Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Umbilical Mesenchymal Stem Cell Culture
2.2. Flow Cytometry
2.3. Cell Proliferation
2.4. Trilineage Differentiation Capabilities
2.4.1. Adipogenesis
2.4.2. Osteogenesis
2.4.3. Chondrogenesis
2.5. Immunohistochemical Staining
2.6. Quantitative Polymerase Chain Reaction
2.7. Transfection of miR-7704
2.8. Extracellular Vesicle Extraction and Characterization
2.9. Extracellular Vesicle Identification
2.10. Western Blot Analysis
2.11. NTA (NanoSight NS300)
2.12. TEM
2.13. Chondrocyte Culture
2.14. Osteoarthritis Mouse Model Induced by Type II Collagen
2.15. Extracellular Vesicle Injection
2.16. Behavioral Assessments
2.17. Tissue Harvesting
2.18. Histological Evaluation
2.19. Immunohistochemical Staining
2.20. Statistical Analysis
3. Results
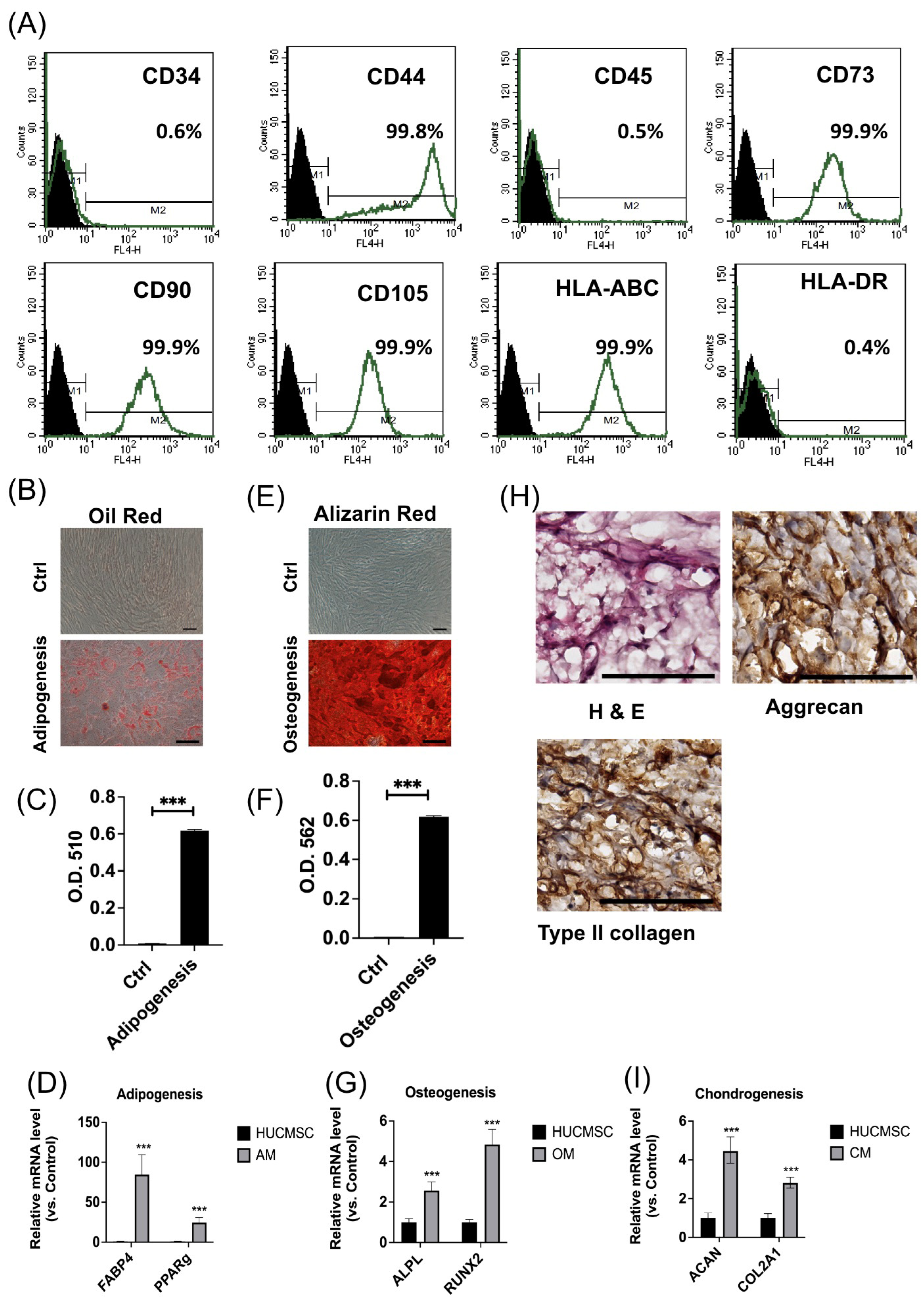
3.1. HUCMSCs Revealed Typical Characteristics of MSCs
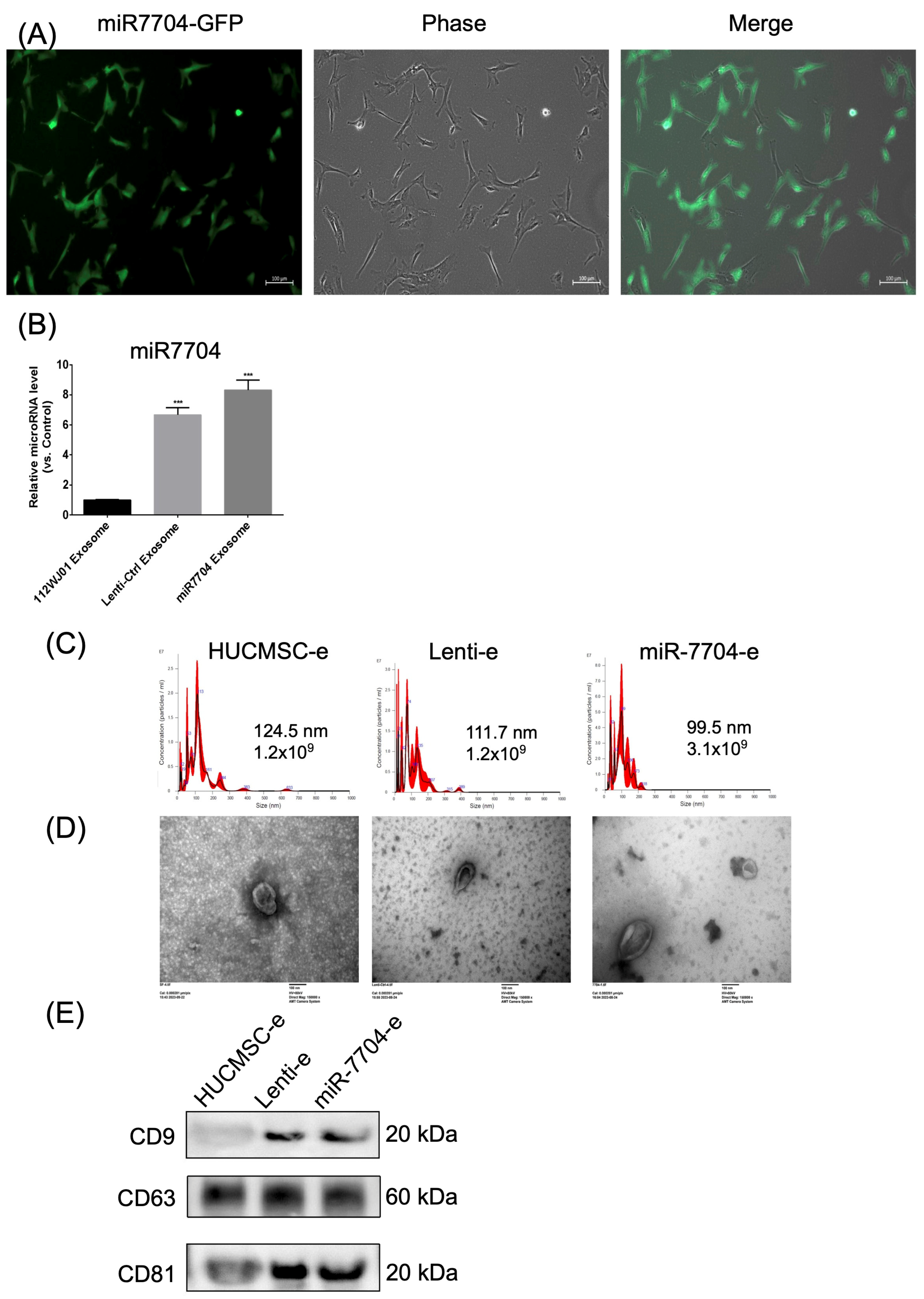
3.2. miR-7704 Successfully Transfected into HUCMSCs
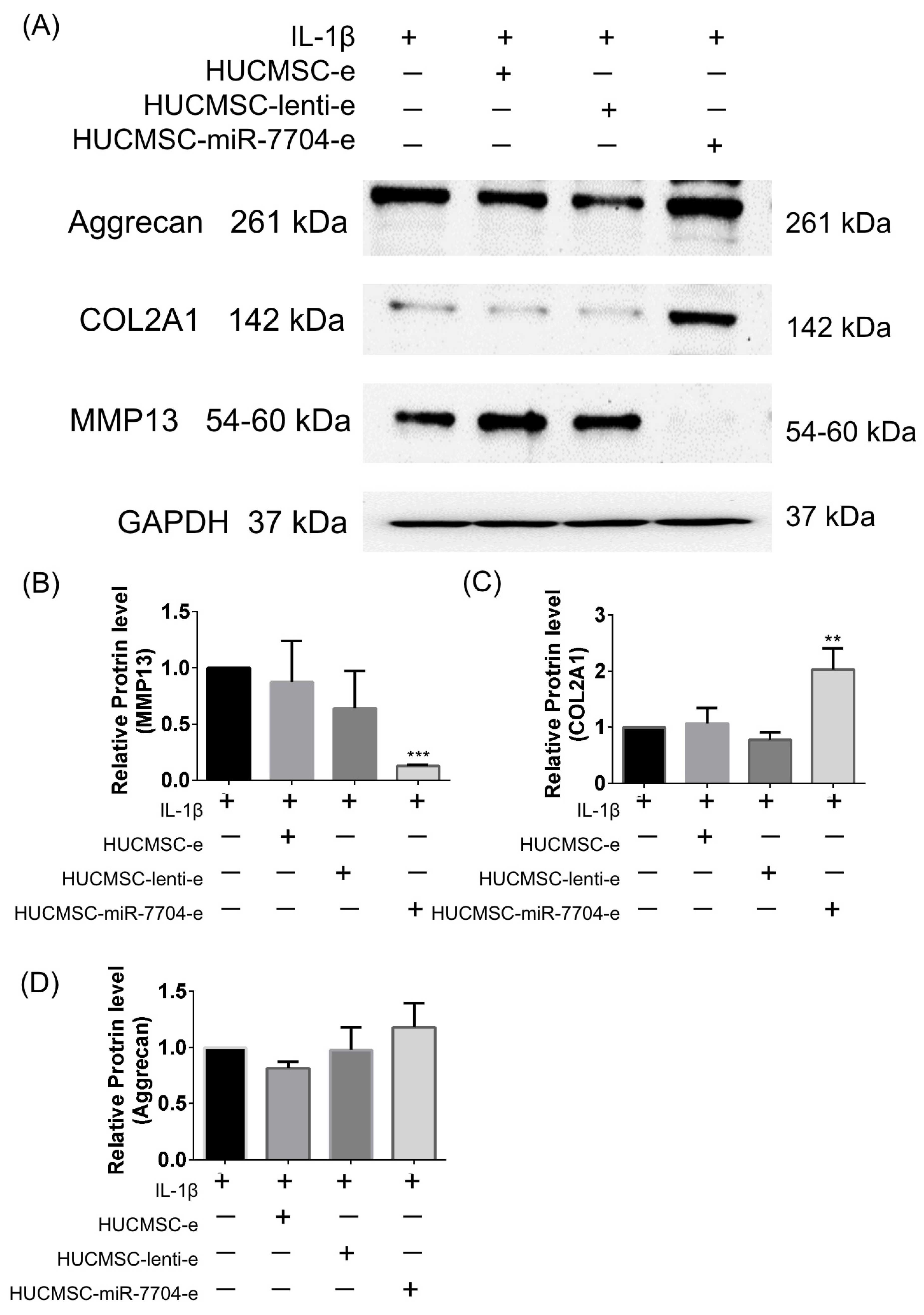
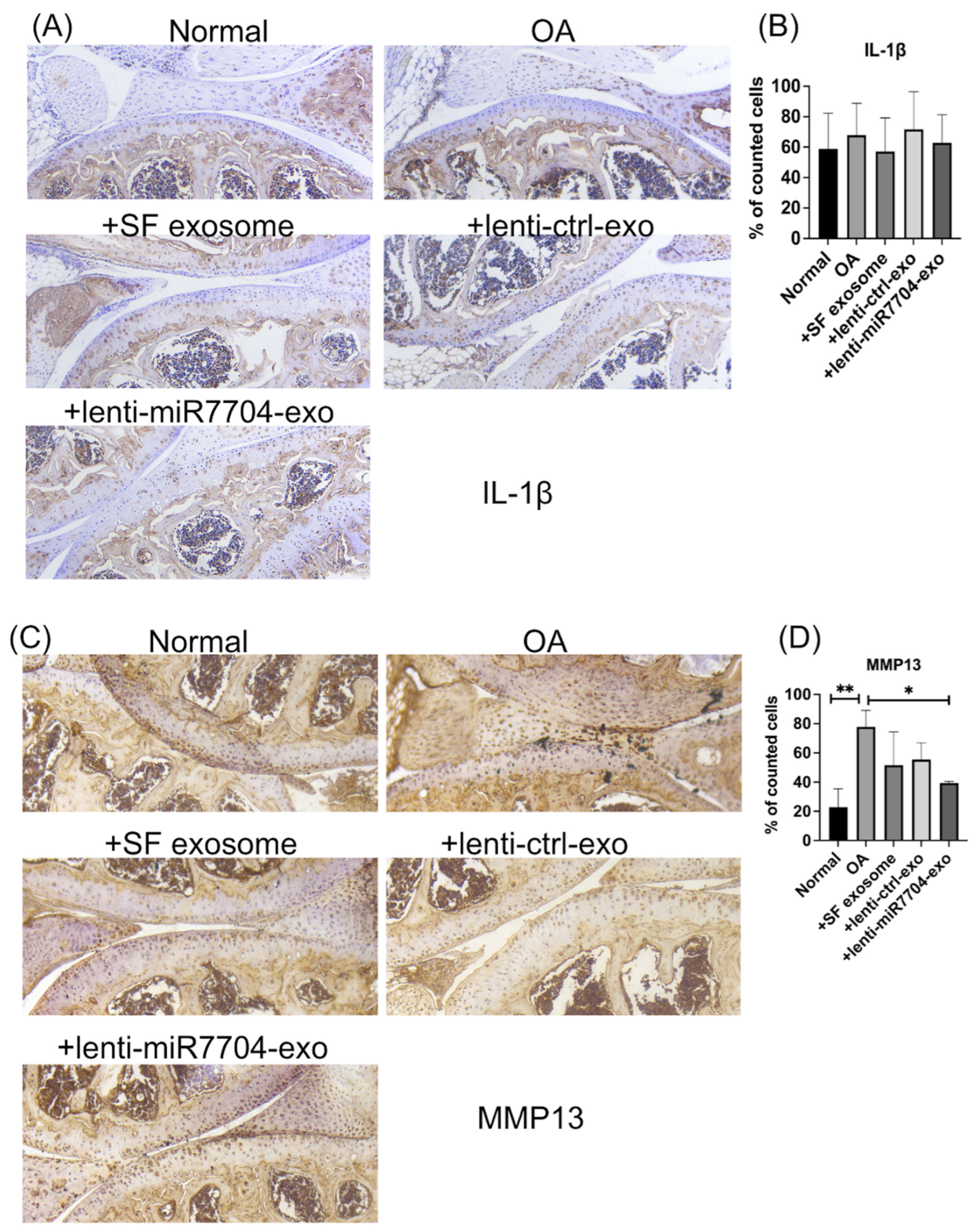
3.3. Impact of HUCMSC-miR-7704-e on Type II Collagen and MMP13 Protein Expression Levels in the IL-1β-Treated Chondrocytes
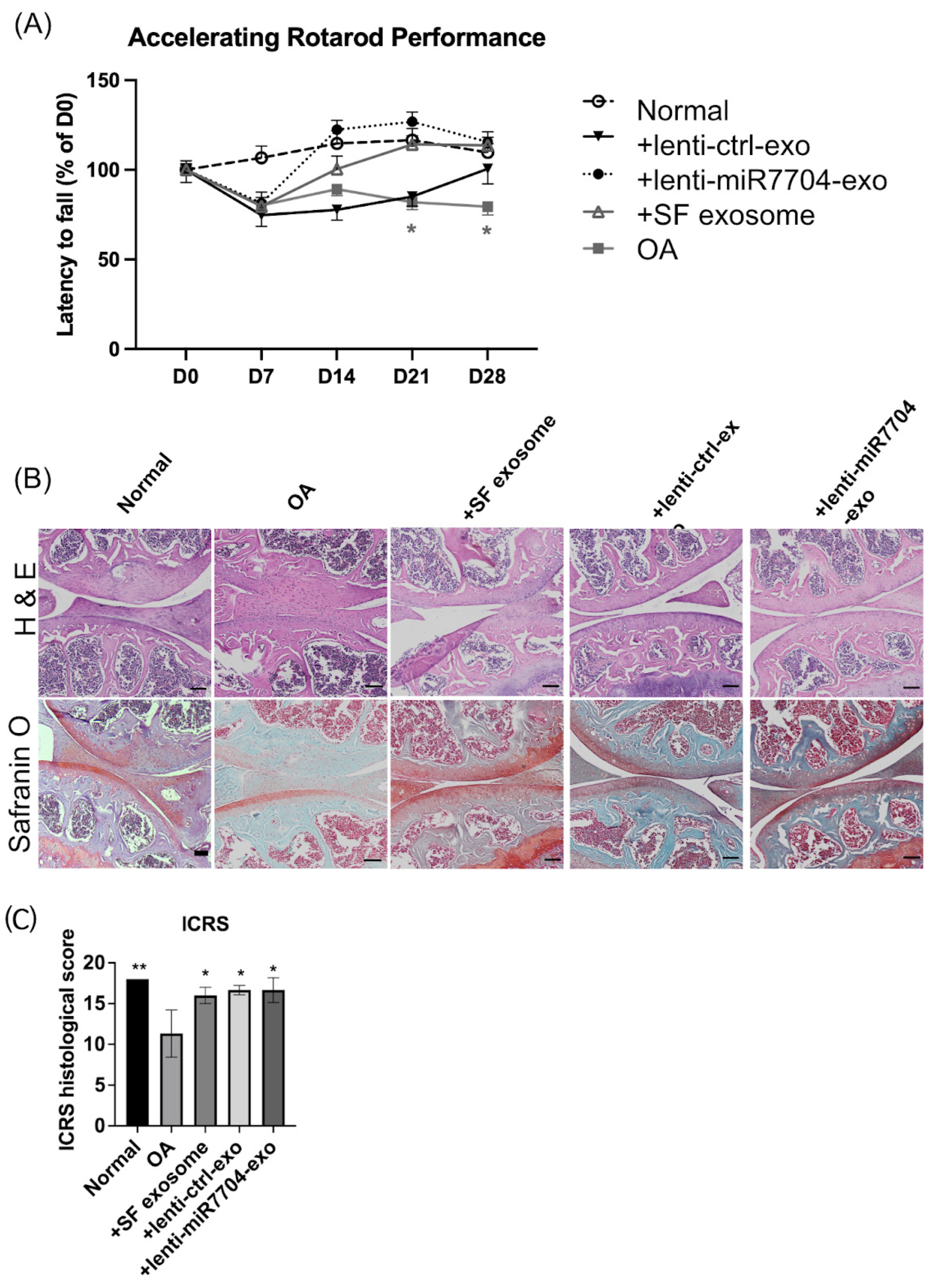
3.4. Rotarod Behavior Improved After EV Treatments
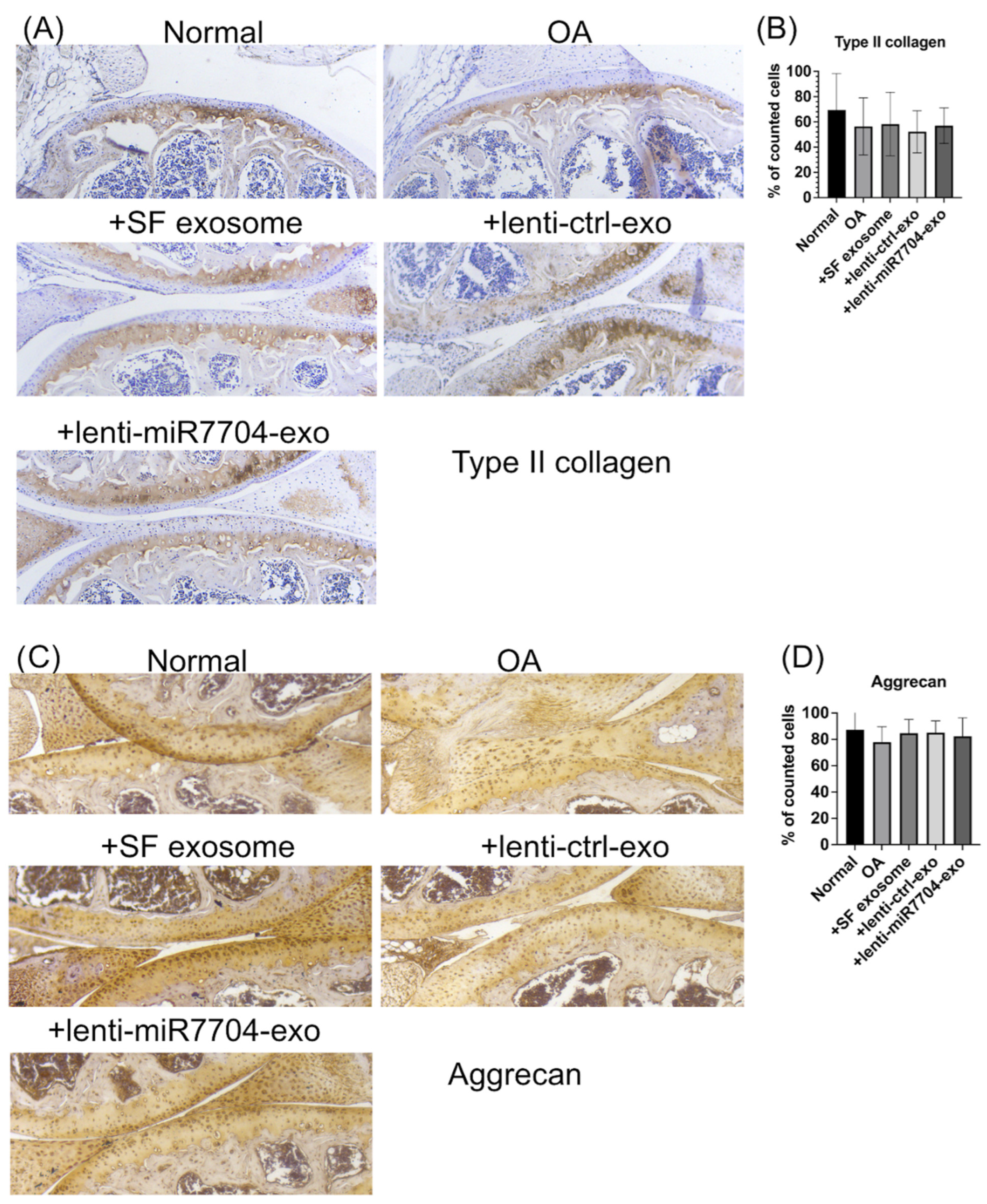
3.5. Histology of Cartilage of Knee Joint
3.6. Comparable Expression of Chondrogenic Proteins Post-Transplantation of EVs
3.7. Decreased MMP13 in EV-Treated Cartilages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Tang, H.; Lin, J.; Zeng, R. Temporal Trends in the Disease Burden of Osteoarthritis from 1990 to 2019, and Projections until 2030. PLoS ONE 2023, 18, e0288561. [Google Scholar] [CrossRef] [PubMed]
- Leifer, V.P.; Katz, J.N.; Losina, E. The Burden of OA-Health Services and Economics. Osteoarthr. Cartil. 2022, 30, 10–16. [Google Scholar] [CrossRef]
