IFN-
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Bovine Uterine Samples
2.2. Cell Culture
2.3. Cell Transfection and Processing
2.4. Cell Viability
2.5. Immunohistochemistry (IHC)
2.6. Immunofluorescence (IF)
2.7. Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.8. Western Blotting
2.9. Dual Luciferase Reporter Assay
2.10. Animal Experiments
2.11. Statistical Analysis
3. Results
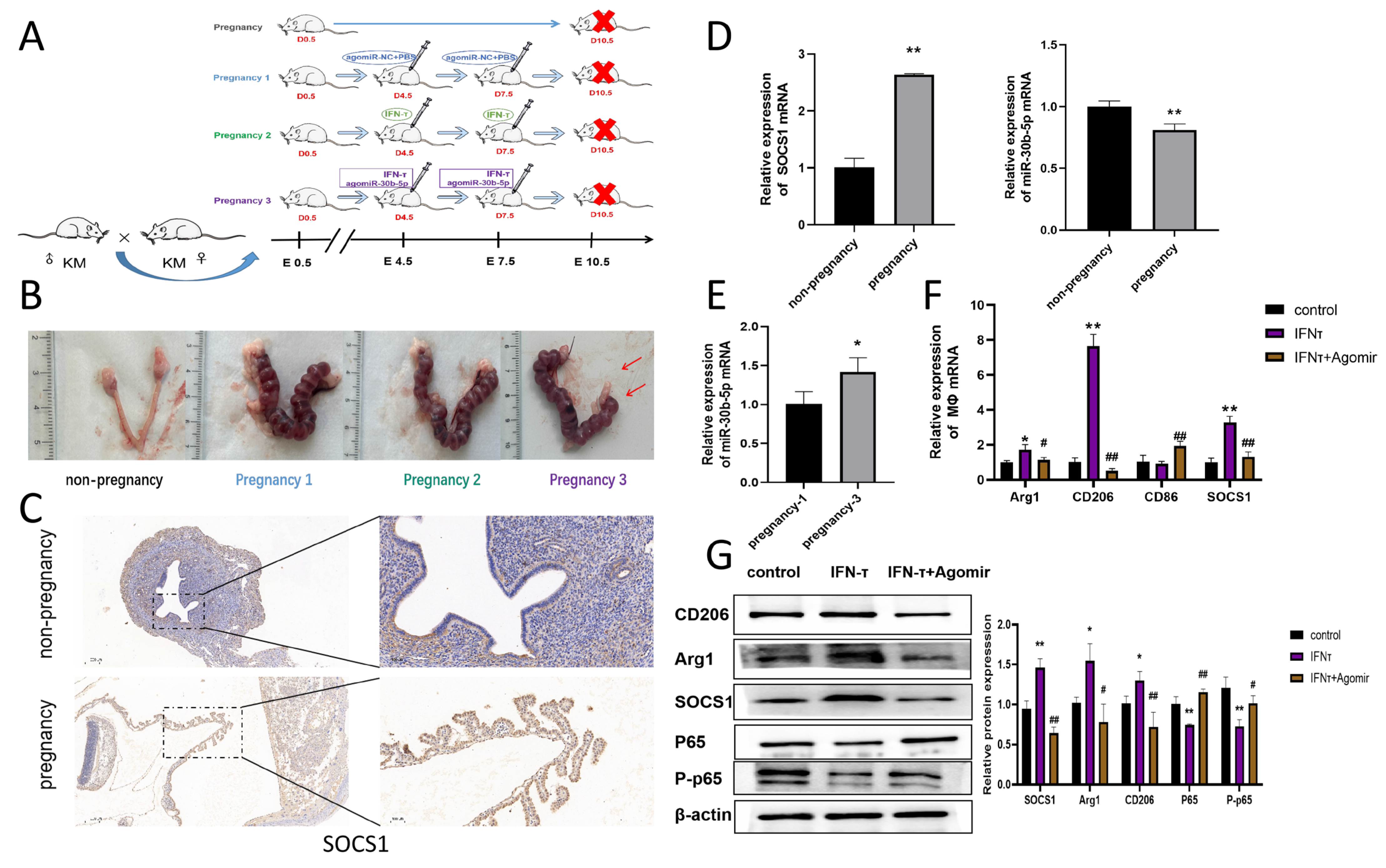
3.1. Recruitment and Bias to the M2 of Macrophages in the Bovine Endometrium in Early Pregnancy and Increased SOCS1 Expression
3.2. Bta-miR-30b-5p Is Negatively Correlated with SOCS1 Expression in Uterine Tissues of Pregnant Cows
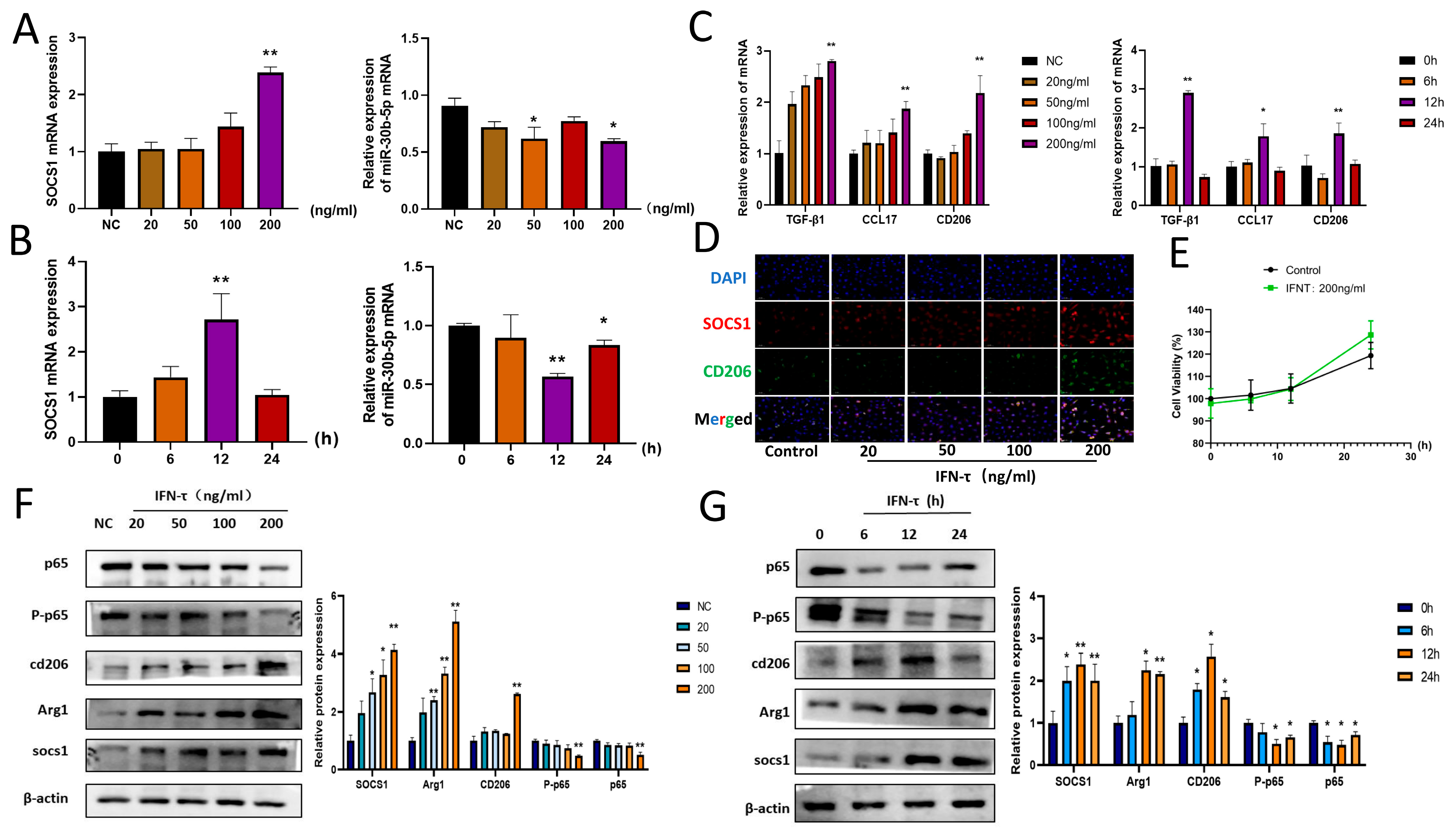
3.3. IFN- Treatment of BoMac Downregulates bta-miR-30b-5p and Upregulates SOCS1
3.4. Effect of IFN- on SOCS1 and Downstream Pathway NF-κB Signaling Pathway
3.5. Bta-miR-30b-5p Has Been Demonstrated to Have a Targeting Relationship with SOCS1
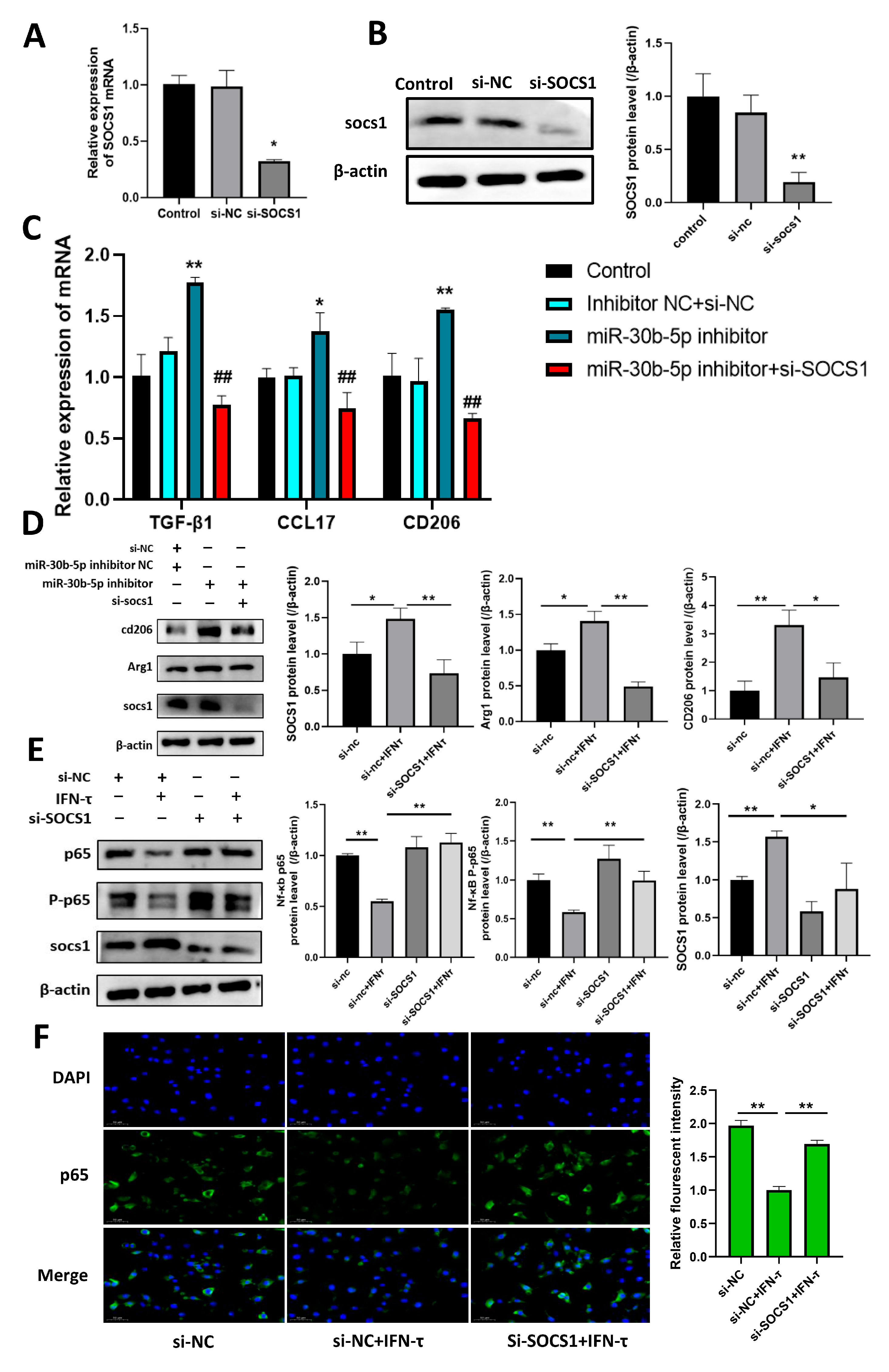
3.6. Bta-miR-30b-5p Inhibits the NF-κB Pathway by Negatively Regulating SOCS1, Thereby Promoting Macrophage Polarization Towards M2 and the Release of Immunosuppressive Factors
3.7. Silencing SOCS1 Prevents bta-miR-30b-5p-Regulated M2 Macrophage Polarization
3.8. Mechanism of IFN- on Macrophage Polarization at the Endometrium in Pregnant Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diskin, M.G.; Morris, D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 260–267. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.E.; Thatcher, W.W.; Chebel, R.C.; Cerri, R.L.; Galvão, K.N. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim. Reprod. Sci. 2004, 82–83, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Niozas, G.; Tsousis, G.; Steinhöfel, I.; Brozos, C.; Römer, A.; Wiedemann, S.; Bollwein, H.; Kaske, M. Extended lactation in high-yielding dairy cows. I. Effects on reproductive measurements. J. Dairy Sci. 2019, 102, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Bigun, Y.; Ezra, E.; Sturman, H.; Hojman, D. The effect of extended calving intervals in high-yielding lactating cows on milk production and profitability. J. Dairy Sci. 2001, 84, 600–608. [Google Scholar] [CrossRef]
