Adenosine Receptors in Neuroinflammation and Neurodegeneration
Abstract
1. Introduction
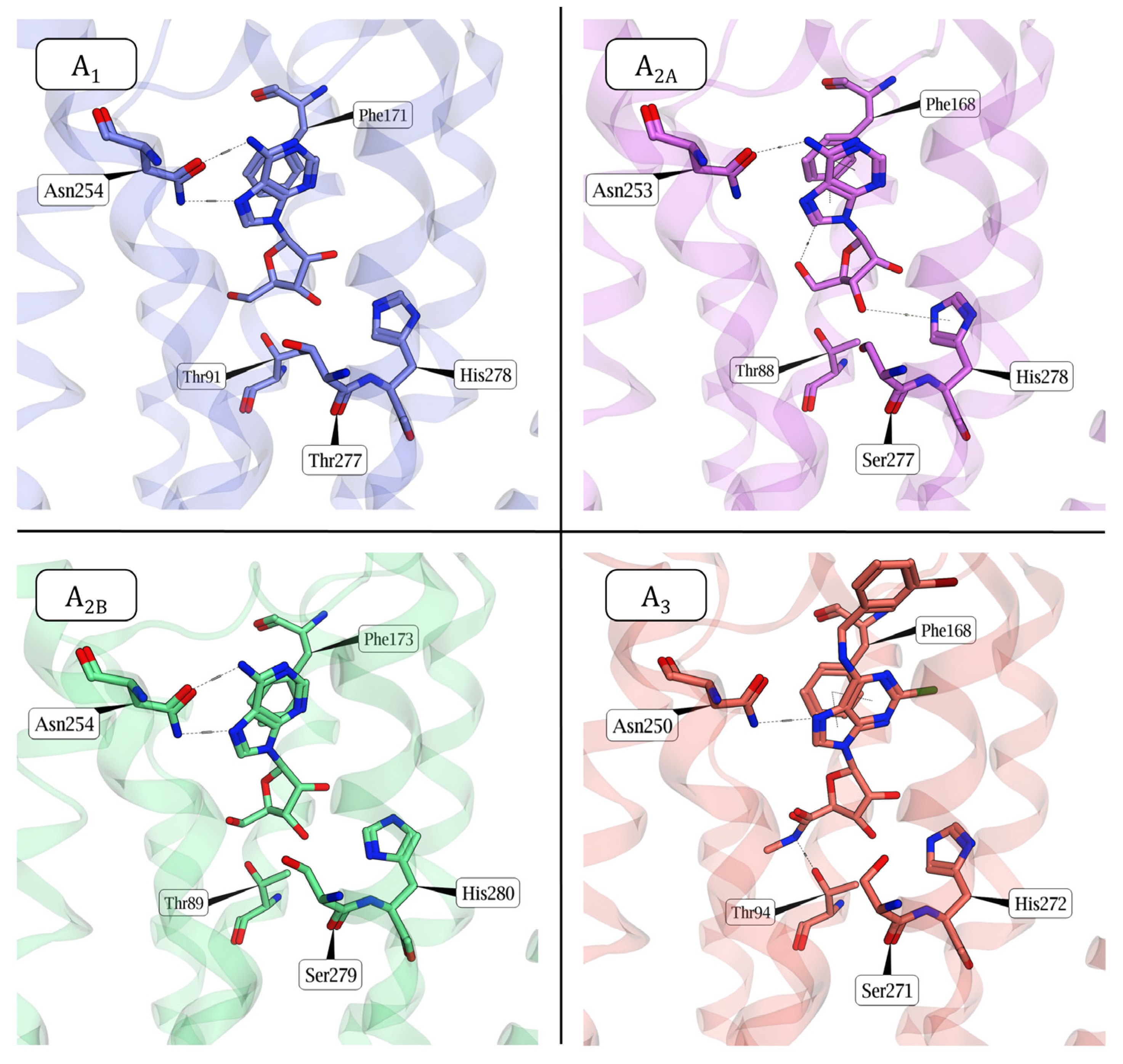
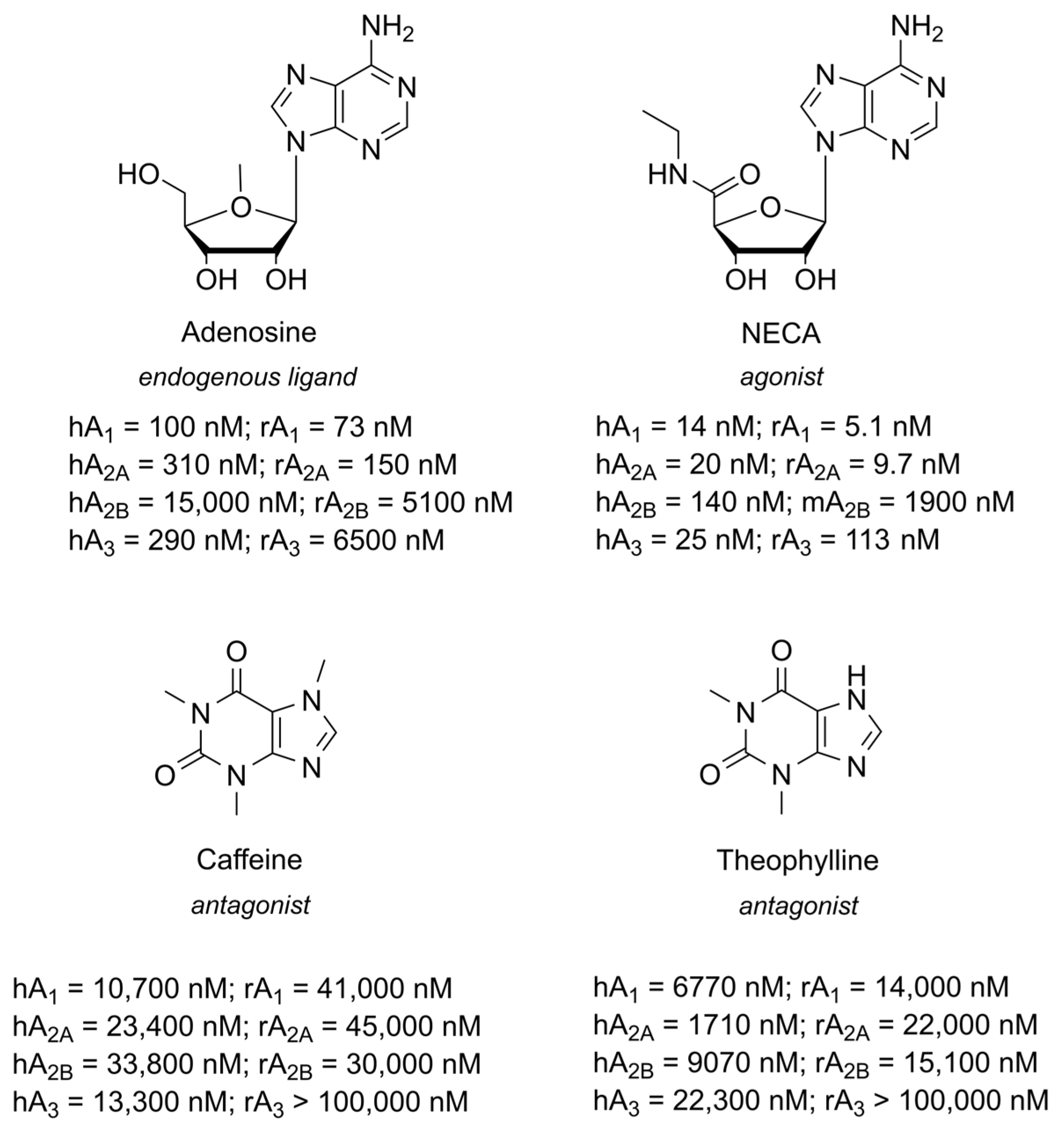
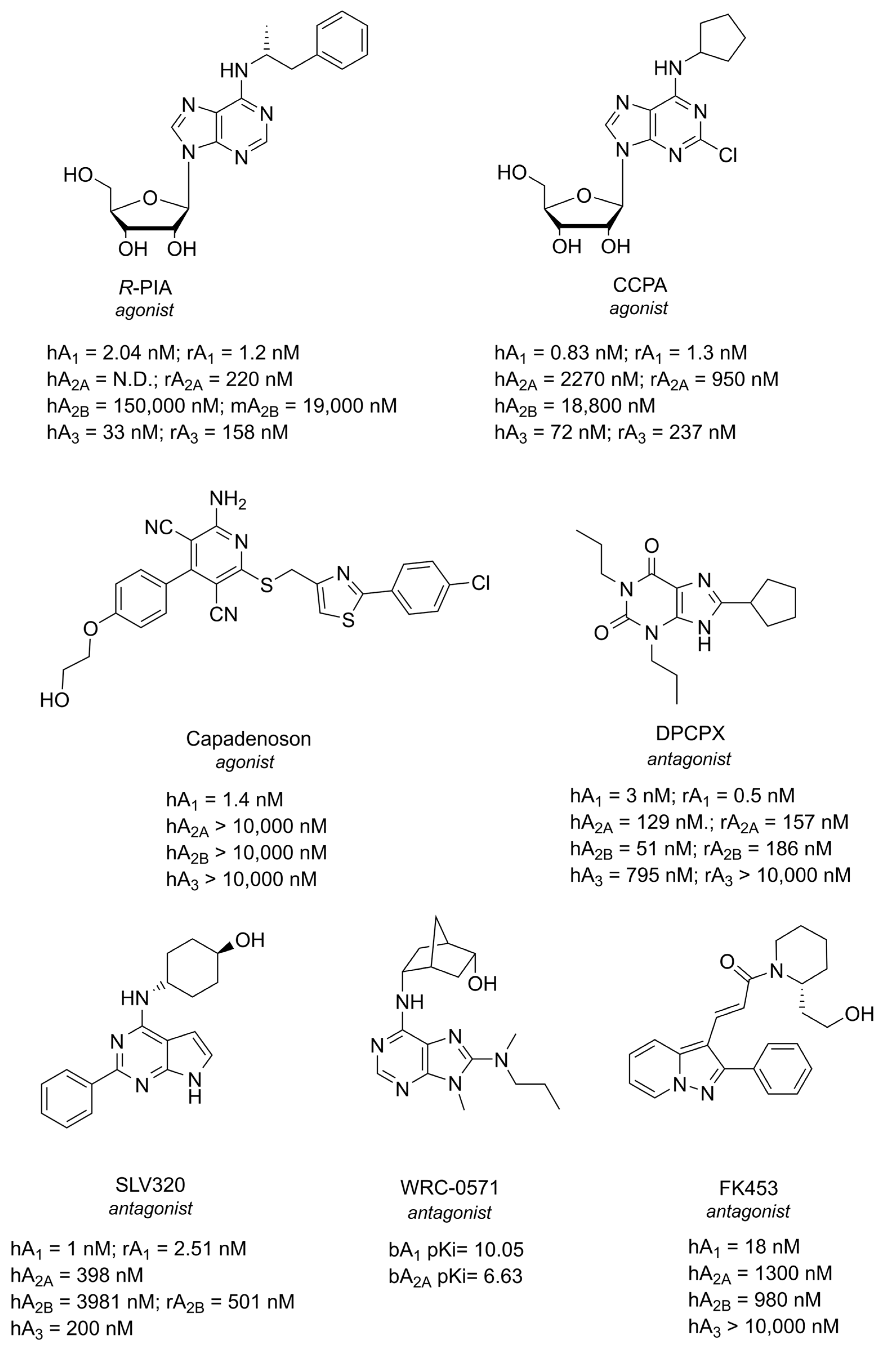
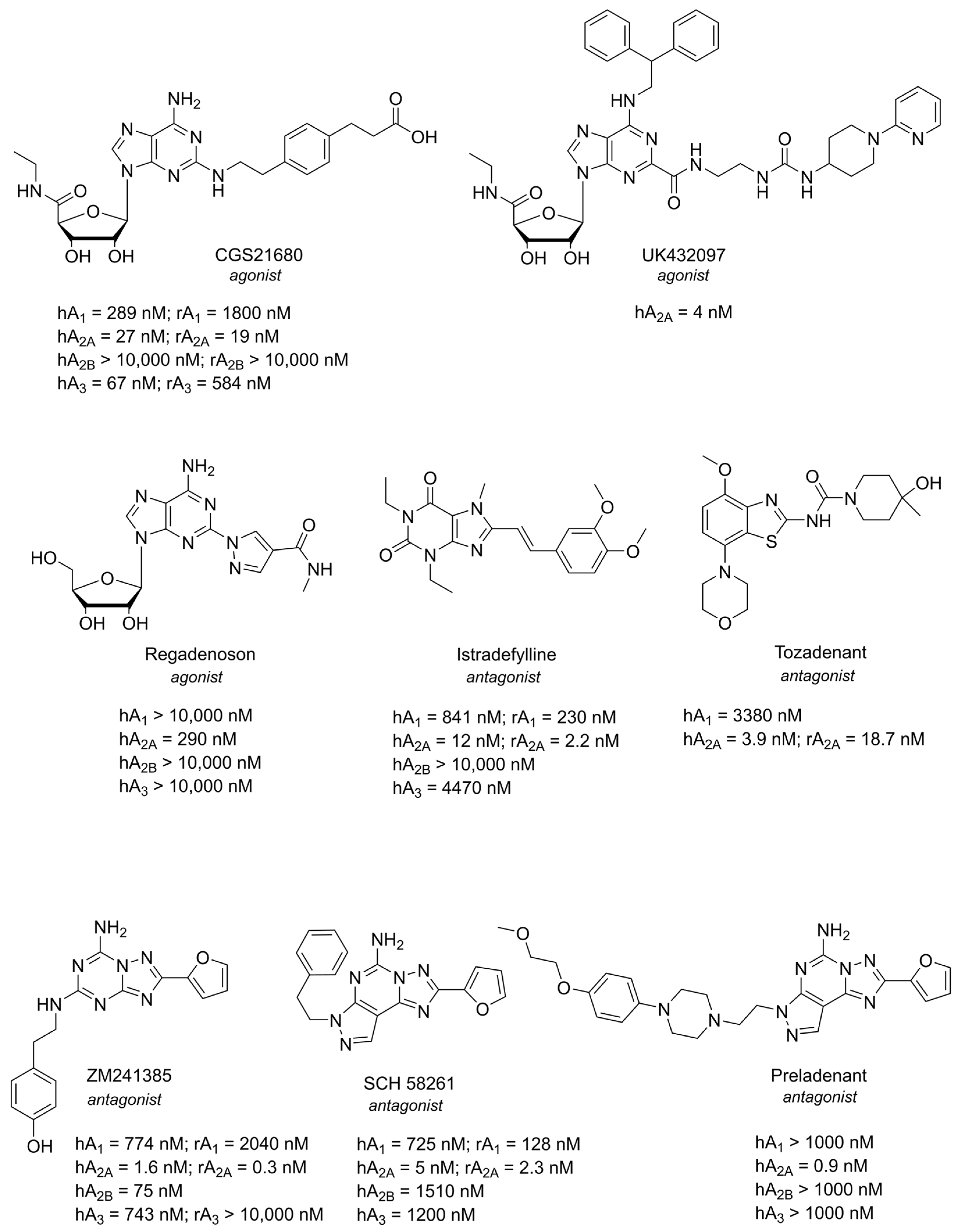
1.1. Adenosine and Adenosine Receptors
1.2. Neuroinflammation
1.3. Neurodegeneration
1.3.1. Alzheimer’s Disease
1.3.2. Parkinson’s Disease
1.3.3. Other Neurodegenerative Disease
2. A1 AR
2.1. Role in Neuroinflammation
2.2. Role in Neurodegenerative Disorders
2.2.1. Alzheimer’s Disease
2.2.2. Parkinson’s Disease
3. A2A AR
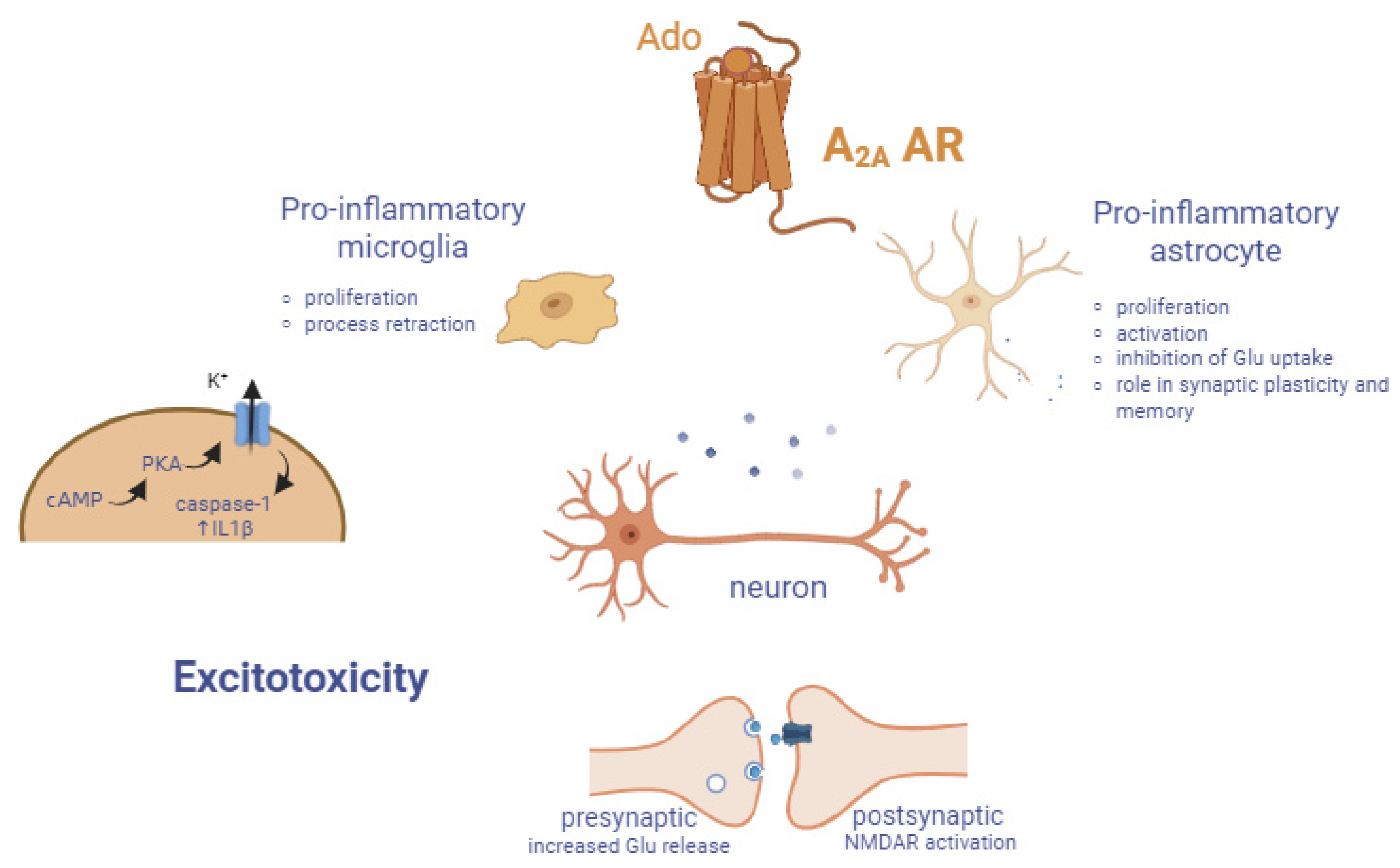
3.1. Role in Neuroinflammation
3.2. Role in Neurodegenerative Disorders
3.2.1. Alzheimer’s Disease
3.2.2. Parkinson’s Disease
3.2.3. Huntington’s Disease
3.2.4. Amyotrophic Lateral Sclerosis
3.2.5. Others
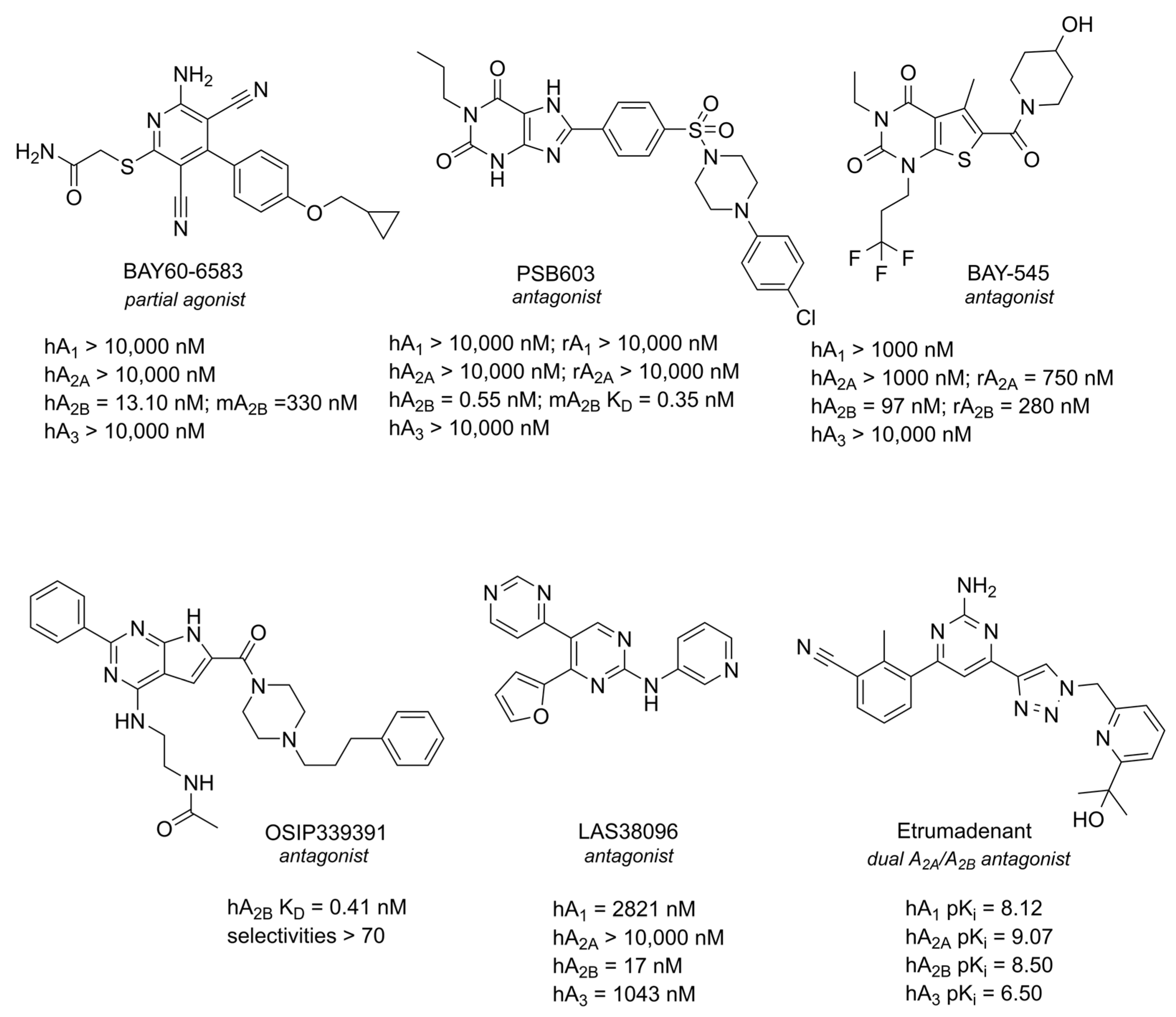
4. A2B AR
4.1. Role in Neuroinflammation
4.2. Role in Neurodegenerative Disorders
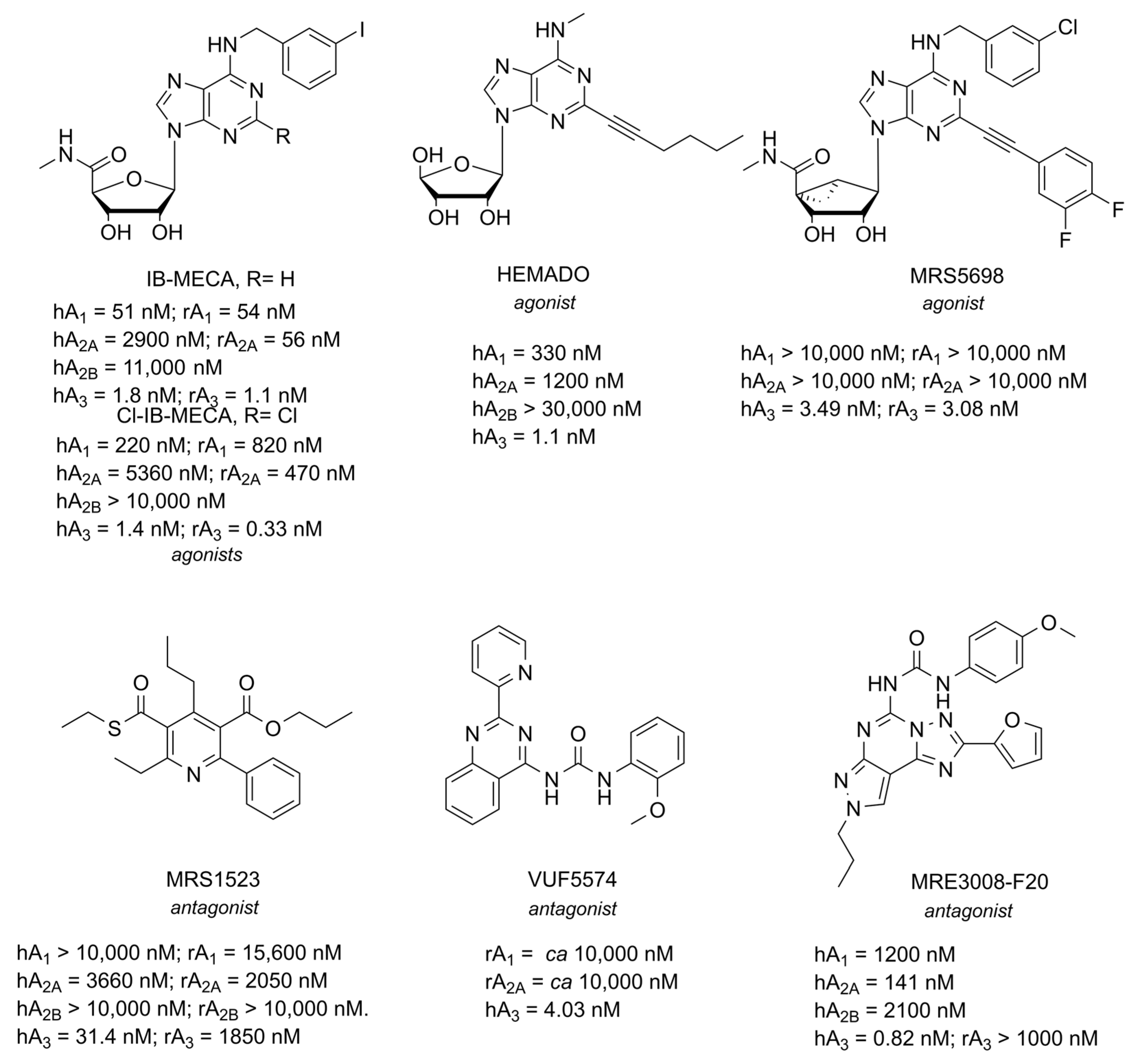
5. A3 AR
5.1. Role in Neuroinflammation
5.2. Role in Neurodegenerative Disorders
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-Syn | α-Synuclein |
| Aβ | Amyloid beta |
| AβPP | Amyloid-beta precursor protein |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| ALSFRS-R | Revised amyotrophic lateral sclerosis functional rating scale |
| APP | Amyloid precursor protein |
| AR | Adenosine receptor |
| ATP | Adenosine triphosphate |
| AV | Atrioventricular |
| BACE1 | β-secretase 1 |
| BBB | Blood brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BFCN | Basal forebrain cholinergic neurons |
| C9orf72 | Chromosome 9 open reading frame 72 |
| CCH | Chronic cerebral hypoperfusion |
| CCL | C-C motif chemokine ligand |
| CLEC7A | C-Type lectin domain containing 7A |
| CNS | Central nervous system |
| COPD | Chronic obstructive pulmonary disease |
| CREB | cAMP response element-binding protein |
| DAMP | Danger-associated molecular pattern |
| DNA | Deoxyribonucleic acid |
| EL | Extracellular loop |
| ERK | Extracellular regulated kinase |
| FDA | Food and drug administration |
| FUS | Fused in Sarcoma, also called translocated in liposarcoma protein (TLP) |
| GABA | Gamma-aminobutyric acid |
| GLT-1 | Glutamate transporter 1 |
| GPCR | G protein-coupled receptor |
| H1 | Histamine 1 |
| HD | Huntington’s disease |
| IFN | Interferon |
| IL | Interleukin |
| JAK | Janus kinase- |
| JNK | C-Jun N-terminal kinase |
| KO | Knock out |
| L-DOPA | Levo-dioxyphenylalanine |
| LPS | Lipopolysaccharide |
| LTD | Long term depression |
| LTP | Long term potentiation |
| MAPK | Mitogen activated protein kinase |
| MAO-B | Monoamine oxidase B |
| mGLU5 | Metabotropic glutamate receptor 5 |
| MPI | Myocardial perfusion imaging |
| MS | Multiple sclerosis |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NECA | N-ethylcarboxamidoadenosine |
| NF-κB | Nuclear factor kappa B |
| NFT | Neurofibrillary tangle |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NMDA | N-methyl-D-aspartate |
| NO | Nitric oxide |
| OGD | Oxygen glucose deprivation |
| PAMP | Pathogen-associated molecular pattern |
| PD | Parkinson’s disease |
| PLC | Phospholipase C |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PRR | Pattern-recognition receptors |
| PS1 | Presenilin-1 |
| RNA | Ribonucleic acid; mRNA (messenger RNA) |
| ROS | Reactive oxygen species |
| SAE | Sepsis associated encephalopathy |
| SAP102 | Synapse-associated protein 102 |
| Shh | Sonic hedgehog |
| SN | Subtantia nigra |
| SOD1 | Superoxide dismutase 1 |
| STAT3 | Signal transducer and transcription activator 3 |
| SVT | Supraventricular tachycardia |
| TBI | Traumatic brain injury |
| TDP-43 | Trans activation response DNA binding protein 43 |
| TGF-β | Transforming growth factor β |
| TLR | Toll-like receptor |
| tMCAo | Transient middle cerebral artery occlusion |
| TNF | Tumor necrosis factor |
| TrKB | Tropomyosin receptor kinase B/tyrosine receptor kinase B |
| tPA | Tissue plasminogen activator |
| USA | Unites States of America |
| VEGF | Vascular endothelial growth factor |
References
- Newby, A.C. Adenosine and the Concept of ‘Retaliatory Metabolites’. Trends Biochem. Sci. 1984, 9, 42–44. [Google Scholar] [CrossRef]
- Fredholm, B.B. Physiological and Pathophysiological Roles of Adenosine. Sleep. Biol. Rhythms 2011, 9, 24–28. [Google Scholar] [CrossRef]
- Pearson, T.; Nuritova, F.; Caldwell, D.; Dale, N.; Frenguelli, B.G. A Depletable Pool of Adenosine in Area CA1 of the Rat Hippocampus. J. Neurosci. 2001, 21, 2298–2307. [Google Scholar] [CrossRef][Green Version]
- Pearson, T.; Damian, K.; Lynas, R.E.; Frenguelli, B.G. Sustained Elevation of Extracellular Adenosine and Activation of A 1 Receptors Underlie the Post-Ischaemic Inhibition of Neuronal Function in Rat Hippocampus in Vitro. J. Neurochem. 2006, 97, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Latini, S.; Pedata, F. Adenosine in the Central Nervous System: Release Mechanisms and Extracellular Concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H. Extracellular Metabolism of ATP and Other Nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 299–309. [Google Scholar] [CrossRef]
- Camici, M.; Garcia-Gil, M.; Tozzi, M. The Inside Story of Adenosine. Int. J. Mol. Sci. 2018, 19, 784. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From Purines to Purinergic Signalling: Molecular Functions and Human Diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Who Is Who in Adenosine Transport. Front. Pharmacol. 2018, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Pándy-Szekeres, G.; Munk, C.; Tsonkov, T.M.; Mordalski, S.; Harpsøe, K.; Hauser, A.S.; Bojarski, A.J.; Gloriam, D.E. GPCRdb in 2018: Adding GPCR Structure Models and Ligands. Nucleic Acids Res. 2018, 46, D440–D446. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Weinstein, H. [19] Integrated Methods for the Construction of Three-Dimensional Models and Computational Probing of Structure-Function Relations in G Protein-Coupled Receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar]
- Seibt, B.F.; Schiedel, A.C.; Thimm, D.; Hinz, S.; Sherbiny, F.F.; Müller, C.E. The Second Extracellular Loop of GPCRs Determines Subtype-Selectivity and Controls Efficacy as Evidenced by Loop Exchange Study at A2 Adenosine Receptors. Biochem. Pharmacol. 2013, 85, 1317–1329. [Google Scholar] [CrossRef]
- Schiedel, A.C.; Hinz, S.; Thimm, D.; Sherbiny, F.; Borrmann, T.; Maaß, A.; Müller, C.E. The Four Cysteine Residues in the Second Extracellular Loop of the Human Adenosine A2B Receptor: Role in Ligand Binding and Receptor Function. Biochem. Pharmacol. 2011, 82, 389–399. [Google Scholar] [CrossRef]
- De Filippo, E.; Namasivayam, V.; Zappe, L.; El-Tayeb, A.; Schiedel, A.C.; Müller, C.E. Role of Extracellular Cysteine Residues in the Adenosine A2A Receptor. Purinergic Signal 2016, 12, 313–329. [Google Scholar] [CrossRef][Green Version]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.G.; Auchampach, J.A.; Jacobson, K.A. Species Dependence of A3 Adenosine Receptor Pharmacology and Function. Purinergic Signal 2023, 19, 523–550. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar] [CrossRef]
- Rosin, D.L.; Robeva, A.; Woodard, R.L.; Guyenet, P.G.; Linden, J. Immunohistochemical Localization of Adenosine A2A Receptors in the Rat Central Nervous System. J. Comp. Neurol. 1998, 401, 163–186. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Song, Y.; Sun, Q.; Deng, L.; Lin, Z.; Zeng, Y.; Qiu, C.; Lin, J.; Guo, H.; et al. Genetic Tagging of the Adenosine A2A Receptor Reveals Its Heterogeneous Expression in Brain Regions. Front. Neuroanat. 2022, 16, 978641. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Merighi, S.; Varani, K.; Borea, P.A.; Baraldi, S.; Aghazadeh Tabrizi, M.; Romagnoli, R.; Baraldi, P.G.; Ciancetta, A.; Tosh, D.K.; et al. A 3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy. Med. Res. Rev. 2018, 38, 1031–1072. [Google Scholar] [CrossRef]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine Receptors: Therapeutic Aspects for Inflammatory and Immune Diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef]
