Fluorescence Lifetime Imaging of NAD(P)H in Patients’ Lymphocytes: Evaluation of Efficacy of Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
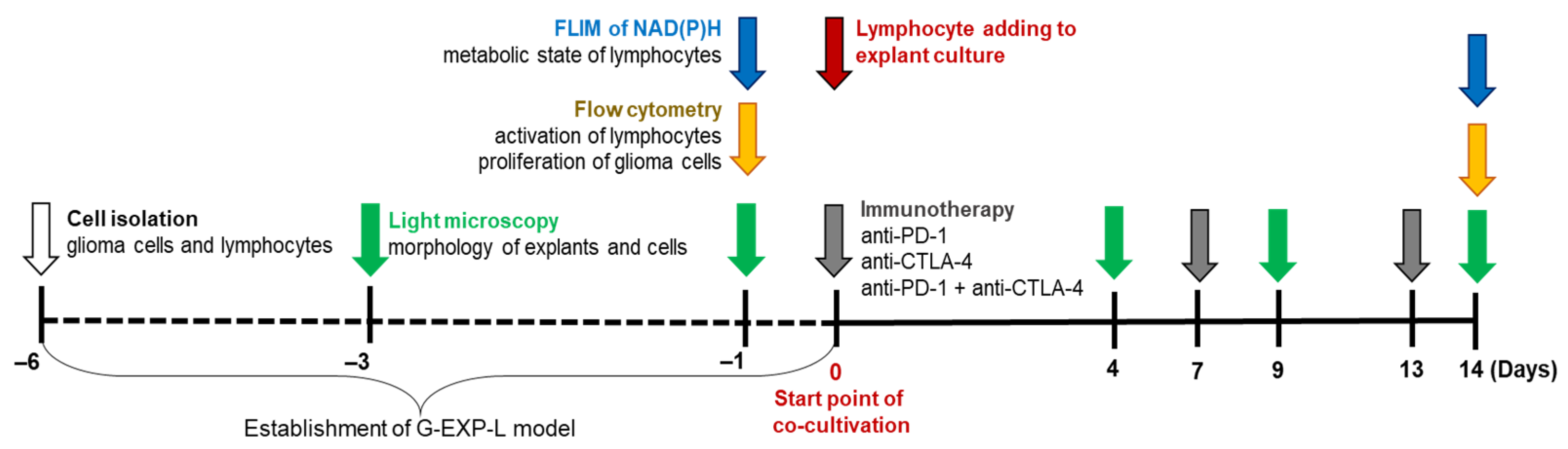
2.2. Experimental Design
2.3. Patient-Derived Explants of Glioma
2.4. Lymphocyte Isolation and Culturing
2.5. Immunotherapy
2.6. FLIM of NAD(P)H in Lymphocytes
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
3.1. Characterization of G-EXP-L Model
3.2. Effects of the Therapy by Immune Checkpoint Inhibitors on T Lymphocytes in the G-EXP-L Model
3.2.1. Morphological Changes in the G-EXP-L Model After Treatment
3.2.2. T-Cell Activation in the G-EXP-L Model After the Treatment
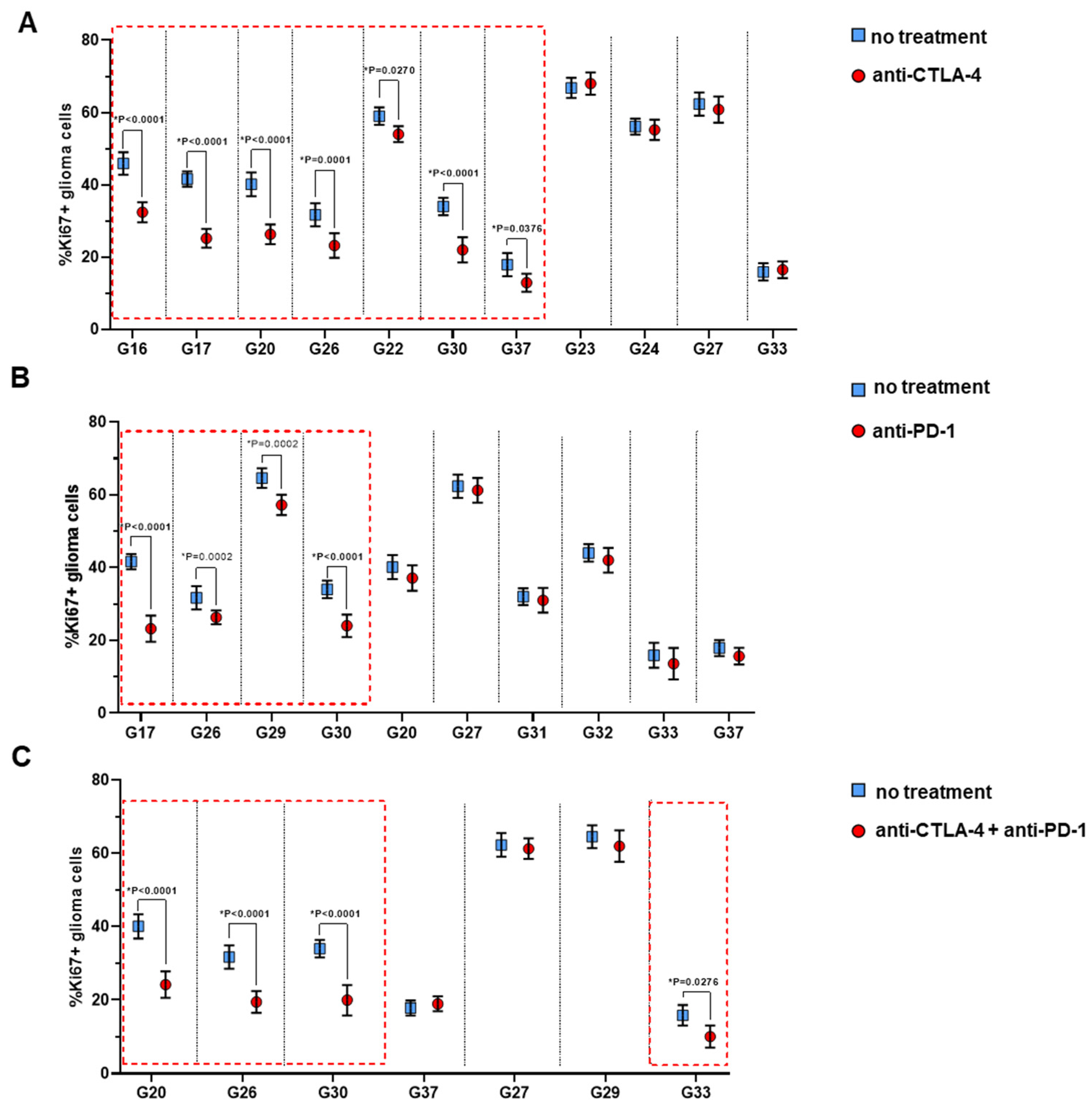
3.2.3. Proliferative Index Ki67 of Glioma Cells in the G-EXP-L Model After Treatment
3.2.4. FLIM of NAD(P)H in Lymphocytes After the Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthukutty, P.; Woo, H.Y.; Ragothaman, M.; Yoo, S.Y. Recent Advances in Cancer Immunotherapy Delivery Modalities. Pharmaceutics 2023, 15, 504. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, M.; Zhang, D.; Chen, M.; Zhu, D. Clinical Cancer Immunotherapy: Current Progress and Prospects. Front. Immunol. 2022, 13, 961805. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of Immune Checkpoints-PD-1, PD-L1, CTLA-4-New Opportunities for Cancer Patients and a New Challenge for Internists and General Practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef]
- Chae, Y.K.; Arya, A.; Iams, W.; Cruz, M.R.; Chandra, S.; Choi, J.; Giles, F. Current Landscape and Future of Dual Anti-CTLA4 and PD-1/PD-L1 Blockade Immunotherapy in Cancer; Lessons Learned from Clinical Trials with Melanoma and Non-Small Cell Lung Cancer (NSCLC). J. Immunother. Cancer 2018, 6, 39. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Cancer Resistance to Immunotherapy: Comprehensive Insights with Future Perspectives. Pharmaceutics 2023, 15, 1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-R.; Wu, X.-L.; Sun, Y.-L. Therapeutic Targets and Biomarkers of Tumor Immunotherapy: Response versus Non-Response. Signal Transduct. Target. Ther. 2022, 7, 331. [Google Scholar] [CrossRef]
