1 and 2 (UCR1 and UCR2), PDE4 isoforms can be divided into four categories: long isoforms harbouring both UCR1 and 2, short isoforms having only UCR2, super-short isoforms with a truncated UCR2, and dead-short isoforms that lack both UCR domains and have a truncated catalytic domain [143]. Various features of the protein, including UCR modules, define many post-translational properties of the enzyme, such as regulation by phosphorylation, dimerization of long forms, and anchoring (or targeting) to subcellular structures (reviewed in [5,143]).
3.5.2. Expression Pattern
PDE4 activity can be revealed by using various selective inhibitors such as rolipram (IC50: 0.05–2 μM), Ro 20-1724 (IC50: 1–2 μM) [144], or roflumilast (IC50: 0.4 nM). PDE4 is generally well detected in PA tissue, but at variable proportions. PDE4 contributes less than half of PDE3 cAMP-hydrolyzing activity [40] in humans but is almost as important as PDE3 in bovine PA [42]. Although cAMP-PDE activity is globally increased in PASMCs from PAH patients, the PDE4 activity does not contribute to this, because PDE4 activity decreases in PAH patients (30% of total) compared to controls (around 45%); meanwhile, there was an increase in activities ascribed to PDE1 and PDE3 [52]. Additionally, PDE4 is clearly a PDE responsible for cAMP hydrolysis in the endothelium, as demonstrated in ECs from porcine PA by using rolipram [48]. A recent study showed that EC was one major cell type expressing PDE4B in the lung. This isoform was the only one whose expression was significantly upregulated in several models of experimental PH and in PH patients [93].
3.5.3. Functional Role and Therapeutic Potential
In human PA, PDE4 inhibitors alone exert only poor vasorelaxant effects unless PDE3 is simultaneously inhibited [40]. This suggests that the overlapping activities of both enzymes are responsible for regulating vascular tone. By contrast, rolipram potently relaxes rat PA [42]. The discrepancy between these results may originate from the species used or the agonist used to obtain precontraction of the arterial rings (PGF2α vs. phenylephrine). High concentration of rolipram can relax the rat PA contracted by acute hypoxia in the perfused lung [51]. PDE4 inhibitors also exhibit anti-proliferative effects in rat and human PASMCs [44,51,52]. These effects, however, were not increased in PASMCs from PAH patients compared with controls [52]. Taken together, the data show that although PDE4 is a substantial contributor to cAMP hydrolysis in PASMCs, limiting vasorelaxation and promoting cell proliferation, it is not significantly altered in PAH.
On the contrary, PDE4 located in PAECs may be a more likely contributor to PA tissue remodelling. PDE4 inhibition by rolipram was shown to limit endothelial permeability induced by H2O2 [48], synergizing with PGE1. More specifically, Xing et al. provided data showing that PDE4B participates in experimental PH (see below). This process involves attenuation of the PKA-CREB-BMPR2 axis as a possible mechanism [93].
In vivo studies conducted to test the efficacy of PDE4 inhibition in PH models yielded mixed results. In the rat CHx model, rolipram attenuated neither mPAP, RV hypertrophy, nor the distal vessel muscularization, except when combined with iloprost or a PDE3 inhibitor [51]. Another PDE4 inhibitor, roflumilast, used at high dose (1.5 mg/kg), prevented both MCT- and CHx-induced PH phenotype in rats [94]. Moreover, roflumilast partly reversed the established PH phenotype induced by MCT (curative protocol) [94]. A lower dose (0.5 mg/kg) was only effective in preventing MCT rather than CHx-induced PH phenotype [94].
More recently, curative effects of roflumilast at high dose (2 mg/kg/day) were confirmed in the SuHx model in mice [93]. The same group demonstrated that genetic invalidation of PDE4B protected mice against salient alterations evoked by the SuHx model. Interestingly, the salutary effects of roflumilast were not observed in PDE4B−/− mice, confirming that the pharmacological action of roflumilast could be ascribed to selective inhibition of PDE4B isoform. Likewise, another PDE4B-selective inhibitor (BI 1015550, 3.24 mg/kg/day, per os) improved the SuHx-induced rat PH phenotype [93].
Because endothelial PDE4B was one major PDE isoform that was upregulated in experimental PH, Xing et al. investigated mice with PDE4B deletion restricted to ECs, using expression of Cre recombinase under the control of the Tek gene promoter. It was found that PDE4BEC−/− mice showed less severe PH phenotype in SuHx models, compared to PDE4BEC+/+ “floxed” mice. In this context, the authors examined the EndoMT process, which is characterized by a decrease in endothelial markers and a concomitant increase in mesenchymal markers such as the α-smooth muscle actin gene (Acta2) expression. While EndoMT increased in the SuHx model, it was limited by the PDE4B ablation. Taken together, while PDE4 is quantitatively an important cAMP-hydrolyzing PDE in pulmonary circulation, PDE4B in ECs may be a potential target for future therapeutic development.
3.5.4. Perspectives and Limitations
These advances need to be translated into human cells and tissues, while the PDE4B inhibitor BI 1015550 has so far only been tested in patients with idiopathic fibrosis [145,146]. Supported by the rationale that activity spectra of PDE4 and PDE3 overlap, combined inhibition of PDE3 and PDE4 provided efficacy when administered with iloprost in the rat MCT and CHx models [51,147]. Data obtained from PH patients show that acute tolafentrine (a dual PDE3/PDE4 inhibitor) could serve as a useful adjuvant to iloprost [148]. Nevertheless, further investigations are necessary to explore the long-term potential of this therapy. One may speculate that similar limitations may apply to PDE4 inhibitors as to PDE3 inhibitors, although the level of expression of PDE4 is much lower than PDE3 in human cardiac tissue, thereby limiting the potential inotropic and proarrhythmic effects of a possible PDE4 inhibition-based therapy [24].
Conversely, the well-characterized anti-inflammatory action of PDE4 inhibitors may complement therapies acting more specifically on tissue remodelling [5]. More specifically, PDE4 is expressed in immune cells and promotes cell activity by lowering cAMP levels. In addition, chronic inflammation is an accepted component of various forms of PH (review in [149,150]), although its causal role in the pathogenesis remains unclear. Inflammation primarily manifests as the infiltration of immune cells into vascular lesions and of increased levels of circulating pro-inflammatory mediators. While PDE4 inhibition limited the increase in some cytokines in MCT-induced PH [94], data in other relevant PH models are needed.
The clinical development of PDE4 inhibition has been hindered by adverse side effects, mainly in the gastrointestinal area [5]. Off-targeting of PDE4D located in the area postrema may be responsible for emetic symptoms [5]. Targeting PDE4B more selectively than PDE4D may increase the tolerability of PDE4 inhibitors therapies by preserving the efficacy of inhibition of the most relevant PDE isoform. The use of isoform-selective inhibitors, such as the anti-fibrotic nerandomilast (BI 101555), may offer therapeutic insight through future dedicated clinical investigation.
3.6. Phosphodiesterase 5 (PDE5)
3.6.1. Enzymatic Properties
PDE5 is a cGMP-specific PDE, and is coded only by one gene, PDE5A, that can yield three variants (PDE5A1, PDE5A2, and PDE5A3). Structurally, the PDE5 N-terminal region contains two allosteric cGMP-binding domains (GAF-A and GAF-B) and one phosphorylation site (at Ser92 in bovine and Ser102 in human) [151,152]. High-affinity (Kd < 40 nM) cGMP binding occurs only to the GAF-A domain, which stimulates enzyme activity 9 to 11-fold [153]. The role of GAF-B is currently unclear. Phosphorylation at Ser92 by PKG or PKA not only activates the catalytic function but also further increases the allosteric cGMP-binding affinity, thus working as positive feedback to mitigate cGMP levels [102,151].
3.6.2. Expression Pattern
Compared with other PDEs, PDE5 has the highest expression and contribution in GMP hydrolysis in the lungs, pulmonary arterial vessels, and PASMCs, both from animals [42,104,154] and humans [40,52]. PDE5 expression and activity are strongly increased in the lungs and/or PA from animal models or PAH patients [49,50,89]. Expression markedly localizes in the thickened media layer [89]. This suggests that PDE5 represents a marker of vascular remodelling in PAH development. Furthermore, the expression of PDE5 was reported to increase in the hypertrophied RV compared with the normal RV both in PAH patients and MCT-induced PAH rat models [155], indicating that cardiac pharmacodynamics may also account for the therapeutic action of PDE5i.
3.6.3. Functional Role and Therapeutic Potential
Several studies addressed the role of PDE5 in PAH, providing a compelling basis for the treatment of PH [9,35]. Ex vivo studies have consistently shown that PDE5i relaxes PA in both humans [40,156] and animal models of PAH [56,74,104]. Furthermore, the inhibition of PDE5 increases cGMP levels in a concentration-dependent manner, producing anti-proliferative and pro-apoptotic effects in PASMCs from patients with PAH. These effects can be augmented synergistically or additively by NO donors or NP analogues [89,156]. Moreover, in vivo studies show that PDE5i not only can prevent the development of various PAH models [74,157,158,159], but also partially reverse the established remodelling of the PA wall [50,158]. This translates into a decrease in mean PAP or RV systolic pressure, a reduction in RV hypertrophy, and mitigation of PA remodelling or of distal vascular muscularization, without affecting systemic blood pressure. Regarding the cardiac effects, acute inhibition of PDE5 in isolated hypertrophied RV exerts positive inotropism, which is mediated by increased cAMP levels, resulting from the inhibition of PDE3 activity by increasing cGMP [155].
The beneficial effects of PDE5 inhibition obtained in preclinical models warranted the assessment of three compounds in clinical trials: sildenafil, tadalafil, and vardenafil. These inhibitors have demonstrated efficacy in improving clinical endpoints such as hemodynamics, six-minute walk distance, and quality of life in PAH patients [160,161,162,163]. In the long term, PDE5i improve the time-to-clinical worsening and mortality [164].
3.6.4. Perspectives and Limitations
PDE5i are currently a cornerstone of the management of PAH patients (see Section 1.3 above), as a part of first-line therapy, regardless of risk status [4,6]. PDE5i are generally well-tolerated, with common systemic side effects ascribed to PDE inhibition in various tissues. These effects include headaches, flushing, vision problems, gastrointestinal discomfort, and muscle and joint pain [165,166].