- Ye, H.; Zhao, J.; Xu, X.; Zhang, D.; Shen, H.; Wang, S. Role of Adenosine A2a Receptor in Cancers and Autoimmune Diseases. Immun. Inflamm. Dis. 2023, 11, e826. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Role of Adenosine A2b Receptor Overexpression in Tumor Progression. Life Sci. 2016, 166, 92–99. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Buccioni, M. Current Understanding of the Role of Adenosine Receptors in Cancer. Molecules 2024, 29, 3501. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Xiong, W.; Zhang, D.; Soylu, H.; Sun, C.; Albensi, B.C.; Parkinson, F.E. Regulation of Adenosine Levels during Cerebral Ischemia. Acta Pharmacol. Sin. 2013, 34, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Gobbo, D.; Zhao, N.; Zhang, H.; Awuku, N.O.; Liu, Q.; Fang, L.P.; Gampfer, T.M.; Meyer, M.R.; Zhao, R.; et al. Adenosine Triggers Early Astrocyte Reactivity That Provokes Microglial Responses and Drives the Pathogenesis of Sepsis-Associated Encephalopathy in Mice. Nat. Commun. 2024, 15, 6340. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.-G. Adenosine Receptors as Therapeutic Targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Elson, G.; Eisenberg, M.; Garg, C.; Outram, S.; Ferrante, C.J.; Hasko, G.; Leibovich, S.J. Induction of Murine Adenosine A 2A Receptor Expression by LPS: Analysis of the 5′ Upstream Promoter. Genes. Immun. 2013, 14, 147–153. [Google Scholar] [CrossRef]
- Sereda, M.J.; Bradding, P.; Vial, C. Adenosine Potentiates Human Lung Mast Cell Tissue Plasminogen Activator Activity. J. Immunol. 2011, 186, 1209–1217. [Google Scholar] [CrossRef]
- Chen, J.-F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine Receptors as Drug Targets—What Are the Challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef]
- Kutryb-Zając, B.; Kawecka, A.; Nasadiuk, K.; Braczko, A.; Stawarska, K.; Caiazzo, E.; Koszałka, P.; Cicala, C. Drugs Targeting Adenosine Signaling Pathways: A Current View. Biomed. Pharmacother. 2023, 165, 115184. [Google Scholar] [CrossRef]
- Rankin, A.C.; Brooks, R.; Ruskin, J.N.; McGovern, B.A. Adenosine and the Treatment of Supraventricular Tachycardia. Am. J. Med. 1992, 92, 655–664. [Google Scholar] [CrossRef]
- Derry, C.J.; Derry, S.; Moore, R.A. Caffeine as an Analgesic Adjuvant for Acute Pain in Adults. Cochrane Database Syst. Rev. 2014, 2014, CD009281. [Google Scholar] [CrossRef]
- Spina, D.; Page, C.P. Xanthines and Phosphodiesterase Inhibitors. Handb. Exp. Pharmacol. 2016, 237, 63–91. [Google Scholar] [CrossRef]
- da Fonseca, A.C.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R.S. The Impact of Microglial Activation on Blood-Brain Barrier in Brain Diseases. Front. Cell Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The Role of Neuroinflammation in Neurodegenerative Diseases: Current Understanding and Future Therapeutic Targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Tan, J.; Miao, Y.; Zhang, Q. The Role of Autophagy in Hypoxia-Induced Neuroinflammation. DNA Cell Biol. 2021, 40, 733–739. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, C.X. From Chronic Cerebral Hypoperfusion to Alzheimer-Like Brain Pathology and Neurodegeneration. Cell Mol. Neurobiol. 2015, 35, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ciacciarelli, A.; Sette, G.; Giubilei, F.; Orzi, F. Chronic Cerebral Hypoperfusion: An Undefined, Relevant Entity. J. Clin. Neurosci. 2020, 73, 8–12. [Google Scholar] [CrossRef]
- Breteler, M.M.B.; van Swieten, J.C.; Bots, M.L.; Grobbee, D.E.; Claus, J.J.; van den Hout, J.H.W.; van Harskamp, F.; Tanghe, H.L.J.; de Jong, P.T.V.M.; van Gijn, J.; et al. Cerebral White Matter Lesions, Vascular Risk Factors, and Cognitive Function in a Population-based Study. Neurology 1994, 44, 1246. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Ohh, M.; Park, C.W.; Ivan, M.; Hoffman, M.A.; Kim, T.Y.; Huang, L.E.; Pavletich, N.; Chau, V.; Kaelin, W.G. Ubiquitination of Hypoxia-Inducible Factor Requires Direct Binding to the β-Domain of the von Hippel—Lindau Protein. Nat. Cell Biol. 2000, 2, 423–427. [Google Scholar] [CrossRef]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-Inducible Nuclear Factors Bind to an Enhancer Element Located 3′ to the Human Erythropoietin Gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef] [PubMed]
- Bowser, J.L.; Lee, J.W.; Yuan, X.; Eltzschig, H.K. The Hypoxia-Adenosine Link during Inflammation. J. Appl. Physiol. 2017, 123, 1303–1320. [Google Scholar] [CrossRef]
- Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Ransohoff, R.M. A Polarizing Question: Do M1 and M2 Microglia Exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Pivoriūnas, A. Astroglia Support, Regulate and Reinforce Brain Barriers. Neurobiol. Dis. 2023, 179, 106054. [Google Scholar] [CrossRef]
- Fakhoury, M. Role of Immunity and Inflammation in the Pathophysiology of Neurodegenerative Diseases. Neurodegener. Dis. 2015, 15, 63–69. [Google Scholar] [CrossRef]
- Kurtishi, A.; Rosen, B.; Patil, K.S.; Alves, G.W.; Møller, S.G. Cellular Proteostasis in Neurodegeneration. Mol. Neurobiol. 2019, 56, 3676–3689. [Google Scholar] [CrossRef]
- Sheppard, O.; Coleman, M. Alzheimer’s Disease: Etiology, Neuropathology and Pathogenesis. In Alzheimer’s Disease: Drug Discovery; Exon Publications: Brisbane, Australia, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current Understanding of Alzheimer’s Disease Diagnosis and Treatment. F1000Res 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Rios-Romenets, S.; Lopera, F.; Sink, K.M.; Hu, N.; Lian, Q.; Guthrie, H.; Smith, J.; Cho, W.; Mackey, H.; Langbaum, J.B.; et al. Baseline Demographic, Clinical, and Cognitive Characteristics of the Alzheimer’s Prevention Initiative (API) Autosomal-Dominant Alzheimer’s Disease Colombia Trial. Alzheimer’s Dement. 2020, 16, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Decourt, B.; D’Souza, G.X.; Shi, J.; Ritter, A.; Suazo, J.; Sabbagh, M.N. The Cause of Alzheimer’s Disease: The Theory of Multipathology Convergence to Chronic Neuronal Stress. Aging Dis. 2022, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s Disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of Microglia and Astrocytes: A Roadway to Neuroinflammation and Alzheimer’s Disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Lopes, C.R.; Cunha, R.A.; Agostinho, P. Astrocytes and Adenosine A2A Receptors: Active Players in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 666710. [Google Scholar] [CrossRef]
- Gholami, A.; Mortezaee, K. Neurotransmitters in Neural Circuits and Neurological Diseases. ACS Chem. Neurosci. 2025, 16, 3653–3664. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The Scientific and Clinical Basis for the Treatment of Parkinson Disease (2009). Neurology 2009, 72, S1–S136. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E.; Shulman, G. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Tenreiro, S.; Eckermann, K.; Outeiro, T.F. Protein Phosphorylation in Neurodegeneration: Friend or Foe? Front. Mol. Neurosci. 2014, 7, 42. [Google Scholar] [CrossRef]
- Li, D.W.; Liu, Z.Q.; Chen, W.; Yao, M.; Li, G.R. Association of Glycogen Synthase Kinase-3β with Parkinson’s Disease (Review). Mol. Med. Rep. 2014, 9, 2043–2050. [Google Scholar] [CrossRef]
- Leak, R.K.; Clark, R.N.; Abbas, M.; Xu, F.; Brodsky, J.L.; Chen, J.; Hu, X.; Luk, K.C. Current Insights and Assumptions on α-Synuclein in Lewy Body Disease. Acta Neuropathol. 2024, 148, 1–21. [Google Scholar] [CrossRef]
- Bono, N.; Fruzzetti, F.; Farinazzo, G.; Candiani, G.; Marcuzzo, S. Perspectives in Amyotrophic Lateral Sclerosis: Biomarkers, Omics, and Gene Therapy Informing Disease and Treatment. Int. J. Mol. Sci. 2025, 26, 5671. [Google Scholar] [CrossRef]
- Koski, L.; Ronnevi, C.; Berntsson, E.; Wärmländer, S.K.T.S.; Roos, P.M. Metals in ALS TDP-43 Pathology. Int. J. Mol. Sci. 2021, 22, 12193. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Lee, S.; Jeon, Y.M.; Kim, S.; Kwon, Y.; Kim, H.J. The Role of TDP-43 Propagation in Neurodegenerative Diseases: Integrating Insights from Clinical and Experimental Studies. Exp. Mol. Med. 2020, 52, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E.; et al. TDP-43 Mutations in Familial and Sporadic Amyotrophic Lateral Sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef]