- Sankar, K.; Ye, J.C.; Li, Z.; Zheng, L.; Song, W.; Hu-Lieskovan, S. The Role of Biomarkers in Personalized Immunotherapy. Biomark. Res. 2022, 10, 32. [Google Scholar] [CrossRef]
- Shindo, Y.; Hazama, S.; Tsunedomi, R.; Suzuki, N.; Nagano, H. Novel Biomarkers for Personalized Cancer Immunotherapy. Cancers 2019, 11, 1223. [Google Scholar] [CrossRef]
- Shelton, S.E.; Nguyen, H.T.; Barbie, D.A.; Kamm, R.D. Engineering Approaches for Studying Immune-Tumor Cell Interactions and Immunotherapy. iScience 2021, 24, 101985. [Google Scholar] [CrossRef]
- Boucherit, N.; Gorvel, L.; Olive, D. 3D Tumor Models and Their Use for the Testing of Immunotherapies. Front. Immunol. 2020, 11, 603640. [Google Scholar] [CrossRef]
- Mu, P.; Zhou, S.; Lv, T.; Xia, F.; Shen, L.; Wan, J.; Wang, Y.; Zhang, H.; Cai, S.; Peng, J.; et al. Newly Developed 3D in Vitro Models to Study Tumor-Immune Interaction. J. Exp. Clin. Cancer Res. 2023, 42, 81. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wells, G.; Rantala, J.K.; Helleday, T.; Muthana, M.; Danson, S.J. In-Vitro Assays for Immuno-Oncology Drug Efficacy Assessment and Screening for Personalized Cancer Therapy: Scopes and Challenges. Expert Rev. Clin. Immunol. 2024, 20, 821–838. [Google Scholar] [CrossRef]
- Paillon, N.; Ung, T.P.L.; Dogniaux, S.; Stringari, C.; Hivroz, C. Label-Free Single-Cell Live Imaging Reveals Fast Metabolic Switch in T Lymphocytes. Mol. Biol. Cell 2024, 35, ar11. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Szmacinski, H.; Nowaczyk, K.; Johnson, M.L. Fluorescence Lifetime Imaging of Free and Protein-Bound NADH. Proc. Natl. Acad. Sci. USA 1992, 89, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Kolenc, O.I.; Quinn, K.P. Evaluating Cell Metabolism Through Autofluorescence Imaging of NAD(P)H and FAD. Antioxid. Redox Signal 2019, 30, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Gillette, A.; Stefely, M.; Skala, M.C. Recent Innovations in Fluorescence Lifetime Imaging Microscopy for Biology and Medicine. J. Biomed. Opt. 2021, 26, 070603. [Google Scholar] [CrossRef]
- Powley, I.R.; Patel, M.; Miles, G.; Pringle, H.; Howells, L.; Thomas, A.; Kettleborough, C.; Bryans, J.; Hammonds, T.; MacFarlane, M.; et al. Patient-Derived Explants (PDEs) as a Powerful Preclinical Platform for Anti-Cancer Drug and Biomarker Discovery. Br. J. Cancer 2020, 122, 735–744. [Google Scholar] [CrossRef]
