Neonatal Febrile Seizures in Rats Induce Long-Term Region-Specific Alterations in the Glutamatergic System of Hippocampal–Prefrontal Circuits and Lead to Behavioral Deficits
Highlights
- This study provides the first comprehensive analysis of long-term, region-specific changes in the entire glutamatergic system (iGluRs, mGluRs, EAATs) after neonatal febrile seizures.
- The most severe and persistent molecular alterations occur in the ventral hippocampus and medial prefrontal cortex, key nodes for emotional regulation and cognition.
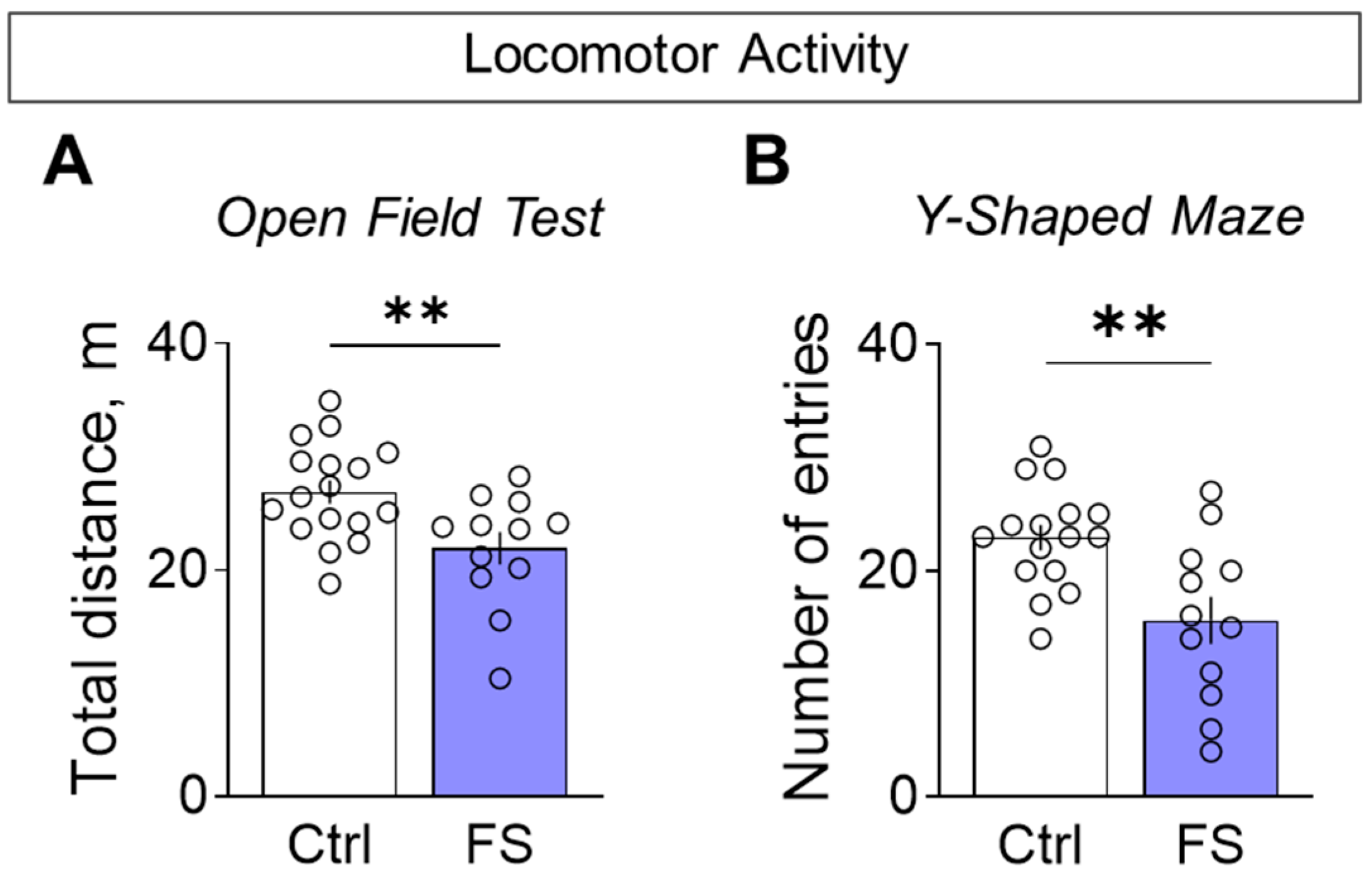
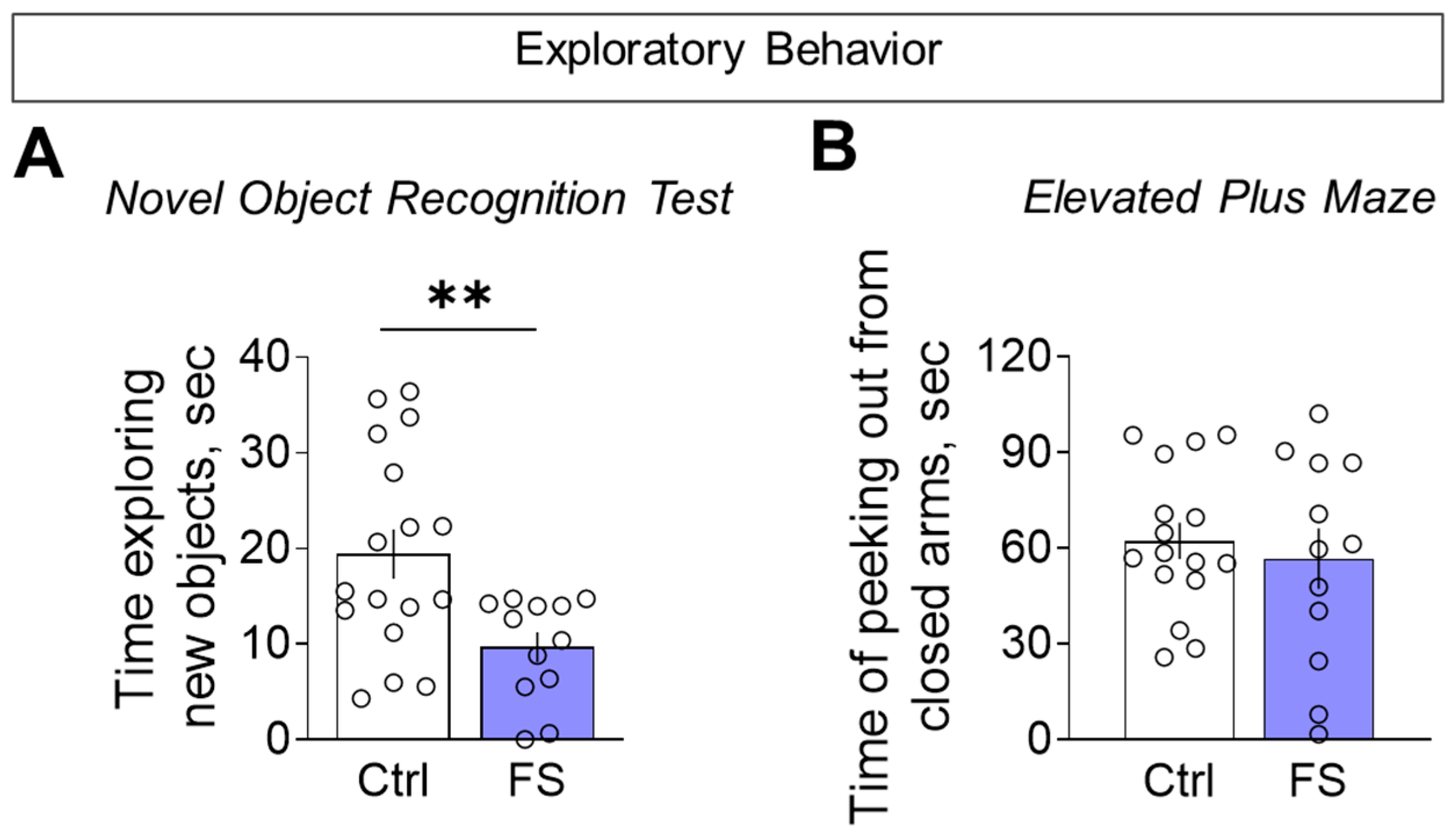
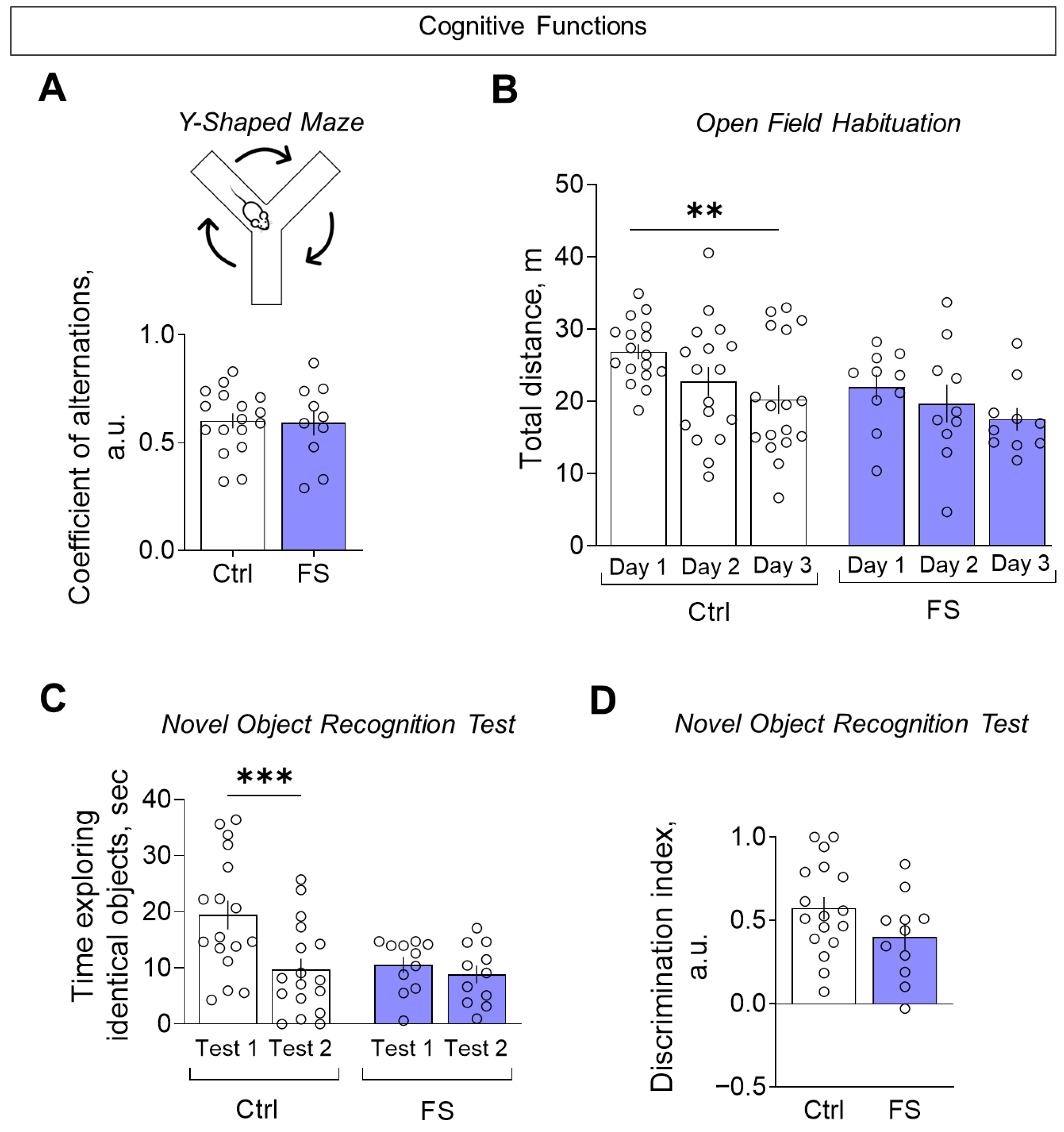
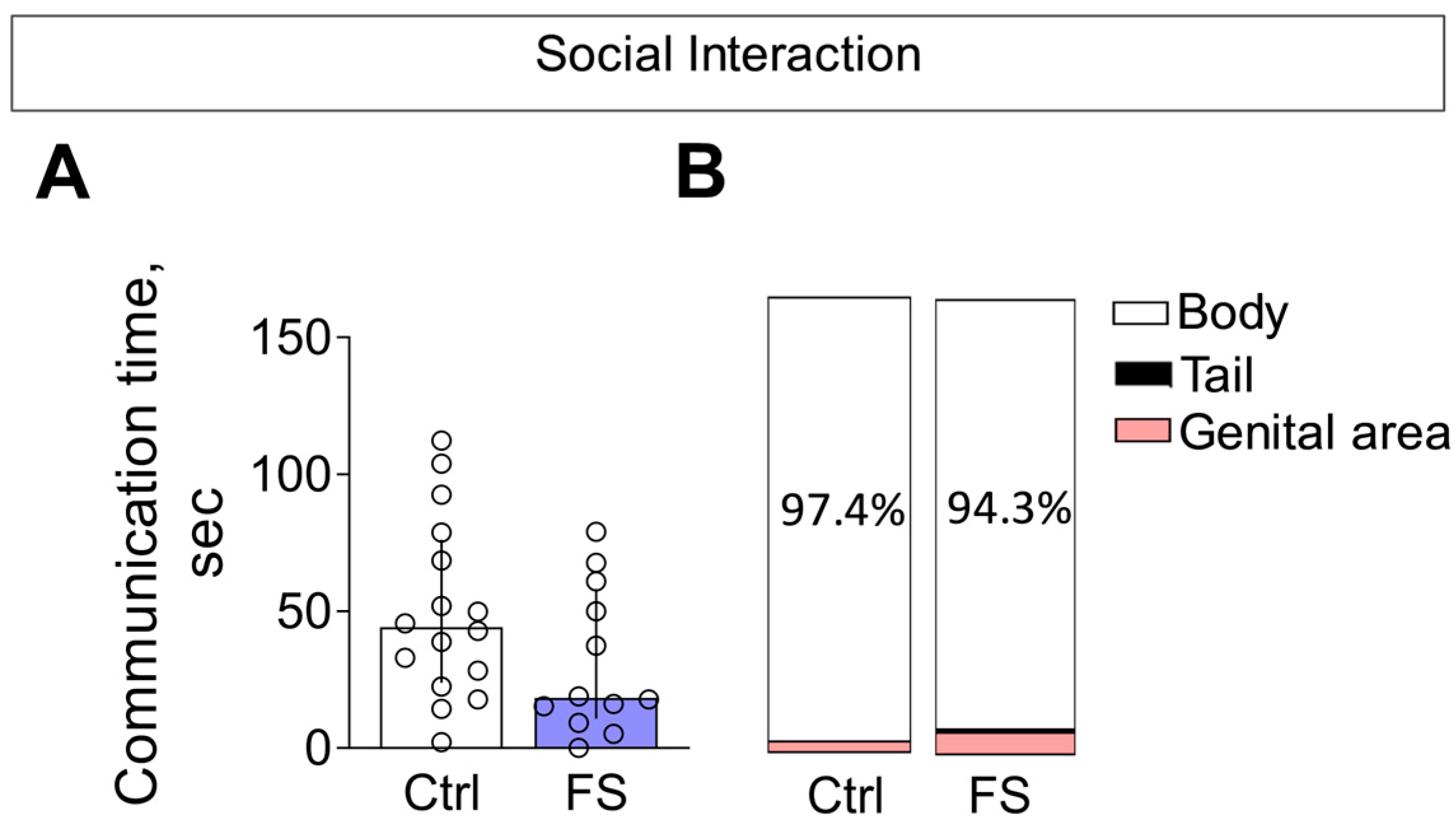
- The behavioral outcome is characterized by a hyperanxious phenotype with locomotor and habituation deficits, but with working memory and sociability remaining unaffected.
- The findings reveal a precise neurobiological mechanism that could underlie the increased risk of anxiety-related disorders following early-life febrile seizures.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
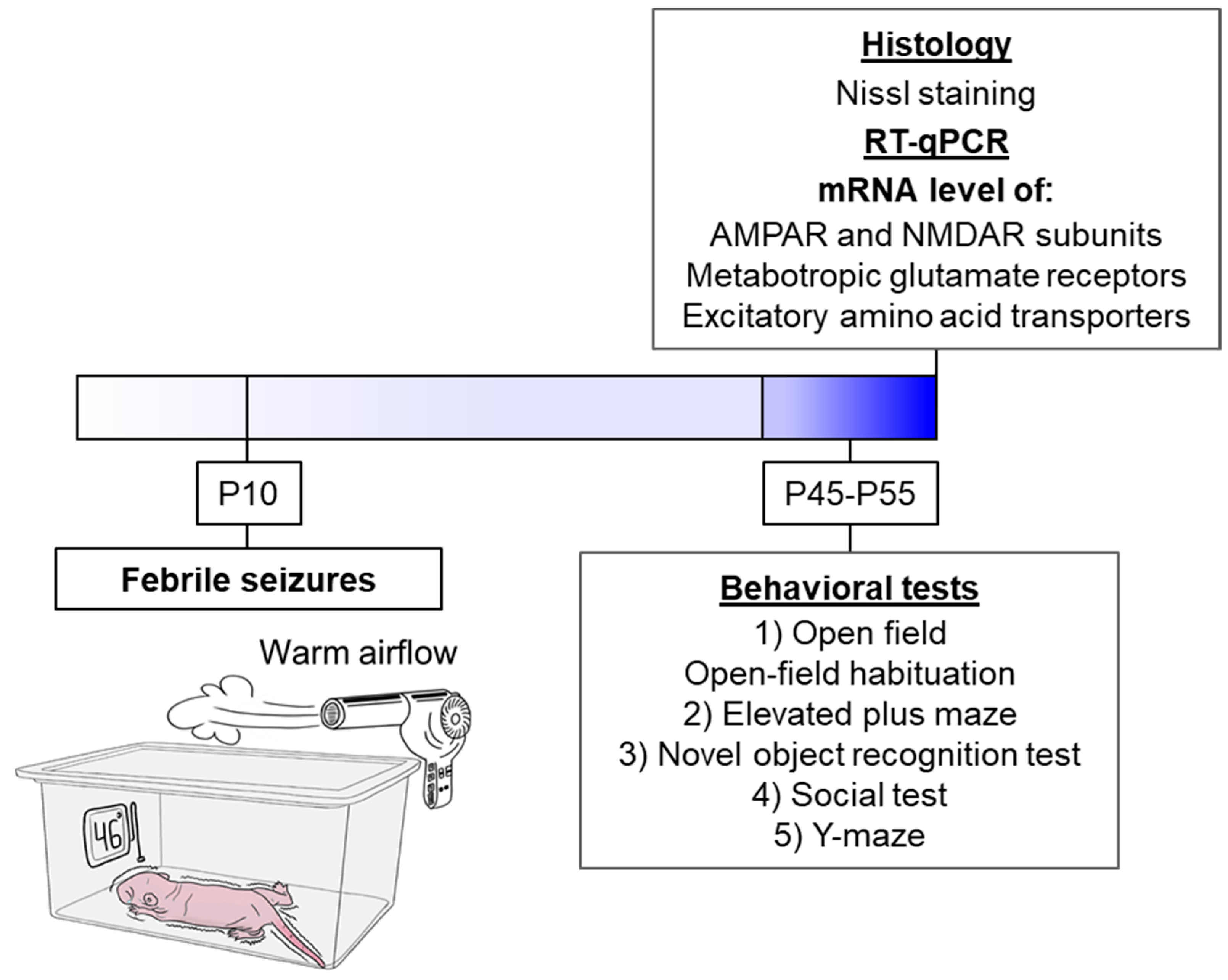
2.2. Febrile Seizure Model
2.3. Behavioral Testing
2.3.1. Open Field Test
2.3.2. Open-Field Habituation
2.3.3. Elevated Plus Maze
2.3.4. Novel Object Recognition Test
2.3.5. Social Interaction Test
2.3.6. Y-Shaped Maze Spontaneous Alternation Test
2.4. mRNA Expression Analysis
| Gene Symbol RefSeq Accession Number | Forward Primer, Reverse Primer, and Probe Sequences (5′→3′) | Final Concentrations, nM | References |
|---|---|---|---|
| Actb NM_031144 | TGTCACCAACTGGGACGATA GGGGTGTTGAAGGTCTCAAA FAM-CGTGTGGCCCCTGAGGAGCAC-BHQ1 | 200 200 | [58] (primers) [55] (probe) |
| Gapdh NM_017008 | TGCACCACCAACTGCTTAG GGATGCAGGGATGATGTTC R6G-ATCACGCCACAGCTTTCCAGAGGG-BHQ2 | 200 100 | [59] |
| B2m NM_012512 | TGCCATTCAGAAAACTCCCC GAGGAAGTTGGGCTTCCCATT ROX-ATTCAAGTGTACTCTCGCCATCCACCG-BHQ1 | 200 100 | [60] |
| Rpl13a NM_173340 | GGATCCCTCCACCCTATGACA CTGGTACTTCCACCCGACCTC FAM-CTGCCCTCAAGGTTGTGCGGCT-BHQ1 | 200 100 | [61] (primers) [55] (probe) |
| Sdha NM_130428 | AGACGTTTGACAGGGGAATG TCATCAATCCGCACCTTGTA R6G-ACCTGGTGGAGACGCTGGAGCT-BHQ2 | 200 100 | [62] (primers) [55] (probe) |
| Ppia NM_017101 | AGGATTCATGTGCCAGGGTG CTCAGTCTTGGCAGTGCAGA ROX-CACGCCATAATGGCACTGGTGGCA-BHQ1 | 200 100 | [63] |
| Hprt1 NM_012583 | TCCTCAGACCGCTTTTCCCGC TCATCATCACTAATCACGACGCTGG FAM-CCGACCGGTTCTGTCATGTCGACCCT-BHQ1 | 200 100 | [64] (primers) [55] (probe) |
| Pgk1 NM_053291 | ATGCAAAGACTGGCCAAGCTAC AGCCACAGCCTCAGCATATTTC R6G-TGCTGGCTGGATGGGCTTGGA-BHQ2 | 200 100 | [65] (primers) [55] (probe) |
| Ywhaz NM_013011 | GATGAAGCCATTGCTGAACTTG GTCTCCTTGGGTATCCGATGTC ROX-TGAAGAGTCGTACAAAGACAGCACGC-BHQ1 | 200 100 | [65] (primers) [55] (probe) |
| Grm1 NM_001114330.1 | GGGAATGCCAAGAAGAGGCAG CTGCAGTGTGGGGGTTTTCAA FAM-GCCCAGCAGCCAGTGTCCGTCGGC-BHQ1 | 400 200 | [66] |
| Grm2 NM_001105711.1 | TCCAGTGATTATCGGGTGCAG AACTTGGGTGCAAAGAGGCA FAM-TGCGTGTCCGTCAGCCTCAGTGGCT-BHQ1 | 200 100 | [66] |
| Grm3 NM_001105712.1 | CAGGAGTTGACGGTGCGAG GCCTGTCCTTCAGATAAGGGAG ROX-TCGGTGACGGGCTCTTTCAGCCCAA-BHQ2 | 200 200 | [66] |
| Grm4 NM_022666.1 | GGCAGTGCGAGCAGCTAAGG CCGGTCACTCCTACCAACCG FAM-CTCCCTGAGCTCCCCCGGAGCAGC-BHQ1 | 200 150 | [66] |
| Grm5 NM_017012.1 | ATGCATGTAGGAGACGGCAA TTTCCGTTGGAGCTTAGGGTTT HEX-CGTCCGCTGCCAGCAGATCCAGCA-BHQ2 | 400 200 | [67] (primers) [66] (probe) |
| Grm7 NM_031040.1 | CCAGACAACAAACACAACCAACC GCGTTCCCTTCTGTGTCTTCTTC HEX-TGCAGTGGGGCAAAGGAGTCCGAG-BHQ2 | 200 100 | [68] (primers) [66] (probe) |
| Grm8 NM_022202.1 | TCATCGGGCACTGGACAAAT CACGGTTTTCTTCCTCTCCCC ROX-TGTCTGCAGCCTGCCGTGCAAGCCC-BHQ2 | 300 100 | [66] |
| Grin1 NM_017010 | GTTCTTCCGCTCAGGCTTTG AGGGAAACGTTCTGCTTCCA FAM-CGGCATGCGCAAGGACAGCC-BHQ1 | 200 100 | [69] |
| Grin2a NM_012573 | GCTACACACCCTGCACCAATT CACCTGGTAACCTTCCTCAGTGA FAM-TGGTCAATGTGACTTGGGATGGCAA-BHQ1 | 200 100 | [70] |
| Grin2b NM_012574 | CCCAACATGCTCTCTCCCTTAA CAGCTAGTCGGCTCTCTTGGTT FAM-GACGCCAAACCTCTAGGCGGACAG-BHQ1 | 200 100 | [70] |
| Gria1 NM_031608 | TCAGAACGCCTCAACGCC TGTAGTGGTACCCGATGCCA ROX-TCCTGGGCCAGATCGTGAAGCTAGAAAA-BHQ1 | 200 100 | [71] |
| Gria2 NM_017261 | CAGTGCATTTCGGGTAGGGA TGCGAAACTGTTGGCTACCT FAM-TCGGAGTTCAGACTGACACCCCA-BHQ1 | 200 100 | [71] |
| Slc1a1 NM_013032.3 | CCTGCATCCCTCATCCCAC CTCCTACCACGATGCCCAGTA HEX-CCGCCGCGCTCCCCGATTCC-BHQ2 | 200 100 | [72] |
| Slc1a2 NM_001035233.1 | CCAGTGCTGGAACTTTGCCT TAAAGGGCTGTACCATCCAT FAM-AGCGTGTGACCAGATTCGTCCTCCCA-BHQ1 | 200 150 | [73] (primers) [74] (probe) |
| Slc1a3 NM_019225.2 | GCGCTGTCATTGTGGGTACA CAGAAGCTCCCCAGGAAAGG Cy5-CCTTGGATTTGCCCTCCGACCGT-BHQ3 | 200 100 | [75] |
2.5. Histology
2.5.1. Preparation of Brain Tissue for Histological Examination
2.5.2. Staining of Sections Using the Nissl Method
2.6. Statistical Analysis
3. Results
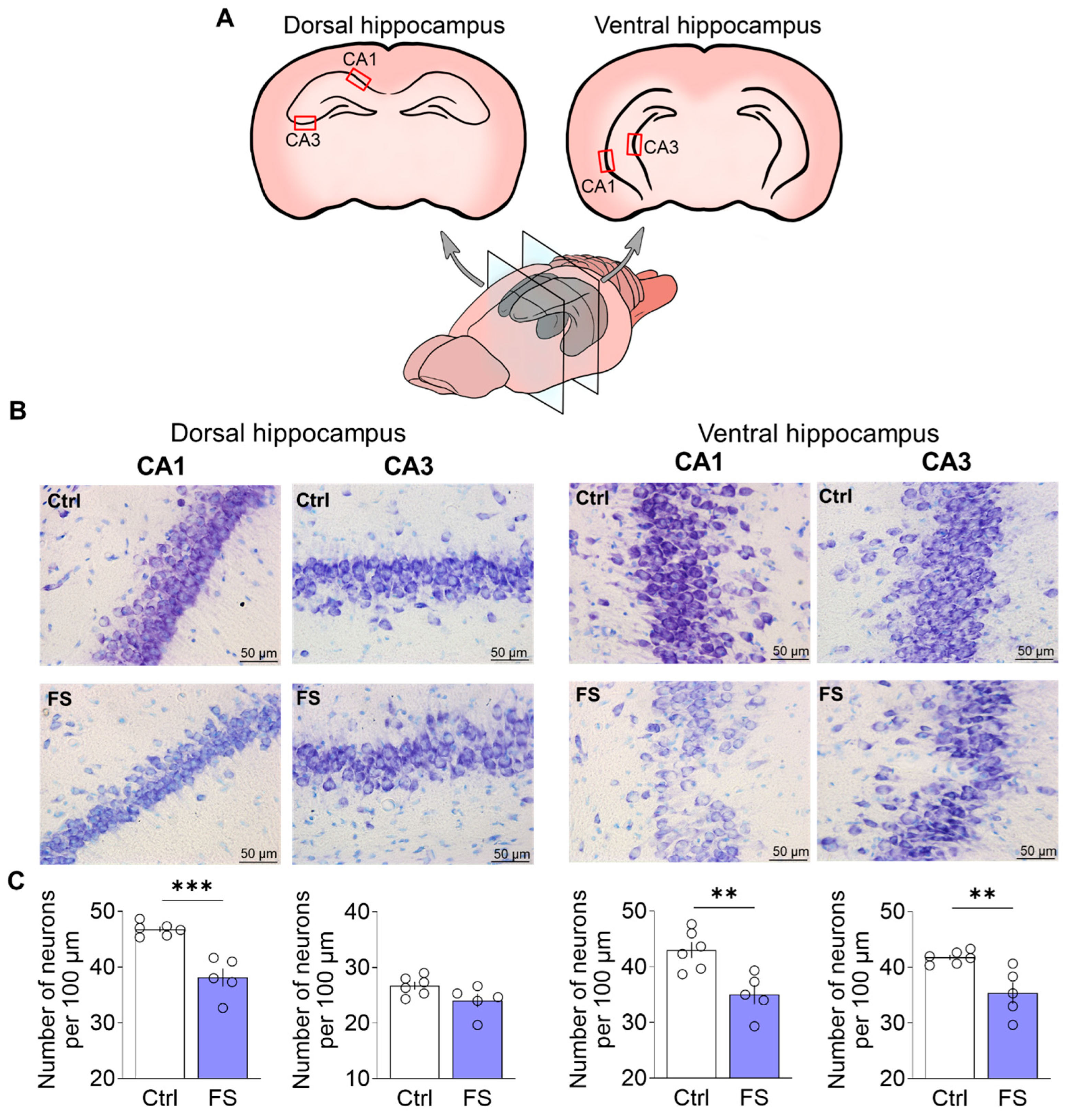
3.1. Febrile Seizures Provoke Neuronal Loss in the Rat Hippocampus
