1. Introduction
The comprehension of genetic contributions to human diseases has experienced significant advancements in recent years. What was once purely theoretical, gene therapy has now materialized as a viable treatment for conditions like primary immunodeficiency and hemophilia, with numerous ongoing clinical trials [
1,
2]. Gene therapy has particularly focused on monogenic diseases with recessive inheritance, given the straightforward nature of the disease-causing genotype [
3]. The retina stands out as a distinctive target for gene therapy due to its immunoprivileged environment, the convenience of subretinal injection, and direct visibility [
4].
One gene that has gained significant attention in the field of genetics is PITPNM3, also known as phosphatidylinositol transfer protein membrane-associated 3. A 20-exon gene spanning 101 kb on human chromosome 17p13 [
5], PITPNM3 codes for calcium-binding proteins with phosphatidylinositol transfer ability [
6], and plays a crucial role in various cellular processes, including signal transduction, vesicle trafficking, and membrane lipid metabolism [
7]. PITPNM3 homologues have long been tied to phototransduction in Drosophila and rat models [
5,
8]. More recently, PITPNM3 mutations have been implicated to several human disorders, particularly those affecting the retina, such as retinitis pigmentosa (RP) [
9], and cone–rod dystrophy [
10].
Previous reports have linked PITPNM3 to autosomal dominant cone-rod dystrophy (CORD5) [
11]. The locus of CORD5 spans a region of 14.3cM and encompasses PITPNM3 as well as AIPL1 and GUCY2D [
11]. Distinguishing itself from other autosomal dominant CORDs, CORD5 is restricted to loss of cone photoreceptor function and appears to spare rod photoreceptors [
10]. Mutations in neighboring gene GUCY2D have been shown to be implicated in CORD5 as well as Leber congenital amaurosis [
12]. Case studies show PITPNM3-associated CORD5 has a varied phenotypic presentation. In one family with identified PITPNM3 mutation, patients presented with reduced visual acuity and light sensitivity from childhood, progressing to legal blindness by early adulthood, while patients in another family showed more mild symptoms with later onset in adulthood [
10].
Mutations in PITPNM3 have also been implicated as a cause of autosomal recessive retinitis pigmentosa (RP). RP is a group of inherited retinal dystrophies characterized by progressive degeneration of photoreceptor cells, leading to visual impairment and eventual blindness [
13]. The mechanism by which PITPNM3 mutations contribute to RP pathogenesis is not fully understood, but PITPNM3′s involvement in lipid metabolism and intracellular signaling pathways may play a possible role. Notably, a missense mutation in PITPNM3 was found in a case report of a patient with autoimmune retinopathy presenting with rod-cone dystrophy and rapidly deteriorating vision [
9]. The involvement of PITPNM3 in numerous retinopathies with high phenotype variation necessitates further investigation.
Preclinical models have been employed to better understand the functional consequences of PITPNM3 mutation and genotype–phenotype interactions. Previous preclinical models have shown that the drosophila homologue of PITPNM3, rdgB, is involved in photoreceptor response and prevention of retinal degeneration [
8]. Expression of PITPNM3 homologues in the retina differs by species. In zebrafish, expression in the retina is limited to cone cell inner segments [
14]. Expression of the rat homologue shows variation by stage of development, strongly expressed in the ganglion cells and outer retina early on and settling in the inner segment in adulthood [
15]. Mouse models have shown that PITPNM3 is expressed in retinal outer nuclear segments [
16].
Despite the progress made in understanding the role of PITPNM3 in human conditions through preclinical models, there are still significant gaps in knowledge that warrant further investigation. First, we have limited understanding of the exact molecular mechanisms by which PITPNM3 mutations lead to retinal degeneration. While studies have implicated lipid metabolism and intracellular signaling pathways [
7], further research is needed to unravel the precise interactions and downstream effects of PITPNM3 dysregulation. Second, there is incomplete genotype–phenotype concordance observed between PITPNM3-related phenotypes in humans and existing preclinical models. Thus, there is a need to develop preclinical models that better recapitulate the human disease phenotype in severity and progression of retinal degeneration and allow for a comprehensive assessment of the genotype–phenotype relationships.
The current study aims to address these gaps by developing a more refined preclinical model in mice for studying PITPNM3-related phenotypes. By bridging the gap between genetic variants and clinical manifestations, the study seeks to generate a more accurate representation of the human condition and pave the way for development of targeted therapeutic interventions.