- Yahaya, I.; Wright, T.; Babatunde, O.O.; Corp, N.; Helliwell, T.; Dikomitis, L.; Mallen, C.D. Prevalence of Osteoarthritis in Lower Middle- and Low-Income Countries: A Systematic Review and Meta-Analysis. Rheumatol. Int. 2021, 41, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kong, W.; Liang, Y.; Sun, H. Burden of osteoarthritis in China, 1990–2019: Findings from the Global Burden of Disease Study 2019. Clin. Rheumatol. 2024, 43, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.J.; Driban, J.B.; McAlindon, T.E. Pharmaceutical Treatment of Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 458–466. [Google Scholar] [CrossRef]
- Cao, P.; Li, Y.; Tang, Y.; Ding, C.; Hunter, D.J. Pharmacotherapy for Knee Osteoarthritis: Current and Emerging Therapies. Expert Opin. Pharmacother. 2020, 21, 797–809. [Google Scholar] [CrossRef]
- Jin, G.-Z. Current Nanoparticle-Based Technologies for Osteoarthritis Therapy. Nanomaterials 2020, 10, 2368. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Zhang, L.; Hu, B.; Hu, S.; Zhang, X.; Hu, J. Small Molecule Inhibitors of Osteoarthritis: Current Development and Future Perspective. Front. Physiol. 2023, 14, 1156913. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Chang, Y.-H.; Shyu, W.-C.; Lin, S.-Z. Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal Stem Cells in Osteoarthritis Therapy: A Review. Am. J. Transl. Res. 2021, 13, 448–461. [Google Scholar] [PubMed]
- Chang, Y.-H.; Wu, K.-C.; Harn, H.-J.; Lin, S.-Z.; Ding, D.-C. Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant. 2018, 27, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Duong, C.M.; Nguyen, X.-H.; Than, U.T.T. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Osteoarthritis Treatment: Extracellular Matrix Protection, Chondrocyte and Osteocyte Physiology, Pain and Inflammation Management. Cells 2021, 10, 2887. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Withrow, J.; Hunter, M.; Liu, Y.; Tang, Y.L.; Fulzele, S.; Hamrick, M.W. Emerging Role of Extracellular Vesicles in Musculoskeletal Diseases. Mol Aspects Med. 2017, 60, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lange, S. Extracellular Vesicles in Phylogeny. Int. J. Mol. Sci. 2023, 24, 10466. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular Vesicles Shuffling Intercellular Messages: For Good or for Bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Chiang, M.-F.; Lin, S.-Z.; Chang, Y.-C.; Wang, H.-J.; Su, C.-Y.; Li, H. Enhancement of Neuroplasticity through Upregulation of beta1-Integrin in Human Umbilical Cord-Derived Stromal Cell Implanted Stroke Model. Neurobiol. Dis. 2007, 27, 339–353. [Google Scholar] [CrossRef]
