Advances in Mitochondrial Dysfunction and Its Role in Cardiovascular Diseases
Abstract
1. Introduction
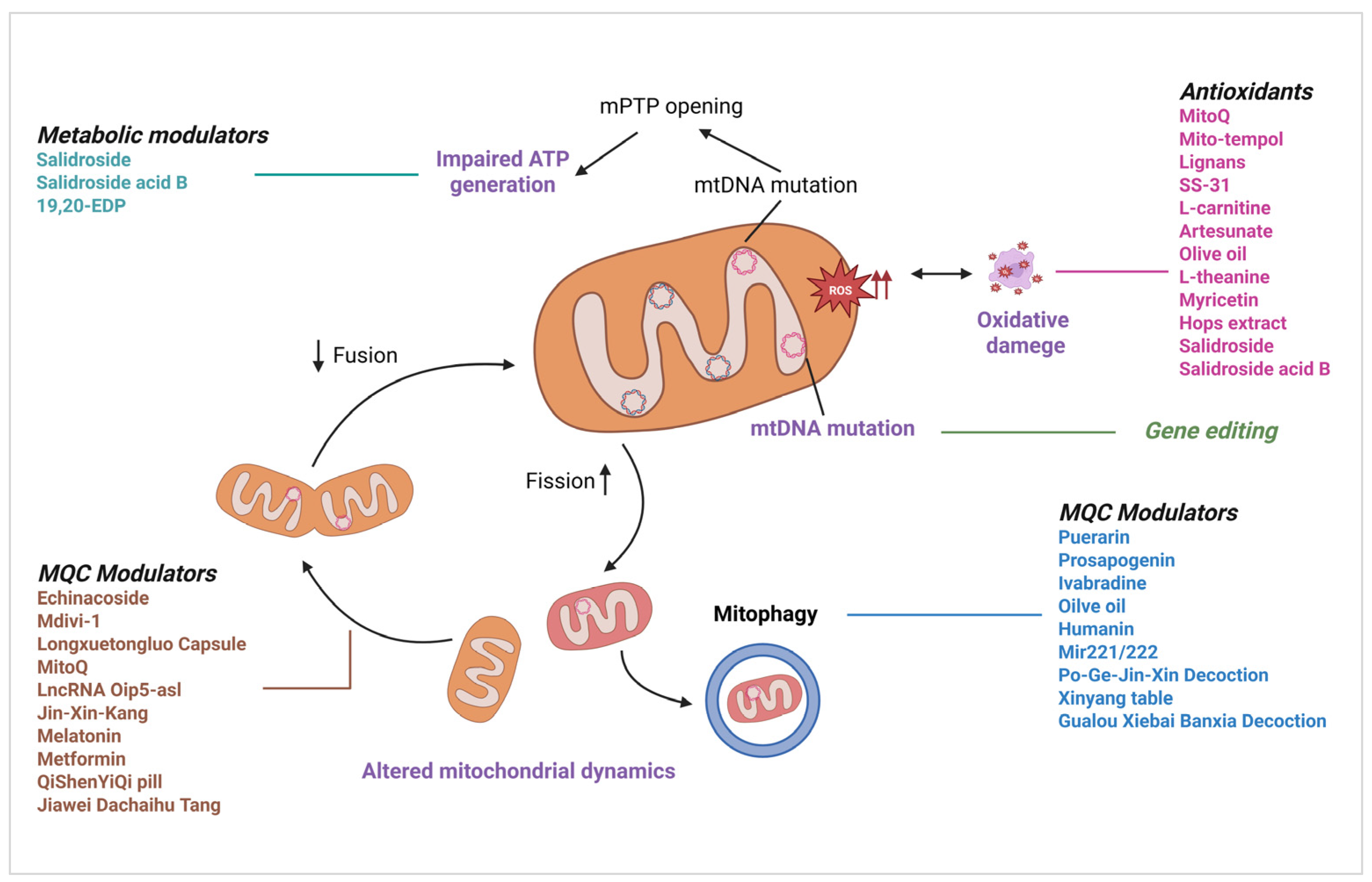
2. Mechanisms of Mitochondrial Dysfunction in CVDs
2.1. Mitochondrial ROS (mtROS) Generation and Amplification
2.2. Metabolic Dysfunction and Energetic Crisis
2.3. Calcium Homeostasis
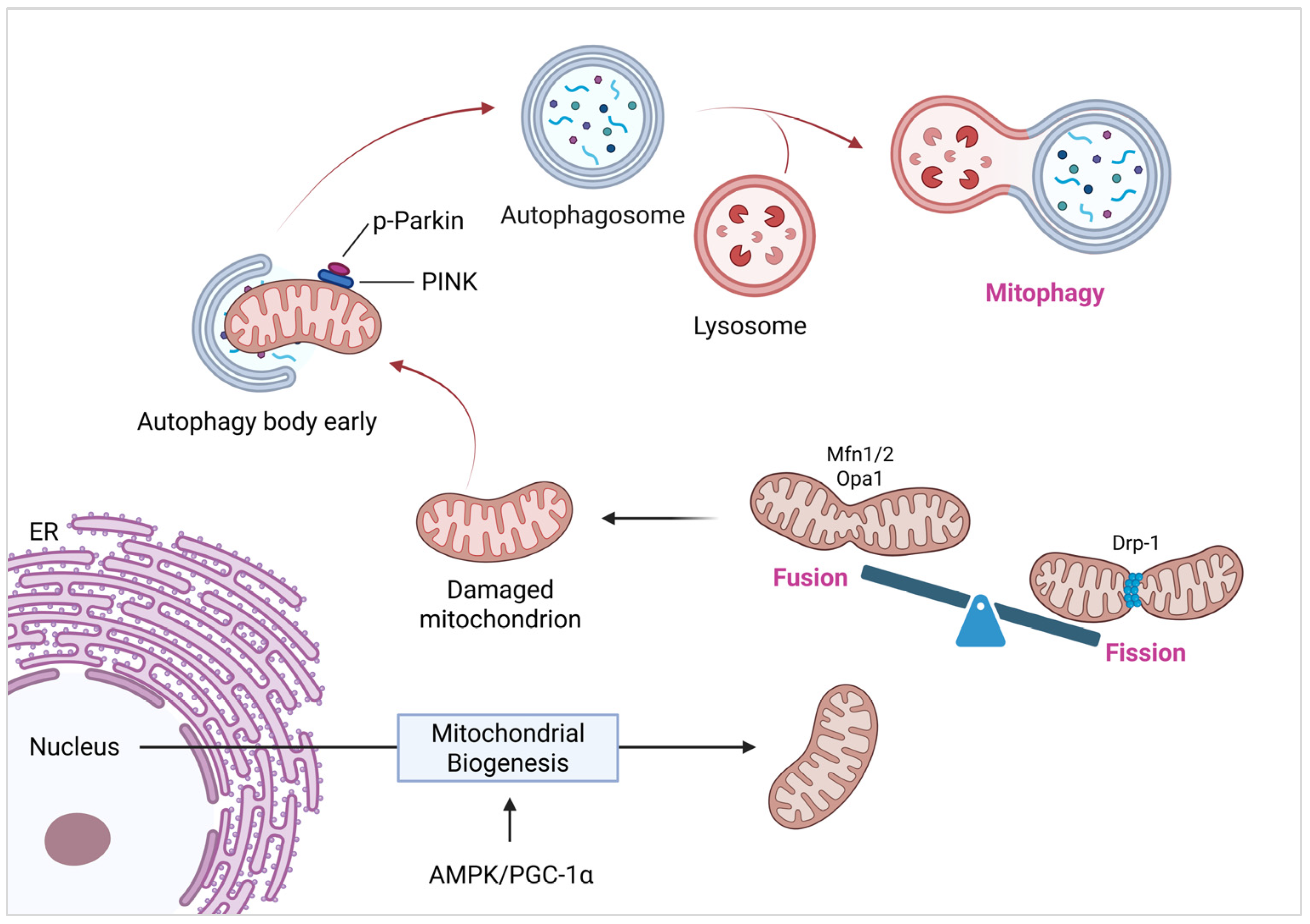
2.4. Mitochondrial Quality Control (MQC)
3. Mitochondrial Dysfunction and Specific Cardiac Conditions
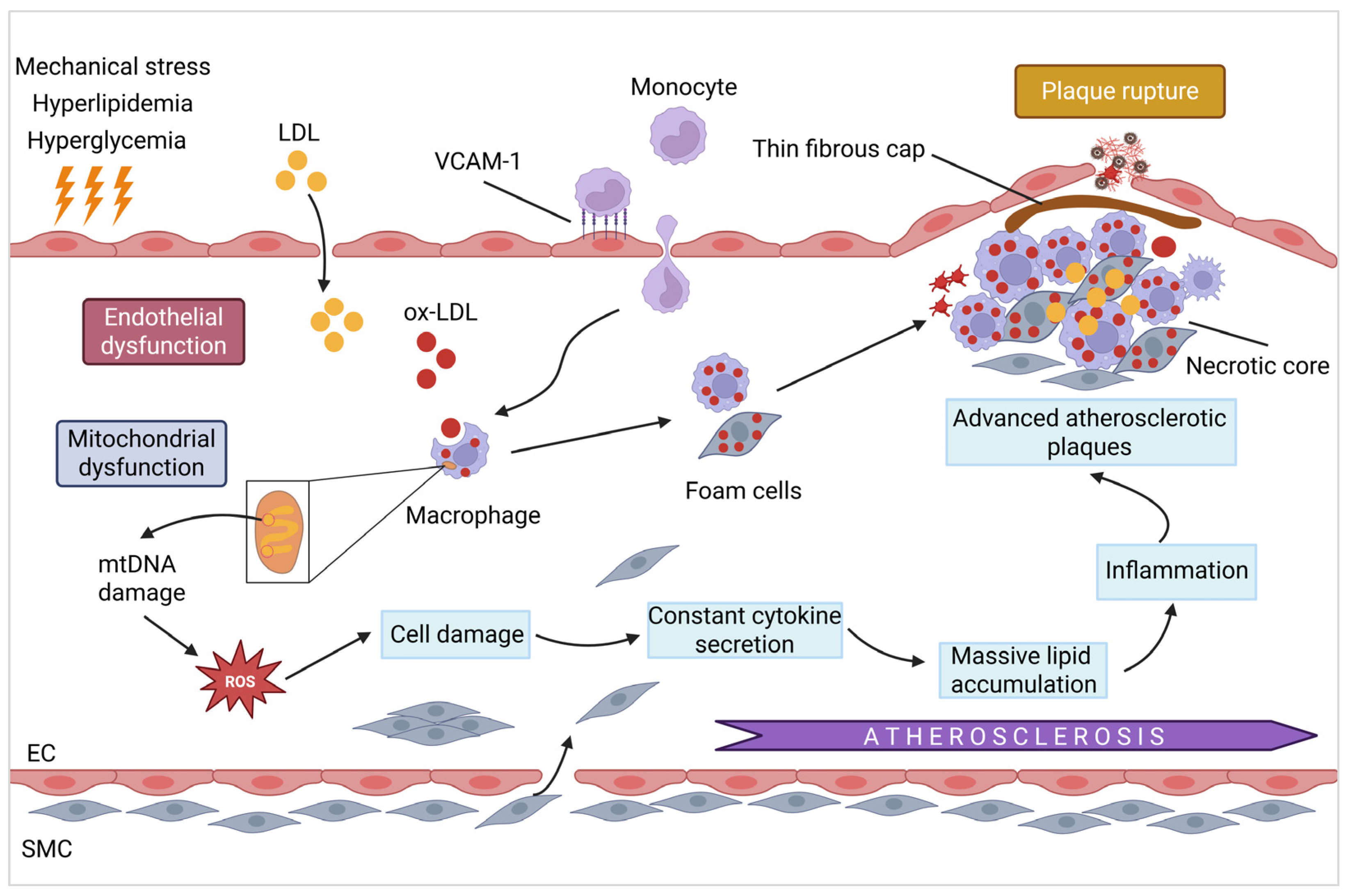
3.1. Atherosclerosis
3.1.1. Mitochondrial Damage and Endothelial Dysfunction
3.1.2. Mitochondrial Dysfunction and Plaque Instability/Rupture
3.1.3. Potential Therapeutic Targets
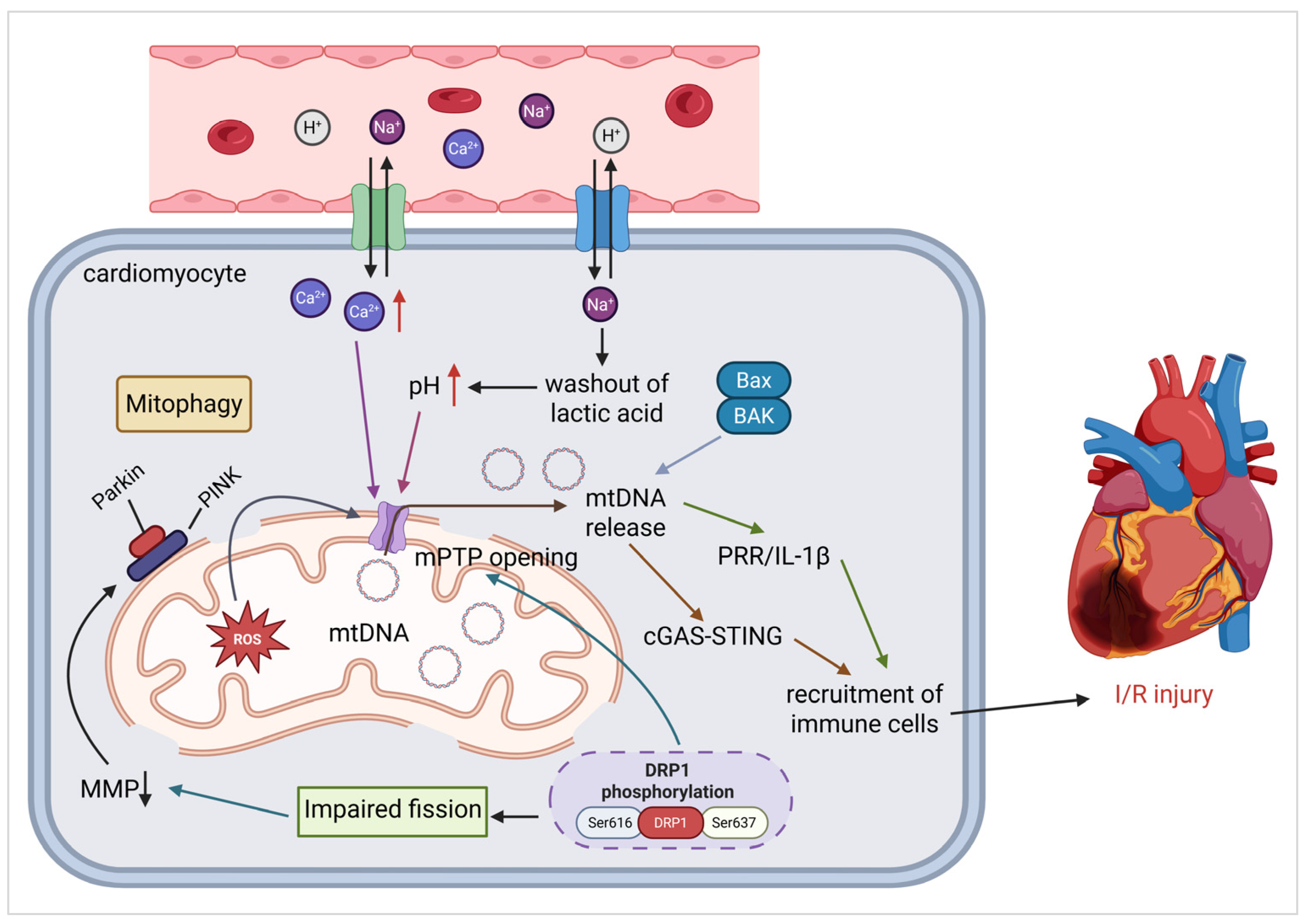
3.2. Ischemia–Reperfusion Injury (IRI)
3.2.1. Oxidative Stress and mPTP Activation
3.2.2. Mitochondrial Fission and Bioenergetic Failure
3.2.3. mtDNA Mutant and Release
3.2.4. Potential Therapeutic Strategies
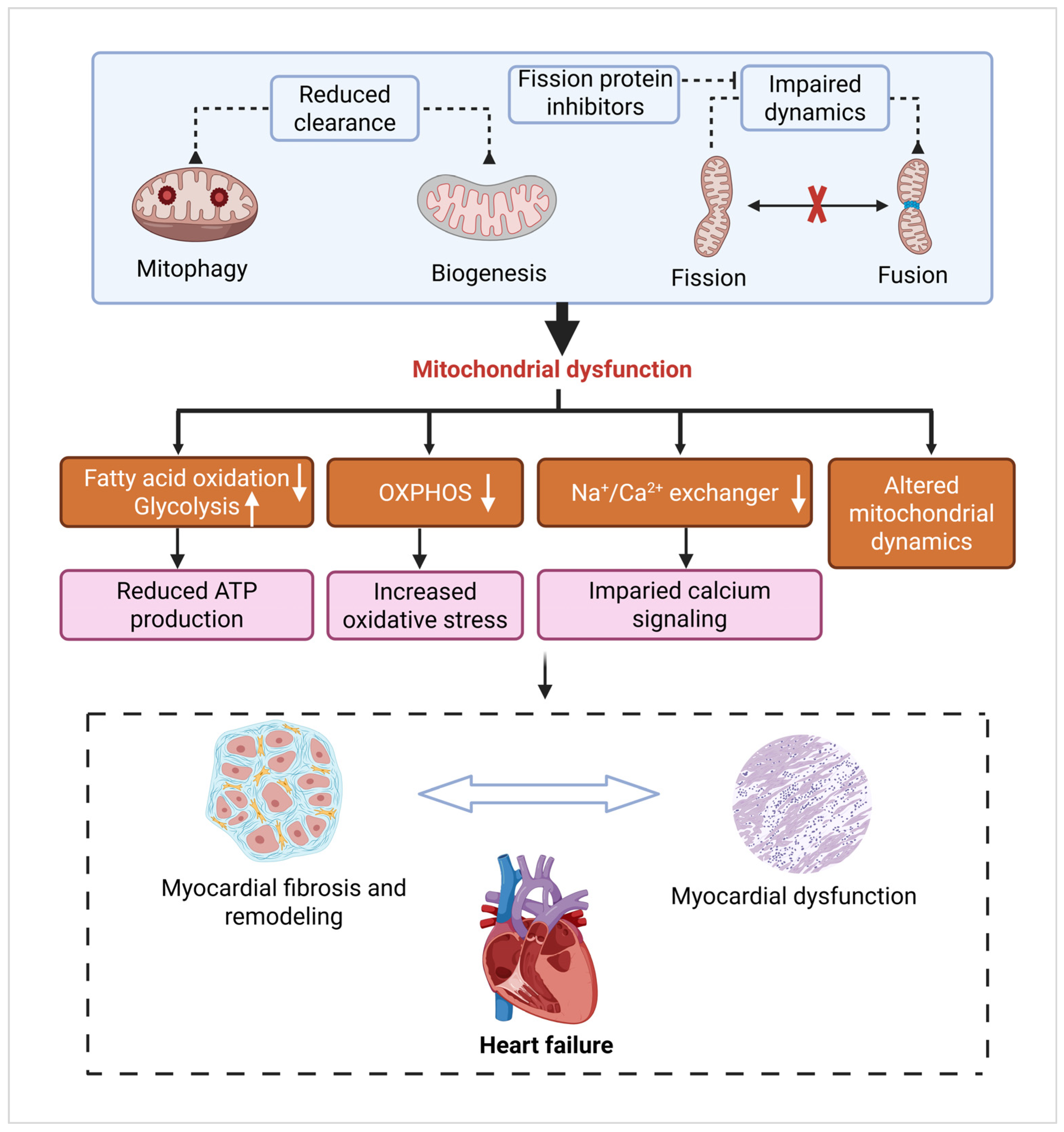
3.3. Heart Failure
3.3.1. Metabolic Dysfunction and Oxidative Stress
3.3.2. Fibrosis and Cardiac Remodeling
3.3.3. Mitochondrial Dynamics and Mitophagy Defects
3.3.4. Potential Therapeutic Targets and Modulators
4. Potential of Mitochondrial Dysfunction as a Biomarker and Therapeutic Target
4.1. Prospects of Mitochondria-Related Indicators in CVD Diagnosis
4.1.1. GDF-15 and FGF-21
4.1.2. mtDNA-CN as a Non-Invasive Diagnostic Tool
4.1.3. Mitochondrial Gene Mutations and CVD Risk
4.2. Exploring Therapeutic Strategies Based on the Regulation of Mitochondrial Function
4.2.1. Mitochondria-Targeted Pharmacological Interventions in CVDs
| Therapeutic Agent | Category | Mechanism of Action | Cardiovascular Application | Ref. |
|---|---|---|---|---|
| MitoQ | Antioxidant | Accumulates in mitochondria; scavenges ROS at their source. | IRI, Atherosclerosis, HF | [82,232,233,234] |
| SS-31 (Elamipretide) | Antioxidant | Stabilizes inner mitochondrial membrane; inhibits respiratory chain uncoupling and ROS production. | IRI, Endothelial Dysfunction | [239,240,241,242] |
| Metformin | MQC Modulator | Activates AMPK/PGC-1α pathway, promoting mitochondrial biogenesis. | Cardiomyocyte protection, endothelial senescence | [164] |
| SGLT2 Inhibitors | Metabolic Modulator | Increases fatty acid oxidation and ketogenesis; may enhance autophagy and mitochondrial turnover. | HF (all phenotypes) | [16,186,187] |
| Mdivi-1 | MQC Modulator | Inhibits the fission protein Drp1, reducing mitochondrial fragmentation. | Post-Myocardial Infarction remodeling | [77] |
| Artesunate/Jiawei Dachaihu Tang | MQC Modulator | Activates SIRT1, leading to deacetylation and activation of PGC-1α and other metabolic regulators. | Atherosclerosis, HF | [33,69] |
| Colchicine | Anti-inflammatory | Inhibits NLRP3 inflammasome assembly, which is linked to mitochondrial DAMPs. | Atherosclerosis (secondary prevention) | [122,123] |
4.2.2. Gene Therapy for Mitochondrial Dysfunction in Coronary Heart Disease: Current Progress and Future Directions
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHR | Aryl Hydrocarbon Receptor |
| ANT | Adenine Nucleotide Translocase |
| AKAP1 | A-Kinase Anchoring Protein 1 |
| ALDH2 | Aldehyde Dehydrogenase 2 |
| AMPK | Adenosine 5′-monophosphate-activated Protein Kinase |
| AS | Atherosclerosis |
| ATP | Adenosine Triphosphate |
| BBC3 | BCL2 Binding Component 3 (also known as PUMA) |
| BDR | Benzodiazepine Receptor |
| BNIP3 | BCL2 Interacting Protein 3 |
| BNP | Brain Natriuretic Peptide |
| BYHWD | Buyang Huanwu Decoction |
| Ca2+ | Calcium ions |
| CABG | Coronary Artery Bypass Grafting |
| CAD | Coronary Artery Disease |
| CaN | Calcineurin |
| CCND1 | Cyclin D1 |
| ccf-mtDNA | Circulating Cell-Free Mitochondrial DNA |
| cGAS | Cyclo-GMP-AMP Synthase |
| CHD | Coronary Heart Disease |
| CHF | Chronic Heart Failure |
| CMD | Coronary Microvascular Disease |
| CoQ10 | Coenzyme Q10 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CUMS | Chronic Unpredictable Mild Stress |
| CVDs | Cardiovascular Diseases |
| CycD | Cyclophilin-D |
| DAMP | Damage-Associated Molecular Pattern |
| DNA | Deoxyribonucleic Acid |
| DNM2 | Dynamin 2 |
| DRP1 | Dynamin-Related Protein 1 |
| ECFCs | Endothelial Colony-Forming Cells |
| ECM | Extracellular Matrix |
| ED | Erectile Dysfunction |
| 19,20-EDP | 19,20-epoxydocosapentaenoic acid |
| eNAMPT | Extracellular Nicotinamide Phosphoribosyltransferase |
| ER | Endoplasmic Reticulum |
| ERRs | Estrogen-Related Receptors |
| ETC | Electron Transport Chain |
| FA | Fatty Acid |
| FAO | Fatty Acid β-oxidation |
| FGF-21 | Fibroblast Growth Factor 21 |
| Fis1 | Fission Protein 1 |
| FOXO3a | Forkhead Box O3a |
| FUNDC1 | FUN14 Domain Containing 1 |
| GAS6 | Growth Arrest-Specific 6 |
| GDF-15 | Growth Differentiation Factor 15 |
| GXBD | Gualou Xiebai Banxia Decoction |
| H2O2 | Hydrogen Peroxide |
| HF | Heart Failure |
| HFmEF | HF with Mid-range Ejection Fraction |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFrEF | HF with Reduced Ejection Fraction |
| HO-1 | Heme Oxygenase-1 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IFN | Interferon |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IMM | Inner Mitochondrial Membrane |
| i-NOS | Inducible Nitric Oxide Synthase |
| I/R | Ischemia–Reperfusion |
| IRI | Ischemia–Reperfusion Injury |
| IVUS | Intravascular Ultrasound |
| JNK | Jun N-Terminal Kinase |
| JPH2 | Junctophilin-2 |
| JXK | Jin-Xin-Kang |
| KLF4 | Krüppel-Like Factor 4 |
| LCLAT1 | Lysocardiolipin Acyltransferase 1 |
| LDL | Low-Density Lipoprotein |
| LETM1 | Leucine Zipper EF-Containing Transmembrane Protein 1 |
| lncRNA | Long Non-Coding RNA |
| LPS | Lipopolysaccharide |
| LTC | Longxuetongluo Capsule |
| MAC | Mitochondrial Apoptosis Channel |
| MAP1LC3B | Microtubule Associated Protein 1 Light Chain 3 Beta |
| MAPK | Mitogen-Activated Protein Kinase |
| MARCHF5 | Membrane Associated Ring-CH-Type Finger 5 |
| MCU | Mitochondrial Calcium Uniporter |
| Mdivi-1 | Mitochondrial Division Inhibitor 1 |
| Mff | Mitochondrial Fission Factor |
| Mfn1/2 | Mitofusins 1/2 |
| MI | Myocardial Infarction |
| MIMP | Mitochondrial Inner Membrane Permeabilization |
| MitoQ | Mitoquinone |
| mito-TALENs | Mitochondrial-Targeted Transcription Activator-Like Effector Nucleases |
| MLKL | Mixed Lineage Kinase Domain-Like Protein |
| MMP | Mitochondrial Membrane Potential |
| MMPs | Matrix Metalloproteinases |
| MnSOD | Manganese Superoxide Dismutase |
| MOMP | Mitochondrial Outer Membrane Permeabilization |
| MPO | Myeloperoxidase |
| MPC | Mitochondrial Pyruvate Carrier |
| mPTP | Mitochondrial Permeability Transition Pore |
| MQC | Mitochondrial Quality Control |
| MRPL12 | Mitochondrial Ribosomal Protein L7/L12 |
| Mst1 | Mammalian Ste20-Like Kinase 1 |
| MTFP1 | Mitochondrial Fission Process 1 |
| mtDNA | Mitochondrial DNA |
| mtDNA-CN | Mitochondrial DNA Copy Number |
| mtROS | Mitochondrial Reactive Oxygen Species |
| Mzb1 | Marginal Zone B And B1 Cell-specific Protein |
| ND1 | NADH Dehydrogenase Subunit 1 |
| NF-κB | Nuclear Factor-κ b |
| NHE | Na+/H+ Exchanger |
| NLRP3 | NOD-Like Receptor Protein 3 |
| NMN | Nicotinamide Mononucleotide |
| NO | Nitric Oxide |
| NOX2 | NADPH Oxidase 2 |
| NRF1/2 | Nuclear Respiratory Factors 1/2 |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| NSmase | Neutral Sphingomyelinase |
| Oip5-as1 | Opa Interacting Protein 5 Antisense RNA 1 |
| OMM | Outer Mitochondrial Membrane |
| OPA1 | Optic Atrophy 1 |
| ox-LDL | Oxidized Low-Density Lipoprotein |
| OXPHOS | Oxidative Phosphorylation |
| p38 MAPK | p38 Mitogen-Activated Protein Kinase |
| PAD | Peripheral Arterial Disease |
| PFOS | Perfluorooctane Sulfonate |
| PFOSA | Perfluorooctane Sulfonamide |
| PGAM5 | Phosphoglycerate Mutase Family Member 5 |
| PGC-1α | Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α |
| PGJXD | Po-Ge-Jiu-Xin Decoction |
| PH-LHD | Pulmonary Hypertension due to Left Heart Disease |
| PHS | Pulmonary Hypertension Syndrome |
| PI3K | Phosphatidylinositol-3-Hydroxykinase |
| PINK1 | PTEN-Induced Kinase 1 |
| PKC | Protein Kinase Cepsilon |
| PM2.5 | Particulate Matter 2.5 |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PRRs | Pattern Recognition Receptors |
| PTSD | Post-Traumatic Stress Disorder |
| PUMA | p53 Upregulated Modulator of Apoptosis (also known as BBC3) |
| QSYQ | QiShenYiQi Pills/Dripping Pills |
| QTc | Corrected QT interval |
| Rap1 | Ras-Related Protein 1 |
| RHO/ROCK1/DRP1 | Ras Homolog Family Member A/Rho-Associated Coiled-Coil Containing Protein Kinase 1/Dynamin-Related Protein 1 pathway |
| RIPK3 | Receptor-Interacting Protein Kinase 3 |
| ROS | Reactive Oxygen Species |
| RYR | Ryanodine Receptor |
| S1PR2 | Sphingosine-1-Phosphate Receptor 2 |
| sGC | Soluble Guanylate Cyclase |
| SGLT2 | Sodium-Glucose Cotransporter-2 |
