Protective Immune Signatures Associated with Latent TB Infection in PLHIV—Insights from an Integrative Prospective Immune Monitoring Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Study Participants
2.3. Assays for Absolute Cell Counts
2.4. Plasma Viral Load Estimation
2.5. Whole-Blood Immunophenotyping
2.6. ELISA and Luminex Assay
2.7. Intracellular Cytokine Staining Assay (ICCSA)
2.8. Antibodies for ICCS Assay
2.9. Statistics
3. Results
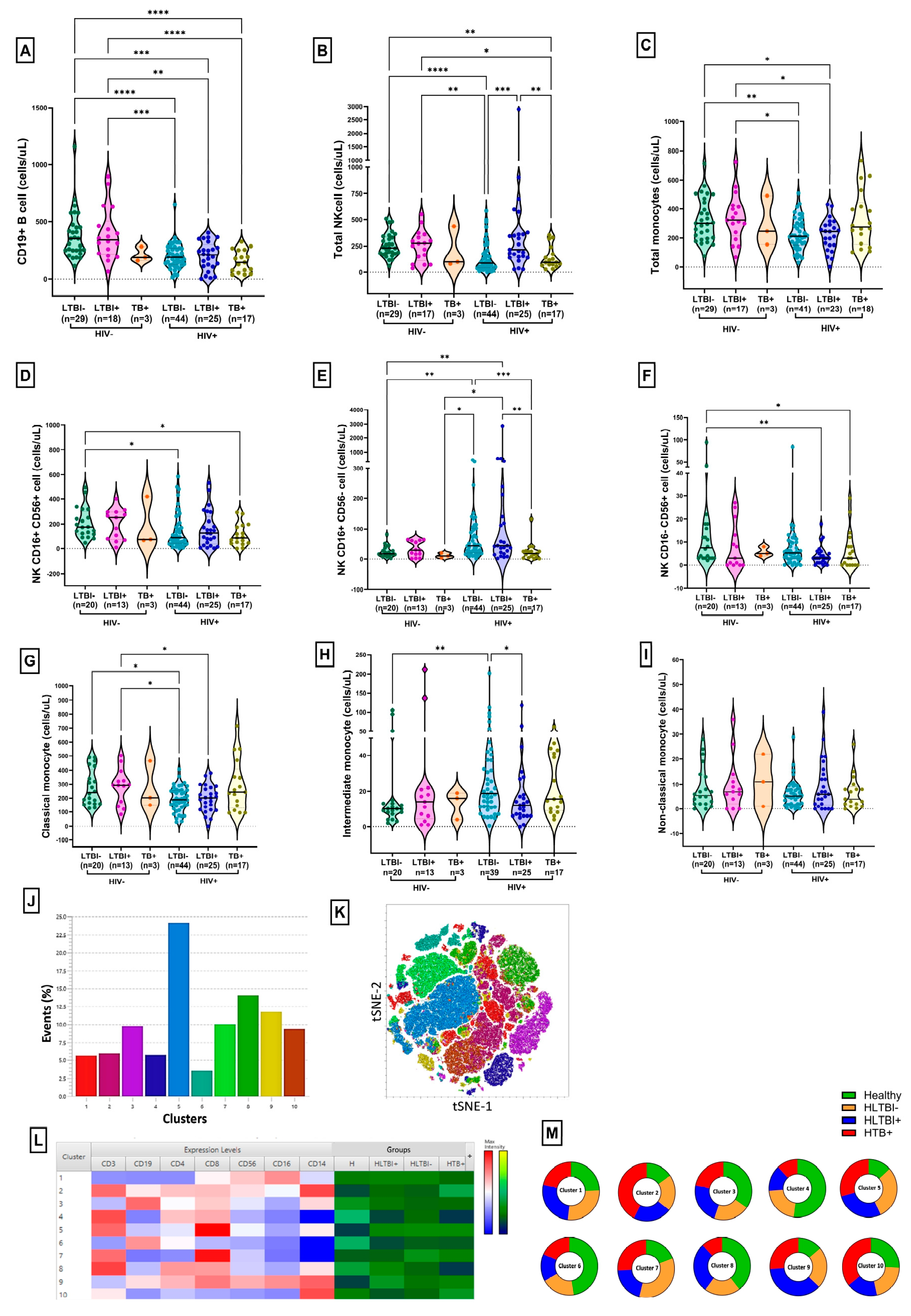
3.1. Demographic, Immunological and Virological Characteristics of Study Population
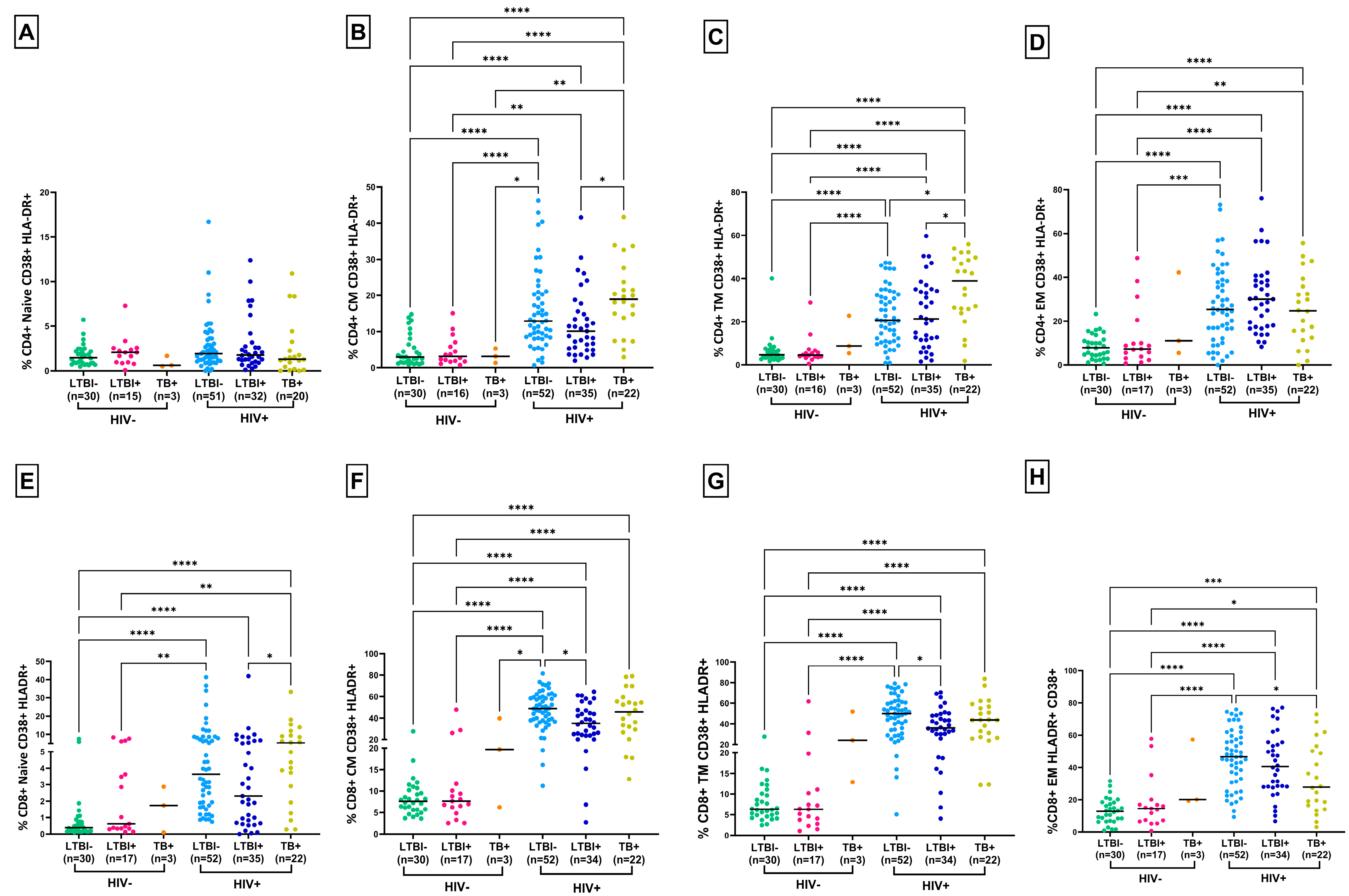
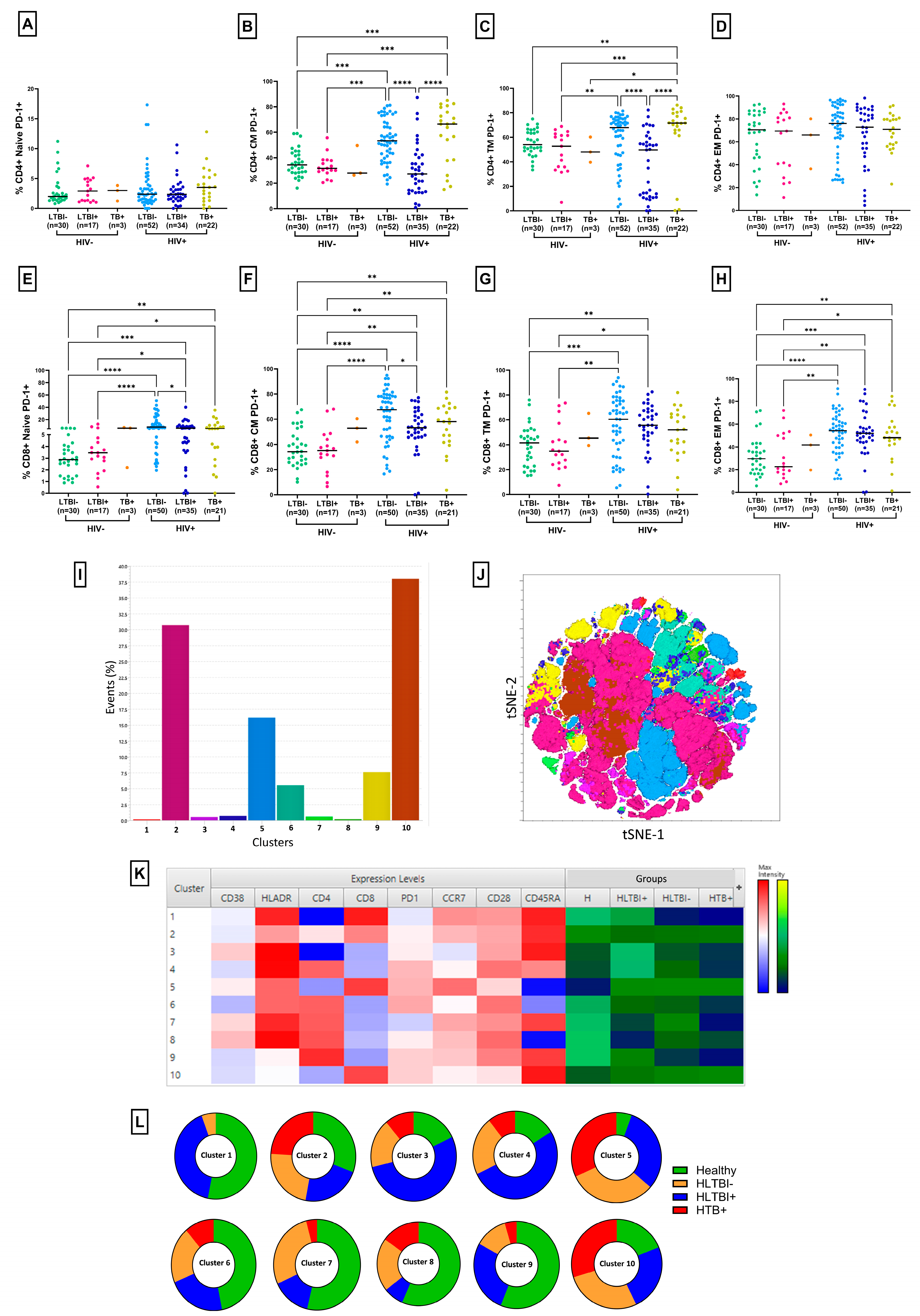
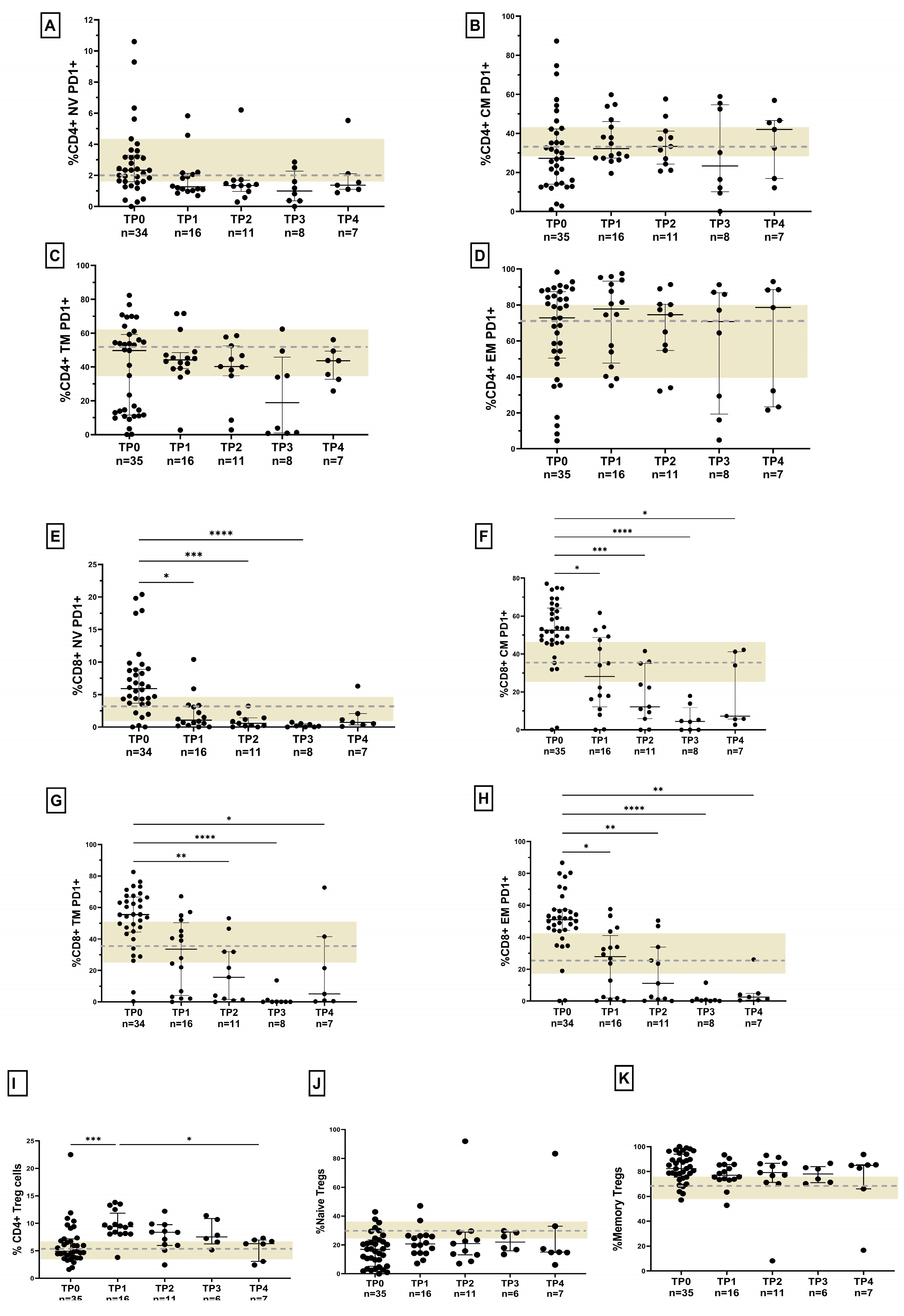
3.2. Lower T-Cell Activation and PD-1 Expression in HLTBI+ Individuals
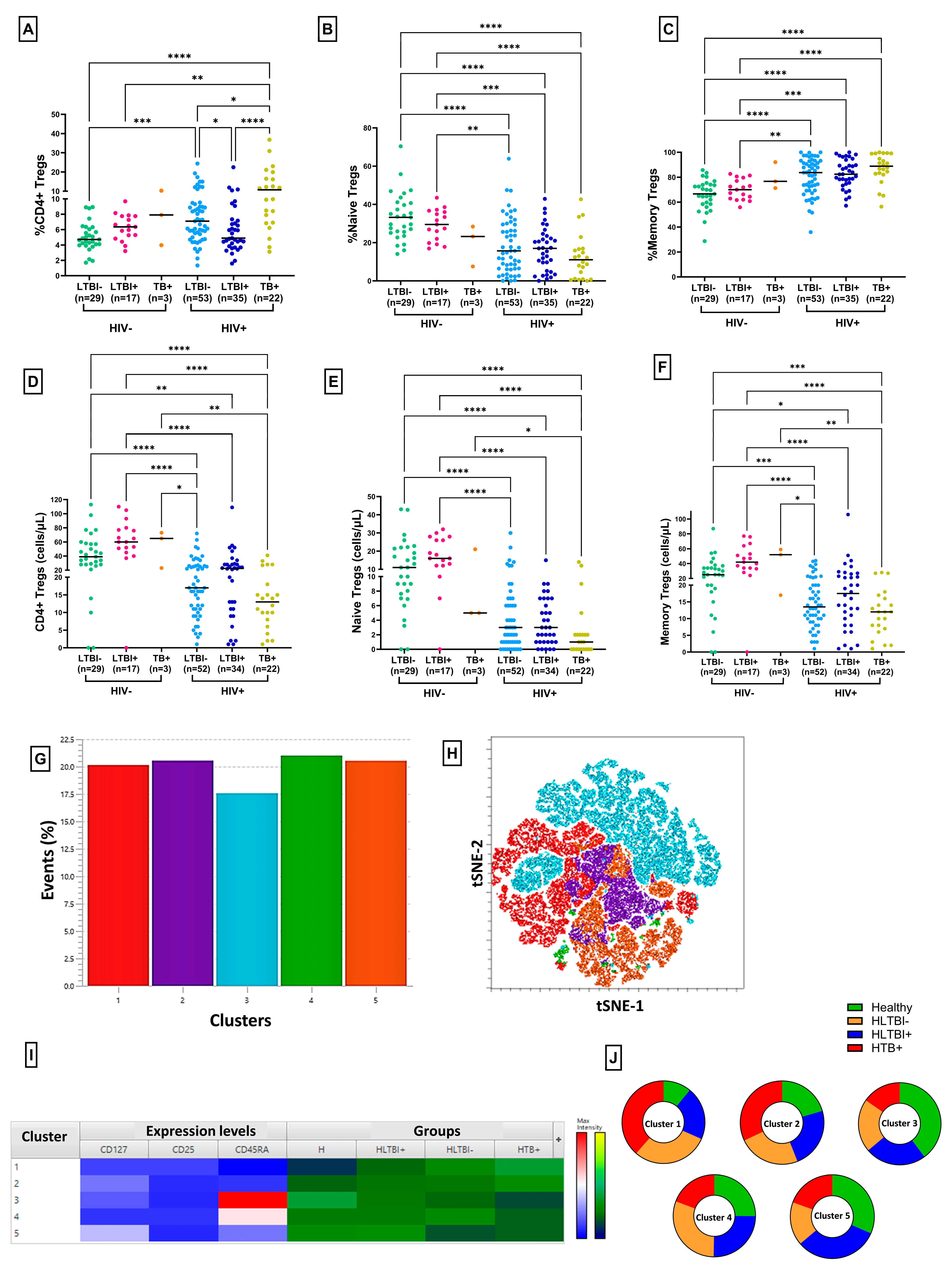
3.3. Reduced Frequency of Regulatory T Cells in HLTBI+ Individuals
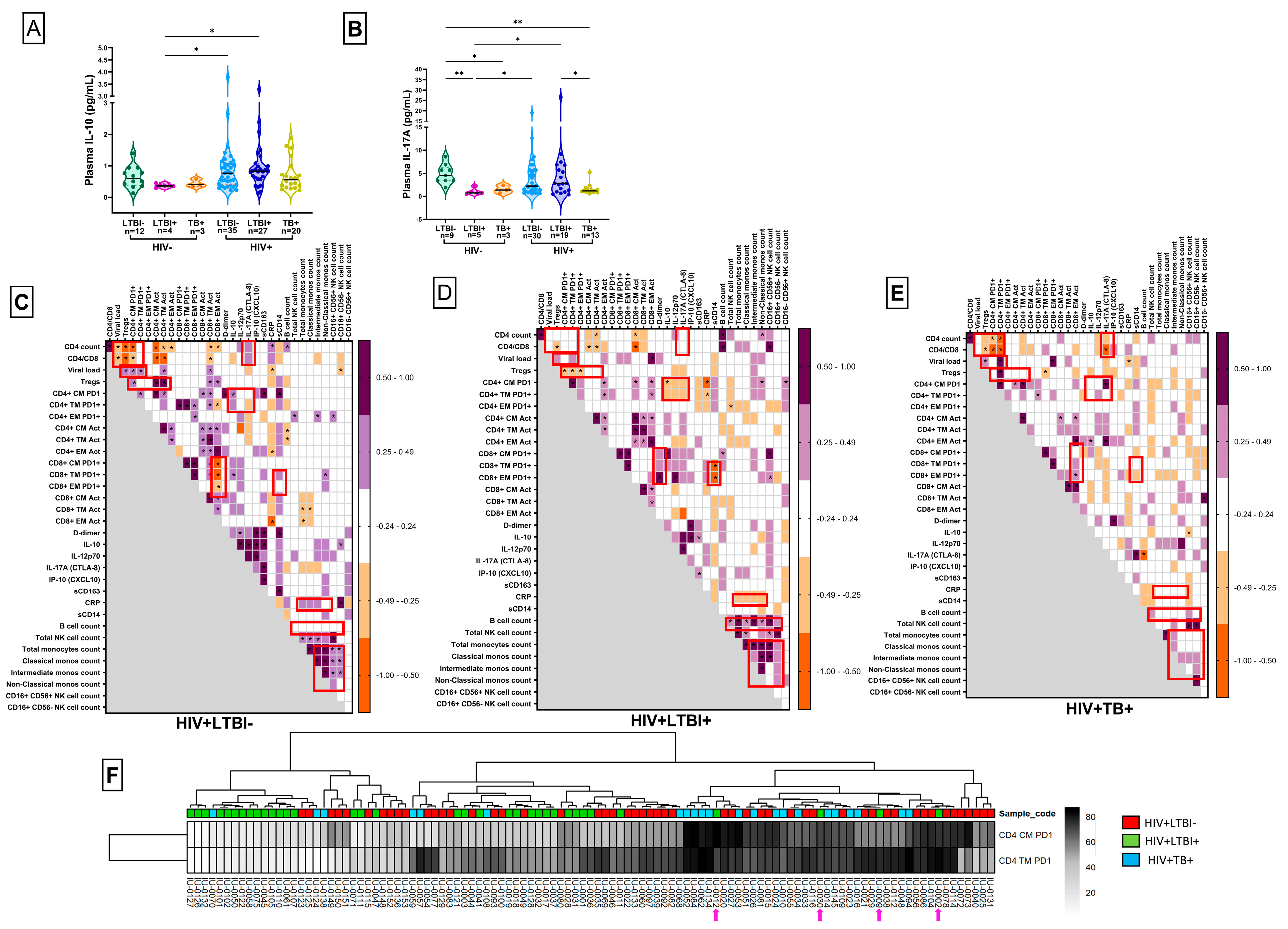
3.4. Plasma Analyte Analysis
