Structural Variants of Dermatan Sulfate Can Affect the Expression of Proteins Involved in Breast Cancer Cell Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. The Used Structural Isoforms of DS
2.2. Cell Cultures
2.3. Quantitative Analysis of Gene Expression on the RNA Level
2.4. Analysis of Protein Expression and Phosphoinositide 3-Kinase Activation by Western Blotting
2.5. Analysis of Protein Level in Breast Cancer Cells by Immunofluorescence
2.6. Statistical Analysis
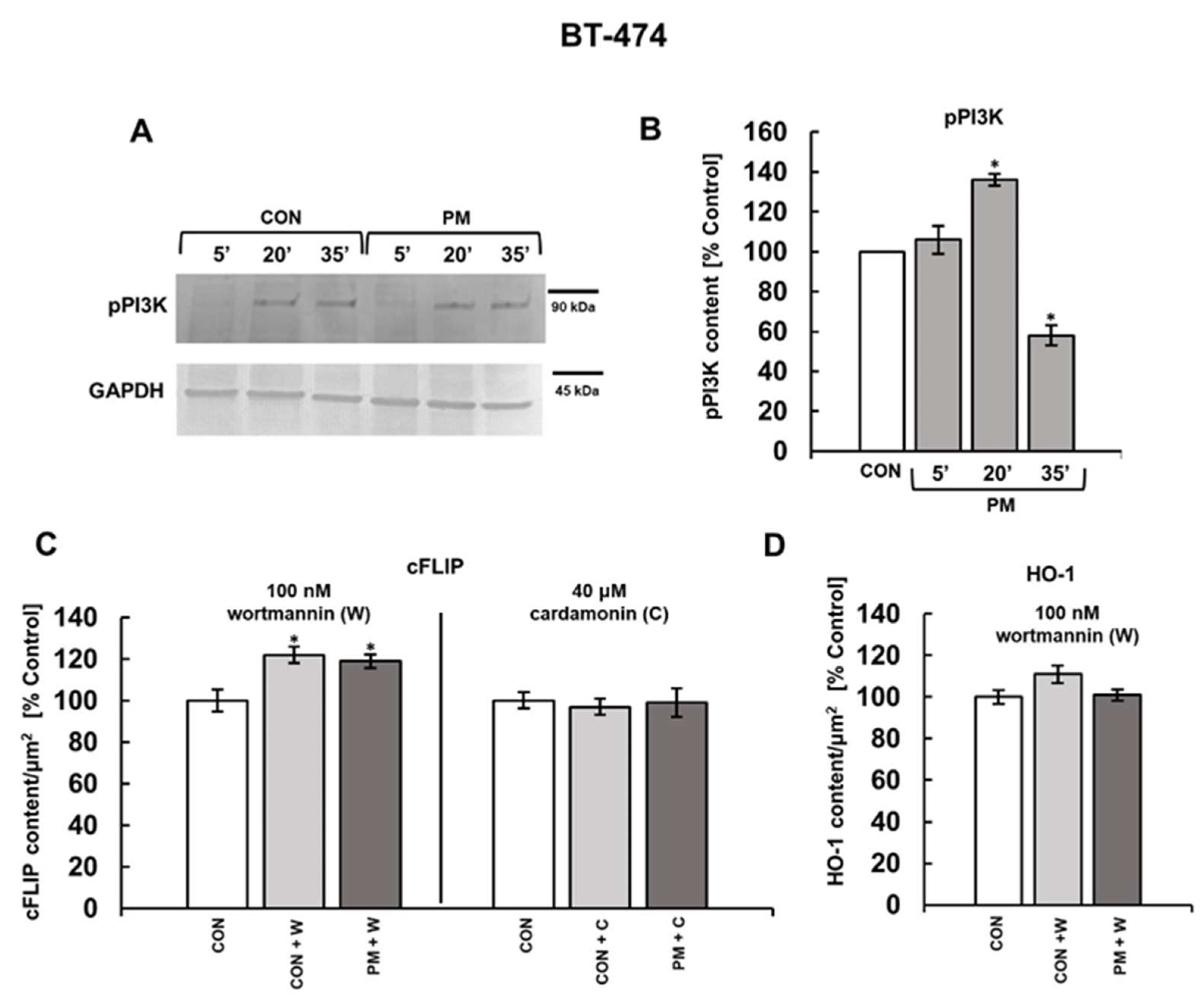
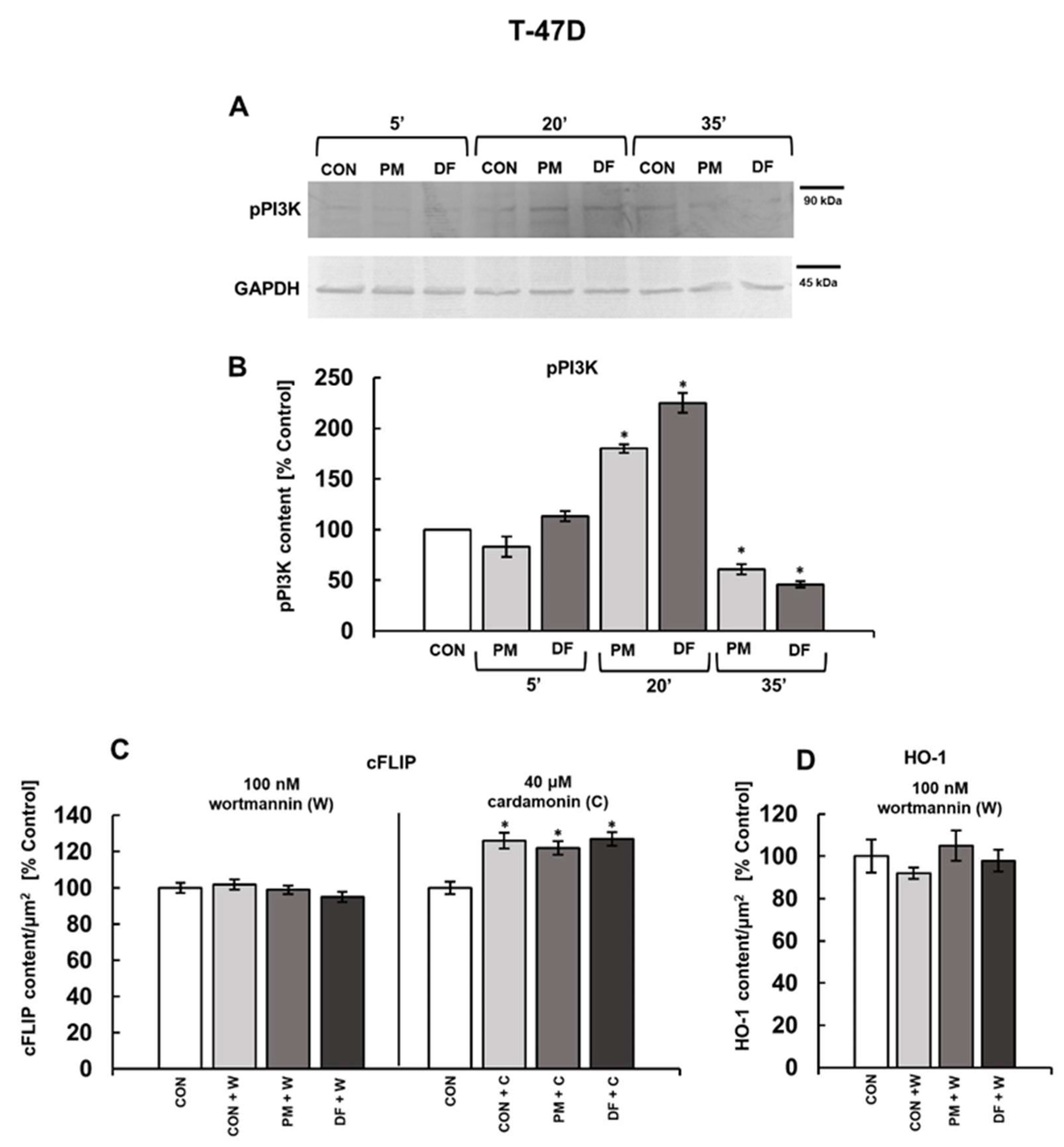
3. Results
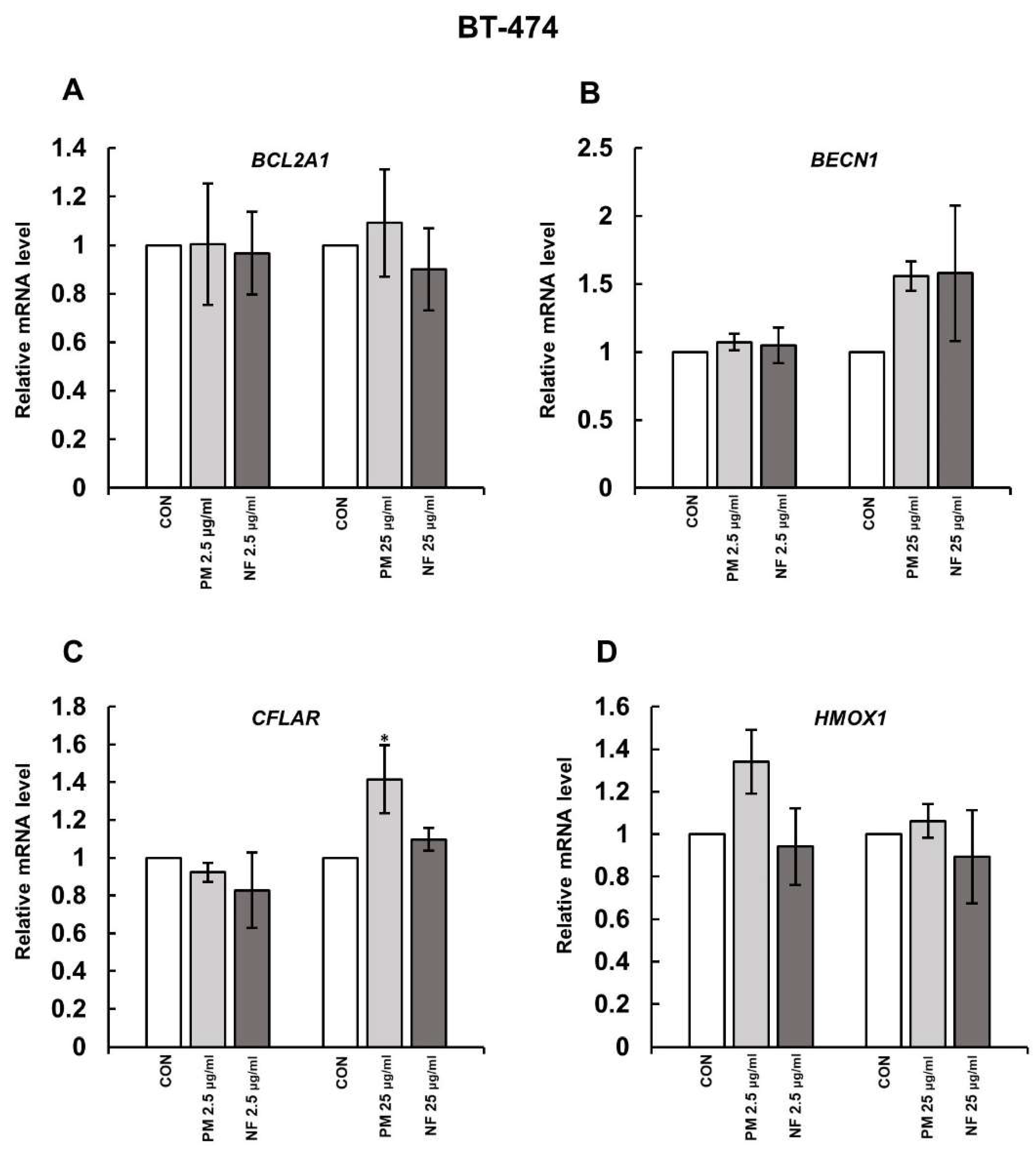
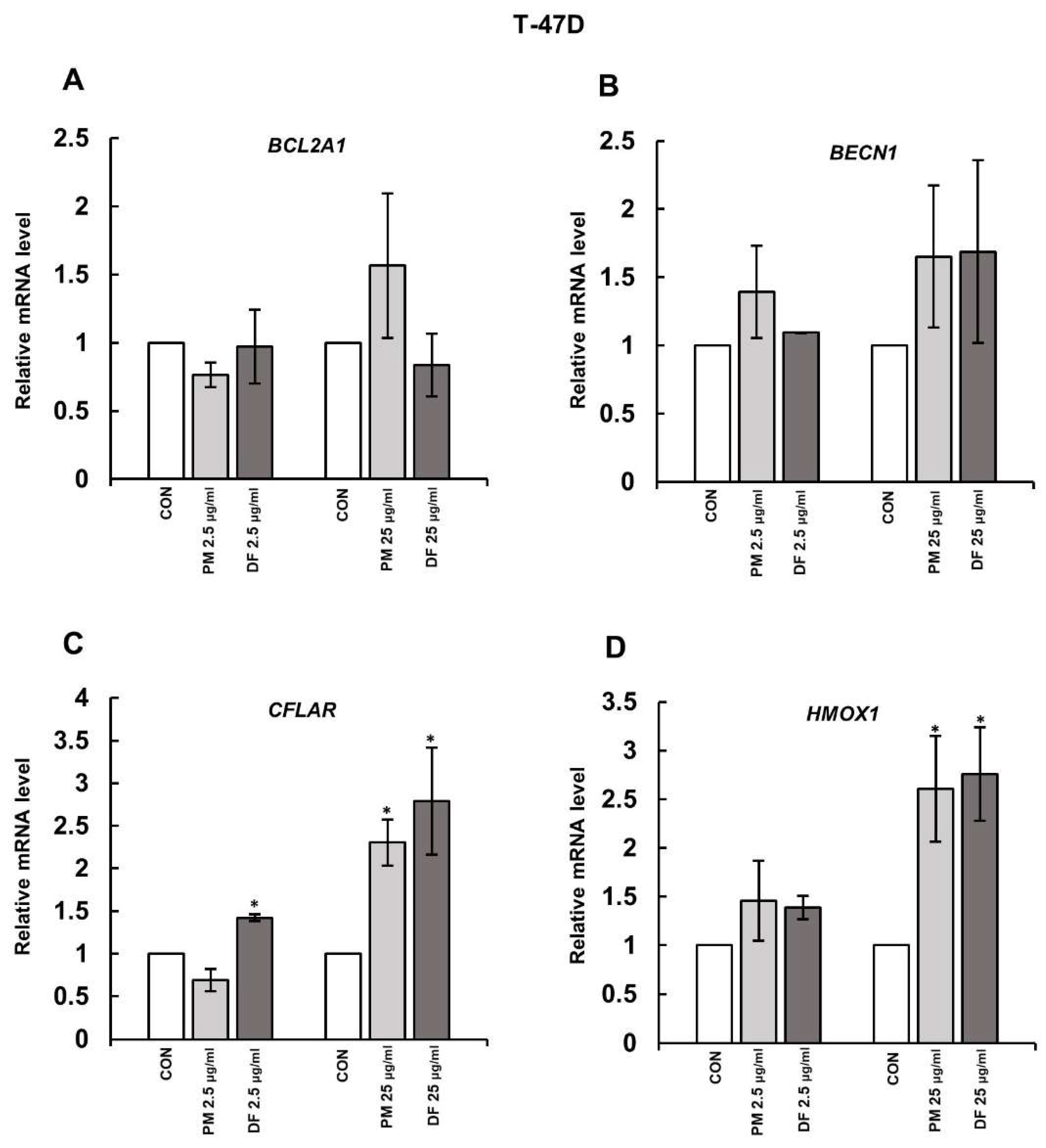
3.1. DS Affects the Expression of Some Genes Involved in Cell Survival
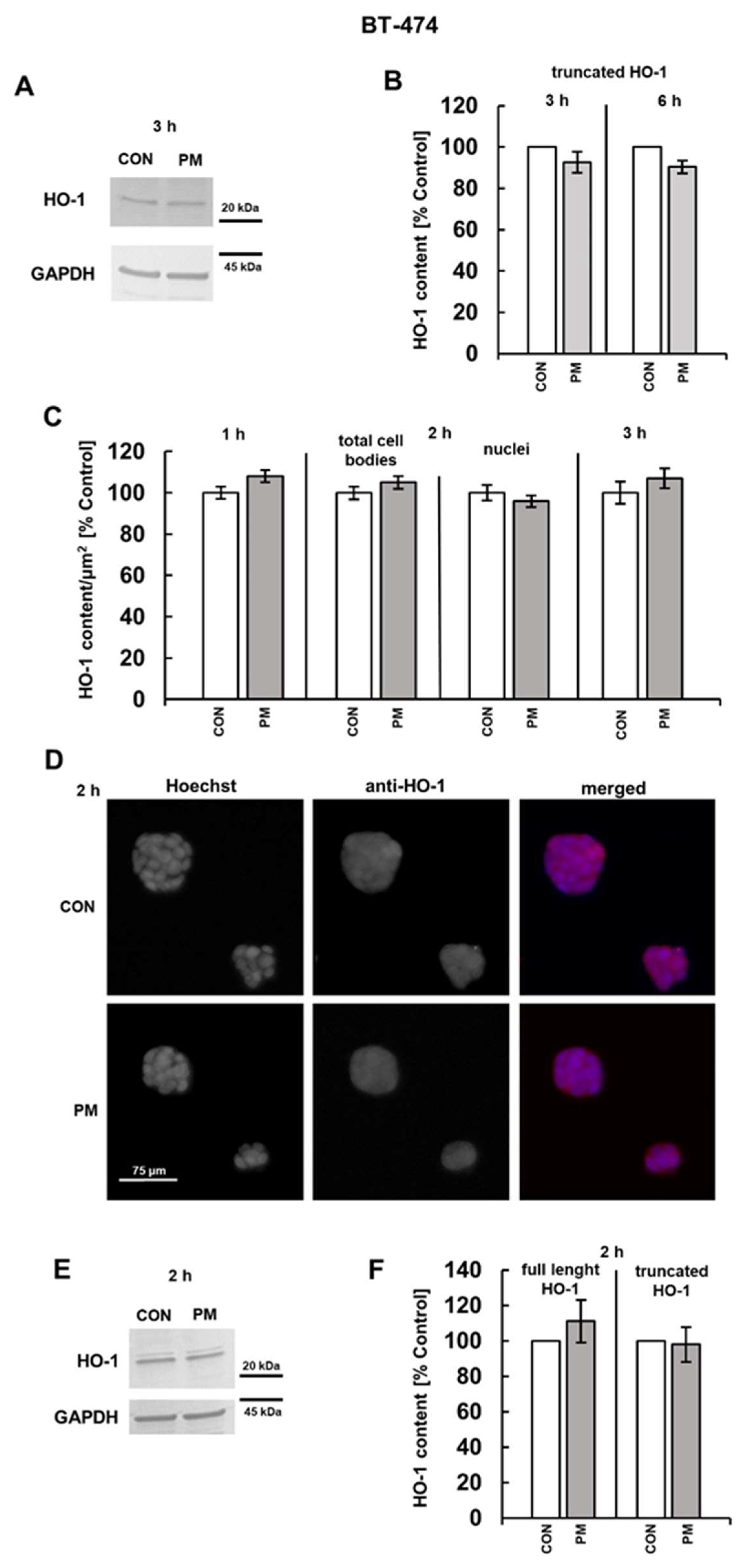
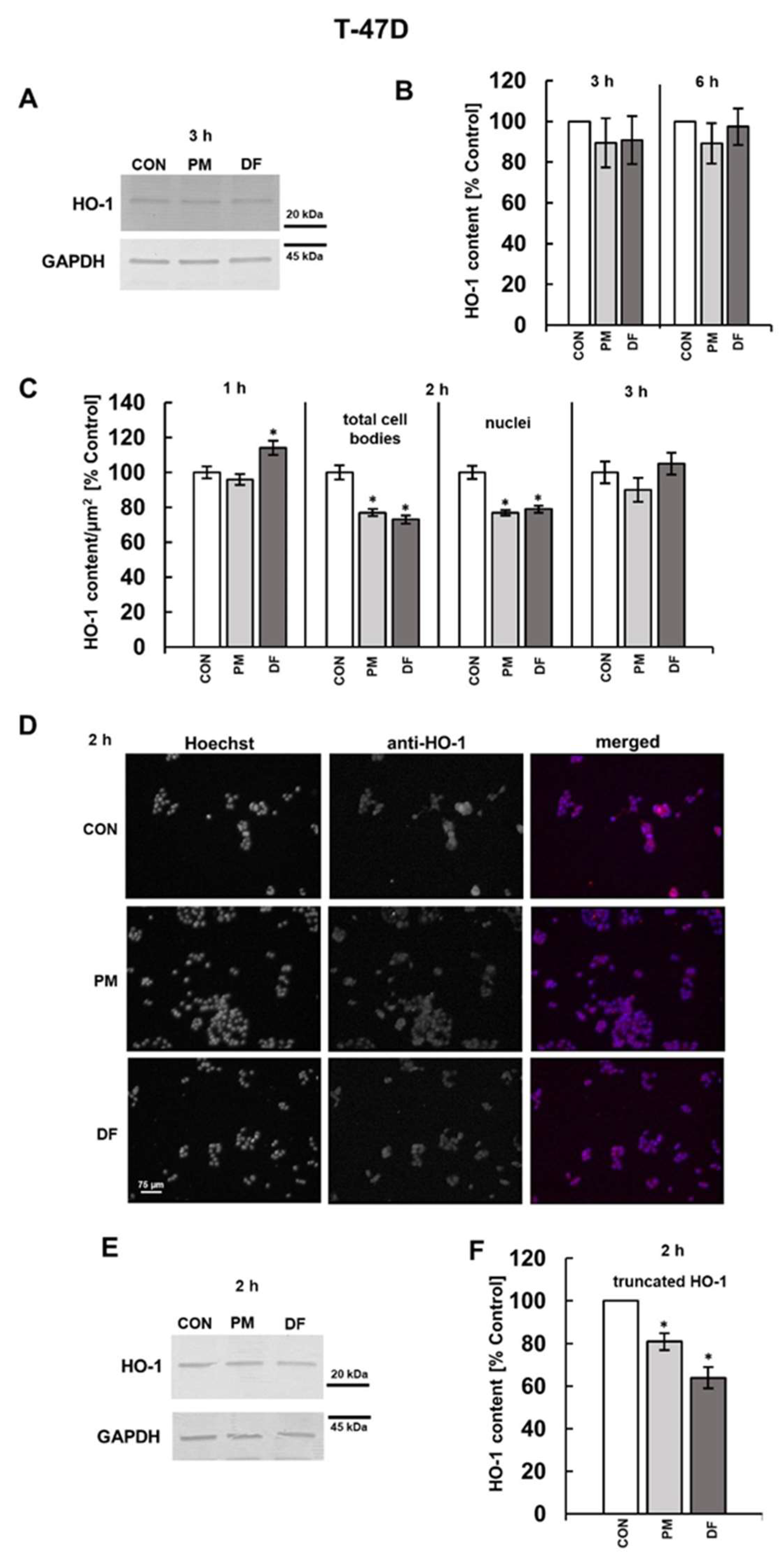
3.2. DS Can Affect the Expression of Both CFLAR and HMOX-1 on the Protein Level
3.3. PI3K and/or NFκB Signaling Pathways Mediate the DS-Induced Expression of cFLIP and HO-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCL-2A1 | B-cell lymphoma-2-related protein A1 |
| cFLIP | cellular FLICE-inhibitory protein |
| CS | chondroitin sulfate |
| DF | dermatan sulfate from fibrosis-affected palmar fascia |
| DS | dermatan sulfate |
| HO-1 | heme oxygenase-1 |
| MLKL | mixed lineage kinase domain-like pseudokinase |
| NF | dermatan sulfate from normal fascia |
| NFκB | nuclear factor κB |
| PI3K | phosphoinositide 3-kinase |
