LT1-3, a Slit2-Derived Peptide, Exhibits Anti-Tumor Activity and Improves Cisplatin Therapy
Highlights
- The LT1-3 peptide inhibits proliferation and invasion of lung cancer cells while being non-toxic to normal cells.
- LT1-3 enhances the efficacy of cisplatin and appears to reduce its side effects.
- LT1-3 is suitable for combination with cisplatin as a first-line treatment for lung cancer and can be added to immunotherapy and platinum doublet therapy.
- LT1-3 can be continued as maintenance therapy, offering a promising new approach to improve treatment outcomes and patient quality of life.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Cell Lines and Cell Culture
2.3. Cell Transfection and Stable Clone Selection
2.4. Cell Proliferation Assay
2.5. Cloning and PCR-Based Site-Directed Mutagenesis
2.6. Western Blot Analysis
2.7. Quantitative Reverse Transcription–PCR
2.8. Wound Healing and Invasion Assays
2.9. Statistical Analysis
3. Results
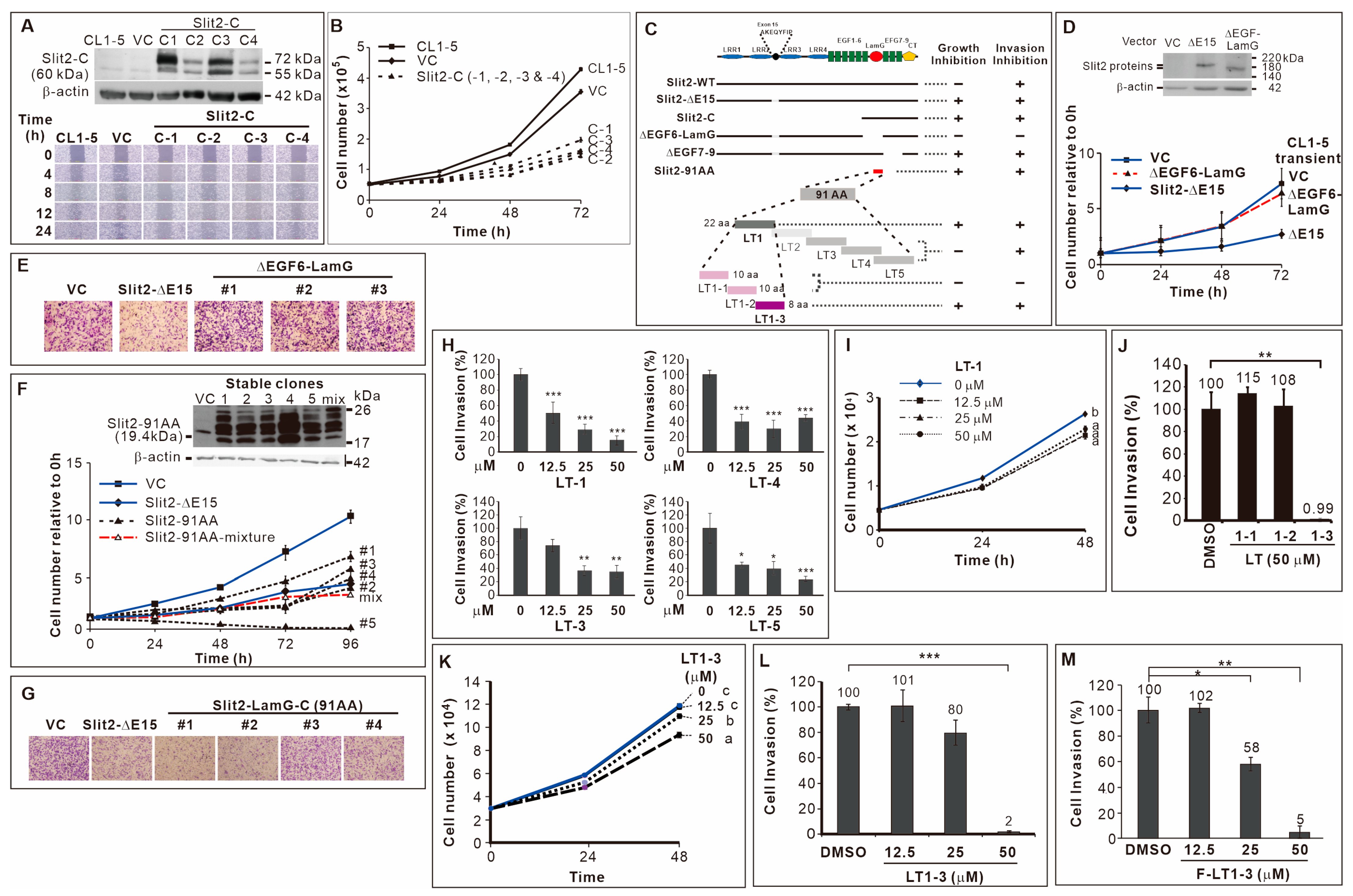
3.1. Effects of Slit2-N- and Slit2-C-Terminal Fragments on Lung Cancer Cell Proliferation and Invasion
3.2. 91 Amino Acid-Long LamG Domain (91 AA) Inhibits Lung Cancer Cell Proliferation and Invasion
3.3. Inhibitory Effect of the 8-Amino Acid Peptide LT1-3 in the LamG-C Domain on Cancer Cell Proliferation and Invasion
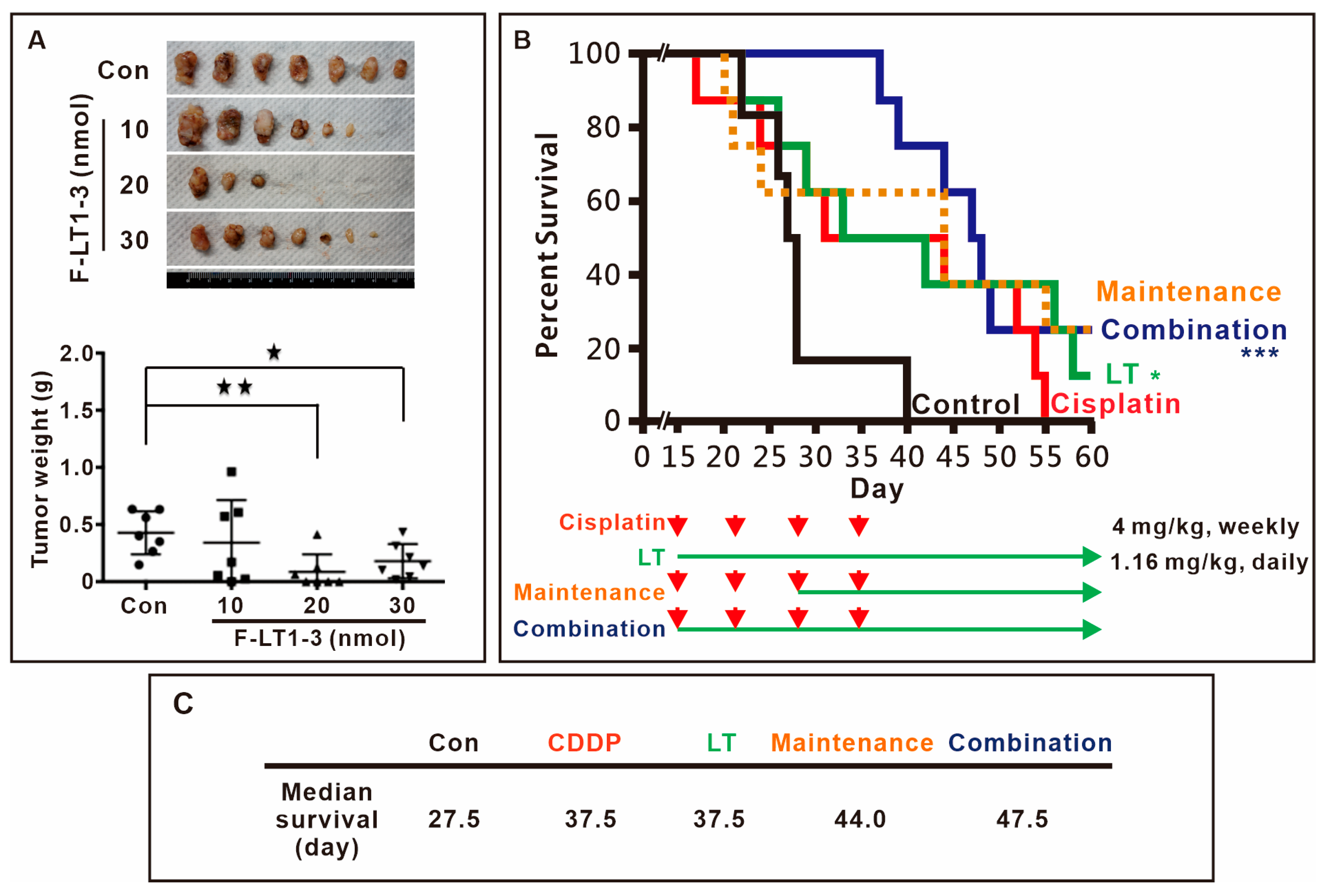
3.4. F-LT1-3 Peptide Inhibits Tumor Progression, Reduces Cisplatin-Induced Side Effects, and Prolongs Animal Survival
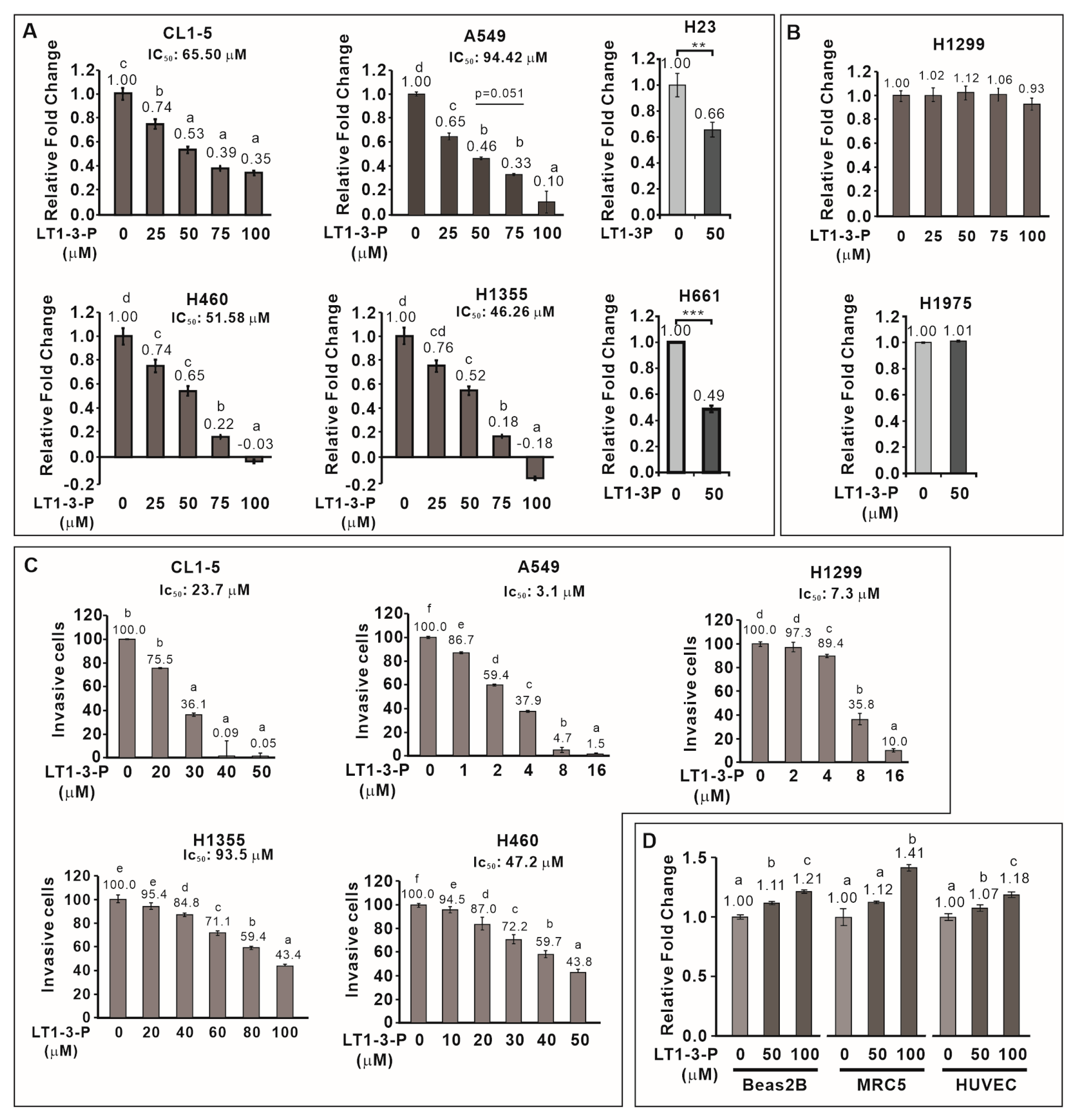
3.5. PEGylation Enhances the Solubility of F-LT1-3 While Retaining Its Proliferation and Invasion Inhibition Activity
3.6. F-LT1-3-PEG Inhibits Lung Cancer Cell Proliferation, but Not Normal Cell Proliferation
