From Adhesion to Invasion: Integrins, Focal Adhesion Signaling, and Actin Binding Proteins in Cervical Cancer Progression—A Scoping Review
Abstract
1. Introduction
2. Methods
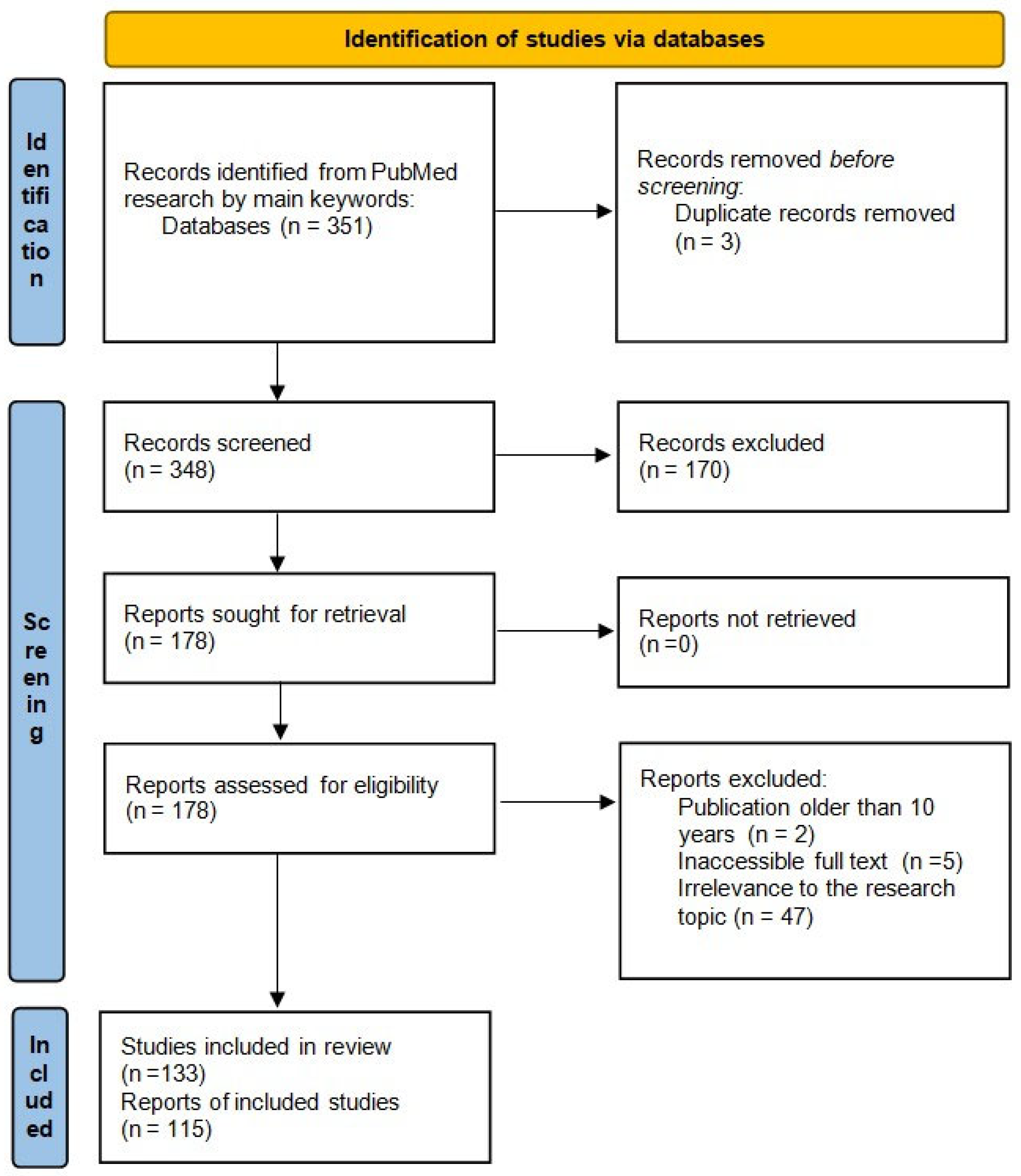
2.1. Protocol and Reporting
2.2. Eligibility Criteria
2.3. Search Strategy and Information Sources
2.4. Selection of Sources of Evidence
2.5. Data Charting Process
- Protein/Target;
- Key role/Function in CC;
- Main mechanisms/Pathways;
- Clinical/Experimental insights;
- References.
2.6. Data Items
2.7. Synthesis of Results
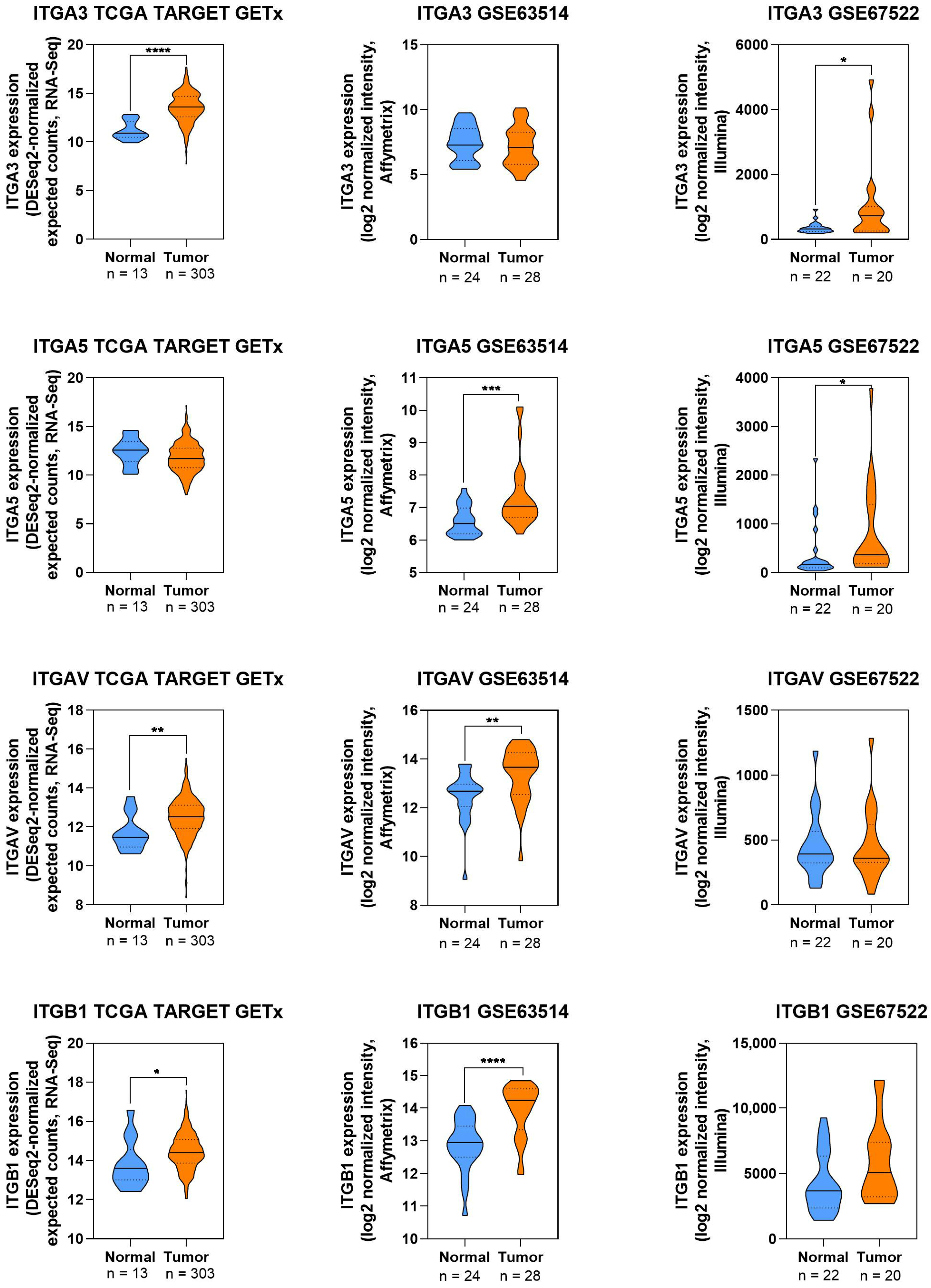
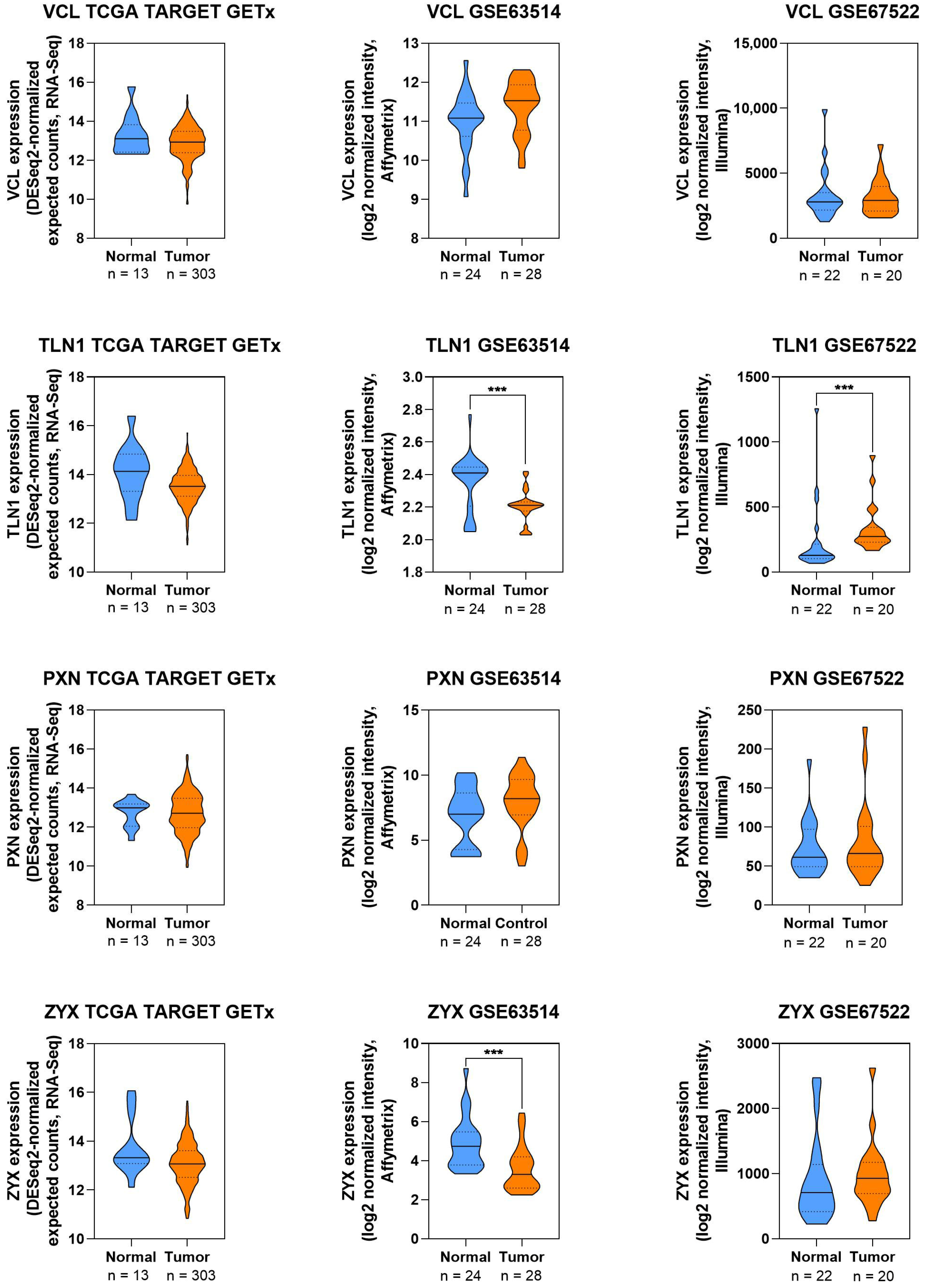
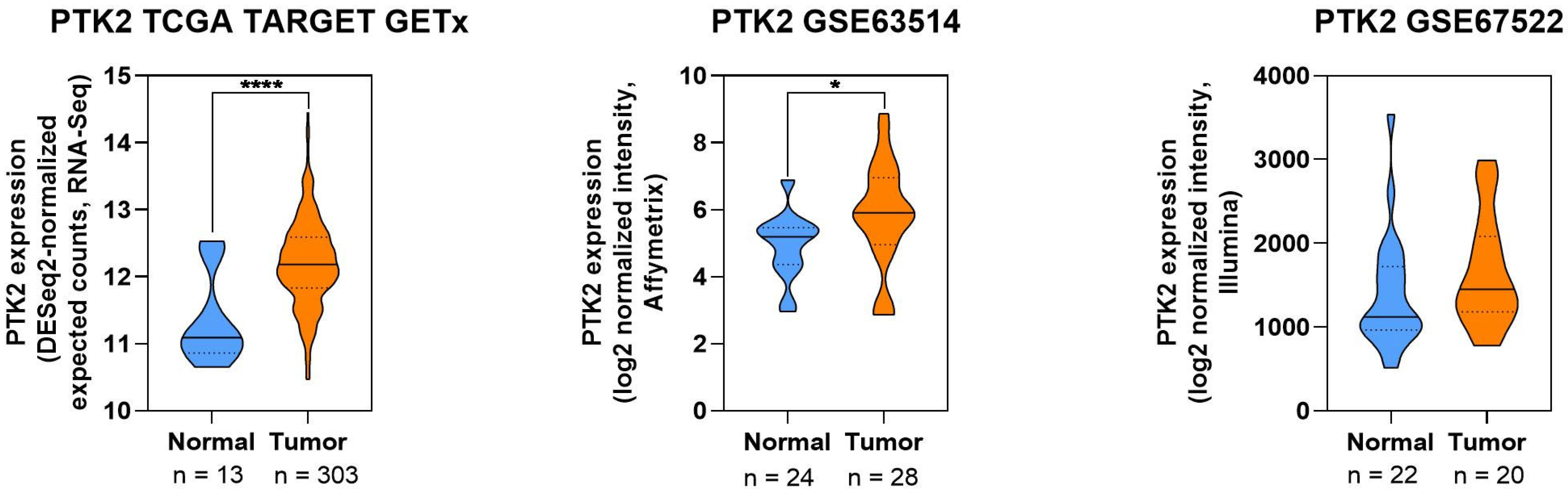
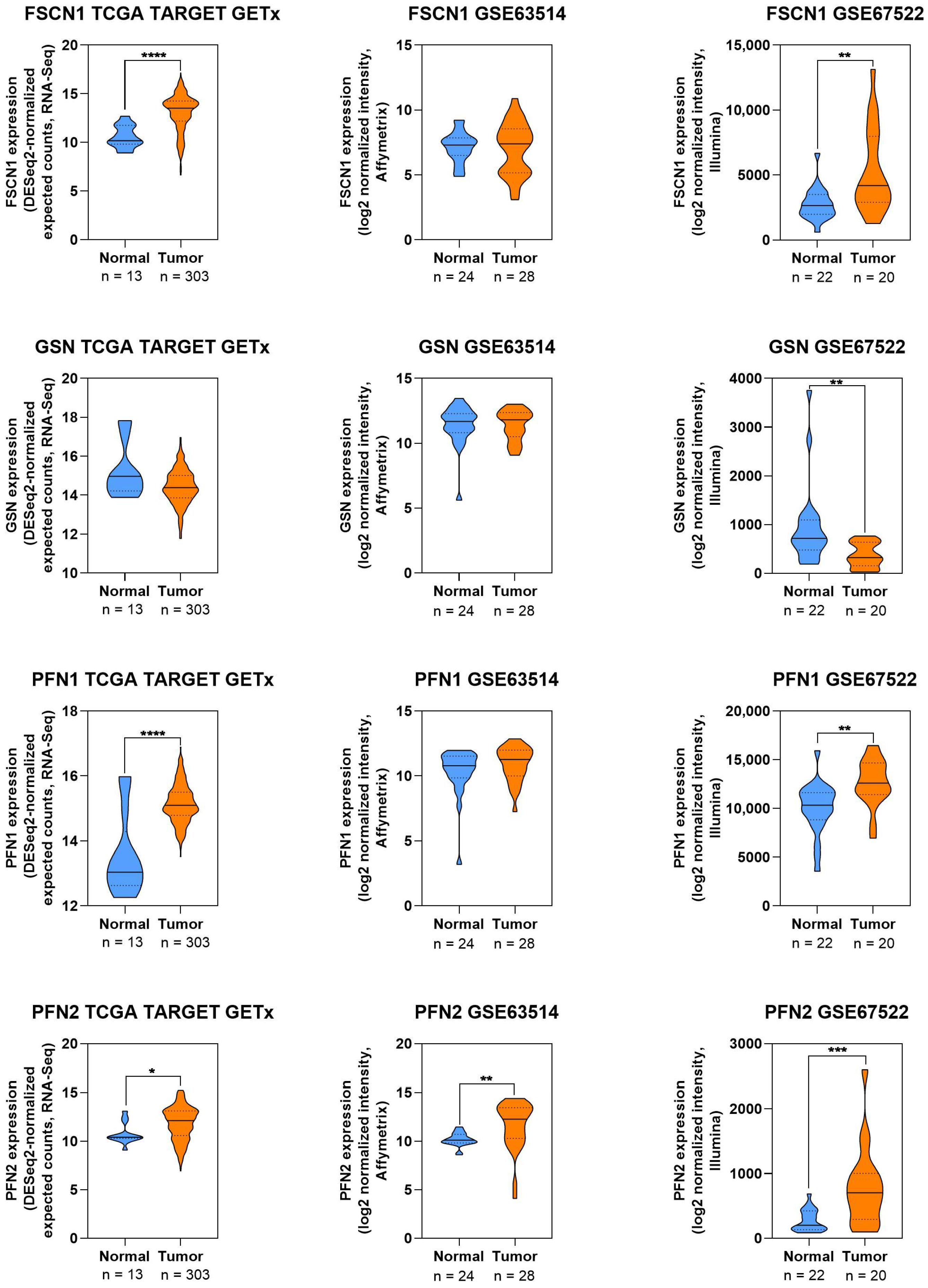
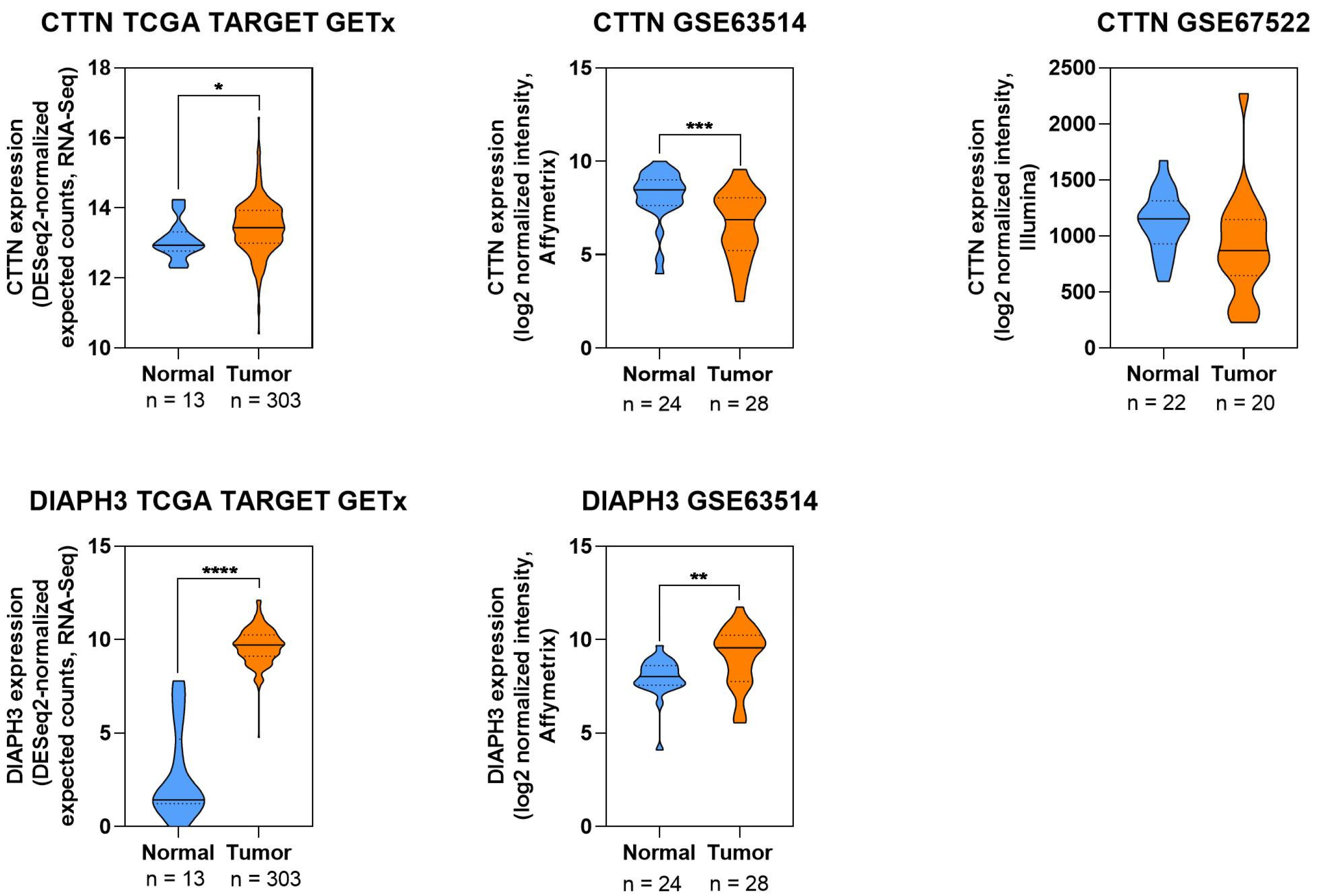
2.8. Visualization of Protein Expression in CC
3. Results
3.1. Integrins in CC
3.1.1. Integrin α3
3.1.2. Integrin α5
3.1.3. Integrin β1
| Protein/Target | Key Role/Function in CC | Main Mechanisms/Pathways | Clinical/Experimental Insights | References |
|---|---|---|---|---|
| ITGA3 | Promotes migration, invasion, angiogenesis | Src/ERK/FAK, PI3K/AKT, ERK phosphorylation | High expression correlates with poor prognosis; targeted by siRNA; affects EMT and MUC1 regulation | [24,27,28,29] |
| ITGA5 | Drives proliferation, migration, invasion, angiogenesis | VEGF/FN1, IMP3-HK2, TGF-β | High expression correlates with poor prognosis; modulated by microRNA-128 & Anabasis setifera; included in prognostic models | [15,26,31,32,33,34,35,36,37,38,39,40,41] |
| ITGB1 | Regulates adhesion, metastasis, response to hypoxia | FN1/MMP9, PI3K-Akt, HIF-1A/ITGB1, FAK/ERK | Overexpressed in advanced CC and HPV-positive cells; targetable via miRNAs or CRABP2 inhibition; correlates with worse OS and therapy resistance | [1,25,35,42,43,44,45,46,47,48] |
3.1.4. Expression Patterns of Integrins in CC
3.2. Adhesive Proteins and Focal Adhesion in CC
3.2.1. Focal Adhesion Proteins
Talin
Vinculin
Paxillin
| Protein/Target | Key Role/Function in CC | Main Mechanisms/Pathways | Clinical/Experimental Insights | References |
|---|---|---|---|---|
| Talin (TLN) | Supports adhesion, motility, metastasis | Integrin activation, focal adhesion signaling | Upregulated in CC; silencing decreases migration and invasion | [52] |
| Vinculin (VCL) | Stabilizes focal adhesions, promotes motility | Integrin-actin linkage, focal adhesion stabilization | Overexpressed in CC; knockdown reduces migration and invasion | [53,54,55] |
| Paxillin (PXN) | Promotes proliferation, migration, invasion | Focal adhesion regulation, actin cytoskeleton remodeling, FAK-PXN-MAPK signaling | Overexpressed in CC; knockdown reduces aggressive phenotypes | [16,56,57,58,59,60,61,62,63] |
Expression Patterns of Focal Adhesion in CC
3.2.2. Focal Adhesion Kinase
| Protein/Target | Key Role/Function in CC | Main Mechanisms/Pathways | Clinical/Experimental Insights | References |
|---|---|---|---|---|
| FAK | Promotes proliferation, migration, invasion, metastasis | Integrin β1/FAK/ERK; Src-dependent FAK; actin/cofilin; FAK/AKT/GSK3β | Overexpressed in CC tissues; silencing reduces migration/proliferation and sensitizes cells to drugs | [64] |
| FAK/SASH1 | Tumor suppressor regulating FAK | FAK-related signaling axis; downregulation of MMP-2/9 | SASH1 downregulated in CC; overexpression inhibits invasion and proliferation | [65] |
| FAK/SPARCL1 | Suppresses CC proliferation/migration | FAK/ERK axis | SPARCL1 overexpression reduces CC cell proliferation and migration; osteopontin increases p-FAK | [66] |
| FAK/LIMK1 | Promotes cytoskeletal reorganization and CC progression | Src-dependent FAK; actin/cofilin activation; oxidative stress | LIMK1 enhances invasive potential Via FAK-mediated cytoskeletal changes | [67] |
| FAK/CRABP2 | Promotes CC progression | Integrin β1/FAK/ERK axis | Silencing CRABP2 reduces p-FAK, p-ERK, and ITGB1; inhibits migration and invasion | [68] |
| FAK/EIF3D | Promotes CC progression | FAK activation via GRP78 | Overexpression activates FAK; knockdown inhibits tumor growth in xenograft model | [48,68] |
| FAK/Natural compounds | Therapeutic inhibition of FAK-driven proliferation and migration | FAK/integrin/AKT/GSK3β pathway | Bufalin, shikonin, parthenolide, tretinoin, luteolin, etc., reduce proliferation/migration/invasion in vitro and in vivo; combination with paclitaxel enhances effect | [60,61,62,63,69,70,71,72] |
Expression Pattern of FAK in CC
3.3. Actin Binding Proteins in CC
3.3.1. Actinins
3.3.2. Cofilin
3.3.3. Cortactin
3.3.4. Diaphanous-Related Formin 3
3.3.5. Ezrin/Radixin/Moesin
3.3.6. Fascin
3.3.7. Gelsolin
3.3.8. Transgelin
3.3.9. Tropomodulin
| Protein/Target | Key Role/Function in CC | Main Mechanisms/Pathways | Clinical/Experimental Insights | References |
|---|---|---|---|---|
| Actinin 4 (ACTN4) | Promotes proliferation, migration, invasion, EMT | Wnt/β-catenin, PI3K/AKT/mTOR, Snail/MMP-9 | Overexpressed in CC tissues and serum; correlated with stage and metastasis; knockdown reduces stemness and invasion; potential diagnostic marker | [74,75,76,77,78,79,80,81] |