| PM | dermatan sulfate from intestinal mucosa |
| RIPK | receptor-interacting protein kinase |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
References
- Thelin, M.A.; Bartolini, B.; Axelsson, J.; Gustafsson, R.; Tykesson, E.; Pera, E.; Oldberg, Å.; Maccarana, M.; Malmstrom, A. Biological functions of iduronic acid in chondroitin/dermatan sulfate. FEBS J. 2013, 280, 2431–2446. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, X.; Zhang, W.; Ding, D.; Loka, R.S.; Zhao, K.; Ling, P.; Wang, S. Dermatan Sulfate: Structure, Biosynthesis, and Biological Roles. Biomolecules 2025, 15, 1158. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Vivès, R.R.; Schaefer, L.; Götte, M.; Merline, R.; Passi, A.; Heldin, P.; Magalhães, A.; Reis, C.A.; Skandalis, S.S.; et al. A biological guide to glycosaminoglycans: Current perspectives and pending questions. FEBS J. 2024, 291, 3331–3366. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Berthollier, C.; Ricard-Blum, S. The glycosaminoglycan interactome 2.0. Am. J. Physiol. Cell Physiol. 2022, 322, C1271–C1278. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Wang, Q.; Chi, L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers 2022, 14, 5014. [Google Scholar] [CrossRef]
- Pudełko, A.; Wisowski, G.; Olczyk, K.; Koźma, E.M. The dual role of the glycosaminoglycan chondroitin-6-sulfate in the development, progression and metastasis of cancer. FEBS J. 2019, 286, 1815–1837. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Piperigkou, Z.; Passi, A.; Götte, M.; Rousselle, P.; Vlodavsky, I. Extracellular matrix-based cancer targeting. Trends Mol. Med. 2021, 27, 1000–1013. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Kyriakopoulou, K.; Koutsakis, C.; Mastronikolis, S.; Karamanos, N.K. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Wisowski, G.; Pudełko, A.; Olczyk, K.; Paul-Samojedny, M.; Koźma, E.M. Dermatan sulfate affects breast cancer cell function via the induction of necroptosis. Cells 2022, 11, 173. [Google Scholar] [CrossRef]
- Wisowski, G.; Pudełko, A.; Paul-Samojedny, M.; Komosińska-Vassev, K.; Koźma, E.M. Dermatan Sulfate Affects the Activation of the Necroptotic Effector MLKL in Breast Cancer Cell Lines via the NFκB Pathway and Rac-Mediated Oxidative Stress. Biomolecules 2024, 14, 829. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M. BCL2A1: The underdog in the BCL2 family. Cell Death Differ. 2012, 19, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Shang, N.; Gui, M.; Yin, J.; Li, P. Sturgeon (Acipenser)-Derived Chondroitin Sulfate Suppresses Human Colon Cancer HCT-116 Both In Vitro and In Vivo by Inhibiting Proliferation and Inducing Apoptosis. Nutrients 2020, 12, 1130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Qian, J.; Luo, X.; Zhou, A.; Zhang, Z.; Fang, Q. CHSY1 promoted proliferation and suppressed apoptosis in colorectal cancer through regulation of the NFκB and/or caspase-3/7 signaling pathway. Oncol. Lett. 2018, 16, 6140–6146. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nakabayashi, O.; Nakano, H. FLIP the Switch: Regulation of Apoptosis and Necroptosis by cFLIP. Int. J. Mol. Sci. 2015, 16, 30321–30341. [Google Scholar] [CrossRef]
- Humphreys, L.; Espona-Fiedler, M.; Longley, D.B. FLIP as a therapeutic target in cancer. FEBS J. 2018, 285, 4104–4123. [Google Scholar] [CrossRef]
- French, R.; Hayward, O.; Jones, S.; Yang, W.; Clarkson, R. Cytoplasmic levels of cFLIP determine a broad susceptibility of breast cancer stem/progenitor-like cells to TRAIL. Mol. Cancer 2015, 14, 209. [Google Scholar] [CrossRef]
- Holmgren, C.; Sunström Thörnberg, E.; Granqvist, V.; Larsson, C. Induction of Breast Cancer Cell Apoptosis by TRAIL and Smac Mimetics: Involvement of RIP1 and cFLIP. Curr. Issues Mol. Biol. 2022, 44, 4803–4821. [Google Scholar] [CrossRef] [PubMed]
- Buraschi, S.; Neill, T.; Goyal, A.; Poluzzi, C.; Smythies, J.; Owens, R.T.; Schaefer, L.; Torres, A.; Iozzo, R.V. Decorin causes autophagy in endothelial cells via Peg3. Proc. Natl. Acad. Sci. USA 2013, 110, E2582–E2591. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Pirozzi, M.; Auricchio, A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics 2009, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation. Cells 2021, 10, 515. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HO-1/CO system in tumor growth, angiogenesis and metabolism—Targeting HO-1 as an anti-tumor therapy. Vascul. Pharmacol. 