Short-Term Cryopreservation Preserved the Function of MSCs from Bone Marrow Aspirate Concentrate
Abstract
Highlights
- MSC proliferation and multilineage differentiation were preserved after freezing BMAC at −80 °C for 4 weeks.
- Both fresh and frozen BMAC equally improved histological cartilage scores compared with PBS control in an OA rat model.
- Frozen BMAC retains functional equivalence to fresh BMAC for cartilage repair.
- A single bone marrow harvest with storage for multiple injections may reduce patient burden and expand clinical utility.
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and BMAC Collection
2.2. Preparation of Frozen BMAC and Plasma
2.3. Mononuclear Cell Isolation from Fresh and Frozen BMAC and Platelets Measurement
2.4. Colony Forming Units-Fibroblast (CFU-f) Assay
2.5. Multiplex Analysis to Detect Soluble Factors in the Plasma from BMAC
2.6. In Vitro Adipogenic Differentiation Assay and Oil Red O Staining
2.7. In Vitro Osteogenic Differentiation Assay and Alizarin Red Staining
2.8. In Vitro 3D Pellet Culture for Chondrogenic Differentiation
2.9. Immunohistochemistry
2.10. q-PCR Analysis
2.11. Surgical Induction of Osteoarthritis and Human BMAC Treatment
2.12. Micro-CT Scanning
2.13. Histology
2.14. Statistical Analysis
3. Results
3.1. Effect of Freezing on Platelets in the BMAC
3.2. Effect of Freezing on CFU-f Formation
3.3. Effect of Freezing on Adipogenic and Osteogenic Differentiation of MSCs and Markers of MSC
3.4. Effects of Freezing on Chondrogenic Differentiation of MSCs
3.5. Effect of Freezing on Soluble Factors in the Plasma from BMAC
3.6. No Significant Difference Between Fresh and Frozen in the AC Regenerative Potential of BMAC
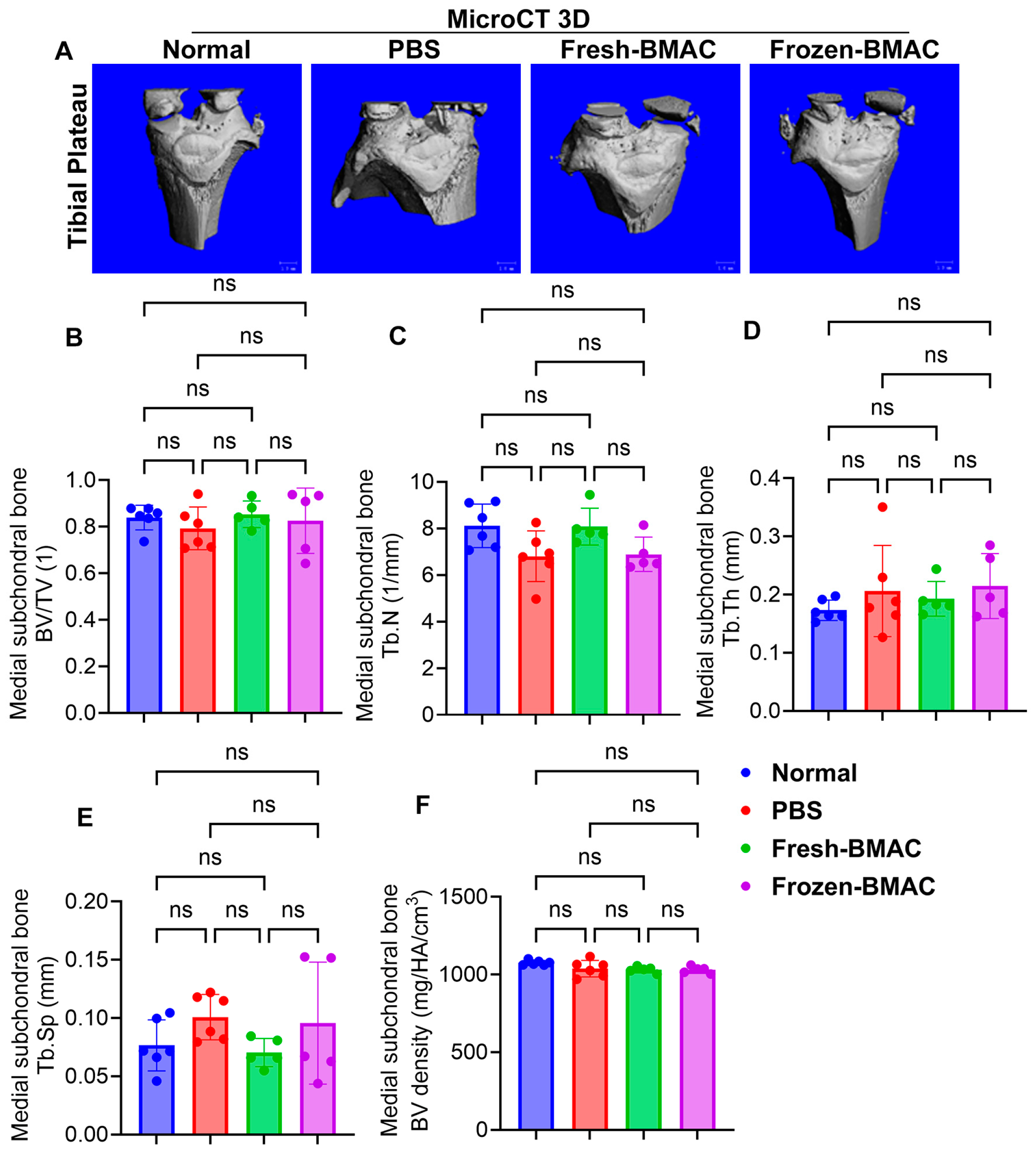
3.7. Neither Fresh nor Frozen BMAC Treatment Significantly Affected Epiphysis and Subchondral Bone
3.8. Both Fresh and Frozen BMAC Treatment Improved the Histology Score of Tibial Plateau Cartilage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yelin, E.; Weinstein, S.; King, T. The burden of musculoskeletal diseases in the United States. Semin. Arthritis Rheum. 2016, 46, 259–260. [Google Scholar] [CrossRef] [PubMed]
- McConaghy, K.; Klika, A.K.; Apte, S.S.; Erdemir, A.; Derwin, K.; Piuzzi, N.S. A Call to Action for Musculoskeletal Research Funding: The Growing Economic and Disease Burden of Musculoskeletal Conditions in the United States Is Not Reflected in Musculoskeletal Research Funding. J. Bone Jt. Surg. Am. 2023, 105, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Guilak, F. Stem cell-based therapies for osteoarthritis: Challenges and opportunities. Curr. Opin. Rheumatol. 2013, 25, 119–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeyaraman, M.; Karthik, K.S.; Choudary, D.; Jeyaraman, N.; Nallakumarasamy, A.; Ramasubramian, S. Autologous Bone Marrow Aspiration Concentrate (BMAC) Therapy for Primary Knee Osteoarthritis-An Observational and Dose Escalation Study. Indian J. Orthop. 