Mechanistically, the efficacy of PDE5i relies on the status of cGMP synthesis by either the NO or NPs pathway [25,97,167]. In smooth muscle cells, PDE5 is distributed to specific subcellular targets and participates in the compartmentalization of both branches of the cGMP signalling. The range of cellular mechanisms targeted by PDE5i encompasses the targets of PKG [17,19], including Ca2+ handling and signalling mechanisms, ion channels, and the RhoA pathway [168,169]. As previously mentioned, the inhibition of PDE5 may lead to the inhibition of PDE3 or the activation of PDE2, thereby establishing a complex cross-talk between cAMP and cGMP pathways. Nevertheless, an accurate description of cellular compartments involving PDE5 and other PDEs is still lacking and needs to be addressed by further research. In particular, the preferential contribution of PDE5 to either membrane- or cytosolic-mediated cGMP pools is uncertain in PA. The combination of PDE5i with riociguat promotes vasodilation, suggesting that PDE5i potentiates cGC activity [170] and that PDE5 connects with the NO–sGC–cGMP axis. Moreover, PDE5i synergize with NP-enhancing drugs, indicating that PDE5 also mitigates the NP receptor-generated cGMP pool [74]. Thus, PDE5 is likely to be involved in the control of global cGMP in the smooth muscle cells.
The role of PDE5 as a master controller of cGMP limits the use of further combinational therapy with other cGMP enhancers in clinics. This would lead to excesses in pharmacodynamic responses. The association of a PDE5i with riociguat is therefore contraindicated due to the risk of hypotension and other side effects [171]. In addition, the selectivity of PDE5i is relative, and other PDE isoforms may be affected, depending on the reached plasma concentration [166]. Recently, novel, highly selective PDE5i have been reported, such as dihydroquinoline-2(1H)-ones [99] and DDCI [98,172]. These new compounds may offer improved selectivity compared to sildenafil or tadalafil, improving the tolerability of PDEi among patients.
3.7. Phosphodiesterase 9 (PDE9)
3.7.1. Enzymatic Properties
PDE9 was discovered in the late 1990s and is coded by a single gene, PDE9A [173,174,175]. PDE9A was reported to encompass more than 21 splice variants, possibly displaying differential subcellular localizations and properties [176,177]. PDE9 is highly selective for cGMP hydrolysis with a Km of about 70–170 nM for cGMP and only 230 µM for cAMP [175]. Importantly, the affinity of PDE9 for cGMP is 40–170 times higher than two other cGMP-specific PDEs, PDE5 and PDE6, respectively. The maximum velocity of the catalytic site is similar to PDE5. All these properties make PDE9 a potential sensitive tuner of cellular cGMP signalling.
3.7.2. Expression Pattern
The tissue distribution of PDE9 is ubiquitous, as PDE9 mRNA has been detected in many organs. The highest levels are reported in the brain, kidney, spleen, with relatively lower levels found in the lung [173,174,175], and the heart [174,175,178]. Studies accounting for the expression of PDE9 in the pulmonary vasculature are scarce. PDE9 was only mentioned to be expressed at the mRNA level in fetal rat and human PASMCs in a couple of reports [52,179]. Another report mentioned that PDE9 inhibition decreased leucocyte adhesion in arterioles [180]. Therefore, expression of PDE9 in ECs cannot be ruled out. Regarding PDE9 expression in the context of PH, a study reported that PDE9 mRNA was not altered in PASMCs from iPAH patients but was upregulated in Group III PH [52].
3.7.3. Functional Role and Therapeutic Potential
To date, several selective PDE9 inhibitors have been characterized. Studies mainly focused on their ability to increase cGMP in the brain and rescue cognitive function [181,182,183,184,185,186] (reviewed in [5,35]). Importantly, PDE9 was also explored as a therapeutic target in experimental heart failure. PDE9 expression in the myocardium was reported to increase in subjects with dilated cardiomyopathy and heart failure, or in cardiomyocytes submitted to a pro-hypertrophic stimulus [178]. Several studies demonstrated that PDE9 global ablation or pharmacological inhibition (PF-04449613, PF-04447943, CRD-733) can improve cardiac hypertrophy, diastolic dysfunction, and coronary microvascular rarefaction in models of hypertrophy and myocardium dysfunction [178,185,187]. In addition to the direct effects on cardiac myocytes, PDE9 inhibition may also produce pharmacodynamic effects on the vasculature. Acute PDE9 inhibition with PF-047499982 dose-dependently diminished systemic blood pressure and peripheral vascular resistance in a heart failure model in sheep, indicating a vasodilatory action [188,189,190]. Moreover, the PDE9 inhibitor decreased pulmonary arterial resistance, although not to the same degree as sildenafil [190]. Therefore, PDE9 inhibition may induce the relaxation of PASMCs in vivo, although direct evidence is still lacking. Although the selectivity indexes of PF-04449613 and PF-047499982 towards PDE9 are at least 40 and 300 times over other PDEs, a non-selective effect on other PDEs cannot be ruled out in these in vivo studies [182,191].
Like other PDEs, the contribution of PDE9 to cGMP compartmentalization has been explored [167]. By following a pharmacological approach combined with cGMP measurements, by means of an intracellular FRET-based biosensor, data by Zhang et al. suggested that PDE9 selectively regulates NO-related cGMP levels in rat aorta smooth muscle cells [192]. This conclusion is at odds with other results obtained from cardiac myocytes, since Lee et al. demonstrated that PDE9 mitigates only cGMP pathways related to NP rather than the NO pathways [178]. In addition, in sheep with heart failure, PF-047499982 produced additional improvement in promoting the hemodynamic effects of NPs when combined with neprilysin inhibition [188].
Altogether, while the expression and role of PDE9 in vasculature remain unclear, PDE9 inhibition may produce vasodilatory responses that could be useful in fine-tuning cardiac preload and afterload and may also oppose the progression of PH. Nevertheless, one study reported that PDE9A-deficient mice were not protected from CHx-induced PH [101]. A recent study demonstrated that PDE9 expression increased in the lungs of aged mice and that treatment with PF-04447943 reversed the age-related increase in pulmonary vascular resistance. It also improved the vasodilatory response to sodium nitroprusside in isolated, perfused lung [187]. These data suggest that the physiological role of PDE9 may be more prominent in aged animals.
3.7.4. Perspectives and Limitations
Several PDE9 inhibitors have been tested in humans, with early clinical trials, such as PF-04447943 (clinical trial Ib) [193] and BI 409306 [194]. Ongoing clinical trials are evaluating the therapeutic effects of CRD-740 (NCT05409183) and tovinontrine in heart failure (NCT06215586, NCT06215586). Despite possible promising hemodynamic properties, the usefulness of PDE9 inhibition in improving pulmonary vasculature or RV dysfunction remains unclear and warrants further non-clinical studies in order to support future clinical investigation in PAH patients.
3.8. Phosphodiesterase 10 (PDE10)
3.8.1. Enzymatic Properties
PDE10 is coded by a single gene, PDE10A. PDE10 hydrolyzes both cAMP and cGMP, with a higher affinity for cAMP than for cGMP. Vmax, however, is 2–5-fold lower for cAMP compared to cGMP. Therefore, cAMP inhibits cGMP hydrolysis, thus making this enzyme a cAMP-inhibited cGMP PDE [38]. PDE10 harbours two GAF domains in the N-terminal region. In contrast to other PDEs, only cAMP can bind to the GAF domain, which activates PDE10 [195,196]. Papaverine has been used as a potent inhibitor of PDE10A, although it also inhibits PDE3 with a selectivity ratio < 10 [53,197].
3.8.2. Expression Pattern
Expression of PDE10 was reported in human PASMCs, but no significant variation in transcript levels was reported in iPAH PASMCs [52]. Tian et al. explored PDE10 in the MCT-induced PH model in rats [53]. They found that PDE10 transcripts and protein levels were more abundant in PASMCs, PA, and lung isolated from rats with PH, compared to control rats. In particular, immunolabelling indicated a strong PDE10 signal in the remodelled media layer from iPAH donors and MCT-exposed rats [53].
By using 10 µM papaverine to reveal the activity of PDE10, surprisingly high levels were reported in PASMCs. Indeed, PDE10 contributed to 38% and 53% of cAMP hydrolysis in PASMCs from control and MCT rats, respectively. However, papaverine is probably not selective at this concentration, and activities of other PDEs, such as PDE3, may also be included in these measurements. Thus, PDE10 seems to be highly expressed in the remodelling PA media. However, further exploration of animal models and human tissues is needed to ascertain the contribution of PDE10 to cAMP activity.
3.8.3. Functional Role and Therapeutic Potential
Papaverine was shown to increase cAMP levels and to activate downstream phosphorylation of the transcription factor CREB and exert an anti-proliferative effect on PASMCs isolated from MCT-exposed or control rats [53]. Papaverine and, importantly, other more recent synthetic PDE10 inhibitors were tested for the prevention of MCT-induced PAH [53,198,199]. Interestingly, these compounds decreased mPAP or RV systolic pressure, RV hypertrophy, PA thickness, and distal vascular muscularization [53,198,199]. The interpretation of the MCT model, however, is of limited translational value as it does not recapitulate all the severe features of PAH in humans [200]. Additional studies should be performed in other PH models to confirm the salutary effects of PDE10 inhibition.
In addition, PDE10 expression increased in mouse and human failing hearts, and PDE10A inhibition was found to improve cardiac remodelling and dysfunction [201]. It may be speculated that PDE10 inhibition would also protect against RV hypertrophy and fibrosis, although additional studies are required to verify this.
3.8.4. Perspectives and Limitations
As discussed previously, the selectivity of papaverine for PDE10 is limited. Therefore, efforts using highly selective inhibitors of PDE10 may help demonstrate the translational potential in PH. Several PDE10 inhibitors have been developed in the central nervous system area, some being tested in schizophrenia, Huntington’s disease, and cancer [5,35,202]. Such growing diversity of selective PDE10 inhibitors may afford opportunities for potent pharmacological approaches to treat PAH. Nevertheless, because PDE10 is abundant in the brain, strategies that favour pulmonary and cardiac selectivity would be valuable to preserve the tolerability of future drug candidates.
3.9. Other PDEs
Other PDEs include PDE6, PDE7, PDE8, and PDE11, for which there is little information, if any, regarding their potential role in PA. The PDE6 family is restricted to the retina and works as a photoreceptor. Transcripts of PDE7A were reported to be more abundant in PASMCs from PAH patients [52] and rat models [53], whereas PDE7B, PDE8A, PDE8B, and PDE11A showed no significant changes in PASMCs from iPAH patients [52]. These PDEs are, for now, mostly studied in diseases of the central nervous system [5,35].