2015, 74, 11–22. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Gulisano, M.; Spampinato, M.; Vanella, L. Navigating heme pathways: The breach of heme oxygenase and hemin in breast cancer. Mol. Cell Biochem. 2025, 480, 1495–1518. [Google Scholar] [CrossRef]
- Ciaffaglione, V.; Intagliata, S.; Pittalà, V.; Marrazzo, A.; Sorrenti, V.; Vanella, L.; Rescifina, A.; Floresta, G.; Sultan, A.; Greish, K.; et al. New Arylethanolimidazole Derivatives as HO-1 Inhibitors with Cytotoxicity against MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1923. [Google Scholar] [CrossRef]
- Shao, W.; Wang, X.; Liu, Z.; Song, X.; Wang, F.; Liu, X.; Yu, Z. Cyperotundone combined with adriamycin induces apoptosis in MCF-7 and MCF-7/ADR cancer cells by ROS generation and NRF2/ARE signaling pathway. Sci. Rep. 2023, 13, 1384. [Google Scholar] [CrossRef]
- Cañas, N.; Valero, T.; Villarroya, M.; Montell, E.; Vergés, J.; García, A.G.; López, M.G. Chondroitin sulfate protects SH-SY5Y cells from oxidative stress by inducing heme oxygenase-1 via phosphatidylinositol 3-kinase/Akt. J. Pharmacol. Exp. Ther. 2007, 323, 946–953. [Google Scholar] [CrossRef]
- Katayama, R.; Ishioka, T.; Takada, S.; Takada, R.; Fujita, N.; Tsuruo, T.; Naito, M. Modulation of Wnt signaling by the nuclear localization of cellular FLIP-L. J. Cell Sci. 2010, 123, 23–28. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Huang, Q.; Cheng, W.; Kang, Y.; Shu, L.; Yin, W.; Hua, Z.C. Nuclear localization of c-FLIP-L and its regulation of AP-1 activity. Int. J. Biochem. Cell Biol. 2009, 41, 1678–1684. [Google Scholar] [CrossRef]