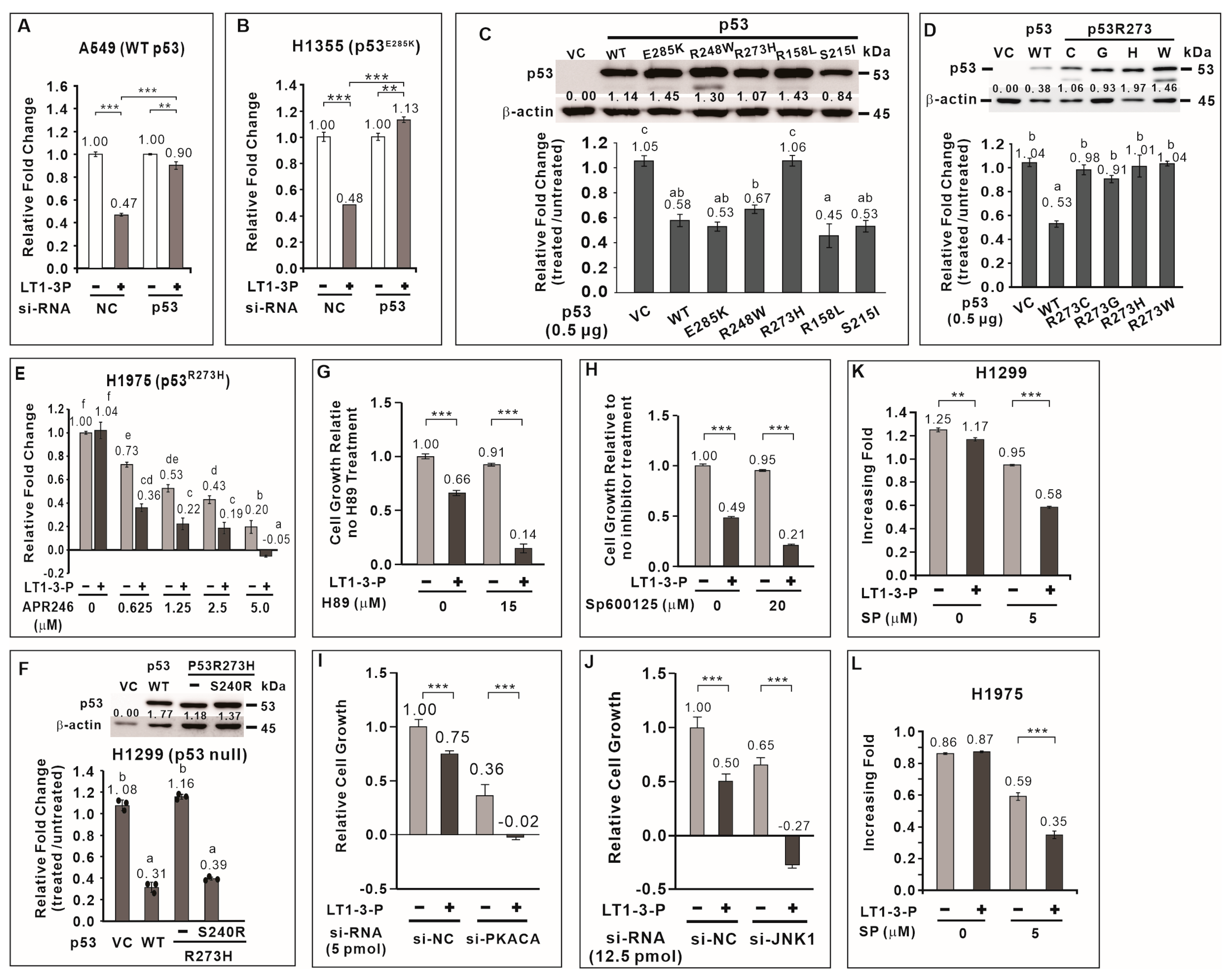
3.7. TP53 Positively Regulates F-LT1-3-PEG-Mediated Inhibition of Lung Cancer Cell Proliferation
3.8. Most p53 Mutants Retain the Ability to Mediate F-LT1-3-PEG-Induced Inhibition of Lung Cancer Cell Proliferation
3.9. APR-246 Restores Sensitivity to F-LT1-3-Mediated Inhibition of Cell Proliferation in p53R273H-Expressing Cells
3.10. JNK1 and PKA Negatively Regulate F-LT1-3-PEG-Mediated Inhibition of Cell Proliferation
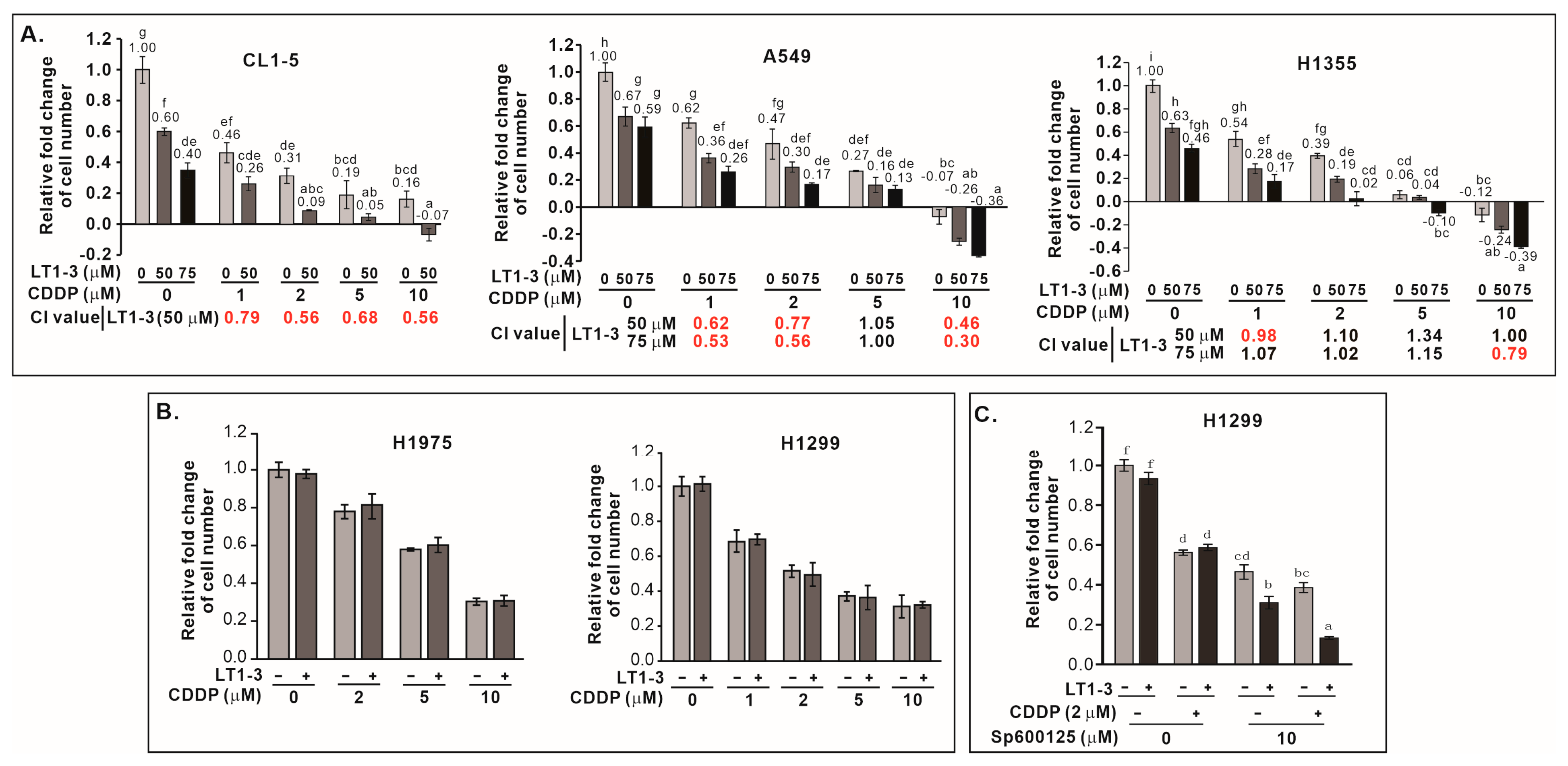
3.11. F-LT1-3-PEG Enhances Cisplatin Efficacy in Lung Cancer Cells
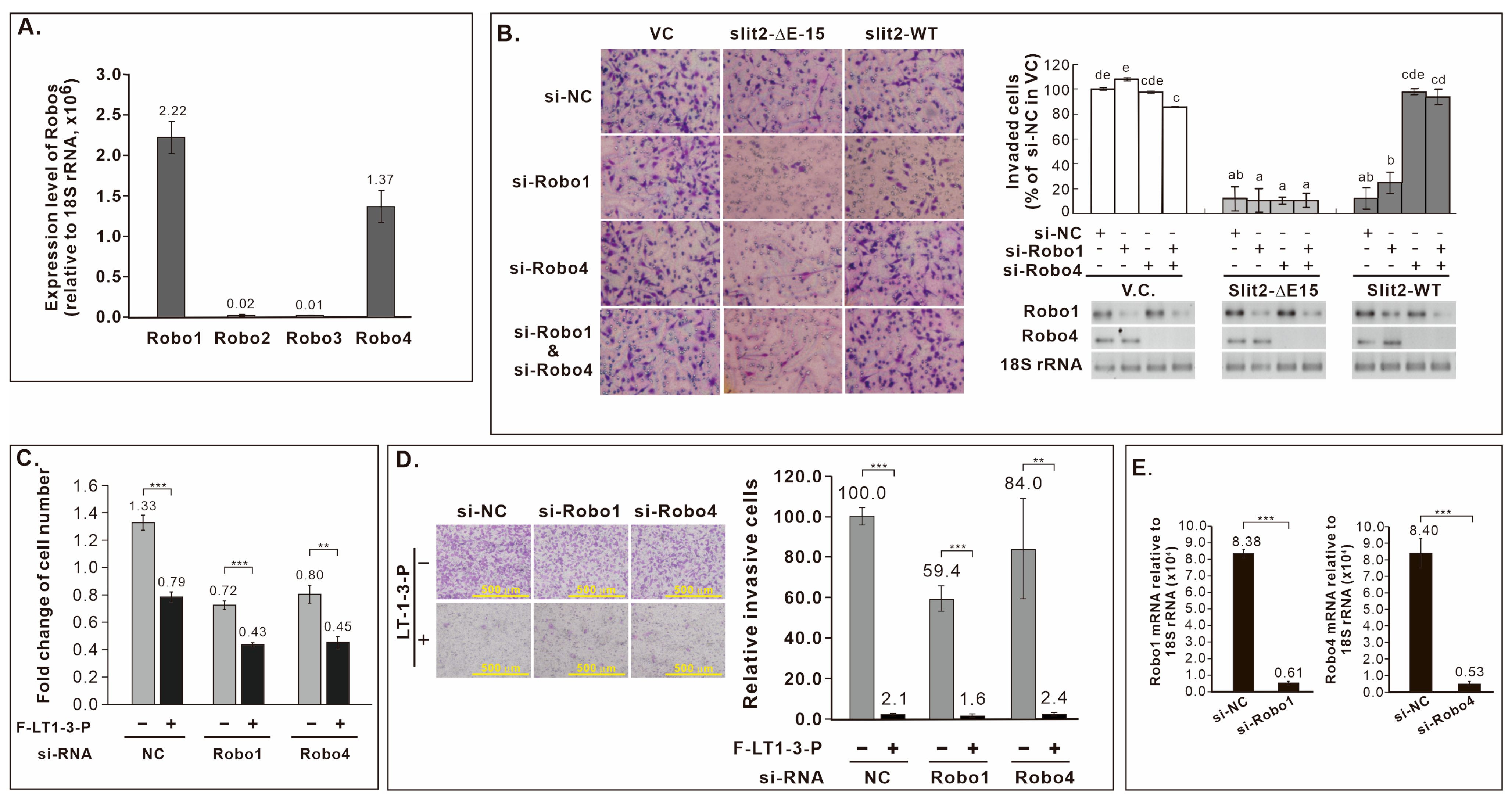
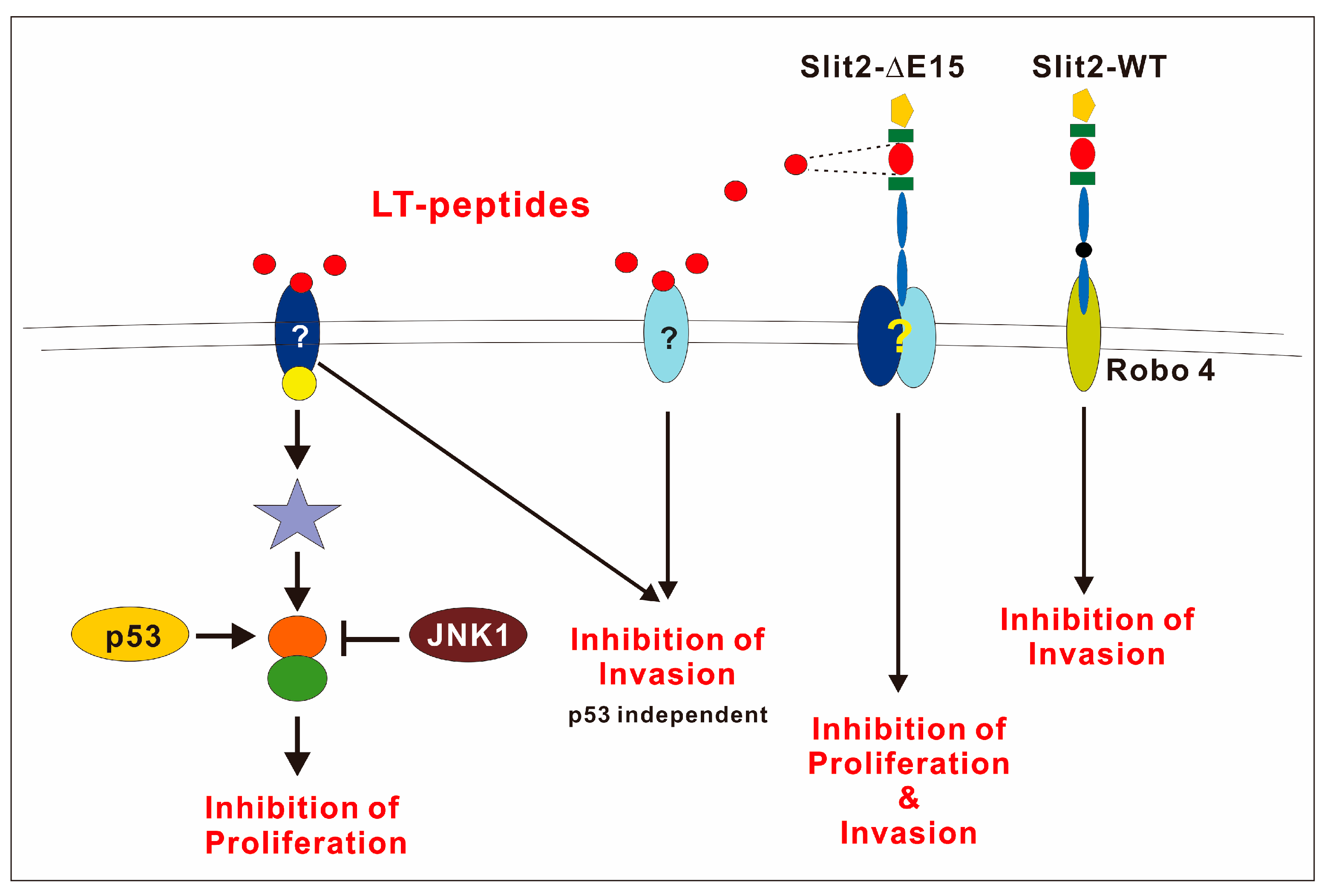
3.12. Robo Receptors Are Not Involved in F-LT1-3-PEG’s Inhibition of Lung Cancer Cell Proliferation and Invasion
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMP | Epithelial–mesenchymal transition |
| Fmoc | Fluorenylmethyloxycarbonyl |
| NSCLC | Non-small cell lung caner |
| PEG | Polyethylene glycol |
| Q-RT-PCR | Quantitative reverse transcription polymerase chain reaction |
References
- Wang, K.H.; Brose, K.; Arnott, D.; Kidd, T.; Goodman, C.S.; Henzel, W.; Tessier-Lavigne, M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 1999, 96, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Brose, K.; Bland, K.S.; Wang, K.H.; Arnott, D.; Henzel, W.; Goodman, C.S.; Tessier-Lavigne, M.; Kidd, T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999, 96, 795–806. [Google Scholar] [CrossRef]
- Niclou, S.P.; Jia, L.; Raper, J.A. Slit2 is a repellent for retinal ganglion cell axons. J. Neurosci. 2000, 20, 4962–4974. [Google Scholar] [CrossRef]
- Nguyen-Ba-Charvet, K.T.; Plump, A.S.; Tessier-Lavigne, M.; Chedotal, A. Slit1 and slit2 proteins control the development of the lateral olfactory tract. J. Neurosci. 2002, 22, 5473–5480. [Google Scholar] [CrossRef]
