Author Contributions
Conceptualization, methodology, validation, investigation, writing—original draft, data interpretation, visualization, supervision, project administration, J.S.; investigation, visualization, R.S.; resources, writing—review and editing, funding acquisition, J.H.; methodology, supervision, writing—review and editing, T.S.; methodology, resources, supervision, writing—review and editing, Z.G. All authors have read and agreed to the published version of the manuscript.
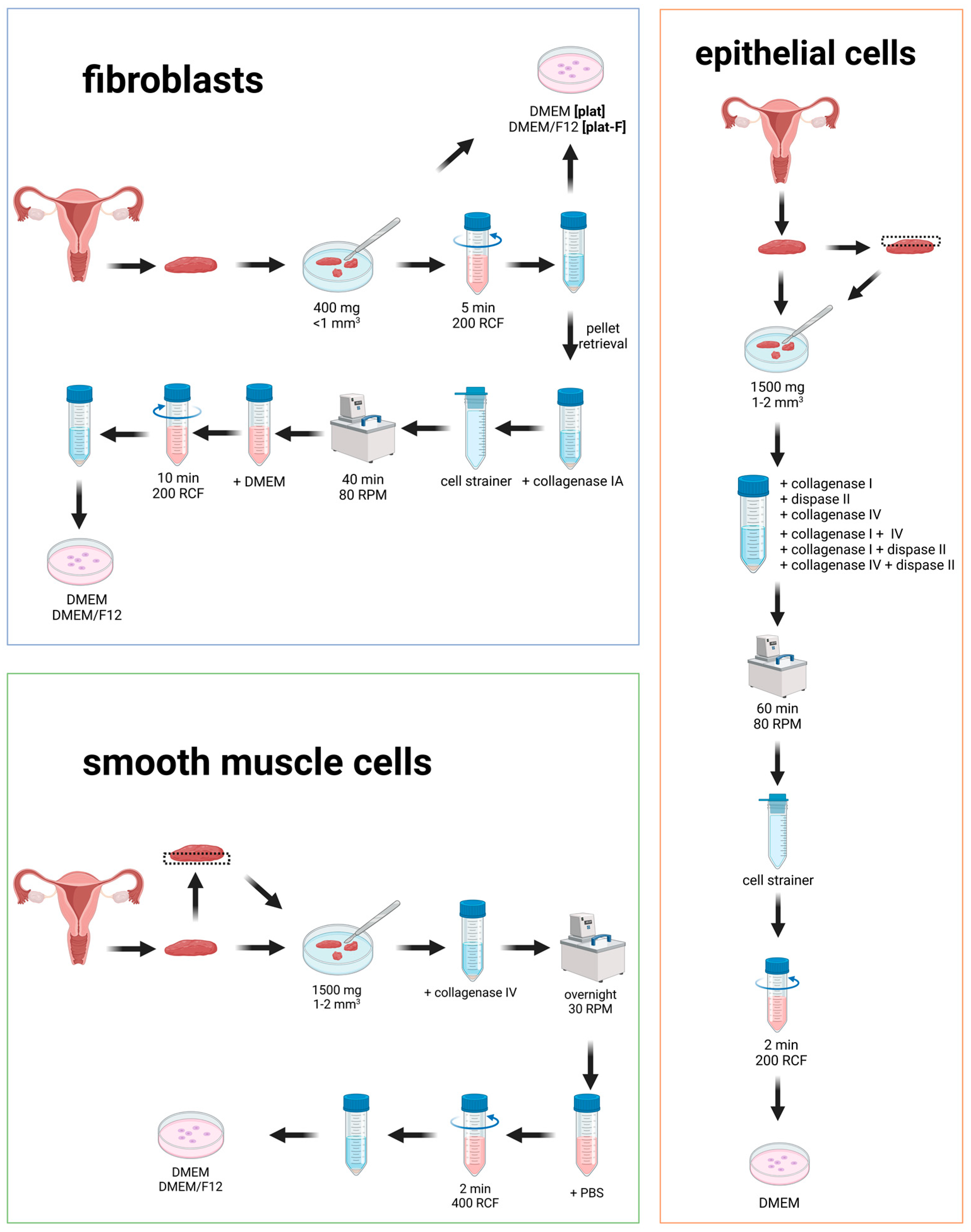
Figure 1.
Isolation protocols were tested for fibroblasts (blue), smooth muscle cells (green), and epithelial cells (orange) from human vaginal wall tissue. This illustration provides a schematic overview of the tested protocols.
Figure 1.
Isolation protocols were tested for fibroblasts (blue), smooth muscle cells (green), and epithelial cells (orange) from human vaginal wall tissue. This illustration provides a schematic overview of the tested protocols.