2. Materials and Methods
2.1. Generation and Genotyping of PITPNM3 Mouse Lines
To establish a mouse model that closely parallels the human genetic context of PITPNM3-related retinal disease, heterozygous mice carrying PITPNM3 mutations were bred together. This approach aimed to generate offspring that were homozygous for the PITPNM3 mutation, thereby recapitulating the biallelic loss-of-function scenario found in patients with severe forms of the disease. All mice were housed and cared for in a controlled environment designed to minimize extraneous variables, thus ensuring consistency and reliability in both animal health and experimental outcomes. Following breeding, genomic DNA was extracted from tail biopsies of each mouse.
The PITPNM3 mutant mouse line carries a targeted knock-in of the V367M variant, corresponding precisely to a missense mutation (c.1099G>A, p.Val367Met) previously described in human patients with autosomal dominant cone-rod dystrophy (CORD5) [REF]. This mutation site was confirmed by Sanger sequencing in all experimental animals. We chose this humanized mutation to maximize translational relevance between the model and patient phenotype. PITPNM3 expression, as assessed by in situ hybridization and immunostaining, is highest within rod and cone photoreceptors, as well as the retinal pigment epithelium. Thus, affected cell types in our model include both photoreceptors and RPE, as in human disease. The precise genotype was confirmed through polymerase chain reaction (PCR) amplification and Sanger sequencing, verifying the presence and zygosity of the intended PITPNM3 mutation. This rigorous screening process was crucial in attributing observed phenotypes solely to alterations in PITPNM3, effectively eliminating potential confounders arising from genetic drift or background mutations.
2.2. Electroretinography (ERG)
Functional assessment of retinal integrity was conducted using full-field electroretinography (
Appendix A.1), a highly sensitive technique to measure the electrical responses of photoreceptors and downstream retinal cells to light stimulation. Mice were dark-adapted overnight to maximize retinal responsiveness and then anesthetized to ensure immobilization during data acquisition. Gold wire or contact lens electrodes were gently placed on the surface of each cornea, while reference and ground electrodes were positioned subcutaneously at predetermined sites. Mice were then exposed to a stepwise protocol of light flashes spanning a range of intensities. As previously described, pulses of 0.00130 cd/m
2 and 3 cd/m
2 (White-6500K) were employed [
17,
18,
19]. This allowed the specific testing of rod and cone functionality so that the individual and combined function could be analyzed. Retinal responses were recorded as waveforms consisting of an initial negative deflection (A-wave, photoreceptor response) and subsequent positive deflection (B-wave, reflecting activity of bipolar and Müller cells). Both the amplitude and the latency of these signals were analyzed. It was thus possible to detect even subtle functional deficits in retinal processing, providing detailed functional genotype–phenotype correlation and facilitating comparisons to clinical findings in human PITPNM3-associated retinopathies.
2.3. Histological Analysis
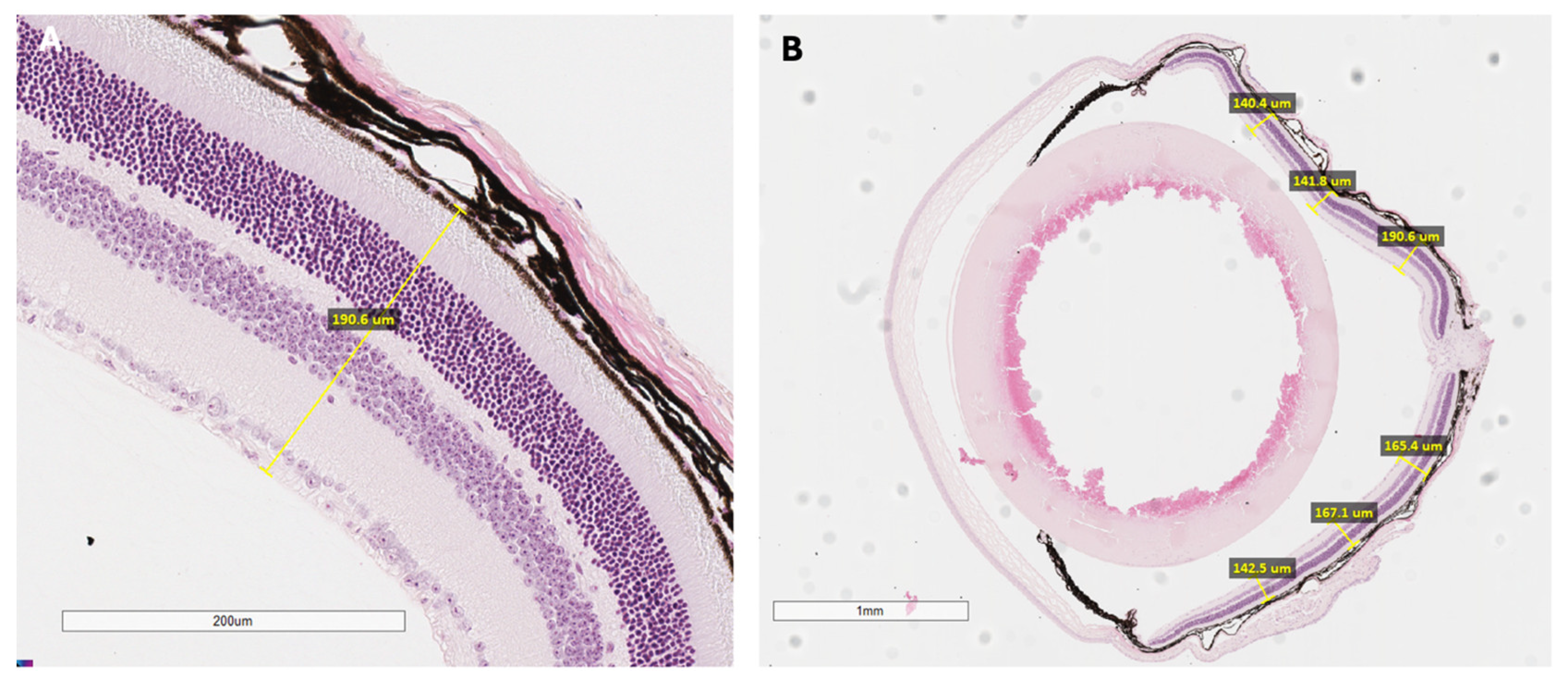