- Ma, Z.-J.; Yang, J.-J.; Lu, Y.-B.; Liu, Z.-Y.; Wang, X.-X. Mesenchymal Stem Cell-Derived Exosomes: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. World J. Stem Cells 2020, 12, 814–840. [Google Scholar] [CrossRef]
- Ding, D.-C.; Chou, H.-L.; Chang, Y.-H.; Hung, W.-T.; Liu, H.-W.; Chu, T.-Y. Characterization of HLA-G and Related Immunosuppressive Effects in Human Umbilical Cord Stroma-Derived Stem Cells. Cell Transplant. 2016, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-C.; Chang, Y.-H.; Liu, H.-W.; Ding, D.-C. Transplanting Human Umbilical Cord Mesenchymal Stem Cells and Hyaluronate Hydrogel Repairs Cartilage of Osteoarthritis in the Minipig Model. Ci Ji Yi Xue Za Zhi 2019, 31, 11–19. [Google Scholar]
- Gu, J.; Rao, W.; Huo, S.; Fan, T.; Qiu, M.; Zhu, H.; Chen, D.; Sheng, X. MicroRNAs and Long Non-Coding RNAs in Cartilage Homeostasis and Osteoarthritis. Front. Cell Dev. Biol. 2022, 10, 1092776. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M. MicroRNAs: Exploring New Horizons in Osteoarthritis. Osteoarthr. Cartil. 2016, 24, 573–580. [Google Scholar] [CrossRef]
- Trzeciak, T.; Czarny-Ratajczak, M. MicroRNAs: Important Epigenetic Regulators in Osteoarthritis. Curr. Genom. 2014, 15, 481–484. [Google Scholar] [CrossRef][Green Version]
- Cong, L.; Zhu, Y.; Tu, G. A Bioinformatic Analysis of microRNAs Role in Osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1362–1371. [Google Scholar] [CrossRef]
- Lin, W.-T.; Wu, H.-H.; Lee, C.-W.; Chen, Y.-F.; Huang, L.; Hui-Chun Ho, J.; Kuang-Sheng Lee, O. Modulation of Experimental Acute Lung Injury by Exosomal miR-7704 from Mesenchymal Stromal Cells Acts through M2 Macrophage Polarization. Mol. Ther. Nucleic Acids 2024, 35, 102102. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Wang, M.; Sampson, E.R.; Jin, H.; Li, J.; Ke, Q.H.; Im, H.-J.; Chen, D. MMP13 Is a Critical Target Gene during the Progression of Osteoarthritis. Arthritis Res. Ther. 2013, 15, R5. [Google Scholar] [CrossRef]