2024, 58, 1016–1026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keeling, L.E.; Belk, J.W.; Kraeutler, M.J.; Kallner, A.C.; Lindsay, A.; McCarty, E.C.; Postma, W.F. Bone Marrow Aspirate Concentrate for the Treatment of Knee Osteoarthritis: A Systematic Review. Am. J. Sports Med. 2022, 50, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Themistocleous, G.S.; Chloros, G.D.; Kyrantzoulis, I.M.; Georgokostas, I.A.; Themistocleous, M.S.; Papagelopoulos, P.J.; Savvidou, O.D. Effectiveness of a single intra-articular bone marrow aspirate concentrate (BMAC) injection in patients with grade 3 and 4 knee osteoarthritis. Heliyon 2018, 4, e00871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kon, E.; Boffa, A.; Andriolo, L.; Di Martino, A.; Di Matteo, B.; Magarelli, N.; Marcacci, M.; Onorato, F.; Trenti, N.; Zaffagnini, S.; et al. Subchondral and intra-articular injections of bone marrow concentrate are a safe and effective treatment for knee osteoarthritis: A prospective, multi-center pilot study. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 4232–4240. [Google Scholar] [CrossRef] [PubMed]
- Linch, D.C.; Knott, L.J.; Patterson, K.G.; Cowan, D.A.; Harper, P.G. Bone marrow processing and cryopreservation. J. Clin. Pathol. 1982, 35, 186–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, G.B.; Seo, M.S.; Park, W.T.; Lee, G.W. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lana, J.; da Fonseca, L.F.; Macedo, R.D.R.; Mosaner, T.; Murrell, W.; Kumar, A.; Purita, J.; de Andrade, M.A.P. Platelet-rich plasma vs bone marrow aspirate concentrate: An overview of mechanisms of action and orthobiologic synergistic effects. World J. Stem Cells 2021, 13, 155–167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayala-Cuellar, A.P.; Kang, J.H.; Jeung, E.B.; Choi, K.C. Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol. Ther. 2019, 27, 25–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lana, J.F.; da Fonseca, L.F.; Azzini, G.; Santos, G.; Braga, M.; Cardoso Junior, A.M.; Murrell, W.D.; Gobbi, A.; Purita, J.; Percope de Andrade, M.A. Bone Marrow Aspirate Matrix: A Convenient Ally in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 2762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brozovich, A.; Sinicrope, B.J.; Bauza, G.; Niclot, F.B.; Lintner, D.; Taraballi, F.; McCulloch, P.C. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared With Traditional Bone Marrow Aspiration Technique. Orthop. J. Sports Med. 2021, 9, 23259671211058459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yong, K.W.; Wan Safwani, W.K.; Xu, F.; Wan Abas, W.A.; Choi, J.R.; Pingguan-Murphy, B. Cryopreservation of Human Mesenchymal Stem Cells for Clinical Applications: Current Methods and Challenges. Biopreserv. Biobank 2015, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Kuçi, S.; Schäfer, R. Production and quality testing of multipotent mesenchymal stromal cell therapeutics for clinical use. Transfusion 