| SIRT1/2/3/5 | Sirtuin 1/2/3/5 |
| siRNA | Small Interfering RNA |
| SMCs | Smooth Muscle Cells |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| SOD | Superoxide Dismutase |
| SOD2 | Superoxide Dismutase 2 |
| SR | Sarcoplasmic Reticulum |
| SS-31 | Elamipretide (also known as Bendavia) |
| STING | Stimulator of Interferon Genes |
| TFAM | Mitochondrial Transcription Factor A |
| TLR4/9 | Toll-Like Receptor 4/9 |
| TNF-α | Tumor Necrosis Factor-alpha |
| tRNA | Transfer Ribonucleic Acid |
| TWAS | Transcriptome-Wide Association Studies |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VDAC | Voltage-Dependent Anion Channel |
| VDAC1 | Voltage-Dependent Anion Channel 1 |
| VSMCs | Vascular Smooth Muscle Cells |
| XYT | Xinyang Tablet |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiong, W.; Li, C.; Zhao, R.; Lu, H.; Song, S.; Zhou, Y.; Hu, Y.; Shi, B.; Ge, J. Hypoxia-Induced Signaling in the Cardiovascular System: Pathogenesis and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 431. [Google Scholar] [CrossRef]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease and Cancer. SAGE Open Med. 2020, 8, 2050312120965752. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Zhang, L.; Nepliouev, I.; Brian, L.; Huang, T.; Snow, K.P.; Schickling, B.M.; Hauser, E.R.; Miller, F.J.; Freedman, N.J.; et al. Drebrin Attenuates Atherosclerosis by Limiting Smooth Muscle Cell Transdifferentiation. Cardiovasc. Res. 2022, 118, 772–784. [Google Scholar] [CrossRef]
- Bornstein, R.; Gonzalez, B.; Johnson, S.C. Mitochondrial Pathways in Human Health and Aging. Mitochondrion 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Smeitink, J.; van den Heuvel, L.; DiMauro, S. The Genetics and Pathology of Oxidative Phosphorylation. Nat. Rev. Genet. 2001, 2, 342–352. [Google Scholar] [CrossRef]
- Szabo, I.; Zoratti, M. Mitochondrial Channels: Ion Fluxes and More. Physiol. Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef]
- Xu, M.; Wang, W.; Cheng, J.; Qu, H.; Xu, M.; Wang, L. Effects of Mitochondrial Dysfunction on Cellular Function: Role in Atherosclerosis. Biomed. Pharmacother. 2024, 174, 116587. [Google Scholar] [CrossRef]
- Qi, X.; Zhu, Z.; Wang, Y.; Wen, Z.; Jiang, Z.; Zhang, L.; Pang, Y.; Lu, J. Research Progress on the Relationship between Mitochondrial Function and Heart Failure: A Bibliometric Study from 2002 to 2021. Front. Mol. Biosci. 2022, 9, 1036364. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial Ros. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W., 2nd; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement from the American Heart Association. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Huynh, D.T.N.; Heo, K.S. Role of Mitochondrial Dynamics and Mitophagy of Vascular Smooth Muscle Cell Proliferation and Migration in Progression of Atherosclerosis. Arch. Pharm. Res. 2021, 44, 1051–1061. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a Therapeutic Target for Common Pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (Sglt2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Empagliflozin’s Fuel Hypothesis: Not So Soon. Cell Metab. 2016, 24, 200–202. [Google Scholar] [CrossRef]
- Gammage, P.A.; Viscomi, C.; Simard, M.L.; Costa, A.S.H.; Gaude, E.; Powell, C.A.; Van Haute, L.; McCann, B.J.; Rebelo-Guiomar, P.; Cerutti, R.; et al. Genome Editing in Mitochondria Corrects a Pathogenic Mtdna Mutation in Vivo. Nat. Med. 2018, 24, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.L.; Kuo, L.T.; Sung, F.C.; Yeh, C.C. Association between Polymorphisms of Antioxidant Gene (Mnsod, Cat, and Gpx1) and Risk of Coronary Artery Disease. BioMed Res. Int. 2018, 2018, 5086869. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial Dysfunction and Oxidative Stress in Aging and Cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, C.; Jin, Y.; Wu, O.; Chen, L.; Guo, Z.; Wang, X.; Chen, Q.; Kwan, K.Y.H.; Li, Y.M.; et al. Role of Oxidative Stress in Mitochondrial Dysfunction and Their Implications in Intervertebral Disc Degeneration: Mechanisms and Therapeutic Strategies. J. Orthop. Translat. 2024, 49, 181–206. [Google Scholar] [CrossRef]
- Shemiakova, T.; Ivanova, E.; Grechko, A.V.; Gerasimova, E.V.; Sobenin, I.A.; Orekhov, A.N. Mitochondrial Dysfunction and DNA Damage in the Context of Pathogenesis of Atherosclerosis. Biomedicines 2020, 8, 166. [Google Scholar] [CrossRef]
- Ashar, F.N.; Zhang, Y.; Longchamps, R.J.; Lane, J.; Moes, A.; Grove, M.L.; Mychaleckyj, J.C.; Taylor, K.D.; Coresh, J.; Rotter, J.I.; et al. Association of Mitochondrial DNA Copy Number with Cardiovascular Disease. JAMA Cardiol. 2017, 2, 1247–1255. [Google Scholar] [CrossRef]
- Xia, D.; Liu, Y.; Wu, P.; Wei, D. Current Advances of Mitochondrial Dysfunction and Cardiovascular Disease and Promising Therapeutic Strategies. Am. J. Pathol. 2023, 193, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial Control of Inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Sinyov, V.V.; Margarita, A.S.; Anastasia, I.R.; Elena, V.G.; Alexsandra, A.M.; Anton, Y.P.; Alexander, N.O.; Andrey, V.G.; Igor, A.S. Potential Use of Buccal Epithelium for Genetic Diagnosis of Atherosclerosis Using Mtdna Mutations. Vessel. Plus 2017, 1, 145–150. [Google Scholar] [CrossRef]
- Lee, W.E.; Genetzakis, E.; Figtree, G.A. Novel Strategies in the Early Detection and Treatment of Endothelial Cell-Specific Mitochondrial Dysfunction in Coronary Artery Disease. Antioxidants 2023, 12, 1359. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.; Sofiou, F.-I.; Lembessis, P.; Traikov, L.L.; Karela, N.-R.; Angouras, D.C.; Philippou, A. Mitochondrial Mutations in Cardiovascular Diseases: Preliminary Findings. Genes 2024, 15, 1442. [Google Scholar] [CrossRef]
- Tolstik, T.V.; Kirichenko, T.V.; Bogatyreva, A.I.; Markina, Y.V.; Kalmykov, V.A.; Markin, A.M. The Relationship between Mitochondrial Genome Mutations in Monocytes and the Development of Obesity and Coronary Heart Disease. Front. Biosci. 2024, 16, 6. [Google Scholar] [CrossRef]
- Tolstik, T.V.; Kirichenko, T.V.; Markin, A.M.; Bogatyreva, A.I.; Markina, Y.V.; Kiseleva, D.G.; Shaposhnikova, N.N.; Starodubova, A.V.; Orekhov, A.N. The Association of Tnf-Alpha Secretion and Mtdna Copy Number in Cd14(+) Monocytes of Patients with Obesity and CHD. Front. Mol. Biosci. 2024, 11, 1362955. [Google Scholar] [CrossRef]
- Lee, W.E.; Genetzakis, E.; Barsha, G.; Vescovi, J.; Mifsud, C.; Vernon, S.T.; Nguyen, T.V.; Gray, M.P.; Grieve, S.M.; Figtree, G.A. Expression of Myeloperoxidase in Patient-Derived Endothelial Colony-Forming Cells-Associations with Coronary Artery Disease and Mitochondrial Function. Biomolecules 2024, 14, 1308. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, Q.; Wu, J.; Zhou, C.; Liu, X.; Yue, J.; Chen, X.; Liu, J.; Zhang, Q.; Zhang, Y.; et al. A Detrimental Role of Endothelial S1pr2 in Cardiac Ischemia-Reperfusion Injury via Modulating Mitochondrial Dysfunction, Nlrp3 Inflammasome Activation, and Pyroptosis. Redox Biol. 2024, 75, 103244. [Google Scholar] [CrossRef]
- Li, Y.; Hu, P. Artesunate Inhibits Ros Production and Ameliorates Mitochondrial Damage through the Sirt1/Foxo3a/Mnsod Pathway to Alleviate Heart Failure. Int. J. Mol. Sci. 2024, 20, 2912. [Google Scholar]
- Ma, T.; Jiang, Y.; Chen, P.; Xiao, F.; Zhang, J.; Ma, Y.; Chen, T. Pfos and Pfosa Induce Oxidative Stress-Mediated Cardiac Defects in Zebrafish Via Pparγ and Ahr Pathways, Respectively. Sci. Total Environ. 2024, 951, 175716. [Google Scholar] [CrossRef]
- Bertozzi, G.; Ferrara, M.; Di Fazio, A.; Maiese, A.; Delogu, G.; Di Fazio, N.; Tortorella, V.; La Russa, R.; Fineschi, V. Oxidative Stress in Sepsis: A Focus on Cardiac Pathology. Int. J. Mol. Sci. 2024, 25, 2912. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, B.; Kurian, G.A. Mitigating Pm(2.5) Induced Myocardial Metal Deposition through Sodium Thiosulfate Resulted in Reduction of Cardiotoxicity and Physiological Recovery from Ischemia-Reperfusion via Mitochondrial Preservation. Environ. Toxicol. 2025, 40, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Ramezani-Aliakbari, K.; Sarihi, A.; Heshmati, A.; Shiri, E.; Nosrati, S.; Hashemi, S.P.; Bahrami, M.; Ramezani-Aliakbari, F. Protective Effects of Olive Oil against Cardiac Aging through Mitophagy and Apoptosis. Vet. Res. Forum 2025, 16, 27–33. [Google Scholar] [PubMed]
- Rudokas, M.W.; McKay, M.; Toksoy, Z.; Eisen, J.N.; Bögner, M.; Young, L.H.; Akar, F.G. Mitochondrial Network Remodeling of the Diabetic Heart: Implications to Ischemia Related Cardiac Dysfunction. Cardiovasc. Diabetol. 2024, 23, 261. [Google Scholar] [CrossRef]