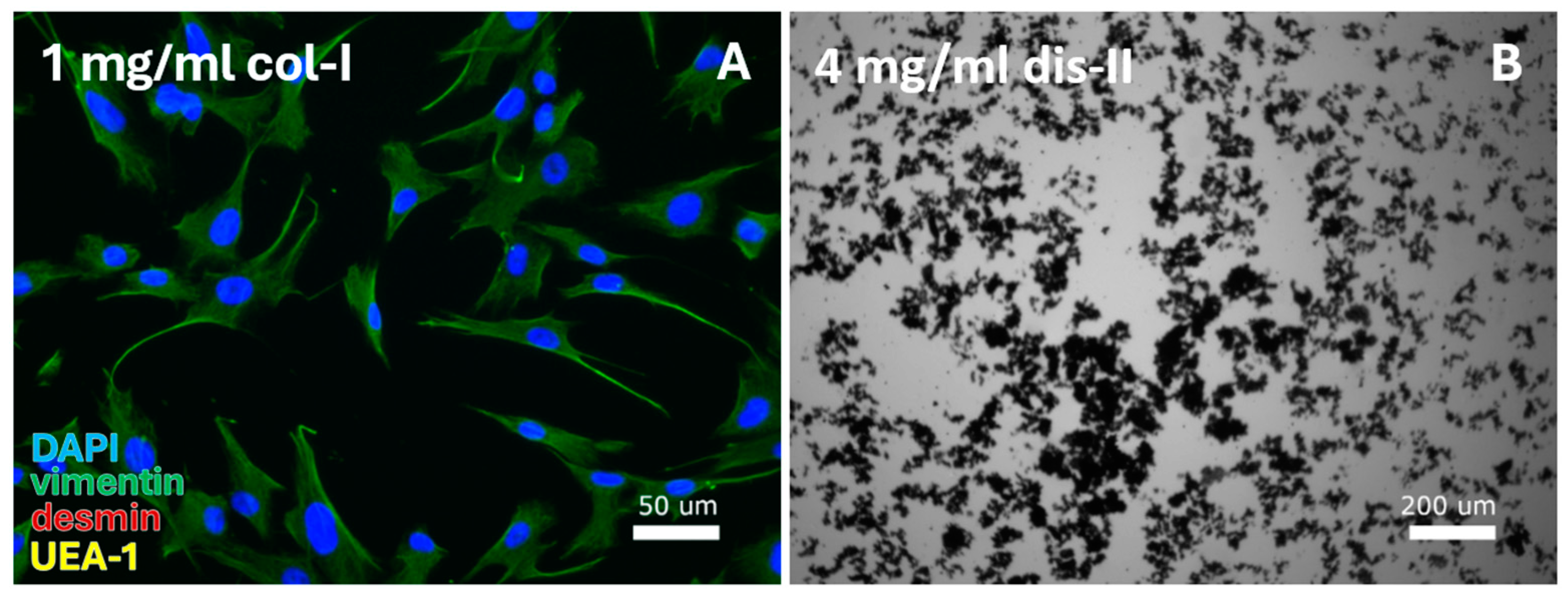
Figure 2.
VEC-specific protocols were tested for digestion by a range of collagenase-I (col-I), collagenase-IV, and dispase-II (dis-II) concentrations. This resulted in (A) isolation of fibroblasts from the cell pellet after digestion with 1 mg/mL collagenase-I and (B) cell debris for use of high (2 or 4 mg/mL) dispase-II concentrations or combined use of dispase-II, collagenase-I, and collagenase-IV. DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
Figure 2.
VEC-specific protocols were tested for digestion by a range of collagenase-I (col-I), collagenase-IV, and dispase-II (dis-II) concentrations. This resulted in (A) isolation of fibroblasts from the cell pellet after digestion with 1 mg/mL collagenase-I and (B) cell debris for use of high (2 or 4 mg/mL) dispase-II concentrations or combined use of dispase-II, collagenase-I, and collagenase-IV. DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
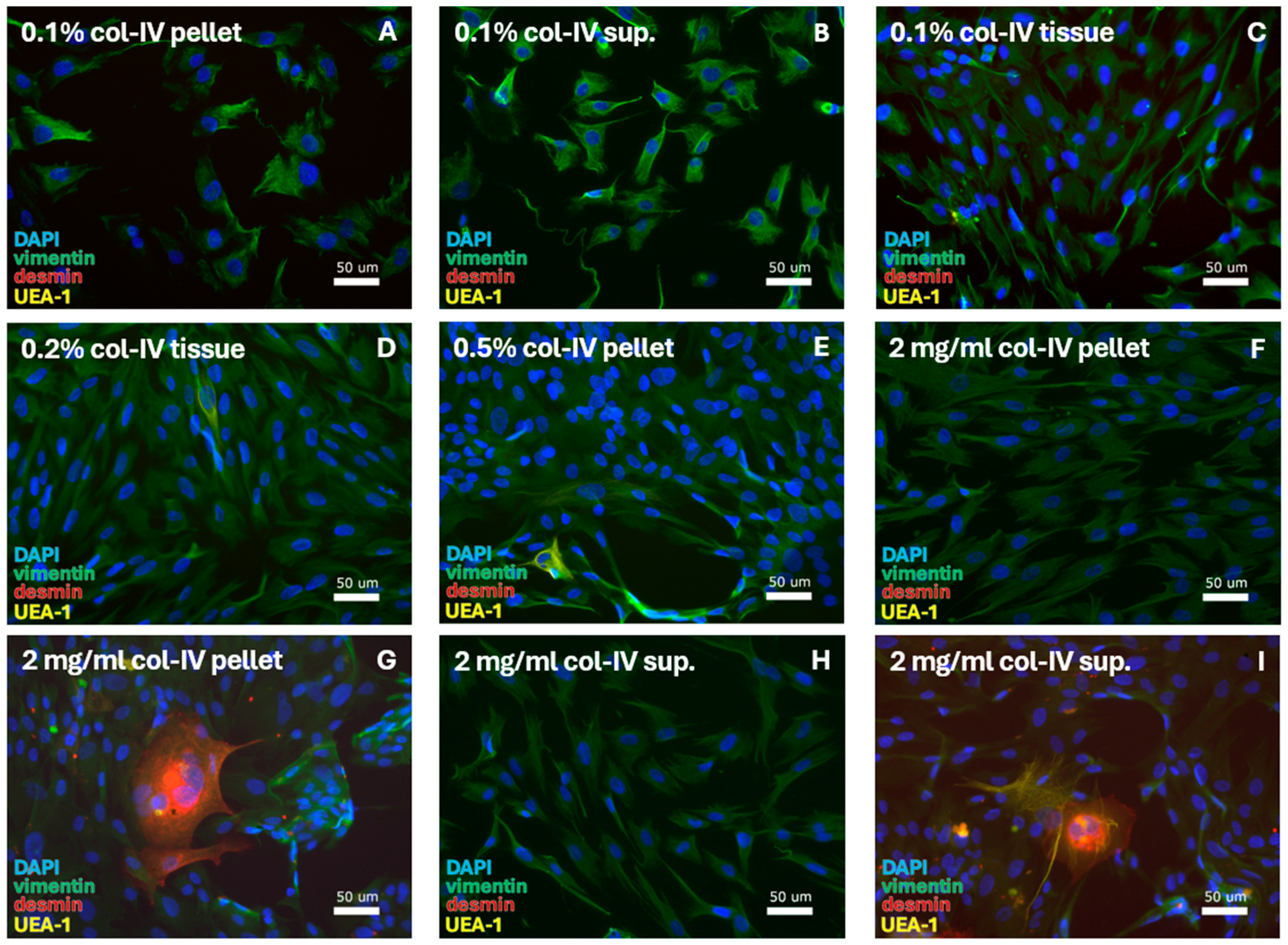
Figure 3.
SMC-specific protocols were tested for digestion using a range of collagenase-IV (col-IV) concentrations. This resulted in isolation of FBs. With 0.1% col-IV and culturing in DMEM, FBs were retrieved from the (A) pellet, (B) supernatant (sup.), and (C) tissue. FBs were also retrieved through culturing in DMEM/F12 after isolation with (D) 0.2% col-IV from the tissue and (E) 0.5% collagenase-IV from the pellet. Digestion with 2 mg/mL col-IV resulted in FBs from (F) the pellet, with (G) the sporadic detection of muscle cells at quantities <5%, and from (H) the supernatant, with (I) the sporadic detection of muscle and epithelial cells at quantities <5%. DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
Figure 3.
SMC-specific protocols were tested for digestion using a range of collagenase-IV (col-IV) concentrations. This resulted in isolation of FBs. With 0.1% col-IV and culturing in DMEM, FBs were retrieved from the (A) pellet, (B) supernatant (sup.), and (C) tissue. FBs were also retrieved through culturing in DMEM/F12 after isolation with (D) 0.2% col-IV from the tissue and (E) 0.5% collagenase-IV from the pellet. Digestion with 2 mg/mL col-IV resulted in FBs from (F) the pellet, with (G) the sporadic detection of muscle cells at quantities <5%, and from (H) the supernatant, with (I) the sporadic detection of muscle and epithelial cells at quantities <5%. DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
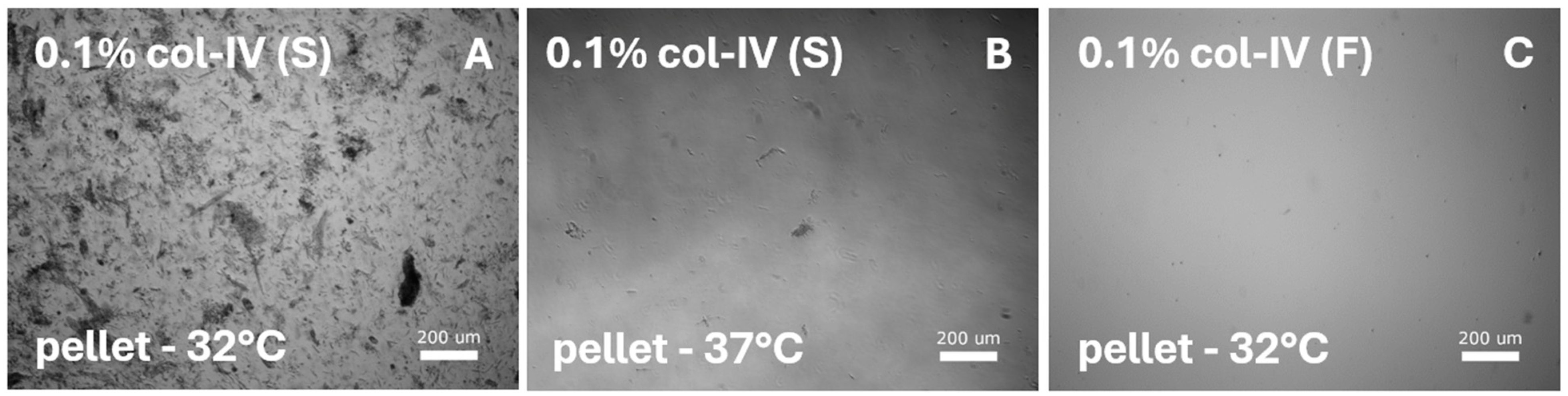
Figure 4.