3.5. Identification of HLTBI+-Specific Systemic Immune Signatures
3.6. PD-1 Expression on CD4+ Memory T Cells: An Independent Discriminatory Marker
3.7. Response to Anti-Retroviral Therapy (ART) in HLTBI+ Individuals: Impaired Immune Restoration
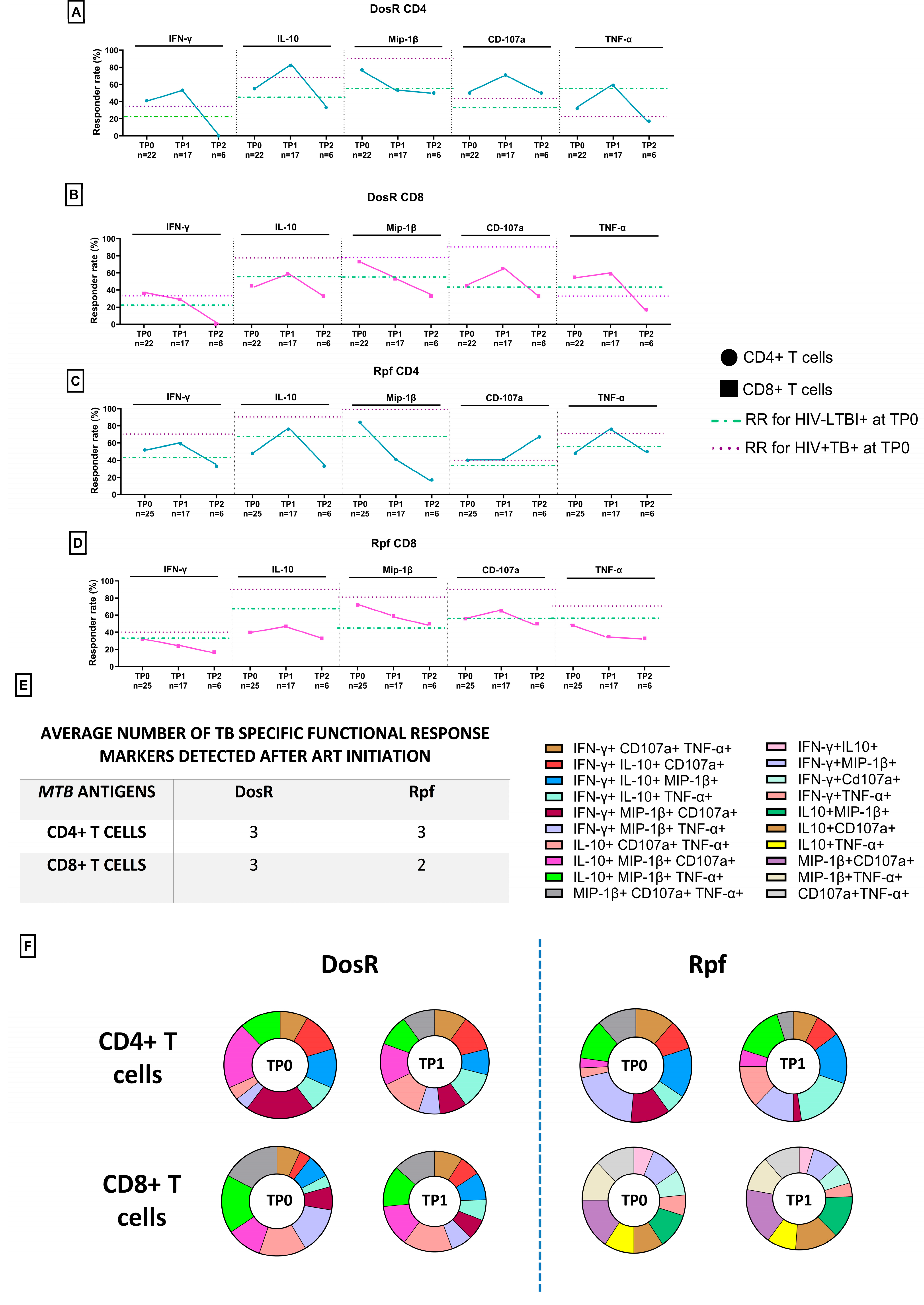
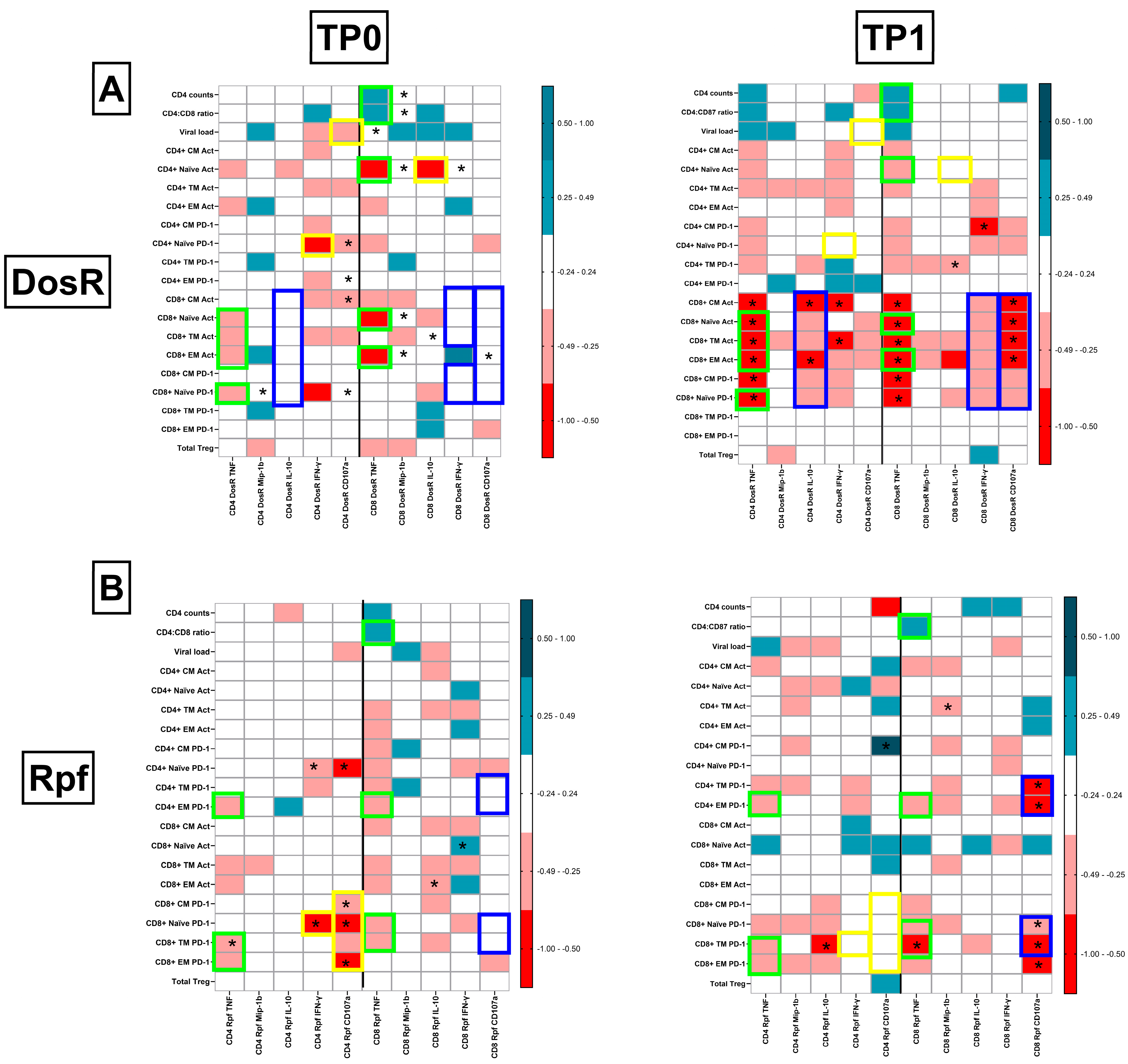
3.8. TB-Specific Responses in ART-Naïve PLHIV with Co-Infection
3.9. TB-Specific Responses in PLHIV with LTBI Following Initiation of ART