| Actinin 1 (ACTN1) | Regulates cytoskeleton and motility | miR-129-5p targeting | miR-129-5p suppresses ACTN1, inhibiting migration, invasion, and angiogenesis | [77] |
| Cofilin 1 (CFL1) | Controls actin turnover, migration, invasion | LIMK1/2–p-cofilin, ROS/Src, WWC2/YAP axis | Upregulated in CC; phosphorylation regulates motility; LIMK and YAP signaling modulate activity; inhibitors reduce invasion | [67,82,84,85,86,87,88] |
| Cortactin | Promotes invasion, lesion progression | VEGF-C/c-Src ↓miR-326 → ↑Cortactin; cytoskeletal remodeling | Expression increases with lesion grade; potential biomarker for progression | [89,90] |
| DIAPH3 | Enhances proliferation, tumor growth | mTOR activation, modulation of immune cell infiltration | Overexpressed in CC; knockdown reduces proliferation and tumor size in vitro and in vivo | [91,92,93] |
| Ezrin (EZR) | Supports migration, EMT, immune evasion | PI3K/Akt signaling; scaffolding of CD47 and PD-L1 | Overexpression correlates with poor prognosis; knockdown reduces invasion and colony formation | [85,94,95,96,97,98,99,100,101,102,103,104,105,106,107,110] |
| Radixin (RDX) | Promotes proliferation, invasion | c-Jun/HIF1A-AS2/miR-34b-5p axis | Overexpression associated with CC progression | [108] |
| Moesin (MSN) | Regulates immune evasion | Membrane localization of PD-L1 | Knockdown reduces PD-L1 surface expression | [109] |
| Fascin (FSCN1) | Enhances motility, invasion | Filopodia formation, miR-145/FSCN1 axis, Wnt/β-catenin, angiogenesis (ANGPTL4) | Overexpressed in CC and HPV16+ lesions; correlates with poor prognosis and radiotherapy resistance | [111,112,113,114,115,116,117,118,119,120] |
| Gelsolin (GSN) | Mediates cytoskeletal remodeling, EMT | HPV16 E7 interaction → ↑F-actin, HIPPO pathway suppression; USP7/UHRF1 ↓GSN | Downregulated in CC; knockdown reduces invasion; therapeutic target potential | [82,121,122,123,124] |
| Transgelin 2 (TAGLN2) | Tumor suppressor | ↑E-cadherin, ↓MMP-2/MMP-9, NF-κB modulation | Downregulated in advanced CC; overexpression inhibits migration and invasion | [125,126,127] |
| Tropomodulin 1 (TMOD1) | Tumor suppressor, regulates actin dynamics | Capping actin filaments; controls motility | Knockdown ↑migration/invasion; high expression associated with early-stage CC | [128,129] |
3.3.10. Expression Patterns of ABP in CC
4. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yi, Y.; Fang, Y.; Wu, K.; Liu, Y.; Zhang, W. Comprehensive Gene and Pathway Analysis of Cervical Cancer Progression. Oncol. Lett. 2020, 19, 3316–3332. [Google Scholar] [CrossRef]
- Kalita, B.; Coumar, M.S. Deciphering Molecular Mechanisms of Metastasis: Novel Insights into Targets and Therapeutics. Cell. Oncol. 2021, 44, 751–775. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Song, H.; Jiang, H.; Hu, W.; Hai, Y.; Cai, Y.; Li, H.; Liao, Y.; Huang, Y.; Lv, X.; Zhang, Y.; et al. Cervical Extracellular Matrix Hydrogel Optimizes Tumor Heterogeneity of Cervical Squamous Cell Carcinoma Organoids. Sci. Adv. 2024, 10, eadl3511. [Google Scholar] [CrossRef]
- Fan, C.; Xiong, F.; Zhang, S.; Gong, Z.; Liao, Q.; Li, G.; Guo, C.; Xiong, W.; Huang, H.; Zeng, Z. Role of Adhesion Molecules in Cancer and Targeted Therapy. Sci. China Life Sci. 2024, 67, 940–957. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting Integrin Pathways: Mechanisms and Advances in Therapy. Sig. Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Molika, P.; Leetanaporn, K.; Chiangjong, W.; Choochuen, P.; Navakanitworakul, R. Proteomic Analysis Reveals Cadherin, Actin, and Focal Adhesion Molecule-Mediated Formation of Cervical Cancer Spheroids. Cells 2024, 13, 2004. [Google Scholar] [CrossRef] [PubMed]
- Renukadevi, J.; Mridula, D.S.; Nimithasree, K.; Sanjay, V. Cofilin in Cancer: A Molecular Review of Its Role in Tumor Plasticity and Progression. J. Bio-X Res. 2025, 8, 0048. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Pavelescu, L.A.; Mititelu-Zafiu, N.L.; Mindru, D.E.; Vladareanu, R.; Curici, A. Molecular Insights into HPV-Driven Cervical Cancer: Oncoproteins, Immune Evasion, and Epigenetic Modifications. Microorganisms 2025, 13, 1000. [Google Scholar] [CrossRef]
- Yu, L.; Majerciak, V.; Lobanov, A.; Mirza, S.; Band, V.; Liu, H.; Cam, M.; Hughes, S.H.; Lowy, D.R.; Zheng, Z.-M. HPV Oncogenes Expressed from Only One of Multiple Integrated HPV DNA Copies Drive Clonal Cell Expansion in Cervical Cancer. mBio 2024, 15, e00729-24. [Google Scholar] [CrossRef]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and Cancer: Regulators of Cancer Stemness, Metastasis, and Drug Resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef]
- Tai, Y.-L.; Chen, L.-C.; Shen, T.-L. Emerging Roles of Focal Adhesion Kinase in Cancer. Biomed. Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef]
- Li, S.; Sampson, C.; Liu, C.; Piao, H.; Liu, H.-X. Integrin Signaling in Cancer: Bidirectional Mechanisms and Therapeutic Opportunities. Cell Commun. Signal. 2023, 21, 266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, X.; Yang, S. Clinical Significance of Focal Adhesion Kinase (FAK) in Cervical Cancer Progression and Metastasis. Int. J. Clin. Exp. Pathol. 2020, 13, 2586–2592. [Google Scholar]