- Macias, H.; Moran, A.; Samara, Y.; Moreno, M.; Compton, J.E.; Harburg, G.; Strickland, P.; Hinck, L. SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by limiting basal cell number. Dev. Cell 2011, 20, 827–840. [Google Scholar] [CrossRef]
- Fan, X.; Yang, H.; Kumar, S.; Tumelty, K.E.; Pisarek-Horowitz, A.; Rasouly, H.M.; Sharma, R.; Chan, S.; Tyminski, E.; Shamashkin, M.; et al. SLIT2/ROBO2 signaling pathway inhibits nonmuscle myosin IIA activity and destabilizes kidney podocyte adhesion. JCI Insight 2016, 1, e86934. [Google Scholar] [CrossRef]
- Zhao, J.; Mommersteeg, M.T.M. Slit-Robo signalling in heart development. Cardiovasc. Res. 2018, 114, 794–804. [Google Scholar] [CrossRef]
- Anselmo, M.A.; Dalvin, S.; Prodhan, P.; Komatsuzaki, K.; Aidlen, J.T.; Schnitzer, J.J.; Wu, J.Y.; Kinane, T.B. Slit and robo: Expression patterns in lung development. Gene Expr. Patterns 2003, 3, 13–19. [Google Scholar] [CrossRef]
- Gara, R.K.; Kumari, S.; Ganju, A.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Slit/Robo pathway: A promising therapeutic target for cancer. Drug Discov. Today 2015, 20, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Ba-Charvet, K.T.; Brose, K.; Ma, L.; Wang, K.H.; Marillat, V.; Sotelo, C.; Tessier-Lavigne, M.; Chedotal, A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J. Neurosci. 2001, 21, 4281–4289. [Google Scholar] [CrossRef] [PubMed]
- Morlot, C.; Thielens, N.M.; Ravelli, R.B.; Hemrika, W.; Romijn, R.A.; Gros, P.; Cusack, S.; McCarthy, A.A. Structural insights into the Slit-Robo complex. Proc. Natl. Acad. Sci. USA 2007, 104, 14923–14928. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Paruchuri, V.; Preet, A.; Latif, F.; Ganju, R.K. Slit-2 induces a tumor-suppressive effect by regulating beta-catenin in breast cancer cells. J. Biol. Chem. 2008, 283, 26624–26633. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.C.; Lee, S.H.; Hsu, H.S.; Chen, B.H.; Tsai, W.C.; Tzao, C.; Wang, Y.C. SLIT2 attenuation during lung cancer progression deregulates beta-catenin and E-cadherin and associates with poor prognosis. Cancer Res. 2010, 70, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Yiin, J.J.; Hu, B.; Jarzynka, M.J.; Feng, H.; Liu, K.W.; Wu, J.Y.; Ma, H.I.; Cheng, S.Y. Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro-Oncology 2009, 11, 779–789. [Google Scholar] [CrossRef]
- Tseng, R.C.; Chang, J.M.; Chen, J.H.; Huang, W.R.; Tang, Y.A.; Kuo, I.Y.; Yan, J.J.; Lai, W.W.; Wang, Y.C. Deregulation of SLIT2-mediated Cdc42 activity is associated with esophageal cancer metastasis and poor prognosis. J. Thorac. Oncol. 2015, 10, 189–198. [Google Scholar] [CrossRef]
- Chen, W.F.; Gao, W.D.; Li, Q.L.; Zhou, P.H.; Xu, M.D.; Yao, L.Q. SLIT2 inhibits cell migration in colorectal cancer through the AKT-GSK3beta signaling pathway. Int. J. Color. Dis. 2013, 28, 933–940. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, Y.; Ding, B.B.; Zhang, N.; Yuan, X.; Gui, L.; Qian, K.X.; Duan, S.; Chen, Z.; Rao, Y.; et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003, 4, 19–29. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhou, D.L.; Lei, Y.; Zheng, L.; Chen, S.X.; Gou, H.J.; Gu, Q.L.; He, X.D.; Lan, T.; Qi, C.L.; et al. Slit2/Robo1 signaling promotes intestinal tumorigenesis through Src-mediated activation of the Wnt/beta-catenin pathway. Oncotarget 2015, 6, 3123–3135. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, F.L.; Li, W.P.; Wang, J.; Wang, L.J. Slit2Robo1 signaling promotes the adhesion, invasion and migration of tongue carcinoma cells via upregulating matrix metalloproteinases 2 and 9, and downregulating Ecadherin. Mol. Med. Rep. 2016, 14, 1901–1906. [Google Scholar] [CrossRef][Green Version]
- Zhao, S.J.; Shen, Y.F.; Li, Q.; He, Y.J.; Zhang, Y.K.; Hu, L.P.; Jiang, Y.Q.; Xu, N.W.; Wang, Y.J.; Li, J.; et al. SLIT2/ROBO1 axis contributes to the Warburg effect in osteosarcoma through activation of SRC/ERK/c-MYC/PFKFB2 pathway. Cell Death Dis. 2018, 9, 390. [Google Scholar] [CrossRef]
- Tan, Q.; Liang, X.J.; Lin, S.M.; Cheng, Y.; Ding, Y.Q.; Liu, T.F.; Zhou, W.J. Engagement of Robo1 by Slit2 induces formation of a trimeric complex consisting of Src-Robo1-E-cadherin for E-cadherin phosphorylation and epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2020, 522, 757–762. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Yang, C.H.; Sheu, G.T.; Huang, C.Y.; Wu, Y.C.; Chuang, S.M.; Fann, M.J.; Chang, H.; Lee, H.; Chang, J.T. A novel exon 15-deleted, splicing variant of Slit2 shows potential for growth inhibition in addition to invasion inhibition in lung cancer. Cancer 2011, 117, 3404–3415. [Google Scholar] [CrossRef]
- Sun, S.J.; Wu, C.C.; Sheu, G.T.; Chang, H.Y.; Chen, M.Y.; Lin, Y.Y.; Chuang, C.Y.; Hsu, S.L.; Chang, J.T. Integrin beta3 and CD44 levels determine the effects of the OPN-a splicing variant on lung cancer cell growth. Oncotarget 2016, 7, 55572–55584. [Google Scholar] [CrossRef]
- Zhang, Q.; Bykov, V.J.N.; Wiman, K.G.; Zawacka-Pankau, J. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Baroni, T.E.; Wang, T.; Qian, H.; Dearth, L.R.; Truong, L.N.; Zeng, J.; Denes, A.E.; Chen, S.W.; Brachmann, R.K. A global suppressor motif for p53 cancer mutants. Proc. Natl. Acad. Sci. USA 2004, 101, 4930–4935. [Google Scholar] [CrossRef]