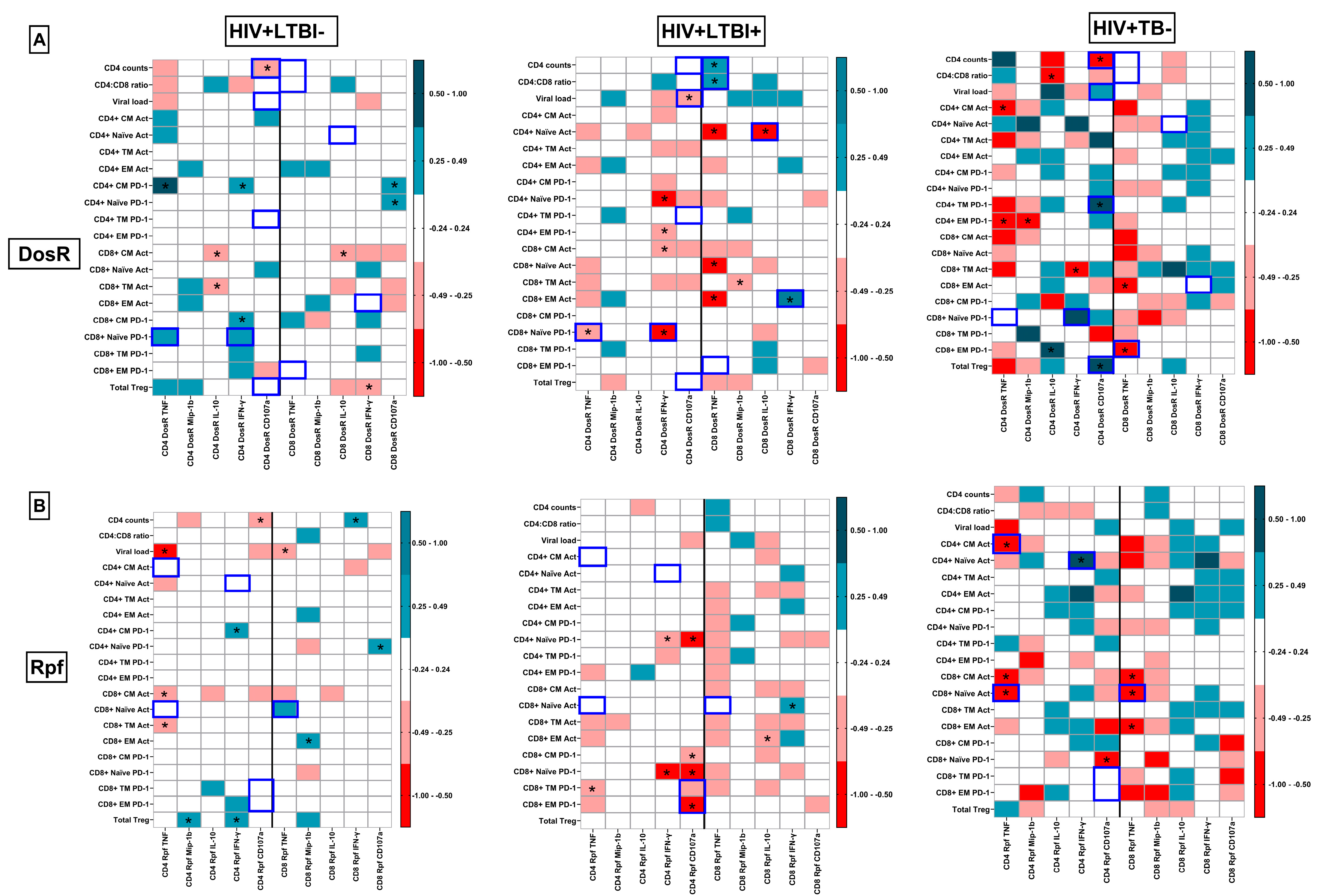
3.10. Integrated Pathogenic Signatures Delineating Latent and Active TB Co-Infection
3.11. Preservation of Protective TB-Specific Signatures Following ART Initiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HIV | Human immunodeficiency virus |
| TB | Tuberculosis |
| MTB | Mycobacterium tuberculosis |
| LTBI | Latent TB infection |
| HLTBI− | HIV+, LTBI− |
| HLTBI+ | HIV+, LTBI+ |
| HTB+ | HIV+, TB+ |
| DosR | Dormancy survival regulon |
| Rpf | Resuscitation promoting factor |
| ART | Anti-retroviral therapy |
| IPT | INH prophylaxis therapy |
| ATT | Anti-tuberculosis treatment |
| RR | Responder rate |
References
- Joint United Nations Programme on HIV/AIDS. Global AIDS Monitoring 2024: Guidelines. UNAIDS. 2024. Available online: http://www.aidsdatahub.org/sites/default/files/resource/global-aids-monitoring-2024-guidelines-en.pdf (accessed on 1 September 2025).