- Pajohande, K.; Amirabadi Farahani, T.; Farsuni, N.E. Increased incidence of reproductive disorders associated with short gestation length in Holstein dairy cows. Theriogenology 2023, 205, 9–17. [Google Scholar] [CrossRef]
- Druker, S.A.; Sicsic, R.; van Straten, M.; Goshen, T.; Kedmi, M.; Raz, T. Cytological endometritis diagnosis in primiparous versus multiparous dairy cows. J. Dairy Sci. 2022, 105, 665–683. [Google Scholar] [CrossRef]
- Lima, F.S.; Silvestre, F.T.; Peñagaricano, F.; Thatcher, W.W. Early genomic prediction of daughter pregnancy rate is associated with improved reproductive performance in Holstein dairy cows. J. Dairy Sci. 2020, 103, 3312–3324. [Google Scholar] [CrossRef]
- Seneda, M.M.; Costa, C.B.; Zangirolamo, A.F.; Dos Anjos, M.M.; de Paula, G.R.; Morotti, F. From the laboratory to the field: How to mitigate pregnancy losses in embryo transfer programs? Anim. Reprod. 2024, 21, e20240032. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Baez, G.M.; Garcia-Guerra, A.; Toledo, M.Z.; Monteiro, P.L.; Melo, L.F.; Ochoa, J.C.; Santos, J.E.; Sartori, R. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 2016, 86, 239–253. [Google Scholar] [CrossRef]
- Chelmońskasoyta, A. Interferon tau and its immunobiological role in ruminant reproduction. Arch. Immunol. Ther. Exp. 2002, 50, 47–52. [Google Scholar]
- Thatcher, W.W.; Guzeloglu, A.; Mattos, R.; Binelli, M.; Hansen, T.R.; Pru, J.K. Uterine-conceptus interactions and reproductive failure in cattle. Theriogenology 2001, 56, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Bazer, F.W. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front. Biosci. 2002, 7, d1879–d1898. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Reviews. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Jiang, K.; Cai, J.; Jiang, Q.; Loor, J.J.; Deng, G.; Li, X.; Yang, J. Interferon-tau protects bovine endometrial epithelial cells against inflammatory injury by regulating the PI3K/AKT/β-catenin/FoxO1 signaling axis. J. Dairy Sci. 2024, 107, 555–572. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, G.; Jiang, K.; Chen, X.; Rui, G.; Qiu, C.; Guo, M.; Deng, G. IFN- Alleviates Lipopolysaccharide-Induced Inflammation by Suppressing NF-κB and MAPKs Pathway Activation in Mice. Inflammation 2016, 39, 1141–1150. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Hansen, P.J. Deviations in populations of peripheral blood mononuclear cells and endometrial macrophages in the cow during pregnancy. Reproduction 2008, 136, 481–490. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Hansen, P.J. Phenotypic characterization of macrophages in the endometrium of the pregnant cow. Am. J. Reprod. Immunol. 2009, 62, 418–426. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Guenther, S.; Vrekoussis, T.; Heublein, S.; Bayer, B.; Anz, D.; Knabl, J.; Navrozoglou, I.; Dian, D.; Friese, K.; Makrigiannakis, A.; et al. Decidual macrophages are significantly increased in spontaneous miscarriages and over-express FasL: A potential role for macrophages in trophoblast apoptosis. Int. J. Mol. Sci. 2012, 13, 9069–9080. [Google Scholar] [CrossRef]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Shirasuna, K.; Usui, F.; Karasawa, T.; Mizushina, Y.; Kimura, H.; Kawashima, A.; Ohkuchi, A.; Matsuyama, S.; Kimura, K.; et al. Interferon-tau attenuates uptake of nanoparticles and secretion of interleukin-1β in macrophages. PLoS ONE 2014, 9, e113974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wu, H.; Jiang, K.; Chen, X.; Wang, X.; Qiu, C.; Guo, M.; Deng, G. The Anti-Inflammatory Effects of Interferon Tau by Suppressing NF-κB/MMP9 in Macrophages Stimulated with Staphylococcus aureus. J. Interferon Cytokine Res. 2016, 36, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Bidarimath, M.; Lingegowda, H.; Miller, J.E.; Koti, M.; Tayade, C. Insights Into Extracellular Vesicle/Exosome and miRNA Mediated Bi-Directional Communication During Porcine Pregnancy. Front. Vet. Sci. 2021, 8, 654064. [Google Scholar] [CrossRef]