4. Future Directions and Limitations
4.1. Potential Systemic Adverse Effects of PDE Inhibition (e.g., Hypotension, Cardiac Effects)
There are several potential limitations in the use of PDE inhibitors in PH and PAH. These include redundancy of PDE activity, difficulty in selectively targeting discrete PDE isoforms in specific cell types, the lack of selectivity of pharmacological inhibitors, ubiquitous expression of some PDE isoforms, and the insufficient expression of others.
The localization of the PDEs expression across the vascular territories is an important determinant to consider. The success of PDE5i relies on their ability to selectively relax pulmonary circulation, while having little influence on the systemic blood pressure [56,96]. Cyclic-AMP PDEs, such as PDE3, may be more problematic as their inhibitors are potent vasodilators across the vascular system [5,38].
More generally, inaccurate inhibition of PDEs may lead to increases in cAMP and/or cGMP signalling in undesirable organs, or exacerbated pharmacodynamics in the cardiovascular system. For example, the PATENT PLUS study warned that combining sildenafil with riociguat was associated with systemic hypotension, presumably due to the additive effect on cGMP signalling in the systemic vasculature [170].
In addition, because many PDE enzymes are active in cardiac myocytes [24], PDE inhibition aiming at enhancing cAMP signalling in the lung vasculature may also lead to adverse cardiac effects. These effects potentially emerge from alterations in excitation-contraction coupling in the right and possibly the left ventricles [203]. In line with this notion, PDE3 and PDE4 inhibitions were shown to potentiate spontaneous diastolic Ca2+ waves in adult right ventricular myocytes in a model of right ventricular dysfunction in pigs [140]. This may limit the use of bi-selective PDE3-PDE4 inhibitors in PAH, although inhibiting both enzymes may be necessary to provide salutary effects. Refining lung-restricted drug delivery strategies may help overcome this limitation and requires additional studies.
4.2. Challenges in Developing Isoform- or Cell-Type-Selective PDE Inhibitors
4.2.1. Identification of Relevant PDE Isoforms and Subcellular Complexes
It is well accepted that PDEs play a key role in regulating the amplitude, duration, and localization of cAMP and cGMP in vascular cells, thereby affecting the extent of vasodilation and PA wall remodelling. Unfortunately, the understanding of their specific contribution to the various cellular processes still remains limited. Therefore, further knowledge is needed as to the distribution of various PDEs in the relevant subcellular compartments. Indeed, the concept of compartmentalization of cyclic nucleotide signalling is becoming increasingly important in the cardiovascular system [24,204]. It is now well accepted that the signalling pathways are spatially organized around different discrete cAMP and cGMP pools within the cell, each regulating specific cellular functions. PDEs are a key component of this organization, together with other proteins (regulatory subunits, kinases, phosphatases, anchoring proteins, etc.). Increases in PDEs expressions under pathological conditions may jeopardize these signalling scaffolds and thereby promote disease progression. Nevertheless, the delineation of cAMP and cGMP compartments in vascular cells is still in its infancy (reviewed in [20,167]) and needs further research efforts. Additionally, data generated from multi-omics will undoubtedly yield important information on PDEs distribution across various cell types. Research based on single-cell approaches should also provide a more accurate identification of the relevant cell subtypes to be targeted.
Therefore, further knowledge in this area will consolidate the definition of new molecular targets involved in PDE signalling.
4.2.2. Toward High-Resolution Targeting?
While various approaches may be considered to selectively target a relevant PDE isoform, these approaches should also address this targeting in the relevant subcellular compartment. Several strategies may be implemented to achieve this ambitious goal, including the development of selective inhibitors of discrete PDE isoforms, disrupting molecular interactions and protein complexes, or various silencing options (extensively reviewed in [5,35]).
Improved organ or even cell-type-selective targeting may benefit from progress made in drug delivery strategies. Nanoformulations administered through the respiratory route may provide restricted delivery of compounds and limit the side effects. Aerosolization of drugs, as experimented with by Schermuly et al. [141], appears as a good starting point for further optimization. For example, efforts to improve the delivery of sildenafil have been proposed [205], which may be extended to other classes of PDE inhibitors. These strategies will face numerous challenges, including tolerability and efficacy compared to standard medications.
5. Conclusions
It is well accepted that enhancing cAMP and cGMP signalling in the pulmonary vasculature is beneficial in PAH. PDEs, as natural limitation mechanisms of these pathways, are obvious candidates for therapeutic intervention. Currently, only PDE5i are successfully used in PAH treatment and are still included in the standard battery of first-line therapy. Other PDE families have been the object of characterization in PAH models and tissue from patients. When inhibitors were available, pharmacological therapy was tested with variable efficacy. Research on currently overlooked PDE families, such as PDE10, may benefit from the optimization of pharmacological inhibitors.
Limits in efficacy may arise from redundancy of PDE isoforms, low expression of the targeted PDE, and high severity of the pathological status. In addition, the insufficient selectivity of small molecules may lead to adverse effects due to overly broad PDE inhibition or even off-target effects. Improving understanding of the architecture of the PDE signalling in vascular, cardiac, and inflammatory cells will help define relevant subcellular compartments and enzymes to be targeted. Moreover, the development of nanomedicine and novel drug delivery systems may open up new avenues and accelerate the advancement of novel therapeutic strategies.
Co-targeting of other cyclic nucleotide modulators, such as NP, NO, or G-protein coupled receptors, should be considered to optimize the reshaping of these signalling pathways. Studies focused on possible crosstalk between TGF-β receptor families and cAMP or cGMP will also provide useful translational insights into the understanding of how cyclic nucleotide modulators may interact with sotatercept or future novel therapies.
Author Contributions
Writing—original draft preparation, L.W., B.M., and R.F.; writing—review and editing, B.M., L.W., and R.F.; visualization, L.W., B.M., and R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Grant ANR-10-LABX-33 as a member of the Laboratory of Excellence LERMIT and ANR13BSV10003-02. L.W. was supported by the China Scholarship Council (PhD fellowship) and by a grant from Zhi-Cheng Jing (Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Southern Medical University, No. 106 Zhongshan 2nd Road, Guangzhou 510080, China). A travel grant was awarded to B.M. by the Xu Guangqi Programme 2019 (Campus France).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
Acknowledgments
English editing was revised with the assistance of Michelle Awad, Academic Writing Center, Université Paris-Saclay. Only the authors listed have contributed to the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AC | adenylyl cyclase |
| BMPR2 | bone morphogenetic protein receptor 2 |
| cAMP | 3′, 5′ cyclic adenosine monophosphate |
| cGMP | 3′, 5′ cyclic guanosine monophosphate |
| CHx | chronic hypoxia |
| EC | endothelial cell |
| EndoMT | endothelial-to-mesenchymal transition |
| eNOS | endothelial nitric oxide synthase |
| EPAC1 | exchange protein activated by cAMP 1 |
| ET-1 | endothelin-1 |
| iPAH | idiopathic pulmonary arterial hypertension |
| MCT | monocrotaline |
| mPAP | mean pulmonary arterial pressure |
| MRP4 | multidrug resistance-associated protein 4 |
| NO | nitric oxide |
| PA | pulmonary artery |
| pGC | particulate guanylyl cyclase |
| PGI2 | prostacyclin |
| PKA | cAMP-activated protein kinase |
| PKG | cGMP-activated protein kinase |
| PTGIS | PGI2 synthase |
| PH | pulmonary hypertension |
| PAH | pulmonary arterial hypertension |
| PAEC | pulmonary arterial endothelial cell |
| PASMC | pulmonary arterial smooth muscle cell |
| PDE | phosphodiesterase |
| PDE5i | phosphodiesterase type-5 inhibitors |
| RV | right ventricle |
| sGC | soluble guanylyl cyclase |
| SMC | smooth muscle cell |
| SuHx | Sugen5416– chronic hypoxia |
| TxA2 | thromboxane A2 |
References
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Identification of the most accurate estimates from a systematic literature review. Pulm. Circ. 2021, 11, 2045894020977300. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grunig, E.; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef]
- Sharma, M.; Paudyal, V.; Syed, S.K.; Thapa, R.; Kassam, N.; Surani, S. Management of Pulmonary Arterial Hypertension: Current Strategies and Future Prospects. Life 2025, 15, 430. [Google Scholar] [CrossRef]