- National AIDS Control Organisation. Sankalak: Status of National AIDS Respons, 6th ed.; Ministry of Health & Family Welfare, Government of India: New Delhi, India, 26 December 2024. Available online: http://naco.gov.in/sites/default/files/Sankalak%20Booklet_Electronic%20version_II.pdf (accessed on 1 September 2025).
- Chandrasekaran, P.; Mave, V.; Thiruvengadam, K.; Gupte, N.; Shivakumar, S.V.B.Y.; Hanna, L.E.; Kulkarni, V.; Kadam, D.; Dhanasekaran, K.; Paradkar, M.; et al. Tuberculin skin test and QuantiFERON-Gold in Tube assay for diagnosis of latent TB infection among household contacts of pulmonary TB patients in high TB burden setting. PLoS ONE 2018, 13, e0199360. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Basu, S.; Chopra, K.K. Achieving TB elimination in India: The role of latent TB management. Indian J. Tuberc. 2018, 66, 30–33. [Google Scholar] [CrossRef]
- Selvaraju, S.; Velayutham, B.; Rao, R.; Rade, K.; Thiruvengadam, K.; Asthana, S.; Balachandar, R.; Bangar, S.D.; Bansal, A.K.; Bhat, J. Prevalence and factors associated with tuberculosis infection in India. J. Infect. Public Health 2023, 16, 2058–2065. [Google Scholar] [CrossRef]
- Central TB Division, Ministry of Health & Family Welfare, Government of India. India TB Report 2024 National TB Elimination Programme. 2024. Available online: http://tbcindia.mohfw.gov.in/wp-content/uploads/2024/10/TB-Report_for-Web_08_10-2024-1.pdf (accessed on 1 September 2025).
- World Health Organisation. Global Tuberculosis Report 2024. World Health Organization. 2024. Available online: https://iris.who.int/handle/10665/379339 (accessed on 1 September 2025).
- Kiazyk, S.; Ball, T.B. Latent Tuberculosis Infection: An overview. Can. Commun. Dis. Rep. 2017, 43, 62–66. [Google Scholar] [CrossRef]
- Amelio, C.; Portevin, P.; Hella, D.; Reither, J. HIV Infection Functionally Impairs Mycobacterium tuberculosis-Specific CD4 and CD8 T-Cell Responses. J. Virol. 2019, 93, 1728–1746. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W. Effects of Th17/Treg cell imbalance on HIV replicationin patients with AIDS complicated with tuberculosis. Exp. Ther. Med. 2018, 15, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Heng, X.; Summers, M.F. Structural Determinants and Mechanism of HIV-1 Genome Packaging Kun. J. Mol. Biol. 2011, 410, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.R.; Bucşan, A.N.; Alvarez, X.; Kumar, S.; Chatterjee, A.; Quezada, M.; Fish, A.; Singh, D.K.; Singh, B.; Sharan, R. Antiretroviral therapy does not reduce tuberculosis reactivation in a tuberculosis-HIV coinfection model. J. Clin. Investig. 2020, 130, 5171–5179. [Google Scholar] [CrossRef]
- Kalyan, M.; Sharma, S.; Kaur, P.; Sharma, A.; Verma, I. Antibody response to mycobacterial Rpf B protein and its immunodominant peptides in HIV-TB co-infected individuals. Tuberculosis 2024, 144, 102464. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Xu, J.; Wang, X.; Chan, J.; Tufariello, J.A.M. A Mycobacterium tuberculosis Rpf double-knockout strain exhibits profound defects in reactivation from chronic tuberculosis and innate immunity phenotypes. Infect. Immun. 2008, 76, 4269–4281. [Google Scholar] [CrossRef]
- Rakshit, S.; Hingankar, N.; Alampalli, S.V.; Adiga, V.; Sundararaj, B.K.; Sahoo, P.N.; Finak, G.; J, A.J.U.K.; Dhar, C.; D’souza, G.; et al. HIV Skews a Balanced Mtb-Specific Th17 Response in Latent Tuberculosis Subjects to a Pro-inflammatory Profile Independent of Viral Load. Cell Rep. 2020, 33, 108451. [Google Scholar] [CrossRef]
- Singh, A.K.; Salwe, S.; Padwal, V.; Velhal, S.; Sutar, J.; Bhowmick, S.; Mukherjee, S.; Nagar, V.; Patil, P.; Patel, V. Delineation of Homeostatic Immune Signatures Defining Viremic Non-progression in HIV-1 Infection. Front. Immunol. 2020, 11, 182. [Google Scholar] [CrossRef]
- Salwe, S.; Singh, A.; Padwal, V.; Velhal, S.; Nagar, V.; Patil, P.; Deshpande, A.; Patel, V. Immune signatures for HIV-1 and HIV-2 induced CD4+ T cell dysregulation in an Indian cohort. BMC Infect. Dis. 2019, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Padwal, V.; Palav, H.; Velhal, S.; Nagar, V.; Patil, P.; Patel, V. Highly dampened HIV-specific cytolytic effector T cell responses define viremic non-progression. Immunobiology 2022, 227, 152234. [Google Scholar] [CrossRef]
- Palav, H.C.; Bhonde, G.; Padwal, V.; Velhal, S.; Pereira, J.; Singh, A.K.; Ghosh, S.; Karandikar, K.; Satoskar, P.; Bhor, V.; et al. Integrated immune monitoring of HCMV infection in pregnant women with complications and its association with adverse pregnancy outcomes. Microb. Pathog. 2023, 179, 106109. [Google Scholar] [CrossRef] [PubMed]
- Leyten, E.M.; Lin, M.Y.; Franken, K.L.; Friggen, A.H.; Prins, C.; van Meijgaarden, K.E.; Voskuil, M.I.; Weldingh, K.; Andersen, P.; Schoolnik, G.K.; et al. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect. 2006, 8, 2052–2060. [Google Scholar] [CrossRef]
- Riaño, F.; Arroyo, L.; París, S.; Rojas, M.; Friggen, A.H.; van Meijgaarden, K.E.; Franken, K.L.; Ottenhoff, T.H.; García, L.F.; Barrera, L.F. T cell responses to DosR and Rpf proteins in actively and latently infected individuals from Colombia. Tuberculosis 2012, 92, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, L.; Marín, D.; Franken, K.L.M.C.; Ottenhoff, T.H.M.; Barrera, L.F. Potential of DosR and Rpf antigens from Mycobacterium tuberculosis to discriminate between latent and active tuberculosis in a tuberculosis endemic population of Medellin Colombia. BMC Infect. Dis. 2018, 18, 26. [Google Scholar] [CrossRef]