SMC-specific protocols were tested on diced sections from the smooth muscle layer and lamina propria. Digestion with various collagenase-I, collagenase-IV, and dispase-II concentrations was performed and tested for various media and temperatures. All protocols resulted in cell debris. Representative images illustrate digestion with 0.1% collagenase-IV (col-IV) from the cell pellet after culturing in (A) SMC-specific medium [S] at 32 °C or (B) at 37 °C, and in (C) DMEM/F12 medium [F] at 32 °C.
Figure 4.
SMC-specific protocols were tested on diced sections from the smooth muscle layer and lamina propria. Digestion with various collagenase-I, collagenase-IV, and dispase-II concentrations was performed and tested for various media and temperatures. All protocols resulted in cell debris. Representative images illustrate digestion with 0.1% collagenase-IV (col-IV) from the cell pellet after culturing in (A) SMC-specific medium [S] at 32 °C or (B) at 37 °C, and in (C) DMEM/F12 medium [F] at 32 °C.
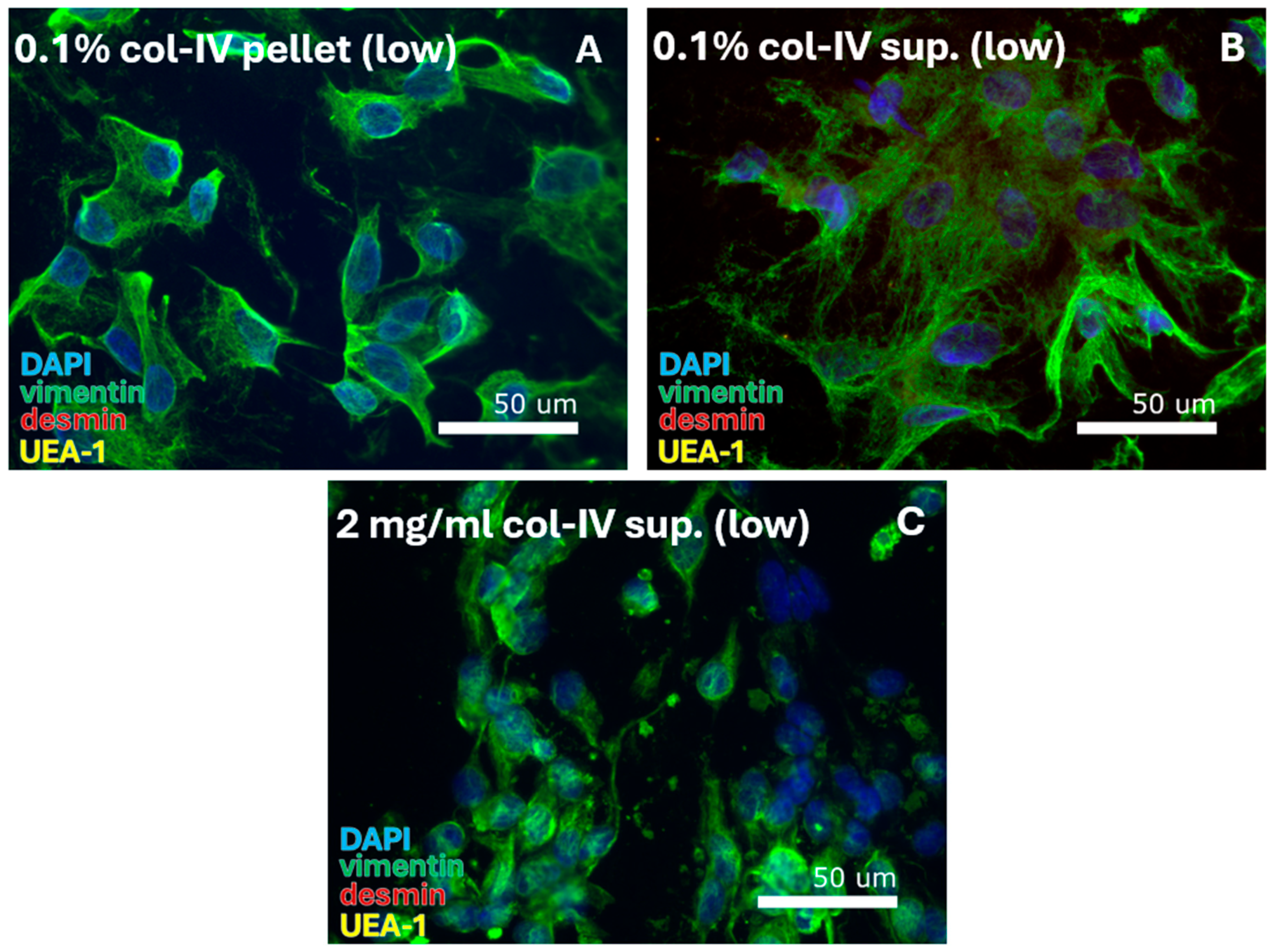
Figure 5.