2019, 59, 2164–2173. [Google Scholar] [CrossRef]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mullen, M.; Nelson, A.L.; Goff, A.; Billings, J.; Kloser, H.; Huard, C.; Mitchell, J.; Hambright, W.S.; Ravuri, S.; Huard, J. Fisetin Attenuates Cellular Senescence Accumulation During Culture Expansion of Human Adipose-Derived Stem Cells. Stem Cells 2023, 41, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Pothiawala, A.; Sahbazoglu, B.E.; Ang, B.K.; Matthias, N.; Pei, G.; Yan, Q.; Davis, B.R.; Huard, J.; Zhao, Z.; Nakayama, N. GDF5+ chondroprogenitors derived from human pluripotent stem cells preferentially form permanent chondrocytes. Development 2022, 149, dev196220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, Z.; Gao, X.; Sun, X.; Amra, S.; Lu, A.; Cui, Y.; Eltzschig, H.K.; Lei, G.; Huard, J. Characterization of articular cartilage homeostasis and the mechanism of superior cartilage regeneration of MRL/MpJ mice. FASEB J. 2019, 33, 8809–8821. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, X.; Fan, Y.; Song, X.; Wu, J.; Fu, Z.; Li, T.; Huang, Y.; Tang, Z.; Meng, S.; et al. Tropoelastin improves adhesion and migration of intra-articular injected infrapatellar fat pad MSCs and reduces osteoarthritis progression. Bioact. Mater. 2022, 10, 443–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Utsunomiya, H.; Gao, X.; Deng, Z.; Cheng, H.; Nakama, G.; Scibetta, A.C.; Ravuri, S.K.; Goldman, J.L.; Lowe, W.R.; Rodkey, W.G.; et al. Biologically Regulated Marrow Stimulation by Blocking TGF-beta1 With Losartan Oral Administration Results in Hyaline-like Cartilage Repair: A Rabbit Osteochondral Defect Model. Am. J. Sports Med. 2020, 48, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Gao, X.; Cheng, H.; Deng, Z.; Nakama, G.; Mascarenhas, R.; Goldman, J.L.; Ravuri, S.K.; Arner, J.W.; Ruzbarsky, J.J.; et al. Intra-articular Injection of Bevacizumab Enhances Bone Marrow Stimulation-Mediated Cartilage Repair in a Rabbit Osteochondral Defect Model. Am. J. Sports Med. 2021, 49, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Usas, A.; Lu, A.; Tang, Y.; Wang, B.; Chen, C.W.; Li, H.; Tebbets, J.C.; Cummins, J.H.; Huard, J. BMP2 is superior to BMP4 for promoting human muscle-derived stem cell-mediated bone regeneration in a critical-sized calvarial defect model. Cell Transplant. 2013, 22, 2393–2408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, X.; Usas, A.; Lu, A.; Kozemchak, A.; Tang, Y.; Poddar, M.; Sun, X.; Cummins, J.H.; Huard, J. Cyclooxygenase-2 deficiency impairs muscle-derived stem cell-mediated bone regeneration via cellular autonomous and non-autonomous mechanisms. Hum. Mol. Genet. 