- Yuzhakova, D.V.; Sachkova, D.A.; Shirmanova, M.V.; Mozherov, A.M.; Izosimova, A.V.; Zolotova, A.S.; Yashin, K.S. Measurement of Patient-Derived Glioblastoma Cell Response to Temozolomide Using Fluorescence Lifetime Imaging of NAD(P)H. Pharmaceuticals 2023, 16, 796. [Google Scholar] [CrossRef] [PubMed]
- Shekarian, T.; Zinner, C.P.; Bartoszek, E.M.; Duchemin, W.; Wachnowicz, A.T.; Hogan, S.; Etter, M.M.; Flammer, J.; Paganetti, C.; Martins, T.A.; et al. Immunotherapy of Glioblastoma Explants Induces Interferon-γ Responses and Spatial Immune Cell Rearrangements in Tumor Center, but Not Periphery. Sci. Adv. 2022, 8, eabn9440. [Google Scholar] [CrossRef] [PubMed]
- Izosimova, A.V.; Shirmanova, M.V.; Shcheslavskiy, V.I.; Sachkova, D.A.; Mozherov, A.M.; Sharonov, G.V.; Zagaynova, E.V.; Yuzhakova, D.V. FLIM of NAD(P)H in Lymphatic Nodes Resolves T-Cell Immune Response to the Tumor. Int. J. Mol. Sci. 2022, 23, 15829. [Google Scholar] [CrossRef] [PubMed]
- German, Y.; Vulliard, L.; Kamnev, A.; Pfajfer, L.; Huemer, J.; Mautner, A.-K.; Rubio, A.; Kalinichenko, A.; Boztug, K.; Ferrand, A.; et al. Morphological Profiling of Human T and NK Lymphocytes by High-Content Cell Imaging. Cell Rep. 2021, 36, 109318. [Google Scholar] [CrossRef]
- Lin, W.; Suo, Y.; Deng, Y.; Fan, Z.; Zheng, Y.; Wei, X.; Chu, Y. Morphological Change of CD4+ T Cell during Contact with DC Modulates T-Cell Activation by Accumulation of F-Actin in the Immunology Synapse. BMC Immunol. 2015, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- M Kholosy, W.; Derieppe, M.; van den Ham, F.; Ober, K.; Su, Y.; Custers, L.; Schild, L.; M J van Zogchel, L.; M Wellens, L.; R Ariese, H.; et al. Neuroblastoma and DIPG Organoid Coculture System for Personalized Assessment of Novel Anticancer Immunotherapies. J. Pers. Med. 2021, 11, 869. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-Culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e12. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xuan, Y.; Zhang, X.; Liu, Y.; Yang, S.; Yang, K. Immune Cell Metabolism and Metabolic Reprogramming. Mol. Biol. Rep. 2022, 49, 9783–9795. [Google Scholar] [CrossRef]