- Chin, K.M.; Gaine, S.P.; Gerges, C.; Jing, Z.C.; Mathai, S.C.; Tamura, Y.; McLaughlin, V.V.; Sitbon, O. Treatment algorithm for pulmonary arterial hypertension. Eur. Respir. J. 2024, 64, 2401325. [Google Scholar] [CrossRef]
- Kelly, M.P.; Nikolaev, V.O.; Gobejishvili, L.; Lugnier, C.; Hesslinger, C.; Nickolaus, P.; Kass, D.A.; Pereira de Vasconcelos, W.; Fischmeister, R.; Brocke, S.; et al. Cyclic nucleotide phosphodiesterases as drug targets. Pharmacol. Rev. 2025, 77, 100042. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart. J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, M.; Guignabert, C.; Humbert, M.; Nicolls, M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Ghofrani, H.A. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2016, 71, 73–83. [Google Scholar] [CrossRef]
- Benza, R.L.; Miller, D.P.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef]
- Boucly, A.; Savale, L.; Jais, X.; Bauer, F.; Bergot, E.; Bertoletti, L.; Beurnier, A.; Bourdin, A.; Bouvaist, H.; Bulifon, S.; et al. Association between Initial Treatment Strategy and Long-Term Survival in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2021, 204, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; Rossano, J.W.; Toll, A.E.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J. Heart Lung Transpl. 2019, 38, 1042–1055. [Google Scholar] [CrossRef]
- Kolaitis, N.A. Lung Transplantation for Pulmonary Arterial Hypertension. Chest 2023, 164, 992–1006. [Google Scholar] [CrossRef]
- Guignabert, C.; Humbert, M. Targeting transforming growth factor-β receptors in pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002341. [Google Scholar] [CrossRef]
- Chen, C.N.; Watson, G.; Zhao, L. Cyclic guanosine monophosphate signalling pathway in pulmonary arterial hypertension. Vasc. Pharmacol. 2013, 58, 211–218. [Google Scholar] [CrossRef]
- Sassi, Y.; Hulot, J.S. Pulmonary hypertension: Novel pathways and emerging therapies inhibitors of cGMP and cAMP metabolism. Handb. Exp. Pharmacol. 2013, 218, 513–529. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Surapisitchat, J.; Beavo, J.A. Regulation of endothelial barrier function by cyclic nucleotides: The role of phosphodiesterases. In Handb Exp Pharmacol; Springer: Berlin/Heidelberg, Germany, 2011; pp. 193–210. [Google Scholar]
- Manoury, B.; Idres, S.; Leblais, V.; Fischmeister, R. Ion channels as effectors of cyclic nucleotide pathways: Functional relevance for arterial tone regulation. Pharmacol. Ther. 2020, 209, 107499. [Google Scholar] [CrossRef]
- Vina, D.; Seoane, N.; Vasquez, E.C.; Campos-Toimil, M. cAMP Compartmentalization in Cerebrovascular Endothelial Cells: New Therapeutic Opportunities in Alzheimer’s Disease. Cells 2021, 10, 1951. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Newby, A.C.; Bond, M. Ending Restenosis: Inhibition of Vascular Smooth Muscle Cell Proliferation by cAMP. Cells 2019, 8, 1447. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Al-Lamki, R.S.; Wu, C.; Weiss, A.; Berk, J.; Schermuly, R.T.; Morrell, N.W. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arter. Thromb. Vasc. Biol. 2013, 33, 34–42. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Al-Lamki, R.S.; Southwood, M.; Zhao, J.; Lever, A.M.; Grimminger, F.; Schermuly, R.T.; Morrell, N.W. Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ. Res. 2010, 107, 252–262. [Google Scholar] [CrossRef]
- Kamel, R.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiac hypertrophy and heart failure. Nat. Rev. Cardiol. 2022, 20, 90–108. [Google Scholar] [CrossRef]
- Benza, R.L.; Grunig, E.; Sandner, P.; Stasch, J.P.; Simonneau, G. The nitric oxide-soluble guanylate cyclase-cGMP pathway in pulmonary hypertension: From PDE5 to soluble guanylate cyclase. Eur. Respir. Rev. 2024, 33, 230183. [Google Scholar] [CrossRef]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef]
- Bobin, P.; Belacel-Ouari, M.; Bedioune, I.; Zhang, L.; Leroy, J.; Leblais, V.; Fischmeister, R.; Vandecasteele, G. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch. Cardiovasc. Dis. 2016, 109, 431–443. [Google Scholar] [CrossRef]
- Baliga, R.S.; Macallister, R.J.; Hobbs, A.J. Vasoactive peptides and the pathogenesis of pulmonary hypertension: Role and potential therapeutic application. Handb. Exp. Pharmacol. 2013, 218, 477–511. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Cooper, D.M. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 2007, 87, 965–1010. [Google Scholar] [CrossRef]
- Jourdan, K.B.; Mason, N.A.; Long, L.; Philips, P.G.; Wilkins, M.R.; Morrell, N.W. Characterization of adenylyl cyclase isoforms in rat peripheral pulmonary arteries. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L1359–L1369. [Google Scholar] [CrossRef] [PubMed]
- El-Haroun, H.; Bradbury, D.; Clayton, A.; Knox, A.J. Interleukin-1beta, transforming growth factor-beta1, and bradykinin attenuate cyclic AMP production by human pulmonary artery smooth muscle cells in response to prostacyclin analogues and prostaglandin E2 by cyclooxygenase-2 induction and downregulation of adenylyl cyclase isoforms 1, 2, and 4. Circ. Res. 2004, 94, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Mathie, A.; Peters, J.A. Guide to Receptors and Channels (GRAC), 5th edition. Br. J. Pharmacol. 2011, 164 (Suppl. S1), S1–S324. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3’,5’-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Sassi, Y.; Lipskaia, L.; Vandecasteele, G.; Nikolaev, V.O.; Hatem, S.N.; Cohen Aubart, F.; Russel, F.G.; Mougenot, N.; Vrignaud, C.; Lechat, P.; et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J. Clin. Investig. 2008, 118, 2747–2757. [Google Scholar] [CrossRef]
- Hara, Y.; Sassi, Y.; Guibert, C.; Gambaryan, N.; Dorfmuller, P.; Eddahibi, S.; Lompre, A.M.; Humbert, M.; Hulot, J.S. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J. Clin. Investig. 2011, 121, 2888–2897. [Google Scholar] [CrossRef]
- Omori, K.; Kotera, J. Overview of PDEs and their regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef]
- Maclean, M.R.; Johnston, E.D.; McCulloch, K.M.; Pooley, L.; Houslay, M.D.; Sweeney, G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: Changes in pulmonary hypertension. J. Pharmacol. Exp. Ther. 1997, 283, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Tenor, H.; Dent, G.; Schudt, C.; Na-kashima, M.; Magnussen, H. Identification of PDE isozymes in human pulmonary artery and effect of selective PDE inhibitors. Am. J. Physiol. 1994, 266, 536–543. [Google Scholar] [CrossRef]
- Crosswhite, P.; Sun, Z. Inhibition of phosphodiesterase-1 attenuates cold-induced pulmonary hypertension. Hypertension 2013, 61, 585–592. [Google Scholar] [CrossRef]
- Pauvert, O.; Salvail, D.; Rousseau, E.; Lugnier, C.; Marthan, R.; Savineau, J.P. Characterisation of cyclic nucleotide phosphodiesterase isoforms in the media layer of the main pulmonary artery. Biochem. Pharmacol. 2002, 63, 1763–1772. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Pullamsetti, S.S.; Kwapiszewska, G.; Dumitrascu, R.; Tian, X.; Weissmann, N.; Ghofrani, H.A.; Kaulen, C.; Dunkern, T.; Schudt, C.; et al. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: Target for reverse-remodeling therapy. Circulation 2007, 115, 2331–2339. [Google Scholar] [CrossRef]
- Growcott, E.J.; Spink, K.G.; Ren, X.; Afzal, S.; Banner, K.H.; Wharton, J. Phosphodiesterase type 4 expression and anti-proliferative effects in human pulmonary artery smooth muscle cells. Respir. Res. 2006, 7, 9. [Google Scholar] [CrossRef]
- Kimura, M.; Tamura, Y.; Guignabert, C.; Takei, M.; Kosaki, K.; Tanabe, N.; Tatsumi, K.; Saji, T.; Satoh, T.; Kataoka, M.; et al. A genome-wide association analysis identifies PDE1A|DNAJC10 locus on chromosome 2 associated with idiopathic pulmonary arterial hypertension in a Japanese population. Oncotarget 2017, 8, 74917–74926. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haynes, J., Jr.; Killilea, D.W.; Peterson, P.D.; Thompson, W.J. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic-3’,5’-guanosine monophosphate-stimulated phosphodiesterase to reverse hypoxic pulmonary vasoconstriction in the perfused rat lung. J. Pharmacol. Exp. Ther. 1996, 276, 752–757. [Google Scholar] [CrossRef]
- Bubb, K.J.; Trinder, S.L.; Baliga, R.S.; Patel, J.; Clapp, L.H.; MacAllister, R.J.; Hobbs, A.J. Inhibition of Phosphodiesterase 2 Augments cGMP and cAMP Signaling to Ameliorate Pulmonary Hypertension. Circulation 2014, 130, 496–507. [Google Scholar] [CrossRef]
- Suttorp, N.; Weber, U.; Welsch, T.; Schudt, C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J. Clin. Investig. 1993, 91, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; MacLean, M.R.; Pyne, N.J. Increased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertension. Br. J. Pharmacol. 2002, 137, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Sebkhi, A.; Strange, J.W.; Phillips, S.C.; Wharton, J.; Wilkins, M.R. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation 2003, 107, 3230–3235. [Google Scholar] [CrossRef]
- Phillips, P.G.; Long, L.; Wilkins, M.R.; Morrell, N.W. cAMP phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L103–L115. [Google Scholar] [CrossRef][Green Version]