2016, 25, 3216–3231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, X.; Cheng, H.; Awada, H.; Tang, Y.; Amra, S.; Lu, A.; Sun, X.; Lv, G.; Huard, C.; Wang, B.; et al. A comparison of BMP2 delivery by coacervate and gene therapy for promoting human muscle-derived stem cell-mediated articular cartilage repair. Stem Cell Res. Ther. 2019, 10, 346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cordeiro-Spinetti, E.; de Mello, W.; Trindade, L.S.; Taub, D.D.; Taichman, R.S.; Balduino, A. Human bone marrow mesenchymal progenitors: Perspectives on an optimized in vitro manipulation. Front. Cell Dev. Biol. 2014, 2, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berger, D.R.; Aune, E.T.; Centeno, C.J.; Steinmetz, N.J. Cryopreserved bone marrow aspirate concentrate as a cell source for the colony-forming unit fibroblast assay. Cytotherapy 2020, 22, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Wan Safwani, W.K.; Makpol, S.; Sathapan, S.; Chua, K.H. The changes of stemness biomarkers expression in human adipose-derived stem cells during long-term manipulation. Biotechnol. Appl. Biochem. 2011, 58, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, D.; Koh, H.S.; Choi, Y.H.; Park, I. Bone Marrow Aspirate Concentrate (BMAC) for Knee Osteoarthritis: A Narrative Review of Clinical Efficacy and Future Directions. Medicina 2025, 61, 853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holton, J.; Imam, M.; Ward, J.; Snow, M. The Basic Science of Bone Marrow Aspirate Concentrate in Chondral Injuries. Orthop. Rev. 2016, 8, 6659. [Google Scholar] [CrossRef]
- Milaras, C.; Lepetsos, P.; Dafou, D.; Potoupnis, M.; Tsiridis, E. Association of Matrix Metalloproteinase (MMP) Gene Polymorphisms With Knee Osteoarthritis: A Review of the Literature. Cureus 2021, 13, e18607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuroda, Y.; Kitada, M.; Wakao, S.; Dezawa, M. Bone marrow mesenchymal cells: How do they contribute to tissue repair and are they really stem cells? Arch. Immunol. Ther. Exp. 2011, 59, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Das, B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slovacek, H.; Khanna, R.; Poredos, P.; Poredos, P.; Jezovnik, M.; Hoppensteadt, D.; Fareed, J.; Hopkinson, W. Interrelationship of MMP-9, Proteoglycan-4, and Inflammation in Osteoarthritis Patients Undergoing Total Hip Arthroplasty. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029621995569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Wang, H.; Zhang, Y.; Qiao, R.; Xia, P.; Kong, Z.; Zhao, H.; Yin, L. COL3A1 and MMP9 Serve as Potential Diagnostic Biomarkers of Osteoarthritis and Are Associated With Immune Cell Infiltration. Front. Genet. 2021, 12, 721258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farag, A.; Ngeun, S.K.; Kaneda, M.; Aboubakr, M.; Elhaieg, A.; Hendawy, H.; Tanaka, R. Exploring the Potential Effects of Cryopreservation on the Biological Characteristics and Cardiomyogenic Differentiation of Rat Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 9908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ginis, I.; Grinblat, B.; Shirvan, M.H. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng. Part. C Methods 2012, 18, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Verdanova, M.; Pytlik, R.; Kalbacova, M.H. Evaluation of sericin as a fetal bovine serum-replacing cryoprotectant during freezing of human mesenchymal stromal cells and human osteoblast-like cells. Biopreserv. Biobank 2014, 12, 99–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muthu, S.; Jeyaraman, M.; Narula, A.; Ravi, V.R.; Gandi, A.; Khanna, M.; Maffulli, N.; Gupta, A. Factors Influencing the Yield of Progenitor Cells in Bone Marrow Aspiration Concentrate-A Retrospective Analysis of 58 Patients. Biomedicines 2023, 11, 738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gobbi, A.; Whyte, G.P. One-Stage Cartilage Repair Using a Hyaluronic Acid-Based Scaffold With Activated Bone Marrow-Derived Mesenchymal Stem Cells Compared With Microfracture: Five-Year Follow-up. Am. J. Sports Med. 2016, 44, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.D.; Kucharik, M.P.; Abraham, P.F.; Nazal, M.R.; Meek, W.M.; Varady, N.H. Functional Outcomes of Arthroscopic Acetabular Labral Repair with and without Bone Marrow Aspirate Concentrate. J. Bone Jt. Surg. Am. 2022, 104, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Anz, A.W.; Plummer, H.A.; Cohen, A.; Everts, P.A.; Andrews, J.R.; Hackel, J.G. Bone Marrow Aspirate Concentrate Is Equivalent to Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis at 2 Years: A Prospective Randomized Trial. Am. J. Sports Med. 2022, 50, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Lim, J.J.; Keeter, C.; McCulloch, P.C.; Houck, D.A.; McCarty, E.C.; Frank, R.M.; Kraeutler, M.J. Patients With Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-analysis. Arthroscopy 2023, 39, 1714–1734. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Zwingenberger, S. Concentrated autologous bone marrow aspirate is not “stem cell” therapy in the repair of nonunions and bone defects. Biomater. Biosyst. 2021, 2, 100017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruan, G.; Xu, J.; Wang, K.; Zheng, S.; Wu, J.; Bian, F.; Chang, B.; Zhang, Y.; Meng, T.; Zhu, Z.; et al. Associations between serum IL-8 and knee symptoms, joint structures, and cartilage or bone biomarkers in patients with knee osteoarthritis. Clin. Rheumatol. 2019, 38, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manrique, M.; Calvet, J.; Orellana, C.; Berenguer-Llergo, A.; Garcia-Cirera, S.; Llop, M.; Albinana-Gimenez, N.; Galisteo-Lencastre, C.; Gratacos, J. Synovial fluid but not plasma interleukin-8 is associated with clinical severity and inflammatory markers in knee osteoarthritis women with joint effusion. Sci. Rep. 2021, 11, 5258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, J.M.; Sun, L.Z.; Liu, J.; Su, B.H.; Shi, L. Serum interleukin-15 levels are associated with severity of pain in patients with knee osteoarthritis. Dis. Markers 2013, 35, 203–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warner, S.C.; Nair, A.; Marpadga, R.; Chubinskaya, S.; Doherty, M.; Valdes, A.M.; Scanzello, C.R. IL-15 and IL15RA in Osteoarthritis: Association With Symptoms and Protease Production, but Not Structural Severity. Front. Immunol. 