- Leone, R.D.; Powell, J.D. Metabolism of Immune Cells in Cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.; Kluger, H.; Ruppin, E.; Hu-Lieskovan, S. Immune Resistance Mechanisms and the Road to Personalized Immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390290. [Google Scholar] [CrossRef]
- Yost, K.E.; Chang, H.Y.; Satpathy, A.T. Recruiting T Cells in Cancer Immunotherapy. Science 2021, 372, 130–131. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant Anti-PD-1 Immunotherapy Promotes a Survival Benefit with Intratumoral and Systemic Immune Responses in Recurrent Glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Yasinjan, F.; Xing, Y.; Geng, H.; Guo, R.; Yang, L.; Liu, Z.; Wang, H. Immunotherapy: A Promising Approach for Glioma Treatment. Front. Immunol. 2023, 14, 1255611. [Google Scholar] [CrossRef]
- Schalper, K.A.; Rodriguez-Ruiz, M.E.; Diez-Valle, R.; López-Janeiro, A.; Porciuncula, A.; Idoate, M.A.; Inogés, S.; de Andrea, C.; López-Diaz de Cerio, A.; Tejada, S.; et al. Neoadjuvant Nivolumab Modifies the Tumor Immune Microenvironment in Resectable Glioblastoma. Nat. Med. 2019, 25, 470–476. [Google Scholar] [CrossRef]
- Ma, S.; Ming, Y.; Wu, J.; Cui, G. Cellular Metabolism Regulates the Differentiation and Function of T-Cell Subsets. Cell Mol. Immunol. 2024, 21, 419–435. [Google Scholar] [CrossRef]
- Aksoylar, H.-I.; Tijaro-Ovalle, N.M.; Boussiotis, V.A.; Patsoukis, N. T Cell Metabolism in Cancer Immunotherapy. Immunometabolism 2020, 2, e200020. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 Alters T-Cell Metabolic Reprogramming by Inhibiting Glycolysis and Promoting Lipolysis and Fatty Acid Oxidation. Nat. Commun. 2015, 6, 6692. [Google Scholar] [CrossRef]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef]
- Yuzhakova, D.V.; Lukina, M.M.; Sachkova, D.A.; Yusubalieva, G.M.; Baklaushev, V.P.; Mozherov, A.M.; Dudenkova, V.V.; Gavrina, A.I.; Yashin, K.S.; Shirmanova, M.V. Development of a 3D Tumor Spheroid Model from the Patient’s Glioblastoma Cells and Its Study by Metabolic Fluorescence Lifetime Imaging. Sovrem. Tekhnologii Med. 2023, 15, 28–38. [Google Scholar] [CrossRef]