- Rakshit, S.; Adiga, V.; Nayak, S.; Sahoo, P.N.; Sharma, P.K.; van Meijgaarden, K.E.; J, A.J.U.; Dhar, C.; Souza, G.D.; Finak, G.; et al. Circulating Mycobacterium tuberculosis DosR latency antigen-specific, polyfunctional, regulatory IL10+ Th17 CD4 T-cells differentiate latent from active tuberculosis. Sci. Rep. 2017, 7, 11948. [Google Scholar] [CrossRef]
- Horsburgh, C.R., Jr.; O’Donnell, M.; Chamblee, S.; Moreland, J.L.; Johnson, J.; Marsh, B.J.; Narita, M.; Johnson, L.S.; von Reyn, C.F. Revisiting rates of reactivation tuberculosis: A population-based approach. Am. J. Respir. Crit. Care Med. 2010, 182, 420–425. [Google Scholar] [CrossRef]
- Wong, N.S.; Leung, C.C.; Chan, K.C.W.; Chan, W.K.; Lin, A.W.C.; Lee, S.S. A longitudinal study on latent TB infection screening and its association with TB incidence in HIV patients. Sci. Rep. 2019, 9, 10093. [Google Scholar] [CrossRef]
- Vázquez García, J.C.; Sada Díaz, E.; Rivera Martínez, E.; Narváez Porras, O.; Salazar Lezama, M.A. Tuberculosis associated with HIV infection. Rev. Invest. Clin. 1994, 46, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Bucşan, A.N.; Chatterjee, A.; Singh, D.K.; Foreman, T.W.; Lee, T.-H.; Threeton, B.; Kirkpatrick, M.G.; Ahmed, M.; Golden, N.; Alvarez, X.; et al. Mechanisms of reactivation of latent tuberculosis infection due to SIV coinfection. J. Clin. Investig. 2019, 129, 5254–5260. [Google Scholar] [CrossRef] [PubMed]
- Lalvani, A.; Pareek, M. A 100 year update on diagnosis of tuberculosis infection. Br. Med. Bull. 2010, 93, 69–84. [Google Scholar] [CrossRef]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef]
- Salwe, S.; Padwal, V.; Nagar, V.; Patil, P.; Patel, V. T cell functionality in HIV-1, HIV-2 and dually infected individuals: Correlates of disease progression and immune restoration. Clin. Exp. Immunol. 2019, 198, 233–250. [Google Scholar] [CrossRef]
- Scanga, C.A.; Mohan, V.P.; Yu, K.; Joseph, H.; Tanaka, K.; Chan, J.; Flynn, J.L. Depletion of CD4(+) T Cells Causes Reactivation of Murine Persistent Tuberculosis Despite Continued Expression of Interferon and Nitric Oxide Synthase 2. J. Exp. Med. 2000, 192, 347–358. [Google Scholar] [CrossRef]
- Mupfumi, L.; Mpande, C.A.; Reid, T.; Moyo, S.; Shin, S.S.; Zetola, N.; Mogashoa, T.; Musonda, R.M.; Kasvosve, I.; Scriba, T.J.; et al. Immune phenotype and functionality of Mtb-specific T-cells in HIV/TB co-infected patients on antiretroviral treatment. Pathogens 2020, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, A.; Jansson, M.; Sköld, M.; Rottenberg, M.E.; Källenius, G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012, 8, e1002464. [Google Scholar] [CrossRef]
- Klatt, N.R.; Brenchley, J.M. Th17 cell dynamics in HIV infection. Curr. Opin. HIV AIDS 2010, 5, 135–140. [Google Scholar] [CrossRef]
- Adamopoulos, I.E.; Kuchroo, V. IL-17A and IL-17F in tissue homeostasis, inflammation and regeneration. Nat. Rev. Rheumatol. 2023, 19, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Li, H.X.; Zheng, C.; Han, J.; Zhu, J.; Liu, S.; Jin, T. PD-1/PD-L1 Axis as a Potential Therapeutic Target for Multiple Sclerosis: A T Cell Perspective. Front. Cell Neurosci. 2021, 15, 716747. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Gao, X.; Wu, C.; Wang, X.; Xu, H.; Wu, Y.; Li, Y.; Jia, Y.; Wei, C.; He, W.; Wang, Y.; et al. The DosR antigen Rv1737c from Mycobacterium tuberculosis confers inflammation regulation in tuberculosis infection. Scand. J. Immunol. 2019, 89, e12729. [Google Scholar] [CrossRef]
- Mohamed, R.; Jayakumar, C.; Chen, F.; Fulton, D.; Stepp, D.; Gansevoort, R.T.; Ramesh, G. Low-dose IL-17 therapy prevents and reverses diabetic nephropathy, metabolic syndrome, and associated organ fibrosis. J. Am. Soc. Nephrol. 2016, 27, 745–765. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef] [PubMed]
- Sawoo, R.; Dey, R.; Ghosh, R.; Bishayi, B. Exogenous IL-10 posttreatment along with TLR4 and TNFR1 blockade improves tissue antioxidant status by modulating sepsis-induced macrophage polarization. J. Appl. Toxicol. 2023, 43, 1549–1572. [Google Scholar] [CrossRef]
- Murray, L.W.; Satti, I.; Meyerowitz, J.; Jones, M.; Willberg, C.B.; Ussher, J.E.; Goedhals, D.; Hurst, J.; Phillips, R.E.; McShane, H.; et al. Human immunodeficiency virus infection impairs Th1 and Th17 mycobacterium tuberculosis–specific T-Cell responses. J. Infect. Dis. 2018, 217, 1782–1792. [Google Scholar] [CrossRef]
- Shey, M.S.; Balfour, A.; Masina, N.; Bekiswa, A.; Schutz, C.; Goliath, R.; Dielle, R.; Katoto, P.D.; Wilkinson, K.A.; Lewinsohn, D.; et al. Mycobacterial-specific secretion of cytokines and chemokines in healthcare workers with apparent resistance to infection with Mycobacterium tuberculosis. Front. Immunol. 2023, 14, 1176615. [Google Scholar] [CrossRef] [PubMed]
- Biketov, S.; Potapov, V.; Ganina, E.; Downing, K.; Kana, B.D.; Kaprelyants, A. The role of resuscitation promoting factors in pathogenesis and reactivation of Mycobacterium tuberculosis during intra-peritoneal infection in mice. BMC Infect. Dis. 2007, 7, 146. [Google Scholar] [CrossRef]
- Rosser, A.; Stover, C.; Pareek, M.; Mukamolova, G.V. Resuscitation-promoting factors are important determinants of the pathophysiology in Mycobacterium tuberculosis infection. Crit. Rev. Microbiol. 2017, 43, 621–630. [Google Scholar] [CrossRef]
- Lawn, S.D.; Myer, L.; Bekker, L.-G.; Wood, R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: Impact on treatment outcomes and implications for tuberculosis control. AIDS 2006, 20, 1605–1612. [Google Scholar] [CrossRef]
- Johansen, I.S.; Roen, A.; Kraef, C.; Martín-Iguacel, R.; Nemeth, J.; Fenner, L.; Zangerle, R.; Llibre, J.M.; Miller, R.F.; Suarez, I.; et al. Risk of tuberculosis after initiation of antiretroviral therapy among persons with HIV in Europe. Int. J. Infect. Diseases 2024, 147, 107199. [Google Scholar] [CrossRef]