- Lycoudi, A.; Mavreli, D.; Mavrou, A.; Papantoniou, N.; Kolialexi, A. miRNAs in pregnancy-related complications. Expert Rev. Mol. Diagn. 2015, 15, 999–1010. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Zhang, B.; Chan, H.Y.; Sharkey, D.J.; Fullston, T.; Robertson, S.A. miRNA Regulation of Immune Tolerance in Early Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 272–280. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Doucha, J.; Dlouha, K.; Krofta, L. Absolute and relative quantification of placenta-specific micrornas in maternal circulation with placental insufficiency-related complications. J. Mol. Diagn. JMD 2012, 14, 160–167. [Google Scholar] [CrossRef]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef]
- Rumpel, N.; Riechert, G.; Schumann, J. miRNA-Mediated Fine Regulation of TLR-Induced M1 Polarization. Cells 2024, 13, 701. [Google Scholar] [CrossRef]
- Qin, L.; Yang, J.; Su, X.; Xilan, L.; Lei, Y.; Dong, L.; Chen, H.; Chen, C.; Zhao, C.; Zhang, H.; et al. The miR-21-5p enriched in the apoptotic bodies of M2 macrophage-derived extracellular vesicles alleviates osteoarthritis by changing macrophage phenotype. Genes Dis. 2023, 10, 1114–1129. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Ediriweera, H.N.; Gundra, U.M.; Sheedy, F.J.; Ramkhelawon, B.; Hutchison, S.B.; Rinehold, K.; van Solingen, C.; Fullerton, M.D.; Cecchini, K.; et al. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Investig. 2015, 125, 4334–4348. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Sakurai, T.; Fujiwara, H.; Ideta, A.; Aoyagi, Y.; Godkin, J.D.; Imakawa, K. Functions of interferon tau as an immunological regulator for establishment of pregnancy. Reprod. Med. Biol. 2012, 11, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A. Interferons and uterine receptivity. Semin. Reprod. Med. 2009, 27, 90–102. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Guo, S.; Zhang, T.; Ma, X.; Jiang, K.; Guo, X.; Deng, G. IFN- Attenuates LPS-Induced Endometritis by Restraining HMGB1/NF-κB Activation in bEECs. Inflammation 2021, 44, 1478–1489. [Google Scholar] [CrossRef]