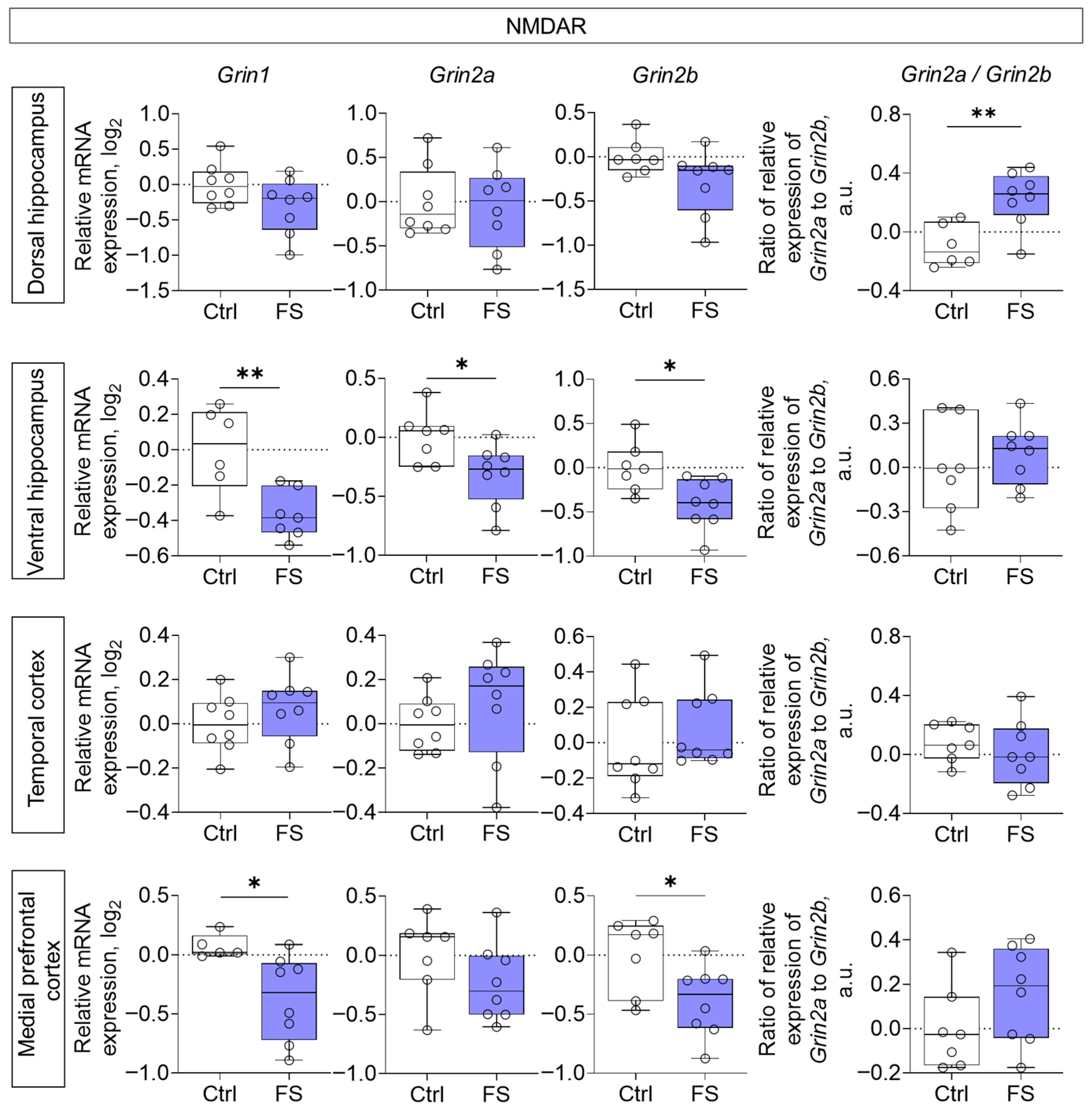
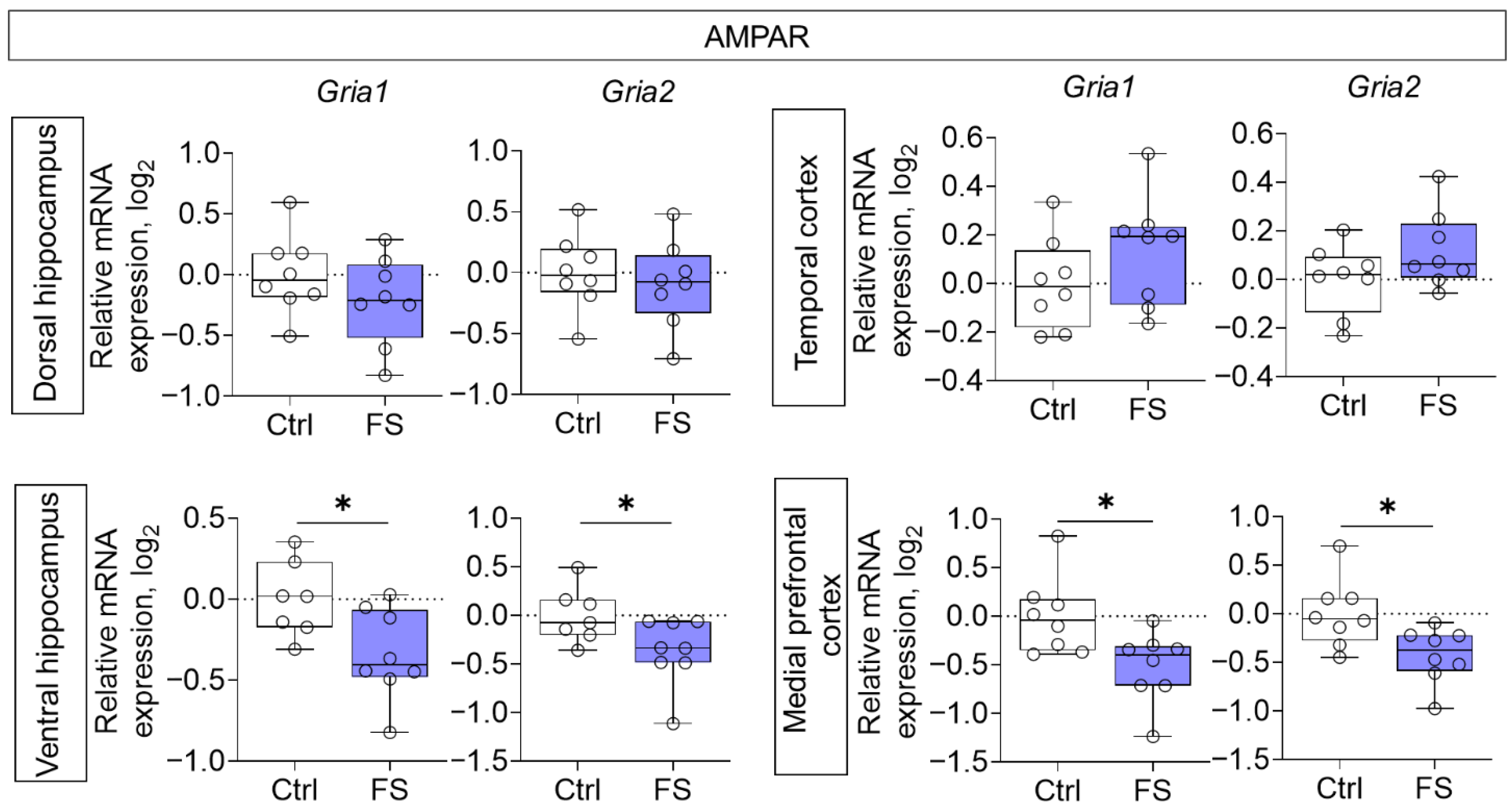
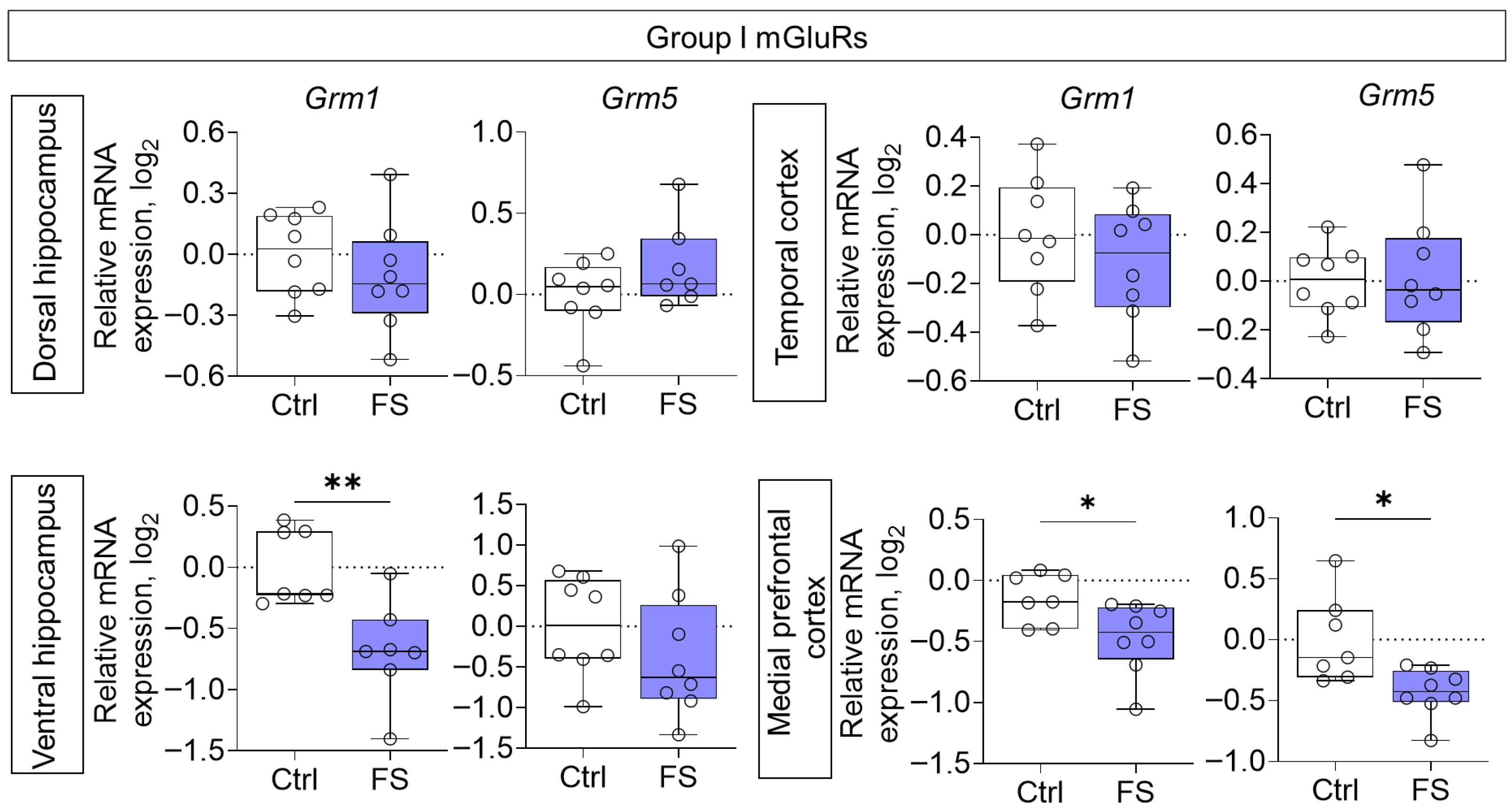
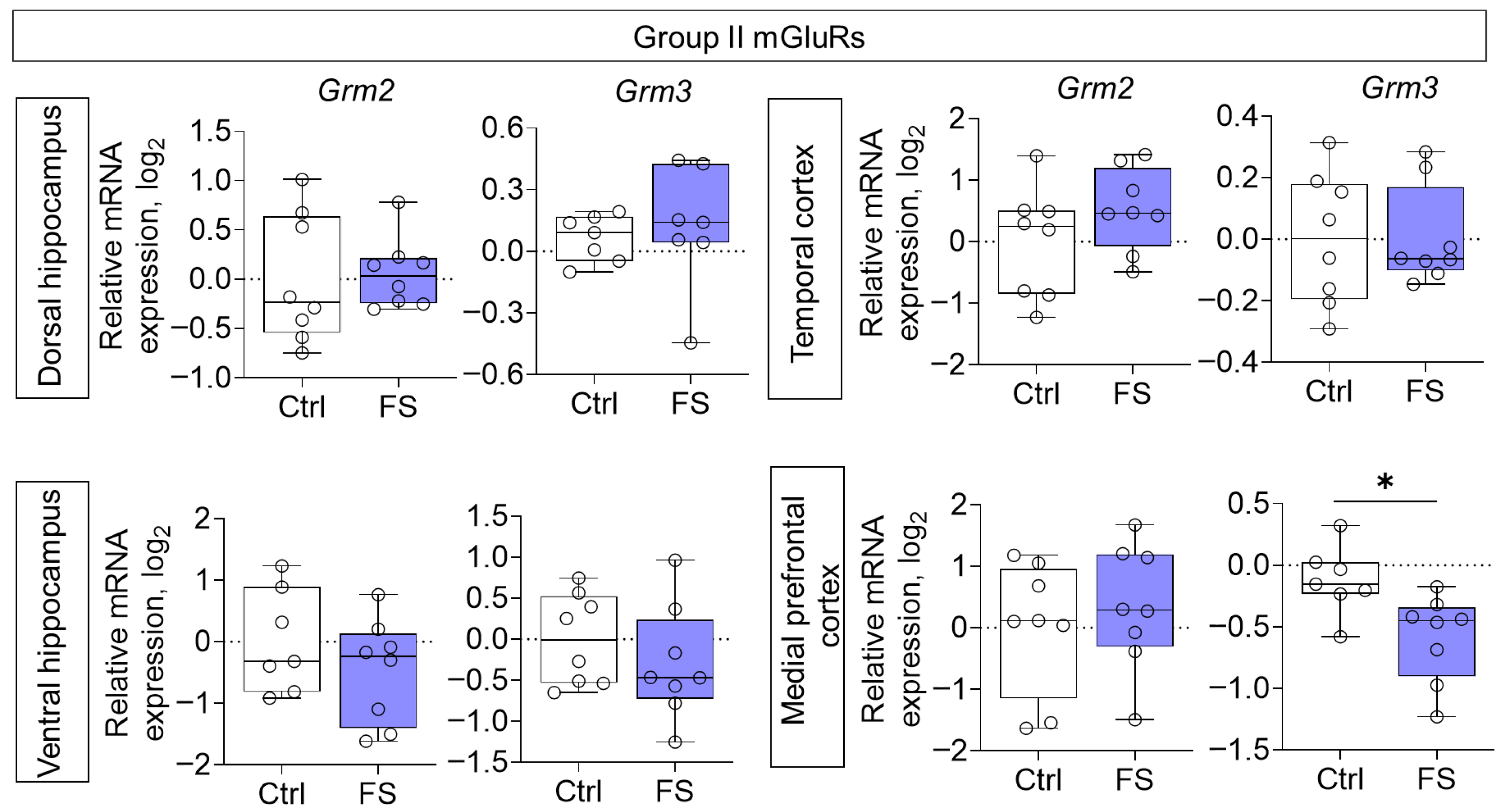
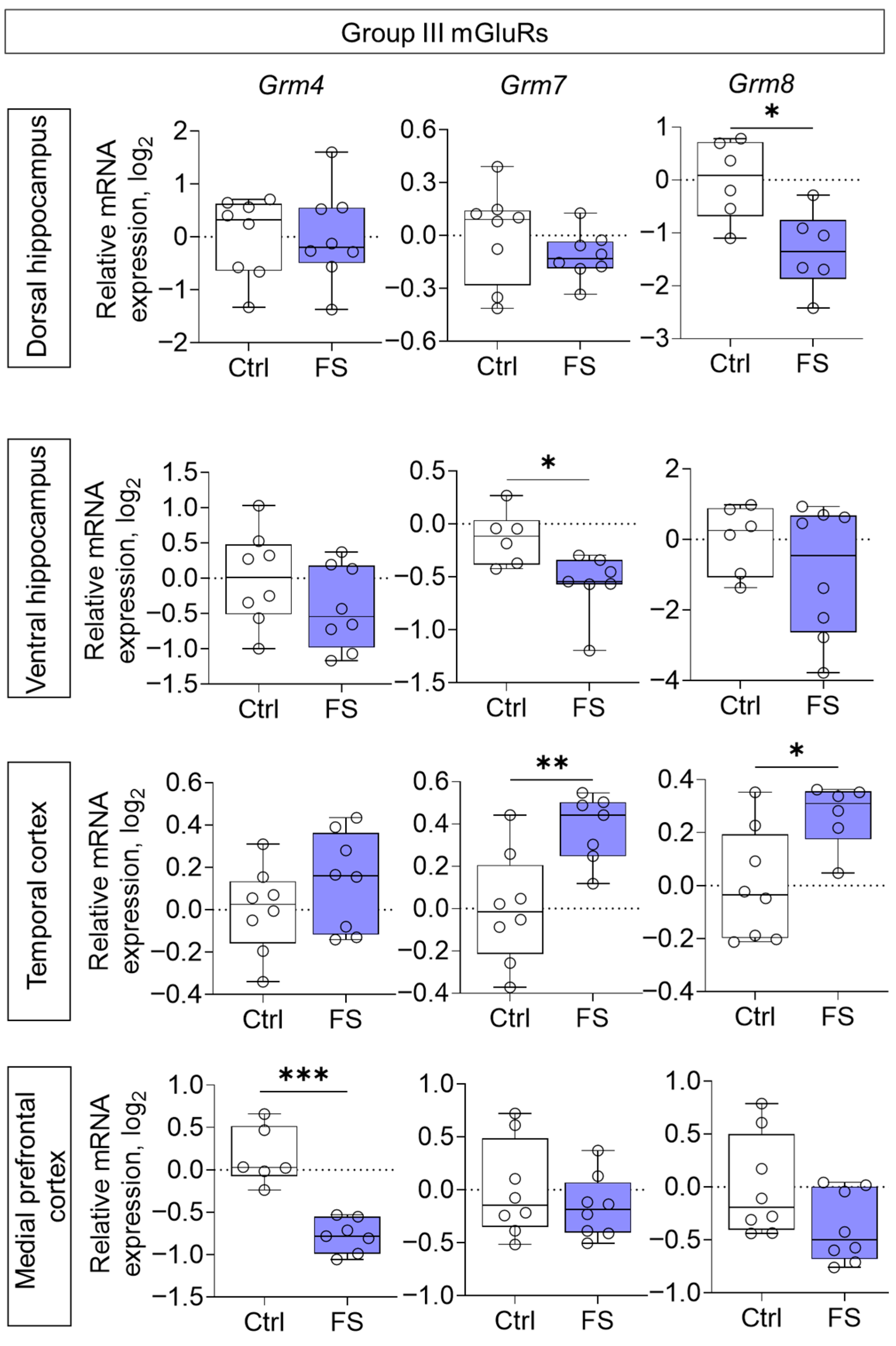
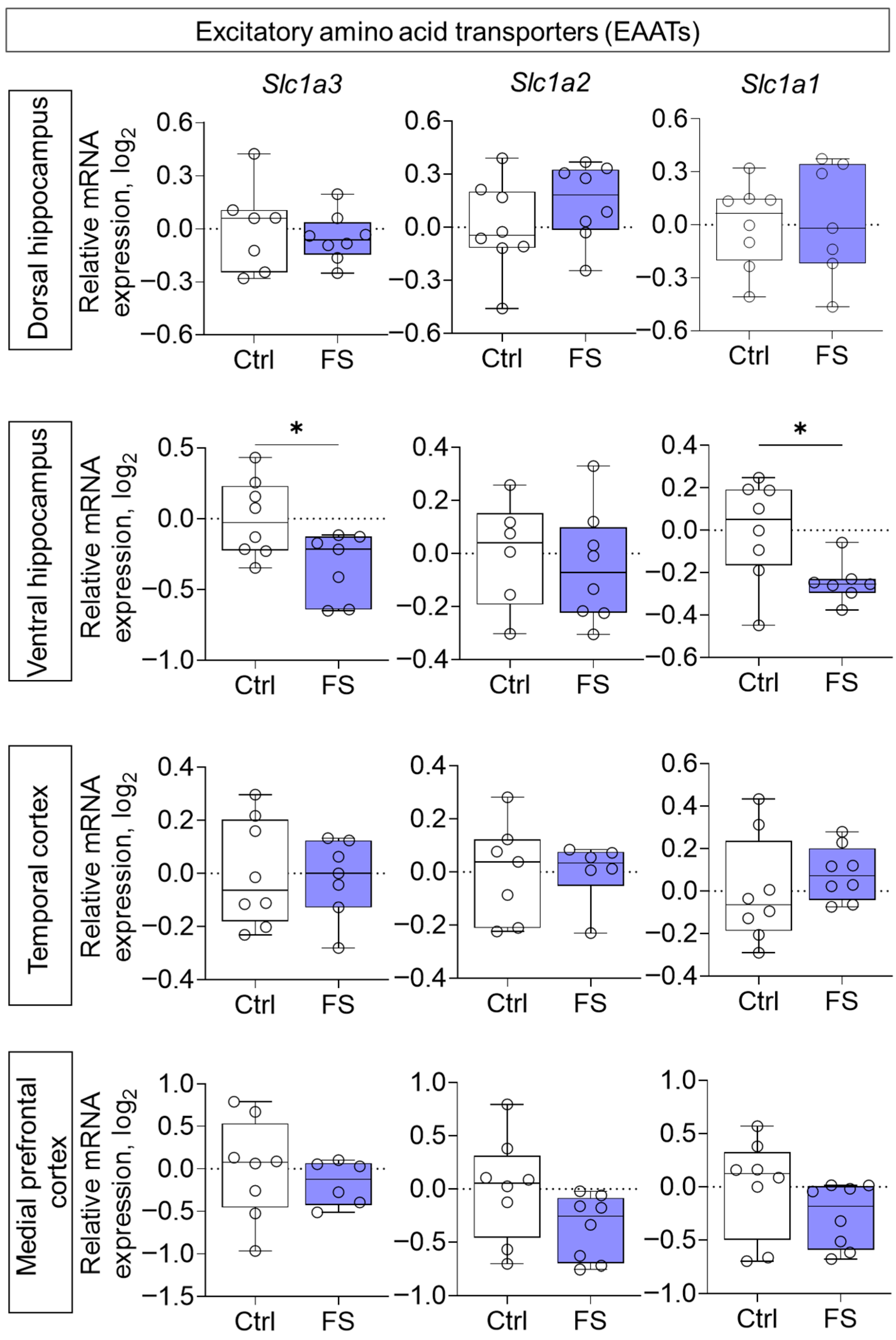
3.2. Changes in Glutamate Receptors and Excitatory Amino Acid Transporters Gene Expression in Rat Brain After Febrile Seizures
3.3. Behavioral Alterations
3.3.1. Decline in Locomotor Activity and Exploratory Behavior
3.3.2. Cognitive Functions
3.3.3. Social Interaction
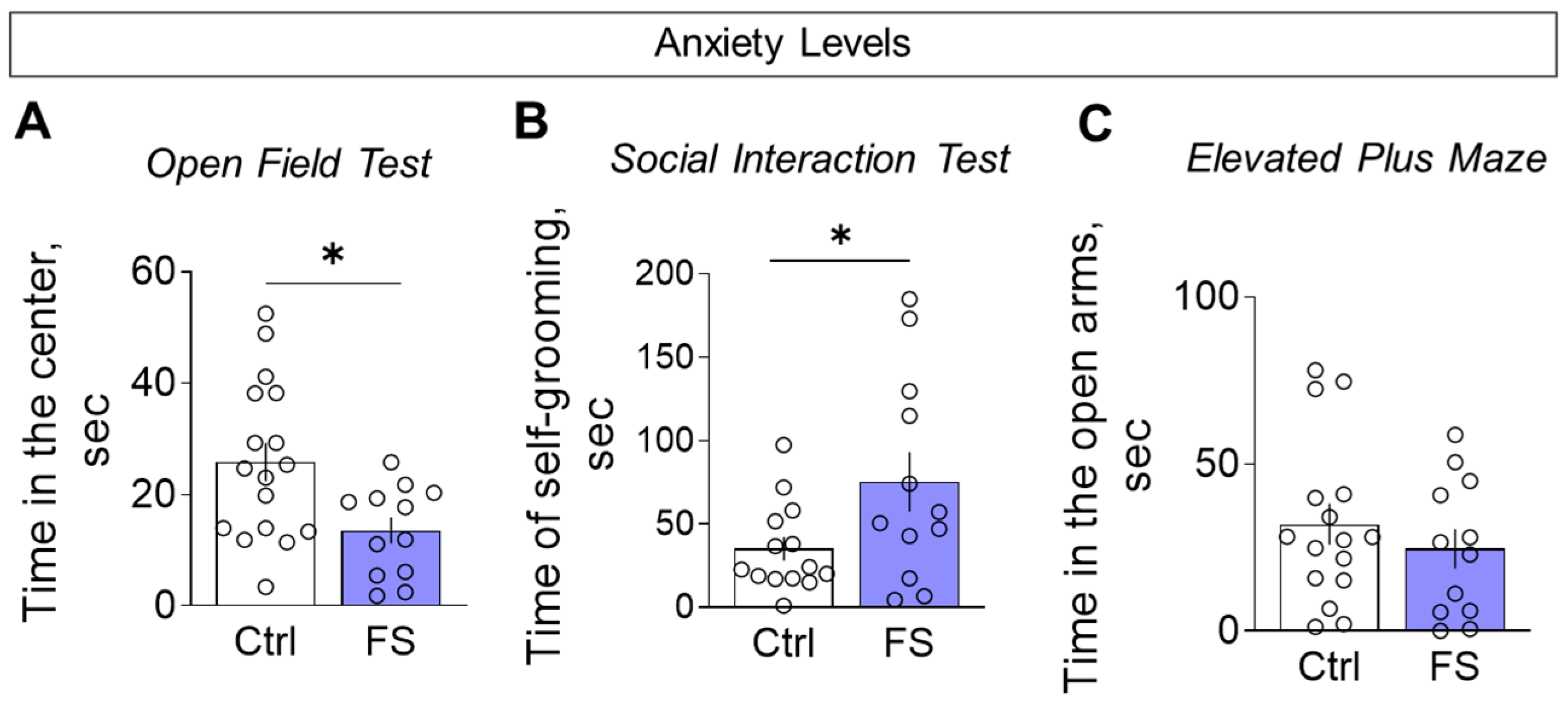
3.3.4. Anxiety Levels
4. Discussion
4.1. The Histopathological Changes
4.2. The Molecular Changes
4.3. Linking Molecular Disruptions to Behavioral Deficits
4.4. Conclusion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ANOVA | Analysis of Variance |
| CA | Cornu Ammonis (followed by number: CA1, CA3) |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| cDNA | complementary DNA |
| Ctrl | Control |
| DI | Discrimination Index |
| DNA | Deoxyribonucleic acid |
| EAAT | Excitatory amino acid transporter (followed by number: EAAT1, EAAT2, EAAT3) |
| EEG | Electroencephalography |
| FS | Febrile Seizures |
| Gria1/2 | Gene names for AMPA receptor subunits (GluA1/2 proteins) |
| Grin1 | Gene name for obligatory NMDA receptor subunit (GluN1 protein) |
| Grin2a/2b | Gene names for NMDA receptor subunits (GluN2A/2B proteins) |
| Grm1-8 | Gene names for metabotropic glutamate receptors (mGluR1-8 proteins) |
| iGluR | Ionotropic glutamate receptor |
| IL-1β | Interleukin-1 beta |
| LTP | Long-Term Potentiation |
| mGluR | Metabotropic glutamate receptor (Groups: I, II, III) |
| mPFC | medial Prefrontal Cortex |
| mRNA | Messenger RNA |
| NMDA | N-methyl-D-aspartate |
| p | Postnatal day (followed by number: P10, P50) |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| RT-qPCR | Reverse Transcription quantitative Polymerase Chain Reaction |
| Slc1a1 | Gene name for EAAT3 (Solute carrier family 1 member 1) |
| Slc1a2 | Gene name for EAAT2 (Solute carrier family 1 member 2) |
| Slc1a3 | Gene name for EAAT1 (Solute carrier family 1 member 3) |
Appendix A
| Dorsal Hippocampus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | BestKeeper | NormFinder | GeNorm | Comprehensive Ranking | ||||||
| Rank | Gene | Average of STDV | Gene | Std Dev | Gene | Stability Value | Gene | Stability Value | Gene | Geomean of Ranking Values |
| 1 | Pgk1 | 0.23 | Pgk1 | 0.09 | Rpl13a | 0.131 | Pgk1 | 0.090 | Pgk1 | 1.19 |
| 2 | Rpl13a | 0.23 | Ywhaz | 0.14 | Pgk1 | 0.134 | Ywhaz | 0.090 | Ywhaz | 2.06 |
| 3 | Ywhaz | 0.24 | Rpl13a | 0.16 | Ywhaz | 0.160 | Ppia | 0.198 | Rpl13a | 2.21 |
| 4 | Ppia | 0.26 | Ppia | 0.17 | Ppia | 0.193 | Rpl13a | 0.210 | Ppia | 3.94 |
| 5 | Actb | 0.29 | Actb | 0.17 | Actb | 0.224 | Actb | 0.235 | Actb | 4.73 |
| 6 | Hprt1 | 0.31 | Hprt1 | 0.25 | Hprt1 | 0.256 | Hprt1 | 0.260 | Hprt1 | 6.00 |
| Ventral Hippocampus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | BestKeeper | NormFinder | GeNorm | Comprehensive Ranking | ||||||
| Rank | Gene | Average of STDV | Gene | Std Dev | Gene | Stability Value | Gene | Stability Value | Gene | Geomean of Ranking Values |
| 1 | Ppia | 0.29 | Rpl13a | 0.22 | Ppia | 0.085 | Actb | 0.093 | Ppia | 1.68 |
| 2 | Gapdh | 0.30 | Ppia | 0.36 | Gapdh | 0.182 | Gapdh | 0.093 | Gapdh | 1.86 |
| 3 | Actb | 0.30 | Gapdh | 0.38 | Actb | 0.186 | Pgk1 | 0.208 | Actb | 2.59 |
| 4 | Pgk1 | 0.33 | Pgk1 | 0.41 | Pgk1 | 0.227 | Ppia | 0.231 | Pgk1 | 3.72 |
| 5 | B2m | 0.41 | Actb | 0.43 | B2m | 0.334 | B2m | 0.294 | Rpl13a | 3.83 |
| 6 | Rpl13a | 0.45 | B2m | 0.44 | Rpl13a | 0.400 | Rpl13a | 0.347 | B2m | 5.23 |
| Temporal Cortex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | BestKeeper | NormFinder | GeNorm | Comprehensive Ranking | ||||||
| Rank | Gene | Average of STDV | Gene | Std Dev | Gene | Stability Value | Gene | Stability Value | Gene | Geomean of Ranking Values |
| 1 | Gapdh | 0.19 | Actb | 0.14 | Gapdh | 0.102 | Actb | 0.069 | Gapdh | 1.19 |
| 2 | Actb | 0.20 | Gapdh | 0.18 | Actb | 0.124 | Gapdh | 0.069 | Actb | 1.41 |
| 3 | Hprt1 | 0.22 | Rpl13a | 0.20 | Hprt1 | 0.152 | Rpl13a | 0.158 | Rpl13a | 3.46 |
| 4 | Rpl13a | 0.23 | Pgk1 | 0.23 | Rpl13a | 0.169 | Hprt1 | 0.193 | Hprt1 | 3.66 |
| 5 | Ppia | 0.24 | Hprt1 | 0.23 | Ppia | 0.188 | Ppia | 0.206 | Ppia | 5.23 |
| 6 | Pgk1 | 0.25 | Ppia | 0.26 | Pgk1 | 0.206 | Pgk1 | 0.221 | Pgk1 | 5.42 |
| Medial Prefrontal Cortex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | BestKeeper | NormFinder | GeNorm | Comprehensive Ranking | ||||||
| Rank | Gene | Average of STDV | Gene | Std Dev | Gene | Stability Value | Gene | Stability Value | Gene | Geomean of Ranking Values |
| 1 | Pgk1 | 0.30 | Rpl13a | 0.20 | Pgk1 | 0.070 | Pgk1 | 0.155 | Pgk1 | 1.32 |