- Liu, M.; Lv, J.; Pan, Z.; Wang, D.; Zhao, L.; Guo, X. Mitochondrial Dysfunction in Heart Failure and Its Therapeutic Implications. Front. Cardiovasc. Med. 2022, 9, 945142. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Dhalla, N.S. Status of Mitochondrial Oxidative Phosphorylation During the Development of Heart Failure. Antioxidants 2023, 12, 1941. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Rai, A.K.; Sanghvi, S.; Muthukumaran, N.S.; Chandrasekera, D.; Kadam, A.; Kishore, J.; Kyriazis, I.D.; Tomar, D.; Ponnalagu, D.; Shettigar, V.; et al. Role of Mitochondrial Ribosomal Protein L7/L12 (Mrpl12) in Diabetic Ischemic Heart Disease. Free Radic. Biol. Med. 2024, 222, 531–538. [Google Scholar] [CrossRef]
- Le, D.E.; Alkayed, N.J.; Cao, Z.; Chattergoon, N.N.; Garcia-Jaramillo, M.; Thornburg, K.; Kaul, S. Metabolomics of Repetitive Myocardial Stunning in Chronic Multivessel Coronary Artery Stenosis: Effect of Non-Selective and Selective Β1-Receptor Blockers. J. Physiol. 2024, 602, 3423–3448. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, A.M.; Fang, L.; Altamimi, T.R.; Jamieson, K.L.; Bassiouni, W.; Valencia, R.; Huang, A.; Wang, F.; Zhang, H.; Ahmed, M.; et al. Cardioprotective Effect of 19,20-Epoxydocosapentaenoic Acid (19,20-Edp) in Ischaemic Injury Involves Direct Activation of Mitochondrial Sirtuin 3. Cardiovasc. Res. 2025, 121, 267–282. [Google Scholar] [CrossRef]
- Yan, T.; Li, X.; Wang, X.; He, B.; Jia, Y.; Xiao, W. Salidroside Alleviates Myocardial Ischemia Reperfusion by Balancing Mitochondrial Homeostasis Via Nrf2. J. Food Biochem. 2024, 2024, 9971510. [Google Scholar] [CrossRef]
- de Carmo, J.M.; Omoto, A.C.M.; Hall, J.E.; Dai, X.; Ladnier, E.C.; Mouro, M.C.; Tosta, O.E.S.; Wang, Z.; Li, X.; da Silva, A.A. Parental Obesity Predisposes Male and Female Offspring to Exacerbated Cardiac Dysfunction and Increased Mortality after Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H1039–H1050. [Google Scholar] [CrossRef]
- Ruan, L.; Wang, Y.; Zhang, X.; Tomaszewski, A.; McNamara, J.T.; Li, R. Mitochondria-Associated Proteostasis. Annu. Rev. Biophys. 2020, 49, 41–67. [Google Scholar] [CrossRef]
- De Stefani, D.; Rizzuto, R.; Pozzan, T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annu. Rev. Biochem. 2016, 85, 161–192. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wen, Y.; Li, S.; Lu, X.; Xu, R.; Li, C. Endoplasmic Reticulum-Mitochondria Contacts: A Potential Therapy Target for Cardiovascular Remodeling-Associated Diseases. Front. Cell Dev. Biol. 2021, 9, 774989. [Google Scholar] [CrossRef]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial Calcium Overload Is a Key Determinant in Heart Failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Ramaccini, D.; Morciano, G.; Pedriali, G.; Kahsay, A.E.; Bouhamida, E.; Giorgi, C.; Wieckowski, M.R.; Pinton, P. Physiopathology of the Permeability Transition Pore: Molecular Mechanisms in Human Pathology. Biomolecules 2020, 10, 998. [Google Scholar] [CrossRef]
- Xu, H.X.; Cui, S.M.; Zhang, Y.M.; Ren, J. Mitochondrial Ca(2+) Regulation in the Etiology of Heart Failure: Physiological and Pathophysiological Implications. Acta Pharmacol. Sin. 2020, 41, 1301–1309. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, R.; Li, M.; Yu, Y.; Liang, Y.; Han, F.; Qin, S.; Chen, X.; Su, Y.; Ge, J. Mitochondrial Calcium Uniporter Inhibition Provides Cardioprotection in Pressure Overload-Induced Heart Failure through Autophagy Enhancement. Int. J. Cardiol. 2018, 271, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhao, L.; Clish, C.B.; Clapham, D.E. Letm1, the Mitochondrial Ca2+/H+ Antiporter, Is Essential for Normal Glucose Metabolism and Alters Brain Function in Wolf-Hirschhorn Syndrome. Proc. Natl. Acad. Sci. USA 2013, 110, E2249–E2254. [Google Scholar] [CrossRef]
- Wang, L.; Manson, J.E.; Sesso, H.D. Calcium Intake and Risk of Cardiovascular Disease: A Review of Prospective Studies and Randomized Clinical Trials. Am. J. Cardiovasc. Drugs 2012, 12, 105–116. [Google Scholar] [CrossRef]
- Pentti, K.; Tuppurainen, M.T.; Honkanen, R.; Sandini, L.; Kröger, H.; Alhava, E.; Saarikoski, S. Use of Calcium Supplements and the Risk of Coronary Heart Disease in 52-62-Year-Old Women: The Kuopio Osteoporosis Risk Factor and Prevention Study. Maturitas 2009, 63, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Avenell, A.; Baron, J.A.; Grey, A.; MacLennan, G.S.; Gamble, G.D.; Reid, I.R. Effect of Calcium Supplements on Risk of Myocardial Infarction and Cardiovascular Events: Meta-Analysis. BMJ 2010, 341, c3691. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, S.; Peng, Y.; Shi, Y.; Tsauo, J.Y.; Chen, M. Serum Calcium Levels Correlates with Coronary Artery Disease Outcomes. Open Med. 2020, 15, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Sripusanapan, A.; Piriyakulthorn, C.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Ivabradine Ameliorates Doxorubicin-Induced Cardiotoxicity through Improving Mitochondrial Function and Cardiac Calcium Homeostasis. Biochem. Pharmacol. 2025, 236, 116881. [Google Scholar] [CrossRef]
- Xie, A.; Kang, G.-J.; Kim, E.J.; Liu, H.; Feng, F.; Dudley, S.C., Jr. C-Src Is Responsible for Mitochondria-Mediated Arrhythmic Risk in Ischemic Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2024, 17, e013054. [Google Scholar] [CrossRef]
- Lahiri, S.K.; Lu, J.; Aguilar-Sanchez, Y.; Li, H.; Moreira, L.M.; Hulsurkar, M.M.; Mendoza, A.; Paredes, M.R.T.; Navarro-Garcia, J.A.; Munivez, E.; et al. Targeting Calpain-2-Mediated Junctophilin-2 Cleavage Delays Heart Failure Progression Following Myocardial Infarction. J. Mol. Cell Cardiol. 2024, 194, 85–95. [Google Scholar] [CrossRef]
- Hohendanner, F.; Boegner, M.; Huettemeister, J.; Zhang, K.; Dreysse, S.; Knosalla, C.; Falk, V.; Schoenrath, F.; Just, I.A.; Stawowy, P. Microvascular Dysfunction in Heart Transplantation Is Associated with Altered Cardiomyocyte Mitochondrial Structure and Unimpaired Excitation-Contraction Coupling. PLoS ONE 2024, 19, e0303540. [Google Scholar] [CrossRef]
- Popov, L.D. Mitochondrial Biogenesis: An Update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Yokokawa, T.; Kido, K.; Suga, T.; Isaka, T.; Hayashi, T.; Fujita, S. Exercise-Induced Mitochondrial Biogenesis Coincides with the Expression of Mitochondrial Translation Factors in Murine Skeletal Muscle. Physiol. Rep. 2018, 6, e13893. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. Amp-Activated Protein Kinase (Ampk) Action in Skeletal Muscle via Direct Phosphorylation of Pgc-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Guarente, L. Sirtuins in Aging and Disease. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 483–488. [Google Scholar] [CrossRef]
- Song, Y.; Li, S.; Geng, W.; Luo, R.; Liu, W.; Tu, J.; Wang, K.; Kang, L.; Yin, H.; Wu, X.; et al. Sirtuin 3-Dependent Mitochondrial Redox Homeostasis Protects against Ages-Induced Intervertebral Disc Degeneration. Redox Biol. 2018, 19, 339–353. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Wang, J.; Wu, F.; Chen, Y.; Zhang, H.; Guo, Y.; Lin, Y.; Li, L.; Yu, X.; et al. Spermidine Alleviates Cardiac Aging by Improving Mitochondrial Biogenesis and Function. Aging 2020, 12, 650–671. [Google Scholar] [CrossRef]
- Shi, H.; Sun, M.; Wang, S.; He, F.; Yang, R.; Li, Z.; Chen, W.; Wang, F. Jiawei Dachaihu Decoction Protects against Mitochondrial Dysfunction in Atherosclerosis (as) Mice with Chronic Unpredictable Mild Stress (Cums) Via Sirt1/Pgc-1α/Tfam/Lon Signaling Pathway. J. Ethnopharmacol. 2024, 330, 118150. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Prudent, J.; McBride, H.M. Mitochondrial Dynamics: Er Actin Tightens the Drp1 Noose. Curr. Biol. 2016, 26, R207–R209. [Google Scholar] [CrossRef]
- Lee, J.E.; Westrate, L.M.; Wu, H.; Page, C.; Voeltz, G.K. Multiple Dynamin Family Members Collaborate to Drive Mitochondrial Division. Nature 2016, 540, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, A.; Cipolat, S.; Chen, Y.; Dorn, G.W., 2nd; Scorrano, L. Mitochondrial Fusion Directs Cardiomyocyte Differentiation Via Calcineurin and Notch Signaling. Science 2013, 342, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Leboucher, G.P.; Tsai, Y.C.; Yang, M.; Shaw, K.C.; Zhou, M.; Veenstra, T.D.; Glickman, M.H.; Weissman, A.M. Stress-Induced Phosphorylation and Proteasomal Degradation of Mitofusin 2 Facilitates Mitochondrial Fragmentation and Apoptosis. Mol. Cell 2012, 47, 547–557. [Google Scholar] [CrossRef]
- Chung, K.K.; Thomas, B.; Li, X.; Pletnikova, O.; Troncoso, J.C.; Marsh, L.; Dawson, V.L.; Dawson, T.M. S-Nitrosylation of Parkin Regulates Ubiquitination and Compromises Parkin’s Protective Function. Science 2004, 304, 1328–1331. [Google Scholar] [CrossRef]
- Qiu, X.; Feng, Y. Echinacoside Activates Nrf2/Pparγ Signaling Pathway to Modulate Mitochondrial Fusion-Fission Balance to Ameliorate Ox-Ldl-Induced Dysfunction of Coronary Artery Endothelial Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 9767–9776. [Google Scholar] [CrossRef]