- Liu, Z.; Zhang, X.; Ben, T.; Li, M.; Jin, Y.; Wang, T.; Song, Y. Focal Adhesion in the Tumour Metastasis: From Molecular Mechanisms to Therapeutic Targets. Biomark. Res. 2025, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Padežnik, T.; Oleksy, A.; Cokan, A.; Takač, I.; Sobočan, M. Changes in the Extracellular Matrix in Endometrial and Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 5463. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Wang, S.; Yang, J.-M.; Huang, C.-H. Targeting Signaling Excitability in Cervical and Pancreatic Cancer Cells Through Combined Inhibition of FAK and PI3K. Int. J. Mol. Sci. 2025, 26, 3040. [Google Scholar] [CrossRef]
- Biber, G.; Ben-Shmuel, A.; Sabag, B.; Barda-Saad, M. Chapter Three—Actin Regulators in Cancer Progression and Metastases: From Structure and Function to Cytoskeletal Dynamics. In International Review of Cell and Molecular Biology; Thomas, C., Galluzzi, L., Eds.; Actin Cytoskeleton in Cancer Progression and Metastasis—Part B; Academic Press: Cambridge, MA, USA, 2020; Volume 356, pp. 131–196. [Google Scholar]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical Cancer Therapies: Current Challenges and Future Perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Kabir, I.M.; Ribeiro, R.I.M.D.A. HPV and Cervical Cancer: From Molecular Diagnostics to Emerging Treatment Approaches (Review). World Acad. Sci. J. 2025, 7, 58. [Google Scholar] [CrossRef]
- Backer-Meurke, S.; Agyemang, A.; Castellano, T.; Jernigan, A. Management of Recurrent and Metastatic Cervical Cancer: A Review of Current Practice. Oncology 2025, 2, 126–138. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Tan, W.; Chen, G.; Ci, Q.; Deng, Z.; Gu, R.; Yang, D.; Dai, F.; Liu, H.; Cheng, Y. Elevated ITGA3 Expression Serves as a Novel Prognostic Biomarker and Regulates Tumor Progression in Cervical Cancer. Sci. Rep. 2024, 14, 27063. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Lee, H.R.; Lee, J.H.; Seo, C.; Ha, M.; Roh, J.; Kim, Y.H.; Jang, J.Y. Identification of Differentially Expressed Genes and Pathways for Risk Stratification in HPV-Associated Cancers Governing Different Anatomical Sites. Front. Biosci. (Landmark Ed.) 2022, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shen, L.; Li, W.; Liu, X.; Yang, P.; Cai, J. ITGA5 Promotes Tumor Angiogenesis in Cervical Cancer. Cancer Med. 2023, 12, 11983–11999. [Google Scholar] [CrossRef]
- Harryman, W.L.; Pond, E.; Singh, P.; Little, A.S.; Eschbacher, J.M.; Nagle, R.B.; Cress, A.E. Laminin-Binding Integrin Gene Copy Number Alterations in Distinct Epithelial-Type Cancers. Am. J. Transl. Res. 2016, 8, 940–954. [Google Scholar]
- Du, Q.; Wang, W.; Liu, T.; Shang, C.; Huang, J.; Liao, Y.; Qin, S.; Chen, Y.; Liu, P.; Liu, J.; et al. High Expression of Integrin A3 Predicts Poor Prognosis and Promotes Tumor Metastasis and Angiogenesis by Activating the C-Src/Extracellular Signal-Regulated Protein Kinase/Focal Adhesion Kinase Signaling Pathway in Cervical Cancer. Front. Oncol. 2020, 10, 36. [Google Scholar] [CrossRef]
- Zhao, A.; Pan, Y.; Gao, Y.; Zhi, Z.; Lu, H.; Dong, B.; Zhang, X.; Wu, M.; Zhu, F.; Zhou, S.; et al. MUC1 Promotes Cervical Squamous Cell Carcinoma through ERK Phosphorylation-Mediated Regulation of ITGA2/ITGA3. BMC Cancer 2024, 24, 559. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nie, S.; Li, S.; Meng, H.; Sun, R.; Yang, J.; Cheng, W. Methylation-Driven Genes and Their Prognostic Value in Cervical Squamous Cell Carcinoma. Ann. Transl. Med. 2020, 8, 868. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Zhang, Y.; Hu, F.; Shen, L.; Hou, S. ITGA5 Promotes Cervical Cancer Progression by Regulating IMP3 Recruitment of HK2 mRNA. Am. J. Transl. Res. 2025, 17, 3031–3049. [Google Scholar] [CrossRef]
- Qu, H.; Zhao, J.; Zuo, X.; He, H.; Wang, X.; Li, H.; Zhang, K. TGF-β-Mediated Activation of Fibroblasts in Cervical Cancer: Implications for Tumor Microenvironment and Prognosis. PeerJ 2025, 13, e19072. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Huang, W.; Yu, Q.; Wang, K.; Xiong, Y.; Wang, Q.; Qin, Y.; Kuang, X.; Tang, J. Single-Cell RNA Sequencing Reveals the Landscape of Biomarker in Allergic Rhinitis Patient Undergoing Intracervical Lymphatic Immunotherapy and Related Pan-Cancer Analysis. Environ. Toxicol. 2024, 39, 2817–2829. [Google Scholar] [CrossRef]
- Huang, T.; Cao, R.; Gao, C.; Luo, J.; Zhou, Z.; Ma, K. Construction and Validation of a Chemokine-Related Gene Signature Associated with Prognosis, Clinical Significance, and Immune Microenvironment Characteristics in Cervical Cancer. Discov. Oncol. 2025, 16, 1114. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, K.; Yao, D. Comprehensive Analysis of Angiogenesis Associated Genes and Tumor Microenvironment Infiltration Characterization in Cervical Cancer. Heliyon 2024, 10, e33277. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhuang, X.; Liang, M.; Sheng, L.; Huang, L.; Li, Y.; Ke, Y. Identification of an Inflammatory Response-Related Gene Prognostic Signature and Immune Microenvironment for Cervical Cancer. Front. Mol. Biosci. 2024, 11, 1394902. [Google Scholar] [CrossRef]