| 2 | Ywhaz | 0.32 | Ppia | 0.33 | Ywhaz | 0.167 | Ywhaz | 0.155 | Ywhaz | 2.00 |
| 3 | Ppia | 0.35 | Pgk1 | 0.34 | Ppia | 0.218 | Hprt1 | 0.187 | Ppia | 2.91 |
| 4 | Hprt1 | 0.36 | Ywhaz | 0.40 | Hprt1 | 0.258 | Ppia | 0.238 | Rpl13a | 3.34 |
| 5 | Rpl13a | 0.42 | Hprt1 | 0.46 | Rpl13a | 0.320 | Rpl13a | 0.298 | Hprt1 | 3.94 |
| 6 | B2m | 0.55 | B2m | 0.46 | B2m | 0.501 | B2m | 0.381 | B2m | 6.00 |
References
- Leung, A.K.C.; Hon, K.L.; Leung, T.N.H. Febrile Seizures: An Overview. Drugs Context 2018, 7, 212536. [Google Scholar] [CrossRef]
- Vestergaard, M.; Pedersen, C.B.; Christensen, J.; Madsen, K.M.; Olsen, J.; Mortensen, P.B. Febrile Seizures and Risk of Schizophrenia. Schizophr. Res. 2005, 73, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, E.N.; Larsen, J.T.; Petersen, L.; Christensen, J.; Dalsgaard, S. Childhood Epilepsy, Febrile Seizures, and Subsequent Risk of ADHD. Pediatrics 2016, 138, e20154654. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Yousefichaijan, P.; Safi Arian, S.; Ebrahimi, S.; Naziri, M. Comparison of Relation between Attention Deficit Hyperactivity Disorder in Children with and without Simple Febrile Seizure Admitted in Arak Central Iran. Iran. J. Child Neurol. 2016, 10, 56–61. [Google Scholar]
- Gillberg, C.; Lundström, S.; Fernell, E.; Nilsson, G.; Neville, B. Febrile Seizures and Epilepsy: Association with Autism and Other Neurodevelopmental Disorders in the Child and Adolescent Twin Study in Sweden. Pediatr. Neurol. 2017, 74, 80–86.e2. [Google Scholar] [CrossRef]
- Dubé, C.M.; Zhou, J.-L.; Hamamura, M.; Zhao, Q.; Ring, A.; Abrahams, J.; McIntyre, K.; Nalcioglu, O.; Shatskih, T.; Baram, T.Z.; et al. Cognitive Dysfunction after Experimental Febrile Seizures. Exp. Neurol. 2009, 215, 167–177. [Google Scholar] [CrossRef]
- Griflyuk, A.V.; Postnikova, T.Y.; Zaitsev, A.V. Prolonged Febrile Seizures Impair Synaptic Plasticity and Alter Developmental Pattern of Glial Fibrillary Acidic Protein (GFAP)-Immunoreactive Astrocytes in the Hippocampus of Young Rats. Int. J. Mol. Sci. 2022, 23, 12224. [Google Scholar] [CrossRef]
- Kloc, M.L.; Marchand, D.H.; Holmes, G.L.; Pressman, R.D.; Jeremy, M. Cognitive Impairment Following Experimental Febrile Seizures Is Determined by Sex and Seizure Duration. Epilepsy Behav. 2023, 126, 108430. [Google Scholar] [CrossRef]
- Dai, Y.-J.; Wu, D.-C.; Feng, B.; Chen, B.; Tang, Y.-S.; Jin, M.-M.; Zhao, H.-W.; Dai, H.-B.; Wang, Y.; Chen, Z. Prolonged Febrile Seizures Induce Inheritable Memory Deficits in Rats through DNA Methylation. CNS Neurosci. Ther. 2019, 25, 601–611. [Google Scholar] [CrossRef]
- Yagoubi, N.; Jomni, Y.; Sakly, M. Hyperthermia-Induced Febrile Seizures Have Moderate and Transient Effects on Spatial Learning in Immature Rats. Behav. Neurol. 2015, 2015, 924303. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kim, S.-W.; Im, H.; Song, Y.; Kim, S.J.; Lee, Y.R.; Kim, G.W.; Hwang, C.; Park, D.-K.; Kim, D.-S. Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats. Cells 2022, 11, 3228. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Early-Life Hyperthermic Seizures Upregulate Adenosine A(2A) Receptors in the Cortex and Promote Depressive-like Behavior in Adult Rats. Epilepsy Behav. 2018, 86, 173–178. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical Periods of Vulnerability for the Developing Nervous System: Evidence from Humans and Animal Models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar]
- Hamelin, S.; Vercueil, L. A Simple Febrile Seizure with Focal Onset. Epileptic Disord. 2014, 16, 112–115. [Google Scholar] [CrossRef]
- Neville, B.; Gindner, D. Febrile Seizures Are a Syndrome of Secondarily Generalized Hippocampal Epilepsy. Dev. Med. Child Neurol. 2010, 52, 1151–1153. [Google Scholar] [CrossRef]
- Baram, T.Z.; Gerth, A.; Schultz, L. Febrile Seizures: An Appropriate-Aged Model Suitable for Long-Term Studies. Dev. Brain Res. 1997, 98, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.B.; Moser, E.I. Functional Differentiation in the Hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar] [CrossRef]
- Barkus, C.; McHugh, S.B.; Sprengel, R.; Seeburg, P.H.; Rawlins, J.N.P.; Bannerman, D.M. Hippocampal NMDA Receptors and Anxiety: At the Interface between Cognition and Emotion. Eur. J. Pharmacol. 2010, 626, 49–56. [Google Scholar] [CrossRef]
- Grigoryan, G.; Segal, M. Lasting Differential Effects on Plasticity Induced by Prenatal Stress in Dorsal and Ventral Hippocampus. Neural Plast. 2016, 2016, 2540462. [Google Scholar] [CrossRef]
- Zubareva, O.E.; Postnikova, T.Y.; Grifluk, A.V.; Schwarz, A.P.; Smolensky, I.V.; Karepanov, A.A.; Vasilev, D.S.; Veniaminova, E.A.; Rotov, A.Y.; Kalemenev, S.V.; et al. Exposure to Bacterial Lipopolysaccharide in Early Life Affects the Expression of Ionotropic Glutamate Receptor Genes and Is Accompanied by Disturbances in Long-Term Potentiation and Cognitive Functions in Young Rats. Brain Behav. Immun. 2020, 90, 3–15. [Google Scholar] [CrossRef]