- Murray, F.; Patel, H.H.; Suda, R.Y.; Zhang, S.; Thistlethwaite, P.A.; Yuan, J.X.; Insel, P.A. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: Role for PDE1. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L294–L303. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Vroom, C.; Ghofrani, H.A.; Weissmann, N.; Bieniek, E.; Grimminger, F.; Seeger, W.; Schermuly, R.T.; Pullamsetti, S.S. Phosphodiesterase 10A upregulation contributes to pulmonary vascular remodeling. PLoS ONE 2011, 6, e18136. [Google Scholar] [CrossRef] [PubMed]
- MacLean, M.R.; Sweeney, G.; Baird, M.; McCulloch, K.M.; Houslay, M.; Morecroft, I. 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br. J. Pharmacol. 1996, 119, 917–930. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Bhatia, V.; Hinton, M.; Singh, N.; Khan, M.W.; Chelikani, P.; Dakshinamurti, S. Adenylyl Cyclase Isoform 6 in the Pulmonary Artery Is Inhibited by Hypoxia via Cysteine Nitrosylation. Am. J. Respir. Cell Mol. Biol. 2024, 72, 82–96. [Google Scholar] [CrossRef]
- Cohen, A.H.; Hanson, K.; Morris, K.; Fouty, B.; McMurty, I.F.; Clarke, W.; Rodman, D.M. Inhibition of cyclic 3’-5’-guanosine monophosphate-specific phosphodiesterase selectively vasodilates the pulmonary circulation in chronically hypoxic rats. J. Clin. Investig. 1996, 97, 172–179. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Stasch, J.P.; Pullamsetti, S.S.; Middendorff, R.; Muller, D.; Schluter, K.D.; Dingendorf, A.; Hackemack, S.; Kolosionek, E.; Kaulen, C.; et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur. Respir. J. 2008, 32, 881–891. [Google Scholar] [CrossRef]
- Champion, H.C.; Bivalacqua, T.J.; Greenberg, S.S.; Giles, T.D.; Hyman, A.L.; Kadowitz, P.J. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13248–13253. [Google Scholar] [CrossRef]
- Grego, A.; Fernandes, C.; Fonseca, I.; Dias-Neto, M.; Costa, R.; Leite-Moreira, A.; Oliveira, S.M.; Trindade, F.; Nogueira-Ferreira, R. Endothelial dysfunction in cardiovascular diseases: Mechanisms and in vitro models. Mol. Cell Biochem. 2025, 480, 4671–4695. [Google Scholar] [CrossRef]
- Giaid, A.; Saleh, D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995, 333, 214–221. [Google Scholar] [CrossRef]
- Cella, G.; Bellotto, F.; Tona, F.; Sbarai, A.; Mazzaro, G.; Motta, G.; Fareed, J. Plasma markers of endothelial dysfunction in pulmonary hypertension. Chest 2001, 120, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Girgis, R.E.; Champion, H.C.; Diette, G.B.; Johns, R.A.; Permutt, S.; Sylvester, J.T. Decreased exhaled nitric oxide in pulmonary arterial hypertension: Response to bosentan therapy. Am. J. Respir. Crit. Care Med. 2005, 172, 352–357. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Higenbottam, T.W.; Dinh-Xuan, A.T.; Stone, D.; Wallwork, J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 1991, 338, 1173–1174. [Google Scholar] [CrossRef]
- Turanlahti, M.; Pesonen, E.; Pohjavuori, M.; Lassus, P.; Fyhrquist, F.; Andersson, S. Plasma cyclic guanosine monophosphate reflecting the severity of persistent pulmonary hypertension of the newborn. Biol. Neonate 2001, 80, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D., Jr.; Lang, P.; Bigatello, L.M.; Vlahakes, G.J.; Zapol, W.M. Inhaled nitric oxide in congenital heart disease. Circulation 1993, 87, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Beghetti, M.; Habre, W.; Friedli, B.; Berner, M. Continuous low dose inhaled nitric oxide for treatment of severe pulmonary hypertension after cardiac surgery in paediatric patients. Br. Heart J. 1995, 73, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Galie, N.; Grimminger, F.; Grunig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.P. Soluble Guanylate Cyclase Stimulators and Activators. Handb. Exp. Pharmacol. 2021, 264, 355–394. [Google Scholar] [CrossRef]
- Casserly, B.; Klinger, J.R. Brain natriuretic peptide in pulmonary arterial hypertension: Biomarker and potential therapeutic agent. Drug Des. Dev. Ther. 2009, 3, 269–287. [Google Scholar] [CrossRef]
- Klinger, J.R.; Warburton, R.R.; Pietras, L.A.; Smithies, O.; Swift, R.; Hill, N.S. Genetic disruption of atrial natriuretic peptide causes pulmonary hypertension in normoxic and hypoxic mice. Am. J. Physiol. 1999, 276, L868–L874. [Google Scholar] [CrossRef]
- Zhao, L.; Long, L.; Morrell, N.W.; Wilkins, M.R. NPR-A-Deficient mice show increased susceptibility to hypoxia-induced pulmonary hypertension. Circulation 1999, 99, 605–607. [Google Scholar] [CrossRef]
- Klinger, J.R.; Thaker, S.; Houtchens, J.; Preston, I.R.; Hill, N.S.; Farber, H.W. Pulmonary hemodynamic responses to brain natriuretic peptide and sildenafil in patients with pulmonary arterial hypertension. Chest 2006, 129, 417–425. [Google Scholar] [CrossRef]
- Michaels, A.D.; Chatterjee, K.; De Marco, T. Effects of intravenous nesiritide on pulmonary vascular hemodynamics in pulmonary hypertension. J. Card. Fail. 2005, 11, 425–431. [Google Scholar] [CrossRef]
- Baliga, R.S.; Zhao, L.; Madhani, M.; Lopez-Torondel, B.; Visintin, C.; Selwood, D.; Wilkins, M.R.; MacAllister, R.J.; Hobbs, A.J. Synergy between natriuretic peptides and phosphodiesterase 5 inhibitors ameliorates pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 861–869. [Google Scholar] [CrossRef]
- Clements, R.T.; Vang, A.; Fernandez-Nicolas, A.; Kue, N.R.; Mancini, T.J.; Morrison, A.R.; Mallem, K.; McCullough, D.J.; Choudhary, G. Treatment of Pulmonary Hypertension with Angiotensin II Receptor Blocker and Neprilysin Inhibitor Sacubitril/Valsartan. Circ. Heart Fail. 2019, 12, e005819. [Google Scholar] [CrossRef]
- Hobbs, A.J.; Moyes, A.J.; Baliga, R.S.; Ghedia, D.; Ochiel, R.; Sylvestre, Y.; Dore, C.J.; Chowdhury, K.; Maclagan, K.; Quartly, H.L.; et al. Neprilysin inhibition for pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled, proof-of-concept trial. Br. J. Pharmacol. 2019, 176, 1251–1267. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef] [PubMed]
- Adatia, I.; Barrow, S.E.; Stratton, P.D.; Miall-Allen, V.M.; Ritter, J.M.; Haworth, S.G. Thromboxane A2 and prostacyclin biosynthesis in children and adolescents with pulmonary vascular disease. Circulation 1993, 88, 2117–2122. [Google Scholar] [CrossRef]
- Christman, B.W.; McPherson, C.D.; Newman, J.H.; King, G.A.; Bernard, G.R.; Groves, B.M.; Loyd, J.E. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N. Engl. J. Med. 1992, 327, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Cool, C.D.; Geraci, M.W.; Wang, J.; Abman, S.H.; Wright, L.; Badesch, D.; Voelkel, N.F. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 159, 1925–1932. [Google Scholar] [CrossRef]
- Shaul, P.W.; Kinane, B.; Farrar, M.A.; Buja, L.M.; Magness, R.R. Prostacyclin production and mediation of adenylate cyclase activity in the pulmonary artery. Alterations after prolonged hypoxia in the rat. J. Clin. Investig. 1991, 88, 447–455. [Google Scholar] [CrossRef]
- Higenbottam, T.; Wheeldon, D.; Wells, F.; Wallwork, J. Long-term treatment of primary pulmonary hypertension with continuous intravenous epoprostenol (prostacyclin). Lancet 1984, 1, 1046–1047. [Google Scholar] [CrossRef]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; McGoon, M.D.; Rich, S.; Badesch, D.B.; Groves, B.M.; Tapson, V.F.; Bourge, R.C.; Brundage, B.H.; et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N. Engl. J. Med. 1996, 334, 296–301. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Shillington, A.; Rich, S. Survival in primary pulmonary hypertension: The impact of epoprostenol therapy. Circulation 2002, 106, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Zolty, R. Pulmonary arterial hypertension specific therapy: The old and the new. Pharmacol. Ther. 2020, 214, 107576. [Google Scholar] [CrossRef]
- Kuwano, K.; Hashino, A.; Asaki, T.; Hamamoto, T.; Yamada, T.; Okubo, K.; Kuwabara, K. 2-[4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy]-N-(methylsulfonyl)acetam ide (NS-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J. Pharmacol. Exp. Ther. 2007, 322, 1181–1188. [Google Scholar] [CrossRef]
- Simonneau, G.; Torbicki, A.; Hoeper, M.M.; Delcroix, M.; Karlocai, K.; Galie, N.; Degano, B.; Bonderman, D.; Kurzyna, M.; Efficace, M.; et al. Selexipag: An oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur. Respir. J. 2012, 40, 874–880. [Google Scholar] [CrossRef]
- Sitbon, O.; Channick, R.; Chin, K.M.; Frey, A.; Gaine, S.; Galie, N.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Preiss, R.; et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 2522–2533. [Google Scholar] [CrossRef] [PubMed]
- Wharton, J.; Strange, J.W.; Moller, G.M.; Growcott, E.J.; Ren, X.; Franklyn, A.P.; Phillips, S.C.; Wilkins, M.R. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am. J. Respir. Crit. Care Med. 2005, 172, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; Maclean, M.R.; Insel, P.A. Role of phosphodiesterases in adult-onset pulmonary arterial hypertension. In Handb Exp Pharmacol; Springer: Berlin/Heidelberg, Germany, 2011; pp. 279–305. [Google Scholar]
- Chang, L.T.; Sun, C.K.; Sheu, J.J.; Chiang, C.H.; Youssef, A.A.; Lee, F.Y.; Wu, C.J.; Yip, H.K. Cilostazol therapy attenuates monocrotaline-induced pulmonary arterial hypertension in rat model. Circ. J. 2008, 72, 825–831. [Google Scholar] [CrossRef]
- Ito, T.; Zhang, E.; Omori, A.; Kabwe, J.; Kawai, M.; Maruyama, J.; Okada, A.; Yokochi, A.; Sawada, H.; Mitani, Y.; et al. Model difference in the effect of cilostazol on the development of experimental pulmonary hypertension in rats. BMC Pulm. Med. 2021, 21, 377. [Google Scholar] [CrossRef]