- Eldar, A.; Rozenberg, H.; Diskin-Posner, Y.; Rohs, R.; Shakked, Z. Structural studies of p53 inactivation by DNA-contact mutations and its rescue by suppressor mutations via alternative protein-DNA interactions. Nucleic Acids Res. 2013, 41, 8748–8759. [Google Scholar] [CrossRef]
- Villalobos, P.; Wistuba, I.I. Lung Cancer Biomarkers. Hematol. Oncol. Clin. N. Am. 2017, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Howitt, J.A.; Clout, N.J.; Hohenester, E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J. 2004, 23, 4406–4412. [Google Scholar] [CrossRef]
- Liu, Z.; Patel, K.; Schmidt, H.; Andrews, W.; Pini, A.; Sundaresan, V. Extracellular Ig domains 1 and 2 of Robo are important for ligand (Slit) binding. Mol. Cell. Neurosci. 2004, 26, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Delloye-Bourgeois, C.; Jacquier, A.; Charoy, C.; Reynaud, F.; Nawabi, H.; Thoinet, K.; Kindbeiter, K.; Yoshida, Y.; Zagar, Y.; Kong, Y.; et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat. Neurosci. 2015, 18, 36–45. [Google Scholar] [CrossRef]
- Reles, A.; Wen, W.H.; Schmider, A.; Gee, C.; Runnebaum, I.B.; Kilian, U.; Jones, L.A.; El-Naggar, A.; Minguillon, C.; Schonborn, I.; et al. Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer. Clin. Cancer Res. 2001, 7, 2984–2997. [Google Scholar] [PubMed]
- Lin, X.; Howell, S.B. DNA mismatch repair and p53 function are major determinants of the rate of development of cisplatin resistance. Mol. Cancer Ther. 2006, 5, 1239–1247. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.I.; Harris, C.C. Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a001016. [Google Scholar] [CrossRef]