- Ruan, G.; Xu, J.; Wang, K.; Wu, J.; Zhu, Q.; Ren, J.; Bian, F.; Chang, B.; Bai, X.; Han, W.; et al. Associations between Knee Structural Measures, Circulating Inflammatory Factors and MMP13 in Patients with Knee Osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1063–1069. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New Insights on the MMP-13 Regulatory Network in the Pathogenesis of Early Osteoarthritis. Arthritis Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, X.; Yuan, Y. MicroRNA-410 Promotes Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells through down-Regulating Wnt3a. Am. J. Transl. Res. 2017, 9, 136–145. [Google Scholar] [PubMed]
- Tornero-Esteban, P.; Hoyas, J.A.; Villafuertes, E.; Garcia-Bullón, I.; Moro, E.; Fernández-Gutiérrez, B.; Marco, F. Study of the Role of miRNA in Mesenchymal Stem Cells Isolated from Osteoarthritis Patients. Rev. Esp. Cir. Ortop. Traumatol. 2014, 58, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Gao, Y.; Jayasuriya, C.T.; Liu, W.; Du, H.; Ding, J.; Feng, M.; Chen, Q. Chondrogenic Induction of Human Osteoarthritic Cartilage-Derived Mesenchymal Stem Cells Activates Mineralization and Hypertrophic and Osteogenic Gene Expression through a mechanomiR. Arthritis Res. Ther. 2019, 21, 167. [Google Scholar] [CrossRef]
- Ham, O.; Lee, C.Y.; Kim, R.; Lee, J.; Oh, S.; Lee, M.Y.; Kim, J.; Hwang, K.-C.; Maeng, L.-S.; Chang, W. Therapeutic Potential of Differentiated Mesenchymal Stem Cells for Treatment of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 14961–14978. [Google Scholar] [CrossRef]
- Li, H.-Z.; Xu, X.-H.; Lin, N.; Wang, D.-W.; Lin, Y.-M.; Su, Z.-Z.; Lu, H.-D. Overexpression of miR-10a-5p Facilitates the Progression of Osteoarthritis. Aging 2020, 12, 5948–5976. [Google Scholar] [CrossRef]
- Li, Z.; Meng, D.; Li, G.; Xu, J.; Tian, K.; Li, Y. Overexpression of microRNA-210 Promotes Chondrocyte Proliferation and Extracellular Matrix Deposition by Targeting HIF-3α in Osteoarthritis. Mol. Med. Rep. 2016, 13, 2769–2776. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rutgers, M.; van Pelt, M.J.P.; Dhert, W.J.A.; Creemers, L.B.; Saris, D.B.F. Evaluation of Histological Scoring Systems for Tissue-Engineered, Repaired and Osteoarthritic Cartilage. Osteoarthr. Cartil. 2010, 18, 12–23. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Gu, J.-H.; Wang, F.-Y.; Shang, X.-S.; Tao, H.-R.; Wang, X. Regulation of Type II Collagen, Matrix Metalloproteinase-13 and Cell Proliferation by Interleukin-1β Is Mediated by Curcumin via Inhibition of NF-κB Signaling in Rat Chondrocytes. Mol. Med. Rep. 2017, 16, 1837–1845. [Google Scholar] [CrossRef]
- Tang, J.; Cui, W.; Song, F.; Zhai, C.; Hu, H.; Zuo, Q.; Fan, W. Effects of Mesenchymal Stem Cells on Interleukin-1β-Treated Chondrocytes and Cartilage in a Rat Osteoarthritic Model. Mol. Med. Rep. 2015, 12, 1753–1760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimo, T.; Takebe, H.; Okui, T.; Kunisada, Y.; Ibaragi, S.; Obata, K.; Kurio, N.; Shamsoon, K.; Fujii, S.; Hosoya, A.; et al. Expression and Role of IL-1β Signaling in Chondrocytes Associated with Retinoid Signaling during Fracture Healing. Int. J. Mol. Sci. 2020, 21, 2365. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A.-M.; Nieminen, P. Extracellular Vesicles and Their Potential Significance in the Pathogenesis and Treatment of Osteoarthritis. Pharmaceuticals 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Mohd Noor, N.A.; Abdullah Nurul, A.; Ahmad Mohd Zain, M.R.; Wan Nor Aduni, W.K.; Azlan, M. Extracellular Vesicles from Mesenchymal Stem Cells as Potential Treatments for Osteoarthritis. Cells 2021, 10, 1287. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.; Tian, G.; Ma, Y.; Ning, C.; Yan, Z.; Wu, J.; Ge, Q.; Sui, X.; Liu, S.; et al. The Role of Extracellular Vesicles in Osteoarthritis Treatment via Microenvironment Regulation. Biomater. Res. 2022, 26, 52. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, Y.; Fang, L.; Yuan, C.; Wang, X.; Lin, K. Breakthrough of Extracellular Vesicles in Pathogenesis, Diagnosis and Treatment of Osteoarthritis. Bioact. Mater. 2023, 22, 423–452. [Google Scholar] [CrossRef]
- Miyaki, S. Cartilage/chondrocyte research and osteoarthritis. The role of microRNAs and extracellular vesicles in osteoarthritis pathogenesis. Clin. Calcium 2018, 28, 783–788. [Google Scholar]