- Suk, T.R.; Rousseaux, M.W.C. The Role of TDP-43 Mislocalization in Amyotrophic Lateral Sclerosis. Mol. Neurodegener. 2020, 15, 45. [Google Scholar] [CrossRef]
- Gasset-rosa, F.; Lu, S.; Yu, H.; Shorter, J.; Cruz, S.D.; Cleveland, D.W.; Gasset-rosa, F.; Lu, S.; Yu, H.; Chen, C.; et al. Cytoplasmic TDP-43 De-Mixing Independent of Stress Granules Drives Inhibition of Nuclear Import, Loss of Nuclear TDP-43, and Cell Death. Neuron 2019, 102, 339–357. [Google Scholar] [CrossRef]
- Fernandes, N.; Nero, L.; Lyons, S.M.; Ivanov, P.; Mittelmeier, T.M.; Bolger, T.A.; Buchan, J.R. Stress Granule Assembly Can Facilitate but Is Not Required for TDP-43 Cytoplasmic Aggregation. Biomolecules 2020, 10, 1367. [Google Scholar] [CrossRef]
- Chen, Y.; Cohen, T.J. Aggregation of the Nucleic Acid–Binding Protein TDP-43 Occurs via Distinct Routes That Are Coordinated with Stress Granule Formation. J. Biol. Chem. 2019, 294, 3696–3706. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Rothstein, J.D. Glial Cells in Amyotrophic Lateral Sclerosis. Exp. Neurol. 2014, 262, 111–120. [Google Scholar] [CrossRef]
- Stoker, T.B.; Mason, S.L.; Greenland, J.C.; Holden, S.T.; Santini, H.; Barker, R.A. Huntington’s Disease: Diagnosis and Management. Pract. Neurol. 2022, 22, 32–41. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s Disease: A Clinical Review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Johnston, J.B.; Silva, C.; Gonzalez, G.; Holden, J.; Warren, K.G.; Metz, L.M.; Power, C. Diminished Adenosine A1 Receptor Expression on Macrophages in Brain and Blood of Patients with Multiple Sclerosis. Ann. Neurol. 2001, 49, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.F.; Xapelli, S.; Miranda-Lourenço, C.; Tanqueiro, S.R.; Fonseca-Gomes, J.; Diógenes, M.J.; Ribeiro, J.A.; Sebastião, A.M. Purine Nucleosides in Neuroregeneration and Neuroprotection. Neuropharmacology 2016, 104, 226–242. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P.; Vizi, E.S.; Illes, P. Adenosine Receptor Signaling in the Brain Immune System. Trends Pharmacol. Sci. 2005, 26, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Beamer, E.; Gölöncsér, F.; Horváth, G.; Beko, K.; Otrokocsi, L.; Koványi, B.; Sperlágh, B. Purinergic Mechanisms in Neuroinflammation: An Update from Molecules to Behavior. Neuropharmacology 2016, 104, 94–104. [Google Scholar] [CrossRef]
- Tsutsui, S.; Schnermann, J.; Noorbakhsh, F.; Henry, S.; Yong, V.W.; Winston, B.W.; Warren, K.; Power, C. A1 Adenosine Receptor Upregulation and Activation Attenuates Neuroinflammation and Demyelination in a Model of Multiple Sclerosis. J. Neurosci. 2004, 24, 1521–1529. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Haselkorn, M.L.; Shellington, D.K.; Jackson, E.K.; Vagni, V.A.; Janesko-Feldman, K.; Dubey, R.K.; Gillespie, D.G.; Cheng, D.; Bell, M.J.; Jenkins, L.W.; et al. Adenosine A1 Receptor Activation as a Brake on the Microglial Response after Experimental Traumatic Brain Injury in Mice. J. Neurotrauma 2010, 27, 901. [Google Scholar] [CrossRef]
- Synowitz, M.; Glass, R.; Färber, K.; Markovic, D.; Kronenberg, G.; Herrmann, K.; Schnermann, J.; Nolte, C.; Van Rooijen, N.; Kiwit, J.; et al. A1 Adenosine Receptors in Microglia Control Glioblastoma-Host Interaction. Cancer Res. 2006, 66, 8550–8557. [Google Scholar] [CrossRef]
- Luongo, L.; Guida, F.; Imperatore, R.; Napolitano, F.; Gatta, L.; Cristino, L.; Giordano, C.; Siniscalco, D.; Di Marzo, V.; Bellini, G.; et al. The A1 Adenosine Receptor as a New Player in Microglia Physiology. Glia 2014, 62, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, W.; Guo, J.; Kong, F.; Zhou, S.; Chen, S.; Wang, Z.; Zang, D. Adenosine Binds Predominantly to Adenosine Receptor A1 Subtype in Astrocytes and Mediates an Immunosuppressive Effect. Brain Res. 2018, 1700, 47–55. [Google Scholar] [CrossRef]
- Liu, S.L.; Tang, Y. Astrocytic Adenosine A1 Receptors: A New Potential Target for Treating Sepsis-Associated Encephalopathy. Purinergic Signal 2024, 21, 1–3. [Google Scholar] [CrossRef]
- De Mendonça, A.; Sebastião, A.M.; Ribeiro, J.A. Adenosine: Does It Have a Neuroprotective Role after All? Brain Res. Rev. 2000, 33, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, K.; Krasnow, M.A. The Hypoxic Response: Huffing and HIFing. Cell 1997, 89, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.J.; Schmitt, L.I.; Hines, R.M.; Moss, S.J.; Haydon, P.G. Antidepressant Effects of Sleep Deprivation Require Astrocyte-Dependent Adenosine Mediated Signaling. Transl. Psychiatry 2013, 3, e212. [Google Scholar] [CrossRef]
- Kaster, M.P.; Budni, J.; Santos, A.R.S.; Rodrigues, A.L.S. Pharmacological Evidence for the Involvement of the Opioid System in the Antidepressant-like Effect of Adenosine in the Mouse Forced Swimming Test. Eur. J. Pharmacol. 2007, 576, 91–98. [Google Scholar] [CrossRef]
- Nguyen, A.T.N.; Tran, Q.L.; Baltos, J.A.; McNeill, S.M.; Nguyen, D.T.N.; May, L.T. Small Molecule Allosteric Modulation of the Adenosine A1 Receptor. Front. Endocrinol. 2023, 14, 1184360. [Google Scholar] [CrossRef] [PubMed]
- Albrecht-Küpper, B.E.; Leineweber, K.; Nell, P.G. Partial Adenosine A1 Receptor Agonists for Cardiovascular Therapies. Purinergic Signal 2012, 8, 91–99. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What Is the Evidence? Front. Neurosci. 2015, 9, 170294. [Google Scholar] [CrossRef]
- Juvenal, G.; Higa, G.S.V.; Bonfim Marques, L.; Tessari Zampieri, T.; Costa Viana, F.J.; Britto, L.R.; Tang, Y.; Illes, P.; di Virgilio, F.; Ulrich, H.; et al. Regulation of GABAergic Neurotransmission by Purinergic Receptors in Brain Physiology and Disease. Purinergic Signal. 2024, 21, 149–177. [Google Scholar] [CrossRef]
- Boison, D. Adenosine Kinase: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2013, 65, 906–943. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sebastião, A.M.; De Mendonça, A. Adenosine Receptors in the Nervous System: Pathophysiological Implications. Prog. Neurobiol. 2002, 68, 377–392. [Google Scholar] [CrossRef]
- Dunwiddie, T.V.; Masino, S.A. The Role and Regulation of Adenosine in the Central Nervous System. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef]
- von Lubitz, D.K.J.E.; Carter, M.F.; Beenhakker, M.; Lin, R.C.; Jacobson, K.A. Adenosine: A Prototherapeutic Concept in Neurodegeneration. Ann. N. Y. Acad. Sci. 1995, 765, 163–178. [Google Scholar] [CrossRef]
- Fredholm, B.B. Chapter 11 Adenosine and Neuroprotection. Int. Rev. Neurobiol. 1996, 40, 259–280. [Google Scholar] [CrossRef]
- Lopes, C.R.; Lourenço, V.S.; Tomé, Â.R.; Cunha, R.A.; Canas, P.M. Use of Knockout Mice to Explore CNS Effects of Adenosine. Biochem. Pharmacol. 2021, 187, 114367. [Google Scholar] [CrossRef] [PubMed]
- Daval, J.L.; Nicolas, F. Non-Selective Effects of Adenosine A1 Receptor Ligands on Energy Metabolism and Macromolecular Biosynthesis in Cultured Central Neurons. Biochem. Pharmacol. 1998, 55, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Håberg, A.; Qu, H.; Haraldseth, O.; Unsgård, G.; Sonnewald, U. In Vivo Effects of Adenosine A1 Receptor Agonist and Antagonist on Neuronal and Astrocytic Intermediary Metabolism Studied with Ex Vivo 13C NMR Spectroscopy. J. Neurochem. 2000, 74, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Canals, S.; Larrosa, B.; Pintor, J.; Mena, M.A.; Herreras, O. Metabolic Challenge to Glia Activates an Adenosine-Mediated Safety Mechanism That Promotes Neuronal Survival by Delaying the Onset of Spreading Depression Waves. J. Cereb. Blood Flow Metab. 2008, 28, 1835–1844. [Google Scholar] [CrossRef]
- Duarte, J.M.N.; Cunha, R.A.; Carvalho, R.A. Adenosine A1 Receptors Control the Metabolic Recovery after Hypoxia in Rat Hippocampal Slices. J. Neurochem. 2016, 136, 947–957. [Google Scholar] [CrossRef]
- Chang, C.P.; Wu, K.C.; Lin, C.Y.; Chern, Y. Emerging Roles of Dysregulated Adenosine Homeostasis in Brain Disorders with a Specific Focus on Neurodegenerative Diseases. J. Biomed. Sci. 2021, 28, 70. [Google Scholar] [CrossRef]