- Piamsiri, C.; Maneechote, C.; Jinawong, K.; Arunsak, B.; Chunchai, T.; Nawara, W.; Kerdphoo, S.; Chattipakorn, S.C.; Chattipakorn, N. Chronic Mitochondrial Dynamic-Targeted Therapy Alleviates Left Ventricular Dysfunction by Reducing Multiple Programmed Cell Death in Post-Myocardial Infarction Rats. Eur. J. Pharmacol. 2024, 977, 176736. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Ye, X.; Jiang, M.; Wu, X.; Tang, Z.; Hu, L.; Zhang, H.; Li, Y.; Pan, J. Disturbance of Mitochondrial Dynamics in Myocardium of Broilers with Pulmonary Hypertension Syndrome. Br. Poult. Sci. 2024, 65, 154–164. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Q.; Chen, T.; Zhou, Z.; Li, S.; Li, Z.; Zhang, D. The Possible Association of Mitochondrial Fusion and Fission in Copper Deficiency-Induced Oxidative Damage and Mitochondrial Dysfunction of the Heart. J. Trace Elem. Med. Biol. 2024, 85, 127483. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Hou, L.; Liu, B.-Y.; Fan, Y.-M.; Zhang, Y.; Wang, F.; Jia, K.; Li, X.; Tang, Z.; et al. Proteomic Analysis Reveals Qishenyiqi Pills Ameliorates Ischemia-Induced Heart Failure through Inhibition of Mitochondrial Fission. Phytomedicine 2025, 138, 156435. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.X.; Fan, X.X.; Liu, M.X.; Zhang, X.Z.; Cao, L.; Wang, Z.Z.; Tian, J.Z.; Zhang, Y.W.; Xiao, W. Longxuetongluo Capsule Alleviate Ischemia/Reperfusion Induced Cardiomyocyte Apoptosis through Modulating Oxidative Stress and Mitochondrial Dysfunction. Phytomedicine 2024, 134, 155993. [Google Scholar] [CrossRef]
- Oskuye, Z.Z.; Mehri, K.; Mokhtari, B.; Bafadam, S.; Nemati, S.; Badalzadeh, R. Cardioprotective Effect of Antioxidant Combination Therapy: A Highlight on Mitoq Plus Alpha-Lipoic Acid Beneficial Impact on Myocardial Ischemia-Reperfusion Injury in Aged Rats. Heliyon 2024, 10, e28158. [Google Scholar] [CrossRef]
- Niu, X.; Zhang, J.; Hu, S.; Dang, W.; Wang, K.; Bai, M. Lncrna Oip5-As1 Inhibits Excessive Mitochondrial Fission in Myocardial Ischemia/Reperfusion Injury by Modulating Drp1 Phosphorylation. Cell Mol. Biol. Lett. 2024, 29, 72. [Google Scholar] [CrossRef]
- Lin, L.; Xu, H.; Yao, Z.; Zeng, X.; Kang, L.; Li, Y.; Zhou, G.; Wang, S.; Zhang, Y.; Cheng, D.; et al. Jin-Xin-Kang Alleviates Heart Failure by Mitigating Mitochondrial Dysfunction through the Calcineurin/Dynamin-Related Protein 1 Signaling Pathway. J. Ethnopharmacol. 2024, 335, 118685. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, J.; Huan, L.; Sheng, S.; Xu, F. Mitophagy in Atherosclerosis: From Mechanism to Therapy. Front. Immunol. 2023, 14, 1165507. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, Q.; Huang, Y.; Liu, J.; Wang, Y.; Guan, X.; Wu, Q.; Liu, Z.; Liu, R. Quercetin Inhibits Necroptosis in Cardiomyocytes after Ischemia-Reperfusion Via DNA-Pkcs-Sirt5-Orchestrated Mitochondrial Quality Control. Phytother. Res. 2024, 38, 2496–2517. [Google Scholar] [CrossRef]
- Yan, L.; Yang, H.; Li, Y.; Duan, H.; Wu, J.; Qian, P.; Li, B.; Wang, S. Regulator of Calcineurin 1-1l Protects Cardiomyocytes against Hypoxia-Induced Apoptosis Via Mitophagy. J. Cardiovasc. Pharmacol. 2014, 64, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Choi, S.E.; Koh, H.C. Pgam5 Regulates Pink1/Parkin-Mediated Mitophagy Via Drp1 in Cccp-Induced Mitochondrial Dysfunction. Toxicol. Lett. 2018, 284, 120–128. [Google Scholar] [CrossRef]
- Xue, J.; Ren, H.; Zhang, Q.; Gu, J.; Xu, Q.; Sun, J.; Zhang, L.; Zhou, M.S. Puerarin Attenuates Myocardial Ischemic Injury and Endoplasmic Reticulum Stress by Upregulating the Mzb1 Signal Pathway. Front. Pharmacol. 2024, 15, 1442831. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Li, C.; Wang, L.; Zhou, B.; Guo, Z.; Huang, Y.; Deng, B.; Ouyang, Y.; Qiu, J.; Chang, X.; et al. High-Throughput Screening of an Fda-Approved Compound Library Reveals a Novel Gas6 Receptor Agonist for Therapeutic Intervention in Septic Myocardial and Microvascular Injury Via Modulation of Danger-Associated Molecular Patterns. Int. J. Biol. Sci. 2024, 20, 6222–6240. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Wang, K.; Guo, L.; Zou, C. Humanin Inhibits Lymphatic Endothelial Cells Dysfunction to Alleviate Myocardial Infarction-Reperfusion Injury Via Bnip3-Mediated Mitophagy. Free Radic. Res. 2024, 58, 180–193. [Google Scholar] [CrossRef]
- Lee, T.-L.; Shen, W.-C.; Chen, Y.-C.; Lai, T.-C.; Lin, S.-R.; Lin, S.-W.; Yu, I.-S.; Yeh, Y.-H.; Li, T.-K.; Lee, I.-T.; et al. Mir221- and Mir222-Enriched Adsc-Exosomes Mitigate Pm Exposure-Exacerbated Cardiac Ischemia-Reperfusion Injury through the Modulation of the Bnip3-Map1lc3b-Bbc3/Puma Pathway. Autophagy 2025, 21, 374–393. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Dong, C.; Miao, K.; Jiang, B.; Zhou, D.; Dong, K.; Wang, Y.; Zhang, Z. Po-Ge-Jiu-Xin Decoction Alleviate Sepsis-Induced Cardiomyopathy Via Regulating Phosphatase and Tensin Homolog-Induced Putative Kinase 1 /Parkin-Mediated Mitophagy. J. Ethnopharmacol. 2025, 337, 118952. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhuang, H.W.; Wen, R.J.; Huang, Y.S.; Liang, B.X.; Li, H.; Xian, S.X.; Li, C.; Wang, L.J.; Wang, J.Y. Xinyang Tablet Alleviated Cardiac Dysfunction in a Cardiac Pressure Overload Model by Regulating the Receptor-Interacting Serum/Three-Protein Kinase 3/Fun14 Domain Containing 1-Mediated Mitochondrial Unfolded Protein Response and Mitophagy. J. Ethnopharmacol. 2024, 330, 118152. [Google Scholar] [CrossRef]
- Peng, W.; Cai, G.; Xia, Y.; Chen, J.; Wu, P.; Wang, Z.; Li, G.; Wei, D. Mitochondrial Dysfunction in Atherosclerosis. DNA Cell Biol. 2019, 38, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Zhelankin, A.V.; Mitrofanov, K.Y.; Sinyov, V.V.; Sazonova, M.A.; Postnov, A.Y.; Orekhov, A.N. Mutations of Mitochondrial DNA in Atherosclerosis and Atherosclerosis-Related Diseases. Curr. Pharm. Des. 2015, 21, 1158–1163. [Google Scholar] [CrossRef]
- Barthelmes, J.; Nägele, M.P.; Ludovici, V.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Endothelial Dysfunction in Cardiovascular Disease and Flammer Syndrome-Similarities and Differences. EPMA J. 2017, 8, 99–109. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam Cells in Atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef]
- Xue, W.; Deng, L. Ep300 Improves Endothelial Injury and Mitochondrial Dysfunction in Coronary Artery Disease by Regulating Histone Acetylation of Socs1 Promoter Via Inhibiting Jak/Stat Pathway. Cytokine 2024, 176, 156507. [Google Scholar] [CrossRef] [PubMed]
- SenthilKumar, G.; Katunaric, B.; Zirgibel, Z.; Lindemer, B.; Jaramillo-Torres, M.J.; Bordas-Murphy, H.; Schulz, M.E.; Pearson, P.J.; Freed, J.K. Necessary Role of Ceramides in the Human Microvascular Endothelium During Health and Disease. Circ. Res. 2024, 134, 81–96. [Google Scholar] [CrossRef]
- Rhee, M.; Lee, J.; Lee, E.Y.; Yoon, K.-H.; Lee, S.H. Lipid Variability Induces Endothelial Dysfunction by Increasing Inflammation and Oxidative Stress. Endocrinol. Metab. 2024, 39, 511–520. [Google Scholar] [CrossRef]
- He, T.; Pu, J.; Ge, H.; Liu, T.; Lv, X.; Zhang, Y.; Cao, J.; Yu, H.; Lu, Z.; Du, F. Elevated Circulating Lncrna Norad Fosters Endothelial Cell Growth and Averts Ferroptosis by Modulating the Mir-106a/Ccnd1 Axis in Cad Patients. Sci. Rep. 2024, 14, 24223. [Google Scholar] [CrossRef]
- Gao, L.; Ramirez, F.J.; Cabrera, J.T.O.; Varghese, M.V.; Watanabe, M.; Tsuji-Hosokawa, A.; Zheng, Q.; Yang, M.; Razan, R.; Kempf, C.L.; et al. Enampt Is a Novel Therapeutic Target for Mitigation of Coronary Microvascular Disease in Type 2 Diabetes. Diabetologia 2024, 67, 1998–2011. [Google Scholar] [CrossRef]
- Becker, P.H.; Thérond, P.; Gaignard, P. Targeting Mitochondrial Function in Macrophages: A Novel Treatment Strategy for Atherosclerotic Cardiovascular Disease? Pharmacol. Ther. 2023, 247, 108441. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial Dysfunction in Vascular Endothelial Cells and Its Role in Atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, G.; Conte, S.; Cimmino, G.; Maiorano, P.; Morrione, A.; Giordano, A. Mitochondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int. J. Mol. Sci. 2023, 24, 1086. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A Review on Coronary Artery Disease, Its Risk Factors, and Therapeutics. J. Cell Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef]