- Vertes, R.P. Interactions among the Medial Prefrontal Cortex, Hippocampus and Midline Thalamus in Emotional and Cognitive Processing in the Rat. Neuroscience 2006, 142, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Bai, W.; Xia, M.; Tian, X. Directional Hippocampal-Prefrontal Interactions during Working Memory. Behav. Brain Res. 2018, 338, 1–8. [Google Scholar] [CrossRef]
- Jin, J.; Maren, S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front. Syst. Neurosci. 2015, 9, 170. [Google Scholar] [CrossRef]
- Furtak, S.C.; Wei, S.-M.; Agster, K.L.; Burwell, R.D. Functional Neuroanatomy of the Parahippocampal Region in the Rat: The Perirhinal and Postrhinal Cortices. Hippocampus 2007, 17, 709–722. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.L.; Clark, R.E. The Medial Temporal Lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef]
- Agster, K.L.; Burwell, R.D. Hippocampal and Subicular Efferents and Afferents of the Perirhinal, Postrhinal, and Entorhinal Cortices of the Rat. Behav. Brain Res. 2013, 254, 50–64. [Google Scholar] [CrossRef]
- Dubé, C.M.; Brewster, A.L.; Richichi, C.; Zha, Q.; Baram, T.Z. Fever, Febrile Seizures and Epilepsy. Trends Neurosci. 2007, 30, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Sawires, R.; Buttery, J.; Fahey, M. A Review of Febrile Seizures: Recent Advances in Understanding of Febrile Seizure Pathophysiology and Commonly Implicated Viral Triggers. Front. Pediatr. 2021, 9, 801321. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Vezzani, A.; Bartfai, T. Chapter 12—Mechanisms of Fever and Febrile Seizures: Putative Role of the Interleukin-1 System. In Febrile Seizures; Baram, T.Z., Shinnar, S., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 169–188. ISBN 978-0-12-078141-6. [Google Scholar]
- Dubé, C.M.; Brewster, A.L.; Baram, T.Z. Febrile Seizures: Mechanisms and Relationship to Epilepsy. Brain Dev. 2009, 31, 366–371. [Google Scholar] [CrossRef]
- Reid, A.Y.; Galic, M.A.; Teskey, G.C.; Pittman, Q.J. Febrile Seizures: Current Views and Investigations. Can. J. Neurol. Sci. 2009, 36, 679–686. [Google Scholar] [CrossRef]
- Millichap, J.G.J.; Millichap, J.G.J. Role of Viral Infections in the Etiology of Febrile Seizures. Pediatr. Neurol. 2006, 35, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D. Early-Life Infection Is a Vulnerability Factor for Aging-Related Glial Alterations and Cognitive Decline. Neurobiol. Learn. Mem. 2010, 94, 57–64. [Google Scholar] [CrossRef]
- Yi, Y.; Zhong, C.; Hu, W. The Long-Term Neurodevelopmental Outcomes of Febrile Seizures and Underlying Mechanisms. Front. Cell Dev. Biol. 2023, 11, 1186050. [Google Scholar] [CrossRef]
- Griflyuk, A.V.; Postnikova, T.Y.; Malkin, S.L.; Zaitsev, A.V. Alterations in Rat Hippocampal Glutamatergic System Properties after Prolonged Febrile Seizures. Int. J. Mol. Sci. 2023, 24, 16875. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Glutamatergic System Is Affected in Brain from an Hyperthermia-Induced Seizures Rat Model. Cell. Mol. Neurobiol. 2021, 42, 1501–1512. [Google Scholar] [CrossRef]
- Bellone, C.; Lüscher, C. Drug-Evoked Plasticity: Do Addictive Drugs Reopen a Critical Period of Postnatal Synaptic Development? Front. Mol. Neurosci. 2012, 5, 75. [Google Scholar] [CrossRef]
- Barker-Haliski, M.; White, H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef]
- Green, J.L.; Dos Santos, W.F.; Fontana, A.C.K. Role of Glutamate Excitotoxicity and Glutamate Transporter EAAT2 in Epilepsy: Opportunities for Novel Therapeutics Development. Biochem. Pharmacol. 2021, 193, 114786. [Google Scholar] [CrossRef]
- Wasterlain, C.G.; Shirasaka, Y. Seizures, Brain Damage and Brain Development. Brain Dev. 1994, 16, 279–295. [Google Scholar] [CrossRef]
- Swann, J.W.; Le, J.T.; Lam, T.T.; Owens, J.; Mayer, A.T. The Impact of Chronic Network Hyperexcitability on Developing Glutamatergic Synapses. Eur. J. Neurosci. 2007, 26, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Kubová, H.; Bendová, Z.; Moravcová, S.; Pačesová, D.; Rocha, L.L.; Mareš, P. Neonatal Clonazepam Administration Induces Long-Lasting Changes in Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 382. [Google Scholar] [CrossRef]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Na(+)/K(+)- and Mg(2+)-ATPases and Their Interaction with AMPA, NMDA and D(2) Dopamine Receptors in an Animal Model of Febrile Seizures. Int. J. Mol. Sci. 2022, 23, 14638. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kuo, Y.; Huang, A.; Huang, C. Repetitive Febrile Seizures in Rat Pups Cause Long-Lasting Deficits in Synaptic Plasticity and NR2A Tyrosine Phosphorylation. Neurobiol. Dis. 2005, 18, 466–475. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marceau, K.; Ruttle, P.L.; Shirtcliff, E.A.; Essex, M.J.; Susman, E.J. Developmental and Contextual Considerations for Adrenal and Gonadal Hormone Functioning during Adolescence: Implications for Adolescent Mental Health. Dev. Psychobiol. 2015, 57, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Trotman, H.D.; Holtzman, C.W.; Ryan, A.T.; Shapiro, D.I.; MacDonald, A.N.; Goulding, S.M.; Brasfield, J.L.; Walker, E.F. The Development of Psychotic Disorders in Adolescence: A Potential Role for Hormones. Horm. Behav. 2013, 64, 411–419. [Google Scholar] [CrossRef]