- Nehlig, A. Effects of Coffee/Caffeine on Brain Health and Disease: What Should I Tell My Patients? Pract. Neurol. 2016, 16, 89–95. [Google Scholar] [CrossRef]
- Johansson, B.; Georgiev, V.; Lindström, K.; Fredholm, B.B. A1 and A2A Adenosine Receptors and A1 MRNA in Mouse Brain: Effect of Long-Term Caffeine Treatment. Brain Res. 1997, 762, 153–164. [Google Scholar] [CrossRef]
- Park, S.W.; Chen, S.W.C.; Kim, M.; D’Agati, V.D.; Lee, H.T. Selective Intrarenal Human A1 Adenosine Receptor Overexpression Reduces Acute Liver and Kidney Injury after Hepatic Ischemia Reperfusion in Mice. Lab. Investig. 2010, 90, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. How Does Adenosine Control Neuronal Dysfunction and Neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Coelho, J.E.; Rebola, N.; Fragata, I.; Ribeiro, J.A.; De Mendonça, A.; Cunha, R.A. Hypoxia-Induced Desensitization and Internalization of Adenosine A1 Receptors in the Rat Hippocampus. Neuroscience 2006, 138, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.E.; Corrêa, S.A.L.; Irving, A.J.; Frenguelli, B.G. Differential Trafficking of Adenosine Receptors in Hippocampal Neurons Monitored Using GFP- and Super-Ecliptic PHluorin-Tagged Receptors. Neuropharmacology 2011, 61, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Plamondon, H.; Blondeau, N.; Heurteaux, C.; Lazdunski, M. Mutually Protective Actions of Kainic Acid Epileptic Preconditioning and Sublethal Global Ischemia on Hippocampal Neuronal Death: Involvement of Adenosine A1 Receptors and K(ATP) Channels. J. Cereb. Blood Flow Metab. 1999, 19, 1296–1308. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Chen, J.F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.M. Adenosine and Brain Function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar] [CrossRef]
- Trendelenburg, G.; Dirnagl, U. Neuroprotective Role of Astrocytes in Cerebral Ischemia: Focus on Ischemic Preconditioning. Glia 2005, 50, 307–320. [Google Scholar] [CrossRef]
- Ułas, J.; Brunner, L.C.; Nguyen, L.; Cotman, C.W. Reduced Density of Adenosine A1 Receptors and Preserved Coupling of Adenosine A1 Receptors to G Proteins in Alzheimer Hippocampus: A Quantitative Autoradiographic Study. Neuroscience 1993, 52, 843–854. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Sromek, S.; Wilcox, B.J.; Unnerstall, J.R. Hippocampal Adenosine A1 Receptors Are Decreased in Alzheimer’s Disease. Neurosci. Lett. 1990, 118, 257–260. [Google Scholar] [CrossRef]
- Jansen, K.L.R.; Faull, R.L.M.; Dragunow, M.; Synek, B.L. Alzheimer’s Disease: Changes in Hippocampal N-Methyl-d-Aspartate, Quisqualate, Neurotensin, Adenosine, Benzodiazepine, Serotonin and Opioid Receptors—An Autoradiographic Study. Neuroscience 1990, 39, 613–627. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Evolution of the Neuropathology of Alzheimer’s Disease. Acta Neurol. Scand. 1996, 94, 3–12. [Google Scholar] [CrossRef]
- Deckert, J.; Abel, F.; Künig, G.; Hartmann, J.; Senitz, D.; Maier, H.; Ransmayr, G.; Riederer, P. Loss of Human Hippocampal Adenosine A1 Receptors in Dementia: Evidence for Lack of Specificity. Neurosci. Lett. 1998, 244, 1–4. [Google Scholar] [CrossRef]
- Angulo, E.; Casadó, V.; Mallol, J.; Canela, E.I.; Viñals, F.; Ferrer, I.; Lluis, C.; Franco, R. A1 Adenosine Receptors Accumulate in Neurodegenerative Structures in Alzheimer Disease and Mediate Both Amyloid Precursor Protein Processing and Tau Phosphorylation and Translocation. Brain Pathol. 2003, 13, 440–451. [Google Scholar] [CrossRef]
- Stone, T.W.; Ceruti, S.; Abbracchio, M.P. Adenosine Receptors and Neurological Disease: Neuroprotection and Neurodegeneration. Handb. Exp. Pharmacol. 2009, 193, 535–587. [Google Scholar] [CrossRef]
- Burnstock, G. An Introduction to the Roles of Purinergic Signalling in Neurodegeneration, Neuroprotection and Neuroregeneration. Neuropharmacology 2016, 104, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Albasanz, J.L.; Perez, S.; Barrachina, M.; Ferrer, I.; Martín, M. Up-Regulation of Adenosine Receptors in the Frontal Cortex in Alzheimer’s Disease. Brain Pathol. 2008, 18, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, E.; Rohani, M.; Shahidi, G.A.; Nafissi, S.; Arefian, E.; Soleimani, M.; Moghadam, A.; Arzenani, M.K.; Keramatian, F.; Klotzle, B.; et al. Mutation in ADORA1 Identified as Likely Cause of Early-Onset Parkinsonism and Cognitive Dysfunction. Mov. Disord. 2016, 31, 1004–1011. [Google Scholar] [CrossRef]
- Roberts, B.M.; Lambert, E.; Livesey, J.A.; Wu, Z.; Li, Y.; Cragg, S.J. Dopamine Release in Nucleus Accumbens Is under Tonic Inhibition by Adenosine A1 Receptors Regulated by Astrocytic ENT1 and Dysregulated by Ethanol. J. Neurosci. 2022, 42, 1738–1751. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Yang, G. Adenosinergic Pathway in Parkinson’s Disease: Recent Advances and Therapeutic Perspective. Mol. Neurobiol. 2023, 60, 3054–3070. [Google Scholar] [CrossRef]
- Ferré, S.; Fredholm, B.B.; Morelli, M.; Popoli, P.; Fuxe, K. Adenosine–Dopamine Receptor–Receptor Interactions as an Integrative Mechanism in the Basal Ganglia. Trends Neurosci. 1997, 20, 482–487. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Borroto-Escuela, D.O.; Guescini, M.; Fernández-Dueñas, V.; Tanganelli, S.; Rivera, A.; Ciruela, F.; Agnati, L.F. Adenosine–Dopamine Interactions in the Pathophysiology and Treatment of CNS Disorders. CNS Neurosci. Ther. 2010, 16, e18–e42. [Google Scholar] [CrossRef]
- Ferré, S.; Torvinen, M.; Antoniou, K.; Irenius, E.; Civelli, O.; Arenas, E.; Fredholm, B.B.; Fuxe, K. Adenosine A1 Receptor-Mediated Modulation of Dopamine D1 Receptors in Stably Cotransfected Fibroblast Cells. J. Biol. Chem. 1998, 273, 4718–4724. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.L.; Federico, S.; Spalluto, G.; Klotz, K.N.; Pastorin, G. The Current Status of Pharmacotherapy for the Treatment of Parkinson’s Disease: Transition from Single-Target to Multitarget Therapy. Drug Discov. Today 2019, 24, 1769–1783. [Google Scholar] [CrossRef]
- Ongini, E.; Fredholm, B.B. Pharmacology of Adenosine A2A Receptors. Trends Pharmacol. Sci. 1996, 17, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.C.; Gao, A.B.; Yang, J.; Zhong, L.Y.; Jia, B.; Ouyang, S.H.; Gui, L.; Peng, T.H.; Sun, S.; Cayabyab, F. Long-Term Adenosine A1 Receptor Activation-Induced Sortilin Expression Promotes α-Synuclein Upregulation in Dopaminergic Neurons. Neural Regen. Res. 2020, 15, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Jakova, E.; Moutaoufik, M.T.; Lee, J.S.; Babu, M.; Cayabyab, F.S. Adenosine A1 Receptor Ligands Bind to α-Synuclein: Implications for α-Synuclein Misfolding and α-Synucleinopathy in Parkinson’s Disease. Transl. Neurodegener. 2022, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- IJzerman, A.P.; Jacobson, K.A.; Müller, C.E.; Cronstein, B.N.; Cunha, R.A. International Union of Basic and Clinical Pharmacology. CXII: Adenosine Receptors: A Further Update. Pharmacol. Rev. 2022, 74, 340–372. [Google Scholar] [CrossRef]
- Renk, D.R.; Skraban, M.; Bier, D.; Schulze, A.; Wabbals, E.; Wedekind, F.; Neumaier, F.; Neumaier, B.; Holschbach, M. Design, Synthesis and Biological Evaluation of Tozadenant Analogues as Adenosine A2A Receptor Ligands. Eur. J. Med. Chem. 2021, 214, 113214. [Google Scholar] [CrossRef]
- Chiu, G.S.; Darmody, P.T.; Walsh, J.P.; Moon, M.L.; Kwakwa, K.A.; Bray, J.K.; McCusker, R.H.; Freund, G.G. Adenosine through the A2A Adenosine Receptor Increases IL-1β in the Brain Contributing to Anxiety. Brain Behav. Immun. 2014, 41, 218–231. [Google Scholar] [CrossRef]
- Bernier, L.P. Purinergic Regulation of Inflammasome Activation after Central Nervous System Injury. J. Gen. Physiol. 2012, 140, 571–575. [Google Scholar] [CrossRef]