- Mascaró, M.; Alonso, E.N.; Alonso, E.G.; Lacunza, E.; Curino, A.C.; Facchinetti, M.M. Nuclear Localization of Heme Oxygenase-1 in Pathophysiological Conditions: Does It Explain the Dual Role in Cancer? Antioxidants 2021, 10, 87. [Google Scholar] [CrossRef]
- Khezri, M.R.; Jafari, R.; Yousefi, K.; Zolbanin, N.M. The PI3K/AKT signaling pathway in cancer: Molecular mechanisms and possible therapeutic interventions. Exp. Mol. Pathol. 2022, 127, 104787. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Hong, E.M.; Kim, M.; Kim, J.H.; Jang, J.; Park, S.W.; Byun, H.W.; Koh, D.H.; Choi, M.H.; Kae, S.H.; et al. Simvastatin induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2) activation through ERK and PI3K/Akt pathway in colon cancer. Oncotarget 2016, 7, 46219–46229. [Google Scholar] [CrossRef] [PubMed]
- Ivanisenko, N.V.; Seyrek, K.; Hillert-Richter, L.K.; König, C.; Espe, J.; Bose, K.; Lavrik, I.N. Regulation of extrinsic apoptotic signaling by c-FLIP: Towards targeting cancer networks. Trends Cancer 2022, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Panner, A.; James, C.D.; Berger, M.S.; Pieper, R.O. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol. Cell Biol. 2005, 25, 8809–8823. [Google Scholar] [CrossRef]