- Bidgood, G.M.; Keating, N.; Doggett, K.; Nicholson, S.E. SOCS1 is a critical checkpoint in immune homeostasis, inflammation and tumor immunity. Front. Immunol. 2024, 15, 1419951. [Google Scholar] [CrossRef]
- Kile, B.T.; Alexander, W.S. The suppressors of cytokine signalling (SOCS). Cell. Mol. Life Sci. 2001, 58, 1627–1635. [Google Scholar] [CrossRef]
- Kubo, M.; Hanada, T.; Yoshimura, A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003, 4, 1169–1176. [Google Scholar] [CrossRef]
- Ahmed, C.M.; Larkin, J., 3rd; Johnson, H.M. SOCS1 Mimetics and Antagonists: A Complementary Approach to Positive and Negative Regulation of Immune Function. Front. Immunol. 2015, 6, 183. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, X.; Tian, Y.; Gao, X.; Liu, Z.; Dong, X.; Wang, L.; He, F.; Zhou, J. Downregulation of microRNA-155-5p prevents immune thrombocytopenia by promoting macrophage M2 polarization via the SOCS1-dependent PD1/PDL1 pathway. Life Sci. 2020, 257, 118057. [Google Scholar] [CrossRef]
- Körholz, J.; Gabrielyan, A.; Sowerby, J.M.; Boschann, F.; Chen, L.S.; Paul, D.; Brandt, D.; Kleymann, J.; Kolditz, M.; Toepfner, N.; et al. One Gene, Many Facets: Multiple Immune Pathway Dysregulation in SOCS1 Haploinsufficiency. Front. Immunol. 2021, 12, 680334. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Xia, Y. Defective Suppressor of Cytokine Signaling 1 Signaling Contributes to the Pathogenesis of Systemic Lupus Erythematosus. Front. Immunol. 2017, 8, 1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bai, X.; Wang, B.; Yi, Q.; Yu, W.; Zhang, X.; Tian, R.; Zhang, X.; Li, C.; Chen, Y.; et al. CTLA4Ig/VISTAIg combination therapy selectively induces CD4(+) T cell-mediated immune tolerance by targeting the SOCS1 signaling pathway in porcine islet xenotransplantation. Immunology 