Subsets of mice underwent histological analysis to directly examine microscopic retinal structure and cellular organization (
Appendix A.2). After functional testing, mice were euthanized, and eyes were promptly enucleated. Each globe was fixed in paraformaldehyde to preserve tissue architecture, embedded in paraffin, and serially sectioned at defined thicknesses suitable for mouse ocular tissue. These sections were then stained, typically with hematoxylin and eosin, to allow for clear visualization of individual retinal layers under light microscopy. Particular attention was paid to the thickness and organization of the outer nuclear layer, the integrity of photoreceptor cells, and the structure of the retinal pigment epithelium (RPE). Quantitative measurements of retinal thickness were obtained at standard positions relative to the optic nerve head, which permitted precise spatial mapping of degenerative or dysplastic changes (
Figure A1). This meticulous approach enabled a robust structural assessment, complementing the functional and imaging-based findings in the study.
2.4. Retinal Phenotyping in PITPNM3 Mouse Model and Human Disease
Comprehensive retinal imaging and functional analyses were performed to characterize the effects of PITPNM3 mutations in both the preclinical mouse model and human patients. This approach integrated color fundus photography, fundus autofluorescence (FAF), optical coherence tomography (OCT), and histological assessment in the animal model for robust genotype–phenotype correlation.
2.5. Integrated Rationale for Multimodal Phenotyping
Employing this multimodal, layered phenotyping strategy—combining quantitative imaging, electrophysiological measurement, and direct histological examination—ensured a comprehensive and nuanced characterization of PITPNM3-related retinal disease in the mouse model. This design enabled the detection not only of overt manifestations but also of subtle, incipient changes that precede clinically significant degeneration. Through detailed genotype–phenotype correlation, the approach facilitated benchmarking of the mouse model against clinical features observed in human patients, enhancing our understanding of disease mechanisms and progression. All animal procedures conformed to ethical guidelines promulgated by institutional review boards and adhered strictly to best practices for animal research.
4. Discussion
The differences observed in the mouse model compared with human phenotypes associated with PITPNM3 underscore the need for further investigation. The results of the study revealed subtle differences in the generated mouse model compared to human phenotypes associated with PITPNM3 mutations. Full-field electroretinogram (ERG) analysis indicated a reduced cone response in the heterozygous and homozygous mice, although the severity was not as pronounced as observed in humans with PITPNM3-related conditions. Histological examinations of selected cases showed no major morphological changes in the retinal structure. Ultimately, the preclinical mouse model generated showed weak genotype–phenotype concordance and does not fully recapitulate the spectrum of human phenotypes caused by PITPNM3 mutations. The study highlights the complex and nuanced nature of PITPNM3-related retinal disorders. There are several possible causes for the discrepancy between the mouse model and human PITPNM3 mutation associated conditions.