- Shang, X.; Fang, Y.; Xin, W.; You, H. The Application of Extracellular Vesicles Mediated miRNAs in Osteoarthritis: Current Knowledge and Perspective. J. Inflamm. Res. 2022, 15, 2583–2599. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl. Mater. Interfaces 2020, 12, 36938–36947. [Google Scholar] [CrossRef]
- Lara-Barba, E.; Araya, M.J.; Hill, C.N.; Bustamante-Barrientos, F.A.; Ortloff, A.; García, C.; Galvez-Jiron, F.; Pradenas, C.; Luque-Campos, N.; Maita, G.; et al. Role of microRNA Shuttled in Small Extracellular Vesicles Derived from Mesenchymal Stem/stromal Cells for Osteoarticular Disease Treatment. Front. Immunol. 2021, 12, 768771. [Google Scholar] [CrossRef]
- Wang, H.; Shu, J.; Zhang, C.; Wang, Y.; Shi, R.; Yang, F.; Tang, X. Extracellular Vesicle-Mediated miR-150-3p Delivery in Joint Homeostasis: A Potential Treatment for Osteoarthritis? Cells 2022, 11, 2766. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, A.; Li, X.; Sun, Z.; Sun, Z.; Liu, Y.; Wang, G.; Huang, D.; Xiong, H.; Yu, S.; et al. Dual-Engineered Cartilage-Targeting Extracellular Vesicles Derived from Mesenchymal Stem Cells Enhance Osteoarthritis Treatment via miR-223/NLRP3/pyroptosis Axis: Toward a Precision Therapy. Bioact. Mater. 2023, 30, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-C.; Chang, Y.-H.; Ding, D.-C.; Lin, S.-Z. Mesenchymal Stromal Cells for Aging Cartilage Regeneration: A Review. Int. J. Mol. Sci. 2024, 25, 12911. [Google Scholar] [CrossRef]
- Jimenez, D.E.; Tahir, M.; Faheem, M.; Alves, W.B.D.S.; Correa, B.d.L.; Andrade, G.R.d.; Larsen, M.R.; Oliveira, G.P.d., Jr.; Pereira, R.W. Comparison of Four Purification Methods on Serum Extracellular Vesicle Recovery, Size Distribution, and Proteomics. Proteomes 2023, 11, 23. [Google Scholar] [CrossRef]
- Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Rivas, M.; Oliva, P.; Villafani, J.; Navarro, A.; Blanco-López, M.C.; Cernuda-Morollón, E. Characterization of Plasma-Derived Extracellular Vesicles Isolated by Different Methods: A Comparison Study. Bioengineering 2019, 6, 8. [Google Scholar] [CrossRef]