- Rebola, N.; Simões, A.P.; Canas, P.M.; Tomé, A.R.; Andrade, G.M.; Barry, C.E.; Agostinho, P.M.; Lynch, M.A.; Cunha, R.A. Adenosine A2A Receptors Control Neuroinflammation and Consequent Hippocampal Neuronal Dysfunction. J. Neurochem. 2011, 117, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Cheng, W.; Wang, Y.; Chen, X.; Zhang, L.; Li, Y.; Shen, F.; Yuan, D.; Hong, P.; Huang, W. The Dual Role of A2aR in Neuroinflammation: Modulating Microglial Polarization in White Matter Lesions. eNeuro 2025, 12, ENEURO.0579-24.2025. [Google Scholar] [CrossRef] [PubMed]
- Pedata, F.; Pugliese, A.M.; Coppi, E.; Dettori, I.; Maraula, G.; Cellai, L.; Melani, A. Adenosine A2A Receptors Modulate Acute Injury and Neuroinflammation in Brain Ischemia. Mediat. Inflamm. 2014, 2014, 805198. [Google Scholar] [CrossRef]
- Reece, T.B.; Okonkwo, D.O.; Ellman, P.I.; Warren, P.S.; Smith, R.L.; Hawkins, A.S.; Linden, J.; Kron, I.L.; Tribble, C.G.; Kern, J.A. The Evolution of Ischemic Spinal Cord Injury in Function, Cytoarchitecture, and Inflammation and the Effects of Adenosine A2A Receptor Activation. J. Thorac. Cardiovasc. Surg. 2004, 128, 925–932. [Google Scholar] [CrossRef][Green Version]
- Guerrero, A. A2A Adenosine Receptor Agonists and Their Potential Therapeutic Applications. An Update. Curr. Med. Chem. 2018, 25, 3597–3612. [Google Scholar] [CrossRef] [PubMed]
- Boknik, P.; Eskandar, J.; Hofmann, B.; Zimmermann, N.; Neumann, J.; Gergs, U. Role of Cardiac A2A Receptors Under Normal and Pathophysiological Conditions. Front. Pharmacol. 2021, 11, 627838. [Google Scholar] [CrossRef]
- Boison, D. Adenosinergic Signaling in Epilepsy. Neuropharmacology 2016, 104, 131–139. [Google Scholar] [CrossRef]
- Sebastião, A.M.; Ribeiro, J.A. Adenosine A2 Receptor-Mediated Excitatory Actions on the Nervous System. Prog. Neurobiol. 1996, 48, 167–189. [Google Scholar] [CrossRef]
- Dai, S.S.; Zhou, Y.G. Adenosine 2A Receptor: A Crucial Neuromodulator with Bidirectional Effect in Neuroinflammation and Brain Injury. Rev. Neurosci. 2011, 22, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.G.; Orr, A.L.; Li, X.J.; Gross, R.E.; Traynelis, S.F. Adenosine A2A Receptor Mediates Microglial Process Retraction. Nat. Neurosci. 2009, 12, 872–878. [Google Scholar] [CrossRef]
- Gomes, C.A.R.V.; Vaz, S.H.; Ribeiro, J.A.; Sebastião, A.M. Glial Cell Line-Derived Neurotrophic Factor (GDNF) Enhances Dopamine Release from Striatal Nerve Endings in an Adenosine A2A Receptor-Dependent Manner. Brain Res. 2006, 1113, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Gyoneva, S.; Shapiro, L.; Lazo, C.; Garnier-Amblard, E.; Smith, Y.; Miller, G.W.; Traynelis, S.F. Adenosine A2A Receptor Antagonism Reverses Inflammation-Induced Impairment of Microglial Process Extension in a Model of Parkinson’s Disease. Neurobiol. Dis. 2014, 67, 191–202. [Google Scholar] [CrossRef]
- Minghetti, L.; Greco, A.; Potenza, R.L.; Pezzola, A.; Blum, D.; Bantubungi, K.; Popoli, P. Effects of the Adenosine A2A Receptor Antagonist SCH 58621 on Cyclooxygenase-2 Expression, Glial Activation, and Brain-Derived Neurotrophic Factor Availability in a Rat Model of Striatal Neurodegeneration. J. Neuropathol. Exp. Neurol. 2007, 66, 363–371. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Biber, K.; Lieb, K.; Van Calker, D.; Berger, M.; Bauer, J.; Gebicke-Haerter, P.J. Cyclooxygenase-2 Expression in Rat Microglia Is Induced by Adenosine A2a-Receptors. Glia 1996, 18, 152–180. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S.; Müller, V. From Homeostasis to Neuroinflammation: Insights into Cellular and Molecular Interactions and Network Dynamics. Cells 2025, 14, 54. [Google Scholar] [CrossRef]
- Colella, M.; Zinni, M.; Pansiot, J.; Cassanello, M.; Mairesse, J.; Ramenghi, L.; Baud, O. Modulation of Microglial Activation by Adenosine A2a Receptor in Animal Models of Perinatal Brain Injury. Front. Neurol. 2018, 9, 355877. [Google Scholar] [CrossRef]
- Merighi, S.; Borea, P.A.; Varani, K.; Vincenzi, F.; Jacobson, K.A.; Gessi, S. A2A Adenosine Receptor Antagonists in Neurodegenerative Diseases. Curr. Med. Chem. 2022, 29, 4138. [Google Scholar] [CrossRef]
- Pedata, F.; Gianfriddo, M.; Turchi, D.; Melani, A. The Protective Effect of Adenosine A2A Receptor Antagonism in Cerebral Ischemia. Neurol. Res. 2005, 27, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Cottini, L.; Fumagalli, M.; Ceruti, S.; Abbracchio, M.P. Blockade of A2A Adenosine Receptors Prevents Basic Fibroblast Growth Factor-Induced Reactive Astrogliosis in Rat Striatal Primary Astrocytes. Glia 2003, 43, 190–194. [Google Scholar] [CrossRef]
- Hindley, S.; Herman, M.A.R.; Rathbone, M.P. Stimulation of Reactive Astrogliosis in Vivo by Extracellular Adenosine Diphosphate or an Adenosine A2 Receptor Agonist. J. Neurosci. Res. 1994, 38, 399–406. [Google Scholar] [CrossRef]
- Paiva, I.; Carvalho, K.; Santos, P.; Cellai, L.; Pavlou, M.A.S.; Jain, G.; Gnad, T.; Pfeifer, A.; Vieau, D.; Fischer, A.; et al. A2AR-Induced Transcriptional Deregulation in Astrocytes: An in Vitro Study. Glia 2019, 67, 2329–2342. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Shen, H.Y.; Augusto, E.; Wang, Y.; Wei, C.J.; Wang, Y.T.; Agostinho, P.; Boison, D.; Cunha, R.A.; Chen, J.F. Deletion of Adenosine A2A Receptors From Astrocytes Disrupts Glutamate Homeostasis Leading to Psychomotor and Cognitive Impairment: Relevance to Schizophrenia. Biol. Psychiatry 2015, 78, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, L.; Wang, J.; Xia, S.; Huang, J.; Zhou, L.; Feng, C.; Hu, X.; Zhou, Z.; Ran, H. Adenosine A2A Receptor Suppressed Astrocyte-Mediated Inflammation Through the Inhibition of STAT3/YKL-40 Axis in Mice With Chronic Cerebral Hypoperfusion-Induced White Matter Lesions. Front. Immunol. 2022, 13, 841290. [Google Scholar] [CrossRef]
- Pugliese, A.M.; Traini, C.; Cipriani, S.; Gianfriddo, M.; Mello, T.; Giovannini, M.G.; Galli, A.; Pedata, F. The Adenosine A 2A Receptor Antagonist ZM241385 Enhances Neuronal Survival after Oxygen-Glucose Deprivation in Rat CA1 Hippocampal Slices. Br. J. Pharmacol. 2009, 157, 818–830. [Google Scholar] [CrossRef]
- Congreve, M.; Brown, G.A.; Borodovsky, A.; Lamb, M.L. Targeting Adenosine A 2A Receptor Antagonism for Treatment of Cancer. Expert. Opin. Drug Discov. 2018, 13, 997–1003. [Google Scholar] [CrossRef]
- Hodgson, R.A.; Bertorelli, R.; Varty, G.B.; Lachowicz, J.E.; Forlani, A.; Fredduzzi, S.; Cohen-Williams, M.E.; Higgins, G.A.; Impagnatiello, F.; Nicolussi, E.; et al. Characterization of the Potent and Highly Selective A2A Receptor Antagonists Preladenant and SCH 412348 [7-[2-[4-2,4-Difluorophenyl]-1-Piperazinyl]Ethyl]-2-(2-Furanyl)-7H-Pyrazolo[4,3-e][1,2,4]Triazolo[1,5-c]Pyrimidin-5-Amine] in Rodent Models of Movement Disorders and Depression. J. Pharmacol. Exp. Ther. 2009, 330, 294–303. [Google Scholar] [CrossRef]
- Pechlivanova, D.M.; Tchekalarova, J.D.; Alova, L.H.; Petkov, V.V.; Nikolov, R.P.; Yakimova, K.S. Effect of Long-Term Caffeine Administration on Depressive-like Behavior in Rats Exposed to Chronic Unpredictable Stress. Behav. Pharmacol. 2012, 23, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Thurner, P.; Gsandtner, I.; Kudlacek, O.; Choquet, D.; Nanoff, C.; Freissmuth, M.; Zezula, J. A Two-State Model for the Diffusion of the A 2A Adenosine Receptor in Hippocampal Neurons. J. Biol. Chem. 2014, 289, 9263–9274. [Google Scholar] [CrossRef]
- Chen, J.F.; Sonsalla, P.K.; Pedata, F.; Melani, A.; Domenici, M.R.; Popoli, P.; Geiger, J.; Lopes, L.V.; de Mendonça, A. Adenosine A2A Receptors and Brain Injury: Broad Spectrum of Neuroprotection, Multifaceted Actions and “Fine Tuning” Modulation. Prog. Neurobiol. 2007, 83, 310–331. [Google Scholar] [CrossRef]
- Boussadia, Z.; Chiodi, V.; Pazienti, A.; Martire, A. A Major Role for Adenosine A2A Receptor in the Interaction between Astrocytes and Myelinated Neurons: Possible Implications for the Therapy of Neurodegenerative Disorders. Purinergic Signal 2022, 18, 5–7. [Google Scholar] [CrossRef]
- Merighi, S.; Battistello, E.; Casetta, I.; Gragnaniello, D.; Poloni, T.E.; Medici, V.; Cirrincione, A.; Varani, K.; Vincenzi, F.; Borea, P.A.; et al. Upregulation of Cortical A2A Adenosine Receptors Is Reflected in Platelets of Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 80, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.G.; Matricon, P.; Eddy, M.T.; Carlsson, J. Adenosine A2A Receptor Antagonists: From Caffeine to Selective Non-Xanthines. Br. J. Pharmacol. 2022, 179, 3496–3511. [Google Scholar] [CrossRef]