2020, 11, 1385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez, A.G.; Lana, J.F.; Rodrigues, A.A.; Luzo, A.C.; Belangero, W.D.; Santana, M.H. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014, 2014, 176060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waters, L.; Marks, D.C.; Johnson, L. Strategies to improve platelet cryopreservation: A narrative review. Transfusion 2025, 65, 740–749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Thirumala, S.; Goebel, W.S.; Woods, E.J. Clinical grade adult stem cell banking. Organogenesis 2009, 5, 143–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuschong, L.; Soenen, S.L.; Blaese, R.M.; Candotti, F.; Muul, L.M. Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum. Gene Ther. 2002, 13, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, A.; Poggi, G.; Da Prada, G.; Pedrazzoli, P.; Cuomo, A.; Miotti, D.; Perotti, C.; Preti, P.; Robustelli della Cuna, G. Clinical toxicity of cryopreserved circulating progenitor cells infusion. Anticancer Res. 1998, 18, 4705–4708. [Google Scholar] [PubMed]





| Gene Name | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| Gapdh | Forward: GTATCGGACGCCTGGTTACC Reverse: ACCAGCTTCCCATTCTCAGC | 166 |
| CD90 | Forward: CAGCAGTTCACCCATCCAGT Reverse: GATGCCCTCACACTTGACCA | 271 |
| Sox2 | Forward: GCTACAGCATGATGCAGGACCA Reverse: TCTGCGAGCTGGTCATGGAGTT | 134 |
| OCT4 | Forward: GCAAAGCAGAAACCCTCGTG Reverse: AACCACACTCGGACCACATC | 172 |
| MMP9 | Forward: ACAGCGAGACACTAAAGGCC Reverse: GGCAAGTCTTCGGTGTAGCT | 139 |
| MMP13 | Forward: TCCATCCCGAGACCTCATGT Reverse: CACACGTGGTTCCCTGAGAA | 189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, J.; Nishimura, H.; Xiao, Z.; Gao, X.; Knezic, N.; Chubb, L.; Layne, J.E.; Guo, P.; Lu, A.; Huard, J. Short-Term Cryopreservation Preserved the Function of MSCs from Bone Marrow Aspirate Concentrate. Cells 2025, 14, 1569. https://doi.org/10.3390/cells14191569
Singer J, Nishimura H, Xiao Z, Gao X, Knezic N, Chubb L, Layne JE, Guo P, Lu A, Huard J. Short-Term Cryopreservation Preserved the Function of MSCs from Bone Marrow Aspirate Concentrate. Cells. 2025; 14(19):1569. https://doi.org/10.3390/cells14191569
Chicago/Turabian StyleSinger, Jacob, Haruki Nishimura, Zuokui Xiao, Xueqin Gao, Noah Knezic, Laura Chubb, Jonathan E. Layne, Ping Guo, Aiping Lu, and Johnny Huard. 2025. "Short-Term Cryopreservation Preserved the Function of MSCs from Bone Marrow Aspirate Concentrate" Cells 14, no. 19: 1569. https://doi.org/10.3390/cells14191569
APA StyleSinger, J., Nishimura, H., Xiao, Z., Gao, X., Knezic, N., Chubb, L., Layne, J. E., Guo, P., Lu, A., & Huard, J. (2025). Short-Term Cryopreservation Preserved the Function of MSCs from Bone Marrow Aspirate Concentrate. Cells, 14(19), 1569. https://doi.org/10.3390/cells14191569