- Yao, S.; Zhao, L.; Chen, S.; Wang, H.; Gao, Y.; Shao, N.-Y.; Dai, M.; Cai, H. Cervical Cancer Immune Infiltration Microenvironment Identification, Construction of Immune Scores, Assisting Patient Prognosis and Immunotherapy. Front. Immunol. 2023, 14, 1135657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, Y.; Yang, L.; Dou, X.; Jiang, J.; Wang, L. Long Non-Coding RNA Activated by Transforming Growth Factor-β Promotes Proliferation and Invasion of Cervical Cancer Cells by Regulating the miR-144/ITGA6 Axis. Exp. Physiol. 2019, 104, 837–844. [Google Scholar] [CrossRef]
- Ebadi, M.; Kavousi, M.; Farahmand, M. Investigation of the Apoptotic and Antimetastatic Effects of Nano-Niosomes Containing the Plant Extract Anabasis Setifera on HeLa: In Vitro Cervical Cancer Study. Chem. Biodivers. 2025, 22, e202402599. [Google Scholar] [CrossRef]
- Chuang, P.-C.; Lu, C.-W.; Tsai, C.-C.; Tseng, S.-H.; Su, W.-H. MicroRNA-128 Confers Anti-Endothelial Adhesion and Anti-Migration Properties to Counteract Highly Metastatic Cervical Cancer Cells’ Migration in a Parallel-Plate Flow Chamber. Int. J. Mol. Sci. 2020, 22, 215. [Google Scholar] [CrossRef]
- Ma, L.-L.; Zhao, L.-J.; Zhang, Y.; Ma, Y.-F.; Shang, Q.; Liu, X.-L. Development and Validation of a Prognostic Model for Efferocytosis-Associated Genes in Cervical Squamous Cell Carcinoma. Discov. Oncol. 2025, 16, 1224. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Liu, L.; Jin, D.; Wang, P.; Hu, J. MicroRNA-183-5p Inhibits Aggressiveness of Cervical Cancer Cells by Targeting Integrin Subunit Beta 1 (ITGB1). Med. Sci. Monit. 2018, 24, 7137–7145. [Google Scholar] [CrossRef]
- Xiang, X.; Kang, J.; Jiang, J.; Zhang, Y.; Zhang, Y.; Li, L.; Peng, X. A Novel DNA Damage Repair-Related Gene Signature Predicting Survival, Immune Infiltration and Drug Sensitivity in Cervical Cancer Based on Single Cell Sequencing. Front. Immunol. 2023, 14, 1198391. [Google Scholar] [CrossRef]
- Zou, C.; Lyu, Y.; Jiang, J.; Cao, Y.; Wang, M.; Sang, C.; Zhang, R.; Li, H.; Liew, C.-C.; Cheng, C.; et al. Use of Peripheral Blood Transcriptomic Biomarkers to Distinguish High-Grade Cervical Squamous Intraepithelial Lesions from Low-Grade Lesions. Oncol. Lett. 2020, 20, 2280–2290. [Google Scholar] [CrossRef]
- Meng, L.; Chen, S.; Shi, G.; He, S.; Wang, Z.; Shen, J.; Wang, J.; Sooranna, S.R.; Zhao, J.; Song, J. Use of Single Cell Transcriptomic Techniques to Study the Role of High-Risk Human Papillomavirus Infection in Cervical Cancer. Front. Immunol. 2022, 13, 907599. [Google Scholar] [CrossRef]
- Yang, W.; Xie, T. Hsa_circ_CSPP1/MiR-361-5p/ITGB1 Regulates Proliferation and Migration of Cervical Cancer (CC) by Modulating the PI3K-Akt Signaling Pathway. Reprod. Sci. 2020, 27, 132–144. [Google Scholar] [CrossRef]
- Li, Z.; Wei, R.; Yao, S.; Meng, F.; Kong, L. HIF-1A as a Prognostic Biomarker Related to Invasion, Migration and Immunosuppression of Cervical Cancer. Heliyon 2024, 10, e24664. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, L.; Chu, W.; Wei, L. Cellular Retinoic Acid Binding Protein 2 (CRABP2), Up-Regulated by HPV E6/E7, Leads to Aberrant Activation of the Integrin Β1/FAK/ERK Signaling Pathway and Aggravates the Malignant Phenotypes of Cervical Cancer. Biochem. Genet. 2024, 62, 2686–2701. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Ven, K.; Chastney, M.; Kokate, S.B.; Peränen, J.; Aaron, J.; Kogan, K.; Almeida-Souza, L.; Kremneva, E.; Poincloux, R.; et al. Focal Adhesions Contain Three Specialized Actin Nanoscale Layers. Nat. Commun. 2024, 15, 2547. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, A.; Gui, J.; Zhou, H.; Zhu, L.; Mi, Y. TLN1: An Oncogene Associated with Tumorigenesis and Progression. Discov. Oncol. 2024, 15, 716. [Google Scholar] [CrossRef]
- Malla, R.R.; Vempati, R.K. Talin: A Potential Drug Target for Cancer Therapy. Curr. Drug Metab. 2020, 21, 25–32. [Google Scholar] [CrossRef]
- Rothenberg, K.E.; Scott, D.W.; Christoforou, N.; Hoffman, B.D. Vinculin Force-Sensitive Dynamics at Focal Adhesions Enable Effective Directed Cell Migration. Biophys. J. 2018, 114, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Sun, W.; Wang, Y.; Liu, X.; Wang, A.; Liu, L.; Han, S.; Sun, Y.; Zhang, J.; Guo, L.; et al. Cervical Cancer-Derived Exosomal miR-663b Promotes Angiogenesis by Inhibiting Vinculin Expression in Vascular Endothelial Cells. Cancer Cell Int. 2021, 21, 684. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Kong, J.; Liu, Y.; Xu, Q.; Wang, K.; Huang, D.; Wei, Y.; Chen, W.; Mao, H. The Measurement and Analysis of Impedance Response of HeLa Cells to Distinct Chemotherapy Drugs. Micromachines 2021, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, S.; Chen, K.; Ji, L.; Cui, S. Magnolol and 5-Fluorouracil Synergy Inhibition of Metastasis of Cervical Cancer Cells by Targeting PI3K/AKT/mTOR and EMT Pathways. Chin. Herb. Med. 2024, 16, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wen, J. Abnormal Level of Paxillin in Cervical Cancer Cells Is Involved in Tumor Progression and Invasion. Acta Biochim. Pol. 2021, 68, 49–53. [Google Scholar] [CrossRef]