- Griflyuk, A.V.; Postnikova, T.Y.; Zaitsev, A.V. Animal Models of Febrile Seizures: Limitations and Recent Advances in the Field. Cells 2024, 13, 1895. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A Critical Review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of Open:Closed Arm Entries in an Elevated plus-Maze as a Measure of Anxiety in the Rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Herrman, L.; Palmatier, M.I.; Bevins, R.A. Rats’ Novel Object Interaction as a Measure of Environmental Familiarity. Learn. Motiv. 2006, 37, 131–148. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- File, S.E.; Hyde, J.R. Can Social Interaction Be Used to Measure Anxiety? Br. J. Pharmacol. 1978, 62, 19–24. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780080475134. [Google Scholar]
- Schwarz, A.P.; Malygina, D.A.; Kovalenko, A.A.; Trofimov, A.N.; Zaitsev, A.V. Multiplex QPCR Assay for Assessment of Reference Gene Expression Stability in Rat Tissues/Samples. Mol. Cell. Probes 2020, 53, 101611. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kovalenko, A.A.; Zakharova, M.V.; Schwarz, A.P.; Zubareva, O.E.; Zaitsev, A.V. Identification of Reliable Reference Genes for Use in Gene Expression Studies in Rat Febrile Seizure Model. Int. J. Mol. Sci. 2024, 25, 11125. [Google Scholar] [CrossRef]
- Bonefeld, B.E.; Elfving, B.; Wegener, G. Reference Genes for Normalization: A Study of Rat Brain Tissue. Synapse 2008, 62, 302–309. [Google Scholar] [CrossRef]
- Lin, W.; Burks, C.A.; Hansen, D.R.; Kinnamon, S.C.; Gilbertson, T.A. Taste Receptor Cells Express PH-Sensitive Leak K + Channels. J. Neurophysiol. 2004, 92, 2909–2919. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yamauchi, A.; Nishimura, M.; Ueda, N.; Naito, S. Soybean Oil Fat Emulsion Prevents Cytochrome P450 MRNA Down-Regulation Induced by Fat-Free Overdose Total Parenteral Nutrition in Infant Rats. Biol. Pharm. Bull. 2005, 28, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Swijsen, A.; Nelissen, K.; Janssen, D.; Rigo, J.-M.; Hoogland, G. Validation of Reference Genes for Quantitative Real-Time PCR Studies in the Dentate Gyrus after Experimental Febrile Seizures. BMC Res. Notes 2012, 5, 685. [Google Scholar] [CrossRef] [PubMed]
- Pohjanvirta, R.; Niittynen, M.; Lindén, J.; Boutros, P.C.; Moffat, I.D.; Okey, A.B. Evaluation of Various Housekeeping Genes for Their Applicability for Normalization of MRNA Expression in Dioxin-Treated Rats. Chem. Biol. Interact. 2006, 160, 134–149. [Google Scholar] [CrossRef]
- Malkin, S.L.; Amakhin, D.V.; Veniaminova, E.A.; Kim, K.K.; Zubareva, O.E.; Magazanik, L.G.; Zaitsev, A.V. Changes of AMPA Receptor Properties in the Neocortex and Hippocampus Following Pilocarpine-Induced Status Epilepticus in Rats. Neuroscience 2016, 327, 146–155. [Google Scholar] [CrossRef]
- Cook, N.L.; Vink, R.; Donkin, J.J.; van den Heuvel, C. Validation of Reference Genes for Normalization of Real-Time Quantitative RT-PCR Data in Traumatic Brain Injury. J. Neurosci. Res. 2009, 87, 34–41. [Google Scholar] [CrossRef]
- Langnaese, K.; John, R.; Schweizer, H.; Ebmeyer, U.; Keilhoff, G. Selection of Reference Genes for Quantitative Real-Time PCR in a Rat Asphyxial Cardiac Arrest Model. BMC Mol. Biol. 2008, 9, 53. [Google Scholar] [CrossRef]
- Kovalenko, A.A.; Zakharova, M.V.; Schwarz, A.P.; Dyomina, A.V.; Zubareva, O.E.; Zaitsev, A.V. Changes in Metabotropic Glutamate Receptor Gene Expression in Rat Brain in a Lithium-Pilocarpine Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2022, 23, 2725. [Google Scholar] [CrossRef] [PubMed]
- Dulman, R.S.; Auta, J.; Teppen, T.; Pandey, S.C. Acute Ethanol Produces Ataxia and Induces Fmr1 Expression via Histone Modifications in the Rat Cerebellum. Alcohol. Clin. Exp. Res. 2019, 43, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, C.-H.; Wu, S.-H.; Yu, X.-D.; Yu, X.-G.; Shen, X.-M. Developmental Lead Exposure Alters Gene Expression of Metabotropic Glutamate Receptors in Rat Hippocampal Neurons. Neurosci. Lett. 2007, 413, 222–226. [Google Scholar] [CrossRef]
- Giza, C.C.; Maria, N.S.S.; Hovda, D.A. N-Methyl-D-Aspartate Receptor Subunit Changes after Traumatic Injury to the Developing Brain. J. Neurotrauma 2006, 23, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Floyd, D.W.; Jung, K.-Y.; McCool, B.A. Chronic Ethanol Ingestion Facilitates N-Methyl-D-Aspartate Receptor Function and Expression in Rat Lateral/Basolateral Amygdala Neurons. J. Pharmacol. Exp. Ther. 2003, 307, 1020–1029. [Google Scholar] [CrossRef]