- Yu, E.P.K.; Reinhold, J.; Yu, H.; Starks, L.; Uryga, A.K.; Foote, K.; Finigan, A.; Figg, N.; Pung, Y.F.; Logan, A.; et al. Mitochondrial Respiration Is Reduced in Atherosclerosis, Promoting Necrotic Core Formation and Reducing Relative Fibrous Cap Thickness. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2322–2332. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Yu, E.; Calvert, P.A.; Mercer, J.R.; Harrison, J.; Baker, L.; Figg, N.L.; Kumar, S.; Wang, J.C.; Hurst, L.A.; Obaid, D.R.; et al. Mitochondrial DNA Damage Can Promote Atherosclerosis Independently of Reactive Oxygen Species through Effects on Smooth Muscle Cells and Monocytes and Correlates with Higher-Risk Plaques in Humans. Circulation 2013, 128, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, Z.; Dong, Z.; Wang, C.; Cao, Q.; Fan, F.; Zhao, J.; Liu, X.; Yuan, M.; Sun, X.; et al. Aldehyde Dehydrogenase 2 Deficiency Promotes Atherosclerotic Plaque Instability through Accelerating Mitochondrial Ros-Mediated Vascular Smooth Muscle Cell Senescence. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1782–1792. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Stevenson, M.D.; Alahari, S.; Pan, H.; Wickline, S.A.; Madamanchi, N.R.; Runge, M.S. Attenuated Superoxide Dismutase 2 Activity Induces Atherosclerotic Plaque Instability During Aging in Hyperlipidemic Mice. J. Am. Heart Assoc. 2017, 6, 006775. [Google Scholar] [CrossRef]
- Ma, S.; Xie, X.; Yuan, R.; Xin, Q.; Miao, Y.; Leng, S.X.; Chen, K.; Cong, W. Vascular Aging and Atherosclerosis: A Perspective on Aging. Aging Dis. 2024, 16, 33–48. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. From Mitochondria to Atherosclerosis: The Inflammation Path. Biomedicines 2021, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A Role for Mitochondria in Nlrp3 Inflammasome Activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Coppi, L.; Ligorio, S.; Mitro, N.; Caruso, D.; De Fabiani, E.; Crestani, M. Pgc1s and Beyond: Disentangling the Complex Regulation of Mitochondrial and Cellular Metabolism. Int. J. Mol. Sci. 2021, 22, 6913. [Google Scholar] [CrossRef]
- Kadlec, A.O.; Chabowski, D.S.; Ait-Aissa, K.; Gutterman, D.D. Role of Pgc-1α in Vascular Regulation: Implications for Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1467–1474. [Google Scholar] [CrossRef]
- Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Patra, S.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Singh, A.; Patil, S.; Dhiman, R.; et al. Mitochondrial Dysfunction as a Driver of Nlrp3 Inflammasome Activation and Its Modulation through Mitophagy for Potential Therapeutics. Int. J. Biochem. Cell Biol. 2021, 136, 106013. [Google Scholar] [CrossRef]
- Swiader, A.; Nahapetyan, H.; Faccini, J.; D’aNgelo, R.; Mucher, E.; Elbaz, M.; Boya, P.; Vindis, C. Mitophagy Acts as a Safeguard Mechanism against Human Vascular Smooth Muscle Cell Apoptosis Induced by Atherogenic Lipids. Oncotarget 2016, 7, 28821–28835. [Google Scholar] [CrossRef]
- Nahapetyan, H.; Moulis, M.; Grousset, E.; Faccini, J.; Grazide, M.H.; Mucher, E.; Elbaz, M.; Martinet, W.; Vindis, C. Altered Mitochondrial Quality Control in Atg7-Deficient Vsmcs Promotes Enhanced Apoptosis and Is Linked to Unstable Atherosclerotic Plaque Phenotype. Cell Death Dis. 2019, 10, 119. [Google Scholar] [CrossRef]
- Alfehaid, L.S.; Farah, S.; Omer, A.; Weber, B.N.; Alkhezi, O.; Tawfik, Y.M.K.; Shah, A.M.; Libby, P.; Buckley, L.F. Drug-Drug Interactions and the Clinical Tolerability of Colchicine among Patients with COVID-19: A Secondary Analysis of the Colcorona Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2431309. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; A Bax, W.; A Budgeon, C.; Tijssen, J.G.P.; Mosterd, A.; Cornel, J.H.; et al. The Effect of Low-Dose Colchicine in Patients with Stable Coronary Artery Disease: The Lodoco2 Trial Rationale, Design, and Baseline Characteristics. Am. Heart J. 2019, 218, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-Atp Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015, 95, 1111–1155. [Google Scholar] [CrossRef]
- Webster, K.A. Mitochondrial Death Channels. Am. Sci. 2009, 97, 384–391. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Schulz, R.; Girao, H.; Kwak, B.R.; De Stefani, D.; Rizzuto, R.; Bernardi, P.; Di Lisa, F. Mitochondrial Ion Channels as Targets for Cardioprotection. J. Cell Mol. Med. 2020, 24, 7102–7114. [Google Scholar] [CrossRef]
- Ramachandra, C.J.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.-H.; Hausenloy, D.J. Mitochondria in Acute Myocardial Infarction and Cardioprotection. eBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef]
- Whelan, R.S.; Konstantinidis, K.; Wei, A.C.; Chen, Y.; Reyna, D.E.; Jha, S.; Yang, Y.; Calvert, J.W.; Lindsten, T.; Thompson, C.B.; et al. Bax Regulates Primary Necrosis through Mitochondrial Dynamics. Proc. Natl. Acad. Sci. USA 2012, 109, 6566–6571. [Google Scholar] [CrossRef]
- Whelan, R.S.; Kaplinskiy, V.; Kitsis, R.N. Cell Death in the Pathogenesis of Heart Disease: Mechanisms and Significance. Annu. Rev. Physiol. 2010, 72, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.A. Programmed Death as a Therapeutic Target to Reduce Myocardial Infarction. Trends Pharmacol. Sci. 2007, 28, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M. The Pathobiology of Acute Coronary Syndromes: Clinical Implications and Central Role of the Mitochondria. Tex. Heart Inst. J. 2013, 40, 221–228. [Google Scholar]
- Kwong, J.Q.; Molkentin, J.D. Physiological and Pathological Roles of the Mitochondrial Permeability Transition Pore in the Heart. Cell Metab. 2015, 21, 206–214. [Google Scholar] [CrossRef]
- Xu, S.; Wang, P.; Zhang, H.; Gong, G.; Cortes, N.G.; Zhu, W.; Yoon, Y.; Tian, R.; Wang, W. Camkii Induces Permeability Transition through Drp1 Phosphorylation During Chronic Β-Ar Stimulation. Nat. Commun. 2016, 7, 13189. [Google Scholar] [CrossRef]
- Sharp, W.W.; Fang, Y.H.; Han, M.; Zhang, H.J.; Hong, Z.; Banathy, A.; Morrow, E.; Ryan, J.J.; Archer, S.L. Dynamin-Related Protein 1 (Drp1)-Mediated Diastolic Dysfunction in Myocardial Ischemia-Reperfusion Injury: Therapeutic Benefits of Drp1 Inhibition to Reduce Mitochondrial Fission. FASEB J. 2014, 28, 316–326. [Google Scholar] [CrossRef]
- Tsushima, K.; Bugger, H.; Wende, A.R.; Soto, J.; Jenson, G.A.; Tor, A.R.; McGlauflin, R.; Kenny, H.C.; Zhang, Y.; Souvenir, R.; et al. Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of Akap121, Drp1, and Opa1 That Promote Mitochondrial Fission. Circ. Res. 2018, 122, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Toan, S. Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules 2020, 10, 85. [Google Scholar] [CrossRef]
- Jin, S.M.; Lazarou, M.; Wang, C.; Kane, L.A.; Narendra, D.P.; Youle, R.J. Mitochondrial Membrane Potential Regulates Pink1 Import and Proteolytic Destabilization by Parl. J. Cell Biol. 2010, 191, 933–942. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, P.; Wang, J.; Zhu, H.; Ren, J.; Chen, Y. Pathogenesis of Cardiac Ischemia Reperfusion Injury Is Associated with Ck2α-Disturbed Mitochondrial Homeostasis Via Suppression of Fundc1-Related Mitophagy. Cell Death Differ. 2018, 25, 1080–1093. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Wang, Y.; Qian, H.; Zhu, C.; Dong, H.; Hao, C.; Zhang, Y.; Ji, Z.; Li, X.; et al. Cardiac Fibroblast-Derived Mitochondria-Enriched Sevs Regulate Tissue Inflammation and Ventricular Remodeling Post-Myocardial Infarction through Nlrp3 Pathway. Pharmacol. Res. 2025, 214, 107676. [Google Scholar] [CrossRef] [PubMed]
- Patrushev, M.; Kasymov, V.; Patrusheva, V.; Ushakova, T.; Gogvadze, V.; Gaziev, A. Mitochondrial Permeability Transition Triggers the Release of Mtdna Fragments. Cell Mol. Life Sci. 2004, 61, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.; Shteinfer-Kuzmine, A.; Wang, K.; Zhu, J.; Yoon, H.E.; Wang, X.; Kerkhofs, M.; et al. Vdac Oligomers Form Mitochondrial Pores to Release Mtdna Fragments and Promote Lupus-Like Disease. Science 2019, 366, 1531–1536. [Google Scholar] [CrossRef]
- Riley, J.S.; Quarato, G.; Cloix, C.; Lopez, J.; O’Prey, J.; Pearson, M.; Chapman, J.; Sesaki, H.; Carlin, L.M.; Passos, J.F.; et al. Mitochondrial Inner Membrane Permeabilisation Enables Mtdna Release During Apoptosis. EMBO J. 2018, 37, e99238. [Google Scholar] [CrossRef]
- White, M.J.; McArthur, K.; Metcalf, D.; Lane, R.M.; Cambier, J.C.; Herold, M.J.; van Delft, M.F.; Bedoui, S.; Lessene, G.; Ritchie, M.E.; et al. Apoptotic Caspases Suppress Mtdna-Induced Sting-Mediated Type I Ifn Production. Cell 2014, 159, 1549–1562. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Ong, S.-B.; Yellon, D.M. The Mitochondrial Permeability Transition Pore as a Target for Preconditioning and Postconditioning. Basic. Res. Cardiol. 2009, 104, 189–202. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Duchen, M.R.; Yellon, D.M. Inhibiting Mitochondrial Permeability Transition Pore Opening at Reperfusion Protects against Ischaemia-Reperfusion Injury. Cardiovasc. Res. 2003, 60, 617–625. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Correa, F.; Zúñiga-Muñoz, A.M.; José-Rodríguez, A.; Castañeda-Gómez, P.; Mejía-Díaz, E. L-Theanine Abates Oxidative Stress and Mitochondrial Dysfunction in Myocardial Ischemia-Reperfusion Injury by Positively Regulating the Antioxidant Response. Toxicol. Appl. Pharmacol. 2024, 486, 116940. [Google Scholar] [CrossRef] [PubMed]