- Qin, K.; Hou, H.; Liang, Y.; Zhang, X. Prognostic value of TP53 concurrent mutations for EGFR- TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: A meta-analysis. BMC Cancer 2020, 20, 328. [Google Scholar] [CrossRef]
- Ahrendt, S.A.; Hu, Y.; Buta, M.; McDermott, M.P.; Benoit, N.; Yang, S.C.; Wu, L.; Sidransky, D. p53 mutations and survival in stage I non-small-cell lung cancer: Results of a prospective study. J. Natl. Cancer Inst. 2003, 95, 961–970. [Google Scholar] [CrossRef]
- Cho, Y.; Gorina, S.; Jeffrey, P.D.; Pavletich, N.P. Crystal structure of a p53 tumor suppressor-DNA complex: Understanding tumorigenic mutations. Science 1994, 265, 346–355. [Google Scholar] [CrossRef]
- Kotler, E.; Shani, O.; Goldfeld, G.; Lotan-Pompan, M.; Tarcic, O.; Gershoni, A.; Hopf, T.A.; Marks, D.S.; Oren, M.; Segal, E. A Systematic p53 Mutation Library Links Differential Functional Impact to Cancer Mutation Pattern and Evolutionary Conservation. Mol. Cell 2018, 71, 178–190. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Gaur, S.; Zhang, K.; Wu, X.; Yuan, Y.C.; Li, H.; Hu, S.; Weng, Y.; Yen, Y. Mutants TP53 p.R273H and p.R273C but not p.R273G enhance cancer cell malignancy. Hum. Mutat. 2014, 35, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.Y.; Adler, V.; Pincus, M.R.; Ronai, Z. MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl. Acad. Sci. USA 1998, 95, 10541–10546. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.N.; Jiang, H.; Yang, Y.; Zhu, X.; Wang, X.; Schimmer, A.D.; Qiu, L.; Chang, H. Targeting p53 via JNK pathway: A novel role of RITA for apoptotic signaling in multiple myeloma. PLoS ONE 2012, 7, e30215. [Google Scholar] [CrossRef]