- Yoo, S.M.; Latifkar, A.; Cerione, R.A.; Antonyak, M.A. Cool-Associated Tyrosine-Phosphorylated Protein 1 Is Required for the Anchorage-Independent Growth of Cervical Carcinoma Cells by Binding Paxillin and Promoting AKT Activation. J. Biol. Chem. 2017, 292, 3947–3957. [Google Scholar] [CrossRef]
- Das, J.; Maiti, T.K. Fluid Shear Stress Influences Invasiveness of HeLa Cells through the Induction of Autophagy. Clin. Exp. Metastasis 2022, 39, 495–504. [Google Scholar] [CrossRef]
- Chen, T.; Wang, J.; Li, M.; Wu, Q.; Cui, S. Genistein Inhibits Proliferation and Metastasis in Human Cervical Cancer Cells through the Focal Adhesion Kinase Signaling Pathway: A Network Pharmacology-Based In Vitro Study in HeLa Cells. Molecules 2023, 28, 1919. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, J.-X.; Yang, S.-F.; Yang, C.-K.; Chen, T.-H.; Hsiao, Y.-H. Anticancer Effects and Molecular Mechanisms of Apigenin in Cervical Cancer Cells. Cancers 2022, 14, 1824. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, J.-X.; Yang, S.-F.; Hsiao, Y.-H. Synergistic Combination of Luteolin and Asiatic Acid on Cervical Cancer In Vitro and In Vivo. Cancers 2023, 15, 548. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Liu, Y.; Li, C.; He, B.; Zhou, G.; Cui, Y.; Huang, H. Arctigenin Hinders the Invasion and Metastasis of Cervical Cancer Cells via the FAK/Paxillin Pathway. Heliyon 2023, 9, e16683. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Chen, S.; Ruan, X.; Liao, H.; Zhang, Y.; Sun, J.; Gao, J.; Deng, G. Genistein Inhibits Migration and Invasion of Cervical Cancer HeLa Cells by Regulating FAK-Paxillin and MAPK Signaling Pathways. Taiwan. J. Obs. Gynecol. 2020, 59, 403–408. [Google Scholar] [CrossRef]
- Chuang, H.-H.; Zhen, Y.-Y.; Tsai, Y.-C.; Chuang, C.-H.; Hsiao, M.; Huang, M.-S.; Yang, C.-J. FAK in Cancer: From Mechanisms to Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 1726. [Google Scholar] [CrossRef]
- Chen, H.; Wang, D.; Liu, Y. SASH1 Inhibits Cervical Cancer Cell Proliferation and Invasion by Suppressing the FAK Pathway. Mol. Med. Rep. 2016, 13, 3613–3618. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, F.; Feng, L. The Inhibition of HeLa Cells Proliferation through SPARCL1 Mediated by SPP1. Cytotechnology 2021, 73, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, Y.; Du, N.; Zhao, W.; Liu, Y. LIMK1 Promotes the Development of Cervical Cancer by Up-Regulating the ROS/Src-FAK/Cofilin Signaling Pathway. Aging 2024, 16, 11090–11102. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lan, J. Overexpression of Eukaryotic Translation Initiation Factor 3D Induces Stem Cell-like Properties and Metastasis in Cervix Cancer by Activating FAK through Inhibiting Degradation of GRP78. Bioengineered 2022, 13, 1952–1961. [Google Scholar] [CrossRef]
- Liu, F.; Tong, D.; Li, H.; Liu, M.; Li, J.; Wang, Z.; Cheng, X. Bufalin Enhances Antitumor Effect of Paclitaxel on Cervical Tumorigenesis via Inhibiting the Integrin A2/Β5/FAK Signaling Pathway. Oncotarget 2016, 7, 8896–8907. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, L.; Zhang, T.; Liu, Y.; Fang, F.; Wu, X.; Chen, W.; Lan, L.; Zhang, Y.; Li, N.; et al. Shikonin Inhibits the Proliferation of Cervical Cancer Cells via FAK/AKT/GSK3β Signalling. Oncol. Lett. 2022, 24, 304. [Google Scholar] [CrossRef]
- Huang, L.; Liu, F.; Liu, X.; Niu, L.; Sun, L.; Fang, F.; Ma, K.; Hu, P. Parthenolide Inhibits the Proliferation and Migration of Cervical Cancer Cells via FAK/GSK3β Pathway. Cancer Chemother. Pharmacol. 2024, 93, 203–213. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, L.; Liu, J. Protective Effect of Tretinoin on Cervical Cancer Growth and Proliferation through Downregulation of pFAK2 Expression. Environ. Toxicol. 2024, 39, 2732–2740. [Google Scholar] [CrossRef]
- Pratiwi, L.; Elisa, E.; Sutanto, H. Probing the Protrusions: Lamellipodia and Filopodia in Cancer Invasion and Beyond. Mechanobiol. Med. 2024, 2, 100064. [Google Scholar] [CrossRef]
- Ma, X.; Xue, H.; Zhong, J.; Feng, B.; Zuo, Y. Serum Actinin-4 Levels as a Potential Diagnostic and Prognostic Marker in Cervical Cancer. Dis. Markers 2020, 2020, 5327378. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, S.; An, H.-T.; Ko, J. α-Actinin-4 Regulates Cancer Stem Cell Properties and Chemoresistance in Cervical Cancer. Carcinogenesis 2020, 41, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Dong, B.; Hong, S.; Wang, M.; Dai, W.; Zheng, Q.; Wu, D.; Cao, Y. Combined Detection of ACTN4 and SCC-Ag Is a Promising Serological Biomarker for Cervical Intraepithelial Neoplasia 3 or Worse: A Case–Control Study. Risk Manag. Heal. Policy 2020, 13, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Huseinovic, A.; Xu, M.; Jaspers, A.; Bais, B.; Steenbergen, R.D.M. miR-129-5p Inhibits Anchorage-Independent Growth through Silencing of ACTN1 and the ELK4/c-FOS Axis in HPV-Transformed Keratinocytes. J. Med. Virol. 2024, 96, e29580. [Google Scholar] [CrossRef]
- Li, J.; Wang, H. H3K27ac-Activated EGFR-AS1 Promotes Cell Growth in Cervical Cancer through ACTN4-Mediated WNT Pathway. Biol. Direct 2022, 17, 3. [Google Scholar] [CrossRef]