- Proudnikov, D.; Yuferov, V.; Zhou, Y.; LaForge, K.S.; Ho, A.; Kreek, M.J. Optimizing Primer—Probe Design for Fluorescent PCR. J. Neurosci. Methods 2003, 123, 31–45. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Kovalenko, A.A.; Zubareva, O.E.; Schwarz, A.P.; Postnikova, T.Y.; Trofimova, A.M.; Zaitsev, A.V. Pentylenetetrazole-Induced Seizures Cause Short-Term Changes in the Phenotype of Microglial and Astroglial Cells in the Hippocampus and Temporal Cortex of Young Male Wistar Rats. J. Neurosci. Res. 2024, 102, e25385. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Hasselfeld, K.; Bauer, D.; Simmons, M.; Roussos, P.; Haroutunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Glutamate Transporter Splice Variant Expression in an Enriched Pyramidal Cell Population in Schizophrenia. Transl. Psychiatry 2015, 5, e579. [Google Scholar] [CrossRef]
- Dyomina, A.V.; Zubareva, O.E.; Smolensky, I.V.; Vasilev, D.S.; Zakharova, M.V.; Kovalenko, A.A.; Schwarz, A.P.; Ischenko, A.M.; Zaitsev, A.V. Anakinra Reduces Epileptogenesis, Provides Neuroprotection, and Attenuates Behavioral Impairments in Rats in the Lithium–Pilocarpine Model of Epilepsy. Pharmaceuticals 2020, 13, 340. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Dyomina, A.V.; Kovalenko, A.A.; Zubareva, O.E.; Ischenko, A.M.; Zaitsev, A.V. Anakinra Promotes M2 Microglia Activation during the Latent Phase of the Lithium-Pilocarpine Model of Temporal Lobe Epilepsy. J. Evol. Biochem. Physiol. 2024, 60, 672–689. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Griflyuk, A.V.; Amakhin, D.V.; Kovalenko, A.A.; Soboleva, E.B.; Zubareva, O.E.; Zaitsev, A.V. Early Life Febrile Seizures Impair Hippocampal Synaptic Plasticity in Young Rats. Int. J. Mol. Sci. 2021, 22, 8218. [Google Scholar] [CrossRef] [PubMed]
- Karantysh, G.V.; Fomenko, M.P.; Menzheritskii, A.M.; Prokof’ev, V.N.; Ryzhak, G.A.; Butenko, E.V. Effect of Pinealon on Learning and Expression of NMDA Receptor Subunit Genes in the Hippocampus of Rats with Experimental Diabetes. Neurochem. J. 2020, 14, 314–320. [Google Scholar] [CrossRef]
- Bar-Shira, O.; Maor, R.; Chechik, G. Gene Expression Switching of Receptor Subunits in Human Brain Development. PLoS Comput. Biol. 2015, 11, e1004559. [Google Scholar] [CrossRef]
- Mewasingh, L.D.; Chin, R.F.M.; Scott, R.C. Current Understanding of Febrile Seizures and Their Long-Term Outcomes. Dev. Med. Child Neurol. 2020, 62, 1245–1249. [Google Scholar] [CrossRef]
- Gould, L.; Delavale, V.; Plovnick, C.; Wisniewski, T.; Devinsky, O. Are Brief Febrile Seizures Benign? A Systematic Review and Narrative Synthesis. Epilepsia 2023, 64, 2539–2549. [Google Scholar] [CrossRef]
- Scott, R.C. What Are the Effects of Prolonged Seizures in the Brain? Epileptic Disord. 2014, 16, S6–S11. [Google Scholar] [CrossRef]
- Cendes, F. Febrile Seizures and Mesial Temporal Sclerosis. Curr. Opin. Neurol. 2004, 17, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sokol, D.K.; Demyer, W.E.; Edwards-Brown, M.; Sanders, S.; Garg, B. From Swelling to Sclerosis: Acute Change in Mesial Hippocampus after Prolonged Febrile Seizure. Seizure 2003, 12, 237–240. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Functional Neurochemistry of the Ventral and Dorsal Hippocampus: Stress, Depression, Dementia and Remote Hippocampal Damage. Neurochem. Res. 2019, 44, 1306–1322. [Google Scholar] [CrossRef]
- Nazem, A.; Jafarian, A.H.; Sadraie, S.H.; Gorji, A.; Kheradmand, H.; Radmard, M.; Haghir, H. Neuronal Injury and Cytogenesis after Simple Febrile Seizures in the Hippocampal Dentate Gyrus of Juvenile Rat. Child’s Nerv. Syst. 2012, 28, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Hino, H.; Takahashi, H.; Suzuki, Y.; Tanaka, J.; Ishii, E.; Fukuda, M. Anticonvulsive Effect of Paeoniflorin on Experimental Febrile Seizures in Immature Rats: Possible Application for Febrile Seizures in Children. PLoS ONE 2012, 7, e42920. [Google Scholar] [CrossRef]
- Mosili, P.; Maikoo, S.; Mabandla, M.V.; Qulu, L. The Pathogenesis of Fever-Induced Febrile Seizures and Its Current State. Neurosci. Insights 2020, 15, 2633105520956973. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, Function, and Allosteric Modulation of NMDA Receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef]
- Chen, B.; Feng, B.; Tang, Y.; You, Y.; Wang, Y.; Hou, W.; Hu, W.; Chen, Z. Blocking GluN2B Subunits Reverses the Enhanced Seizure Susceptibility after Prolonged Febrile Seizures with a Wide Therapeutic Time-Window. Exp. Neurol. 2016, 283, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA Receptor Subunit Diversity: Impact on Receptor Properties, Synaptic Plasticity and Disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Acutain, M.F.; Baez, M.V. Reduced Expression of GluN2A Induces a Delay in Neuron Maturation. J. Neurochem. 2024, 168, 4001–4013. [Google Scholar] [CrossRef]