- Das, M.; Jiang, F.; Sluss, H.K.; Zhang, C.; Shokat, K.M.; Flavell, R.A.; Davis, R.J. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 15759–15764. [Google Scholar] [CrossRef]
| A549 | H460 | H1355 | H23 | CL1-5 | H661 | H1299 | H1975 | |
|---|---|---|---|---|---|---|---|---|
| P53 | WT | WT | E285K | M246I | R248W | R158L a | Null | R273H |
| kRas | G12S | Q61H | G13C | G12C | WT | WT | WT | WT |
| Growth inhibition | + | + | + | + | + | + | − | − |
| Invasion inhibition | + | + | + | NA | + | NA | + | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-C.; Liao, C.-Y.; Lin, Y.-Y.; Chuang, S.-M.; Liu, S.-Y.; Wang, C.-H.; Su, S.-E.; Wu, S.-W.; Wang, L.-I.; Chen, W.-T.; et al. LT1-3, a Slit2-Derived Peptide, Exhibits Anti-Tumor Activity and Improves Cisplatin Therapy. Cells 2025, 14, 1654. https://doi.org/10.3390/cells14211654
Wu T-C, Liao C-Y, Lin Y-Y, Chuang S-M, Liu S-Y, Wang C-H, Su S-E, Wu S-W, Wang L-I, Chen W-T, et al. LT1-3, a Slit2-Derived Peptide, Exhibits Anti-Tumor Activity and Improves Cisplatin Therapy. Cells. 2025; 14(21):1654. https://doi.org/10.3390/cells14211654
Chicago/Turabian StyleWu, Ting-Chien, Chen-Yi Liao, Yu-Ying Lin, Shu-Ming Chuang, Szu-Yu Liu, Chi-Hsiang Wang, Shang-Er Su, Siang-Wei Wu, Ling-I Wang, Wei-Ting Chen, and et al. 2025. "LT1-3, a Slit2-Derived Peptide, Exhibits Anti-Tumor Activity and Improves Cisplatin Therapy" Cells 14, no. 21: 1654. https://doi.org/10.3390/cells14211654
APA StyleWu, T.-C., Liao, C.-Y., Lin, Y.-Y., Chuang, S.-M., Liu, S.-Y., Wang, C.-H., Su, S.-E., Wu, S.-W., Wang, L.-I., Chen, W.-T., Cheng, S.-W., Huang, Y.-T., Zheng, Y.-B., Chuang, C.-Y., Lung, F.-D., & Chang, J. T. (2025). LT1-3, a Slit2-Derived Peptide, Exhibits Anti-Tumor Activity and Improves Cisplatin Therapy. Cells, 14(21), 1654. https://doi.org/10.3390/cells14211654