- Wang, X. Isolation of Extracellular Vesicles from Breast Milk. Methods Mol. Biol. 2017, 1660, 351–353. [Google Scholar]
- Arakelyan, A.; Fitzgerald, W.; Vagida, M.; Vasilieva, E.; Margolis, L.; Grivel, J.-C. Addition of Thrombin Reduces the Recovery of Extracellular Vesicles from Blood Plasma. J. Circ. Biomark. 2016, 5, 1849454416663648. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.-M.; Wang, Z. Preserving Extracellular Vesicles for Biomedical Applications: Consideration of Storage Stability before and after Isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1. [Google Scholar] [CrossRef]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The Impact of Storage on Extracellular Vesicles: A Systematic Study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef]
- Lőrincz, Á.M.; Timár, C.I.; Marosvári, K.A.; Veres, D.S.; Otrokocsi, L.; Kittel, Á.; Ligeti, E. Effect of Storage on Physical and Functional Properties of Extracellular Vesicles Derived from Neutrophilic Granulocytes. J. Extracell. Vesicles 2014, 3, 25465. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.K.E.; Lim, J.; Foong, L.H.; Mok, C.Y.; Tan, H.Y.; Tung, X.Y.; Ramasamy, T.S.; Govindasamy, V.; Then, K.-Y.; Das, A.K.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Progress and Remaining Hurdles in Developing Regulatory Compliant Quality Control Assays. Adv. Exp. Med. Biol. 2022, 1401, 191–211. [Google Scholar] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying Extracellular Vesicles Based Therapeutics in Clinical Trials—An ISEV Position Paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Stawarska, A.; Bamburowicz-Klimkowska, M.; Runden-Pran, E.; Dusinska, M.; Cimpan, M.R.; Rios-Mondragon, I.; Grudzinski, I.P. Extracellular Vesicles as next-Generation Diagnostics and Advanced Therapy Medicinal Products. Int. J. Mol. Sci. 2024, 25, 6533. [Google Scholar] [CrossRef]
- Lai, J.J.; Hill, J.J.; Huang, C.Y.; Lee, G.C.; Mai, K.W.; Shen, M.Y.; Wang, S.K. Unveiling the Complex World of Extracellular Vesicles: Novel Characterization Techniques and Manufacturing Considerations. Chonnam Med. J. 2024, 60, 1–12. [Google Scholar] [CrossRef]