2022, 166, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Aki, D.; Ito, M. SOCS, SPRED, and NR4a: Negative regulators of cytokine signaling and transcription in immune tolerance. Proc. Jpn. Academy. Ser. B 2021, 97, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, T.; Kashiwagi, I.; Takahashi, R.; Yasukawa, H.; Yoshimura, A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: Regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 980–985. [Google Scholar] [CrossRef]
- Gulyaeva, L.F.; Kushlinskiy, N.E. Regulatory mechanisms of microRNA expression. J. Transl. Med. 2016, 14, 143. [Google Scholar] [CrossRef]
- van Wijk, N.; Zohar, K.; Linial, M. Challenging Cellular Homeostasis: Spatial and Temporal Regulation of miRNAs. Int. J. Mol. Sci. 2022, 23, 16152. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Q.; Yu, L.; Zhu, D.; Li, Y.; Xue, Z.; Hua, Z.; Luo, X.; Song, Z.; Lu, C.; et al. The Role of miRNA in Tumor Immune Escape and miRNA-Based Therapeutic Strategies. Front. Immunol. 2021, 12, 807895. [Google Scholar] [CrossRef]
- Yang, T.; Ge, B. miRNAs in immune responses to Mycobacterium tuberculosis infection. Cancer Lett. 2018, 431, 22–30. [Google Scholar] [CrossRef]
- Moradi-Chaleshtori, M.; Bandehpour, M.; Heidari, N.; Mohammadi-Yeganeh, S.; Mahmoud Hashemi, S. Exosome-mediated miR-33 transfer induces M1 polarization in mouse macrophages and exerts antitumor effect in 4T1 breast cancer cell line. Int. Immunopharmacol. 2021, 90, 107198. [Google Scholar] [CrossRef]
- Khan, M.J.; Singh, P.; Dohare, R.; Jha, R.; Rahmani, A.H.; Almatroodi, S.A.; Ali, S.; Syed, M.A. Inhibition of miRNA-34a Promotes M2 Macrophage Polarization and Improves LPS-Induced Lung Injury by Targeting Klf4. Genes 2020, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Maurya, M.; Maurya, P.; Barthwal, M.K. Lin28B Regulates Angiotensin II-Mediated Let-7c/miR-99a MicroRNA Formation Consequently Affecting Macrophage Polarization and Allergic Inflammation. Inflammation 2020, 43, 1846–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, Y.; Wang, J.X.; Chen, M.H.; Xu, J.J.; Jiang, M.H.; Feng, Y.L.; Gu, Y.F. miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. 