- Wang, Q.; Song, R.; Zhao, C.; Liu, H.; Yang, Y.; Gu, S.; Feng, D.; He, J. HPV16 E6 Promotes Cervical Cancer Cell Migration and Invasion by Downregulation of NHERF1. Int. J. Cancer 2019, 144, 1619–1632. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Q.; Song, R.; Zhao, C.; Liu, H.; Yang, Y.; Gu, S.; Zhou, D.; He, J. NHERF1 Inhibits Beta-Catenin-Mediated Proliferation of Cervical Cancer Cells through Suppression of Alpha-Actinin-4 Expression. Cell Death Dis. 2018, 9, 668. [Google Scholar] [CrossRef]
- An, H.-T.; Yoo, S.; Ko, J. α-Actinin-4 Induces the Epithelial-to-Mesenchymal Transition and Tumorigenesis via Regulation of Snail Expression and β-Catenin Stabilization in Cervical Cancer. Oncogene 2016, 35, 5893–5904. [Google Scholar] [CrossRef] [PubMed]
- Hezinger, L.; Bauer, S.; Ellwanger, K.; Piotrowsky, A.; Biber, F.; Venturelli, S.; Kufer, T.A. NOD1 Cooperates with HAX-1 to Promote Cell Migration in a RIPK2- and NF-ĸB-Independent Manner. FEBS J. 2023, 290, 5295–5312. [Google Scholar] [CrossRef] [PubMed]
- Pappa, K.I.; Lygirou, V.; Kontostathi, G.; Zoidakis, J.; Makridakis, M.; Vougas, K.; Daskalakis, G.; Polyzos, A.; Anagnou, N.P. Proteomic Analysis of Normal and Cancer Cervical Cell Lines Reveals Deregulation of Cytoskeleton-Associated Proteins. Cancer Genom. Proteom. 2017, 14, 253–266. [Google Scholar] [CrossRef]
- Du, N.; Li, D.; Zhao, W.; Liu, Y. Stratifin (SFN) Regulates Cervical Cancer Cell Proliferation, Apoptosis, and Cytoskeletal Remodeling and Metastasis Progression Through LIMK2/Cofilin Signaling. Mol. Biotechnol. 2023, 66, 3369. [Google Scholar] [CrossRef]
- Mercier, A.E.; Joubert, A.M.; Prudent, R.; Viallet, J.; Desroches-Castan, A.; De Koning, L.; Mabeta, P.; Helena, J.; Pepper, M.S.; Lafanechère, L. Sulfamoylated Estradiol Analogs Targeting the Actin and Microtubule Cytoskeletons Demonstrate Anti-Cancer Properties In Vitro and In Ovo. Cancers 2024, 16, 2941. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Tian, J.; Yan, W.; Qi, C.; Liu, W.; Xuan, S.; Shang, A. Cervical Cancer Cells-Derived Extracellular Vesicles Containing microRNA-146a-5p Affect Actin Dynamics to Promote Cervical Cancer Metastasis by Activating the Hippo-YAP Signaling Pathway via WWC2. J. Oncol. 2022, 2022, 4499876. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L. MiR-29a Inhibits Cell Proliferation and Migration by Targeting the CDC42/PAK1 Signaling Pathway in Cervical Cancer. Anticancer Drugs 2019, 30, 579–587. [Google Scholar] [CrossRef]
- Xu, J.; Ma, X.; Yang, H.; Zhang, J.; Cai, G.; Yao, N. MiR-509-3p Induces Apoptosis and Affects the Chemosensitivity of Cervical Cancer Cells by Targeting the RAC1/PAK1/LIMK1/Cofilin Pathway. Chem. Pharm. Bull. 2021, 69, 325–332. [Google Scholar] [CrossRef]
- Bumrungthai, S.; Ekalaksananan, T.; Kleebkaow, P.; Pongsawatkul, K.; Phatnithikul, P.; Jaikan, J.; Raumsuk, P.; Duangjit, S.; Chuenchai, D.; Pientong, C. Mathematical Modelling of Cervical Precancerous Lesion Grade Risk Scores: Linear Regression Analysis of Cellular Protein Biomarkers and Human Papillomavirus E6/E7 RNA Staining Patterns. Diagnostics 2023, 13, 1084. [Google Scholar] [CrossRef]
- Cheng, Y.; Jiang, S.; Yuan, J.; Liu, J.; Simoncini, T. Vascular Endothelial Growth Factor C Promotes Cervical Cancer Cell Invasiveness via Regulation of microRNA-326/Cortactin Expression. Gynecol. Endocrinol. 2018, 34, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Z.; Xue, L.; Li, G.; Zhang, C.; Cai, Z.; Li, H.; Guo, R. DIAPH3 Promoted the Growth, Migration and Metastasis of Hepatocellular Carcinoma Cells by Activating Beta-Catenin/TCF Signaling. Mol. Cell Biochem. 2018, 438, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhu, J.; Wu, Q. Knockdown of DIAPH3 Inhibits the Proliferation of Cervical Cancer Cells through Inactivating mTOR Signaling Pathway. J. Oncol. 2021, 2021, 4228241. [Google Scholar] [CrossRef]
- Chen, X.; Xie, L.; Qiao, K.; Zhu, X.; Ren, J.; Tan, Y. The Pan-Cancer Analysis Identified DIAPH3 as a Diagnostic Biomarker of Clinical Cancer. Aging 2023, 15, 689. [Google Scholar] [CrossRef] [PubMed]
- Kobori, T.; Ito, Y.; Urashima, Y.; Ito, T.; Takagaki, N.; Hotta, K.; Obata, T. Ezrin Works as a Scaffold Protein for a Macrophage Checkpoint Molecule CD47, Leading to a Poor Prognosis for Patients with Uterine Cervical Squamous Cell Carcinoma. Taiwan. J. Obs. Gynecol. 2025, 64, 239–247. [Google Scholar] [CrossRef]
- Carvalho, M.F.L.; Calicchio, C.S.; de Almeida, B.O.; de Miranda, L.B.L.; Lipreri da Silva, J.C.; Lima, K.; Machado-Neto, J.A. Transcriptomics Analysis Identified Ezrin as a Potential Druggable Target in Cervical and Gastric Cancer Cells. Clinics 2024, 79, 100422. [Google Scholar] [CrossRef]
- Fadiel, A.; Choi, S.D.; Park, B.; Kim, T.-H.; Buldo-Licciardi, J.; Ahmadi, M.; Arslan, A.; Mittal, K.; Naftolin, F. Expression of Ezrin and Estrogen Receptors During Cervical Carcinogenesis. Reprod. Sci. 2017, 24, 706–712. [Google Scholar] [CrossRef]
- Li, M.; Feng, Y.M.; Fang, S.Q. Overexpression of Ezrin and Galectin-3 as Predictors of Poor Prognosis of Cervical Cancer. Braz. J. Med. Biol. Res. 2017, 50, e5356. [Google Scholar] [CrossRef]