- Piffoux, M.; Volatron, J.; Cherukula, K.; Aubertin, K.; Wilhelm, C.; Silva, A.K.A.; Gazeau, F. Engineering and Loading Therapeutic Extracellular Vesicles for Clinical Translation: A Data Reporting Frame for Comparability. Adv. Drug Deliv. Rev. 2021, 178, 113972. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, S.; Grippin, A.J.; Teng, L.; Lee, A.S.; Kim, B.Y.S.; Jiang, W. Engineering Therapeutical Extracellular Vesicles for Clinical Translation. Trends Biotechnol. 2004, 43, 61–82. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Song, J.; Song, B.; Yuan, L.; Yang, G. Multiplexed Strategies toward Clinical Translation of Extracellular Vesicles. Theranostics 2022, 12, 6740–6761. [Google Scholar] [CrossRef]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles as Nanomedicine: Hopes and Hurdles in Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef]
| Gene Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Product Size (bp) |
|---|---|---|---|
| PPARγ | AGCCTCATGAAGAGCCTTCCA | TCCGGAAGAAACCCTTGCA | 120 |
| FABP4 | ATGGGATGGAAAATCAACCA | GTGGAAGTGACGCCTTTCAT | 87 |
| ALPL | CCACGTCTTCACATTTGGTG | GCAGTGAAGGGCTTCTTGTC | 96 |
| RUNX2 | CGGAATGCCTCTGCTGTTAT | TTCCCGAGGTCCATCTACTG | 174 |
| ACAN | GAGATGGAG GGTGAGGTC | ACGCTGCCTCGGGCTTC | 443 |
| COL2A1 | GGACTTTTCTCCCCTCTCT | GACCCGAAGGTCTTACAGGA | 104 |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC | 172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-C.; Yang, H.-I.; Chang, Y.-H.; Chiang, R.Y.-S.; Ding, D.-C. Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13. Cells 2025, 14, 82. https://doi.org/10.3390/cells14020082
Wu K-C, Yang H-I, Chang Y-H, Chiang RY-S, Ding D-C. Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13. Cells. 2025; 14(2):82. https://doi.org/10.3390/cells14020082
Chicago/Turabian StyleWu, Kun-Chi, Hui-I Yang, Yu-Hsun Chang, Raymond Yuh-Shyan Chiang, and Dah-Ching Ding. 2025. "Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13" Cells 14, no. 2: 82. https://doi.org/10.3390/cells14020082
APA StyleWu, K.-C., Yang, H.-I., Chang, Y.-H., Chiang, R. Y.-S., & Ding, D.-C. (2025). Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13. Cells, 14(2), 82. https://doi.org/10.3390/cells14020082