- Reid, A.Y.; Riazi, K.; Campbell Teskey, G.; Pittman, Q.J. Increased Excitability and Molecular Changes in Adult Rats after a Febrile Seizure. Epilepsia 2013, 54, e45–e48. [Google Scholar] [CrossRef]
- Herring, B.E.; Nicoll, R.A. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu. Rev. Physiol. 2016, 78, 351–365. [Google Scholar] [CrossRef]
- Jaskova, K.; Pavlovicova, M.; Jurkovicova, D. Calcium Transporters and Their Role in the Development of Neuronal Disease and Neuronal Damage. Gen. Physiol. Biophys. 2012, 31, 375–382. [Google Scholar] [CrossRef]
- Tang, X.-J.; Xing, F. Calcium-Permeable AMPA Receptors in Neonatal Hypoxic-Ischemic Encephalopathy (Review). Biomed. Rep. 2013, 1, 828–832. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-M.; Bodepudi, A.; Chu, X.-P.; Wang, J.Q. Group I Metabotropic Glutamate Receptors and Interacting Partners: An Update. Int. J. Mol. Sci. 2022, 23, 840. [Google Scholar] [CrossRef]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic Glutamate Receptors: From the Workbench to the Bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, A.V.; Smolensky, I.V.; Jorratt, P.; Ovsepian, S.V. Neurobiology, Functions, and Relevance of Excitatory Amino Acid Transporters (EAATs) to Treatment of Refractory Epilepsy. CNS Drugs 2020, 34, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Holmseth, S.; Dehnes, Y.; Huang, Y.H.; Follin-Arbelet, V.V.; Grutle, N.J.; Mylonakou, M.N.; Plachez, C.; Zhou, Y.; Furness, D.N.; Bergles, D.E.; et al. The Density of EAAC1 (EAAT3) Glutamate Transporters Expressed by Neurons in the Mammalian CNS. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6000–6013. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P.; et al. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Bjørn-Yoshimoto, W.E.; Underhill, S.M. The Importance of the Excitatory Amino Acid Transporter 3 (EAAT3). Neurochem. Int. 2016, 98, 4–18. [Google Scholar] [CrossRef]
- Tzingounis, A.V.; Wadiche, J.I. Glutamate Transporters: Confining Runaway Excitation by Shaping Synaptic Transmission. Nat. Rev. Neurosci. 2007, 8, 935–947. [Google Scholar] [CrossRef]
- Cercato, M.C.; Vázquez, C.A.; Kornisiuk, E.; Aguirre, A.I.; Colettis, N.; Snitcofsky, M.; Jerusalinsky, D.A.; Baez, M.V. GluN1 and GluN2A NMDA Receptor Subunits Increase in the Hippocampus during Memory Consolidation in the Rat. Front. Behav. Neurosci. 2016, 10, 242. [Google Scholar] [CrossRef]
- Daenen, E.W.; Van der Heyden, J.A.; Kruse, C.G.; Wolterink, G.; Van Ree, J.M. Adaptation and Habituation to an Open Field and Responses to Various Stressful Events in Animals with Neonatal Lesions in the Amygdala or Ventral Hippocampus. Brain Res. 2001, 918, 153–165. [Google Scholar] [CrossRef]
- Leussis, M.P.; Bolivar, V.J. Habituation in Rodents: A Review of Behavior, Neurobiology, and Genetics. Neurosci. Biobehav. Rev. 2006, 30, 1045–1064. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Wang, T.; Chen, L. Dopamine D1 Receptor in the Medial Prefrontal Cortex Mediates Anxiety-like Behaviors Induced by Blocking Glutamatergic Activity of the Ventral Hippocampus in Rats. Brain Res. 2019, 1704, 59–67. [Google Scholar] [CrossRef]
- Chao, O.Y.; de Souza Silva, M.A.; Yang, Y.-M.; Huston, J.P. The Medial Prefrontal Cortex-Hippocampus Circuit That Integrates Information of Object, Place and Time to Construct Episodic Memory in Rodents: Behavioral, Anatomical and Neurochemical Properties. Neurosci. Biobehav. Rev. 2020, 113, 373–407. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, B.; van Kerkoerle, T.; Vartak, D.; Roelfsema, P.R. The Contribution of AMPA and NMDA Receptors to Persistent Firing in the Dorsolateral Prefrontal Cortex in Working Memory. J. Neurosci. 2020, 40, 2458–2470. [Google Scholar] [CrossRef]
- Davies, D.A.; Greba, Q.; Howland, J.G. GluN2B-Containing NMDA Receptors and AMPA Receptors in Medial Prefrontal Cortex Are Necessary for Odor Span in Rats. Front. Behav. Neurosci. 2013, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Morici, J.F.; Bekinschtein, P.; Weisstaub, N.V. Medial Prefrontal Cortex Role in Recognition Memory in Rodents. Behav. Brain Res. 2015, 292, 241–251. [Google Scholar] [CrossRef]
- Mesquita, A.R.; Tavares, H.B.; Silva, R.; Sousa, N. Febrile Convulsions in Developing Rats Induce a Hyperanxious Phenotype Later in Life. Epilepsy Behav. 2006, 9, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.; Wood, C.M.; Roberts, A.C. The Ventromedial Prefrontal Cortex and Emotion Regulation: Lost in Translation? J. Physiol. 2023, 601, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Mercado, D.; Padilla-Coreano, N.; Quirk, G.J. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology 2011, 36, 529–538. [Google Scholar] [CrossRef]