- Colareda, G.A.; Díaz, R.G.; Lojano-Gutierrez, L.C.; Matera, S.I.; Córdoba, O.L.; Flores, M.L.; Consolini, A.E. Cardioprotective and Antispasmodic Effects of Humulus Lupulus (Hops) in an Oral Subacute Treatment in Rats. J. Biol. Act. Prod. Nat. 2024, 14, 463–480. [Google Scholar] [CrossRef]
- Qu, F.X.; Guo, X.; Liu, X.J.; Zhang, S.W.; Xin, Y.; Li, J.Y.; Wang, R.; Xu, C.J.; Li, H.Y.; Lu, C.H. Treatment with a Combination of Myricitrin and Exercise Alleviates Myocardial Infarction in Rats Via Suppressing Nrf2/Ho-1 Antioxidant Pathway. Arch. Biochem. Biophys. 2024, 761, 110153. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Shao, C.; Zhou, H.; Yu, L.; Bao, Y.; Zhao, Y.; Yang, J.; Wan, H. Exploring the Mechanism of Salvianolic Acid B against Myocardial Ischemia-Reperfusion Injury Based on Network Pharmacology. Pharmaceuticals 2024, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Payne, F.M.; Dabb, A.R.; Harrison, J.C.; Sammut, I.A. Inhibitors of Nlrp3 Inflammasome Formation: A Cardioprotective Role for the Gasotransmitters Carbon Monoxide, Nitric Oxide, and Hydrogen Sulphide in Acute Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 9247. [Google Scholar] [CrossRef]
- Golla, M.S.G.; Shams, P. Heart Failure with Preserved Ejection Fraction (Hfpef). In StatPearls; Treasure Island (FL) with ineligible companies. Disclosure: Pirbhat Shams declares no relevant financial relationships with ineligible companies; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kemp, C.D.; Conte, J.V. The Pathophysiology of Heart Failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial Dysfunction in Pathophysiology of Heart Failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Sheeran, F.L.; Pepe, S. Mitochondrial Bioenergetics and Dysfunction in Failing Heart. Adv. Exp. Med. Biol. 2017, 982, 65–80. [Google Scholar]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Glatz, J.F.; Nabben, M.; Young, M.E.; Schulze, P.C.; Taegtmeyer, H.; Luiken, J.J. Re-Balancing Cellular Energy Substrate Metabolism to Mend the Failing Heart. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165579. [Google Scholar] [CrossRef] [PubMed]
- Abozguia, K.; Clarke, K.; Lee, L.; Frenneaux, M. Modification of Myocardial Substrate Use as a Therapy for Heart Failure. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 490–498. [Google Scholar] [CrossRef]
- Gupte, A.A.; Hamilton, D.J. Mitochondrial Function in Non-Ischemic Heart Failure. Adv. Exp. Med. Biol. 2017, 982, 113–126. [Google Scholar]
- McCommis, K.S.; Kovacs, A.; Weinheimer, C.J.; Shew, T.M.; Koves, T.R.; Ilkayeva, O.R.; Kamm, D.R.; Pyles, K.D.; King, M.T.; Veech, R.L.; et al. Nutritional Modulation of Heart Failure in Mitochondrial Pyruvate Carrier-Deficient Mice. Nat. Metab. 2020, 2, 1232–1247. [Google Scholar] [CrossRef]
- Cluntun, A.A.; Badolia, R.; Lettlova, S.; Parnell, K.M.; Shankar, T.S.; Diakos, N.A.; Olson, K.A.; Taleb, I.; Tatum, S.M.; Berg, J.A.; et al. The Pyruvate-Lactate Axis Modulates Cardiac Hypertrophy and Heart Failure. Cell Metab. 2021, 33, 629–648.e10. [Google Scholar] [CrossRef]
- Fernandez-Caggiano, M.; Kamynina, A.; Francois, A.A.; Prysyazhna, O.; Eykyn, T.R.; Krasemann, S.; Crespo-Leiro, M.G.; Vieites, M.G.; Bianchi, K.; Morales, V.; et al. Mitochondrial Pyruvate Carrier Abundance Mediates Pathological Cardiac Hypertrophy. Nat. Metab. 2020, 2, 1223–1231. [Google Scholar] [CrossRef]
- Kumar, A.A.; Kelly, D.P.; Chirinos, J.A. Mitochondrial Dysfunction in Heart Failure with Preserved Ejection Fraction. Circulation 2019, 139, 1435–1450. [Google Scholar] [CrossRef]
- Tatekoshi, Y.; Mahmoodzadeh, A.; Shapiro, J.S.; Liu, M.; Bianco, G.M.; Tatekoshi, A.; Camp, S.D.; De Jesus, A.; Koleini, N.; De La Torre, S.; et al. Protein O-Glcnacylation and Hexokinase Mitochondrial Dissociation Drive Heart Failure with Preserved Ejection Fraction. Cell Metab. 2025, 37, 1584–1600.e10. [Google Scholar] [CrossRef]
- Bhattarai, N.; Scott, I. In the Heart and Beyond: Mitochondrial Dysfunction in Heart Failure with Preserved Ejection Fraction (Hfpef). Curr. Opin. Pharmacol. 2024, 76, 102461. [Google Scholar] [CrossRef]
- Scandalis, L.; Kitzman, D.W.; Nicklas, B.J.; Lyles, M.; Brubaker, P.; Nelson, M.B.; Gordon, M.; Stone, J.; Bergstrom, J.; Neufer, P.D.; et al. Skeletal Muscle Mitochondrial Respiration and Exercise Intolerance in Patients with Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2023, 8, 575–584. [Google Scholar] [CrossRef]
- Anderson, M.; Parrott, C.F.; Haykowsky, M.J.; Brubaker, P.H.; Ye, F. Skeletal Muscle Abnormalities in Heart Failure with Preserved Ejection Fraction. Heart Fail. Rev. 2023, 28, 157–168. [Google Scholar]
- Guo, Y.; Wen, J.; He, A.; Qu, C.; Peng, Y.; Luo, S.; Wang, X. Inos Contributes to Heart Failure with Preserved Ejection Fraction through Mitochondrial Dysfunction and Akt S-Nitrosylation. J. Adv. Res. 2023, 43, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.; Hughie, H.H.; Lucchesi, P.A. Regulation of Hypertrophic and Apoptotic Signaling Pathways by Reactive Oxygen Species in Cardiac Myocytes. Antioxid. Redox Signal 2003, 5, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.-M.; Papageorgiou, N.; Antoniades, C.; Siasos, G.; Latsios, G.; Tsiamis, E.; Stefanadis, C. Matrix Metallopropteinases in Heart Failure. Curr. Top. Med. Chem. 2012, 12, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Coker, M.L.; Thomas, C.V.; Walker, J.D.; Mukherjee, R.; Hebbar, L. Time-Dependent Changes in Matrix Metalloproteinase Activity and Expression During the Progression of Congestive Heart Failure: Relation to Ventricular and Myocyte Function. Circ. Res. 1998, 82, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N.; Gupta, R.C.; Singh-Gupta, V.; Zhang, K.; Lanfear, D.E. Abnormalities of Mitochondrial Dynamics in the Failing Heart: Normalization Following Long-Term Therapy with Elamipretide. Cardiovasc. Drugs Ther. 2018, 32, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Givvimani, S.; Pushpakumar, S.; Veeranki, S.; Tyagi, S.C. Dysregulation of Mfn2 and Drp-1 Proteins in Heart Failure. Can. J. Physiol. Pharmacol. 2014, 92, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Caron, C.; Bertolin, G. Cristae Shaping and Dynamics in Mitochondrial Function. J. Cell Sci. 2024, 137, jcs260986. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Tran, A.; Lu, X.; Tomilov, A.A.; Davies, V.; Cortopassi, G.; Chiamvimonvat, N.; Bers, D.M.; Votruba, M.; et al. Opa1 Mutation and Late-Onset Cardiomyopathy: Mitochondrial Dysfunction and Mtdna Instability. J. Am. Heart Assoc. 2012, 1, e003012. [Google Scholar] [CrossRef]
- Kondadi, A.K.; Anand, R.; Hänsch, S.; Urbach, J.; Zobel, T.; Wolf, D.M.; Segawa, M.; Liesa, M.; Shirihai, O.S.; Weidtkamp-Peters, S.; et al. Cristae Undergo Continuous Cycles of Membrane Remodelling in a Micos-Dependent Manner. EMBO Rep. 2020, 21, e49776. [Google Scholar] [CrossRef]
- Eramo, M.J.; Lisnyak, V.; E Formosa, L.; Ryan, M.T. The ‘Mitochondrial Contact Site and Cristae Organising System’ (Micos) in Health and Human Disease. J. Biochem. 2020, 167, 243–255. [Google Scholar] [CrossRef]
- Saku, T.; Takashio, S.; Tsuruta, Y.; Otsuka, Y.; Takae, M.; Kiyama, T.; Yamamoto, E.; Kaikita, K.; Hotta, T.; Matsumoto, S.; et al. Comparison of Electron Microscopic Findings and Clinical Presentation in Three Patients with Mitochondrial Cardiomyopathy Caused by the Mitochondrial DNA Mutation M.3243a>G. Med. Mol. Morphol. 2021, 54, 181–186. [Google Scholar] [CrossRef]
- Korge, P.; John, S.A.; Calmettes, G.; Weiss, J.N. Reactive Oxygen Species Production Induced by Pore Opening in Cardiac Mitochondria: The Role of Complex Ii. J. Biol. Chem. 2017, 292, 9896–9905. [Google Scholar] [CrossRef]
- Bhandari, P.; Song, M.; Chen, Y.; Burelle, Y.; Dorn, G.W., 2nd. Mitochondrial Contagion Induced by Parkin Deficiency in Drosophila Hearts and Its Containment by Suppressing Mitofusin. Circ. Res. 2014, 114, 257–265. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, B. Mitochondrial Dysfunction in Cardiac Diseases and Therapeutic Strategies. Biomedicines 2023, 11, 1500. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Zhang, W.; Liu, X. Mitochondrial Dysfunction and Mitochondrial Therapies in Heart Failure. Pharmacol. Res. 2022, 175, 106038. [Google Scholar] [CrossRef]
- Omori, Y.; Ohtani, T.; Sakata, Y.; Mano, T.; Takeda, Y.; Tamaki, S.; Tsukamoto, Y.; Kamimura, D.; Aizawa, Y.; Miwa, T.; et al. L-Carnitine Prevents the Development of Ventricular Fibrosis and Heart Failure with Preserved Ejection Fraction in Hypertensive Heart Disease. J. Hypertens. 2012, 30, 1834–1844. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, H.; Wang, Y.; Gao, X.; Liu, H.; Liu, W. Effects of Levocarnitine on Cardiac Function, Urinary Albumin, Hs-Crp, Bnp, and Troponin in Patients with Coronary Heart Disease and Heart Failure. Hell. J. Cardiol. 2020, 61, 99–102. [Google Scholar] [CrossRef]