- Morelli, M.; Lessi, F.; Barachini, S.; Liotti, R.; Montemurro, N.; Perrini, P.; Santonocito, O.S.; Gambacciani, C.; Snuderl, M.; Pieri, F.; et al. Metabolic-Imaging of Human Glioblastoma Live Tumors: A New Precision-Medicine Approach to Predict Tumor Treatment Response Early. Front. Oncol. 2022, 12, 969812. [Google Scholar] [CrossRef] [PubMed]
- Kovács, S.A.; Fekete, J.T.; Győrffy, B. Predictive Biomarkers of Immunotherapy Response with Pharmacological Applications in Solid Tumors. Acta Pharmacol. Sin. 2023, 44, 1879–1889. [Google Scholar] [CrossRef]
- Roy, S.; Dukic, T.; Bhandary, B.; Tu, K.J.; Molitoris, J.; Ko, Y.H.; Shukla, H.D. 3-Bromopyruvate Inhibits Pancreatic Tumor Growth by Stalling Glycolysis, and Dismantling Mitochondria in a Syngeneic Mouse Model. Am. J. Cancer Res. 2022, 12, 4977–4987. [Google Scholar] [PubMed]
- Walsh, A.J.; Mueller, K.P.; Tweed, K.; Jones, I.; Walsh, C.M.; Piscopo, N.J.; Niemi, N.M.; Pagliarini, D.J.; Saha, K.; Skala, M.C. Classification of T-Cell Activation via Autofluorescence Lifetime Imaging. Nat. Biomed. Eng. 2020, 5, 77–88. [Google Scholar] [CrossRef] [PubMed]





| Sample Code | Age | Sex | Diagnosis | Grade | IDH- Status | Primary/ Recurrent | Treatment Before Surgery |
|---|---|---|---|---|---|---|---|
| G16 | 34 | M | Oligoastrocytoma | 3 | Mutant | Primary | No |
| G17 | 46 | F | Glioblastoma | 4 | Wild-type | Primary | No |
| G20 | 54 | F | Glioblastoma | 4 | Wild-type | Recurrent | SR+RT+ TMZ |
| G22 | 40 | F | Astrocytoma | 3 | Mutant | Primary | No |
| G23 | 72 | F | Glioblastoma | 4 | Wild-type | Primary | No |
| G24 | 64 | M | Glioblastoma | 4 | Wild-type | Primary | No |
| G26 | 50 | F | Glioblastoma | 4 | Wild-type | Primary | No |
| G27 | 73 | F | Oligodendroglioma | 3 | Mutant | Primary | No |
| G29 | 72 | F | Glioblastoma | 4 | Wild-type | Primary | No |
| G30 | 49 | M | Astrocytoma | 4 | Mutant | Recurrent | SR |
| G31 | 32 | M | Astrocytoma | 2 | Mutant | Primary | No |
| G32 | 67 | M | Astrocytoma | 4 | Mutant | Primary | No |
| G33 | 37 | M | Astrocytoma | 2 | Mutant | Primary | No |
| G37 | 28 | F | Oligodendroglioma | 3 | Mutant | Primary | No |
| Sample Code | Type of Treatment | Light Microscopy | Flow Cytometry | FLIM | |||
|---|---|---|---|---|---|---|---|