- Zacapala-Gómez, A.E.; Navarro-Tito, N.; del Carmen Alarcón-Romero, L.; Ortuño-Pineda, C.; Illades-Aguiar, B.; Castañeda-Saucedo, E.; Ortiz-Ortiz, J.; Garibay-Cerdenares, O.L.; Jiménez-López, M.A.; Mendoza-Catalán, M.A. Ezrin and E-Cadherin Expression Profile in Cervical Cytology: A Prognostic Marker for Tumor Progression in Cervical Cancer. BMC Cancer 2018, 18, 349. [Google Scholar] [CrossRef]
- Qin, R.; Cao, L.; Wang, J.; Liu, J. Promoter Methylation of Ezrin and Its Impact on the Incidence and Prognosis of Cervical Cancer. Cell Physiol. Biochem. 2018, 50, 277–287. [Google Scholar] [CrossRef]
- Kong, J.; Di, C.; Piao, J.; Sun, J.; Han, L.; Chen, L.; Yan, G.; Lin, Z. Ezrin Contributes to Cervical Cancer Progression through Induction of Epithelial-Mesenchymal Transition. Oncotarget 2016, 7, 19631–19642. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Tang, W. Knockdown of Ezrin Inhibited Migration and Invasion of Cervical Cancer Cells in Vitro. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420930899. [Google Scholar] [CrossRef]
- Hałas-Wiśniewska, M.; Arendt, W.; Grzanka, A.; Izdebska, M. Downregulation of Ezrin Suppresses Migration Potential in Cervical Cancer Cells. Pharmaceuticals 2025, 18, 3. [Google Scholar] [CrossRef]
- Xu, W.; Xie, S.; Chen, X.; Pan, S.; Qian, H.; Zhu, X. Effects of Quercetin on the Efficacy of Various Chemotherapeutic Drugs in Cervical Cancer Cells. Drug Des. Dev. Ther. 2021, 15, 577–588. [Google Scholar] [CrossRef]
- Wang, L.; Qi, Y.; Xiong, Y.; Peng, Z.; Ma, Q.; Zhang, Y.; Song, J.; Zheng, J. Ezrin-Radixin-Moesin Binding Phosphoprotein 50 (EBP50) Suppresses the Metastasis of Breast Cancer and HeLa Cells by Inhibiting Matrix Metalloproteinase-2 Activity. Anticancer Res. 2017, 37, 4353–4360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peng, Z.; Wang, Q.; Zhang, Y.; He, J.; Zheng, J. EBP50 Interacts with EGFR and Regulates EGFR Signaling to Affect the Prognosis of Cervical Cancer Patients. Int. J. Oncol. 2016, 49, 1737–1745. [Google Scholar] [CrossRef]
- Mubthasima, P.P.; Kannan, A. Unraveling the Role of EPHA2 in Regulating Migration and Immunomodulation Processes in Cervical Cancer: Exploring the Synergic Effect of 17β-Estradiol on Cancer Progression. Med. Oncol. 2024, 41, 255. [Google Scholar] [CrossRef]
- Xue, F.; Xu, Y.; Song, Y.; Zhang, W.; Li, R.; Zhu, X. The Effects of Sevoflurane on the Progression and Cisplatinum Sensitivity of Cervical Cancer Cells. Drug Des. Dev. Ther. 2019, 13, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Chen, C.; Roy, B.; Li, Q.; Zhang, W.; Zhang, X.; Pu, J.; Li, Y.; Liu, Y.; et al. lncRNA HIF1A-AS2 Acts as an Oncogene to Regulate Malignant Phenotypes in Cervical Cancer. Front. Oncol. 2025, 15, 1530677. [Google Scholar] [CrossRef] [PubMed]
- Doukuni, R.; Kobori, T.; Tanaka, C.; Tameishi, M.; Urashima, Y.; Ito, T.; Obata, T. Moesin Serves as Scaffold Protein for PD-L1 in Human Uterine Cervical Squamous Carcinoma Cells. J. Clin. Med. 2022, 11, 3830. [Google Scholar] [CrossRef]
- Tanaka, C.; Kobori, T.; Tameishi, M.; Urashima, Y.; Ito, T.; Obata, T. Ezrin Modulates the Cell Surface Expression of Programmed Cell Death Ligand-1 in Human Cervical Adenocarcinoma Cells. Molecules 2021, 26, 5648. [Google Scholar] [CrossRef]
- yousefi Ghalejoogh, Z.; Mirakhor Samani, S.; Shatizadeh Malekshahi, S.; Shahsiah, R.; Yavarian, J.; KianI, S.J. Human Papilloma Virus Infection and Fascin Over-Expression in Squamous Cell Carcinoma of the Cervix. Med. J. Islam. Repub. Iran. 2018, 32, 134. [Google Scholar] [CrossRef][Green Version]
- Tian, Q.; Huang, J.; Zhang, Q.; Zhao, J. N6-Methyladenosine Methylation on FSCN1 Mediated by METTL14/IGF2BP3 Contributes to Human Papillomavirus Type 16-Infected Cervical Squamous Cell Carcinoma. Clin. Exp. Pharmacol. Physiol. 2024, 51, e13864. [Google Scholar] [CrossRef]
- Csizmár, S.; Výbohová, D.; Mešt’anová, V.; Krajňáková, B.; Kajo, K.; Kunertová, L.; Adamkov, M. Expression of Fascin in Association with P16 and Ki-67 in Cervical Lesions: Immunohistochemical Study. Pol. J. Pathol. 2022, 73, 208–214. [Google Scholar] [CrossRef]
- Ma, L.; Li, L.-L. miR-145 Contributes to the Progression of Cervical Carcinoma by Directly Regulating FSCN1. Cell Transpl. 2019, 28, 1299–1305. [Google Scholar] [CrossRef]
- He, S.; Yu, G.; Peng, K.; Liu, S. MicroRNA-145-5p Suppresses Fascin to Inhibit the Invasion and Migration of Cervical Carcinoma Cells. Mol. Med. Rep. 2020, 22, 5282–5292. [Google Scholar] [CrossRef]
- Chetry, M.; Song, Y.; Pan, C.; Li, R.; Zhang, J.; Zhu, X. Effects of Galectin-1 on Biological Behavior in Cervical Cancer. J. Cancer 2020, 11, 1584–1595. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Wang, M.; Chen, D.; Li, M.; Zhu, Y.; Yu, Y.; Zheng, L.; Qi, B.; Liu, J. FSCN1 Promotes Radiation Resistance in Patients with PIK3CA Gene Alteration. Front. Oncol. 2021, 11, 653005. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, W.; Chu, D.-M. Role of Fascin-1 in Cervical Cancer Metastasis via Wnt/β-Catenin Pathway Activation. Biomol. Biomed. 2025, 25, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Du, S.; Chen, X.; Min, X.; Dong, Z.; Wang, Y.; Zhu, C.; Wei, F.; Gao, S.; Cai, Q. Lactate-Mediated Fascin Protrusions Promote Cell Adhesion and Migration in Cervical Cancer. Theranostics 2023, 13, 2368–2383. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, Y.; Cheng, Y.; Zhang, Q.; Quan, W.; Wei, Y.; Hong, L. Transcriptome Analysis Reveals the Potential Biological Function of FSCN1 in HeLa Cervical Cancer Cells. PeerJ 2022, 10, e12909. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M.J.; Kim, Y.S.; Choi, M.Y.; Cho, G.J.; Choi, W.S. UHRF1 Silences Gelsolin to Inhibit Cell Death in Early Stage Cervical Cancer. Biochem. Biophys. Res. Commun. 2020, 526, 1061–1068. [Google Scholar] [CrossRef]