- Xing, Y.; Hou, Y.; Fan, T.; Gao, R.; Feng, X.; Li, B.; Pang, J.; Guo, W.; Shu, T.; Li, J.; et al. Endothelial phosphodiesterase 4B inactivation ameliorates endothelial-to-mesenchymal transition and pulmonary hypertension. Acta Pharm. Sin. B 2024, 14, 1726–1741. [Google Scholar] [CrossRef]
- Izikki, M.; Raffestin, B.; Klar, J.; Hatzelmann, A.; Marx, D.; Tenor, H.; Zadigue, P.; Adnot, S.; Eddahibi, S. Effects of roflumilast, a phosphodiesterase-4 inhibitor, on hypoxia- and monocrotaline-induced pulmonary hypertension in rats. J. Pharmacol. Exp. Ther. 2009, 330, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Kreisselmeier, K.P.; Ghofrani, H.A.; Yilmaz, H.; Butrous, G.; Ermert, L.; Ermert, M.; Weissmann, N.; Rose, F.; Guenther, A.; et al. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2004, 169, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mason, N.A.; Morrell, N.W.; Kojonazarov, B.; Sadykov, A.; Maripov, A.; Mirrakhimov, M.M.; Aldashev, A.; Wilkins, M.R. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation 2001, 104, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mason, N.A.; Strange, J.W.; Walker, H.; Wilkins, M.R. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic Peptide activity. Circulation 2003, 107, 234–237. [Google Scholar] [CrossRef]
- Li, A.; Zhu, Z.; He, Y.; Dong, Q.; Tang, D.; Chen, Z.; Huang, W. DDCI-01, a novel long acting phospdiesterase-5 inhibitor, attenuated monocrotaline-induced pulmonary hypertension in rats. Pulm. Circ. 2020, 10, 2045894020939842. [Google Scholar] [CrossRef]
- Zhang, B.; Zou, Z.K.; Cai, J.F.; Tan, W.M.; Chen, J.W.; Li, W.E.; Liang, J.N.; Wu, W.P.; Wang, G.; Ruan, X.H.; et al. Discovery and Optimization of Dihydroquinolin-2(1H)-ones as Novel Highly Selective and Orally Bioavailable Phosphodiesterase 5 Inhibitors for the Treatment of Pulmonary Arterial Hypertension. J. Med. Chem. 2024, 67, 22134–22144. [Google Scholar] [CrossRef]
- Murray, F.; MacLean, M.R.; Pyne, N.J. An assessment of the role of the inhibitory gamma subunit of the retinal cyclic GMP phosphodiesterase and its effect on the p42/p44 mitogen-activated protein kinase pathway in animal and cellular models of pulmonary hypertension. Br. J. Pharmacol. 2003, 138, 1313–1319. [Google Scholar] [CrossRef]
- Kolb, T.M.; Johnston, L.; Damarla, M.; Kass, D.A.; Hassoun, P.M. PDE9A deficiency does not prevent chronic-hypoxic pulmonary hypertension in mice. Physiol. Rep. 2021, 9, e15057. [Google Scholar] [CrossRef]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef]
- Roks, A.J.M. Phosphodiesterase-1 in the cardiovascular system. Cell Signal 2022, 92, 110251. [Google Scholar] [CrossRef]
- Pauvert, O.; Lugnier, C.; Keravis, T.; Marthan, R.; Rousseau, E.; Savineau, J.P. Effect of sildenafil on cyclic nucleotide phosphodiesterase activity, vascular tone and calcium signaling in rat pulmonary artery. Br. J. Pharmacol. 2003, 139, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Nagel, D.J.; Zhou, Q.; Cygnar, K.D.; Zhao, H.; Li, F.; Pi, X.; Knight, P.A.; Yan, C. Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ. Res. 2015, 116, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Rybalkin, S.D.; Rybalkina, I.; Beavo, J.A.; Bornfeldt, K.E. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ. Res. 2002, 90, 151–157. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Busch, C.J.; Evgenov, N.V.; Liu, R.; Petersen, B.; Falkowski, G.E.; Petho, B.; Vas, A.; Bloch, K.D.; Zapol, W.M.; et al. Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs with acute pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L723–L729. [Google Scholar] [CrossRef]
- Ahn, H.S.; Crim, W.; Romano, M.; Sybertz, E.; Pitts, B. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem. Pharmacol. 1989, 38, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.N.; Miller, J.R. Methylxanthine inhibitors of phosphodiesterases. Methods Enzym. 1988, 159, 489–496. [Google Scholar] [CrossRef]
- Bonoczk, P.; Gulyas, B.; Adam-Vizi, V.; Nemes, A.; Karpati, E.; Kiss, B.; Kapas, M.; Szantay, C.; Koncz, I.; Zelles, T.; et al. Role of sodium channel inhibition in neuroprotection: Effect of vinpocetine. Brain Res. Bull. 2000, 53, 245–254. [Google Scholar] [CrossRef]
- Jeon, K.I.; Xu, X.; Aizawa, T.; Lim, J.H.; Jono, H.; Kwon, D.S.; Abe, J.; Berk, B.C.; Li, J.D.; Yan, C. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 9795–9800. [Google Scholar] [CrossRef]
- Yan, C.; Zhao, A.Z.; Bentley, J.K.; Beavo, J.A. The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific manner. J. Biol. Chem. 1996, 271, 25699–25706. [Google Scholar] [CrossRef]
- Laursen, M.; Beck, L.; Kehler, J.; Christoffersen, C.T.; Bundgaard, C.; Mogensen, S.; Mow, T.J.; Pinilla, E.; Knudsen, J.S.; Hedegaard, E.R.; et al. Novel selective PDE type 1 inhibitors cause vasodilatation and lower blood pressure in rats. Br. J. Pharmacol. 2017, 174, 2563–2575. [Google Scholar] [CrossRef]
- Le, M.L.; Jiang, M.Y.; Han, C.; Yang, Y.Y.; Wu, Y. PDE1 inhibitors: A review of the recent patent literature (2008-present). Expert. Opin. Ther. Pat. 2022, 32, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Nadur, N.F.; de Azevedo, L.L.; Caruso, L.; Graebin, C.S.; Lacerda, R.B.; Kummerle, A.E. The long and winding road of designing phosphodiesterase inhibitors for the treatment of heart failure. Eur. J. Med. Chem. 2020, 212, 113123. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.B.; Katugampola, S.; Able, S.; Napier, C.; Harding, S.E. Profiling of cAMP and cGMP phosphodiesterases in isolated ventricular cardiomyocytes from human hearts: Comparison with rat and guinea pig. Life Sci. 2012, 90, 328–336. [Google Scholar] [CrossRef]
- Beavo, J.A.; Francis, S.H.; Houslay, K.F. Cyclic Nucleotide Phosphodiesterases in Health and Disease; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sadek, M.S.; Cachorro, E.; El-Armouche, A.; Kammerer, S. Therapeutic Implications for PDE2 and cGMP/cAMP Mediated Crosstalk in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7462. [Google Scholar] [CrossRef]
- Martinez, S.E.; Wu, A.Y.; Glavas, N.A.; Tang, X.B.; Turley, S.; Hol, W.G.; Beavo, J.A. The two GAF domains in phosphodiesterase 2A have distinct roles in dimerization and in cGMP binding. Proc. Natl. Acad. Sci. USA 2002, 99, 13260–13265. [Google Scholar] [CrossRef]
- Wu, A.Y.; Tang, X.B.; Martinez, S.E.; Ikeda, K.; Beavo, J.A. Molecular determinants for cyclic nucleotide binding to the regulatory domains of phosphodiesterase 2A. J. Biol. Chem. 2004, 279, 37928–37938. [Google Scholar] [CrossRef]
- Wang, L.; Hubert, F.; Idres, S.; Belacel-Ouari, M.; Domergue, V.; Domenichini, S.; Lefebvre, F.; Mika, D.; Fischmeister, R.; Leblais, V.; et al. Phosphodiesterases type 2, 3 and 4 promote vascular tone in mesenteric arteries from rats with heart failure. Eur. J. Pharmacol. 2023, 944, 175562. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, K.; Hensley, K.; Florio, V.A.; Wolda, S.L. Differential expression of the cyclic GMP-stimulated phosphodiesterase PDE2A in human venous and capillary endothelial cells. J. Histochem. Cytochem. 1999, 47, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Podzuweit, T.; Nennstiel, P.; Muller, A. Isozyme selective inhibition of cGMP-stimulated cyclic nucleotide phosphodiesterases by erythro-9-(2-hydroxy-3-nonyl) adenine. Cell Signal 1995, 7, 733–738. [Google Scholar] [CrossRef]
- Mery, P.F.; Pavoine, C.; Pecker, F.; Fischmeister, R. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic GMP-stimulated phosphodiesterase in isolated cardiac myocytes. Mol. Pharmacol. 1995, 48, 121–130. [Google Scholar] [CrossRef]
- Boess, F.G.; Hendrix, M.; van der Staay, F.J.; Erb, C.; Schreiber, R.; van Staveren, W.; de Vente, J.; Prickaerts, J.; Blokland, A.; Koenig, G. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 2004, 47, 1081–1092. [Google Scholar] [CrossRef]
- Seybold, J.; Thomas, D.; Witzenrath, M.; Boral, S.; Hocke, A.C.; Burger, A.; Hatzelmann, A.; Tenor, H.; Schudt, C.; Krull, M.; et al. Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: Role in endothelial hyperpermeability. Blood 2005, 105, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.R.; Verde, I.; Cooper, D.M.; Fischmeister, R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 2006, 113, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Degerman, E.; Belfrage, P.; Manganiello, V.C. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J. Biol. Chem. 1997, 272, 6823–6826. [Google Scholar] [CrossRef]
- Sudo, T.; Tachibana, K.; Toga, K.; Tochizawa, S.; Inoue, Y.; Kimura, Y.; Hidaka, H. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem. Pharmacol. 2000, 59, 347–356. [Google Scholar] [CrossRef]
- Bardou, M.; Goirand, F.; Marchand, S.; Rouget, C.; Devillier, P.; Dumas, J.P.; Morcillo, E.J.; Rochette, L.; Dumas, M. Hypoxic vasoconstriction of rat main pulmonary artery: Role of endogenous nitric oxide, potassium channels, and phosphodiesterase inhibition. J. Cardiovasc. Pharmacol. 2001, 38, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.S.; Smith, C.J.; Taylor, A.M.; Rhoades, R.A. Phosphodiesterase inhibition improves agonist-induced relaxation of hypertensive pulmonary arteries. J. Pharmacol. Exp. Ther. 1997, 282, 1650–1657. [Google Scholar] [CrossRef]