SMC-specific protocols were tested for culturing under hypoxic conditions of 2% oxygen (low). Digestion with 0.1% collagenase-IV resulted in isolation of FBs from (A) the pellet and (B) the supernatant (sup.). Digestion with 2 mg/mL collagenase-IV resulted in FB isolation from (C) the supernatant. DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
Figure 5.
SMC-specific protocols were tested for culturing under hypoxic conditions of 2% oxygen (low). Digestion with 0.1% collagenase-IV resulted in isolation of FBs from (A) the pellet and (B) the supernatant (sup.). Digestion with 2 mg/mL collagenase-IV resulted in FB isolation from (C) the supernatant. DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
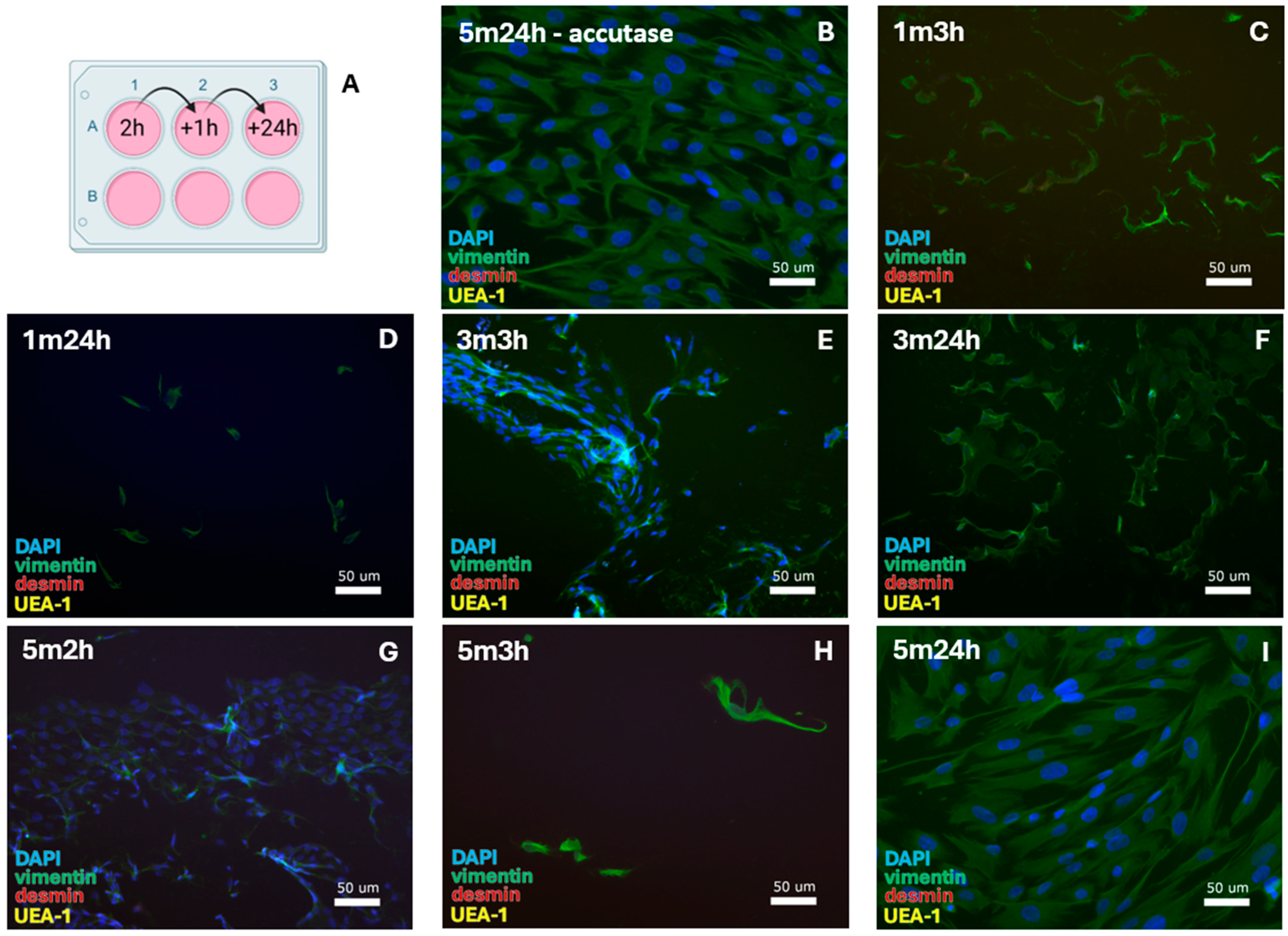
Figure 6.