- Yin, W.; Wang, C.; Peng, Y.; Yuan, W.; Zhang, Z.; Liu, H.; Xia, Z.; Ren, C.; Qian, J. Dexmedetomidine alleviates H2O2-induced oxidative stress and cell necroptosis through activating of α2-adrenoceptor in H9C2 cells. Mol. Biol. Rep. 2020, 47, 3629–363928. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Zhang, Y.; Zhai, J.; Sun, B.; Guo, Y.; Wang, F. The up-regulation of RIPK3 mediated by ac4C modification promotes oxidative stress-induced granulosa cell senescence by inhibiting the Nrf2/HO-1 pathway. IUBMB Life 2025, 77, e2944. [Google Scholar] [CrossRef]
- Kataoka, T.; Budd, R.C.; Holler, N.; Thome, M.; Martinon, F.; Irmler, M.; Burns, K.; Hahne, M.; Kennedy, N.; Kovacsovics, M.; et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr. Biol. 2000, 10, 640–648. [Google Scholar] [CrossRef]
- Wang, W.; Shi, L.; Qin, Y.; Li, F. Research and Application of Chondroitin Sulfate/Dermatan Sulfate-Degrading Enzymes. Front. Cell Dev. Biol. 2020, 8, 560442. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Linhardt, R.J. Analysis of the Glycosaminoglycan Chains of Proteoglycans. J. Histochem. Cytochem. 2021, 69, 121–135. [Google Scholar] [CrossRef]
- Zhang, B.; Chi, L. Chondroitin Sulfate/Dermatan Sulfate-Protein Interactions and Their Biological Functions in Human Diseases: Implications and Analytical Tools. Front. Cell Dev. Biol. 2021, 9, 693563. [Google Scholar] [CrossRef]


| Gene | Primers |
|---|---|