- Woods, L.T.; Ajit, D.; Camden, J.M.; Erb, L.; Weisman, G.A. Purinergic Receptors as Potential Therapeutic Targets in Alzheimer’s Disease. Neuropharmacology 2016, 104, 169–179. [Google Scholar] [CrossRef]
- Matos, M.; Augusto, E.; Agostinho, P.; Cunha, R.A.; Chen, J.F. Antagonistic Interaction between Adenosine A2A Receptors and Na+/K+-ATPase-A2 Controlling Glutamate Uptake in Astrocytes. J. Neurosci. 2013, 33, 18492–18502. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.G.; Hsiao, E.C.; Wang, M.M.; Ho, K.; Kim, D.H.; Wang, X.; Guo, W.; Kang, J.; Yu, G.Q.; Adame, A.; et al. Astrocytic Adenosine Receptor A2A and Gs-Coupled Signaling Regulate Memory. Nat. Neurosci. 2015, 18, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.K.; Zacharia, L.C.; Cracchiolo, J.R.; Shippy, D.; Tan, J. Caffeine Protects Alzheimer’s Mice against Cognitive Impairment and Reduces Brain β-Amyloid Production. Neuroscience 2006, 142, 941–952. [Google Scholar] [CrossRef]
- Dall’lgna, O.P.; Porciúncula, L.O.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Neuroprotection by Caffeine and Adenosine A 2A Receptor Blockade of β-Amyloid Neurotoxicity. Br. J. Pharmacol. 2003, 138, 1207–1209. [Google Scholar] [CrossRef]
- Cao, C.; Cirrito, J.R.; Lin, X.; Wang, L.; Verges, D.K.; Dickson, A.; Mamcarz, M.; Zhang, C.; Mori, T.; Arendash, G.W.; et al. Caffeine Suppresses Amyloid-β Levels in Plasma and Brain of Alzheimer’s Disease Transgenic Mice. J. Alzheimer’s Dis. 2009, 17, 681–697. [Google Scholar] [CrossRef]
- Canas, P.M.; Porciúncula, L.O.; Cunha, G.M.A.; Silva, C.G.; Machado, N.J.; Oliveira, J.M.A.; Oliveira, C.R.; Cunha, R.A. Adenosine A2A Receptor Blockade Prevents Synaptotoxicity and Memory Dysfunction Caused by β-Amyloid Peptides via P38 Mitogen-Activated Protein Kinase Pathway. J. Neurosci. 2009, 29, 14741–14751. [Google Scholar] [CrossRef]
- Canas, P.M.; Cunha, R.A.; Agostinho, P. Adenosine Receptors in Alzheimer’s Disease. Receptors 2018, 34, 259–280. [Google Scholar] [CrossRef]
- Da Silva, S.V.; Haberl, M.G.; Zhang, P.; Bethge, P.; Lemos, C.; Gonçalves, N.; Gorlewicz, A.; Malezieux, M.; Gonçalves, F.Q.; Grosjean, N.; et al. Early Synaptic Deficits in the APP/PS1 Mouse Model of Alzheimer’s Disease Involve Neuronal Adenosine A2A Receptors. Nat. Commun. 2016, 7, 11915. [Google Scholar] [CrossRef]
- Chen, J.F. Adenosine Receptor Control of Cognition in Normal and Disease. Int. Rev. Neurobiol. 2014, 119, 257–307. [Google Scholar] [CrossRef]
- Temido-Ferreira, M.; Ferreira, D.G.; Batalha, V.L.; Marques-Morgado, I.; Coelho, J.E.; Pereira, P.; Gomes, R.; Pinto, A.; Carvalho, S.; Canas, P.M.; et al. Age-Related Shift in LTD Is Dependent on Neuronal Adenosine A2A Receptors Interplay with MGluR5 and NMDA Receptors. Mol. Psychiatry 2020, 25, 1876–1900. [Google Scholar] [CrossRef]
- Gomez-Murcia, V.; Launay, A.; Carvalho, K.; Burgard, A.; Meriaux, C.; Caillierez, R.; Eddarkaoui, S.; Kilinc, D.; Siedlecki-Wullich, D.; Besegher, M.; et al. Neuronal A2A Receptor Exacerbates Synapse Loss and Memory Deficits in APP/PS1 Mice. Brain 2024, 147, 2691–2705. [Google Scholar] [CrossRef]
- Launay, A.; Carvalho, K.; Genin, A.; Gauvrit, T.; Nobili, P.; Gomez-Murcia, V.; Augustin, E.; Burgard, A.; Gambi, J.; Fourmy, D.; et al. Upregulation of Adenosine A2A Receptor in Astrocytes Is Sufficient to Trigger Hippocampal Multicellular Dysfunctions and Memory Deficits. Mol. Psychiatry 2025, 1–15. [Google Scholar] [CrossRef]
- Morelli, M.; Carta, A.R.; Kachroo, A.; Schwarzschild, M.A. Pathophysiological Roles for Purines: Adenosine, Caffeine and Urate. Prog. Brain Res. 2010, 183, 183–208. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, S.N.; Fisone, G.; Moresco, R.; Cunha, R.A.; Ferré, S. Adenosine A2A Receptors and Basal Ganglia Physiology. Prog. Neurobiol. 2007, 83, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Ferre, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C.; et al. Adenosine A2A and Dopamine D2 Heteromeric Receptor Complexes and Their Function. J. Mol. Neurosci. 2005, 26, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.; Carta, A.R.; Jenner, P. Adenosine A2A Receptors and Parkinson’s Disease. Handb. Exp. Pharmacol. 2009, 193, 589–615. [Google Scholar] [CrossRef]
- Pinna, A. Adenosine A2A Receptor Antagonists in Parkinson’s Disease: Progress in Clinical Trials from the Newly Approved Istradefylline to Drugs in Early Development and Those Already Discontinued. CNS Drugs 2014, 28, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Uchida, S. ichi Clinical/Pharmacological Aspect of Adenosine A2A Receptor Antagonist for Dyskinesia. Int. Rev. Neurobiol. 2014, 119, 127–150. [Google Scholar] [CrossRef]
- Ferreira, D.G.; Batalha, V.L.; Miranda, H.V.; Coelho, J.E.; Gomes, R.; Gonçalves, F.Q.; Real, J.I.; Rino, J.; Albino-Teixeira, A.; Cunha, R.A.; et al. Adenosine A2A Receptors Modulate α-Synuclein Aggregation and Toxicity. Cereb. Cortex 2017, 27, 718–730. [Google Scholar] [CrossRef]
- Uchida, S.-i.; Kadowaki-Horita, T.; Kanda, T. Effects of the Adenosine A2A Receptor Antagonist on Cognitive Dysfunction in Parkinson’s Disease. Int. Rev. Neurobiol. 2014, 119, 169–189. [Google Scholar] [CrossRef]
- Yamada, K.; Kobayashi, M.; Shiozaki, S.; Ohta, T.; Mori, A.; Jenner, P.; Kanda, T. Antidepressant Activity of the Adenosine A2A Receptor Antagonist, Istradefylline (KW-6002) on Learned Helplessness in Rats. Psychopharmacology 2014, 231, 2839–2849. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s Disease: Its Role in Neuronal Death and Implications for Therapeutic Intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Pinna, A. Novel Investigational Adenosine A2A Receptor Antagonists for Parkinson’s Disease. Expert. Opin. Investig. Drugs 2009, 18, 1619–1631. [Google Scholar] [CrossRef]
- Chen, J.F.; Cunha, R.A. The Belated US FDA Approval of the Adenosine A2A Receptor Antagonist Istradefylline for Treatment of Parkinson’s Disease. Purinergic Signal 2020, 16, 167–174. [Google Scholar] [CrossRef]
- Petzer, J.P.; Steyn, S.; Castagnoli, K.P.; Chen, J.F.; Schwarzschild, M.A.; Van Der Schyf, C.J.; Castagnoli, N. Inhibition of Monoamine Oxidase B by Selective Adenosine A2A Receptor Antagonists. Bioorg Med. Chem. 2003, 11, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Preti, D.; Baraldi, P.G.; Moorman, A.R.; Borea, P.A.; Varani, K. History and Perspectives of A2A Adenosine Receptor Antagonists as Potential Therapeutic Agents. Med. Res. Rev. 2015, 35, 790–848. [Google Scholar] [CrossRef] [PubMed]
- Jaiteh, M.; Zeifman, A.; Saarinen, M.; Svenningsson, P.; Bréa, J.; Loza, M.I.; Carlsson, J. Docking Screens for Dual Inhibitors of Disparate Drug Targets for Parkinson’s Disease. J. Med. Chem. 2018, 61, 5269–5278. [Google Scholar] [CrossRef] [PubMed]
- Stößel, A.; Schlenk, M.; Hinz, S.; Küppers, P.; Heer, J.; Gütschow, M.; Müller, C.E. Dual Targeting of Adenosine A2A Receptors and Monoamine Oxidase B by 4H-3,1-Benzothiazin-4-Ones. J. Med. Chem. 2013, 56, 4580–4596. [Google Scholar] [CrossRef]
- Popoli, P.; Blum, D.; Martire, A.; Ledent, C.; Ceruti, S.; Abbracchio, M.P. Functions, Dysfunctions and Possible Therapeutic Relevance of Adenosine A2A Receptors in Huntington’s Disease. Prog. Neurobiol. 2007, 81, 331–348. [Google Scholar] [CrossRef]
- Varani, K.; Bachoud-Lévi, A.C.; Mariotti, C.; Tarditi, A.; Abbracchio, M.P.; Gasperi, V.; Borea, P.A.; Dolbeau, G.; Gellera, C.; Solari, A.; et al. Biological Abnormalities of Peripheral A2A Receptors in a Large Representation of Polyglutamine Disorders and Huntington’s Disease Stages. Neurobiol. Dis. 2007, 27, 36–43. [Google Scholar] [CrossRef]
- Tarditi, A.; Camurri, A.; Varani, K.; Borea, P.A.; Woodman, B.; Bates, G.; Cattaneo, E.; Abbracchio, M.P. Early and Transient Alteration of Adenosine A2A Receptor Signaling in a Mouse Model of Huntington Disease. Neurobiol. Dis. 2006, 23, 44–53. [Google Scholar] [CrossRef]
- Lee, C.f.; Chern, Y. Adenosine Receptors and Huntington’s Disease. Int. Rev. Neurobiol. 2014, 119, 195–232. [Google Scholar] [CrossRef]