SMC-specific protocols were tested for 0.1% collagenase-IV digestion with (A) alternative cell passaging. This resulted in the isolation of FBs after (B) 5 min of incubation in accutase and 24 h of cell attachment until the first medium refreshment. After 1 min trypsinization, FBs were isolated with cell attachment for (C) 3 h and (D) 24 h. For 3 min trypsinization, FBs formed with (E) 3 h and (F) 24 h cell attachment. For 5 min trypsinization, the same was observed with (G) 2 h, (H) 3 h, and (I) 24 h cell attachment. DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
Figure 6.
SMC-specific protocols were tested for 0.1% collagenase-IV digestion with (A) alternative cell passaging. This resulted in the isolation of FBs after (B) 5 min of incubation in accutase and 24 h of cell attachment until the first medium refreshment. After 1 min trypsinization, FBs were isolated with cell attachment for (C) 3 h and (D) 24 h. For 3 min trypsinization, FBs formed with (E) 3 h and (F) 24 h cell attachment. For 5 min trypsinization, the same was observed with (G) 2 h, (H) 3 h, and (I) 24 h cell attachment. DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
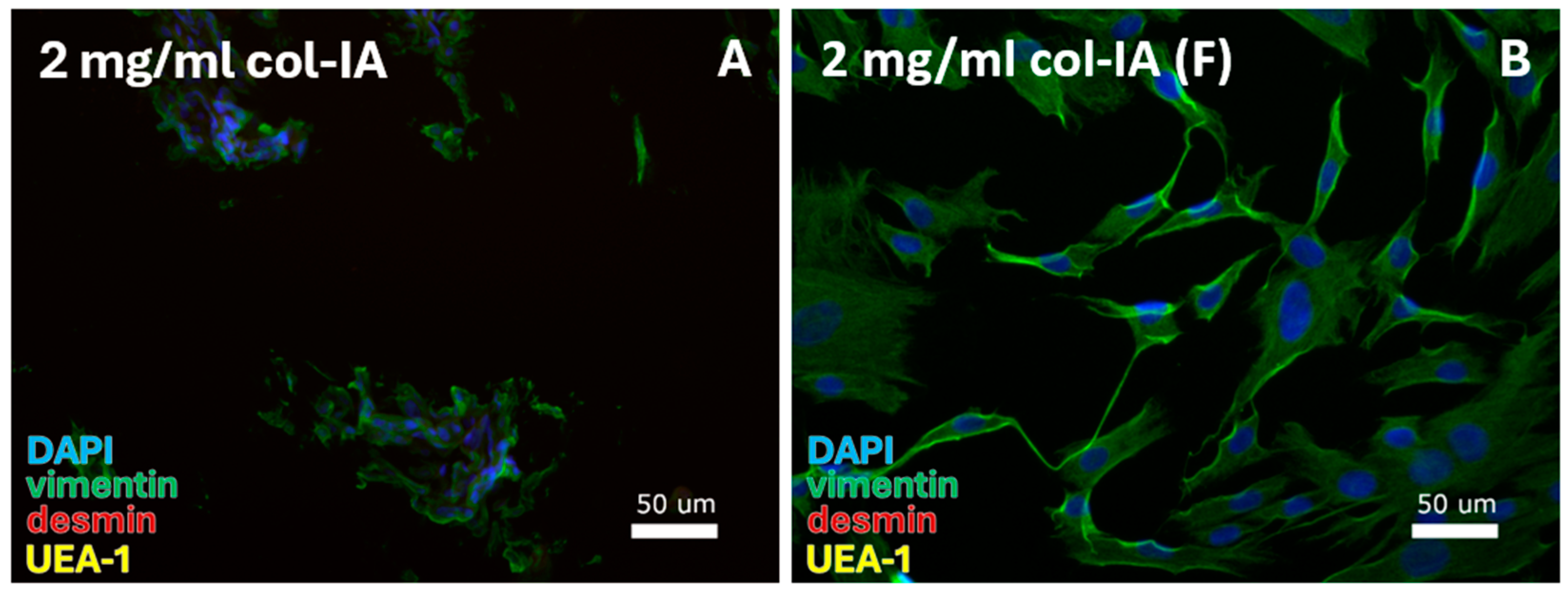
Figure 7.
FB-specific protocols were tested for digestion with 2 mg/mL collagenase-IA. FBs were isolated from the cell pellet with culturing in (A) DMEM and (B) DMEM/F12 (F). DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
Figure 7.
FB-specific protocols were tested for digestion with 2 mg/mL collagenase-IA. FBs were isolated from the cell pellet with culturing in (A) DMEM and (B) DMEM/F12 (F). DAPI = cell nuclei (blue), vimentin = fibroblasts (green), desmin = smooth muscle cells, and UEA-1 = endothelial cells (yellow).
Table 1.
Assessment of published isolation protocols on enzymatic digestion (concentration and time), cell retrieval (method of biopsy/tissue sampling; mechanical digestion before enzymatic digestion), replicate consistency (outcome tested for a number of replicates), and testing of cell viability. √ = reported; - = unreported.
Table 1.