| Morphological Response | ↑ CD8+CD69+ | ↑ CD4+CD69+ | ↓ Ki67+ Glioma Cells | ↑ α1 | ↑ τ2 | ||
| G16 | anti-CTLA-4 | ||||||
| G17 | anti-CTLA-4 | ||||||
| anti-PD-1 | |||||||
| G20 | anti-CTLA-4 | ||||||
| anti-PD-1 | |||||||
| combination | |||||||
| G22 | anti-CTLA-4 | ||||||
| G23 | anti-CTLA-4 | ||||||
| G24 | anti-CTLA-4 | ||||||
| G26 | anti-CTLA-4 | ||||||
| anti-PD-1 | |||||||
| combination | |||||||
| G27 | anti-CTLA-4 | ||||||
| anti-PD-1 | |||||||
| combination | |||||||
| G29 | anti-PD-1 | ||||||
| combination | |||||||
| G30 | anti-CTLA-4 | ||||||
| anti-PD-1 | |||||||
| combination | |||||||
| G31 | anti-PD-1 | ||||||
| G32 | anti-PD-1 | ||||||
| G33 | anti-CTLA-4 | ||||||
| anti-PD-1 | |||||||
| combination | |||||||
| G37 | anti-CTLA-4 | ||||||
| anti-PD-1 | |||||||
| combination | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuzhakova, D.V.; Sachkova, D.A.; Izosimova, A.V.; Yashin, K.S.; Yusubalieva, G.M.; Baklaushev, V.P.; Mozherov, A.M.; Shcheslavskiy, V.I.; Shirmanova, M.V. Fluorescence Lifetime Imaging of NAD(P)H in Patients’ Lymphocytes: Evaluation of Efficacy of Immunotherapy. Cells 2025, 14, 97. https://doi.org/10.3390/cells14020097
Yuzhakova DV, Sachkova DA, Izosimova AV, Yashin KS, Yusubalieva GM, Baklaushev VP, Mozherov AM, Shcheslavskiy VI, Shirmanova MV. Fluorescence Lifetime Imaging of NAD(P)H in Patients’ Lymphocytes: Evaluation of Efficacy of Immunotherapy. Cells. 2025; 14(2):97. https://doi.org/10.3390/cells14020097
Chicago/Turabian StyleYuzhakova, Diana V., Daria A. Sachkova, Anna V. Izosimova, Konstantin S. Yashin, Gaukhar M. Yusubalieva, Vladimir P. Baklaushev, Artem M. Mozherov, Vladislav I. Shcheslavskiy, and Marina V. Shirmanova. 2025. "Fluorescence Lifetime Imaging of NAD(P)H in Patients’ Lymphocytes: Evaluation of Efficacy of Immunotherapy" Cells 14, no. 2: 97. https://doi.org/10.3390/cells14020097
APA StyleYuzhakova, D. V., Sachkova, D. A., Izosimova, A. V., Yashin, K. S., Yusubalieva, G. M., Baklaushev, V. P., Mozherov, A. M., Shcheslavskiy, V. I., & Shirmanova, M. V. (2025). Fluorescence Lifetime Imaging of NAD(P)H in Patients’ Lymphocytes: Evaluation of Efficacy of Immunotherapy. Cells, 14(2), 97. https://doi.org/10.3390/cells14020097