- Rajasuriar, R.; Wright, E.; Lewin, S.R. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr. Opin. HIV AIDS 2015, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ruan, L. Recent advances in poor HIV immune reconstitution: What will the future look like? Front. Microbiol. 2023, 14, 1236460. [Google Scholar] [CrossRef]
- Prasanna, P.; Herrera, B.; Schlesinger, L.S.; Paiardini, M.; Sharan, R. Advances in host-directed therapy for tuberculosis and HIV coinfection: Enhancing immune responses. Trends Microbiol. 2025, 33, 961–975. [Google Scholar] [CrossRef]
- Riou, C.; Jhilmeet, N.; Rangaka, M.X.; Wilkinson, R.J.; Wilkinson, K.A. Tuberculosis Antigen-Specific T-Cell Responses During the First 6 Months of Antiretroviral Treatment. J. Infect. Dis. 2020, 221, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Jhilmeet, N.; Lowe, D.M.; Riou, C.; Scriba, T.J.; Coussens, A.; Goliath, R.; Wilkinson, R.J.; Wilkinson, K.A.; Neyrolles, O. The effect of antiretroviral treatment on selected genes in whole blood from HIV-infected adults sensitised by Mycobacterium tuberculosis. PLoS ONE 2018, 13, e0209516. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, T.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; La Manna, M.P.; Orlando, V.; Pinnetti, C.; Sampaolesi, A.; Antinori, A.; Caccamo, N.; et al. Impact of antiretroviral and tuberculosis therapies on CD4 + and CD8 + HIV/M. tuberculosis-specific T-cell in co-infected subjects. Immunol. Lett. 2018, 198, 33–43. [Google Scholar] [CrossRef]
- Ahmed, A.; Rakshit, S.; Vyakarnam, A. HIV-TB co-infection: Mechanisms that drive reactivation of Mycobacterium tuberculosis in HIV infection. Oral. Dis. 2016, 22, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Saharia, K.K.; Petrovas, C.; Ferrando-Martinez, S.; Leal, M.; Luque, R.; Ive, P.; Luetkemeyer, A.; Havlir, D.; Koup, R.A.; Neyrolles, O. Tuberculosis therapy modifies the cytokine profile, maturation state, and expression of inhibitory molecules on mycobacterium tuberculosis-specific CD4+ T-Cells. PLoS ONE 2016, 11, e0158262. [Google Scholar] [CrossRef] [PubMed]

| HIV-1-Positive | HIV-Seronegative | |||||
|---|---|---|---|---|---|---|
| HLTBI+ | HLTBI− | HTB+ | LTBI+ | LTBI− | TB+ | |
| No. of participants | n = 35 | n = 55 | n = 22 | n = 17 | n = 30 | n = 3 |
| Gender (F/M) | 9/26 | 20/35 | 6/16 | 6/11 * | 17/13 * | 1/2 |
| Age a | 40 (26–59) | 37 (18–58) | 41 (18–59) | 32 (23–48) | 29 (22–56) | 29 (27–48) |
| CD4+ T cells (cells/μL) a | 362 $ (10–1127) | 308 (6–835) | 127 (5–455) | 969 (552–2478) | 837 (468–2019) | 707 (286–1623) |
| CD4/CD8 a | 0.39 # (0.02–1.56) | 0.23 (0.01–1.26) | 0.12 (0.01–0.5) | 1.3 (0.72–2.88) | 1.42 (0.7–2.54) | 1.26 (0.3–2.45) |
| HIV-1 viral load (copies/mL) a | 63,403 (14–6, 21, 950) | 39,905 (22–59, 20, 711) | 1, 63, 566 @,& (67–1, 11, 10, 633) | ---- | ---- | ---- |
| Assay | Reagent Used and Concentration per Reaction | Reagent Volume and Condition Used |
|---|---|---|
| Absolute cell count (stain/lyse/no wash) [Assay volume 577.5 µL] | ||
| --- | 50 µL |
| 0.5 µg/mL | Master mix (27.5 µL) |
| 1× | 450 µL |
| --- | 50 µL |
| Whole-blood immunophenotyping (stain/lyse/wash) [Assay volume 200 µL] | ||
| --- | 200 µL |
| 0.5 µg/mL | Master mix for T-cell subset panel (22.5 µL) Master mix for Treg panel (20 µL) |
| BD FACS lysis buffer (1×) (BD Biosciences, San Jose, CA, USA; catalog no.: 349202) Stain buffer (DPBS + 0.2%FBS) | 200 µL |
| Intracellular cytokine staining assay [Assay volume 200 µL] | ||
| 2 × 106 cells/mL | 200 µL |
| ||
| 10 μg/mL | Individual stimulations |
| 10 μg/mL | |
| 1 μg/mL for each peptide | |
| 50 ng/mL | Used for positive control |
| 1 µg/mL | |
| 1 μL/mL | Used as protein secretion inhibitor in all reactions |
| 0.7 μL/mL | |
| 3. Staining reaction: 200 μL | ||
| 1× per reaction | Used in all reactions |
| 0.5 µg/mL | Master mix (17.5 μL) |
| 200 μL/reaction | Used in all reactions |
| 0.5 µg/mL | Master mix (20 μL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhowmick, S.; Devadiga, P.; Yadav, S.; Mohite, N.; Gurav, P.; Pandey, T.; Padwal, V.; Neman, N.; Suryawanshi, A.; Musale, S.; et al. Protective Immune Signatures Associated with Latent TB Infection in PLHIV—Insights from an Integrative Prospective Immune Monitoring Study. Cells 2025, 14, 1622. https://doi.org/10.3390/cells14201622
Bhowmick S, Devadiga P, Yadav S, Mohite N, Gurav P, Pandey T, Padwal V, Neman N, Suryawanshi A, Musale S, et al. Protective Immune Signatures Associated with Latent TB Infection in PLHIV—Insights from an Integrative Prospective Immune Monitoring Study. Cells. 2025; 14(20):1622. https://doi.org/10.3390/cells14201622
Chicago/Turabian StyleBhowmick, Shilpa, Pratik Devadiga, Sapna Yadav, Nandan Mohite, Pranay Gurav, Tejaswini Pandey, Varsha Padwal, Namrata Neman, Aarya Suryawanshi, Satyajit Musale, and et al. 2025. "Protective Immune Signatures Associated with Latent TB Infection in PLHIV—Insights from an Integrative Prospective Immune Monitoring Study" Cells 14, no. 20: 1622. https://doi.org/10.3390/cells14201622
APA StyleBhowmick, S., Devadiga, P., Yadav, S., Mohite, N., Gurav, P., Pandey, T., Padwal, V., Neman, N., Suryawanshi, A., Musale, S., Singh, A. K., Bhagat, S., Kaginkar, S., Palav, H., Birje, S., Kerkar, S., Idicula-Thomas, S., Nagar, V., Patil, P., ... Patel, V. (2025). Protective Immune Signatures Associated with Latent TB Infection in PLHIV—Insights from an Integrative Prospective Immune Monitoring Study. Cells, 14(20), 1622. https://doi.org/10.3390/cells14201622