| BCL2A1 | Forward 5′-CAAGAAACTTCTACGACAGC-3′ Reverse 5′-AAGCCATTTTCCTCTTCTTG-3′ |
| CFLAR | Forward 5′-AGGGACAAGTTACAGGAATG-3′ Reverse 5′-GAGCCTGAAGTTATTTGAAGG-3′ |
| BECN1 | Forward 5′-ATGAGATTAATGCTGCTTGG-3′ Reverse 5′-AGAGACTCCAGATATGAATGG-3′ |
| HMOX1 | Forward 5′-CAACAAAGTGCAAGATTCTG-3′ Reverse 5′-TGCATTCACATGGCATAAAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisowski, G.; Paul-Samojedny, M.; Komosińska-Vassev, K.; Pudełko, A.; Koźma, E.M. Structural Variants of Dermatan Sulfate Can Affect the Expression of Proteins Involved in Breast Cancer Cell Survival. Cells 2025, 14, 1581. https://doi.org/10.3390/cells14201581
Wisowski G, Paul-Samojedny M, Komosińska-Vassev K, Pudełko A, Koźma EM. Structural Variants of Dermatan Sulfate Can Affect the Expression of Proteins Involved in Breast Cancer Cell Survival. Cells. 2025; 14(20):1581. https://doi.org/10.3390/cells14201581
Chicago/Turabian StyleWisowski, Grzegorz, Monika Paul-Samojedny, Katarzyna Komosińska-Vassev, Adam Pudełko, and Ewa M. Koźma. 2025. "Structural Variants of Dermatan Sulfate Can Affect the Expression of Proteins Involved in Breast Cancer Cell Survival" Cells 14, no. 20: 1581. https://doi.org/10.3390/cells14201581
APA StyleWisowski, G., Paul-Samojedny, M., Komosińska-Vassev, K., Pudełko, A., & Koźma, E. M. (2025). Structural Variants of Dermatan Sulfate Can Affect the Expression of Proteins Involved in Breast Cancer Cell Survival. Cells, 14(20), 1581. https://doi.org/10.3390/cells14201581