- Mojsilovic-Petrovic, J.; Jeong, G.B.; Crocker, A.; Arneja, A.; David, S.; Russell, D.; Kalb, R.G. Protecting Motor Neurons from Toxic Insult by Antagonism of Adenosine A2a and Trk Receptors. J. Neurosci. 2006, 26, 9250–9263. [Google Scholar] [CrossRef]
- Volonté, C.; Apolloni, S.; Parisi, C.; Amadio, S. Purinergic Contribution to Amyotrophic Lateral Sclerosis. Neuropharmacology 2016, 104, 180–193. [Google Scholar] [CrossRef]
- Higashimori, H.; Tolman, M.; Yang, Y. Suppression of Adenosine 2a Receptor (A 2a R)-Mediated Adenosine Signaling Improves Disease Phenotypes in a Mouse Model of Amyotrophic Lateral Sclerosis. Exp. Neurol. 2015, 267, 115–122. [Google Scholar] [CrossRef]
- Wiese, S.; Jablonka, S.; Holtmann, B.; Orel, N.; Rajagopal, R.; Chao, M.V.; Sendtner, M. Adenosine Receptor A2A-R Contributes to Motoneuron Survival by Transactivating the Tyrosine Kinase Receptor TrkB. Proc. Natl. Acad. Sci. USA 2007, 104, 17210–17215. [Google Scholar] [CrossRef]
- Yanpallewar, S.U.; Barrick, C.A.; Buckley, H.; Becker, J.; Tessarollo, L. Deletion of the BDNF Truncated Receptor TrkB.T1 Delays Disease Onset in a Mouse Model of Amyotrophic Lateral Sclerosis. PLoS ONE 2012, 7, e39946. [Google Scholar] [CrossRef]
- Nascimento, F.; Pousinha, P.A.; Correia, A.M.; Gomes, R.; Sebastião, A.M.; Ribeiro, J.A. Adenosine A2A Receptors Activation Facilitates Neuromuscular Transmission in the Pre-Symptomatic Phase of the SOD1(G93A) ALS Mice, but Not in the Symptomatic Phase. PLoS ONE 2014, 9, e104081. [Google Scholar] [CrossRef] [PubMed]
- Rei, N.; Rombo, D.M.; Ferreira, M.F.; Baqi, Y.; Müller, C.E.; Ribeiro, J.A.; Sebastião, A.M.; Vaz, S.H. Hippocampal Synaptic Dysfunction in the SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis: Reversal by Adenosine A2AR Blockade. Neuropharmacology 2020, 171, 108106. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Liu, Y.J.; Lai, H.L.; Chen, H.M.; Kuo, H.C.; Liao, Y.P.; Chern, Y. The D2 Dopamine Receptor Interferes with the Protective Effect of the A2A Adenosine Receptor on TDP-43 Mislocalization in Experimental Models of Motor Neuron Degeneration. Front. Neurosci. 2018, 12, 336030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Ju, T.C.; Chen, H.M.; Jang, Y.S.; Lee, L.M.; Lai, H.L.; Tai, H.C.; Fang, J.M.; Lin, Y.L.; Tu, P.H.; et al. Activation of AMP-Activated Protein Kinase A1 Mediates Mislocalization of TDP-43 in Amyotrophic Lateral Sclerosis. Hum. Mol. Genet. 2015, 24, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Armida, M.; Matteucci, A.; Pèzzola, A.; Baqi, Y.; Müller, C.E.; Popoli, P.; Potenza, R.L. Modulating P1 Adenosine Receptors in Disease Progression of SOD1 G93A Mutant Mice. Neurochem. Res. 2019, 44, 1037–1042. [Google Scholar] [CrossRef]
- Potenza, R.L.; Armida, M.; Ferrante, A.; Pèzzola, A.; Matteucci, A.; Puopolo, M.; Popoli, P. Effects of Chronic Caffeine Intake in a Mouse Model of Amyotrophic Lateral Sclerosis. J. Neurosci. Res. 2013, 91, 585–592. [Google Scholar] [CrossRef]
- Fondell, E.; Oreilly, É.J.; Fitzgerald, K.C.; Falcone, G.J.; Kolonel, L.N.; Park, Y.; Gapstur, S.M.; Ascherio, A. Intakes of Caffeine, Coffee and Tea and Risk of Amyotrophic Lateral Sclerosis: Results from Five Cohort Studies. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 366–371. [Google Scholar] [CrossRef]
- Beghi, E.; Pupillo, E.; Messina, P.; Giussani, G.; Chiò, A.; Zoccolella, S.; Moglia, C.; Corbo, M.; Logroscino, G.; Al-Chalabi, A.; et al. Coffee and Amyotrophic Lateral Sclerosis: A Possible Preventive Role. Am. J. Epidemiol. 2011, 174, 1002–1008. [Google Scholar] [CrossRef]
- Huin, V.; Blum, D.; Delforge, V.; Cailliau, E.; Djeziri, S.; Dujardin, K.; Genet, A.; Viard, R.; Attarian, S.; Bruneteau, G.; et al. Caffeine Consumption Outcomes on Amyotrophic Lateral Sclerosis Disease Progression and Cognition. Neurobiol. Dis. 2024, 199, 106603. [Google Scholar] [CrossRef]
- Vincenzi, F.; Corciulo, C.; Targa, M.; Casetta, I.; Gentile, M.; Granieri, E.; Borea, P.A.; Popoli, P.; Varani, K. A2A Adenosine Receptors Are Up-Regulated in Lymphocytes from Amyotrophic Lateral Sclerosis Patients. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 406–413. [Google Scholar] [CrossRef]
- Chen, J.F.; Huang, Z.; Ma, J.; Zhu, J.M.; Moratalla, R.; Standaert, D.; Moskowitz, M.A.; Fink, J.S.; Schwarzschild, M.A. A2A Adenosine Receptor Deficiency Attenuates Brain Injury Induced by Transient Focal Ischemia in Mice. J. Neurosci. 1999, 19, 9192–9200. [Google Scholar] [CrossRef]
- Hinz, S.; Lacher, S.K.; Seibt, B.F.; Müller, C.E. BAY60-6583 Acts as a Partial Agonist at Adenosine A2B Receptors. J. Pharmacol. Exp. Ther. 2014, 349, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Beukers, M.W.; Meurs, I.; Ijzerman, A.P. Structure-Affinity Relationships of Adenosine A2B Receptor Ligands. Med. Res. Rev. 2006, 26, 667–698. [Google Scholar] [CrossRef]
- Härter, M.; Kalthof, B.; Delbeck, M.; Lustig, K.; Gerisch, M.; Schulz, S.; Kast, R.; Meibom, D.; Lindner, N. Novel Non-Xanthine Antagonist of the A2B Adenosine Receptor: From HTS Hit to Lead Structure. Eur. J. Med. Chem. 2019, 163, 763–778. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Han, S.-W.; Lee, S.; Lee, K.-W.; Kopetz, S.; Mizrahi, J.; Hong, Y.S.; Ghiringhelli, F.; Italiano, A.; Tougeron, D.; et al. ARC-9: A Randomized Study to Evaluate Etrumadenant Based Treatment Combinations in Previously Treated Metastatic Colorectal Cancer (MCRC). J. Clin. Oncol. 2024, 42, 3508. [Google Scholar] [CrossRef]
- Claff, T.; Schlegel, J.G.; Voss, J.H.; Vaaßen, V.J.; Weiße, R.H.; Cheng, R.K.Y.; Markovic-Mueller, S.; Bucher, D.; Sträter, N.; Müller, C.E. Crystal Structure of Adenosine A2A Receptor in Complex with Clinical Candidate Etrumadenant Reveals Unprecedented Antagonist Interaction. Commun. Chem. 2023, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.K.; Gubitz, A.K.; Sirinathsinghji, D.J.S.; Richardson, P.J.; Freeman, T.C. Tissue Distribution of Adenosine Receptor MRNAs in the Rat. Br. J. Pharmacol. 1996, 118, 1461–1468. [Google Scholar] [CrossRef]
- Koscsó, B.; Csóka, B.; Selmeczy, Z.; Himer, L.; Pacher, P.; Virág, L.; Haskó, G. Adenosine Augments IL-10 Production by Microglial Cells through an A2B Adenosine Receptor-Mediated Process. J. Immunol. 2012, 188, 445–453. [Google Scholar] [CrossRef]
- Merighi, S.; Bencivenni, S.; Vincenzi, F.; Varani, K.; Borea, P.A.; Gessi, S. A2B Adenosine Receptors Stimulate IL-6 Production in Primary Murine Microglia through P38 MAPK Kinase Pathway. Pharmacol. Res. 2017, 117, 9–19. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Biber, K.; Gyufko, K.; Berger, M.; Bauer, J.; Van Calker, D. Adenosine A2b Receptors Mediate an Increase in Interleukin (IL)-6 MRNA and IL-6 Protein Synthesis in Human Astroglioma Cells. J. Neurochem. 1996, 66, 1426–1431. [Google Scholar] [CrossRef]
- Rosi, S.; McGann, K.; Hauss-Wegrzyniak, B.; Wenk, G.L. The Influence of Brain Inflammation upon Neuronal Adenosine A2B Receptors. J. Neurochem. 2003, 86, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular Basis of Astrocyte Diversity and Morphology across the CNS in Health and Disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Kopach, O.; Braga, A.; Nizari, S.; Hosford, P.S.; Sagi-Kiss, V.; Hadjihambi, A.; Konstantinou, C.; Esteras, N.; Gutierrez Del Arroyo, A.; et al. Adenosine Signalling to Astrocytes Coordinates Brain Metabolism and Function. Nature 2024, 632, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Fusco, I.; Ugolini, F.; Lana, D.; Coppi, E.; Dettori, I.; Gaviano, L.; Nosi, D.; Cherchi, F.; Pedata, F.; Giovannini, M.G.; et al. The Selective Antagonism of Adenosine A2B Receptors Reduces the Synaptic Failure and Neuronal Death Induced by Oxygen and Glucose Deprivation in Rat CA1 Hippocampus in Vitro. Front. Pharmacol. 2018, 9, 329222. [Google Scholar] [CrossRef]
- Coppi, E.; Dettori, I.; Cherchi, F.; Bulli, I.; Venturini, M.; Lana, D.; Giovannini, M.G.; Pedata, F.; Pugliese, A.M. A2B Adenosine Receptors: When Outsiders May Become an Attractive Target to Treat Brain Ischemia or Demyelination. Int. J. Mol. Sci. 2020, 21, 9697. [Google Scholar] [CrossRef] [PubMed]