- Clarke, W.R.; Uezono, S.; Chambers, A.; Doepfner, P. The type III phosphodiesterase inhibitor milrinone and type V PDE inhibitor dipyridamole individually and synergistically reduce elevated pulmonary vascular resistance. Pulm. Pharmacol. 1994, 7, 81–89. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Ghofrani, H.A.; Enke, B.; Weissmann, N.; Grimminger, F.; Seeger, W.; Schudt, C.; Walmrath, D. Low-dose systemic phosphodiesterase inhibitors amplify the pulmonary vasodilatory response to inhaled prostacyclin in experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 160, 1500–1506. [Google Scholar] [CrossRef]
- Chen, E.P.; Bittner, H.B.; Davis, R.D., Jr.; Van Trigt, P., 3rd. Milrinone improves pulmonary hemodynamics and right ventricular function in chronic pulmonary hypertension. Ann. Thorac. Surg. 1997, 63, 814–821. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Savai, R.; Schaefer, M.B.; Wilhelm, J.; Ghofrani, H.A.; Weissmann, N.; Schudt, C.; Fleming, I.; Mayer, K.; Leiper, J.; et al. cAMP phosphodiesterase inhibitors increases nitric oxide production by modulating dimethylarginine dimethylaminohydrolases. Circulation 2011, 123, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Matot, I.; Gozal, Y. Pulmonary responses to selective phosphodiesterase-5 and phosphodiesterase-3 inhibitors. Chest 2004, 125, 644–651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- James, A.T.; Corcoran, J.D.; McNamara, P.J.; Franklin, O.; El-Khuffash, A.F. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiol. Young 2016, 26, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bassler, D.; Choong, K.; McNamara, P.; Kirpalani, H. Neonatal persistent pulmonary hypertension treated with milrinone: Four case reports. Biol. Neonate 2006, 89, 1–5. [Google Scholar] [CrossRef]
- Mika, D.; Bobin, P.; Lindner, M.; Boet, A.; Hodzic, A.; Lefebvre, F.; Lechene, P.; Sadoune, M.; Samuel, J.L.; Algalarrondo, V.; et al. Synergic PDE3 and PDE4 control intracellular cAMP and cardiac excitation-contraction coupling in a porcine model. J. Mol. Cell Cardiol. 2019, 133, 57–66. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Krupnik, E.; Tenor, H.; Schudt, C.; Weissmann, N.; Rose, F.; Grimminger, F.; Seeger, W.; Walmrath, D.; Ghofrani, H.A. Coaerosolization of phosphodiesterase inhibitors markedly enhances the pulmonary vasodilatory response to inhaled iloprost in experimental pulmonary hypertension. Maintenance of lung selectivity. Am. J. Respir. Crit. Care Med. 2001, 164, 1694–1700. [Google Scholar] [CrossRef]
- Elmi-Sarabi, M.; Jarry, S.; Couture, E.J.; Haddad, F.; Cogan, J.; Sweatt, A.J.; Rousseau-Saine, N.; Beaubien-Souligny, W.; Fortier, A.; Denault, A.Y. Pulmonary Vasodilator Response of Combined Inhaled Epoprostenol and Inhaled Milrinone in Cardiac Surgical Patients. Anesth. Analg. 2023, 136, 282–294. [Google Scholar] [CrossRef]
- Fertig, B.A.; Baillie, G.S. PDE4-Mediated cAMP Signalling. J. Cardiovasc. Dev. Dis. 2018, 5, 8. [Google Scholar] [CrossRef]
- Rich, T.C.; Tse, T.E.; Rohan, J.G.; Schaack, J.; Karpen, J.W. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. J. Gen. Physiol. 2001, 118, 63–78. [Google Scholar] [CrossRef]
- Richeldi, L.; Azuma, A.; Cottin, V.; Kreuter, M.; Maher, T.M.; Martinez, F.J.; Oldham, J.M.; Valenzuela, C.; Clerisme-Beaty, E.; Gordat, M.; et al. Nerandomilast in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2025, 392, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Assassi, S.; Azuma, A.; Cottin, V.; Hoffmann-Vold, A.M.; Kreuter, M.; Oldham, J.M.; Richeldi, L.; Valenzuela, C.; Wijsenbeek, M.S.; et al. Nerandomilast in Patients with Progressive Pulmonary Fibrosis. N. Engl. J. Med. 2025, 392, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Kreisselmeier, K.P.; Ghofrani, H.A.; Samidurai, A.; Pullamsetti, S.; Weissmann, N.; Schudt, C.; Ermert, L.; Seeger, W.; Grimminger, F. Antiremodeling effects of iloprost and the dual-selective phosphodiesterase 3/4 inhibitor tolafentrine in chronic experimental pulmonary hypertension. Circ. Res. 2004, 94, 1101–1108. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Rose, F.; Schermuly, R.T.; Olschewski, H.; Wiedemann, R.; Weissmann, N.; Schudt, C.; Tenor, H.; Seeger, W.; Grimminger, F. Amplification of the pulmonary vasodilatory response to inhaled iloprost by subthreshold phosphodiesterase types 3 and 4 inhibition in severe pulmonary hypertension. Crit. Care Med. 2002, 30, 2489–2492. [Google Scholar] [CrossRef]
- Liu, S.F.; Nambiar Veetil, N.; Li, Q.; Kucherenko, M.M.; Knosalla, C.; Kuebler, W.M. Pulmonary hypertension: Linking inflammation and pulmonary arterial stiffening. Front. Immunol. 2022, 13, 959209. [Google Scholar] [CrossRef]
- Huertas, A.; Tu, L.; Humbert, M.; Guignabert, C. Chronic inflammation within the vascular wall in pulmonary arterial hypertension: More than a spectator. Cardiovasc. Res. 2020, 116, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.D.; Turko, I.V.; Beasley, A.; Francis, S.H. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 2000, 267, 2760–2767. [Google Scholar] [CrossRef]
- Francis, S.H.; Bessay, E.P.; Kotera, J.; Grimes, K.A.; Liu, L.; Thompson, W.J.; Corbin, J.D. Phosphorylation of isolated human phosphodiesterase-5 regulatory domain induces an apparent conformational change and increases cGMP binding affinity. J. Biol. Chem. 2002, 277, 47581–47587. [Google Scholar] [CrossRef]
- Zoraghi, R.; Bessay, E.P.; Corbin, J.D.; Francis, S.H. Structural and functional features in human PDE5A1 regulatory domain that provide for allosteric cGMP binding, dimerization, and regulation. J. Biol. Chem. 2005, 280, 12051–12063. [Google Scholar] [CrossRef][Green Version]
- Corbin, J.D.; Beasley, A.; Blount, M.A.; Francis, S.H. High lung PDE5: A strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem. Biophys. Res. Commun. 2005, 334, 930–938. [Google Scholar] [CrossRef]
- Nagendran, J.; Archer, S.L.; Soliman, D.; Gurtu, V.; Moudgil, R.; Haromy, A.; St Aubin, C.; Webster, L.; Rebeyka, I.M.; Ross, D.B.; et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 2007, 116, 238–248. [Google Scholar] [CrossRef]
- Mauricio, M.D.; Segarra, G.; Medina, P.; Aldasoro, M.; Martinez-Leon, J.B.; Vila, J.M. Relaxation and cyclic GMP levels in response to sildenafil in human pulmonary arteries from donors. Eur. J. Pharmacol. 2006, 530, 259–262. [Google Scholar] [CrossRef]
- Kang, K.K.; Ahn, G.J.; Sohn, Y.S.; Ahn, B.O.; Kim, W.B. DA-8159, a new PDE5 inhibitor, attenuates the development of compensatory right ventricular hypertrophy in a rat model of pulmonary hypertension. J. Int. Med. Res. 2003, 31, 517–528. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.Y.; Guan, Q. Oral sildenafil prevents and reverses the development of pulmonary hypertension in monocrotaline-treated rats. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 608–613. [Google Scholar] [CrossRef]
- Sauzeau, V.; Rolli-Derkinderen, M.; Lehoux, S.; Loirand, G.; Pacaud, P. Sildenafil prevents change in RhoA expression induced by chronic hypoxia in rat pulmonary artery. Circ. Res. 2003, 93, 630–637. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Tymchak, W.; Noga, M.; Webster, L.; Wu, X.C.; Lien, D.; Wang, S.H.; Modry, D.; Archer, S.L. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation 2003, 108, 2066–2069. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A.; et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Brundage, B.H.; Ghofrani, H.A.; Oudiz, R.J.; Simonneau, G.; Safdar, Z.; Shapiro, S.; White, R.J.; Chan, M.; Beardsworth, A.; et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009, 119, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.C.; Yu, Z.X.; Shen, J.Y.; Wu, B.X.; Xu, K.F.; Zhu, X.Y.; Pan, L.; Zhang, Z.L.; Liu, X.Q.; Zhang, Y.S.; et al. Vardenafil in pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2011, 183, 1723–1729. [Google Scholar] [CrossRef]
- Barnes, H.; Brown, Z.; Burns, A.; Williams, T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst. Rev. 2019, 1, CD012621. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Chaumais, M.C.; Savale, L.; Natali, D.; Price, L.C.; Jais, X.; Humbert, M.; Simonneau, G.; Sitbon, O. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv. Ther. 2009, 26, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int. J. Impot. Res. 2004, 16 (Suppl. S1), S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Lorigo, M.; Oliveira, N.; Cairrao, E. PDE-Mediated Cyclic Nucleotide Compartmentation in Vascular Smooth Muscle Cells: From Basic to a Clinical Perspective. J. Cardiovasc. Dev. Dis. 2021, 9, 4. [Google Scholar] [CrossRef]
- Guilluy, C.; Sauzeau, V.; Rolli-Derkinderen, M.; Guerin, P.; Sagan, C.; Pacaud, P.; Loirand, G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br. J. Pharmacol. 2005, 146, 1010–1018. [Google Scholar] [CrossRef]
- Li, S.; Pan, Y.; Ke, R.; Xie, X.; Zhai, C.; Shi, W.; Wang, J.; Yan, X.; Chai, L.; Wang, Q.; et al. Inhibition of phosphodiesterase-5 suppresses calcineurin/NFAT- mediated TRPC6 expression in pulmonary artery smooth muscle cells. Sci. Rep. 2017, 7, 6088. [Google Scholar] [CrossRef]
- Galie, N.; Muller, K.; Scalise, A.V.; Grunig, E. PATENT PLUS: A blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur. Respir. J. 2015, 45, 1314–1322. [Google Scholar] [CrossRef]
- Souza, R.; Kawut, S.M. What is new about Rio? Eur. Respir. J. 2015, 45, 1211–1213. [Google Scholar] [CrossRef]
- Li, Q.; Huang, S.S.; Zhang, D.C.; Zhang, W.Y.; Mao, Y.M.; Chen, R.; Jing, Z.C. Evaluation of Safety and Pharmacokinetics of DDCI-01, a Phosphodiesterase Type 5 Inhibitor, in Healthy Participants. Clin. Pharmacokinet. 2025, 64, 573–583. [Google Scholar] [CrossRef]