- Suzuki, S.; Saitoh, A.; Ohashi, M.; Yamada, M.; Oka, J.-I.; Yamada, M. The Infralimbic and Prelimbic Medial Prefrontal Cortices Have Differential Functions in the Expression of Anxiety-like Behaviors in Mice. Behav. Brain Res. 2016, 304, 120–124. [Google Scholar] [CrossRef]
- Parfitt, G.M.; Nguyen, R.; Bang, J.Y.; Aqrabawi, A.J.; Tran, M.M.; Seo, D.K.; Richards, B.A.; Kim, J.C. Bidirectional Control of Anxiety-Related Behaviors in Mice: Role of Inputs Arising from the Ventral Hippocampus to the Lateral Septum and Medial Prefrontal Cortex. Neuropsychopharmacology 2017, 42, 1715–1728. [Google Scholar] [CrossRef]
- Majidi, J.; Kosari-Nasab, M.; Salari, A.-A. Developmental Minocycline Treatment Reverses the Effects of Neonatal Immune Activation on Anxiety- and Depression-like Behaviors, Hippocampal Inflammation, and HPA Axis Activity in Adult Mice. Brain Res. Bull. 2016, 120, 1–13. [Google Scholar] [CrossRef]
- Remonde, C.G.; Gonzales, E.L.; Adil, K.J.; Jeon, S.J.; Shin, C.Y. Augmented Impulsive Behavior in Febrile Seizure-Induced Mice. Toxicol. Res. 2023, 39, 37–51. [Google Scholar] [CrossRef]
- Sillanpää, M.; Suominen, S.; Rautava, P.; Aromaa, M. Academic and Social Success in Adolescents with Previous Febrile Seizures. Seizure 2011, 20, 326–330. [Google Scholar] [CrossRef][Green Version]
- Klatte, K.; Kirschstein, T.; Otte, D.; Pothmann, L.; Müller, L.; Tokay, T.; Kober, M.; Uebachs, M.; Zimmer, A.; Beck, H. Impaired D-Serine-Mediated Cotransmission Mediates Cognitive Dysfunction in Epilepsy. J. Neurosci. 2013, 33, 13066–13080. [Google Scholar] [CrossRef]
- Nagai, T.; Yu, J.; Kitahara, Y.; Nabeshima, T.; Yamada, K. D-Serine Ameliorates Neonatal PolyI:C Treatment-Induced Emotional and Cognitive Impairments in Adult Mice. J. Pharmacol. Sci. 2012, 120, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Guercio, G.D.; Bevictori, L.; Vargas-Lopes, C.; Madeira, C.; Oliveira, A.; Carvalho, V.F.; d’Avila, J.C.; Panizzutti, R. D-Serine Prevents Cognitive Deficits Induced by Acute Stress. Neuropharmacology 2014, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Otte, D.-M.; Barcena de Arellano, M.L.; Bilkei-Gorzo, A.; Albayram, O.; Imbeault, S.; Jeung, H.; Alferink, J.; Zimmer, A. Effects of Chronic D-Serine Elevation on Animal Models of Depression and Anxiety-Related Behavior. PLoS ONE 2013, 8, e67131. [Google Scholar] [CrossRef]
- Pitsikas, N. The Metabotropic Glutamate Receptors: Potential Drug Targets for the Treatment of Anxiety Disorders? Eur. J. Pharmacol. 2014, 723, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Hovelsø, N.; Sotty, F.; Montezinho, L.P.; Pinheiro, P.S.; Herrik, K.F.; Mørk, A. Therapeutic Potential of Metabotropic Glutamate Receptor Modulators. Curr. Neuropharmacol. 2012, 10, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Henter, I.D.; Park, L.T.; Zarate, C.A. Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status. CNS Drugs 2021, 35, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J.L.; McCarthy, M.M. Evidence for an Extended Duration of GABA-Mediated Excitation in the Developing Male Versus Female Hippocampus. Dev. Neurobiol. 2007, 14, 1879–1890. [Google Scholar] [CrossRef]
| Group | Total Number | Behavioral Testing | mRNA Expression Analysis | Histology |
|---|---|---|---|---|
| Control | 23 | 17 | 8 | 6 |
| FS | 17 | 12 | 8 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griflyuk, A.V.; Zubareva, O.E.; Kovalenko, A.A.; Zakharova, M.V.; Zaitsev, A.V. Neonatal Febrile Seizures in Rats Induce Long-Term Region-Specific Alterations in the Glutamatergic System of Hippocampal–Prefrontal Circuits and Lead to Behavioral Deficits. Cells 2025, 14, 1666. https://doi.org/10.3390/cells14211666
Griflyuk AV, Zubareva OE, Kovalenko AA, Zakharova MV, Zaitsev AV. Neonatal Febrile Seizures in Rats Induce Long-Term Region-Specific Alterations in the Glutamatergic System of Hippocampal–Prefrontal Circuits and Lead to Behavioral Deficits. Cells. 2025; 14(21):1666. https://doi.org/10.3390/cells14211666
Chicago/Turabian StyleGriflyuk, Alexandra V., Olga E. Zubareva, Anna A. Kovalenko, Maria V. Zakharova, and Aleksey V. Zaitsev. 2025. "Neonatal Febrile Seizures in Rats Induce Long-Term Region-Specific Alterations in the Glutamatergic System of Hippocampal–Prefrontal Circuits and Lead to Behavioral Deficits" Cells 14, no. 21: 1666. https://doi.org/10.3390/cells14211666
APA StyleGriflyuk, A. V., Zubareva, O. E., Kovalenko, A. A., Zakharova, M. V., & Zaitsev, A. V. (2025). Neonatal Febrile Seizures in Rats Induce Long-Term Region-Specific Alterations in the Glutamatergic System of Hippocampal–Prefrontal Circuits and Lead to Behavioral Deficits. Cells, 14(21), 1666. https://doi.org/10.3390/cells14211666