Assessment of published isolation protocols on enzymatic digestion (concentration and time), cell retrieval (method of biopsy/tissue sampling; mechanical digestion before enzymatic digestion), replicate consistency (outcome tested for a number of replicates), and testing of cell viability. √ = reported; - = unreported.
| Study | Cell Type (s) | Digestion | Cell Retrieval | Consistency | Viability |
|---|
| conc. | time |
|---|
| De Philippo [34] | SMC/VEC | - | - | - | - | √ |
| Jakubowska [26] | FB/VEC | √ | √ | - | - | - |
| Li [36] | FB/SMC/VEC | √ | √ | √ | - | √ |
| Maseroli [37] | SMC | √ | √ | √ | - | √ |
| Nodale [38] | FB | - | - | - | - | √ |
| Orabi [19] | VEC | √ | √ | - | - | - |
| Ruiz-Zapata [39] | FB | √ | √ | - | - | - |
| Sartoneva [40] | VEC | √ | √ | - | - | √ |
| Skala [41] | FB | - | √ | √ | - | √ |
| Yuan [42] | VEC | √ | √ | - | - | √ |
| Zhu [43] | VEC | - | - | - | - | √ |
Table 2.
VEC isolation protocols were tested for digestion by collagen-I, dispase-II, and collagenase-IV. (A) Overview of the examined enzyme concentrations for full thickness vagina tissue. (B) Isolation protocols performed on diced sections from epithelial layers to increase specificity.
Table 2.
VEC isolation protocols were tested for digestion by collagen-I, dispase-II, and collagenase-IV. (A) Overview of the examined enzyme concentrations for full thickness vagina tissue. (B) Isolation protocols performed on diced sections from epithelial layers to increase specificity.
| A | Layer (s) | Collagenase-I (mg/mL) | Dispase-II (mg/mL) | Collagenase-IV (%) |
| 1 | Full thickness | 1 | - | - |
| 2 | 1 | 2 | - |
| 3 | 1 | 4 | - |
| 4 | 1.5 | 4 | - |
| 5 | 2 | 1 | - |
| 6 | 2 | 2 | - |
| 7 | 2 | 4 | - |
| B | Layer (s) | Collagenase-I (mg/mL) | Dispase-II (mg/mL) | Collagenase-IV (%) |
| S1 | Epidermal layer | 1.5 | - | - |
| S2 | 1.5 | 2 | - |
| S3 | 1 | - | 0.1 |
| S4 | - | 4 | - |
| S5 | - | 2 | 0.25 |
| S6 | - | - | 0.5 |
Table 3.
SMC protocols were tested with collagenase-IV. Overview of the enzyme concentrations with culturing in (A) DMEM and (B) DMEM/F12. Increased cell specificity was tested on diced tissue from (C) smooth muscle and (D) lamina propria.
Table 3.
SMC protocols were tested with collagenase-IV. Overview of the enzyme concentrations with culturing in (A) DMEM and (B) DMEM/F12. Increased cell specificity was tested on diced tissue from (C) smooth muscle and (D) lamina propria.
| A | Medium | Collagenase-IV (%) | Temperature (°C) |
| 1 | DMEM | 0.1 | 37 |
| 2 | 0.2 | 37 |
| 3 | 0.5 | 37 |
| 4 | 2 mg/mL | 37 |
| B | Medium | Collagenase-IV (%) | Temperature (°C) |
| F1 | DMEM/F12 | 0.1 | 37 |
| F2 | 0.2 | 37 |
| F3 | 0.5 | 37 |
| F4 | 2 mg/mL | 37 |
| C | Medium | Collagenase-IV (%) | Temperature (°C) |
| SM1 | DMEM | 0.1 | 37 |
| SM2 | DMEM/F12 |
| SM3 | SMC-specific |
| SM4 | DMEM | 32 |
| SM5 | DMEM/F12 |
| SM6 | SMC-specific |
| D | Medium | Collagenase-IV (%) | Temperature (°C) |
| LP1 | DMEM | 0.1 | 37 |
| LP2 | DMEM/F12 |
| LP3 | SMC-specific |
| LP4 | DMEM | 32 |
| LP5 | DMEM/F12 |
| LP6 | SMC-specific |
Table 4.
Alternative SMC protocols were performed under modified culturing conditions. Overview of the applied modifications, involving (A) digestion with 0.1% collagenase-IV followed by shaking at 5 or 150 RPM, or (B) digestion with 0.1% or 2 mg/mL collagenase-IV followed by culturing at 2% oxygen levels.
Table 4.
Alternative SMC protocols were performed under modified culturing conditions. Overview of the applied modifications, involving (A) digestion with 0.1% collagenase-IV followed by shaking at 5 or 150 RPM, or (B) digestion with 0.1% or 2 mg/mL collagenase-IV followed by culturing at 2% oxygen levels.
| A | Layer (s) | Alteration | Collagenase-IV (%) |
| Full thickness | 5 RPM shaking | 0.1 |
| 150 RPM shaking |
| B | Layer (s) | Alteration | Collagenase-IV (%) |
| Full thickness | 2% oxygen | 0.1 |
| 2 mg/mL |
Table 5.