- Matarrese, P.; Abbruzzese, C.; Mileo, A.M.; Vona, R.; Ascione, B.; Visca, P.; Rollo, F.; Benevolo, M.; Malorni, W.; Paggi, M.G. Interaction between the Human Papillomavirus 16 E7 Oncoprotein and Gelsolin Ignites Cancer Cell Motility and Invasiveness. Oncotarget 2016, 7, 50972–50985. [Google Scholar] [CrossRef][Green Version]
- Matarrese, P.; Vona, R.; Ascione, B.; Paggi, M.G.; Mileo, A.M. Physical Interaction between HPV16E7 and the Actin-Binding Protein Gelsolin Regulates Epithelial-Mesenchymal Transition via HIPPO-YAP Axis. Cancers 2021, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Wüstenhagen, E.; Hampe, L.; Boukhallouk, F.; Schneider, M.A.; Spoden, G.A.; Negwer, I.; Koynov, K.; Kast, W.M.; Florin, L. The Cytoskeletal Adaptor Obscurin-Like 1 Interacts with the Human Papillomavirus 16 (HPV16) Capsid Protein L2 and Is Required for HPV16 Endocytosis. J. Virol. 2016, 90, 10629–10641. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.-M.; Ulloa, L.; Yang, Y.-Q. Transgelin-2: Biochemical and Clinical Implications in Cancer and Asthma. Trends Biochem. Sci. 2019, 44, 885–896. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, X.; Yan, W.; Dou, X. Transgelin 2 Overexpression Inhibits Cervical Cancer Cell Invasion and Migration. Mol. Med. Rep. 2019, 19, 4919–4926. [Google Scholar] [CrossRef]
- Yakabe, K.; Murakami, A.; Kajimura, T.; Nishimoto, Y.; Sueoka, K.; Sato, S.; Nawata, S.; Sugino, N. Functional Significance of Transgelin-2 in Uterine Cervical Squamous Cell Carcinoma. J. Obstet. Gynaecol. Res. 2016, 42, 566–572. [Google Scholar] [CrossRef]
- Lu, F.; Cui, D.; Mu, B.; Zhao, L.; Mu, P. Downregulation of TMOD1 Promotes Cell Motility and Cell Proliferation in Cervical Cancer Cells. Oncol. Lett. 2020, 19, 3339. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kasamatsu, A.; Miyamoto, I.; Saito, T.; Higo, M.; Endo-Sakamoto, Y.; Shiiba, M.; Tanzawa, H.; Uzawa, K. Overexpression of TMOD1 Is Associated with Enhanced Regional Lymph Node Metastasis in Human Oral Cancer. Int. J. Oncol. 2016, 48, 607–612. [Google Scholar] [CrossRef]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. Cells Tissues Organs 2021, 211, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Liang, Q.; Tong, R.; Huang, J.; Yang, X.; Xu, Y.; Wang, W.; Sun, M.; Shi, J. Recent Progress on FAK Inhibitors with Dual Targeting Capabilities for Cancer Treatment. Biomed. Pharmacother. 2022, 151, 113116. [Google Scholar] [CrossRef] [PubMed]
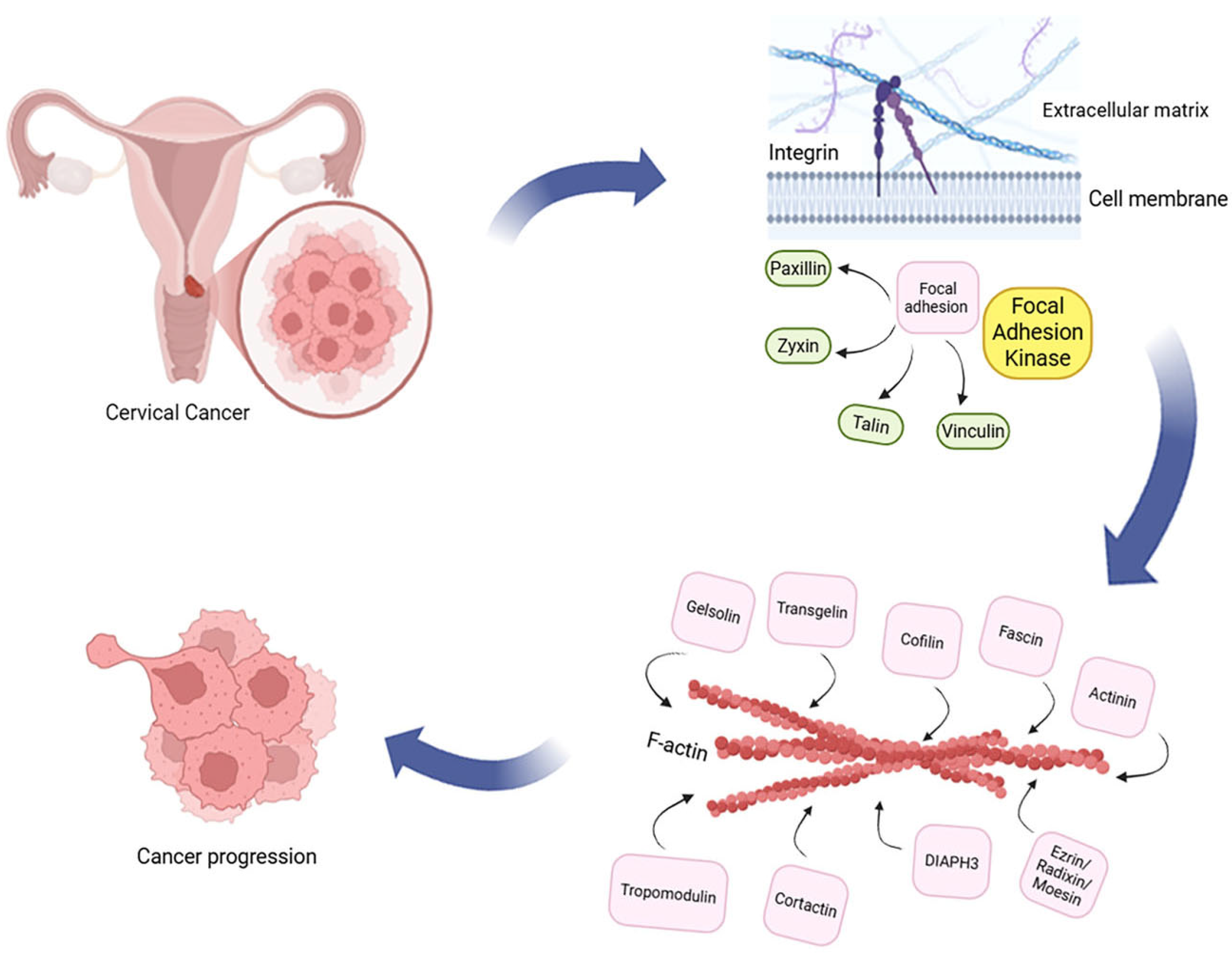
| Protein Group | Examples | Main Function | Role in CC Progression/ Metastasis |
|---|---|---|---|
| Integrins | ITGA3, ITGA5, ITGB1, ITGAV | Receptors linking the cell to the ECM, initiating focal adhesion formation | Adhesion, migration, and pro-oncogenic signaling |
| Focal Adhesion proteins (FAs) | Paxillin, Vinculin, Zyxin, Talin | Structural and adaptor proteins—connect integrins with the cytoskeleton, stabilize focal adhesions | Cytoskeleton reorganization and cancer cell migration |
| Focal Adhesion Kinase (FAK) | FAK | Tyrosine kinase—central signaling hub in focal adhesions | Migration, proliferation, survival, and EMT |
| Actin binding proteins (ABPs) | Filamin, Gelsolin, Profilin, Actinins, Cortactin, Cofilin, Diaphanous-related formin 3, Fascin, Transgelin 2, Tropomodulin, Ezrin/Radixin/Moesin | Link FA and integrins with actin filaments, modulate cytoskeleton dynamics | Cell movement, invasion, and cytoskeleton remodeling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hałas-Wiśniewska, M.; Zawadka, P.; Arendt, W.; Izdebska, M. From Adhesion to Invasion: Integrins, Focal Adhesion Signaling, and Actin Binding Proteins in Cervical Cancer Progression—A Scoping Review. Cells 2025, 14, 1640. https://doi.org/10.3390/cells14201640
Hałas-Wiśniewska M, Zawadka P, Arendt W, Izdebska M. From Adhesion to Invasion: Integrins, Focal Adhesion Signaling, and Actin Binding Proteins in Cervical Cancer Progression—A Scoping Review. Cells. 2025; 14(20):1640. https://doi.org/10.3390/cells14201640
Chicago/Turabian StyleHałas-Wiśniewska, Marta, Patryk Zawadka, Wioletta Arendt, and Magdalena Izdebska. 2025. "From Adhesion to Invasion: Integrins, Focal Adhesion Signaling, and Actin Binding Proteins in Cervical Cancer Progression—A Scoping Review" Cells 14, no. 20: 1640. https://doi.org/10.3390/cells14201640
APA StyleHałas-Wiśniewska, M., Zawadka, P., Arendt, W., & Izdebska, M. (2025). From Adhesion to Invasion: Integrins, Focal Adhesion Signaling, and Actin Binding Proteins in Cervical Cancer Progression—A Scoping Review. Cells, 14(20), 1640. https://doi.org/10.3390/cells14201640