- Li, X.; Flynn, E.R.; Carmo, J.M.D.; Wang, Z.; da Silva, A.A.; Mouton, A.J.; Omoto, A.C.M.; Hall, M.E.; Hall, J.E. Direct Cardiac Actions of Sodium-Glucose Cotransporter 2 Inhibition Improve Mitochondrial Function and Attenuate Oxidative Stress in Pressure Overload-Induced Heart Failure. Front. Cardiovasc. Med. 2022, 9, 859253. [Google Scholar] [CrossRef]
- Jhuo, S.J.; Lin, Y.H.; Liu, I.H.; Lin, T.H.; Wu, B.N.; Lee, K.T.; Lai, W.T. Sodium Glucose Cotransporter 2 (Sglt2) Inhibitor Ameliorate Metabolic Disorder and Obesity Induced Cardiomyocyte Injury and Mitochondrial Remodeling. Int. J. Mol. Sci. 2023, 24, 6842. [Google Scholar] [CrossRef]
- De Blasio, M.J.; Huynh, K.; Qin, C.; Rosli, S.; Kiriazis, H.; Ayer, A.; Cemerlang, N.; Stocker, R.; Du, X.-J.; McMullen, J.R.; et al. Therapeutic Targeting of Oxidative Stress with Coenzyme Q10 Counteracts Exaggerated Diabetic Cardiomyopathy in a Mouse Model of Diabetes with Diminished Pi3k(P110α) Signaling. Free Radic. Biol. Med. 2015, 87, 137–147. [Google Scholar] [CrossRef]
- Hodges, W.T.; Jarasvaraparn, C.; Ferguson, D.; Griffett, K.; Gill, L.E.; Chen, Y.; Ilagan, M.X.G.; Hegazy, L.; Elgendy, B.; Cho, K.; et al. Mitochondrial Pyruvate Carrier Inhibitors Improve Metabolic Parameters in Diet-Induced Obese Mice. J. Biol. Chem. 2022, 298, 101554. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cantrell, A.C.; Williams, Q.A.; Gu, W.; Chen, Y.; Chen, J.X.; Zeng, H. P53 Acetylation Exerts Critical Roles in Pressure Overload–Induced Coronary Microvascular Dysfunction and Heart Failure in Mice. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Deng, Y.; Yang, L.; Ou, W.; Xie, M.; Ding, L.; Jiang, C.; Yu, H.; Li, Q.; et al. Mitochondrial Protein Hyperacetylation Underpins Heart Failure with Preserved Ejection Fraction in Mice. J. Mol. Cell Cardiol. 2022, 165, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Schubert, T.; Welke, C.; Maske, T.; Patschkowski, T.; Donhauser, E.; Heinen-Weiler, J.; Hormann, F.-L.; Heiles, S.; Schulz, T.J.; et al. Nitro-Oleic Acid Enhances Mitochondrial Metabolism and Ameliorates Heart Failure with Preserved Ejection Fraction in Mice. Nat. Commun. 2025, 16, 3933. [Google Scholar] [CrossRef]
- Gao, J.; Guo, H.; Li, J.; Zhan, M.; You, Y.; Xin, G.; Liu, Z.; Fan, X.; Gao, Q.; Liu, J.; et al. Buyang Huanwu Decoction Ameliorates Myocardial Injury and Attenuates Platelet Activation by Regulating the Pi3 Kinase/Rap1/Integrin A(Iib)Β(3) Pathway. Chin. Med. 2024, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.M.; Soni, S.; Liu, S.N.; Rachid, J.-J.; Mast, H.E.; Wiedemeyer, A.; Holody, C.D.; Mah, R.; Woodman, A.G.; Ferdaoussi, M.; et al. Maternal Ketone Supplementation Throughout Gestation Improves Neonatal Cardiac Dysfunction Caused by Perinatal Iron Deficiency. Clin. Sci. 2024, 138, 1249–1264. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, G.; Zeng, Y.; Li, N.; Hu, C.; Pang, M.; Lu, S.; Gu, Y.; Chen, G.; Zhou, Y.; et al. Gualou Xiebai Banxia Decoction Suppresses Cardiomyocyte Apoptosis in Mice after Myocardial Infarction through Activation of Acetaldehyde Dehydrogenase 2. J. Ethnopharmacol. 2025, 339, 119143. [Google Scholar] [CrossRef]
- Tuo, H.; Li, W.; Zhao, W.; Zhao, J.; Li, D.; Jin, L. Shikonin Alleviates Doxorubicin-Induced Cardiotoxicity Via Mst1/Nrf2 Pathway in Mice. Sci. Rep. 2024, 14, 924. [Google Scholar] [CrossRef]
- Ogutveren, M.M.; Satiroglu, O.; Ozden, Z.; Akyildiz, K.; Yilmaz, A.; Mercantepe, F.; Yilmaz, A.S.; Koc, H.; Mercantepe, T. Cardioprotective Effects of Dapagliflozin and Trimetazidine on Doxorubicin-Induced Cardiotoxicity in Streptozotocin-Induced Type 1 Diabetic Rats Via Endoplasmic Reticulum Stress. J. Clin. Med. 2025, 14, 1315. [Google Scholar] [CrossRef]
- Lan, H.; Wang, H.; Chen, C.; Hu, W.; Ai, C.; Chen, L.; Teng, H. Flavonoids and Gastrointestinal Health: Single Molecule for Multiple Roles. Crit. Rev. Food Sci. Nutr. 2024, 64, 10987–11005. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 Aha/Acc/Hfsa Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 Esc Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Whellan, D.J.; Lee, K.L.; Keteyian, S.J.; Cooper, L.S.; Ellis, S.J.; Leifer, E.S.; Kraus, W.E.; Kitzman, D.W.; Blumenthal, J.A.; et al. Efficacy and Safety of Exercise Training in Patients with Chronic Heart Failure: Hf-Action Randomized Controlled Trial. JAMA 2009, 301, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Walker, S.; Smart, N.A.; Piepoli, M.F.; Warren, F.C.; Ciani, O.; O’COnnor, C.; Whellan, D.; Keteyian, S.J.; Coats, A.; et al. Impact of Exercise-Based Cardiac Rehabilitation in Patients with Heart Failure (Extramatch Ii) on Mortality and Hospitalisation: An Individual Patient Data Meta-Analysis of Randomised Trials. Eur. J. Heart Fail. 2018, 20, 1735–1743. [Google Scholar] [CrossRef]
- Ellingsen, Ø.; Halle, M.; Conraads, V.; Støylen, A.; Dalen, H.; Delagardelle, C.; Larsen, A.-I.; Hole, T.; Mezzani, A.; Van Craenenbroeck, E.M.; et al. High-Intensity Interval Training in Patients with Heart Failure with Reduced Ejection Fraction. Circulation 2017, 135, 839–849. [Google Scholar] [CrossRef]
- Budiono, B.P.; Vider, J.; Zaid, A.; Peart, J.N.; Du Toit, E.F.; Headrick, J.P.; Haseler, L.J. Swimming Induces Physiological Cardioprotection Associated with Pro-Growth Versus Anti-Inflammatory Influences in Extracardiac Organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2025, 328, R206–R219. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional Control of Mitochondrial Biogenesis: The Central Role of Pgc-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, J.; Goldenthal, M.J. Mitochondrial Centrality in Heart Failure. Heart Fail. Rev. 2008, 13, 137–150. [Google Scholar] [CrossRef]
- Shayota, B.J. Biomarkers of Mitochondrial Disorders. Neurotherapeutics 2024, 21, e00325. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Qiu, Y.; Zhou, M.; Chen, M.; Hu, Y.; Hong, S.; Jiang, L.; Guo, Y. Circulating Fgf21 and Gdf15 as Biomarkers for Screening, Diagnosis, and Severity Assessment of Primary Mitochondrial Disorders in Children. Front. Pediatr. 2022, 10, 851534. [Google Scholar] [CrossRef]
- Jena, J.; García-Peña, L.M.; Pereira, R.O. The Roles of Fgf21 and Gdf15 in Mediating the Mitochondrial Integrated Stress Response. Front. Endocrinol. 2023, 14, 1264530. [Google Scholar] [CrossRef] [PubMed]
- Hubens, W.; Vallbona-Garcia, A.; de Coo, I.; van Tienen, F.; Webers, C.; Smeets, H.; Gorgels, T. Blood Biomarkers for Assessment of Mitochondrial Dysfunction: An Expert Review. Mitochondrion 2022, 62, 187–204. [Google Scholar] [CrossRef]
- Szögi, T.; Borsos, B.N.; Masic, D.; Radics, B.; Bella, Z.; Bánfi, A.; Ördög, N.; Zsiros, C.; Kiricsi, Á.; Pankotai-Bodó, G.; et al. Novel Biomarkers of Mitochondrial Dysfunction in Long Covid Patients. Geroscience 2024, 47, 2245–2261. [Google Scholar] [CrossRef]
- Liu, L.P.; Cheng, K.; Ning, M.A.; Li, H.H.; Wang, H.C.; Li, F.; Chen, S.Y.; Qu, F.L.; Guo, W.Y. Association between Peripheral Blood Cells Mitochondrial DNA Content and Severity of Coronary Heart Disease. Atherosclerosis 2017, 261, 105–110. [Google Scholar] [CrossRef]
- Koller, A.; Fazzini, F.; Lamina, C.; Rantner, B.; Kollerits, B.; Stadler, M.; Klein-Weigel, P.; Fraedrich, G.; Kronenberg, F. Mitochondrial DNA Copy Number Is Associated with All-Cause Mortality and Cardiovascular Events in Patients with Peripheral Arterial Disease. J. Intern. Med. 2020, 287, 569–579. [Google Scholar] [CrossRef]
- Zhang, Y.; Guallar, E.; Ashar, F.N.; Longchamps, R.J.; A Castellani, C.; Lane, J.; Grove, M.L.; Coresh, J.; Sotoodehnia, N.; Ilkhanoff, L.; et al. Association between Mitochondrial DNA Copy Number and Sudden Cardiac Death: Findings from the Atherosclerosis Risk in Communities Study (Aric). Eur. Heart J. 2017, 38, 3443–3448. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Zhang, Y.; Jiang, W.; Lai, M.; Wiggins, K.L.; Raffield, L.M.; Bielak, L.F.; Zhao, W.; Pitsillides, A.; et al. Association between Whole Blood-Derived Mitochondrial DNA Copy Number, Low-Density Lipoprotein Cholesterol, and Cardiovascular Disease Risk. J. Am. Heart Assoc. 2023, 12, e029090. [Google Scholar] [CrossRef]
- Sundquist, K.; Sundquist, J.; Palmer, K.; Memon, A.A. Role of Mitochondrial DNA Copy Number in Incident Cardiovascular Diseases and the Association between Cardiovascular Disease and Type 2 Diabetes: A Follow-up Study on Middle-Aged Women. Atherosclerosis 2022, 341, 58–62. [Google Scholar] [CrossRef]
- Luo, J.; Noordam, R.; Jukema, J.W.; van Dijk, K.W.; Hägg, S.; Grassmann, F.; le Cessie, S.; van Heemst, D. Low Leukocyte Mitochondrial DNA Abundance Drives Atherosclerotic Cardiovascular Diseases: A Cohort and Mendelian Randomization Study. Cardiovasc. Res. 2023, 119, 998–1007. [Google Scholar] [CrossRef]