2020, 29, 101402. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Reviews. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wang, Y.H.; Peng, Y.J.; Liu, F.C.; Lin, G.J.; Huang, S.H.; Sytwu, H.K.; Cheng, C.P. Interleukin 26 Skews Macrophage Polarization Towards M1 Phenotype by Activating cJUN and the NF-κB Pathway. Cells 2020, 9, 938. [Google Scholar] [CrossRef]
- Fang, C.; Zhong, R.; Lu, S.; Yu, G.; Liu, Z.; Yan, C.; Gao, J.; Tang, Y.; Wang, Y.; Zhao, Q.; et al. TREM2 promotes macrophage polarization from M1 to M2 and suppresses osteoarthritis through the NF-κB/CXCL3 axis. Int. J. Biol. Sci. 2024, 20, 1992–2007. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Panaro, M.A. Understanding the role of SOCS signaling in neurodegenerative diseases: Current and emerging concepts. Cytokine Growth Factor Rev. 2017, 37, 67–79. [Google Scholar] [CrossRef]
- Rogez-Kreuz, C.; Manéglier, B.; Martin, M.; Dereuddre-Bosquet, N.; Martal, J.; Dormont, D.; Clayette, P. Involvement of IL-6 in the anti-human immunodeficiency virus activity of IFN-tau in human macrophages. Int. Immunol. 2005, 17, 1047–1057. [Google Scholar] [CrossRef]
- Alexenko, A.P.; Ealy, A.D.; Roberts, R.M. The cross-species antiviral activities of different IFN-tau subtypes on bovine, murine, and human cells: Contradictory evidence for therapeutic potential. J. Interferon Cytokine Res. 1999, 19, 1335–1341. [Google Scholar] [CrossRef]
- Soos, J.M.; Stüve, O.; Youssef, S.; Bravo, M.; Johnson, H.M.; Weiner, H.L.; Zamvil, S.S. Cutting edge: Oral type I IFN-tau promotes a Th2 bias and enhances suppression of autoimmune encephalomyelitis by oral glatiramer acetate. J. Immunol. 2002, 169, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Soos, J.M.; Subramaniam, P.S.; Hobeika, A.C.; Schiffenbauer, J.; Johnson, H.M. The IFN pregnancy recognition hormone IFN-tau blocks both development and superantigen reactivation of experimental allergic encephalomyelitis without associated toxicity. J. Immunol. 1995, 155, 2747–2753. [Google Scholar] [CrossRef] [PubMed]