First, disease progression may differ between humans and mice, and this study had a relatively short follow-up period of one year. PITPNM3-related retinal degeneration is a progressive condition that develops over time; thus, a relatively short follow-up limits the ability to observe more pronounced phenotypic changes. Our study design included evaluation at 8, 16, and 52 weeks, which captured both early presymptomatic and later degenerative stages. Changes in ERG were seen at 8 and 16 weeks and substantial retinal degeneration was present by 52 weeks, as captured in
Figure 3. The multi-time point approach therefore enabled us to map the progression of pathology and revealed that pronounced phenotypic changes in this model are delayed. In humans, PITPNM3 retinopathies can have decreased visual acuity years before changes are observed on fundus imaging and retinal thickness. In this study, the observed decreases in phototransduction seen on ERG indicate mild degeneration of rod and cone cells in the outer segment, the retina layer in mice where PITPNM3 is expressed, further supporting incomplete manifestation of PITPNM3 phenotype. Future studies should explore longer follow-up to additional functional and structural assessments to gain a more comprehensive understanding of the disease phenotype in the mouse model.
Second, using young animals in the preclinical model may have limited the biological variability or predictability of the observed phenotypic changes. Disease manifestations in humans with PITPNM3-related conditions are influenced by various genetic and environmental factors that may not be fully replicated in the mouse model. In RP, for example, non-genetic factors such as oxidative stress modulate disease progression. After rod cell loss in RP, oxidative stress increases; this has been suggested to play a role in rod and cone degeneration. Oxidative damage is markedly increased in the ocular samples of RP patients and anti-oxidant interventions substantially delay photoreceptor cell death, suggesting that oxidative stress is a key contributor to retinal degeneration in RP. Additionally, RP exhibits diverse heterogeneity in phenotype with young animals.
Lastly, mouse models, while valuable for studying genetic disorders, may not fully recapitulate the complexity of human diseases. Limitations include differences in retinal anatomy, cellular composition, and functional characteristics between mice and humans may contribute to the observed discrepancies in phenotype severity. Expression of PITPNM3 in mice retina is isolated to the outer nuclear segments, while expression of PITPNM3 in humans is highly expressed in the brain and spleen.
These findings highlight the importance of longer follow-up periods in preclinical studies to capture the full spectrum of disease progression and evaluate the long-term effects of PITPNM3 mutations. Additionally, the need to consider genetic variability and different genetic backgrounds in animal models is crucial for understanding the genotype–phenotype relationship. The discrepancies between the mouse model and human phenotypes suggest that caution should be exercised when translating findings from animal studies to human clinical applications. While animal models provide valuable insights into disease mechanisms and therapeutic interventions, the complexity and interplay of genetic and environmental factors in human patients cannot be fully replicated in animal models alone.
While our study contributes valuable insights into phenotype-genotype concordance in PITPNM3, there are several limitations that may impact the interpretation and generalizability of our findings. One limitation is the discrepancy in sample sizes of mice genotypes. This disparity can limit subgroup analyses as smaller sample size groups may not have sufficient statistical power. Additionally, RD1 retinal degeneration mutations in the mouse line are a confounding factor as contamination renders it difficult to isolate the study results to the effects of PITPNM3 mutation. This study also has a limited scope, exploring one PITPNM3 mutation variant, and may not be broadly applicable to other PITPNM3 mutations. Additional limitations include the relatively short follow-up period and use of young animals as previously described.
Future research should extend study durations, involve older animals, and consider diverse mouse strains to better replicate human conditions. The utilization of animal models necessitates meticulous justification, careful evaluation of alternatives, and an assessment of the associated benefits. Whenever feasible, exploring alternatives to animal models is advisable, such as utilizing Drosophila and investigating genetic variants in humans. Furthermore, investigating the impact of oxidative stress and employing alternative models like zebrafish or fruit flies could offer additional insights, contributing to a more holistic understanding of PITPNM3′s involvement in retinal degeneration. The incorporation of additional genetic variants, such as PDE6, which exhibits phenotypic similarities to PITPNM3, and the consideration of various disease stages, could provide complementary insights, leading to a more thorough comprehension of PITPNM3′s role in retinal degeneration.