- Yue, P.; Jing, S.; Liu, L.; Ma, F.; Zhang, Y.; Wang, C.; Duan, H.; Zhou, K.; Hua, Y.; Wu, G.; et al. Association between Mitochondrial DNA Copy Number and Cardiovascular Disease: Current Evidence Based on a Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0206003. [Google Scholar] [CrossRef] [PubMed]
- Rucci, C.; de Simone, G.; Salathia, S.; Casadidio, C.; Censi, R.; Bordoni, L. Exploring Mitochondrial DNA Copy Number in Circulating Cell-Free DNA and Extracellular Vesicles across Cardiovascular Health Status: A Prospective Case-Control Pilot Study. FASEB J. 2024, 38, e23672. [Google Scholar] [CrossRef] [PubMed]
- Blalock, Z.N.; Wu, G.W.Y.; Lindqvist, D.; Trumpff, C.; Flory, J.D.; Lin, J.; Reus, V.I.; Rampersaud, R.; Hammamieh, R.; Gautam, A.; et al. Circulating Cell-Free Mitochondrial DNA Levels and Glucocorticoid Sensitivity in a Cohort of Male Veterans with and without Combat-Related Ptsd. Transl. Psychiatry 2024, 14, 22. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; De Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and Organization of the Human Mitochondrial Genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, M.; He, J.; Zhang, X.; Chen, Y.; Li, H. Maternally Inherited Coronary Heart Disease Is Associated with a Novel Mitochondrial Trna Mutation. BMC Cardiovasc. Disord. 2019, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Vecoli, C.; Borghini, A.; Pulignani, S.; Mercuri, A.; Turchi, S.; Picano, E.; Andreassi, M.G. Independent and Combined Effects of Telomere Shortening and Mtdna(4977) Deletion on Long-Term Outcomes of Patients with Coronary Artery Disease. Int. J. Mol. Sci. 2019, 20, 5508. [Google Scholar] [CrossRef]
- Pei, H.; Peng, Q.; Lan, C.; Liu, B.C. Variations in Mitochondrial Trna(Thr) Gene May Not Be Associated with Coronary Heart Disease. Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 565–568. [Google Scholar] [CrossRef]
- Finsterer, J. Atherosclerosis Can Be Mitochondrial: A Review. Cureus 2020, 12, e6987. [Google Scholar] [CrossRef]
- Tang, C.; Shi, F.; Ji, Y.; Zhu, J.; Gu, X. Aldehyde Dehydrogenase 2 (Aldh2) Rs671 Polymorphism Is a Predictor of Pulmonary Hypertension Due to Left Heart Disease. Heart Lung Circ. 2024, 33, 230–239. [Google Scholar] [CrossRef]
- Zhu, T.; Ma, Y.; Yang, P.; Cao, Z.; Gao, J.; Du, J.; Gao, P.; Jiang, H.; Zhang, X. A Cross-Tissue Transcriptome-Wide Association Study Reveals Novel Susceptibility Genes for Erectile Dysfunction. Andrology 2025. online ahead of print. [Google Scholar]
- Sun, Y.; Jin, L.; Qin, Y.; Ouyang, Z.; Zhong, J.; Zeng, Y. Harnessing Mitochondrial Stress for Health and Disease: Opportunities and Challenges. Biology 2024, 13, 394. [Google Scholar] [CrossRef]
- Kang, E.; Wu, J.; Gutierrez, N.M.; Koski, A.; Tippner-Hedges, R.; Agaronyan, K.; Platero-Luengo, A.; Martinez-Redondo, P.; Ma, H.; Lee, Y.; et al. Mitochondrial Replacement in Human Oocytes Carrying Pathogenic Mitochondrial DNA Mutations. Nature 2016, 540, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.V.E.; Gallia, M.C.; da Luz, J.R.D.; de Rezende, A.A.; Bongiovanni, G.A.; Araujo-Silva, G.; Almeida, M.d.G. Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated Cho-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent. Nutrients 2022, 14, 3265. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.J.; Zeng, S.L.; Li, Y.W.; Wu, Z.; Huang, D.J.; Tang, H.Z. The Effect of Coenzyme Q10 Plus Trimetazidine on Acute Viral Myocarditis Treatment. Am. J. Transl. Res. 2021, 13, 13854–13861. [Google Scholar]
- Ji, Y.; Leng, Y.; Lei, S.; Qiu, Z.; Ming, H.; Zhang, Y.; Zhang, A.; Wu, Y.; Xia, Z. The Mitochondria-Targeted Antioxidant Mitoq Ameliorates Myocardial Ischemia-Reperfusion Injury by Enhancing Pink1/Parkin-Mediated Mitophagy in Type 2 Diabetic Rats. Cell Stress. Chaperones 2022, 27, 353–367. [Google Scholar] [CrossRef]
- Dare, A.J.; Logan, A.; Prime, T.A.; Rogatti, S.; Goddard, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J.; Murphy, M.P.; Saeb-Parsy, K. The Mitochondria-Targeted Anti-Oxidant Mitoq Decreases Ischemia-Reperfusion Injury in a Murine Syngeneic Heart Transplant Model. J. Heart Lung Transplant. 2015, 34, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Junior, R.F.; Dabkowski, E.R.; Shekar, K.C.; O’connell, K.A.; Hecker, P.A.; Murphy, M.P. Mitoq Improves Mitochondrial Dysfunction in Heart Failure Induced by Pressure Overload. Free Radic. Biol. Med. 2018, 117, 18–29. [Google Scholar] [CrossRef]
- Gioscia-Ryan, R.A.; LaRocca, T.J.; Sindler, A.L.; Zigler, M.C.; Murphy, M.P.; Seals, D.R. Mitochondria-Targeted Antioxidant (Mitoq) Ameliorates Age-Related Arterial Endothelial Dysfunction in Mice. J. Physiol. 2014, 592, 2549–2561. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Jiang, J.; Zhang, C.; Zhao, M.; Chen, Z.; Wang, N.; Hu, D.; Liu, X.; Peng, H.; et al. Sonodynamic Therapy in Atherosclerosis by Curcumin Nanosuspensions: Preparation Design, Efficacy Evaluation, and Mechanisms Analysis. Eur. J. Pharm. Biopharm. 2020, 146, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, A.; Budin, S.B.; Fauzi, N.M.; Ritchie, R.H.; Zainalabidin, S. Targeting Mitochondrial Reactive Oxygen Species-Mediated Oxidative Stress Attenuates Nicotine-Induced Cardiac Remodeling and Dysfunction. Sci. Rep. 2021, 11, 13845. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I.; Hu, W.S.; Hung, M.Y.; Ou, H.C.; Huang, S.H.; Hsu, P.T.; Day, C.H.; Lin, K.H.; Viswanadha, V.P.; Kuo, W.W.; et al. Protective Effects of Luteolin against Oxidative Stress and Mitochondrial Dysfunction in Endothelial Cells. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1032–1043. [Google Scholar] [CrossRef]
- Birk, A.V.; Chao, W.M.; Bracken, C.; Warren, J.D.; Szeto, H.H. Targeting Mitochondrial Cardiolipin and the Cytochrome C/Cardiolipin Complex to Promote Electron Transport and Optimize Mitochondrial Atp Synthesis. Br. J. Pharmacol. 2014, 171, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.C.; Moukdar, F.; Frasier, C.R.; Patel, H.D.; Bostian, P.A.; Lust, R.M.; Brown, D.A. Mitochondrial Permeability Transition in the Diabetic Heart: Contributions of Thiol Redox State and Mitochondrial Calcium to Augmented Reperfusion Injury. J. Mol. Cell Cardiol. 2012, 52, 1009–1018. [Google Scholar] [CrossRef]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and Endothelial Function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef]
- Imai, T.; Mishiro, K.; Takagi, T.; Isono, A.; Nagasawa, H.; Tsuruma, K.; Shimazawa, M.; Hara, H. Protective Effect of Bendavia (Ss-31) against Oxygen/Glucose-Deprivation Stress-Induced Mitochondrial Damage in Human Brain Microvascular Endothelial Cells. Curr. Neurovasc Res. 2017, 14, 53–59. [Google Scholar] [CrossRef]
- Karaa, A.; Haas, R.; Goldstein, A.; Vockley, J.; Weaver, W.D.; Cohen, B.H. Randomized Dose-Escalation Trial of Elamipretide in Adults with Primary Mitochondrial Myopathy. Neurology 2018, 90, e1212–e1221. [Google Scholar] [CrossRef]
- Vacante, F.; Senesi, P.; Montesano, A.; Frigerio, A.; Luzi, L.; Terruzzi, I. L-Carnitine: An Antioxidant Remedy for the Survival of Cardiomyocytes under Hyperglycemic Condition. J. Diabetes Res. 2018, 2018, 4028297. [Google Scholar] [CrossRef]
- Qi, X.; Wang, J. Melatonin Improves Mitochondrial Biogenesis through the Ampk/Pgc1α Pathway to Attenuate Ischemia/Reperfusion-Induced Myocardial Damage. Aging 2020, 12, 7299–7312. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Roohbakhsh, A.; Pourbarkhordar, V.; Hayes, A.W.; Karimi, G. Melatonin Regulates Mitochondrial Dynamics and Mitophagy: Cardiovascular Protection. J. Cell. Mol. Med. 2024, 28, e70074. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Ryu, D.; Cho, S.; Jang, Y. Mitochondrial Quality Control in the Heart: New Drug Targets for Cardiovascular Disease. Korean Circ. J. 2020, 50, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Kannan, A. Mitochondrial DNA Modification by Crispr/Cas System: Challenges and Future Direction. Prog. Mol. Biol. Transl. Sci. 2021, 178, 193–211. [Google Scholar]
- Mercer, J.R. Mitochondrial Bioenergetics and Therapeutic Intervention in Cardiovascular Disease. Pharmacol. Ther. 2014, 141, 13–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Chang, S.; Zeng, Y.; Wang, X. Advances in Mitochondrial Dysfunction and Its Role in Cardiovascular Diseases. Cells 2025, 14, 1621. https://doi.org/10.3390/cells14201621
Qiu Y, Chang S, Zeng Y, Wang X. Advances in Mitochondrial Dysfunction and Its Role in Cardiovascular Diseases. Cells. 2025; 14(20):1621. https://doi.org/10.3390/cells14201621
Chicago/Turabian StyleQiu, Yan, Shuo Chang, Ye Zeng, and Xiaoqi Wang. 2025. "Advances in Mitochondrial Dysfunction and Its Role in Cardiovascular Diseases" Cells 14, no. 20: 1621. https://doi.org/10.3390/cells14201621
APA StyleQiu, Y., Chang, S., Zeng, Y., & Wang, X. (2025). Advances in Mitochondrial Dysfunction and Its Role in Cardiovascular Diseases. Cells, 14(20), 1621. https://doi.org/10.3390/cells14201621