SMC isolation protocols were performed with alternative passaging. This involved (A) 1 min trypsinization, (B) 3 min trypsinization, or (C) 5 min trypsinization. Each condition was tested for 2 h, 3 h, and 24 h of cell adherence before a first medium change was performed.
Table 5.
SMC isolation protocols were performed with alternative passaging. This involved (A) 1 min trypsinization, (B) 3 min trypsinization, or (C) 5 min trypsinization. Each condition was tested for 2 h, 3 h, and 24 h of cell adherence before a first medium change was performed.
| A | Layer(s) | Detachment (min) | Adherence (h) |
| 1m2h | Full thickness | 1 | 2 h |
| 1m3h | 3 h |
| 1m24h | 24 h |
| B | Layer(s) | Detachment (min) | Adherence (h) |
| 3m2h | Full thickness | 3 | 2 h |
| 3m3h | 3 h |
| 3m24h | 24 h |
| C | Layer(s) | Detachment (min) | Adherence (h) |
| 5m2h | Full thickness | 5 | 2 h |
| 5m3h | 3 h |
| 5m24h | 24 h |
Table 6.
FB protocols were tested for (A) digestion with 1 mg/mL collagenase-IA or (B) by direct culturing of tissue. Culturing was performed in DMEM and in DMEM/F12.
Table 6.
FB protocols were tested for (A) digestion with 1 mg/mL collagenase-IA or (B) by direct culturing of tissue. Culturing was performed in DMEM and in DMEM/F12.
| A | Layer(s) | Medium | Collagenase-IA (mg/mL) |
| 1 | Full thickness | DMEM | 1 |
| 2 | DMEM/F12 |
| B | Layer(s) | Medium | Collagenase-IA (mg/mL) |
| P1 | Full thickness | DMEM | - |
| P2 | DMEM/F12 |
Table 7.
Overview of all retrieved cell populations. (A) VEC protocols: cells were retrieved by digestion with 1 mg/mL collagen-I from cell pellet. (B) SMC protocols: cells were retrieved by digestion with 0.1%, 0.2%, 0.5%, and 2 mg/mL collagen-IV. (C) FB protocols: cell retrieval by 2 mg/mL collagenase-IV for culturing in DMEM and DMEM/F12. (D) Alternative cell passage tested for SMCs.
Table 7.
Overview of all retrieved cell populations. (A) VEC protocols: cells were retrieved by digestion with 1 mg/mL collagen-I from cell pellet. (B) SMC protocols: cells were retrieved by digestion with 0.1%, 0.2%, 0.5%, and 2 mg/mL collagen-IV. (C) FB protocols: cell retrieval by 2 mg/mL collagenase-IV for culturing in DMEM and DMEM/F12. (D) Alternative cell passage tested for SMCs.
| A | Retrieval | Figure | Collagenase I Concentration | Modification | Consistent | Morphology | Purity | Passage 1 (days) | Cell Viability (days/passage) |
| pellet | 2A | 1 mg/mL | - | yes | Typical FB | 100% | 48 | P6 | 13.5 |
| B | Retrieval | Figure | Collagenase IV Concentration | Modification | Consistent | Morphology | Purity | Passage 1 (days) | Cell Viability (days/passage) |
| pellet | 3A | 0.1% | - | yes | Typical FB | 100% | 56 | P12 | 5.3 |
| supernatant | 3B | P3 | 27 |
| tissue | 3C | P7 | 13 |
| tissue | 3D | 0.2% | - | yes | Typical FB | 100% | 49 | P3 | 10.3 |
| pellet | 3E | 0.5% | - | yes | Typical FB | 100% | 56 | P4 | 23.8 |
| pellet | 3F+G | 2 mg/mL | - | no | Typical FB | >95% | 21 | P6 | 5.8 |
| supernatant | 3H+I |
| pellet | 4A | 0.1% | 2% oxygen | no | Atypical FB | 100% | 2 | P8 | 5.4 |
| supernatant | 4B |
| supernatant | 4C | 2 mg/mL | 2% oxygen | no | Atypical FB | 100% | 11 | P1 | 11 |
| C | Medium | Figure | Enzyme Concentration | Consistent | Morphology | Purity | Passage 1 (days) | Cell Viability (days/passage) |
| DMEM | 7A | 2 mg/mL Collagenase-IA | no | Typical FB | 100% | 10 | P0 | 10 |
| DMEM/F12 | 7B |
| D | Detachment | Figure | Adherence | Consistent | Morphology | Purity | Passage 1 (days) | Cell Viability (days/passage) |
| 1 min | 5C | 3 h | yes | Low density | Atypical FB | 100% | 13 | P7 | 5 |
| 5D | 24 h | 2 | P4 | 4.3 |
| 3 min | 5E | 3 h | yes | Low density | Atypical FB | 100% | 5 | P8 | 3.6 |
| 5F | 24 h | Typical FB | 2 | P4 | 3.5 |
| 5 min | 5G | 2 h | yes | Typical FB | 100% | 9 | P6 | 6.3 |
| 5H | 3 h | Low density | Atypical FB | 2 | P8 | 4.4 |
| 5I | 24 h | P12 | 5.3 |