- Soderling, S.H.; Bayuga, S.J.; Beavo, J.A. Identification and Characterization of a Novel Family of Cyclic Nucleotide Phosphodiesterases. J. Biol. Chem. 1998, 273, 15553–15558. [Google Scholar] [CrossRef] [PubMed]
- Guipponi, M.; Scott, H.S.; Kudoh, J.; Kawasaki, K.; Shibuya, K.; Shintani, A.; Asakawa, S.; Chen, H.; Lalioti, M.D.; Rossier, C.; et al. Identification and characterization of a novel cyclic nucleotide phosphodiesterase gene (PDE9A) that maps to 21q22.3: Alternative splicing of mRNA transcripts, genomic structure and sequence. Hum. Genet. 1998, 103, 386–392. [Google Scholar] [CrossRef]
- Fisher, D.A.; Smith, J.F.; Pillar, J.S.; Denis, S.H.S.; Cheng, J.B.C. Isolation and Characterization of PDE9A, a Novel Human cGMP-specific Phosphodiesterase. J. Biol. Chem. 1998, 273, 15559–15564. [Google Scholar] [CrossRef]
- Patel, N.S.; Klett, J.; Pilarzyk, K.; Lee, D.I.; Kass, D.; Menniti, F.S.; Kelly, M.P. Identification of new PDE9A isoforms and how their expression and subcellular compartmentalization in the brain change across the life span. Neurobiol. Aging 2018, 65, 217–234. [Google Scholar] [CrossRef]
- Rentero, C.; Monfort, A.; Puigdomenech, P. Identification and distribution of different mRNA variants produced by differential splicing in the human phosphodiesterase 9A gene. Biochem. Biophys. Res. Commun. 2003, 301, 686–692. [Google Scholar] [CrossRef]
- Lee, D.I.; Zhu, G.; Sasaki, T.; Cho, G.S.; Hamdani, N.; Holewinski, R.; Jo, S.H.; Danner, T.; Zhang, M.; Rainer, P.P.; et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 2015, 519, 472–476. [Google Scholar] [CrossRef]
- van der Horst, I.W.; Morgan, B.; Eaton, F.; Reiss, I.; Tibboel, D.; Thebaud, B. Expression and function of phosphodiesterases in nitrofen-induced congenital diaphragmatic hernia in rats. Pediatr. Pulmonol. 2010, 45, 320–325. [Google Scholar] [CrossRef]
- Almeida, C.B.; Scheiermann, C.; Jang, J.E.; Prophete, C.; Costa, F.F.; Conran, N.; Frenette, P.S. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood 2012, 120, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Heckman, P.R.; Wouters, C.; Prickaerts, J. Phosphodiesterase inhibitors as a target for cognition enhancement in aging and Alzheimer’s disease: A translational overview. Curr. Pharm. Des. 2015, 21, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, R.J.; Chapin, D.S.; Christoffersen, C.; Freeman, J.; Fonseca, K.R.; Geoghegan, K.F.; Grimwood, S.; Guanowsky, V.; Hajos, M.; Harms, J.F.; et al. Phosphodiesterase 9A regulates central cGMP and modulates responses to cholinergic and monoaminergic perturbation in vivo. J. Pharmacol. Exp. Ther. 2012, 341, 396–409. [Google Scholar] [CrossRef]
- Hutson, P.H.; Finger, E.N.; Magliaro, B.C.; Smith, S.M.; Converso, A.; Sanderson, P.E.; Mullins, D.; Hyde, L.A.; Eschle, B.K.; Turnbull, Z.; et al. The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2H-py ran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology 2011, 61, 665–676. [Google Scholar] [CrossRef]
- Wunder, F.; Tersteegen, A.; Rebmann, A.; Erb, C.; Fahrig, T.; Hendrix, M. Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol. Pharmacol. 2005, 68, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Aronovitz, M.J.; Liu, P.; Martin, G.L.; Tam, K.; Pande, S.; Karas, R.H.; Bloomfield, D.M.; Mendelsohn, M.E.; Blanton, R.M. CRD-733, a Novel PDE9 (Phosphodiesterase 9) Inhibitor, Reverses Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2021, 14, e007300. [Google Scholar] [CrossRef] [PubMed]
- Rosenbrock, H.; Giovannini, R.; Schanzle, G.; Koros, E.; Runge, F.; Fuchs, H.; Marti, A.; Reymann, K.G.; Schroder, U.H.; Fedele, E.; et al. The Novel Phosphodiesterase 9A Inhibitor BI 409306 Increases Cyclic Guanosine Monophosphate Levels in the Brain, Promotes Synaptic Plasticity, and Enhances Memory Function in Rodents. J. Pharmacol. Exp. Ther. 2019, 371, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Buncha, V.; Fopiano, K.A.; Lang, L.; Ilatovskaya, D.V.; Verin, A.; Bagi, Z. Phosphodiesterase 9A inhibition improves aging-related increase in pulmonary vascular resistance in mice. Geroscience 2024, 46, 5191–5202. [Google Scholar] [CrossRef]
- Scott, N.J.A.; Prickett, T.C.R.; Charles, C.J.; Frampton, C.M.; Richards, A.M.; Rademaker, M.T. Augmentation of Natriuretic Peptide Bioactivity via Combined Inhibition of Neprilysin and Phosphodiesterase-9 in Heart Failure. JACC Heart Fail. 2022, 11, 227–239. [Google Scholar] [CrossRef]
- Scott, N.J.A.; Rademaker, M.T.; Charles, C.J.; Espiner, E.A.; Richards, A.M. Hemodynamic, Hormonal, and Renal Actions of Phosphodiesterase-9 Inhibition in Experimental Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 889–901. [Google Scholar] [CrossRef]
- Rademaker, M.T.; Scott, N.J.A.; Charles, C.J.; Richards, A.M. Combined Inhibition of Phosphodiesterases 5 and 9 in Experimental Heart Failure. JACC Heart Fail. 2023, 12, 100–113. [Google Scholar] [CrossRef]
- Claffey, M.M.; Helal, C.J.; Verhoest, P.R.; Kang, Z.; Fors, K.S.; Jung, S.; Zhong, J.; Bundesmann, M.W.; Hou, X.; Lui, S.; et al. Application of structure-based drug design and parallel chemistry to identify selective, brain penetrant, in vivo active phosphodiesterase 9A inhibitors. J. Med. Chem. 2012, 55, 9055–9068. [Google Scholar] [CrossRef]
- Zhang, L.; Bouadjel, K.; Manoury, B.; Vandecasteele, G.; Fischmeister, R.; Leblais, V. Cyclic nucleotide signalling compartmentation by PDEs in cultured vascular smooth muscle cells. Br. J. Pharmacol. 2019, 176, 1780–1792. [Google Scholar] [CrossRef]
- Charnigo, R.J.; Beidler, D.; Rybin, D.; Pittman, D.D.; Tan, B.; Howard, J.; Michelson, A.D.; Frelinger, A.L., III; Clarke, N. PF-04447943, a Phosphodiesterase 9A Inhibitor, in Stable Sickle Cell Disease Patients: A Phase Ib Randomized, Placebo-Controlled Study. Clin. Transl. Sci. 2019, 12, 180–188. [Google Scholar] [CrossRef]
- Moschetti, V.; Boland, K.; Feifel, U.; Hoch, A.; Zimdahl-Gelling, H.; Sand, M. First-in-human study assessing safety, tolerability and pharmacokinetics of BI 409306, a selective phosphodiesterase 9A inhibitor, in healthy males. Br. J. Clin. Pharmacol. 2016, 82, 1315–1324. [Google Scholar] [CrossRef]
- Jager, R.; Russwurm, C.; Schwede, F.; Genieser, H.G.; Koesling, D.; Russwurm, M. Activation of PDE10 and PDE11 phosphodiesterases. J. Biol. Chem. 2012, 287, 1210–1219. [Google Scholar] [CrossRef]
- Gross-Langenhoff, M.; Hofbauer, K.; Weber, J.; Schultz, A.; Schultz, J.E. cAMP is a ligand for the tandem GAF domain of human phosphodiesterase 10 and cGMP for the tandem GAF domain of phosphodiesterase 11. J. Biol. Chem. 2006, 281, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, X.; Xu, J.; Rothfuss, J.; Mach, R.H.; Tu, Z. Synthesis and in vitro evaluation of new analogues as inhibitors for phosphodiesterase 10A. Eur. J. Med. Chem. 2011, 46, 3986–3995. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, S.; Zhou, Q.; Zhang, C.; Gao, Y.; Wang, H.; Li, Z.; Wu, D.; Wu, Y.; Huang, Y.Y.; et al. Discovery of highly selective and orally available benzimidazole-based phosphodiesterase 10 inhibitors with improved solubility and pharmacokinetic properties for treatment of pulmonary arterial hypertension. Acta Pharm. Sin. B 2020, 10, 2339–2347. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Yu, Y.F.; Zhang, C.; Chen, Y.; Zhou, Q.; Li, Z.; Zhou, S.; Li, Z.; Guo, L.; Wu, D.; et al. Validation of Phosphodiesterase-10 as a Novel Target for Pulmonary Arterial Hypertension via Highly Selective and Subnanomolar Inhibitors. J. Med. Chem. 2019, 62, 3707–3721. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arroyo, J.; Saleem, S.J.; Mizuno, S.; Syed, A.A.; Bogaard, H.J.; Abbate, A.; Taraseviciene-Stewart, L.; Sung, Y.; Kraskauskas, D.; Farkas, D.; et al. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L977–L991. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Lighthouse, J.K.; Mickelsen, D.M.; Wu, J.; Yao, P.; Small, E.M.; Yan, C. A Novel Role of Cyclic Nucleotide Phosphodiesterase 10A in Pathological Cardiac Remodeling and Dysfunction. Circulation 2020, 141, 217–233. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Chapin, D.S.; Cianfrogna, J.; Corman, M.L.; Hajos, M.; Harms, J.F.; Hoffman, W.E.; Lebel, L.A.; McCarthy, S.A.; Nelson, F.R.; et al. Preclinical characterization of selective phosphodiesterase 10A inhibitors: A new therapeutic approach to the treatment of schizophrenia. J. Pharmacol. Exp. Ther. 2008, 325, 681–690. [Google Scholar] [CrossRef]
- Molina, C.E.; Johnson, D.M.; Mehel, H.; Spatjens, R.L.; Mika, D.; Algalarrondo, V.; Slimane, Z.H.; Lechene, P.; Abi-Gerges, N.; van der Linde, H.J.; et al. Interventricular differences in beta-adrenergic responses in the canine heart: Role of phosphodiesterases. J. Am. Heart Assoc. 2014, 3, e000858. [Google Scholar] [CrossRef] [PubMed]
- Ghigo, A.; Mika, D. cAMP/PKA signaling compartmentalization in cardiomyocytes: Lessons from FRET-based biosensors. J. Mol. Cell Cardiol. 2019, 131, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abou-Saleh, H.; Kameno, Y.; Marei, I.; de Nucci, G.; Ahmetaj-Shala, B.; Shala, F.; Kirkby, N.S.; Jennings, L.; Al-Ansari, D.E.; et al. Studies on metal-organic framework (MOF) nanomedicine preparations of sildenafil for the future treatment of pulmonary arterial hypertension. Sci. Rep. 2021, 11, 4336. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).