| Mimics | Sense: UGUAAACAUCCUACACUCAGCU Anti-Sense: CUGAGUGUAGGAUGUUUACAUU |
|---|---|
| Mimics NC | Sense: UUCUCCGAACGUGUCACGUTT Anti-sense:ACGUGACACGUUCGGAGAATT |
| Inhibitor | Sense: AGCUGAGUGUAGGAUGUUUACA |
| Inhibitor NC | Sense: CAGUACUUUUGUGUAGUACAA |
| Si-SOCS1 | Sense: GACAGAGGAACUGCUUCUUTT Anti-sense: AAGAAGCAGUUCCUCUGUCTT |
| si-NC | Sense:UUCUCCGAACGUGUCACGUTT Anti-sense:ACGUGACACGUUCGGAGAATT |
| Gene | Sequence |
|---|---|
| RT-U6 | AACGCTTCACGAATTTGCGT |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
| RT-bta-miR-30b-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGCTGAGT |
| bta-miR-30b-5p-F | GCCGAGTGTAAACATCCTAC |
| bta-miR-30b-5p-R | CTCAACTGGTGTCGTGGA |
| bta-CD206-F | GTGATGGATCCCCTGTGTCA |
| bta-CD206-R | GTTGGCTCAGGTTTGGGAGT |
| bta-CCL17-F | CCATTCCCCAAAAGGTGCTG |
| bta-CCL17-R | GCGAGTCACCAGCACAATGG |
| bta-SOCS1-F | CTCGTACCTCCTACCTCTTCATGTT |
| bta-SOCS1-R | ACAGCAGAAAAATAAAGCCAGAGA |
| bta-TGF-β1 | GCCTGCTGAGGCTCAAGTT |
| bta-TGF-β1 | GCCGGAACTGAACCCGTTA |
| bta-GAPDH-F | AAGGTCGGAGTGAACGGATT |
| bta-GAPDH-R | ATGACGAGCTTCCCGTTCTC |
| mus-SOCS1-F | GTCCTGCCGCCAGATGAG |
| mus-SOCS1-R | GAGACAGAGGCAGTGAGCC |
| mus-CD206-F | TTCAGCTATTGGACGCGAGG |
| mus-CD206-R | GAATCTGACACCCAGCGGAA |
| mus-Arg1-F | AGCACTGAGGAAAGCTGGTC |
| mus-Arg1-R | TACGTCTCGCAAGCCAATGT |
| mus-CD86-F | ATGGACCCCAGATGCACCAT |
| mus-CD86-R | CGGCAGATATGCAGTCCCAT |
| mus-GAPDH-F | TGCACCACCAACTGCTTAG |
| mus-GAPDH-R | GGATGCAGGGATGATGTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Yang, C.; Wang, T.; Zhang, J.; Zhou, H.; Ma, B.; Xu, M.; Deng, G.
IFN-
Feng X, Yang C, Wang T, Zhang J, Zhou H, Ma B, Xu M, Deng G.
IFN-
Feng, Xinyu, Cheng Yang, Ting Wang, Jinxin Zhang, Han Zhou, Bin Ma, Ming Xu, and Ganzhen Deng.
2025. "IFN-
Feng, X., Yang, C., Wang, T., Zhang, J., Zhou, H., Ma, B., Xu, M., & Deng, G.
(2025). IFN-