- Popoli, P.; Pepponi, R. Potential Therapeutic Relevance of Adenosine A2B and A2A Receptors in the Central Nervous System. CNS Neurol. Disord. Drug Targets 2012, 11, 664–674. [Google Scholar] [CrossRef]
- Venturini, M.; Cherchi, F.; Santalmasi, C.; Frulloni, L.; Dettori, I.; Catarzi, D.; Pedata, F.; Colotta, V.; Varano, F.; Coppi, E.; et al. Pharmacological Characterization of P626, a Novel Dual Adenosine A2A/A2B Receptor Antagonist, on Synaptic Plasticity and during an Ischemic-like Insult in CA1 Rat Hippocampus. Biomolecules 2023, 13, 894. [Google Scholar] [CrossRef]
- Dettori, I.; Bulli, I.; Venturini, M.; Magni, G.; Cherchi, F.; Rossi, F.; Lee, H.; Pedata, F.; Jacobson, K.A.; Pugliese, A.M.; et al. MRS3997, a Dual Adenosine A2A/A2B Receptor Agonist, Reduces Brain Ischemic Damage and Alleviates Neuroinflammation in Rats. Neuropharmacology 2025, 262, 110214. [Google Scholar] [CrossRef]
- Semwal, B.C.; Garabadu, D. 5-N-Ethyl Carboxamidoadenosine Stimulates Adenosine-2b Receptor-Mediated Mitogen-Activated Protein Kinase Pathway to Improve Brain Mitochondrial Function in Amyloid Beta-Induced Cognitive Deficit Mice. Neuromol. Med. 2020, 22, 542–556. [Google Scholar] [CrossRef]
- Semwal, B.C.; Garabadu, D. Amyloid Beta (1–42) Downregulates Adenosine-2b Receptors in Addition to Mitochondrial Impairment and Cholinergic Dysfunction in Memory-Sensitive Mouse Brain Regions. J. Recept. Signal Transduct. 2020, 40, 531–540. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky Gut: Mechanisms, Measurement and Clinical Implications in Humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Ishioh, M.; Nozu, T.; Miyagishi, S.; Igarashi, S.; Funayama, T.; Ohhira, M.; Okumura, T. Activation of Basal Forebrain Cholinergic Neurons Improves Colonic Hyperpermeability through the Vagus Nerve and Adenosine A2B Receptors in Rats. Biochem. Pharmacol. 2022, 206, 115331. [Google Scholar] [CrossRef] [PubMed]
- Ishioh, M.; Nozu, T.; Miyagishi, S.; Igarashi, S.; Funayama, T.; Ueno, N.; Okumura, T. Brain Histamine Improves Colonic Hyperpermeability through the Basal Forebrain Cholinergic Neurons, Adenosine A2B Receptors and Vagus Nerve in Rats. Biochem. Pharmacol. 2024, 224, 116201. [Google Scholar] [CrossRef] [PubMed]
- Ishioh, M.; Nozu, T.; Igarashi, S.; Tanabe, H.; Kumei, S.; Ohhira, M.; Takakusaki, K.; Okumura, T. Activation of Central Adenosine A2B Receptors Mediate Brain Ghrelin-Induced Improvement of Intestinal Barrier Function through the Vagus Nerve in Rats. Exp. Neurol. 2021, 341, 113708. [Google Scholar] [CrossRef] [PubMed]
- Ishioh, M.; Nozu, T.; Miyagishi, S.; Funayama, T.; Ueno, N.; Takakusaki, K.; Okumura, T. Carnosine Improves Colonic Hyperpermeability through the Brain Histamine H1 Receptor, Basal Forebrain Cholinergic Neurons, Adenosine A2B Receptors and Vagus Nerve in Rats. Eur. J. Pharmacol. 2025, 1002, 177844. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Cacciari, B.; Romagnoli, R.; Merighi, S.; Varani, K.; Borea, P.A.; Spalluto, G. A3 Adenosine Receptor Ligands: History and Perspectives. Med. Res. Rev. 2000, 20, 103–128. [Google Scholar] [CrossRef]
- Moro, S.; Gao, Z.; Jacobson, K.A.; Spalluto, G. Progress in the Pursuit of Therapeutic Adenosine Receptor Antagonists. Med. Res. Rev. 2006, 26, 131–159. [Google Scholar] [CrossRef]
- Tosh, D.K.; Deflorian, F.; Phan, K.; Gao, Z.; Wan, T.C.; Gizewski, E.; Auchampach, J.A.; Jacobson, K.A. Structure-Guided Design of A 3 Adenosine Receptor-Selective Nucleosides: Combination of 2-Arylethynyl and Bicyclo[3.1.0]Hexane Substitutions. J. Med. Chem. 2012, 55, 4847–4860. [Google Scholar] [CrossRef]
- Tosh, D.K.; Padia, J.; Salvemini, D.; Jacobson, K.A. Efficient, Large-Scale Synthesis and Preclinical Studies of MRS5698, a Highly Selective A3 Adenosine Receptor Agonist That Protects against Chronic Neuropathic Pain. Purinergic Signal 2015, 11, 371–387. [Google Scholar] [CrossRef]
- Gao, Z.G.; Suresh, R.R.; Jacobson, K.A. Pharmacological Characterization of DPTN and Other Selective A3 Adenosine Receptor Antagonists. Purinergic Signal 2021, 17, 737–746. [Google Scholar] [CrossRef]
- Ohsawa, K.; Sanagi, T.; Nakamura, Y.; Suzuki, E.; Inoue, K.; Kohsaka, S. Adenosine A3 Receptor Is Involved in ADP-Induced Microglial Process Extension and Migration. J. Neurochem. 2012, 121, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Lee, J.C.; Ju, C.; Hwang, S.; Cho, G.S.; Lee, H.W.; Choi, W.J.; Jeong, L.S.; Kim, W.K. A3 Adenosine Receptor Agonist Reduces Brain Ischemic Injury and Inhibits Inflammatory Cell Migration in Rats. Am. J. Pathol. 2011, 179, 2042–2052. [Google Scholar] [CrossRef]
- Terayama, R.; Tabata, M.; Maruhama, K.; Iida, S. A 3 Adenosine Receptor Agonist Attenuates Neuropathic Pain by Suppressing Activation of Microglia and Convergence of Nociceptive Inputs in the Spinal Dorsal Horn. Exp. Brain Res. 2018, 236, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Coppi, E.; Cherchi, F.; Lucarini, E.; Ghelardini, C.; Pedata, F.; Jacobson, K.A.; Mannelli, L.D.C.; Pugliese, A.M.; Salvemini, D. Uncovering the Mechanisms of Adenosine Receptor-Mediated Pain Control: Focus on the A3 Receptor Subtype. Int. J. Mol. Sci. 2021, 22, 7952. [Google Scholar] [CrossRef]
- Lillo, A.; Serrano-Marín, J.; Lillo, J.; Raïch, I.; Navarro, G.; Franco, R. Gene Regulation in Activated Microglia by Adenosine A3 Receptor Agonists: A Transcriptomics Study. Purinergic Signal 2024, 20, 237–245. [Google Scholar] [CrossRef]
- Fedorova, I.M.; Jacobson, M.A.; Basile, A.; Jacobson, K.A. Behavioral Characterization of Mice Lacking the A3 Adenosine Receptor: Sensitivity to Hypoxic Neurodegeneration. Cell Mol. Neurobiol. 2003, 23, 431–447. [Google Scholar] [CrossRef]
- Maria Pugliese, A.; Coppi, E.; Volpini, R.; Cristalli, G.; Corradetti, R.; Jeong, L.S.; Jacobson, K.A.; Pedata, F. Role of Adenosine A3 Receptors on CA1 Hippocampal Neurotransmission during Oxygen–Glucose Deprivation Episodes of Different Duration. Biochem. Pharmacol. 2007, 74, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.M.; Coppi, E.; Spalluto, G.; Corradetti, R.; Pedata, F. A3 Adenosine Receptor Antagonists Delay Irreversible Synaptic Failure Caused by Oxygen and Glucose Deprivation in the Rat CA1 Hippocampus in Vitro. Br. J. Pharmacol. 2006, 147, 524–532. [Google Scholar] [CrossRef]
- Farr, S.A.; Cuzzocrea, S.; Esposito, E.; Campolo, M.; Niehoff, M.L.; Doyle, T.M.; Salvemini, D. Adenosine A3 Receptor as a Novel Therapeutic Target to Reduce Secondary Events and Improve Neurocognitive Functions Following Traumatic Brain Injury. J. Neuroinflamm. 2020, 17, 339. [Google Scholar] [CrossRef] [PubMed]
- Lillo, A.; Raïch, I.; Lillo, J.; Pérez-Olives, C.; Navarro, G.; Franco, R. Expression of the Adenosine A2A-A3 Receptor Heteromer in Different Brain Regions and Marked Upregulation in the Microglia of the Transgenic APPSw,Ind Alzheimer’s Disease Model. Biomedicines 2022, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Geiger, N.H.; Soliman, M.L.; Hui, L.; Geiger, J.D.; Chen, X. Caffeine, Through Adenosine A3 Receptor-Mediated Actions, Suppresses Amyloid-β Protein Precursor Internalization and Amyloid-β Generation. J. Alzheimer’s Dis. 2015, 47, 73–83. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmaso, V.; Menin, S.; Moro, S.; Spalluto, G.; Federico, S. Adenosine Receptors in Neuroinflammation and Neurodegeneration. Cells 2025, 14, 1585. https://doi.org/10.3390/cells14201585
Salmaso V, Menin S, Moro S, Spalluto G, Federico S. Adenosine Receptors in Neuroinflammation and Neurodegeneration. Cells. 2025; 14(20):1585. https://doi.org/10.3390/cells14201585
Chicago/Turabian StyleSalmaso, Veronica, Silvia Menin, Stefano Moro, Giampiero Spalluto, and Stephanie Federico. 2025. "Adenosine Receptors in Neuroinflammation and Neurodegeneration" Cells 14, no. 20: 1585. https://doi.org/10.3390/cells14201585
APA StyleSalmaso, V., Menin, S., Moro, S., Spalluto, G., & Federico, S. (2025). Adenosine Receptors in Neuroinflammation and Neurodegeneration. Cells, 14(20), 1585. https://doi.org/10.3390/cells14201585









